Transcription Factor Inhibition as a Potential Additional Mechanism of Action of Pyrrolobenzodiazepine (PBD) Dimers
Abstract
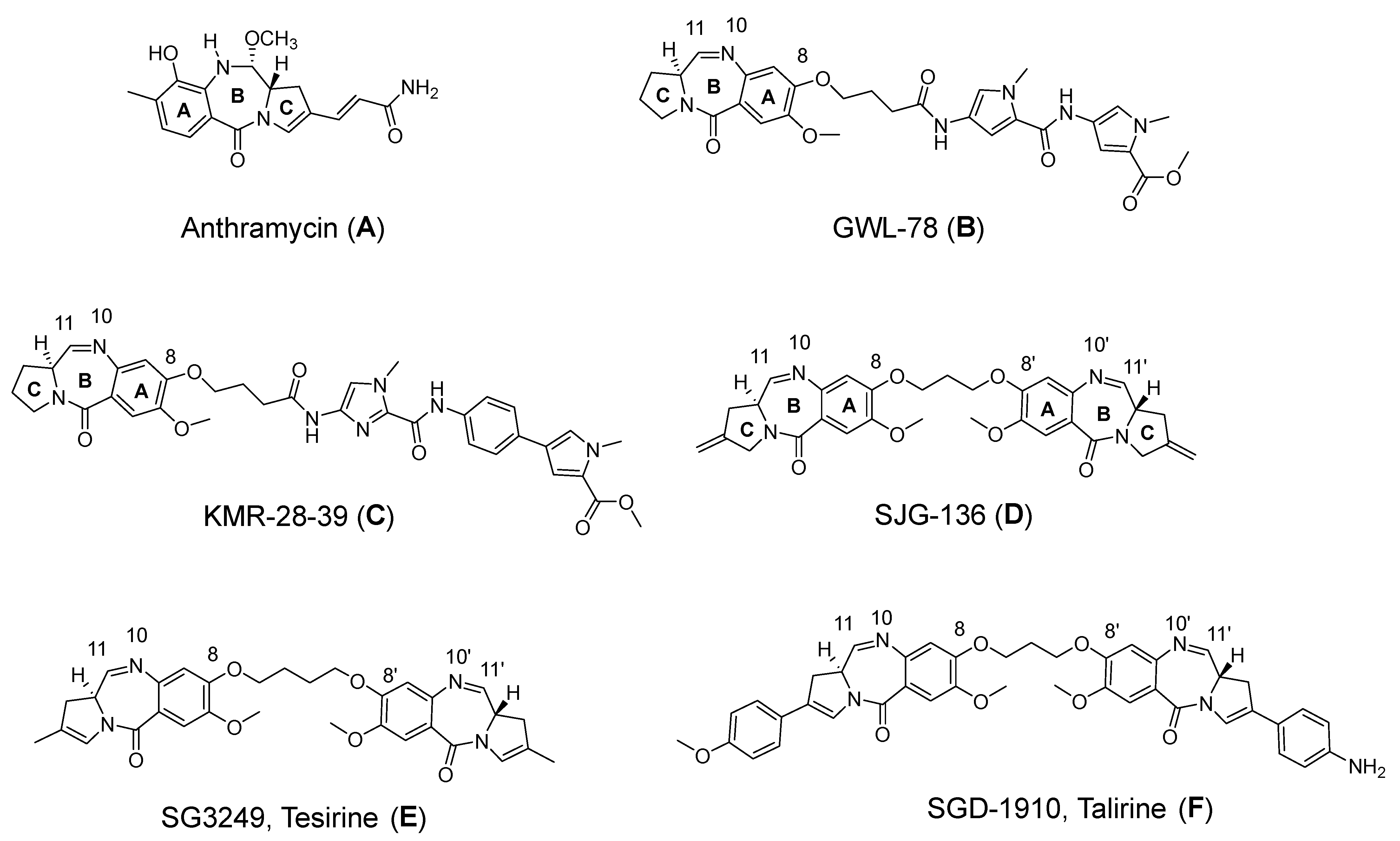
1. Introduction
2. Materials and Methods
2.1. Working Solutions of Oligonucleotides
2.2. Working Solutions of PBDs (GWL-78, KMR-28-39 and SJG-136)
2.3. Preparation of PBD/DNA Complexes
2.4. Ion-Pair Reversed-Phase HPLC
2.5. Matrix-Assisted Laser Desorption/Ionisation Time of Flight (MALDI-TOF) Analysis
2.6. Circular Dichroism (CD) Study
2.7. Molecular Modelling
2.8. Cell Culture
2.9. Drug Treatment
2.10. RNA Extraction and RT-PCR
2.11. Polymerase Chain Reaction (PCR)
2.12. Quantitative Polymerase Chain Reaction (qPCR)
2.13. Statistical Analysis
3. Results
3.1. HPLC/MS Study
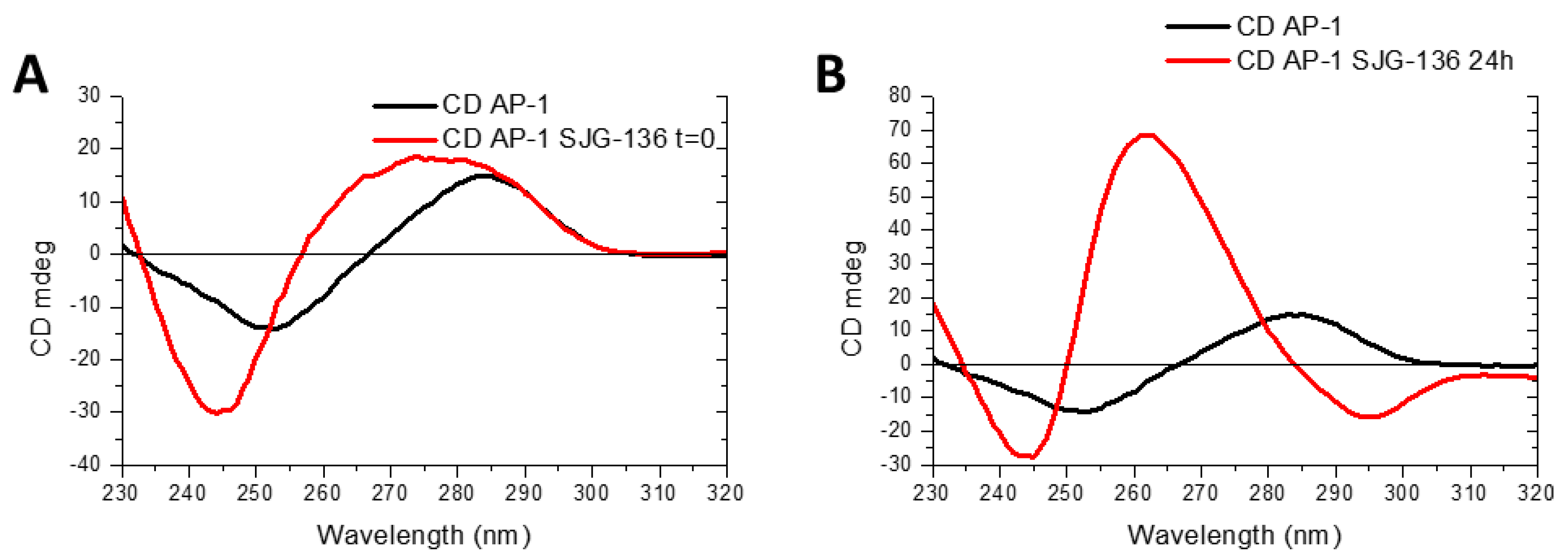
3.2. CD Study
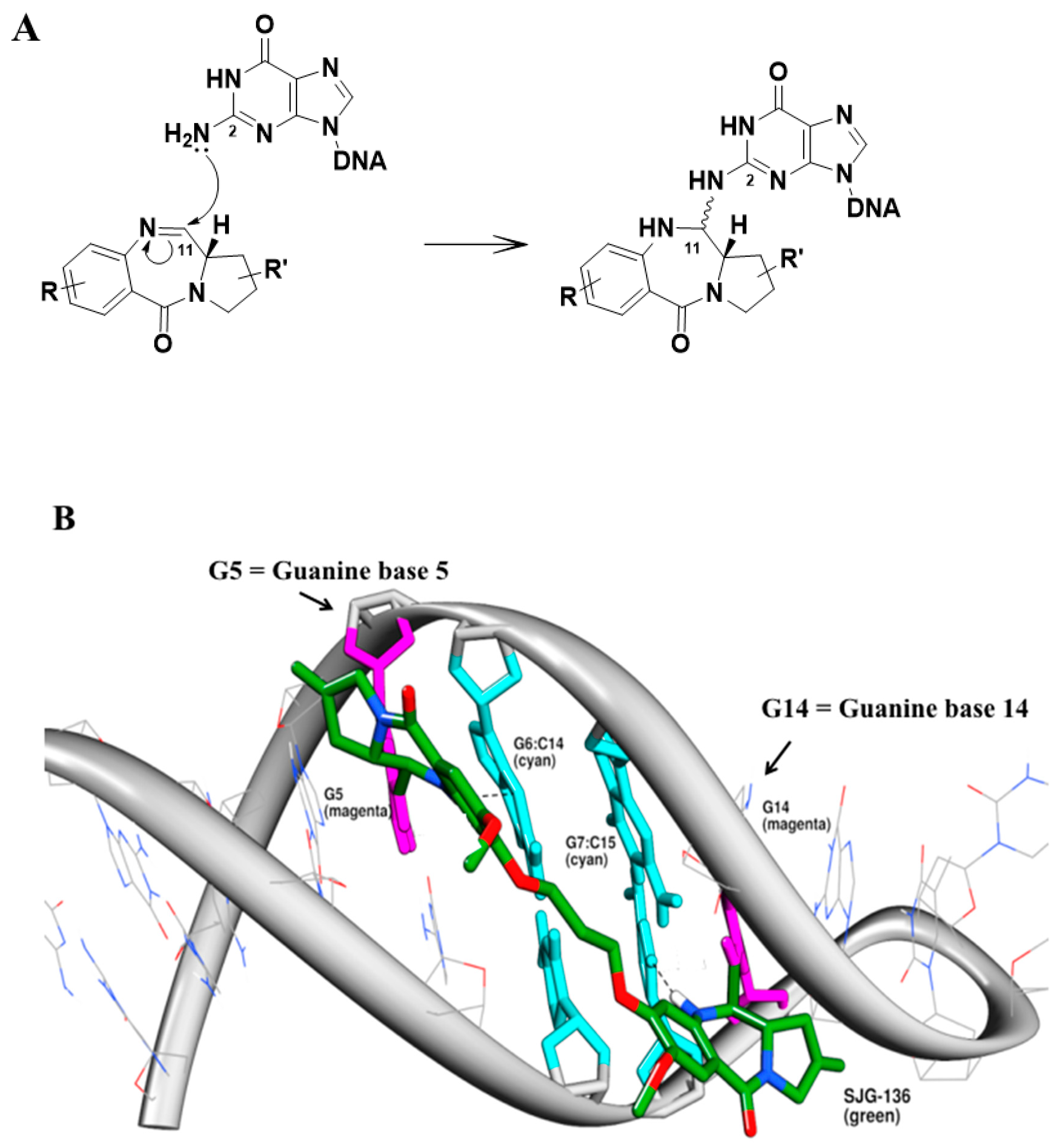
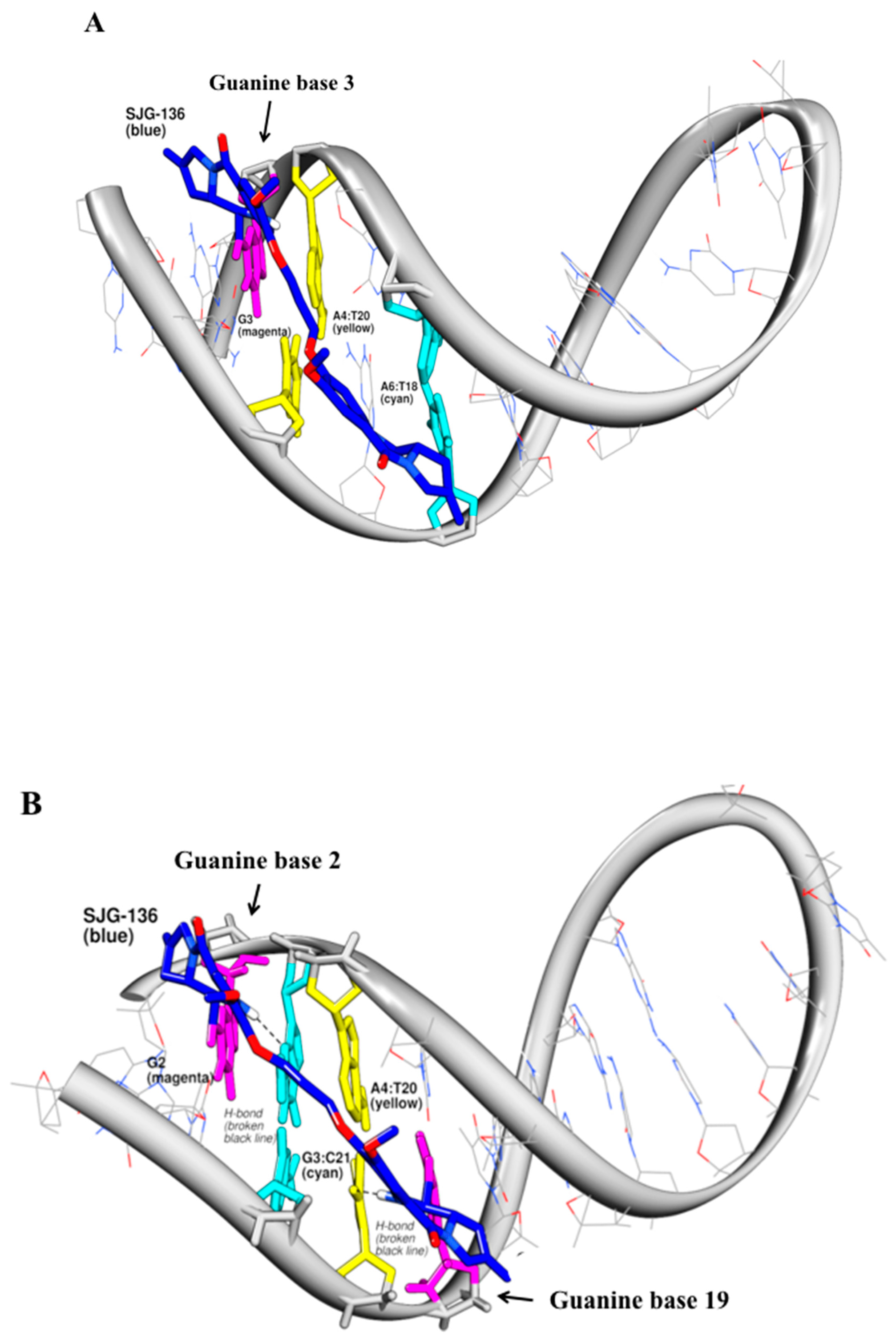
3.3. Molecular Modelling Study
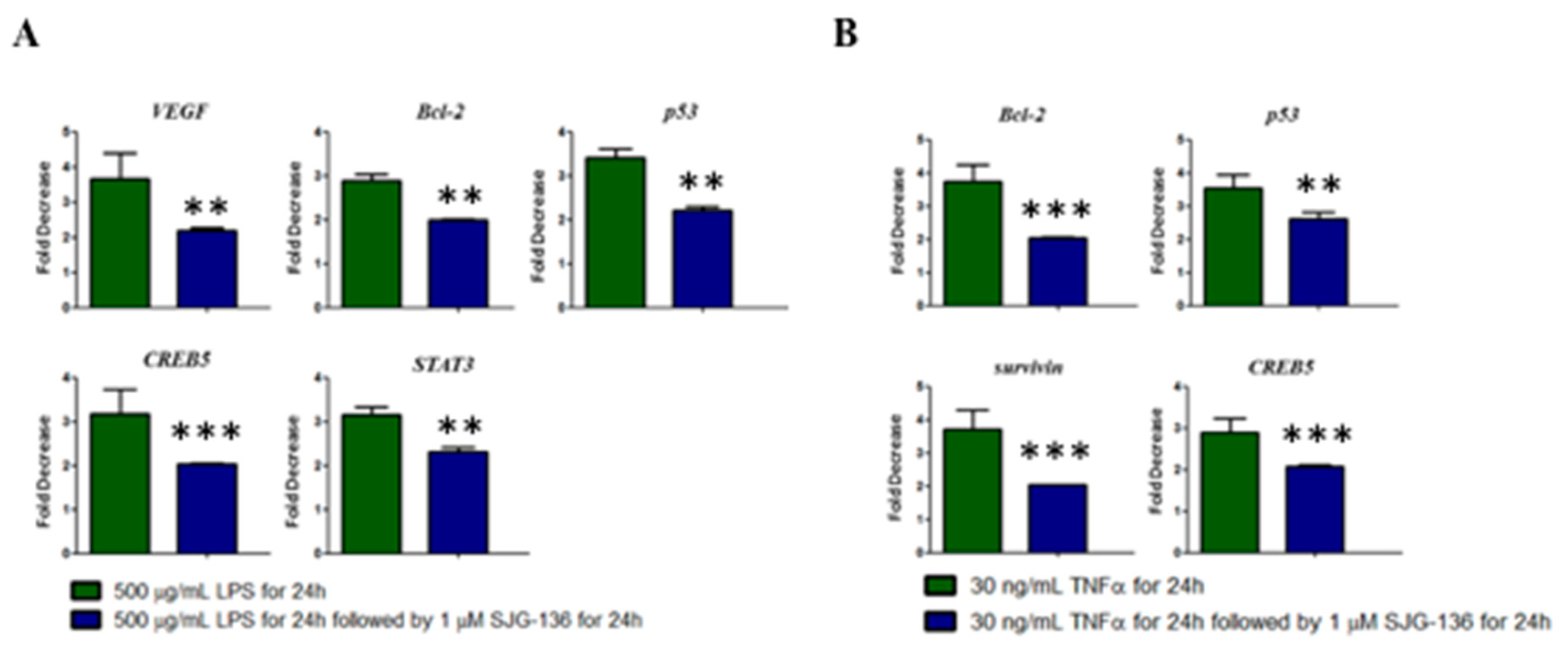
3.4. Effect of SJG-136 on TF-Dependent Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Latchman, D.S. Transcription factors: An overview. Int. J. Biochem. Cell Biol. 1997, 29, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. Too many transcription factors: Positive and negative interactions. New Biol. 1990, 2, 126–131. [Google Scholar] [PubMed]
- Ptashne, M.; Gann, A. Transcriptional activation by recruitment. Nature 1997, 386, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.J.; Tjian, R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science 1989, 245, 371–378. [Google Scholar] [CrossRef]
- Babu, M.M.; Luscombe, N.M.; Aravind, L.; Gerstein, M.; Teichmann, S.A. Structure and evolution of transcriptional regulatory networks. Curr. Opin. Struct. Biol. 2004, 14, 283–291. [Google Scholar] [CrossRef]
- Gill, G. Regulation of the initiation of eukaryotic transcription. Essays Biochem. 2001, 37, 33–43. [Google Scholar]
- Boyadjiev, S.A.; Jabs, E.W. Online Mendelian Inheritance in Man (OMIM) as a knowledgebase for human developmental disorders. Clin. Genet. 2000, 57, 253–266. [Google Scholar] [CrossRef]
