Abstract
Background/Objectives: Lung cancer ranks as the leading cause of cancer-related deaths globally and is highly associated with cisplatin resistance due to both intrinsic and extrinsic mechanisms. Proliferating Cell Nuclear Antigen (PCNA) plays a critical role in molecular processes, such as DNA replication and repair, chromatin structure maintenance, and cell cycle progression. PCNA is known as a molecular marker for proliferation and an excellent inhibition target to shut down highly proliferative cells. One of the mechanisms of cisplatin resistance is the increase in DNA repair, and studies have reported an association between PCNA, lung cancer, and cisplatin treatment. The present study aimed to characterize the absence of PCNA in A549 lung adenocarcinoma cells. Methods: Employing a CRISPR/Cas9 gene-editing approach, we generated a monoclonal cell culture, termed PKO (PCNA knockout). Results: PKO cells exhibited a residual PCNA expression, significantly decreased clonogenic potential and ubiquitylation at K164 residue. IC50 assay suggested that PKO cells could not acquire cisplatin resistance when compared to PX. After cisplatin treatment, PKO cells presented impaired ubiquitylation and did not have increased STAT3 phosphorylation (Tyr705), a previously characterized mechanism of cisplatin resistance. Conclusions: We suggest that PCNA participates in cisplatin resistance in A549, partially by DNA damage tolerance through failure on PCNA monoubiquitylation, and its inhibition may be an approach to circumvent cisplatin resistance.
1. Introduction
Cancer is considered the leading cause of death worldwide and one of the biggest health challenges in socioeconomically developing countries [1]. Lung cancer is the most frequently occurring cancer and the primary cause of cancer-related mortality in men [1], which is mainly related to late diagnosis and low treatment effectiveness [2]. Therapeutic strategies in lung cancer patients include cisplatin-based chemotherapy and more recent immunotherapies, such as pembrolizumab and avelumab [3]. Cisplatin remains one of the most effective drugs for cancer treatment, but it is highly associated with intrinsic and acquired resistance to treatment [4]. In lung cancer cases, patients develop resistance to available therapeutic options, especially cisplatin, and receive a poor outcome, which includes a 5-year survival rate between 10 and 20% [1]. Ongoing research tries to better understand and develop therapeutic opportunities to circumvent chemotherapeutic resistance.
One of the main mechanisms associated with cisplatin resistance is the increase in DNA repair signaling and activation of this machinery with a lower apoptotic response [4,5]. Cisplatin causes intrastrand and interstrand DNA cross-links (ICLs) by forming cisplatin–DNA adducts, which are removed by the nucleotide excision repair (NER), and the overexpression of some of the NER proteins have been linked to cisplatin resistance [6]. In a previous study, using proteomic analysis, we reported increased expression of several proteins, including Proliferating Cell Nuclear Antigen (PCNA), in cisplatin-resistant A549 lung cancer cells, and described increased STAT3 expression and Tyr705 phosphorylation as a marker of cisplatin resistance [7].
PCNA is a DNA sliding clamp characterized by its structural and functional preservation across eukaryotic species. It was first recognized as a facilitator of processivity for DNA polymerase δ, a critical component to drive DNA synthesis during the replication [8]. PCNA is also used as a marker of cell proliferation [9] and is commonly overexpressed in cancer cells. The main post-translational modification in PCNA is ubiquitylation. Polyubiquitylation activates homology repair (HR), while monoubiquitylation at the K164 residue stimulates the translesion synthesis (TLS) mechanism. TLS allows replication to continue over DNA damage, promoting cell survival and perpetuating resistance to damage [10]. Studies also report an association between PCNA expression, lung cancer [11] and cisplatin treatment [12,13], but the molecular mechanisms in lung cancer resistance remain unclear.
To assess whether PCNA is involved with cisplatin resistance, we used a CRISPR approach to inactivate PCNA function in A549 NSCLC cells. With a combination of CRISPR-mediated knockout, overexpression, and functional assays, we characterized that the partial PCNA loss of function decreased clonogenic survival and was sensitized to cisplatin treatment. Furthermore, PKO increased STAT3 phosphorylation at Tyr705, an important pattern of resistance in lung cancer cells. In this study, we propose that PCNA contributes to cisplatin resistance, partially through defective PCNA monoubiquitylation and potentially by modulating STAT3 activity.
2. Materials and Methods
2.1. Cell Culture
A549 cells were cultured in Ham’s-F12 with 10% fetal bovine serum (Gibco; Thermo Scientific, Inc., Waltham, MA USA), penicillin (100 U/mL), and streptomycin (100 μg/mL). Cells were incubated at 37 °C with 10 µM of cisplatin (Sigma-Aldrich; Merck, Burlington, MA, USA) for 72 h to generate the resistant cells (CR).
