DRD2/ANKK1 TaqIA Genetic Variant and Major Depressive Disorder: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Criteria for Articles’ Inclusion and Exclusion
2.2. Article Selection
2.3. Risk of Article Bias
3. Results
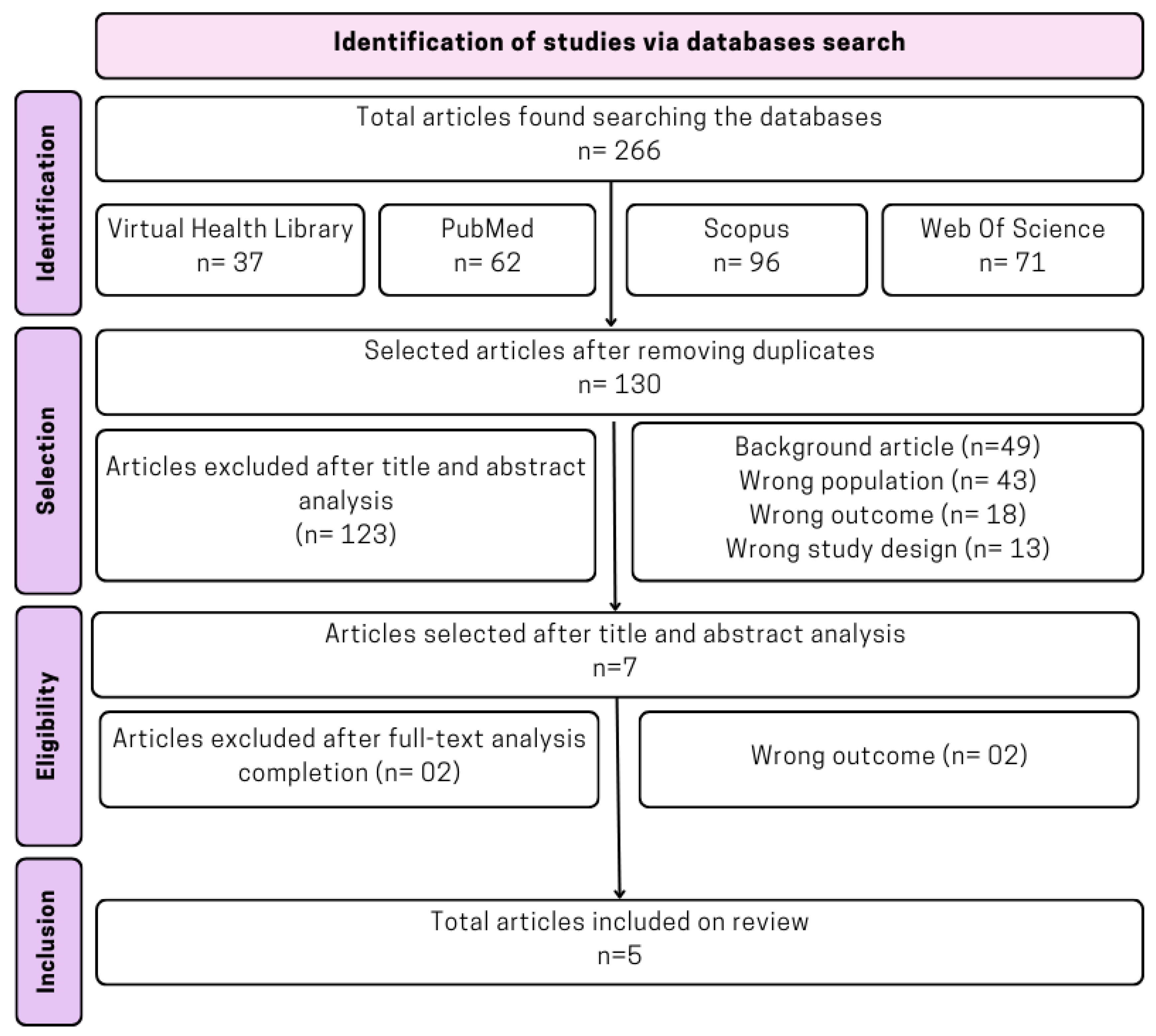
3.1. Articles’ Search and Selection
3.2. Selected Studies’ General Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zuckerman, H.; Pan, Z.; Park, C.; Brietzke, E.; Musial, N.; Shariq, A.S.; Iacobucci, M.; Yim, S.J.; Lui, L.M.W.; Rong, C.; et al. Recognition and Treatment of Cognitive Dysfunction in Major Depressive Disorder. Front. Psychiatry 2018, 9, 655. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Sena, T. Manual Diagnóstico e Estatístico de Transtornos Mentais—DSM-5, Estatísticas e Ciências Humanas: Inflexões sobre normalizações e normatizações. Rev. Int. Interdiscip. INTERthesis 2014, 11, 96–117. [Google Scholar] [CrossRef]
- Bains, N.; Abdijadid, S. Major Depressive Disorder; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://ncbi.nlm.nih.gov/books/NBK559078/ (accessed on 13 September 2024).
