Abstract
DNA damage is induced by exogenous and endogenous sources, creating a variety of lesions. However, the cellular repair machinery that addresses and corrects this damage must contend with the fact that genomic DNA is sequestered in the nucleoprotein complex of chromatin. As the minimal unit of DNA compaction, the nucleosome core particle (NCP) is a major determinant of repair and poses unique barriers to DNA accessibility. This review outlines how the base excision repair (BER) pathway is modulated by the NCP and describes the structural and dynamic factors that influence the ability of BER enzymes to find and repair damage. Structural characteristics of the NCP such as nucleobase positioning and occupancy will be explored along with factors that impact the dynamic nature of NCPs to increase mobilization of nucleosomal DNA. We will discuss how altering the dynamics of NCPs initiates a domino effect that results in the regulation of BER enzymes.
1. Introduction
Genomic integrity is continuously threatened by a variety of DNA-damaging events. Damaging agents, such as the reactive oxygen species that are byproducts of metabolic processes or environmental agents such as ultraviolet light, cigarette smoke, or industrial chemicals, can be generated within the cell [1,2,3]. These agents account for a variety of damage types including single- and double-strand breaks, inter- and intra-strand crosslinks, mismatched nucleobases, and modification of the nucleobases.
When considering modification of the nucleobases, oxidation, alkylation, deamination, and hydrolysis reactions are the most common. In this review, modified nucleobases will be referred to as lesions. Approximately 100 lesions have been identified in vitro and many of these have been detected in cellular DNA [2]. Replication of lesions can have serious consequences for an organism [3]. Lesions can be mutagenic, meaning they are mispaired by a DNA polymerase during replication. They can also be cytotoxic, meaning that they cause a DNA polymerase to stall replication, leading to apoptosis. Genetic stability is essential for cell viability and the mutagenicity and cytotoxicity derived from nucleobase lesions can impact human health with outcomes ranging from neurodegenerative diseases to immune disorders, cancer, and aging [4,5,6,7,8,9,10].
Fortunately, organisms have robust DNA repair processes to assure the quality of further genetic advancement. These include direct reversal repair (DRR), mismatch repair (MMR), homologous recombination (HR), non-homologous end joining (NHEJ), single-strand break repair (SSBR), nucleotide excision repair (NER), and base excision repair (BER). Each of these processes plays an important role in maintaining genomic stability. In this review, we focus on the BER pathway, which functions to correct non-bulky nucleobase lesions that generally do not significantly distort the helical structure of DNA.
2. Base Excision Repair
BER is accomplished by a series of enzymes and can be considered to occur in two parts: excision of the lesion from the sugar–phosphate backbone and filling of the resulting “hole” with the appropriate canonical nucleobase [11,12,13,14]. Initiation of BER is determined by recognition of a lesion by a DNA glycosylase enzyme. There are 11 known human DNA glycosylases each with a specific target lesion(s) [15]. For an in-depth description of the substrate specificity of DNA glycosylases, we refer the reader to a comprehensive review article from the Eichman group [15]. DNA glycosylases use similar catalytic mechanisms for lesion excision [16], however, details of the methods used to search the genome and differentiate lesions from canonical nucleobases are not fully understood. Current data support that DNA glycosylases utilize a combination of short-range sliding and hopping techniques. Through these motions, DNA glycosylases are able to survey ~70,000 base pairs (bp) of DNA [17] in the search for the rare instance of a lesion—a needle in a haystack. Recent work has shown that UV-damaged DNA binding protein (UV-DDB), which serves as a damage sensor in global genomic NER, stimulates DNA glycosylase activity in vitro raising the intriguing possibility that it may contribute to the searching process [18,19].
DNA glycosylases have been described to interrogate a region of DNA and some have been shown use exosite pockets in the enzyme to inspect each nucleobase [17]. Structural and dynamic properties of base pairs are used to differentiate between canonical and damaged bases, collapsing canonical bases back into place in the DNA helix and shifting target lesions into the active site for excision. The nonspecific interactions that control these transfers allow the DNA glycosylase to quickly differentiate damaged from undamaged nucleobases, preventing competitive inhibition of the enzyme by canonical bases which can exist in 30,000-fold excess over the target lesion(s) [17].
When a lesion enters the active site, a DNA glycosylase catalyzes its excision by cleaving the glycosidic bond that attaches the lesion to the sugar–phosphate backbone [11,16,20]. A DNA glycosylase may fall into one of two classifications: monofunctional or bifunctional (Figure 1). A monofunctional DNA glycosylase catalyzes glycosidic bond cleavage through a substitution reaction using an activated water molecule as the nucleophile (Step 1). The resulting abasic (AP) site is further acted upon by apurinic/apyrimidinic endonuclease 1 (APE1) to form a nick in the backbone with 3′-OH and 5′-deoxyribose phosphate (5′-dRP) termini (Step 2). The subsequent enzyme in the pathway, DNA polymerase β (Pol β), has two roles. It catalyzes removal of the 5′-dRP group (Step 3a) and incorporates a canonical deoxynucleotide at the 3′-OH (Step 3b). A DNA ligase seals the resulting nick to complete the repair event (Step 4).
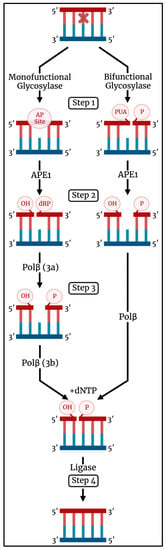
Figure 1.
Schematic illustration of the BER pathway initiated by a monofunctional (left) and bifunctional (right) DNA glycosylase. A lesion is recognized and excised by a DNA glycosylase (Step 1). APE1 processes the product of glycosylase activity by incising the AP site (for a monofunctional glycosylase) or removing the 3′-PUA (for a bifunctional glycosylase) (Step 2). Pol β removes the 5′-dRP (for a monofunctional glycosylase) and incorporates a canonical nucleotide at the 3′-OH (Step 3). A DNA ligase/XRCC1 complex seals the nick to complete the repair. Image created using BioRender (biorender.com (accessed on 29 September 2022)).
A bifunctional DNA glycosylase initiates glycosidic bond cleavage using an amino group in the enzyme active site as the nucleophile. In addition to glycosidic bond cleavage, bifunctional DNA glycosylases can catalyze β-elimination of the DNA backbone by formation of a Schiff base, leading to a break in the backbone with 3′-α,β-unsaturated aldehyde (PUA) and 5′-phosphate termini (Step 1). Some bifunctional glycosylases also catalyze δ-elimination to yield a 3′-phosphate. APE1 can act on the elimination product to form a 3′-OH terminus (Step 2) used by Pol β for deoxynucleotide incorporation (Step 3), and DNA ligase seals the nick and completes the repair (Step 4). In some instances, the β-elimination activity of a bifunctional DNA glycosylase may be bypassed with APE1 acting directly on the abasic site [21,22]. Interestingly, it was recently demonstrated that a small-molecule activator of 8-oxo-7,8-dihydroguanine glycosylase 1 (OGG1), a bifunctional DNA glycosylase, alters the repair process in cells to no longer require APE1 but rather depend on polynucleotide kinase phosphatase activity [23]. XRCC1 is a scaffold protein that plays a role in BER by interacting with Pol β and DNA ligase [24].
The above-described process of recognition, removal, and replacement of a lesion is known as short-patch BER (SP-BER) and is the predominant form of BER. In an instance when chemical modification of the 5′-dRP blocks the dRP lyase activity of Pol β, for example under conditions of oxidative or alkylative stress, a process known as long-patch BER (LP-BER) is used [25,26]. The polymerase performs strand-displacement synthesis incorporating multiple (between two and six) deoxynucleotides at the nick. Flap endonuclease 1 (FEN1) removes the displaced single-stranded flap of DNA, which contains the modified dRP at the 5′-terminus, and the resulting nick is sealed by DNA ligase.
While working to maintain the integrity of the three billion base pairs in the human genome, the above-described BER enzymes function to complete highly complex and vital roles. It is known that these BER enzymes are clinically important, and that deficiencies or inactivity can have detrimental consequences for human health [27,28,29,30,31,32]. However, successful completion of the repair process requires that the repair enzymes can physically access the site of DNA damage, which is not always the case.