2.2. Partial PCNA Loss of Function Establishment Using CRISPR/Cas9 System
To produce cells with loss of PCNA expression, termed PKO, a sgRNA sequence specific for PCNA gene was developed through the CRISPOR platform [14]. The chosen sequence used in this study was 5′ ACTAAGGGCCGAAGATAACG 3′. Oligos containing the sequence were cloned into the PX459 vector (SpCas9(BB)-2A-Puro V2.0, Addgene #62988), and the transfection and clonal selection methods were previously described [7]. Cells were transfected with PX459 or PX459-PCNA vectors, and monoclonal cell cultures were selected for subsequent experiments. Monoclonal cultures of cells transfected with the empty plasmid PX549 were used as experimental controls (termed ’PX’). Expression of PCNA in the resulting monoclonal cultures was examined through Western blotting with an anti-PCNA antibody (Cell Signaling Technology, Danvers, MA, USA #9139). PCR amplification of the PCNA-specific genomic region was performed on genomic DNA, followed by sequencing, to verify the positive monoclonal cell lines. The primers used for genomic screening and identification of the PCNA edition were as follows:
- PCNA _Forward: 5′ TGGCGGGAAAATCAAGGGTT 3′
- PCNA _Reverse: 5′ TATCGTGCCTGTGTACAGCA 3′
2.3. PCNA Overexpression Construct
For FLAG-tagged protein expression, a modified pcDNA 3.1 (+) (#V79020, Thermo Scientific, Waltham, MA USA) was used, consisting of a plasmid containing the FLAG peptide sequence and called pcDNA-FLAG vector, as previously described [15]. Using BamHI (#R3136, New England Biolabs Inc., Ipswich, MA, USA) and XhoI (#R0146, New England Biolabs Inc., Ipswich, MA, USA) restriction sites, the full-length PCNA cDNA (accession number NM_002592) was incorporated into the pcDNA-FLAG plasmid, generating the plasmid referred to as OE-PCNA. An empty pFLAG-GFP plasmid was used as an assay control.
2.4. Cell Viability and Cisplatin Resistance Assay
The A549 WT and PKO cells were seeded in 96-well plates at 6 × 103 cells/well for the CT group (control, cisplatin-sensitive) and 1.2 × 10⁴ cells/well for the CR group (cisplatin-resistant). To determine IC50 and cisplatin sensitivity, the cells were exposed to cisplatin concentrations ranging from 5 to 50 µM. The method performed for viability assessment using MTT was described previously [7].