- Depression in Adults: Treatment and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2022. Available online: https://ncbi.nlm.nih.gov/books/NBK583074/ (accessed on 13 September 2024).
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: Hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Delva, N.C.; Stanwood, G.D. Dysregulation of brain dopamine systems in major depressive disorder. Exp. Biol. Med. 2021, 246, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Belujon, P.; Grace, A.A. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036–1046. [Google Scholar] [CrossRef]
- Kaur, G.; Chavan, B.S.; Gupta, D.; Sinhmar, V.; Prasad, R.; Tripathi, A.; Garg, P.D.; Gupta, R.; Khurana, H.; Gautam, S.; et al. An association study of dopaminergic (DRD2) and serotoninergic (5-HT2) gene polymorphism and schizophrenia in a North Indian population. Asian J. Psychiatr. 2019, 39, 178–184. [Google Scholar] [CrossRef]
- Sznabowicz, M.; Jasiewicz, A.; Iskra-Trifunović, J.; Małecka, I.; Karakiewicz, B.; Kotwas, A.; Samochowiec, J.; Grzywacz, A. Case-control study analysis of DRD2 gene polymorphisms in drug addicted patients. Psychiatr. Polska 2018, 52, 1013–1022. [Google Scholar] [CrossRef]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Frois, T.; Faria, L.O.; de Souza, R.P.; da Silva Cunha, A.E.; Nogueira, N.G.D.H.M.; Lim, V.T.; de Sousa Fortes, L.; de Miranda, D.M.; Bertollo, M.; Albuquerque, M.R. Are COMT Val158Met (rs4680), DRD2 TaqIA (rs1800497), and BDNF Val66Met (rs6265) polymorphisms associated with executive functions performance at rest and during physical exercise? Physiol. Behav. 2022, 257, 113973. [Google Scholar] [CrossRef]
- Petronis, A. The regulation of D2 dopamine receptor gene expression: Epigenetic factors should not be forgotten. Mol. Psychiatry 1999, 4, 212–213. [Google Scholar] [CrossRef]
- Kaminski, J.A.; Schlagenhauf, F.; Rapp, M.; Awasthi, S.; Ruggeri, B.; Deserno, L.; Banaschewski, T.; Bokde, A.L.; Bromberg, U.; Büchel, C.; et al. Epigenetic variance in dopamine D2 receptor: A marker of IQ malleability? Transl. Psychiatry 2018, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Ashford, J.W.; Kateb, B.; Sipple, D.; Braverman, E.; Dennen, C.A.; Baron, D.; Badgaiyan, R.; Elman, I.; Cadet, J.L.; et al. Dopaminergic dysfunction: Role for genetic & epigenetic testing in the new psychiatry. J. Neurol. Sci. 2023, 453, 120809. [Google Scholar] [CrossRef] [PubMed]