3. The Nucleosome Core Particle
To manage the vast amount of genetic material, eukaryotic systems utilize dense packaging known as chromatin, which is organized into arrays of nucleosomes. Nucleosomes were first observed via electron microscopy (EM) and were described as “linear arrays of spherical chromatin particles” [33]. Each of the spherical particles is now known to represent the simplest unit of packaged DNA, the nucleosome core particle (NCP). The first crystallographic analysis of an NCP revealed the wrapping of DNA around a protein core [34]. Subsequent and higher-resolution crystallography structures provided near atomic-level detail of how DNA is bound to and organized by the protein core [35]. In an NCP, 145–147 bp of DNA wrap tightly in a left-handed orientation making 1.65 rotations around a protein core comprised of the four histones H2A, H2B, H3, and H4 (Figure 2). Each histone has a central folded domain, called the histone fold, flanked by disordered N- and C-terminal tail regions. The histone core is organized and formed by two H2A/H2B dimers and an H3/H4 tetramer. A two-fold axis of symmetry runs through the histone core and is referred to as the dyad axis [35].
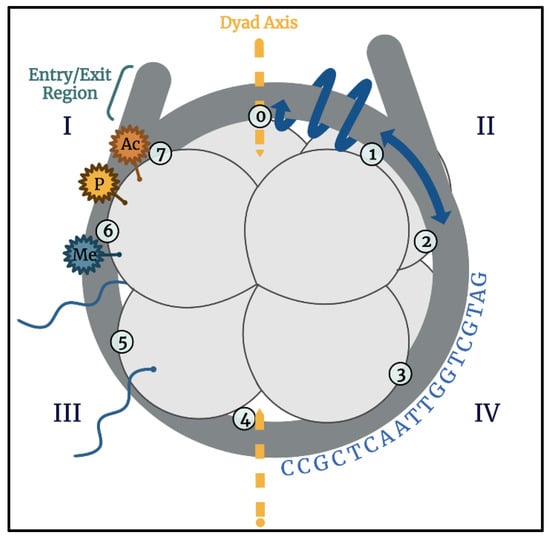
Figure 2.
Representation of a nucleosome core particle (NCP) showing the histone core (light gray) and DNA (dark gray). The dyad axis, entry/exit regions, and seven SHLs (circles numbered 1–7) are labeled. For simplicity, the NCP is divided into four quadrants to depict factors that will be discussed in this review: post translational modifications (region I), rotational and translational nucleobase positioning (region II, blue arrows), unstructured N-terminal histone tails (region III), and DNA-sequence-dependent factors (region IV). Image created using BioRender.
Throughout the nucleoprotein complex of an NCP are various energetically important points of contact. Seven locations of major groove–histone contacts known as super-helical locations (SHLs) are located between each end of histone-bound DNA, referred to as the entry/exit regions, and the dyad axis [35]. These contacts represent landmarks which are used as references to translationally label base pairs throughout the NCP. Similarly, the histone core serves as a reference to rotationally label each DNA nucleobase depending on whether it faces inward towards the histones, outward towards solution, or somewhere in between (Figure 2, II, blue arrows). These translational and rotational positions are commonly identified using chemical and enzymatic footprinting techniques. Defining these geometric and positional relationships provides key information to understand how BER at specific lesion locations may be impacted by structural limitations such as steric hindrance by the histone core and solvent accessibility.
Moving from the NCP to a higher level of packaging, in a nucleosome array, a string of NCPs are spaced at intervals of 200 ± 40 bp and are stabilized by the linker histone H1. Electron cryo-microscopy (cryo-EM) and crystal structures of a 197 bp nucleosome with two linker DNA arms, revealed that H1 binds both linkers, draws the two arms together, and induces a more compact and rigid conformation [36]. Cryo-EM analysis of arrays of multiple nucleosomes has provided mechanistic details of how nucleosomes assemble into even-higher-order chromatin structures [37,38,39,40,41,42,43,44].
The ubiquitous sequestration of DNA presents a conundrum for BER enzymes, which must interact intimately with DNA. Therefore, understanding the physicochemical properties of packaged DNA may unlock information relevant to the methods cells use to regulate DNA access and provide insight on the biological limitations of repair in a genomic context. The next sections of this review will describe the structural and dynamics of NCPs and how these factors may initiate a domino effect to modulate and influence BER.
4. Structural Characteristics Dictate Accessibility of Nucleosomal DNA
Incorporation of DNA into nucleosomes can be described in terms of nucleosome positioning and occupancy. While positioning refers to the location of NCPs on genomic DNA, occupancy reflects how likely a region of genomic DNA is to be bound in an NCP (i.e., density). Neither positioning nor occupancy are random but rather sequences are deliberately included or excluded from nucleosomes [45,46,47].
Chemical footprinting and crystallographic analyses of NCPs have revealed that the wrapping of DNA around the histone core distorts the DNA structure. For example, the periodicity of DNA in an NCP averages 10.2 bp/turn compared to 10.5 bp/turn in the unpackaged duplex [35,43,48], causing an energetic strain which is compensated for by electrostatic interactions between the positively charged histones and the negatively charged DNA [49]. In turn, the flexibility of a DNA sequence has been identified as a major determinant of its ability to incorporate into a nucleosome (Figure 2, IV) [40,45,46,47,49,50].
The Widom 601 DNA sequence provides an example of how sequence determines positioning [50]. This sequence was selected from a library of 5 × 1012 chemically synthetic random DNA molecules as having the highest affinity for the histone octamer [50]. Further analysis revealed that the 601 DNA also has strong positioning ability. The presence of a TA/TT/AA dinucleotide every ~10 bp allows the DNA to be compressed and bend into the minor groove [50]. This periodic distribution of dinucleotides was also observed in nucleosomes isolated from biological sources and is now known to be hallmark of nucleosome positioning. An experimental advantage of using a strong positioning sequence such as 601 DNA is that it has specific, predictable binding to histones and provides a homogeneous population of structurally well-defined NCPs [51,52]. However, data obtained using a strong positioning sequence are likely not broadly reflective of the behavior of all regions of the genome which include strongly-positioning sequences, sequences that excluded NCPs, and everything in between.
In contrast to strong positioning sequences, weak positioning sequences with low flexibility have been observed in arrays with low nucleosome occupancy, in regions of nucleosome depletion, and in regions important for promotor accessibility, transcriptional activity, and other genomic processes. Some polymeric sequences, in particular poly(A:T) tracts, disfavor interaction with the histone core due to their limited flexibility [53] and prevent formation of nucleosomes in the promoter regions of eukaryotic genomes [47,54]. Notably, however, computational analysis was correct in predicting nucleosome positioning only half of the time based on sequence alone, reflecting the complexity of positioning [45]. Though these weaker positioning sequences do not create defined nucleosome populations like 601 DNA, which can complicate interpretation of the results, their biologically-relevant sequence patterns are vital for understanding how sequence alone may influence native systems.
Analysis of genome-wide mutational spectra revealed that nucleosome positioning and occupancy influence mutational patterns [55]. Some mutation types are biased towards nucleotides that are bound in a nucleosome compared to those in linker DNA [55,56]. In turn, these reinforced lesion patterns vary across the genome to modulate DNA accessibility, forming a dependent relationship between sequence and accessibility. This relationship has also been seen to directly impact both the formation and repair of DNA lesions [55,56].
5. Structural Components of the Nucleosome Core Particle Impact BER
Using a number of biochemical strategies and model systems, a variety of research groups have reported that the physical location of a lesion in an NCP impacts how well it can be repaired by BER [13,57,58,59,60,61,62]. These experiments typically exploit the fact that BER enzymes break (or the product can be chemically converted to a break) or make bonds in the sugar–phosphate backbone. Therefore, enzyme activity can be monitored by changes in the size of DNA fragments using sequencing gel electrophoresis. Many of these biochemical experiments were made possible by the use of strong positioning sequences such as Widom 601 DNA. Other positioning sequences such as 5s rDNA sequence, a naturally occurring sequence derived from the 5s ribosomal RNA gene, and α-satellite DNA found at centromeres, have also been used [35]. The use of a positioning sequence allows for the creation of a homogenous population of NCPs with lesions in well-defined rotational and translational positions [50].