- Dervan, P.B. Molecular recognition of DNA by small molecules. Bioorg. Med. Chem. 2001, 9, 2215–2235. [Google Scholar] [CrossRef]
- Neidle, S. DNA minor-groove recognition by small molecules. Nat. Prod. Rep. 2001, 18, 291–309. [Google Scholar] [CrossRef]
- Smellie, M.; Bose, D.S.; Thompson, A.S.; Jenkins, T.C.; Hartley, J.A.; Thurston, D.E. Sequence-selective recognition of duplex DNA through covalent interstrand cross-linking: Kinetic and molecular modeling studies with pyrrolobenzodiazepine dimers. Biochemistry 2003, 42, 8232–8239. [Google Scholar] [CrossRef]
- Leimgruber, W.; Stefanovic, V.; Schenker, F.; Karr, A.; Berger, J. Isolation and characterization of anthramycin, a new antitumor antibiotic. J. Am. Chem. Soc. 1965, 87, 5791–5793. [Google Scholar] [CrossRef] [PubMed]
- Antonow, D.; Thurston, D.E. Synthesis of DNA-interactive pyrrolo[2,1-c][1,4]benzodiazepines (PBDs). Chem. Rev. 2011, 111, 2815–2864. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Martin, C.R.H.; Howard, P.W.; Sands, Z.A.; Laughton, C.A.; Tiberghien, A.; Woo, C.K.; Masterson, L.A.; Stephenson, M.J.; Hartley, J.A.; et al. Design, synthesis, and biophysical and biological evaluation of a series of pyrrolobenzodiazepine-poly(N-methylpyrrole) conjugates. J. Med. Chem. 2006, 49, 5442–5461. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.M.; Jackson, P.J.M.; James, C.H.; Basu, B.P.; Hartley, J.A.; Schatzlein, A.; Robson, M.; Pedley, R.B.; Pepper, C.; Fox, K.R.; et al. GC-targeted C8-linked pyrrolobenzodiazepine-biaryl conjugates with femtomolar in vitro cytotoxicity and in vivo antitumor activity in mouse models. J. Med. Chem. 2013, 56, 2911–2935. [Google Scholar] [CrossRef] [PubMed]
- Gregson, S.J.; Howard, P.W.; Hartley, J.A.; Brooks, N.A.; Adams, L.J.; Jenkins, T.C.; Kelland, L.R.; Thurston, D.E. Design, synthesis, and evaluation of a novel pyrrolobenzodiazepine DNA-interactive agent with highly efficient cross-linking ability and potent cytotoxicity. J. Med. Chem. 2001, 44, 737–748. [Google Scholar] [CrossRef]