2.5. Colony Formation Assay
A549 WT and PKO cells were seeded at a low density (2 × 102 cells/well) in 6-well plates and incubated for 9 days. Following incubation, cells were washed with PBS and stained with 1 mL of crystal violet solution (0.05% crystal violet, 1% formaldehyde, 1% methanol in PBS 1×) for 30 min with mild shaking at room temperature. After washing with deionized water, colonies were counted using ImageJ software v1.53 (National Institutes of Health) by converting images to 8-bit binary mode and classifying colonies into three size categories: smaller than 1 pixel2, between 1 and 25 pixel2, and larger than 25 pixel2, or converted to millimeters measurements.
2.6. Western Blot
Protein samples were extracted using a lysis buffer with protease and phosphatase inhibitor cocktails, and samples were separated by SDS-PAGE (8 and 10% acrylamide gels). Electrotransfer was conducted on 0.45 µm nitrocellulose membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and incubated for 1 h at room temperature with a 5% solution of nonfat powdered milk in TBST (50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 0.1% Tween-20). The primary antibodies were incubated at 4 °C overnight, and secondary antibodies were incubated at room temperature for 1 to 2 h. Protein bands were detected using Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific, Inc., Waltham, MA, USA) in ChemiDoc Imaging System (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and densitometry was performed using ImageJ software v1.53. Primary antibodies Vinculin (#13901), STAT3 (#9139), phospho-STAT3 (Tyr705; #9145), ubiquityl-PCNA (Lys164, #13439) and PCNA (#2586) were purchased from Cell Signaling Technology. α-Tubulin was purchased from Calbiochem (CP06). All antibodies were used 1:2000 in 5% BSA. Secondary antibodies were HRP-conjugated goat anti-mouse IgG (Sigma-Aldrich; AP308P; 1:2000, Burlington, MA, USA) and goat anti-rabbit IgG (Sigma-Aldrich; AP307P; 1:5000, Burlington, MA, USA).
2.7. Statistical Analysis