- Bidwell, L.C.; Karoly, H.C.; Thayer, R.E.; Claus, E.D.; Bryan, A.D.; Weiland, B.J.; YorkWilliams, S.; Hutchison, K.E. DRD2 promoter methylation and measures of alcohol reward: Functional activation of reward circuits and clinical severity. Addict. Biol. 2018, 24, 539–548. [Google Scholar] [CrossRef]
- Noble, E.P. D2 dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2002, 116, 103–125. [Google Scholar] [CrossRef] [PubMed]
- Ohira, K.; Yokota, H.; Hirano, S.; Nishimura, M.; Mukai, H.; Horikoshi, T.; Sawai, S.; Yamanaka, Y.; Yamamoto, T.; Kakeda, S.; et al. DRD2 TaqIA Polymorphism-Related Brain Volume Changes in Parkinson’s Disease: Voxel-Based Morphometry. Park. Dis. 2022, 2022, 8649195. [Google Scholar]
- Dos Santos, E.U.D.; Sampaio, T.F.; Tenório dos Santos, A.D.; Bezerra Leite, F.C.; da Silva, R.C.; Crovella, S.; Asano, A.G.C.; Asano, N.M.J.; de Souza, P.R.E. The influence of SLC6A3 and DRD2 polymorphisms on levodopa-therapy in patients with sporadic Parkinson’s disease. J. Pharm. Pharmacol. 2019, 71, 206–212. [Google Scholar] [CrossRef]
- Pan, Y.-Q.; Qiao, L.; Xue, X.-D.; Fu, J.-H. Association between ANKK1 (rs1800497) polymorphism of DRD2 gene and attention deficit hyperactivity disorder: A meta-analysis. Neurosci. Lett. 2015, 590, 101–105. [Google Scholar] [CrossRef]
- Aliasghari, F.; Nazm, S.A.; Yasari, S.; Mahdavi, R.; Bonyadi, M. Associations of the ANKK1 and DRD2 gene polymorphisms with overweight, obesity and hedonic hunger among women from the Northwest of Iran. Eat. Weight. Disord. Stud. Anorex. Bulim. Obes. 2020, 26, 305–312. [Google Scholar] [CrossRef]
- Rajeevan, H.; Osier, M.V.; Cheung, K.H.; Deng, H.; Druskin, L.; Heinzen, R.; Kidd, J.R.; Stein, S.; Pakstis, A.J.; Tosches, N.P.; et al. ALFRED: The ALelle FREquency Database. Update. Nucleic Acids Res. 2003, 31, 270–271. [Google Scholar] [CrossRef]
- Janssens, A.C.J.; Ioannidis, J.P.; van Duijn, C.M.; Little, J.; Khoury, M.J. Strengthening the reporting of genetic risk prediction studies: The GRIPS statement. Eur. J. Hum. Genet. 2011, 19, 833–836. [Google Scholar] [CrossRef]
- Rafikova, E.I.; Shibalev, D.V.; Shadrina, M.I.; Slominsky, P.A.; Guekht, A.B.; Ryskov, A.P.; Vasilyev, V.A. Influence of Polymorphic Gene Variants of the Dopaminergic System on the Risk of Disorders with Depressive Symptoms. Russ. J. Genet. 2021, 57, 942–948. [Google Scholar] [CrossRef]