- Rahman, K.M.; Thompson, A.S.; James, C.H.; Narayanaswamy, M.; Thurston, D.E. The pyrrolobenzodiazepine dimer SJG-136 forms sequence-dependent intrastrand DNA cross-links and monoalkylated adducts in addition to interstrand cross-links. J. Am. Chem. Soc. 2009, 131, 13756–13766. [Google Scholar] [CrossRef]
- Gregson, S.J.; Howard, P.W.; Gullick, D.R.; Hamaguchi, A.; Corcoran, K.E.; Brooks, N.A.; Hartley, J.A.; Jenkins, T.C.; Patel, S.; Guille, M.J.; et al. Linker length modulates DNA cross-linking reactivity and cytotoxic potency of C8/C8′ ether-linked C2-exo-unsaturated pyrrolo[2,1-c][1,4]benzodiazepine (PBD) dimers. J. Med. Chem. 2004, 47, 1161–1174. [Google Scholar] [CrossRef]
- Bose, D.S.; Thompson, A.S.; Ching, J.S.; Hartley, J.A.; Berardini, M.D.; Jenkins, T.C.; Neidle, S.; Hurley, L.H.; Thurston, D.E. Rational Design of a Highly Efficient Irreversible DNA Interstrand Cross-Linking Agent Based on the Pyrrolobenzodiazepine Ring-System. J. Am. Chem. Soc. 1992, 114, 4939–4941. [Google Scholar] [CrossRef]
- Hartley, J.A.; Spanswick, V.J.; Brooks, N.; Waud, W.R.; Hartley, J.A.; Howard, P.W.; Gregson, S.J.; Thurston, D.E.; Sausville, E.A. SJG-136 (NSC 694501), a novel rationally designed DNA minor groove interstrand cross-linking agent with potent and broad spectrum antitumor activity: Part 1: Cellular pharmacology, in vitro and initial in vivo antitumor activity. Cancer Res. 2004, 64, 6693–6699. [Google Scholar] [CrossRef]
- Rahman, K.M.; Rosado, H.; Moreira, J.B.; Feuerbaum, E.-A.; Fox, K.R.; Stecher, E.; Howard, P.W.; Gregson, S.J.; James, C.H.; de la Fuente, M.; et al. Antistaphylococcal activity of DNA-interactive pyrrolobenzodiazepine (PBD) dimers and PBD-biaryl conjugates. J. Antimicrob. Chemother. 2012, 67, 1683–1696. [Google Scholar] [CrossRef]
- Andriollo, P.; Hind, C.K.; Picconi, P.; Nahar, K.S.; Jamshidi, S.; Varsha, A.; Clifford, M.; Sutton, J.M.; Rahman, K.M. C8-linked pyrrolobenzodiazepine monomers with inverted building blocks show selective activity against multidrug resistant Gram-positive bacteria. ACS Infect. Dis. 2018, 4, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Picconi, P.; Hind, C.K.; Nahar, K.S.; Jamshidi, S.; Di Maggio, L.; Saeed, N.; Evans, B.; Solomons, J.; Wand, M.E.; Sutton, J.M.; et al. New Broad-Spectrum Antibiotics Containing a Pyrrolobenzodiazepine Ring with Activity against Multidrug-Resistant Gram-Negative Bacteria. J. Med. Chem. 2020, 63, 6941–6958. [Google Scholar] [CrossRef] [PubMed]