Data are presented as mean ± S.D. Statistical comparisons were made using GraphPad Prism 8.0.1 software (https://www.graphpad.com/ (accessed on 24 May 2020), with either Student’s unpaired t-test or one-way or two-way ANOVA, followed by Tukey’s or Bonferroni’s post hoc test. Significance was determined with * p < 0.05, ** p < 0.01, and *** p < 0.001.
3. Results
3.1. Generation of A549 PCNA Partial Loss Cells
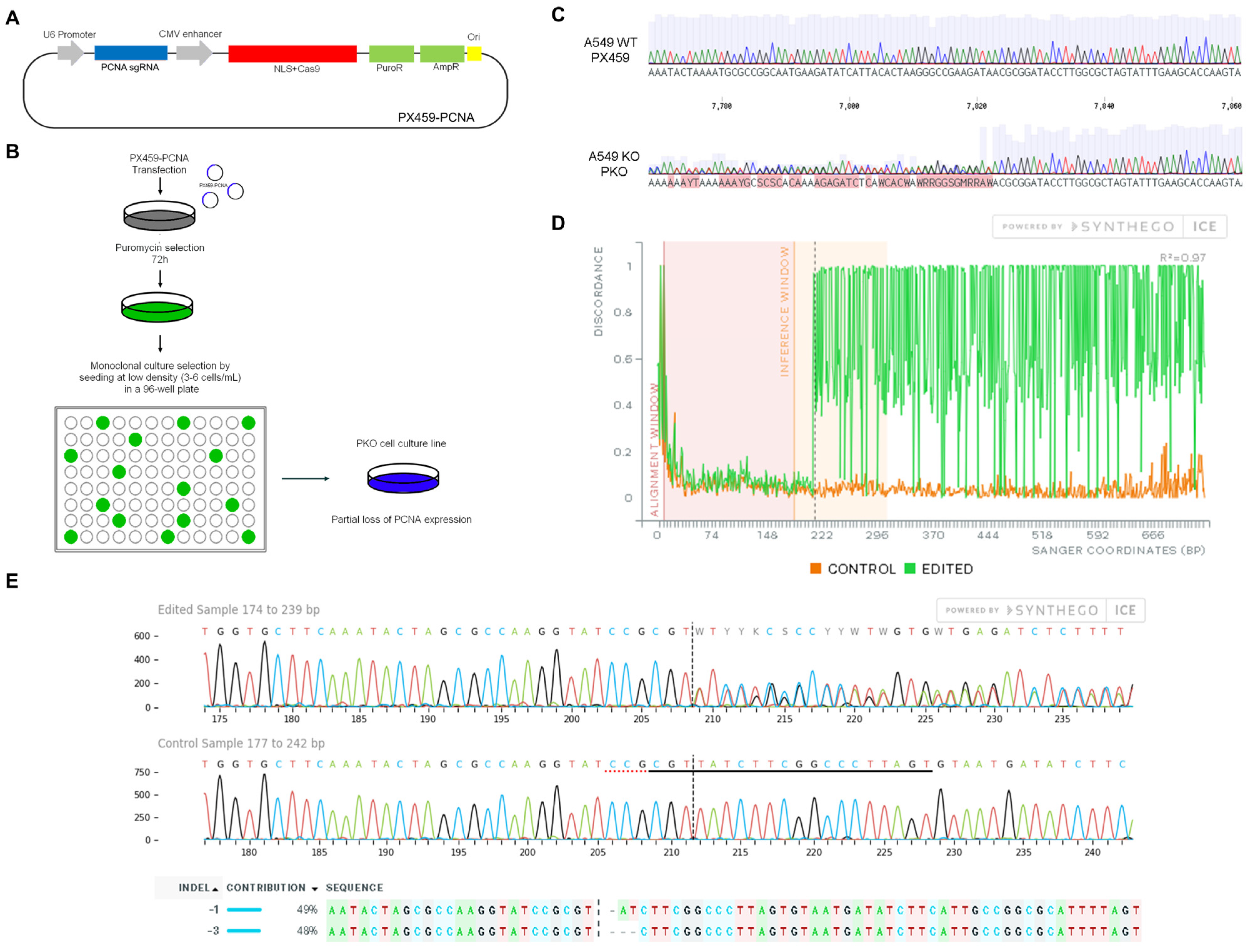
To analyze the role of PCNA in the cisplatin resistance (CR) profile, CRISPR/Cas9 gene editing was employed to introduce genomic mutations in the PCNA gene of A549 cells. The plasmid was constructed by cloning the previously synthesized sgRNA targeting PCNA (Figure 1A). After CRISPR targeting and monoclonal selection, we verified the generation of indels by sequencing (Figure 1B,C). Decomposition of the genomic sequencing through SYNTHEGO ICE v3 software reported that PCNA alleles were heterozygous, edited with two probable types of indels (−1 and −3, Figure 1E). The deletion of three bases may explain why we identified a residual expression of PCNA in the monoclonal population.

Figure 1.
Characterization of PCNA gene editing using the CRISPR/Cas9 system. Design of the plasmid PX459, (Addgene #62988, Watertown, MA, USA), expressing the Cas9 protein and a PCNA single-guide RNA (sgRNA) (A). Experimental design of plasmid transfection and cell cloning selection by serial dilution using 96-well plates to obtain PKO cells (B). The Sanger sequencing of monoclonal PKO cell cultures reports scrambled chromatograms close to the PAM sequence after plasmid transfection and clonal selection, revealing the acquisition of insertions and deletions (Indels) in the PCNA gene sequence (C). Discordance levels (according to ICE SYNTHEGO) between the wild-type and the edited chromatogram show the editing efficiency obtained in the selected clone (D). According to ICE SYNTHEGO v3 software, PKO cells present the most probable indels as 1 (−1) and 3 (−3) deletions (E).
3.2. Partial Loss of PCNA in PKO Cells Impaired Clonogenic Potential
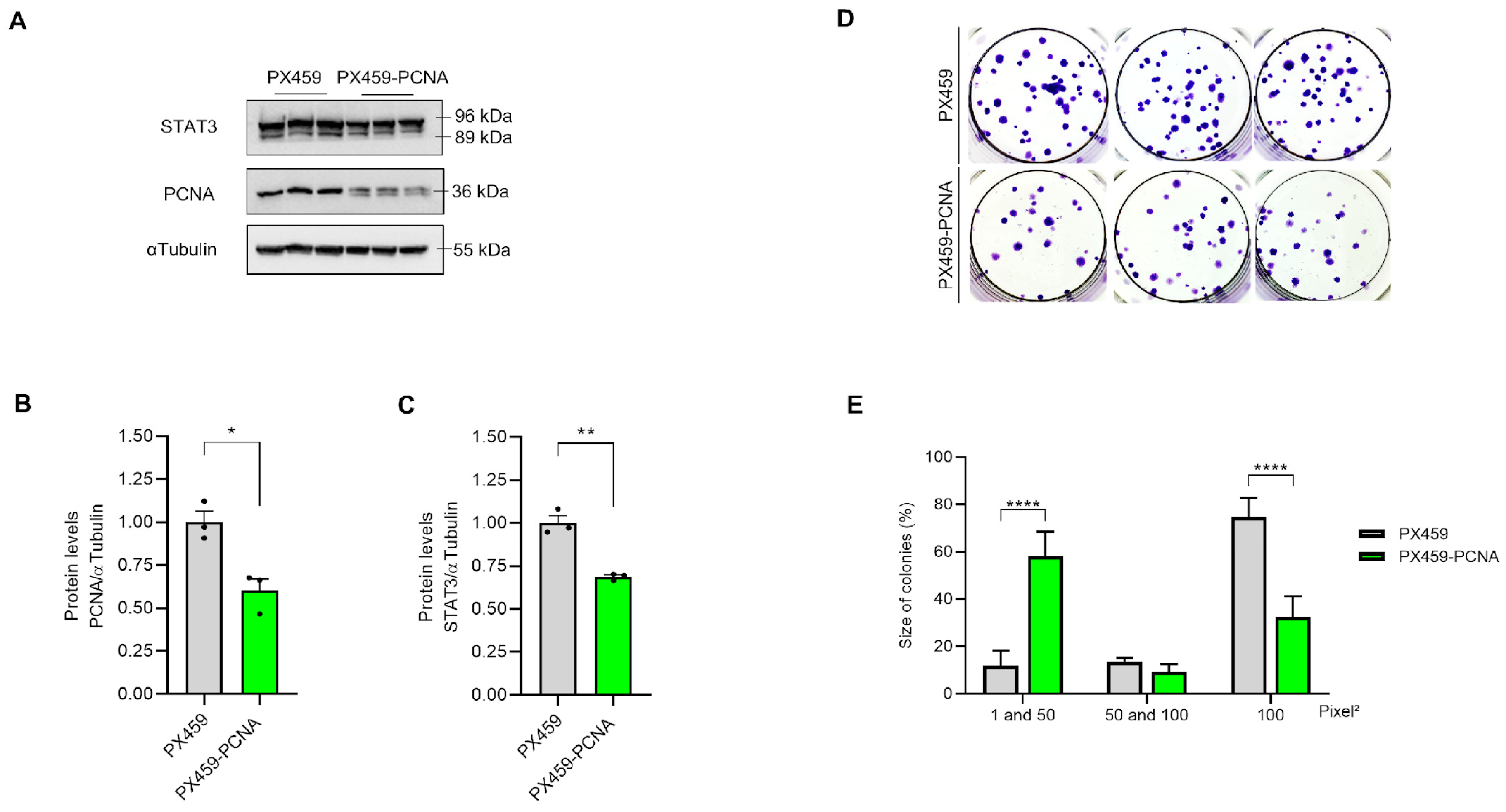