- He, M.; Yan, H.; Duan, Z.X.; Qu, W.; Gong, H.Y.; Fan, Z.L.; Kang, J.Y.; Li, B.C.; Wang, J.M. Genetic distribution and association analysis of DRD2 gene polymorphisms with major depressive disorder in the Chinese Han population. Int. J. Clin. Exp. Pathol. 2013, 6, 1142–1149. [Google Scholar] [PubMed]
- Savitz, J.; Hodgkinson, C.A.; Martin-Soelch, C.; Shen, P.H.; Szczepanik, J.; Nugent, A.C.; Herscovitch, P.; Grace, A.A.; Goldman, D.; Drevets, W.C. DRD2/ANKK1 TaqIA polymorphism (rs1800497) has opposing effects on D2/3 receptor binding in healthy controls and patients with major depressive disorder. Int. J. Neuropsychopharmacol. 2013, 16, 2095–2101. [Google Scholar] [CrossRef]
- Vaske, J.; Makarios, M.; Boisvert, D.; Beaver, K.M.; Wright, J.P. The interaction of DRD2 and violent victimization on depression: An analysis by gender and race. J. Affect. Disord. 2009, 112, 120–125. [Google Scholar] [CrossRef]
- Koks, S.; Nikopensius, T.; Koido, K.; Maron, E.; Altmäe, S.; Heinaste, E.; Vabrit, K.; Tammekivi, V.; Hallast, P.; Kurg, A.; et al. Analysis of SNP profiles in patients with major depressive disorder. Int. J. Neuropsychopharmacol. 2006, 9, 167–174. [Google Scholar] [CrossRef]
- Aguet, F.; Brown, A.A.; Castel, S.E.; Davis, J.R.; He, Y.; Jo, B.; Battle, A.; Brown, C.D.; Engelhardt, B.E.; Montgomery, S.B. Genetic effects on gene expression across human tissues. Nature 2017, 550, 204–213. [Google Scholar]
- Wang, Y.; Liu, L.; Xin, L.; Fan, D.; Ding, N.; Hu, Y.; Cai, G.; Wang, L.; Xia, Q.; Li, X.; et al. The −141C Ins/Del and TaqIA polymorphism in the dopamine D2 receptor gene may confer susceptibility to schizophrenia in Asian populations. J. Clin. Neurosci. 2016, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, Q.; Vallada, H. Association study between the TaqIA (rs1800497) polymorphism and schizophrenia in a Brazilian sample. Arq. Neuropsiquiatr. 2014, 72, 582–586. [Google Scholar] [CrossRef] [PubMed]
- McGuire, V.; Van Den Eeden, S.K.; Tanner, C.M.; Kamel, F.; Umbach, D.M.; Marder, K.; Mayeux, R.; Ritz, B.; Ross, G.W.; Petrovitch, H.; et al. Association of DRD2 and DRD3 polymorphisms with Parkinson’s disease in a multiethnic consortium. J. Neurol. Sci. 2011, 307, 22–29. [Google Scholar] [CrossRef]
- Yu, M.; Huang, F.; Wang, W.; Zhao, C. Association between the DRD2 TaqIA gene polymorphism and Parkinson disease risk: An updated meta-analysis. Medicine 2019, 98, e17136. [Google Scholar] [CrossRef]

| Author | Year | Title | Country | Objective | Sample (n) | Results | Laboratorial Test | p-Value | Genotypic/ Allelic Frequency |