The initiation of BER on NCPs by DNA glycosylases has been extensively studied in reconstituted mononucleosomes and it is generally accepted that lesions that face outward from the histone core are more readily excised than those that are sterically occluded by facing inward towards histones [13,24,57,58,59,60,61,62]. An exception is near the dyad axis where several DNA glycosylases have been shown to have suppressed activity regardless of the rotational positioning of the lesion, which may arise from the underwinding of DNA near SHL 0. Due to transient unwrapping, DNA in the entry–exit regions can, at times, be repaired similarly to unpackaged DNA. Notably, these biochemical observations are consistent with genome-wide patterns of DNA damage accumulation and mutational density; lesions and mutations accumulate near the dyad axis of positioned nucleosomes and at regions of the DNA facing the histone core [63,64,65].
Histone variants are proteins that can substitute for the core histones and have distinct amino acid sequences [66]. These variants confer different structural properties on nucleosomes, influence positioning, affect gene expression and DNA repair, and contribute to disease [66]. Several histone variants have been shown to facilitate DNA glycosylase activity by increasing access to otherwise occluded lesion sites [67,68,69].
Notably, histone N-terminal tails can participate in DNA damage repair by reacting with the AP site product formed by DNA glycosylases. AP sites are known to be chemically labile, and they can be further destabilized in NCPs. Lysine residues in the N-terminal tail regions can form DNA–protein crosslinks with AP sites, which are susceptible to strand cleavage via an elimination reaction [70,71,72,73]. Furthermore, in a process enhanced by histones, the bifunctional DNA glycosylase OGG1 was found to crosslink the 3′-PUA product and hinder the subsequent steps of BER. However, in the presence of APE1 the formation of these crosslinks is suppressed [62].
Enzymes acting downstream of DNA glycosylases can also be impacted by the packaging of DNA into nucleosomes. The ability of APE1 to incise DNA is dependent on the rotational positioning of the AP site [74,75,76,77]. A cryo-EM structure of APE1 bound to an NCP revealed that the enzyme uses a “sculpting mechanism” to bend the nucleosomal DNA and catalyze incision at a solution-accessible AP site [74]. Reports of BER on twelve-nucleosome arrays demonstrated that DNA glycosylase and APE1 activity are inhibited or accelerated in this higher-order packaging depending on the rotational and translational position of the lesion [78,79].
The nucleotide incorporation activity of Pol β on NCPs and nucleosome arrays is decreased on an NCP relative to unpackaged DNA, with the amount of suppression depending on the solution-accessibility of the gap [58,78,80,81,82,83]. In contrast, the dRP lyase activity of Pol β is comparable in unpackaged DNA and an NCP and is not hindered by the presence of the histone core [81]. Interestingly, even the absence of modification of the 5′-dRP, LP-BER can occur on the linker DNA between nucleosomes but was not observed for DNA bound to the histone octamer [84]. The two activities of Pol β are catalyzed by separate domains and the observation that one is hindered in an NCP while the other is not may derive from distinct binding modes and/or interactions with nucleosomes.
The BER enzymes have been shown to work cooperatively and, in some cases, to stimulate each other. Indeed, Pol β nucleotide incorporation activity is enhanced on NCPs in the presence of DNA ligase IIIα-XRCC1 (LigIIIα-XRCC1) [85]. Similarly, Pol β nucleotide incorporation activity is enhanced by the chromatin remodeler SWI/SNF [67,78] and the architectural factor HMGB1 [83]. For the final step of BER, LigIIIα-XRCC1 [85] may require transient unwrapping from the histones to seal a nick [85] whereas DNA ligase I may not [86,87,88].
BER enzymes may also use other unique physical characteristics or be modulated by interactions with proteins from other pathways. Single-strand selective monofunctional uracil DNA glycosylase 1 (SMUG1) uses a helical wedge to distort DNA to recognize and access lesion sites [89]. Alkyladenine DNA glycosylase (AAG) has been shown to interact with transcription machinery such as transcriptional receptors, estrogen receptor α, the transcription elongation complex, and RNA polymerase II. These relationships have been seen to stimulate DNA glycosylase activity, promoting both repair and transcription and developing a dependent relationship between BER and transcription [90,91,92,93,94,95,96,97]. More research is needed regarding the interplay between BER and related processes to understand how DNA glycosylases as well as downstream BER enzymes may be capable of overcoming nucleosomal obstacles.
6. Dynamics of Nucleosome Core Particles
Of the ~146 bp of nucleosomal DNA, ~120 bp are in direct contact with the histone fold domains while the other ~13 bp at each entry/exit region are more loosely bound by alpha-helices unique to H2 [35]. These loosely bound ends undergo spontaneous and transient unwrapping and rewrapping from the histones, often referred to as DNA breathing [98,99,100]. Much of this breathing is controlled by the disordered N-terminal histone tail regions which aid in keeping the nucleosome closed via interactions with one another and with nucleosomal DNA (Figure 2, III). Indeed, studies on NCPs with and without N-terminal histone tails have shown that the lack of histone tails increases DNA breathing by influencing nucleosome opening in a largely sequence-dependent manner [98,99]. These actions enable exposure of regions of DNA that are otherwise inaccessible to protein binding.
Ensemble and single-molecule fluorescence resonance energy transfer (FRET) experiments have shown that nucleosomes unwrap asymmetrically and exist in a partially unwrapped state 2–10% of the time [100]. Spontaneous sliding has also been observed in which the nucleosome repositions itself by altering the translational position of the DNA. This mobility is mainly limited to the entry/exit regions and is significantly reduced by the presence of linker histones [101]. Protein binding to nucleosomal DNA during these windows of partial destabilization further facilitates increased DNA mobility by shifting the unwrapping equilibrium. This observation supports that spontaneous site exposure via DNA breathing may modulate site accessibility for protein binding in locations that may otherwise be translationally or rotationally hindered [102,103].
When considering the mechanism of nucleosome sliding, loop and twist defects have been proposed [101]. The loop method uses a histone core “scooting” technique where an entry/exit region spontaneously unwraps, and while it most often rewraps in the same location, occasionally the DNA is pulled in and binds to the histone core forming a DNA bulge. Through a series of these bulge-intermediate structures, small translational changes can account for a larger positioning change [101].
Structural changes to a nucleosome can also occur through a twist defect, in which a bulge is formed by either an additional or a missing base pair [101]. This twist defect can then translate through the nucleosome promoting a series of either over- or under-twisted intermediate structures. These defects have also been thought to recruit chromatin remodelers which may use these stepwise twist or loop defects to displace previously stabilized nucleosomes [101,104]. Though the loop and twist defects can cause larger changes in nucleosome positioning, they rarely occur across the genome and, more commonly, smaller spontaneous changes are the cause of slight unwrapping [101,104].
In addition to DNA unwrapping, the histone protein core is dynamic with histone exchange and deposition of histone variants [105,106]. Single-molecule FRET experiments have also demonstrated that nucleosomes experience spontaneous gaping, where the two gyres of an NCP separate from each other like the hinged motion of a clam shell [107]. Interestingly, a DNA glycosylase has also been shown to alter inter-nucleosomal interactions by decompacting chromatin fibers and condensing nucleosome arrays, demonstrating that binding of BER enzymes also impacts chromatin structure [108].
7. Energetic Environment Dictates Accessibility of Nucleosomal DNA
One factor that affects the energetic landscape of the NCP is the existence of post-translational modifications (PTMs) (Figure 2, I) [98]. These modifications occur after protein biosynthesis by either enzymatic or non-enzymatic additions to amino acids, creating a diverse population of histones which differ in their chemical makeup. Modification of histones is extensive and commonly known PTMs include acetylation, methylation, phosphorylation, ubiquitylation, ADP-ribosylation, crotonylation, succinylation, and malonylation [109]. Although PTMs occur commonly on N-terminal histone tails, these modifications are known to influence internucleosomal interactions in higher order structures, but do not significantly impact stability at the mononucleosome level. PTMs on the globular histone core, however, have been seen to affect mononucleosomal structure in a variety of ways depending on the electrostatic charge and bulkiness of the PTM [109]. A single PTM can reduce the free energy for nucleosome formation by 2 kcal/mol, increasing the structure’s stability and increasing the probability of an altered structure by a factor of 25 [109,110,111].
Histone PTMs can have a range of structural and dynamic impacts on DNA–histone and histone–histone interactions. To describe the effects of PTMs, one can broadly consider the interactions between the histone tails and nucleosomal DNA, the DNA–histone interactions in the entry/exit regions and near the dyad axis, and the histone–histone interfaces. PTMs have been shown to alter these interactions, often reducing DNA–protein affinity, and destabilizing the nucleosome at large. Electrostatic interactions are very important as their balance plays a major role in maintaining a stable complex between DNA and the histone core [109,110,111,112].