Screening of polyclonal cultures was conducted initially to identify the loss of PCNA expression (Figure 2A,B). Furthermore, STAT3, an important transcription factor directly related to cisplatin resistance [7], significantly decreased expression in PX459-PCNA compared to PX-459 cells (Figure 2C). We then examined the functional effects of polyclonal edition on clonogenic survival of A549 cells. Although the number of PX459-PCNA colonies was similar to the control population (PX459 cells) (Figure 2D), the size of colonies formed by PX459-PCNA cells was significantly smaller when compared to PX459 cells (Figure 2D,E).

Figure 2.
Polyclonal PCNA editing alters clonogenic potential in A549 cells. A549 cells were previously plated in a 6-well plate at the seeding density and collected after 24 h. Partial loss of PCNA and STAT3 in polyclonal PCNA editing culture was observed by Western blot analysis (A), which reported a statistically significant difference when compared to PX459 cells (B,C). The clonogenic survival assay indicates the size of colonies (D,E) measured using the ImageJ v 1.53 software. All data represent the mean ± SD. Statistical analysis of the Western blot was conducted after unpaired t-test and clonogenic survival after 2-way ANOVA followed by Sidak’s post-test (* p < 0.05, ** p < 0.01, **** p < 0001). Clonogenic assay data represent two biological replicates. All values and statistical analyses were included in the raw data.
3.3. Cisplatin Is More Effective Against PKO Cells
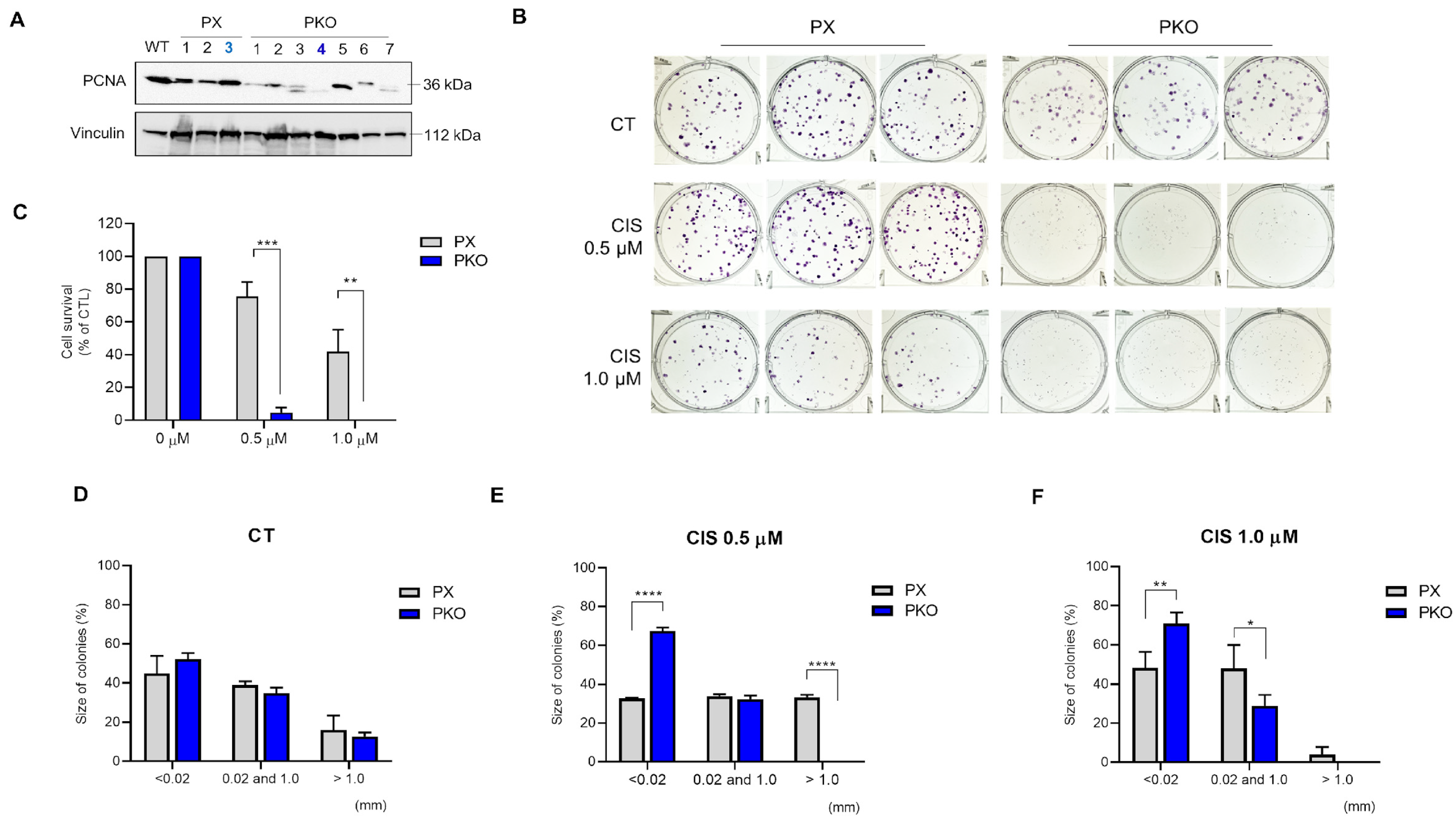
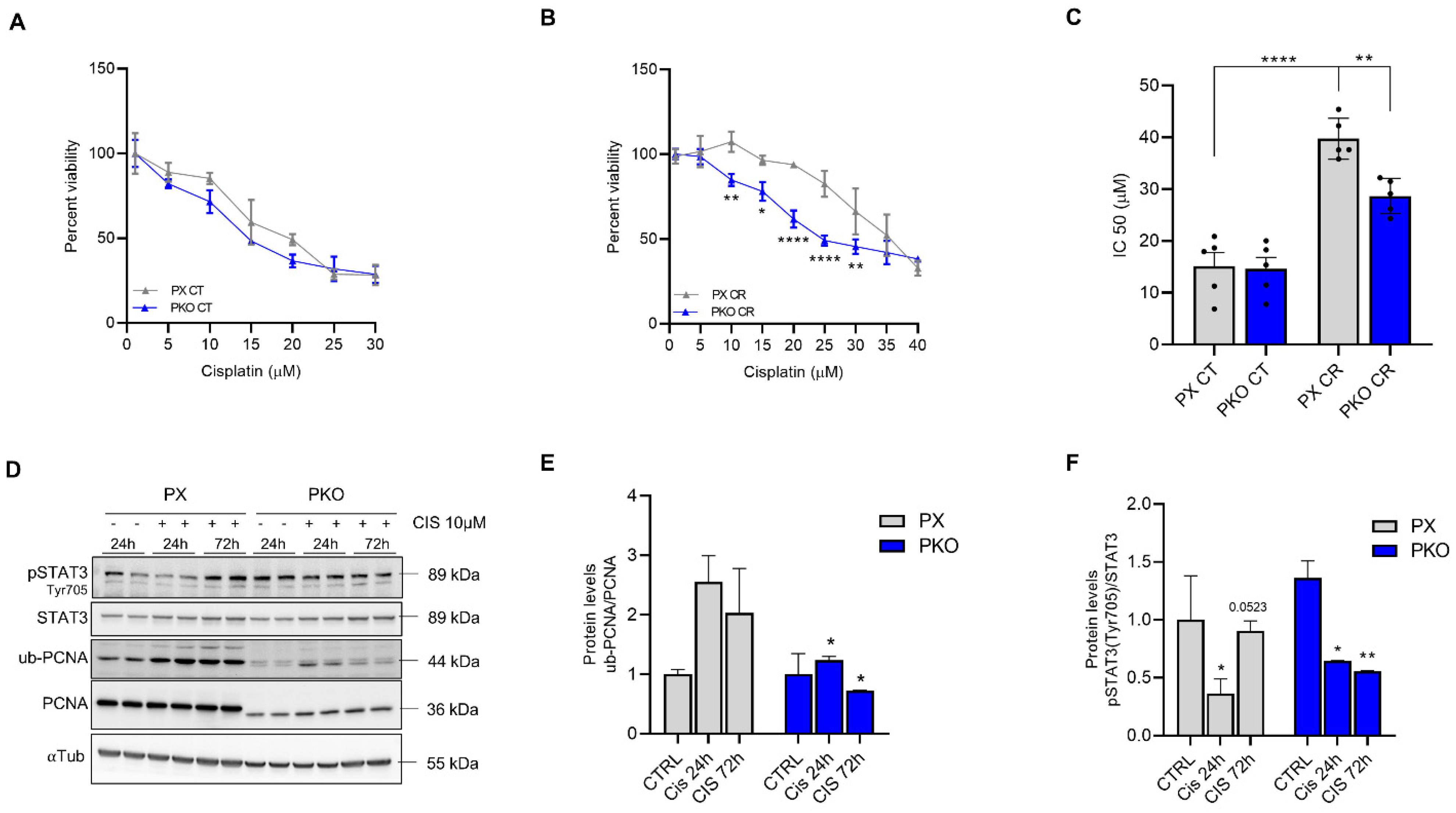
Clonal selection generated different clones, all with residual PCNA expression (Figure 3A). PX 3 and clone 4 were chosen to continue the experiments. To investigate the sensitivity of PKO cells to cisplatin, we performed a clonogenic assay. The clonogenic survival was significantly reduced in response to cisplatin 0.5 and 1.0 µM (Figure 3B,C) when compared to control, showing that PKO cells were more responsive to both cisplatin treatments. Furthermore, the size of colonies was significantly smaller when compared to PX cells (Figure 3D–F). Conversely, IC50 assays were performed, which showed that PKO cells present lower IC50 levels compared to control cells (Figure 4A–C). The data support that PKO cells are more sensitive to cisplatin and have lower proliferative capacity.