|---|---|---|---|---|---|---|---|---|---|
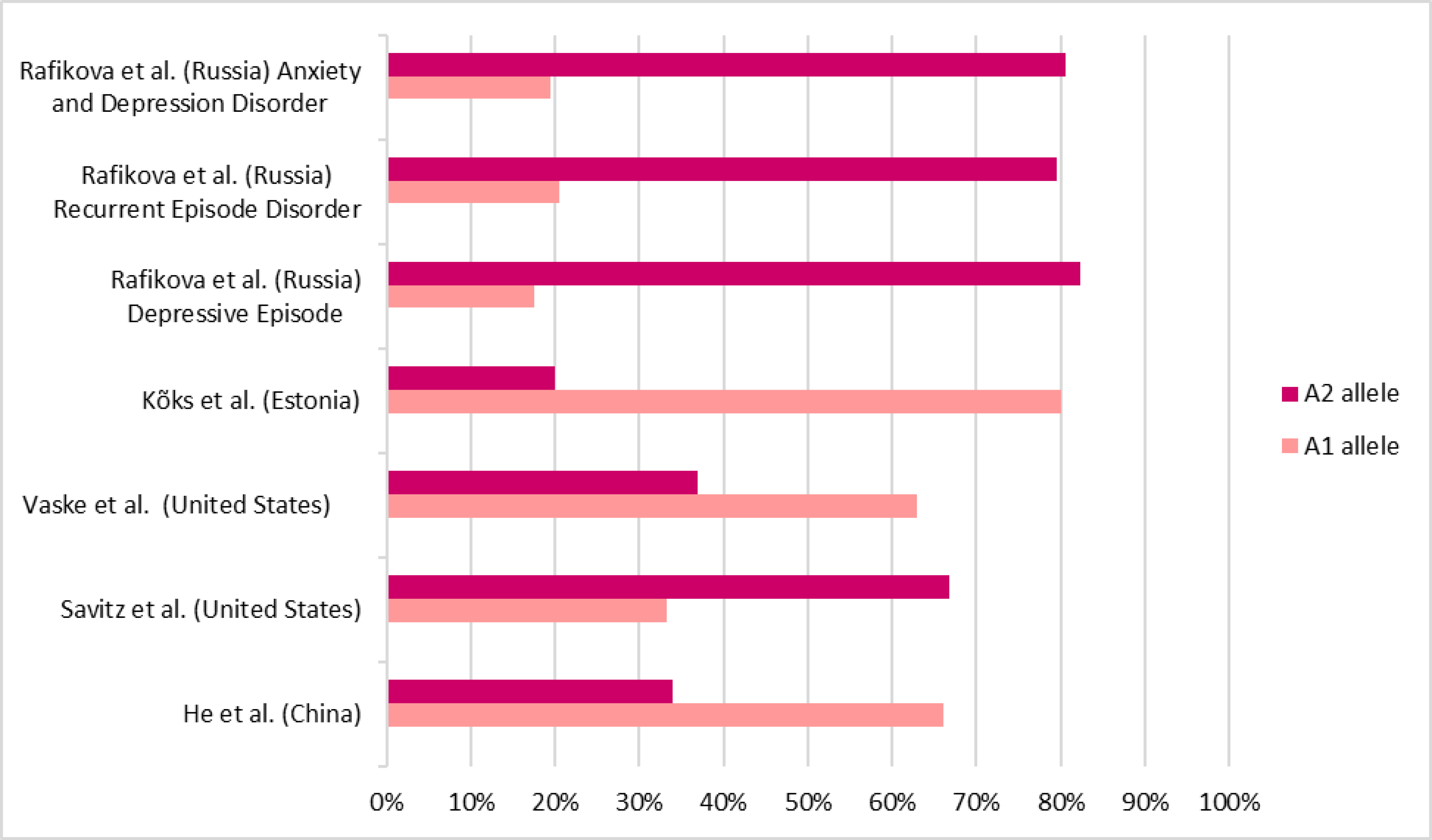
| Rafikova et al. [24] | 2020 | Influence of Polymorphic Gene Variants of the Dopaminergic System on the Risk of Disorders with Depressive Symptoms | Russia | Locate genetic risk factors for individuals diagnosed with a depressive episode, recurrent depression, and anxiety and depressive disorder. | DE *: n = 108 RD *: n = 149 MADD * n = 100 Control group: n = 163 | There was no statistical difference in the DRD2 rs1800497 SNP’s allelic and genotypic distributions and the risk of manifesting disorders with depressive symptoms. | PCR *; Electrophoresis andenzymatic digestion by TaqI. | G *: 0.644 A *:0.432 | DE: A2/A2: 72 (66.7%) A1/A2: 34 (31.5%) A1/A1: 02 (1.9%) A1 allele: 38 (17.6) A2 allele: 178 (82.4%) RD: A2/A2: 96 (64.4%) A1/A2: 45 (30.2%) A1/A1: 08 (5.4%) A1 allele: 61 (20.5%) A2 allele: 237 (79.5%) MADD: A2/A2: 64 (64%) A1/A2: 33 (33%) A1/A1: 03 (3.1%) A1 allele: 39 (19.5%) A2 allele: 161 (80.5%) Control group: A2/A2: 95 (58.3%) A1/A2: 63 (38.6%) A1/A1: 05 (3.1%) A1 allele: 7 (22.4%) A2 allele: 253 (77.6%) |
| He et al. [25] | 2013 | Genetic distribution and association analysis of DRD2 gene polymorphisms with major depressive disorder in the Chinese Han population | China | Screen and analyze the DRD2 gene’s TaqIA, C957T, and -141C polymorphisms among the Chinese population and their association with MDD. | MDD * group: n = 114 Control group: n = 224 | No evidence that the DRD2 gene’s TaqIA SNP * is associated with major depressive disorder was found. | PCR; Electrophoresis and enzymatic digestion by TaqI. | 0.200 | MDD group: A2/A2: 14 (12.2%) A1/A2: 50 (43.9%) A1/A1: 50 (43.9%) A1 allele: 66 (66%) A2 allele: 34 (34%) Control group: A2/A2: 28 (12.5%) A1/A2: 114 (50.9%) A1/A1: 82 (36.6%) A1 allele: 62 (62%) A2 allele: 38 (38%) |
| Savitz et al. [26] | 2013 | DRD2/ANKK1 TaqIA polymorphism (rs1800497) has opposing effects on D2/3 receptor binding in healthy controls and patients with major depressive disorder. | United States | Investigate the TaqIA polymorphism effect on the dopamine D2 receptor in healthy controls and MDD patients during dopamine release. | MDD group: n = 12 Control group: n = 24 | MDD patients with the A1 allele had increased BPND, while controls with the A1 allele had reduced BPND. | PCR; Electrophoresis and enzymatic digestion by TaqI. | MDD group: 0.033 Control group: 0.009 | MDD group: A2/A2: 05 (41.7%) A1/A2: 06 (50.0%) A1/A1: 01 (8.3%) Control group: A2/A2: 14 (53.8%) A1/A2: 10 (38.5%) A1/A1: 02 (7.7%) |