- Kung Sutherland, M.S.; Walter, R.B.; Jeffrey, S.C.; Burke, P.J.; Yu, C.; Kostner, H.; Stone, I.; Ryan, M.C.; Sussman, D.; Lyon, R.P.; et al. SGN-CD33A: A novel CD33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood 2013, 122, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.M.; Buhrow, S.A.; Kuffel, M.J.; Jia, L.; Spanswick, V.J.; Hartley, J.A.; Thurston, D.E.; Tomaszewski, J.E.; Ames, M.M. Pharmacokinetics, pharmacodynamics and metabolism of the dimeric pyrrolobenzodiazepine SJG-136 in rats. Cancer Chemother. Pharmacol. 2011, 68, 777–786. [Google Scholar] [CrossRef]
- Puvvada, M.S.; Hartley, J.A.; Jenkins, T.C.; Thurston, D.E. A quantitative assay to measure the relative DNA-binding affinity of pyrrolo[2,1-c] [1,4]benzodiazepine (PBD) antitumour antibiotics based on the inhibition of restriction endonuclease BamHI. Nucleic Acids Res. 1993, 21, 3671–3675. [Google Scholar] [CrossRef][Green Version]
- Shameem, M.; Kumar, R.; Krishna, S.; Kumar, C.; Siddiqi, M.I.; Kundu, B.; Banerjee, D. Synthetic modified pyrrolo[1,4] benzodiazepine molecules demonstrate anticancer activity by targeting the human ligase 1 enzyme: An in silico and in vitro mechanistic study. Chem. Biol. Interact. 2015, 237, 115–124. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Cacciari, B.; Guiotto, A.; Romagnoli, R.; Spalluto, G.; Leoni, A.; Bianchi, N.; Feriotto, G.; Rutigliano, C.; Mischiati, C.; et al. [2,1-c][1,4]benzodiazepine (PBD)-distamycin hybrid inhibits DNA binding to transcription factor Sp1. Nucleosides Nucleotides Nucleic Acids 2000, 19, 1219–1229. [Google Scholar] [CrossRef]
- Kotecha, M.; Kluza, J.; Wells, G.; Caroline, O.C.; Forni, C.; Mantovani, R.; Howard, P.W.; Morris, P.; Thurston, D.E.; Hartley, J.A.; et al. Inhibition of DNA binding of the NF-Y transcription factor by the pyrrolobenzodiazepine-polyamide conjugate GWL-78. Mol. Cancer Ther. 2008, 7, 1319–1328. [Google Scholar] [CrossRef]