Figure 3.
Cisplatin more effectively inhibits the clonogenic potential in PKO cells. A549 monoclonal cells previously selected were plated in a 6-well plate and collected 24 h later. Partial loss of PCNA expression shown by Western blot analysis provided the PCNA expression in WT (wild-type), PX459, and PX459-PCNA monoclonal selected cells (A). The clonogenic survival assay (B) indicates the size of colonies (D–F) after 0.5 and 1.0 µM cisplatin treatment (C,D). Data were measured using the ImageJ v1.53 software considering more than 0.02 mm as valid colonies. All data represent the mean ± SD. Statistical analysis of clonogenic survival was conducted after 2-way ANOVA followed by Sidak’s post-test (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0001). Clonogenic assay data represent two biological replicates. All values and statistical analyses were included in the raw data.
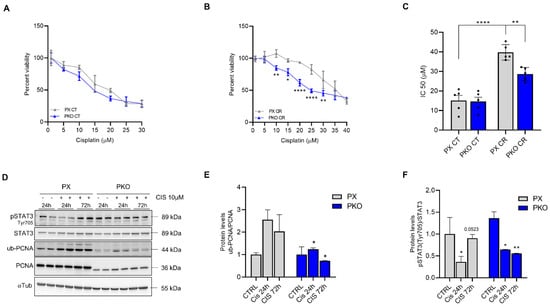
Figure 4.
PKO cells impaired cisplatin resistance while altering STAT3 phosphorylation. A549 cells were previously plated in a 96-well plate and exposed to different concentrations of cisplatin for 72 h. Viability assay showed that PKO cells have the same percent survival and IC50 as PX in control conditions (CT) (A), but do not acquire a cisplatin-resistant (CR) profile after pre-treatment with 10 µM cisplatin for 72 h (B,C). PX and PKO cells were treated with 10 µM for 24 and 72 h, and STAT3 and PCNA were evaluated (D). The graph shows the bands’ densitometry of ub-PCNA/PCNA (E) and p-STAT3tyr705/STAT3 (F). Western blot data represent the mean ± SD (n = 2). The percent survival data (n = 3) represent the mean ± SD and IC50 the mean ± SEM (n = 5). Statistical analysis of percent viability was accessed after 2-way ANOVA followed by Sidak’s post-test and IC50 after one-way ANOVA, followed by Tukey’s post-test (* p < 0.05, ** p < 0.01, **** p < 0001). All values and statistical analyses were included in the raw data.
3.4. STAT3 Is Related to PCNA Expression and Ubiquitylation in Cisplatin Resistance
We previously characterized STAT3 as a crucial factor in cisplatin resistance, which also plays a role in the DNA damage response [7]. Here, we observed that PKO cells tend to not maintain the STAT3 phosphorylationafter 72 h of cisplatin treatment (Figure 4D,F), while maintaining impaired PCNA ubiquitylation (Figure 4E). Furthermore, since PX459-PCNA cells have decreased STAT3 expression, we constructed an overexpression plasmid to assess if the opposite is sustained. As a result, cells with overexpression of PCNA (OE-PCNA) do not show an increase in STAT3 expression, but instead display enhanced phosphorylation at Y705 (Supplementary Figure S1), a residue that signals the STAT3 nuclear localization [16]. Since STAT3 Y705 phosphorylation and nuclear localization are observed in CR cells [7,17], it is suggested that increased phosphorylation of STAT3 in OE-PCNA cells reinforces cisplatin resistance.
Moreover, a TMNPlot analysis [18] demonstrates that increased expression of PCNA is found in cancer samples for almost all types of cancer, including lung adenocarcinoma and squamous cell lung cancer (Supplementary Figure S2A). A paired analysis of PCNA expression in normal vs. tumor samples also reveals increased PCNA expression in lung cancer samples (Figure S2B). Finally, a survival curve analysis derived from CBioPortal [19,20] using TCGA data shows the relevance of PCNA in contributing to cancer progression, presenting a Hazard Ratio of 1.443 (Figure S2C). These analyses contribute to reinforcing the role of PCNA expression in several types of cancer, including LUAD.
4. Discussion
DNA damage tolerance is considered a mechanism of multidrug resistance, especially in cisplatin treatment [6], and PCNA is an integral part of multiple repair pathways, such as MMR (mismatch repair), NER (nucleotide excision repair), and BER (base excision repair) [21]. Strategies to circumvent cisplatin resistance include targeted and immunomodulation therapies, which allow for more effective treatment with fewer side effects [22]. One benefit of targeting PCNA therapeutically lies in the cancer-associated PCNA isoform (caPCNA), which shows high expression in cancer cells and tumor tissues [23]. Here, using CRISPR/Cas9, we reported that PCNA, an essential protein that controls processes such as DNA replication and damage repair [24,25], is related to clonogenic potential and cisplatin resistance, possibly by DNA tolerance mechanisms and STAT3 activation in lung adenocarcinoma cells.
PCNA is a relevant protein for cell growth and survival, especially in cancer cells, and it represents a potential treatment option against cancer [26]. In comparison to normal cells, studies have shown that PCNA is overexpressed and post-translationally modified in malignant cells [8,10,12]. The involvement of PCNA in cellular proliferation and malignant transformation resulted in its wide use as a diagnostic and prognostic cell cycle marker [21,27,28,29].