| Vaske et al. [27] | 2008 | The interaction of DRD2 and violent victimization on depression: An analysis by gender and race | United States | Analyze whether the relationships between DRD2, violent victimization, and depression statistically differ between men and women. | 2380 participants | Although no statistical difference was found between the male and female groups, statistical significance existed between the TaqIA SNP and depressive symptoms in the male group. | PCR; Electrophoresis and enzymatic digestion by TaqI. | Male × female = 0.968 TaqIA male group × depressive symptoms = 0.005 | A1 allele mean: Total = 0.532 ± 0.63 F * = 0.532 ± 0.63 M * = 0.532 ± 0.63 |
| Kõks et al. [28] | 2005 | Analysis of SNP * Profiles in patients with major depressive disorder | Estonia | Find associations with MDD by examining 91 single nucleotide polymorphisms located in 21 genes. | MDD group: n = 177 Control group: n = 160 | There was no statistical significance between the DRD2 TaqIA SNP and MDD manifestation. | PCR and GenoramaTM 4.1 genotyping software (Asper Biotech Ltd.) for identification of polymorphisms. | A1 allele frequency = 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Possatti, I.; Rodrigues Gontijo, B.; Fratelli, C.F.; Sousa Silva Bonasser, L.; de Souza Silva, C.M.; Rodrigues da Silva, I.C. DRD2/ANKK1 TaqIA Genetic Variant and Major Depressive Disorder: A Systematic Review. DNA 2024, 4, 345-354. https://doi.org/10.3390/dna4040024
Possatti I, Rodrigues Gontijo B, Fratelli CF, Sousa Silva Bonasser L, de Souza Silva CM, Rodrigues da Silva IC. DRD2/ANKK1 TaqIA Genetic Variant and Major Depressive Disorder: A Systematic Review. DNA. 2024; 4(4):345-354. https://doi.org/10.3390/dna4040024
Chicago/Turabian StylePossatti, Isabella, Bruna Rodrigues Gontijo, Caroline Ferreira Fratelli, Larissa Sousa Silva Bonasser, Calliandra Maria de Souza Silva, and Izabel Cristina Rodrigues da Silva. 2024. "DRD2/ANKK1 TaqIA Genetic Variant and Major Depressive Disorder: A Systematic Review" DNA 4, no. 4: 345-354. https://doi.org/10.3390/dna4040024
APA StylePossatti, I., Rodrigues Gontijo, B., Fratelli, C. F., Sousa Silva Bonasser, L., de Souza Silva, C. M., & Rodrigues da Silva, I. C. (2024). DRD2/ANKK1 TaqIA Genetic Variant and Major Depressive Disorder: A Systematic Review. DNA, 4(4), 345-354. https://doi.org/10.3390/dna4040024