- Brucoli, F.; Hawkins, R.M.; James, C.H.; Wells, G.; Jenkins, T.C.; Ellis, T.; Hartley, J.A.; Howard, P.W.; David, E. ThurstonNovel C8-linked pyrrolobenzodiazepine (PBD)-heterocycle conjugates that recognize DNA sequences containing an inverted CCAAT box. Bioorg. Med. Chem. Lett. 2011, 21, 3780–3783. [Google Scholar] [CrossRef]
- Chou, Y.W.; Senadi, G.C.; Chen, C.Y.; Kuo, K.K.; Lin, Y.T.; Wang, J.J.; Lee, J.H.; Wang, Y.C.; Hu, W.P. Design and synthesis of pyrrolobenzodiazepine-gallic hybrid agents as p53-dependent and -independent apoptogenic signaling in melanoma cells. Eur. J. Med. Chem. 2016, 109, 59–74. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Hu, W.P.; Yu, H.S.; Wu, W.C.; Chang, L.S.; Kao, Y.H.; Wang, J.J. A DC-81-indole conjugate agent suppresses melanoma A375 cell migration partially via interrupting VEGF production and stromal cell-derived factor-1alpha-mediated signaling. Toxicol. Appl. Pharmacol. 2011, 255, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Thurston, D.E.; Pysz, I. Chemistry and Pharmacology of Anticancer Drugs, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Agarwal, S.; Fang, L.; McGowen, K.; Yin, J.; Bowman, J.; Ku, A.T.; Alilin, A.N.; Corey, E.; Roudier, M.P.; True, L.D.; et al. Tumor-derived biomarkers predict efficacy of B7H3 antibody-drug conjugate treatment in metastatic prostate cancer models. J. Clin. Investig. 2023, 133, e162148. [Google Scholar] [CrossRef] [PubMed]
- Zou, N.; Han, A. Application of Pyrrolobenzodiazepines in Antibody Drug Conjugates. In Contemporary Accounts in Drug Discovery and Development; Willy: Hoboken, NJ, USA, 2022; pp. 293–339. [Google Scholar]
- Lewis, G.; Li, G.; Guo, J.; Yu, S.-F.; Fields, C.; Lee, G.; Zhang, D.; Dragovich, P.; Pillow, T.; Wei, B.; et al. The HER2-directed antibody-drug conjugate DHES0815A in advanced and/or metastatic breast cancer: Preclinical characterization and phase 1 trial results. Nat. Commun. 2024, 15, 466. [Google Scholar]