Decreased DNA–protein affinity can weaken structural regulation, stabilization, and compaction [109,110,111,112]. Crystallography and biochemical experiments on nucleosomes containing histones modified with a single acetylation site, which neutralizes the positive charge on a lysine side chain, have demonstrated increased disassembly specifically in the dyad region [110]. Acetylation of lysines 115 and 122 of histone H3 enhances the efficiency of ATP-dependent disassembly of nucleosomes mediated by chromatin remodelers [110]. These observations have been further supported by FRET studies that captured structural distortion of the nucleosome resulting in destabilization to expose target binding sites buried in the nucleosome with key locations at sites of transcription-factor binding located near the dyad axis and entry/exit regions [45,113]. Establishing this dependent relationship between PTMs and nucleosome unwrapping supported the previous hypothesis that structural factors influence NCP dynamics and reduce DNA–histone association [98,109,110,111,112,113,114,115,116,117,118].
8. Dynamics of the Nucleosome Core Particle Impact DNA Repair
Structural and energetic effects of PTMs can be thought of as a chemical code: providing instructions for cellular components in order to stimulate activity and signal interactors to histone binding sites [109]. In addition to the increased mobility caused by DNA breathing, PTMs that affect the nucleosome’s dynamic environment can further shift the unwrapping equilibrium as a mean of improving DNA accessibility [62,117,119,120]. Some ways in which PTMs influence mobility include unwrapping, rewrapping, sliding, assembly, and disassembly (Figure 3) [109]. Nucleosomes containing histone variants have also been shown to have similar effects on the nucleosome by increasing mobility [50,109].
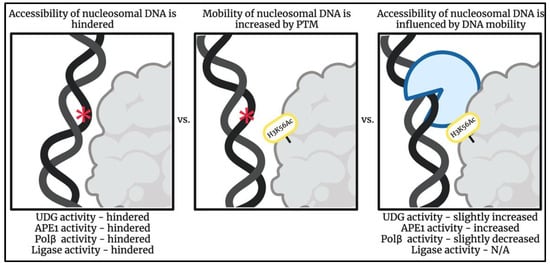
Figure 3.
Schematic illustrating how a post-translational modification, H3K56Ac, can increase DNA mobility allowing for altered binding patterns of BER enzymes in regions otherwise hindered [117,118,121,122,123,124,125,126,127,128,129,130,131]. Impact of H3K56Ac on DNA ligase activity has not been reported. DNA lesion represented by red asterisk symbol. Image created using BioRender.
Many studies utilizing single-site histone modifications have reported that PTMs influence nucleosome dynamics, and eukaryotic systems may exploit these changes to regulate access to DNA. For example, an acetylation site located in the dyad region [110,118] as well as at several locations throughout the nucleosome [117] has been shown to contribute to increased nucleosome disassembly. Increased mobilization affects the binding properties of chaperones, remodelers, and other proteins known to aid in DNA wrapping that impacts nucleosomal processes that are otherwise thermodynamically and/or physiologically unfavored (Figure 3) [109].
Experimental results of destabilization and expansion by charged PTMs have supported a connection between PTMs on the NCP’s histone core and their effects on nucleosomal DNA accessibility [110,117,118]. PTMs that influence nucleosome repositioning, assembly, and disassembly increase site exposure and occur in areas of the nucleosome where repair in the nucleosome may be otherwise hindered. Examples of these modifications include the acetylation of lysine 56 on histone H3 (H3K56) [118,121,122,123,124,125,126], acetylation of lysine 91 on histone H4 [132], and phosphorylation of serine 28 on histone H3 [133].
Acetylation of H3K56 enhances APE1 activity [127] whereas acetylation of H3K56 and H3K14 has been shown to decrease Pol β nucleotide-incorporation activity near the dyad region of a mononucleosome [80]. H3K56 is acetylated during S phase by CBP/p300 once the histone is incorporated into an NCP [128]. In the absence of DNA damage, the deacetylases Hst3 and Hst4 remove the PTM during G2 [129,130]. It is known that defects in this regulation of H3K56 acetylation render cells sensitive to alkylating agents [125], which are known to generate lesions that are repaired by BER. At the molecular level, H3K56 interacts with a phosphate in the DNA backbone [131], and charge neutralization via acetylation likely causes increased dynamics. While H3 is located near the dyad axis it has been reported that acetylation of H3K56 influences DNA dynamics throughout the NCP and its effects are not localized to the dyad axis [117]. The differing impacts of this PTM on APE1 and Pol β may reflect different bind modes of these enzymes.
In studies on twelve-nucleosome arrays, the combined activity of a DNA glycosylase and APE1 were examined with acetylation of H3K18 and H3K27 [134]. Modification of H3K18 resulted in an increase in the incised DNA product while modification of H3K27 had the opposite effect. Given that CBP/p300 is responsible for installing both of these PTMs, this acetyltransferase may play a direct role in modulating the BER pathway in chromatin.
9. Future Outlooks
Genetic compaction into chromatin poses a unique barrier for cellular machinery, including those involved with processes of DNA repair. Understanding the complex dynamics of the NCP will provide vital information to elucidate the physical accessibility of genomic DNA. Appreciating that PTMs are capable of altering the ways that DNA interacts with histones, it is of interest to examine how PTMs alter overall nucleosome dynamics. Data on this subject is often collected using techniques of structural mapping and chemical reactivity in vitro. Although these techniques have allowed for the analysis of nucleosomal interactions in a controlled setting, many currently used systems lack global aspects of native nucleosomes.
To account for these limitations, future studies will benefit from model systems of increased complexity. For example, in vitro systems involving global damage and PTMs as well as those consisting of higher-order structures will allow for a deeper interpretation of processes that occur in nature. In vivo studies will fill major knowledge gaps to identify on a genome-wide scale how the amount and location of lesions are modulated by PTM. Exploring the relationships between BER and other processes such as transcription will also be important to identify any crosstalk or collaboration. These future directions will bypass limitations of current in vitro approaches aimed at exploring complex nucleosome relationships involving PTMs to provide further insight on how histone modifications and other nucleosomal factors, both structural and energetic, may function as teammates to create a domino effect between nucleosome dynamics and the regulation of BER enzymes.
Author Contributions
J.C.C. and S.D. equally contributed to this review. All authors have read and agreed to the published version of the manuscript.