Thakar and colleagues (2020) characterized the role of PCNA functionality in protecting against genotoxic stress. The study aimed to abrogate the PCNA ubiquitylation site and performed this modification by designing an sgRNA close to the ubiquitylation site region. The authors categorically demonstrated that the loss of PCNA ubiquitylation, independent of its expression levels, increased the susceptibility of HEK293 and RPE1 cells to UV-induced and cisplatin-induced damage, which supports our findings. [30].
Wang and colleagues (2018) demonstrate that overexpression of PCNA in A549 and H1975 lung cancer cells significantly increases STAT3 phosphorylation. In both cell lines, silencing STAT3 in cells overexpressing PCNA reduces cell viability to control levels [31]. Wang and colleagues (2006) demonstrated that the translocation of EGFR to the nucleus leads to the phosphorylation of PCNA on tyrosine residue 211 (pY211), explaining the relationship with STAT3 through this regulation [32]. More studies are necessary to assess the effects of PCNA overexpression and STAT3 phosphorylation on increased resistance to cisplatin and, consequently, genotoxic damage.
5. Conclusions
Our study reveals the partial loss of PCNA function as a mechanism contributing to cisplatin sensitivity in A549 lung cancer cells. Analyses from cancer patient samples also reinforce the role of PCNA in cancer progression. Despite the use of a single cell line and methodological limitations, we suggest that STAT3 participates in PCNA activity to generate cisplatin susceptibility, providing primary support to in-depth investigations on the interaction between PCNA and STAT3 activation. Future studies assessing the biochemical interactions between ubiquitinated PCNA and STAT3 may help elucidate mechanisms involved in cisplatin resistance and DNA damage tolerance in lung adenocarcinoma cells.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/dna5010007/s1, Figure S1: PCNA overexpression induces STAT3 phosphorylation at Y705. A549 cells were plated in a 6-well plate and transfected with either the control plasmid (OE-GFP) or the PCNA overexpression plasmid (OE-PCNA) and collected after 48 h. Western blot experiment showing an increase in PCNA and STAT3 phosphorylation at Y705 when compared to OE-GFP and CT cells (A). The graph shows bands densitometry of PCNA/αTubulin (B) and p-STAT3tyr705/STAT3 (C). Data represent the mean ± SD. Statistical analysis was assessed after 1-way ANOVA followed by Tukey’s post-test (* p < 0.05; ** p < 0.01; *** p < 0.001).; Figure S2: PCNA expression is associated with cancer progression in patient samples. TMNPlot analysis shows increased expression of PCNA in several types of cancer, including lung adenocarcinoma (A,C) and squamous cell (SC) lung cancer (A). Paired analysis of PCNA expression in normal vs. tumor samples also shows increased PCNA expression in lung cancer samples (B). Survival curve analysis derived from CBioPortal, using TCGA data, shows that PCNA contributes to cancer progression, HR = Hazard Ratio (C).
Author Contributions
Conceptualization, A.P.M. and F.M.S.; methodology, A.P.M., N.Q.-R., M.C.S.M., I.C.B.P., L.G.S.S., M.B.S. and I.L.F.; validation, N.Q.-R. and A.P.M.; formal analysis, A.P.M., F.M.S., N.Q.-R., I.C.B.P., M.C.S.M., L.G.S.S. and M.B.S.; investigation, A.P.M., N.Q.-R., I.L.F., M.C.S.M., I.C.B.P., M.B.S. and L.G.S.S.; data curation, N.Q.-R., A.P.M. and F.M.S.; writing—original draft preparation, N.Q.-R., A.P.M. and F.M.S., writing—review and editing, N.Q.-R., A.P.M. and F.M.S.; visualization, F.M.S.; resources, F.M.S. and R.M.N.B.; supervision, R.M.N.B. and F.M.S., project administration, F.M.S., funding acquisition, F.M.S. and R.M.N.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the São Paulo Research Foundation, FAPESP (grant number FMS, 2018/14818-9; fellowship numbers: APM, 2019/00607-9; MCSM, 2019/25582-9; ICBP, 2015/00311-1; LGSS, 2020/09527-5, NQR 2020/09133-7) and by the National Council for Scientific and Technological Development, CNPq (FMS, 447553/2014-3).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kakushadze, Z.; Raghubanshi, R.; Yu, W. Estimating Cost Savings from Early Cancer Diagnosis. Data 2017, 2, 30. [Google Scholar] [CrossRef]
- Steven, A.; Fisher, S.A.; Robinson, B.W. Immunotherapy for lung cancer. Respirology 2016, 21, 821–833. [Google Scholar] [CrossRef]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Michels, J.; Brenner, C.; Szabadkai, G.; Harel-Bellan, A.; Castedo, M.; Kroemer, G. Systems biology of cisplatin resistance: Past, present and future. Cell Death Dis. 2014, 5, e1257. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.R.R.; Silva, M.M.; Quinet, A.; Cabral-Neto, J.B.; Menck, C.F.M. DNA repair pathways and cisplatin resistance: An intimate relationship. Clinics 2018, 73, e478s. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.P.; Tortelli, T.C.; Mancini, M.C.S.; Pavan, I.C.B.; Silva, L.G.S.; Severino, M.B.; Granato, D.C.; Pestana, N.F.; Ponte, L.G.S.; Peruca, G.F.; et al. STAT3 contributes to cisplatin resistance, modulating EMT markers, and the mTOR signaling in lung adenocarcinoma. Neoplasia 2021, 23, 1048–1058. [Google Scholar] [CrossRef]