- Hartley, J.A. Antibody-drug conjugates (ADCs) delivering pyrrolobenzodiazepine (PBD) dimers for cancer therapy. Opin. Biol. Ther. 2021, 21, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Tad, D.A.C.; Cheatham, T.E., III; Simmerling, C.L.; Wang, J.; Duke, R.E.; Luo, R.; Walker, R.C.; Zhang, W.; Merz, K.M.; Roberts, B.; et al. AMBER 12; University of California: San Francisco, CA, USA, 2012. [Google Scholar]
- Perez, A.; Marchan, I.; Svozil, D.; Sponer, J.; Cheatham, T.E.; Laughton, C.A.; Orozco, M. Refinement of the AMBER force field for nucleic acids: Improving the description of alpha/gamma conformers. Biophys. J. 2007, 92, 3817–3829. [Google Scholar] [CrossRef]
- Rao, S.N.; Singh, U.C.; Kollman, P.A. Molecular mechanics simulations on covalent complexes between anthramycin and B DNA. J. Med. Chem. 1986, 29, 2484–2492. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Sharif, O.; Bolshakov, V.N.; Raines, S.; Newham, P.; Perkins, N.D. Transcriptional profiling of the LPS induced NF-κB response in macrophages. BMC Immunol. 2007, 8, 1. [Google Scholar] [CrossRef]
- Chen, M.-C.; Chang, W.-W.; Kuan, Y.-D.; Lin, S.-T.; Hsu, H.-C.; Lee, C.-H. Resveratrol inhibits LPS-induced epithelial-mesenchymal transition in mouse melanoma model. Innate Immun. 2012, 18, 685–693. [Google Scholar] [CrossRef]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef]
- Rahman, K.M.; Mussa, V.; Narayanaswamy, M.; James, C.H.; Howard, P.W.; Thurston, D.E. Observation of a dynamic equilibrium between DNA hairpin and duplex forms of covalent adducts of a minor groove binding agent. Chem. Commun. 2009, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Alley, M.C.; Hollingshead, M.G.; Pacula-Cox, C.M.; Hartley, J.A.; Spanswick, V.J.; Brooks, N.; Clingen, P.H.; McHugh, P.J.; Hochhauser, D.; Pedley, R.B.; et al. SJG-136 (NSC 694501), a novel rationally designed DNA minor groove interstrand cross-linking agent with potent and broad spectrum antitumor activity: Part 2: Efficacy evaluations. Cancer Res. 2004, 18, 6700–6706. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.M.; Chen, C.K.; Hou, M.H. Conformational changes in DNA upon ligand binding monitored by circular dichroism. Int. J. Mol. Sci. 2012, 13, 3394–3413. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, M.; Kukreti, R.; Grover, D.; Brahmachari, S.K.; Kukreti, S. Hairpin-duplex equilibrium reflected in the A-->B transition in an undecamer quasi-palindrome present in the locus control region of the human beta-globin gene cluster. Nucleic Acids Res. 2003, 31, 6904–6915. [Google Scholar] [CrossRef]
- Kypr, J.; Kejnovska, I.; Renciuk, D.; Vorlickova, M. Circular dichroism and conformational polymorphism of DNA. Nucleic Acids Res. 2009, 37, 1713–1725. [Google Scholar] [CrossRef]
- Fornasiero, D.; Kurucsev, T. Circular-Dichroism Spectra and the Interaction between Acridine-Dyes and Deoxyribonucleic-Acid. J. Phys. Chem. 1981, 85, 613–618. [Google Scholar] [CrossRef]
- Chun, J.; Li, R.-J.; Cheng, M.-S.; Kim, Y.S. Alantolactone selectively suppresses STAT3 activation and exhibits potent anticancer activity in MDA-MB-231 cells. Cancer Lett. 2015, 357, 393–403. [Google Scholar] [CrossRef]
- McLoughlin, P.; Roengvoraphoj, M.; Gissel, C.; Hescheler, J.; Certa, U.; Sachinidis, A. Transcriptional responses to epigallocatechin-3 gallate in HT 29 colon carcinoma spheroids. Genes Cells 2004, 9, 661–669. [Google Scholar] [CrossRef]
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef]
- Narayanaswamy, M.; Griffiths, W.J.; Howard, P.W.; Thurston, D.E. An assay combining high-performance liquid chromatography and mass spectrometry to measure DNA interstrand cross-linking efficiency in oligonucleotides of varying sequences. Anal. Biochem. 2008, 374, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.M.; James, C.H.; Thurston, D.E. Effect of base sequence on the DNA cross-linking properties of pyrrolobenzodiazepine (PBD) dimers. Nucleic Acids Res. 2011, 39, 5800–5812. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.M.; James, C.H.; Bui, T.T.; Drake, A.F.; Thurston, D.E. Observation of a single-stranded DNA/pyrrolobenzodiazepine adduct. J. Am. Chem. Soc. 2011, 133, 19376–19385. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.M.; James, C.H.; Thurston, D.E. Observation of the reversibility of a covalent pyrrolobenzodiazepine (PBD) DNA adduct by HPLC/MS and CD spectroscopy. Org. Biomol. Chem. 2011, 9, 1632–1641. [Google Scholar] [CrossRef]