Funding
Research in the Delaney laboratory has been supported by the National Science Foundation from awards MCB-1817417 and MCB-2111680. J.C.C. has been supported by a training grant from the National Institute of Environmental Health Sciences (T32ES007272).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lindahl, T. Instability and decay of the primary structure of DNA. Nature 1993, 362, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Gates, K.S. An overview of chemical processes that damage cellular DNA: Spontaneous hydrolysis, alkylation, and reactions with radicals. Chem. Res. Toxicol. 2009, 22, 1747–1760. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.K. DNA Damage, Mutagenesis and Cancer. Int. J. Mol. Sci. 2018, 19, 970. [Google Scholar] [CrossRef] [PubMed]
- Sarno, A.; Lundbæk, M.; Liabakk, N.B.; Aas, P.A.; Mjelle, R.; Hagen, L.; Sousa, M.M.L.; Krokan, H.E.; Kavli, B. Uracil-DNA glycosylase UNG1 isoform variant supports class switch recombination and repairs nuclear genomic uracil. Nucleic Acids Res. 2019, 47, 4569–4585. [Google Scholar] [CrossRef] [PubMed]
- Al-Tassan, N.; Chmiel, N.H.; Maynard, J.; Fleming, N.; Livingston, A.L.; Williams, G.T.; Hodges, A.K.; Davies, D.R.; David, S.S.; Sampson, J.R.; et al. Inherited variants of MYH associated with somatic G:C-->T:A mutations in colorectal tumors. Nat. Genet. 2002, 30, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Markkanen, E.; Fischer, R.; Ledentcova, M.; Kessler, B.M.; Dianov, G.L. Cells deficient in base-excision repair reveal cancer hallmarks originating from adjustments to genetic instability. Nucleic Acids Res. 2015, 43, 3667–3679. [Google Scholar] [CrossRef]
- Qamar, N.; Fuleihan, R.L. The hyper IgM syndromes. Clin. Rev. Allergy Immunol. 2014, 46, 120–130. [Google Scholar] [CrossRef]
- SenGupta, T.; Palikaras, K.; Esbensen, Y.Q.; Konstantinidis, G.; Galindo, F.J.N.; Achanta, K.; Kassahun, H.; Stavgiannoudaki, I.; Bohr, V.A.; Akbari, M.; et al. Base excision repair causes age-dependent accumulation of single-stranded DNA breaks that contribute to Parkinson disease pathology. Cell Rep. 2021, 36, 109668. [Google Scholar] [CrossRef]
- Shah, A.; Gray, K.; Figg, N.; Finigan, A.; Starks, L.; Bennett, M. Defective Base Excision Repair of Oxidative DNA Damage in Vascular Smooth Muscle Cells Promotes Atherosclerosis. Circulation 2018, 138, 1446–1462. [Google Scholar] [CrossRef]
- Weissman, L.; Jo, D.G.; Sørensen, M.M.; de Souza-Pinto, N.C.; Markesbery, W.R.; Mattson, M.P.; Bohr, V.A. Defective DNA base excision repair in brain from individuals with Alzheimer’s disease and amnestic mild cognitive impairment. Nucleic Acids Res. 2007, 35, 5545–5555. [Google Scholar] [CrossRef]
- Schermerhorn, K.M.; Delaney, S. A chemical and kinetic perspective on base excision repair of DNA. Acc. Chem. Res. 2014, 47, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Caldecott, K.W. Mammalian DNA base excision repair: Dancing in the moonlight. DNA Repair 2020, 93, 102921. [Google Scholar] [CrossRef] [PubMed]
- Meas, R.; Wyrick, J.J.; Smerdon, M.J. Nucleosomes Regulate Base Excision Repair in Chromatin. Mutat. Res. Rev. Mutat. Res. 2019, 780, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.S. Base excision repair: A critical player in many games. DNA Repair 2014, 19, 14–26. [Google Scholar] [CrossRef]
- Brooks, S.C.; Adhikary, S.; Rubinson, E.H.; Eichman, B.F. Recent advances in the structural mechanisms of DNA glycosylases. Biochim. Biophys. Acta 2013, 1834, 247–271. [Google Scholar] [CrossRef]
- Mullins, E.A.; Rodriguez, A.A.; Bradley, N.P.; Eichman, B.F. Emerging Roles of DNA Glycosylases and the Base Excision Repair Pathway. Trends Biochem. Sci. 2019, 44, 765–781. [Google Scholar] [CrossRef]
- Friedman, J.I.; Stivers, J.T. Detection of damaged DNA bases by DNA glycosylase enzymes. Biochemistry 2010, 49, 4957–4967. [Google Scholar] [CrossRef]
- Jang, S.; Kumar, N.; Beckwitt, E.C.; Kong, M.; Fouquerel, E.; Rapić-Otrin, V.; Prasad, R.; Watkins, S.C.; Khuu, C.; Majumdar, C.; et al. Damage sensor role of UV-DDB during base excision repair. Nat. Struct. Mol. Biol. 2019, 26, 695–703. [Google Scholar] [CrossRef]
- Jang, S.; Schaich, M.A.; Khuu, C.; Schnable, B.L.; Majumdar, C.; Watkins, S.C.; David, S.S.; Van Houten, B. Single molecule analysis indicates stimulation of MUTYH by UV-DDB through enzyme turnover. Nucleic Acids Res. 2021, 49, 8177–8188. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Coskun, E.; Jaruga, P. Repair of oxidatively induced DNA damage by DNA glycosylases: Mechanisms of action, substrate specificities and excision kinetics. Mutat. Res. Rev. Mutat. Res. 2017, 771, 99–127. [Google Scholar] [CrossRef]
- Vidal, A.E.; Hickson, I.D.; Boiteux, S.; Radicella, J.P. Mechanism of stimulation of the DNA glycosylase activity of hOGG1 by the major human AP endonuclease: Bypass of the AP lyase activity step. Nucleic Acids Res. 2001, 29, 1285–1292. [Google Scholar] [CrossRef]
- Dalhus, B.; Forsbring, M.; Helle, I.H.; Vik, E.S.; Forstrøm, R.J.; Backe, P.H.; Alseth, I.; Bjørås, M. Separation-of-function mutants unravel the dual-reaction mode of human 8-oxoguanine DNA glycosylase. Structure 2011, 19, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Benítez-Buelga, C.; Calvo, P.A.; Hanna, B.M.F.; Mortusewicz, O.; Masuyer, G.; Davies, J.; Wallner, O.; Sanjiv, K.; Albers, J.J.; et al. Small-molecule activation of OGG1 increases oxidative DNA damage repair by gaining a new function. Science 2022, 376, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, H.; Lindahl, T.; Verreault, A. DNA base excision repair of uracil residues in reconstituted nucleosome core particles. EMBO J. 2002, 21, 5943–5952. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.S.; DeMott, M.S.; Demple, B. Long-patch base excision DNA repair of 2-deoxyribonolactone prevents the formation of DNA-protein cross-links with DNA polymerase beta. J. Biol. Chem. 2005, 280, 39095–39103. [Google Scholar] [CrossRef]
- Horton, J.K.; Prasad, R.; Hou, E.; Wilson, S.H. Protection against methylation-induced cytotoxicity by DNA polymerase beta-dependent long patch base excision repair. J. Biol. Chem. 2000, 275, 2211–2218. [Google Scholar] [CrossRef]
- Wallace, S.S.; Murphy, D.L.; Sweasy, J.B. Base excision repair and cancer. Cancer Lett. 2012, 327, 73–89. [Google Scholar] [CrossRef]
- Cleary, S.P.; Cotterchio, M.; Jenkins, M.A.; Kim, H.; Bristow, R.; Green, R.; Haile, R.; Hopper, J.L.; LeMarchand, L.; Lindor, N.; et al. Germline MutY human homologue mutations and colorectal cancer: A multisite case-control study. Gastroenterology 2009, 136, 1251–1260. [Google Scholar] [CrossRef]
- Donigan, K.A.; Sun, K.W.; Nemec, A.A.; Murphy, D.L.; Cong, X.; Northrup, V.; Zelterman, D.; Sweasy, J.B. Human POLB gene is mutated in high percentage of colorectal tumors. J. Biol. Chem. 2012, 287, 23830–23839. [Google Scholar] [CrossRef]
- Lo, Y.L.; Jou, Y.S.; Hsiao, C.F.; Chang, G.C.; Tsai, Y.H.; Su, W.C.; Chen, K.Y.; Chen, Y.M.; Huang, M.S.; Hu, C.Y.; et al. A polymorphism in the APE1 gene promoter is associated with lung cancer risk. Cancer Epidemiol Biomark. Prev. 2009, 18, 223–229. [Google Scholar] [CrossRef]