- Maga, G.; Hubscher, U. Proliferating cell nuclear antigen (PCNA): A dancer with many partners. J. Cell Sci. 2003, 116, 3051–3060. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, M.L.; George, L.K.; Grant, G.D.; Perou, C.M. Common markers of proliferation. Nat. Rev. Cancer 2006, 6, 99–106. [Google Scholar] [CrossRef]
- Mailand, N.; Gibbs-Seymour, I.; Bekker-Jensen, S. Regulation of PCNA-protein interactions for genome stability. Nat. Rev. Mol. Cell Biol. 2013, 14, 269–282. [Google Scholar] [CrossRef]
- Groeger, A.M.; Caputi, M.; Esposito, V.; Baldi, A.; Rossiello, R.; Santini, D.; Mancini, A.; E Kaiser, H.; Baldi, F. Expression of p21 in non small cell lung cancer relationship with PCNA. Anticancer Res. 2000, 20, 3301–3305. [Google Scholar] [PubMed]
- Müller, R.; Misund, K.; Holien, T.; Bachke, S.; Gilljam, K.M.; Våtsveen, T.K.; Rø, T.B.; Bellacchio, E.; Sundan, A.; Otterlei, M. Targeting Proliferating Cell Nuclear Antigen and Its Protein Interactions Induces Apoptosis in Multiple Myeloma Cells. PLoS ONE 2013, 8, e70430. [Google Scholar] [CrossRef]
- Lingeman, R.G.; Hickey, R.J.; Malkas, L.H. Expression of a novel peptide derived from PCNA damages DNA and reverses cisplatin resistance. Cancer Chemother. Pharmacol. 2014, 74, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Concordet, J.P.; Haeussler, M. CRISPOR: Intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 2018, 46, W242–W245. [Google Scholar] [CrossRef] [PubMed]
- Pavan, I.C.B.; Yokoo, S.; Granato, D.C.; Meneguello, L.; Carnielli, C.M.; Tavares, M.R.; Amaral, C.L.D.; de Freitas, L.B.; Leme, A.F.P.; Luchessi, A.D.; et al. Different interactomes for p70-S6K1 and p54-S6K2 revealed by proteomic analysis. Proteomics 2016, 16, 2650–2666. [Google Scholar] [CrossRef]
- Decker, T.; Müller, M. Jak-Stat Signaling: From Basics to Disease; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Liang, F.; Ren, C.; Wang, J.; Wang, S.; Yang, L.; Han, X.; Chen, Y.; Tong, G.; Yang, G. The crosstalk between STAT3 and p53/RAS signaling controls cancer cell metastasis and cisplatin resistance via the Slug/MAPK/PI3K/AKT-mediated regulation of EMT and autophagy. Oncogenesis 2019, 8, 59. [Google Scholar] [CrossRef]
- Bartha, Á.; Győrffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.E.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Stoimenov, I.; Helleday, T. PCNA on the crossroad of cancer. Biochem. Soc. Trans. 2009, 37, 605–613. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.-L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Malkas, L.H.; Herbert, B.S.; Abdel-Aziz, W.; Dobrolecki, L.E.; Liu, Y.; Agarwal, B.; Hoelz, D.; Badve, S.; Schnaper, L.; Arnold, R.J.; et al. A cancer-associated PCNA expressed in breast cancer has implications as a potential biomarker. Proc. Natl. Acad. Sci. USA 2006, 103, 19472–19477. [Google Scholar] [CrossRef]
- González-Magaña, A.; Blanco, F.J. Human PCNA Structure, Function and Interactions. Biomolecules 2020, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Boehm, E.M.; Gildenberg, M.S.; Washington, M.T. The Many Roles of PCNA in Eukaryotic DNA Replication. Enzymes 2016, 39, 231–254. [Google Scholar]
- Cardano, M.; Tribioli, C.; Prosperi, E. Targeting Proliferating Cell Nuclear Antigen (PCNA) as an Effective Strategy to Inhibit Tumor Cell Proliferation. Curr. Cancer Drug Targets 2020, 20, 240–252. [Google Scholar] [CrossRef]
- Haerslev, T.; Jacobsen, G.K.; Zedeler, K. Correlation of growth fraction by Ki-67 and proliferating cell nuclear antigen (PCNA) immunohistochemistry with histopathological parameters and prognosis in primary breast carcinomas. Breast Cancer Res. Treat. 1996, 37, 101–113. [Google Scholar] [CrossRef]
- Lv, Q.; Zhang, J.; Yi, Y.; Huang, Y.; Wang, Y.; Wang, Y.; Zhang, W. Proliferating Cell Nuclear Antigen Has an Association with Prognosis and Risks Factors of Cancer Patients: A Systematic Review. Mol. Neurobiol. 2016, 53, 6209–6217. [Google Scholar] [CrossRef] [PubMed]
- Kordek, R.; Biernat, W.; Debiec-Rychter, M.; Alwasiak, J.; Liberski, P.P. Comparative Evaluation of p53-Protein Expression and the PCNA and Ki-67 Proliferating Cell Indices in Human Astrocytomas. Pathol. Res. Pract. 1996, 192, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Thakar, T.; Leung, W.; Nicolae, C.M.; Clements, K.E.; Shen, B.; Bielinsky, A.-K.; Moldovan, G.-L. Ubiquitinated-PCNA protects replication forks from DNA2-mediated degradation by regulating Okazaki fragment maturation and chromatin assembly. Nat. Commun. 2020, 11, 2147. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kong, W.; Liu, B.; Zhang, X. Proliferating cell nuclear antigen promotes cell proliferation and tumorigenesis by up-regulating STAT3 in non-small cell lung cancer. Biomed. Pharmacother. 2018, 104, 595–602. [Google Scholar] [CrossRef]
- Wang, S.-C.; Nakajima, Y.; Yu, Y.-L.; Xia, W.; Chen, C.-T.; Yang, C.-C.; McIntush, E.W.; Li, L.-Y.; Hawke, D.H.; Kobayashi, R.; et al. Tyrosine phosphorylation controls PCNA function through protein stability. Nat. Cell Biol. 2006, 8, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).