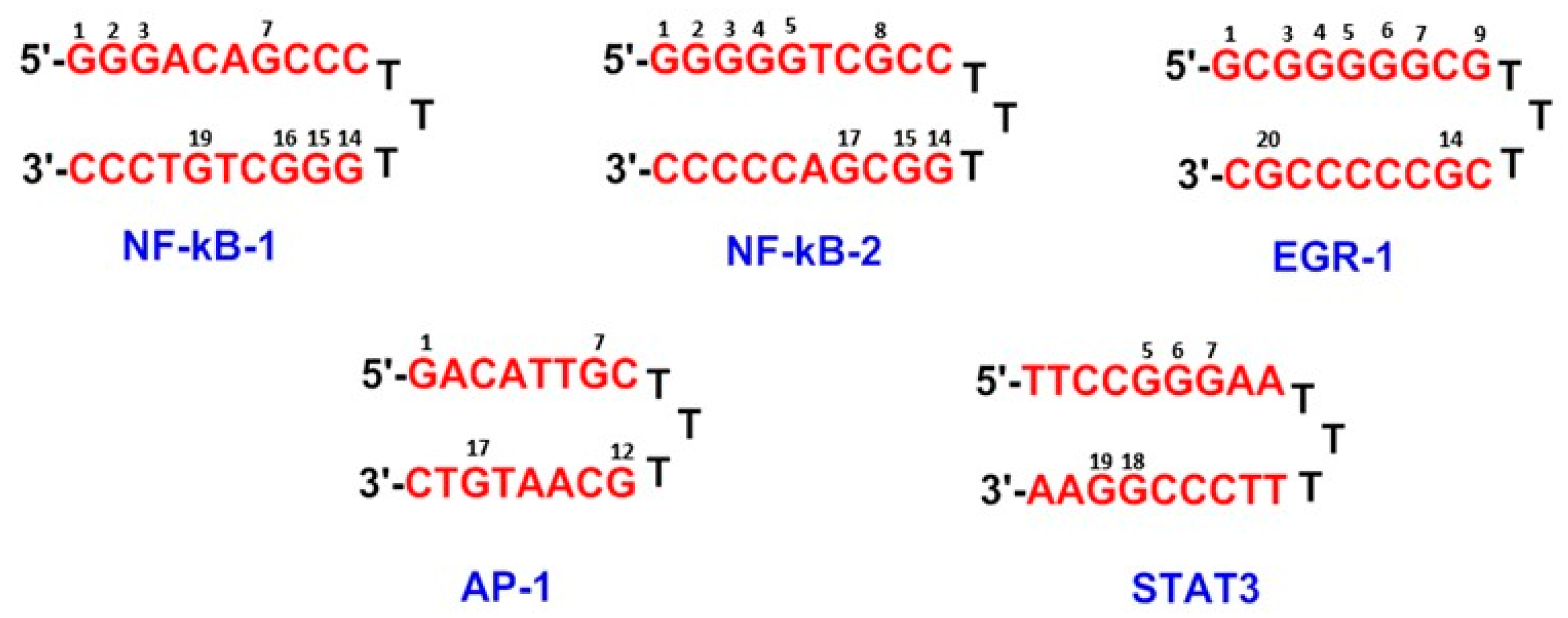
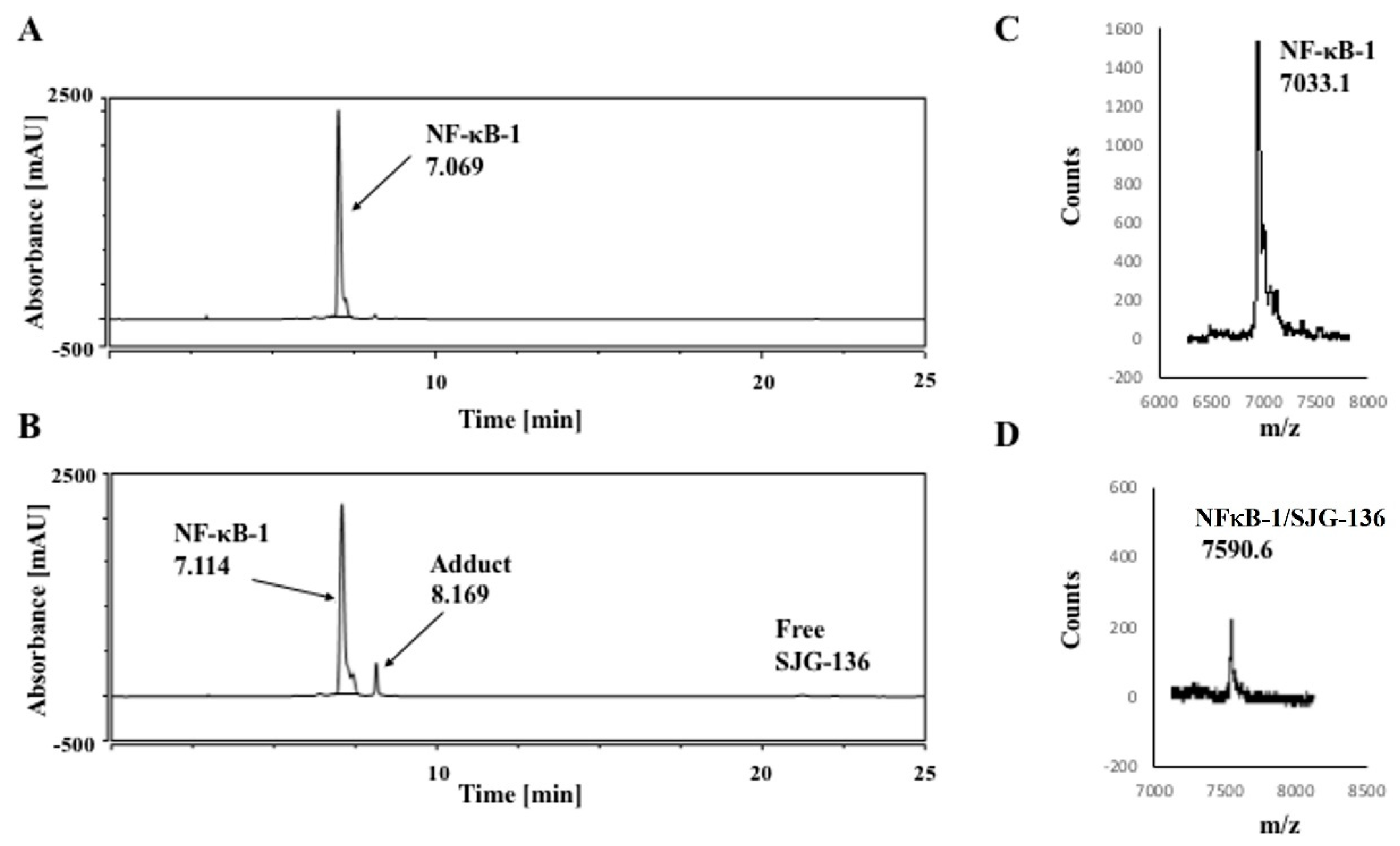
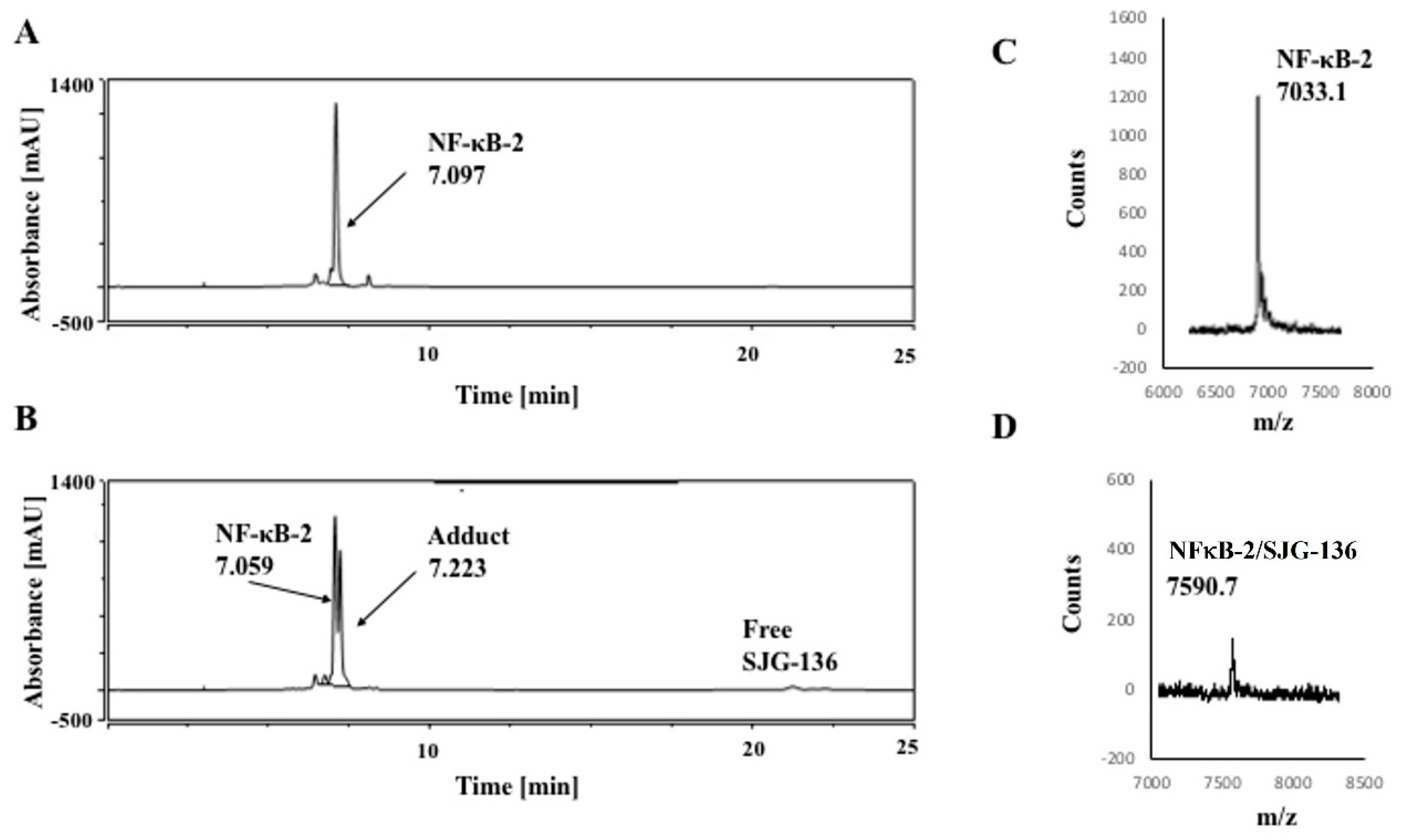
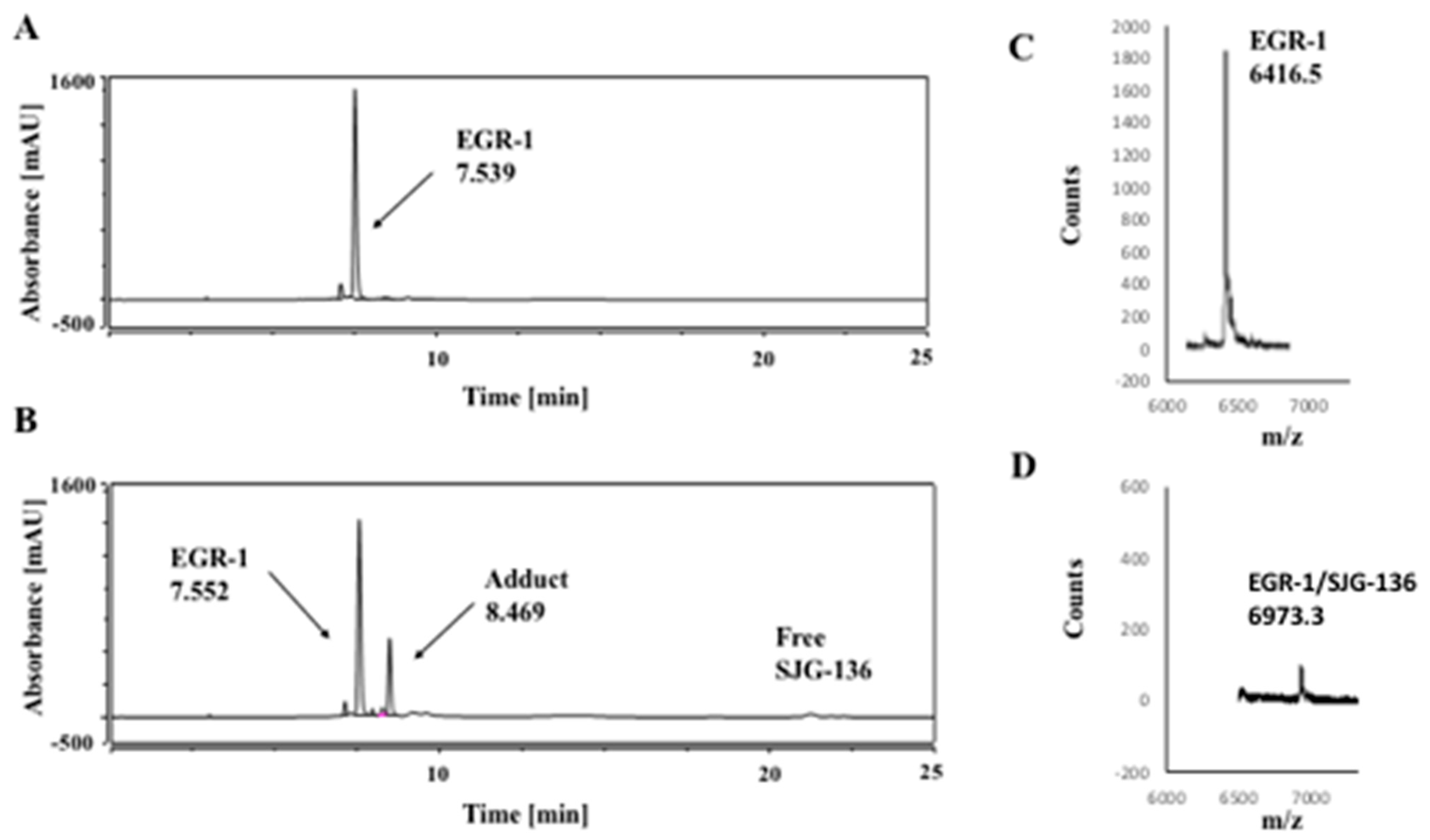
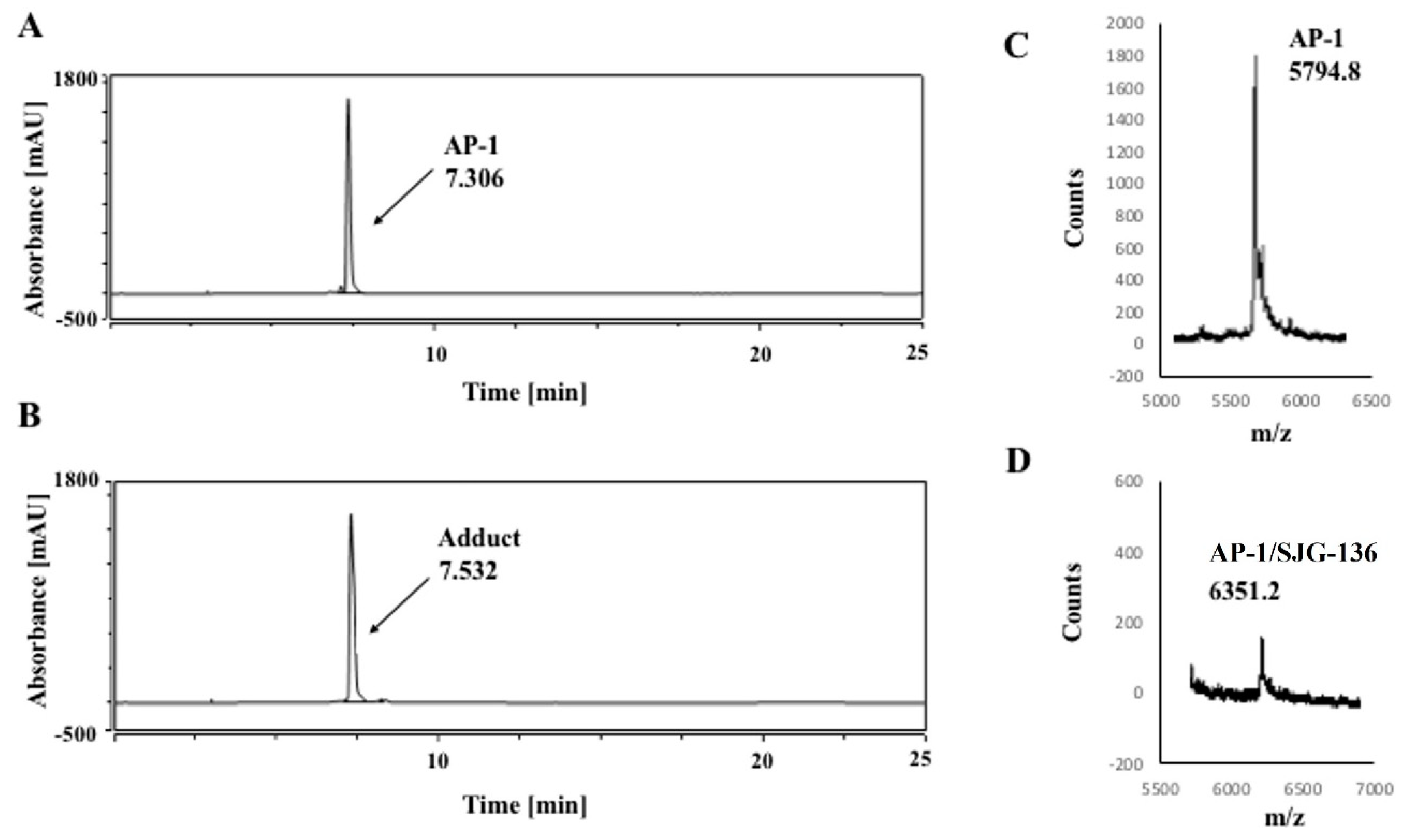
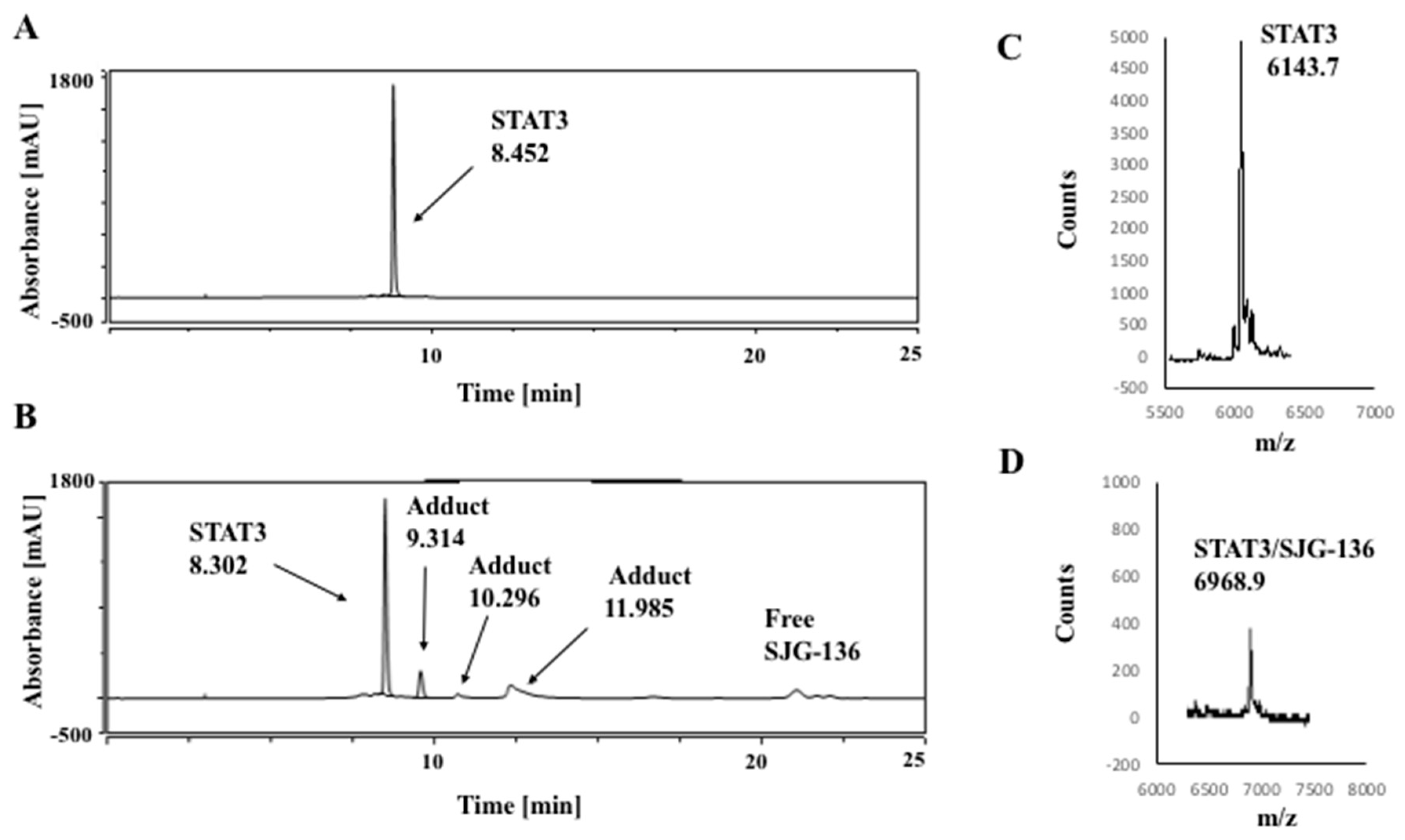
| DNA Sequence | DNA Mass | Theoretical Mass DNA/SJG-136 (1:1) (DNA Mass + 556.64) | Observed DNA/SJG-136 Adduct Mass (1:1) |
|---|---|---|---|
| NF-κB-1 | 7032.6 | 7589.2 | 7590.6 |
| NF-κB-2 | 7033.6 | 7590.2 | 7590.7 |
| EGR-1 | 6416.2 | 6972.8 | 6973.3 |
| AP-1 | 5793.8 | 6350.4 | 6351.2 |
| STAT3 | 6412.2 | 6968.8 | 6968.9 |
| NF-κB-1 | NF-κB-2 | EGR-1 | AP-1 | STAT3 | |
|---|---|---|---|---|---|
| Extent | 16.61 | 42.90 | 27.73 | 100 | 13.94 |
| No of adduct | 1 | 1 | 1 | 1 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantaj, J.; Jackson, P.J.M.; Parsons, R.B.; Bui, T.T.T.; Thurston, D.E.; Rahman, K.M. Transcription Factor Inhibition as a Potential Additional Mechanism of Action of Pyrrolobenzodiazepine (PBD) Dimers. DNA 2025, 5, 8. https://doi.org/10.3390/dna5010008
Mantaj J, Jackson PJM, Parsons RB, Bui TTT, Thurston DE, Rahman KM. Transcription Factor Inhibition as a Potential Additional Mechanism of Action of Pyrrolobenzodiazepine (PBD) Dimers. DNA. 2025; 5(1):8. https://doi.org/10.3390/dna5010008
Chicago/Turabian StyleMantaj, Julia, Paul J. M. Jackson, Richard B. Parsons, Tam T. T. Bui, David E. Thurston, and Khondaker Miraz Rahman. 2025. "Transcription Factor Inhibition as a Potential Additional Mechanism of Action of Pyrrolobenzodiazepine (PBD) Dimers" DNA 5, no. 1: 8. https://doi.org/10.3390/dna5010008
APA StyleMantaj, J., Jackson, P. J. M., Parsons, R. B., Bui, T. T. T., Thurston, D. E., & Rahman, K. M. (2025). Transcription Factor Inhibition as a Potential Additional Mechanism of Action of Pyrrolobenzodiazepine (PBD) Dimers. DNA, 5(1), 8. https://doi.org/10.3390/dna5010008