- Mambo, E.; Chatterjee, A.; de Souza-Pinto, N.C.; Mayard, S.; Hogue, B.A.; Hoque, M.O.; Dizdaroglu, M.; Bohr, V.A.; Sidransky, D. Oxidized guanine lesions and hOgg1 activity in lung cancer. Oncogene 2005, 24, 4496–4508. [Google Scholar] [CrossRef] [PubMed]
- Meas, R.; Burak, M.J.; Sweasy, J.B. DNA repair and systemic lupus erythematosus. DNA Repair 2017, 56, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Olins, A.L.; Olins, D.E. Spheroid Chromatin Units (ν Bodies). Science 1974, 183, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Richmond, T.J.; Finch, J.T.; Rushton, B.; Rhodes, D.; Klug, A. Structure of the nucleosome core particle at 7 A resolution. Nature 1984, 311, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Luger, K.; Mäder, A.W.; Richmond, R.K.; Sargent, D.F.; Richmond, T.J. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 1997, 389, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Bednar, J.; Garcia-Saez, I.; Boopathi, R.; Cutter, A.R.; Papai, G.; Reymer, A.; Syed, S.H.; Lone, I.N.; Tonchev, O.; Crucifix, C.; et al. Structure and Dynamics of a 197 bp Nucleosome in Complex with Linker Histone H1. Mol. Cell 2017, 66, 384–397.e388. [Google Scholar] [CrossRef]
- Dorigo, B.; Schalch, T.; Kulangara, A.; Duda, S.; Schroeder, R.R.; Richmond, T.J. Nucleosome Arrays Reveal the Two-Start Organization of the Chromatin Fiber. Science 2004, 306, 1571–1573. [Google Scholar] [CrossRef]
- Bilokapic, S.; Strauss, M.; Halic, M. Cryo-EM of nucleosome core particle interactions in trans. Sci. Rep. 2018, 8, 7046. [Google Scholar] [CrossRef]
- Cai, S.; Böck, D.; Pilhofer, M.; Gan, L. The in situ structures of mono-, di-, and trinucleosomes in human heterochromatin. Mol. Biol. Cell 2018, 29, 2450–2457. [Google Scholar] [CrossRef]
- Chua, E.Y.; Vasudevan, D.; Davey, G.E.; Wu, B.; Davey, C.A. The mechanics behind DNA sequence-dependent properties of the nucleosome. Nucleic Acids Res. 2012, 40, 6338–6352. [Google Scholar] [CrossRef]
- Dombrowski, M.; Engeholm, M.; Dienemann, C.; Dodonova, S.; Cramer, P. Histone H1 binding to nucleosome arrays depends on linker DNA length and trajectory. Nat. Struct. Mol. Biol. 2022, 29, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Saez, I.; Menoni, H.; Boopathi, R.; Shukla, M.S.; Soueidan, L.; Noirclerc-Savoye, M.; Le Roy, A.; Skoufias, D.A.; Bednar, J.; Hamiche, A.; et al. Structure of an H1-Bound 6-Nucleosome Array Reveals an Untwisted Two-Start Chromatin Fiber Conformation. Mol. Cell 2018, 72, 902–915.e907. [Google Scholar] [CrossRef] [PubMed]
- Schalch, T.; Duda, S.; Sargent, D.F.; Richmond, T.J. X-ray structure of a tetranucleosome and its implications for the chromatin fibre. Nature 2005, 436, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Chen, P.; Sun, D.; Wang, M.; Dong, L.; Liang, D.; Xu, R.-M.; Zhu, P.; Li, G. Cryo-EM Study of the Chromatin Fiber Reveals a Double Helix Twisted by Tetranucleosomal Units. Science 2014, 344, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Arya, G.; Maitra, A.; Grigoryev, S.A. A structural perspective on the where, how, why, and what of nucleosome positioning. J. Biomol. Struct. Dyn. 2010, 27, 803–820. [Google Scholar] [CrossRef]
- Radman-Livaja, M.; Rando, O.J. Nucleosome positioning: How is it established, and why does it matter? Dev. Biol. 2010, 339, 258–266. [Google Scholar] [CrossRef]
- Struhl, K.; Segal, E. Determinants of nucleosome positioning. Nat. Struct. Mol. Biol. 2013, 20, 267–273. [Google Scholar] [CrossRef]
- Hayes, J.J.; Tullius, T.D.; Wolffe, A.P. The structure of DNA in a nucleosome. Proc. Natl. Acad. Sci. USA 1990, 87, 7405–7409. [Google Scholar] [CrossRef]
- Shrader, T.E.; Crothers, D.M. Artificial nucleosome positioning sequences. Proc. Natl. Acad. Sci. USA 1989, 86, 7418–7422. [Google Scholar] [CrossRef]
- Lowary, P.T.; Widom, J. New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J. Mol. Biol. 1998, 276, 19–42. [Google Scholar] [CrossRef]
- Satchwell, S.C.; Drew, H.R.; Travers, A.A. Sequence periodicities in chicken nucleosome core DNA. J. Mol. Biol. 1986, 191, 659–675. [Google Scholar] [CrossRef]
- Ioshikhes, I.; Bolshoy, A.; Trifonov, E.N. Preferred positions of AA and TT dinucleotides in aligned nucleosomal DNA sequences. J. Biomol. Struct. Dyn. 1992, 9, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Segal, E.; Widom, J. Poly(dA:dT) tracts: Major determinants of nucleosome organization. Curr. Opin. Struct. Biol. 2009, 19, 65–71. [Google Scholar] [CrossRef]
- Dechering, K.J.; Cuelenaere, K.; Konings, R.N.; Leunissen, J.A. Distinct frequency-distributions of homopolymeric DNA tracts in different genomes. Nucleic Acids Res. 1998, 26, 4056–4062. [Google Scholar] [CrossRef] [PubMed]
- Barbier, J.; Vaillant, C.; Volff, J.-N.; Brunet, F.G.; Audit, B. Coupling between Sequence-Mediated Nucleosome Organization and Genome Evolution. Genes 2021, 12, 851. [Google Scholar] [CrossRef]
- Prendergast, J.G.; Semple, C.A. Widespread signatures of recent selection linked to nucleosome positioning in the human lineage. Genome Res. 2011, 21, 1777–1787. [Google Scholar] [CrossRef][Green Version]
- Tarantino, M.E.; Dow, B.J.; Drohat, A.C.; Delaney, S. Nucleosomes and the three glycosylases: High, medium, and low levels of excision by the uracil DNA glycosylase superfamily. DNA Repair 2018, 72, 56–63. [Google Scholar] [CrossRef]
- Beard, B.C.; Wilson, S.H.; Smerdon, M.J. Suppressed catalytic activity of base excision repair enzymes on rotationally positioned uracil in nucleosomes. Proc. Natl. Acad. Sci. USA 2003, 100, 7465–7470. [Google Scholar] [CrossRef]
- Bilotti, K.; Tarantino, M.E.; Delaney, S. Human Oxoguanine Glycosylase 1 Removes Solution Accessible 8-Oxo-7,8-dihydroguanine Lesions from Globally Substituted Nucleosomes Except in the Dyad Region. Biochemistry 2018, 57, 1436–1439. [Google Scholar] [CrossRef]
- Cole, H.A.; Tabor-Godwin, J.M.; Hayes, J.J. Uracil DNA glycosylase activity on nucleosomal DNA depends on rotational orientation of targets. J. Biol. Chem. 2010, 285, 2876–2885. [Google Scholar] [CrossRef]
- Hinz, J.M.; Czaja, W. Facilitation of base excision repair by chromatin remodeling. DNA Repair 2015, 36, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Shang, M.; Wang, H.; Xi, Z.; Zhou, C. Histones participate in base excision repair of 8-oxodGuo by transiently cross-linking with active repair intermediates in nucleosome core particles. Nucleic Acids Res. 2021, 49, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Brown, A.J.; Malc, E.P.; Mieczkowski, P.A.; Smerdon, M.J.; Roberts, S.A.; Wyrick, J.J. Genome-wide maps of alkylation damage, repair, and mutagenesis in yeast reveal mechanisms of mutational heterogeneity. Genome Res. 2017, 27, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Pich, O.; Muiños, F.; Sabarinathan, R.; Reyes-Salazar, I.; Gonzalez-Perez, A.; Lopez-Bigas, N. Somatic and Germline Mutation Periodicity Follow the Orientation of the DNA Minor Groove around Nucleosomes. Cell 2018, 175, 1074–1087.e18. [Google Scholar] [CrossRef]
- Bohm, K.A.; Hodges, A.J.; Czaja, W.; Selvam, K.; Smerdon, M.J.; Mao, P.; Wyrick, J.J. Distinct roles for RSC and SWI/SNF chromatin remodelers in genomic excision repair. Genome Res. 2021, 31, 1047–1059. [Google Scholar] [CrossRef]
- Volle, C.; Dalal, Y. Histone variants: The tricksters of the chromatin world. Curr. Opin. Genet. Dev. 2014, 25, 8–14. [Google Scholar] [CrossRef]
- Menoni, H.; Gasparutto, D.; Hamiche, A.; Cadet, J.; Dimitrov, S.; Bouvet, P.; Angelov, D. ATP-dependent chromatin remodeling is required for base excision repair in conventional but not in variant H2A.Bbd nucleosomes. Mol. Cell. Biol. 2007, 27, 5949–5956. [Google Scholar] [CrossRef]
- Li, C.; Delaney, S. Histone H2A Variants Enhance the Initiation of Base Excision Repair in Nucleosomes. ACS Chem. Biol. 2019, 14, 1041–1050. [Google Scholar] [CrossRef]
- Li, C.; Rioux, K.L.; Delaney, S. Histone variants H3.3 and H2A.Z/H3.3 facilitate excision of uracil from nucleosome core particles. DNA Repair 2022, 116, 103355. [Google Scholar] [CrossRef]
- Sczepanski, J.T.; Wong, R.S.; McKnight, J.N.; Bowman, G.D.; Greenberg, M.M. Rapid DNA–Protein cross-linking and strand scission by an abasic site in a nucleosome core particle. Proc. Natl. Acad. Sci. USA 2010, 107, 22475–22480. [Google Scholar] [CrossRef]
- Zhou, C.; Sczepanski, J.T.; Greenberg, M.M. Mechanistic Studies on Histone Catalyzed Cleavage of Apyrimidinic/Apurinic Sites in Nucleosome Core Particles. J. Am. Chem. Soc. 2012, 134, 16734–16741. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, K.; Banerjee, S.; Greenberg, M.M. Rotational Effects within Nucleosome Core Particles on Abasic Site Reactivity. Biochemistry 2018, 57, 3945–3952. [Google Scholar] [CrossRef]
- Yang, K.; Greenberg, M.M. Enhanced Cleavage at Abasic Sites within Clustered Lesions in Nucleosome Core Particles. Chembiochem 2018, 19, 2061–2065. [Google Scholar] [CrossRef] [PubMed]
- Weaver, T.M.; Hoitsma, N.M.; Spencer, J.J.; Gakhar, L.; Schnicker, N.J.; Freudenthal, B.D. Structural basis for APE1 processing DNA damage in the nucleosome. Nat. Commun. 2022, 13, 5390. [Google Scholar] [CrossRef] [PubMed]
- Hinz, J.M. Impact of abasic site orientation within nucleosomes on human APE1 endonuclease activity. Mutat. Res. 2014, 766–767, 19–24. [Google Scholar] [CrossRef]
- Hinz, J.M.; Mao, P.; McNeill, D.R.; Wilson, D.M., 3rd. Reduced Nuclease Activity of Apurinic/Apyrimidinic Endonuclease (APE1) Variants on Nucleosomes: Identification of Access Residues. J. Biol. Chem. 2015, 290, 21067–21075. [Google Scholar] [CrossRef] [PubMed]
- Hinz, J.M.; Rodriguez, Y.; Smerdon, M.J. Rotational dynamics of DNA on the nucleosome surface markedly impact accessibility to a DNA repair enzyme. Proc. Natl. Acad. Sci. USA 2010, 107, 4646–4651. [Google Scholar] [CrossRef]
- Nakanishi, S.; Prasad, R.; Wilson, S.H.; Smerdon, M. Different structural states in oligonucleosomes are required for early versus late steps of base excision repair. Nucleic Acids Res. 2007, 35, 4313–4321. [Google Scholar] [CrossRef]
- Banerjee, D.R.; Deckard, C.E.; Elinski, M.B.; Buzbee, M.L.; Wang, W.W.; Batteas, J.D.; Sczepanski, J.T. Plug-and-Play Approach for Preparing Chromatin Containing Site-Specific DNA Modifications: The Influence of Chromatin Structure on Base Excision Repair. J. Am. Chem. Soc. 2018, 140, 8260–8267. [Google Scholar] [CrossRef]
- Rodriguez, Y.; Hinz, J.M.; Laughery, M.F.; Wyrick, J.J.; Smerdon, M.J. Site-specific Acetylation of Histone H3 Decreases Polymerase β Activity on Nucleosome Core Particles in Vitro. J. Biol. Chem. 2016, 291, 11434–11445. [Google Scholar] [CrossRef]
- Rodriguez, Y.; Howard, M.J.; Cuneo, M.J.; Prasad, R.; Wilson, S.H. Unencumbered Pol β lyase activity in nucleosome core particles. Nucleic Acids Res. 2017, 45, 8901–8915. [Google Scholar] [CrossRef]
- Beard, B.C.; Stevenson, J.J.; Wilson, S.H.; Smerdon, M.J. Base excision repair in nucleosomes lacking histone tails. DNA Repair 2005, 4, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Balliano, A.; Hao, F.; Njeri, C.; Balakrishnan, L.; Hayes, J.J. HMGB1 Stimulates Activity of Polymerase β on Nucleosome Substrates. Biochemistry 2017, 56, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Meas, R.; Smerdon, M.J. Nucleosomes determine their own patch size in base excision repair. Sci. Rep. 2016, 6, 27122. [Google Scholar] [CrossRef] [PubMed]
- Odell, I.D.; Barbour, J.E.; Murphy, D.L.; Della-Maria, J.A.; Sweasy, J.B.; Tomkinson, A.E.; Wallace, S.S.; Pederson, D.S. Nucleosome disruption by DNA ligase III-XRCC1 promotes efficient base excision repair. Mol. Cell. Biol. 2011, 31, 4623–4632. [Google Scholar] [CrossRef]
- Cannan, W.J.; Rashid, I.; Tomkinson, A.E.; Wallace, S.S.; Pederson, D.S. The Human Ligase IIIα-XRCC1 Protein Complex Performs DNA Nick Repair after Transient Unwrapping of Nucleosomal DNA. J. Biol. Chem. 2017, 292, 5227–5238. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.F.; Chafin, D.R.; Aoyagi, S.; Henricksen, L.A.; Bambara, R.A.; Hayes, J.J. Flap endonuclease 1 efficiently cleaves base excision repair and DNA replication intermediates assembled into nucleosomes. Mol. Cell 2002, 10, 1201–1211. [Google Scholar] [CrossRef]
- Chafin, D.R.; Vitolo, J.M.; Henricksen, L.A.; Bambara, R.A.; Hayes, J.J. Human DNA ligase I efficiently seals nicks in nucleosomes. EMBO J. 2000, 19, 5492–5501. [Google Scholar] [CrossRef]
- Raja, S.; Van Houten, B. The Multiple Cellular Roles of SMUG1 in Genome Maintenance and Cancer. Int. J. Mol. Sci. 2021, 22, 1981. [Google Scholar] [CrossRef]
- Montaldo, N.P.; Bordin, D.L.; Brambilla, A.; Rösinger, M.; Fordyce Martin, S.L.; Bjørås, K.Ø.; Bradamante, S.; Aas, P.A.; Furrer, A.; Olsen, L.C.; et al. Alkyladenine DNA glycosylase associates with transcription elongation to coordinate DNA repair with gene expression. Nat. Commun. 2019, 10, 5460. [Google Scholar] [CrossRef]
- Odell, I.D.; Wallace, S.S.; Pederson, D.S. Rules of engagement for base excision repair in chromatin. J. Cell. Physiol. 2013, 228, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Bj Rås, K.; Sousa, M.M.L.; Sharma, A.; Fonseca, D.M.; CK, S.G.; Bj Rås, M.; Otterlei, M. Monitoring of the spatial and temporal dynamics of BER/SSBR pathway proteins, including MYH, UNG2, MPG, NTH1 and NEIL1-3, during DNA replication. Nucleic Acids Res. 2017, 45, 8291–8301. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.L.; Hegde, P.M.; Bellot, L.J.; Mandal, S.M.; Hazra, T.K.; Li, G.M.; Boldogh, I.; Tomkinson, A.E.; Mitra, S. Prereplicative repair of oxidized bases in the human genome is mediated by NEIL1 DNA glycosylase together with replication proteins. Proc. Natl. Acad. Sci. USA 2013, 110, E3090–E3099. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Ichimura, T.; Fujita, N.; Tsuruzoe, S.; Ohki, I.; Shirakawa, M.; Kawasuji, M.; Nakao, M. Methylated DNA-binding domain 1 and methylpurine-DNA glycosylase link transcriptional repression and DNA repair in chromatin. Proc. Natl. Acad. Sci. USA 2003, 100, 12859–12864. [Google Scholar] [CrossRef] [PubMed]
- Likhite, V.S.; Cass, E.I.; Anderson, S.D.; Yates, J.R.; Nardulli, A.M. Interaction of estrogen receptor alpha with 3-methyladenine DNA glycosylase modulates transcription and DNA repair. J. Biol. Chem. 2004, 279, 16875–16882. [Google Scholar] [CrossRef] [PubMed]
- Bjørge, M.D.; Hildrestrand, G.A.; Scheffler, K.; Suganthan, R.; Rolseth, V.; Kuśnierczyk, A.; Rowe, A.D.; Vågbø, C.B.; Vetlesen, S.; Eide, L.; et al. Synergistic Actions of Ogg1 and Mutyh DNA Glycosylases Modulate Anxiety-like Behavior in Mice. Cell Rep. 2015, 13, 2671–2678. [Google Scholar] [CrossRef]
- Huttlin, E.L.; Bruckner, R.J.; Paulo, J.A.; Cannon, J.R.; Ting, L.; Baltier, K.; Colby, G.; Gebreab, F.; Gygi, M.P.; Parzen, H.; et al. Architecture of the human interactome defines protein communities and disease networks. Nature 2017, 545, 505–509. [Google Scholar] [CrossRef]
- Zhou, K.; Gaullier, G.; Luger, K. Nucleosome structure and dynamics are coming of age. Proc. Natl. Acad. Sci. USA 2019, 26, 3–13. [Google Scholar] [CrossRef]
- Fenley, A.T.; Anandakrishnan, R.; Kidane, Y.H.; Onufriev, A.V. Modulation of nucleosomal DNA accessibility via charge-altering post-translational modifications in histone core. Epigenetics Chromatin 2018, 11, 11. [Google Scholar] [CrossRef]
- Li, G.; Levitus, M.; Bustamante, C.; Widom, J. Rapid spontaneous accessibility of nucleosomal DNA. Nat. Struct. Mol. Biol. 2005, 12, 46–53. [Google Scholar] [CrossRef]
- Blossey, R.; Schiessel, H. The dynamics of the nucleosome: Thermal effects, external forces and ATP. FEBS J. 2011, 278, 3619–3632. [Google Scholar] [CrossRef] [PubMed]
- Poirier, M.G.; Bussiek, M.; Langowski, J.; Widom, J. Spontaneous access to DNA target sites in folded chromatin fibers. J. Mol. Biol. 2008, 379, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Poirier, M.G.; Oh, E.; Tims, H.S.; Widom, J. Dynamics and function of compact nucleosome arrays. Nat. Struct. Mol. Biol. 2009, 16, 938–944. [Google Scholar] [CrossRef]
- Brandani, G.B.; Niina, T.; Tan, C.; Takada, S. DNA sliding in nucleosomes via twist defect propagation revealed by molecular simulations. Nucleic Acids Res. 2018, 46, 2788–2801. [Google Scholar] [CrossRef]
- Akey, C.W.; Luger, K. Histone chaperones and nucleosome assembly. Curr. Opin. Struct. Biol. 2003, 13, 6–14. [Google Scholar] [CrossRef]
- Kurumizaka, H.; Kujirai, T.; Takizawa, Y. Contributions of Histone Variants in Nucleosome Structure and Function. J. Mol. Biol. 2020, 433, 166678. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.T.; Ha, T. Nucleosomes undergo slow spontaneous gaping. Nucleic Acids Res. 2015, 43, 3964–3971. [Google Scholar] [CrossRef]
- Deckard, C.E.; Sczepanski, J.T. Reversible chromatin condensation by the DNA repair and demethylation factor thymine DNA glycosylase. Nucleic Acids Res. 2021, 49, 2450–2459. [Google Scholar] [CrossRef]
- Bowman, G.D.; Poirier, M.G. Post-Translational Modifications of Histones That Influence Nucleosome Dynamics. Chem. Rev. 2015, 115, 2274–2295. [Google Scholar] [CrossRef]
- Chatterjee, N.; North, J.A.; Dechassa, M.L.; Manohar, M.; Prasad, R.; Luger, K.; Ottesen, J.J.; Poirier, M.G.; Bartholomew, B. Histone Acetylation near the Nucleosome Dyad Axis Enhances Nucleosome Disassembly by RSC and SWI/SNF. Mol. Cell. Biol. 2015, 35, 4083–4092. [Google Scholar] [CrossRef]
- North, J.A.; Javaid, S.; Ferdinand, M.B.; Chatterjee, N.; Picking, J.W.; Shoffner, M.; Nakkula, R.J.; Bartholomew, B.; Ottesen, J.J.; Fishel, R.; et al. Phosphorylation of histone H3(T118) alters nucleosome dynamics and remodeling. Nucleic Acids Res. 2011, 39, 6465–6474. [Google Scholar] [CrossRef]
- Simon, M.; North, J.A.; Shimko, J.C.; Forties, R.A.; Ferdinand, M.B.; Manohar, M.; Zhang, M.; Fishel, R.; Ottesen, J.J.; Poirier, M.G. Histone fold modifications control nucleosome unwrapping and disassembly. Proc. Natl. Acad. Sci. USA 2011, 108, 12711–12716. [Google Scholar] [CrossRef]
- Chakravarthy, S.; Park, Y.J.; Chodaparambil, J.; Edayathumangalam, R.S.; Luger, K. Structure and dynamic properties of nucleosome core particles. FEBS Lett. 2005, 579, 895–898. [Google Scholar] [CrossRef]
- Allfrey, V.G.; Faulkner, R.; Mirsky, A.E. Acetylation and Methylation of Histones and Their Possible Role in the Regulation of Rna Synthesis. Proc. Natl. Acad. Sci. USA 1964, 51, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.O.; Panchenko, T.; Shabanowitz, J.; Lehman, S.M.; Bai, D.L.; Hunt, D.F.; Black, B.E.; Foltz, D.R. Identification of the Post-translational Modifications Present in Centromeric Chromatin. Mol. Cell. Proteom. 2016, 15, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Garcia, B.A.; Hake, S.B.; Diaz, R.L.; Kauer, M.; Morris, S.A.; Recht, J.; Shabanowitz, J.; Mishra, N.; Strahl, B.D.; Allis, C.D.; et al. Organismal differences in post-translational modifications in histones H3 and H4. J. Biol. Chem. 2007, 282, 7641–7655. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Lee, T.H. Lysine Acetylation Facilitates Spontaneous DNA Dynamics in the Nucleosome. J. Phys. Chem. B 2015, 119, 15001–15005. [Google Scholar] [CrossRef] [PubMed]
- Manohar, M.; Mooney, A.M.; North, J.A.; Nakkula, R.J.; Picking, J.W.; Edon, A.; Fishel, R.; Poirier, M.G.; Ottesen, J.J. Acetylation of histone H3 at the nucleosome dyad alters DNA-histone binding. J. Biol. Chem. 2009, 284, 23312–23321. [Google Scholar] [CrossRef]
- Lee, J.; Lee, T.H. How Protein Binding Sensitizes the Nucleosome to Histone H3K56 Acetylation. ACS Chem. Biol. 2019, 14, 506–515. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.; Yue, H.; Lee, T.H. Dynamics of nucleosome assembly and effects of DNA methylation. J. Biol. Chem. 2015, 290, 4291–4303. [Google Scholar] [CrossRef]
- Downs, J.A. Histone H3 K56 acetylation, chromatin assembly, and the DNA damage checkpoint. DNA Repair 2008, 7, 2020–2024. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Carson, J.J.; Feser, J.; Tamburini, B.; Zabaronick, S.; Linger, J.; Tyler, J.K. Acetylated lysine 56 on histone H3 drives chromatin assembly after repair and signals for the completion of repair. Cell 2008, 134, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, H.; Hawke, D.; Kobayashi, R.; Verreault, A. A role for cell-cycle-regulated histone H3 lysine 56 acetylation in the DNA damage response. Nature 2005, 436, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.K.; Truong, D.; Tyler, J.K. Acetylation in the globular core of histone H3 on lysine-56 promotes chromatin disassembly during transcriptional activation. Proc. Natl. Acad. Sci. USA 2008, 105, 9000–9005. [Google Scholar] [CrossRef] [PubMed]
- Wurtele, H.; Kaiser, G.S.; Bacal, J.; St-Hilaire, E.; Lee, E.H.; Tsao, S.; Dorn, J.; Maddox, P.; Lisby, M.; Pasero, P.; et al. Histone H3 lysine 56 acetylation and the response to DNA replication fork damage. Mol. Cell. Biol. 2012, 32, 154–172. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, Q.; Zhang, K.; Xie, W.; Grunstein, M. Sir2 deacetylates histone H3 lysine 56 to regulate telomeric heterochromatin structure in yeast. Mol. Cell 2007, 27, 890–900. [Google Scholar] [CrossRef]
- Rodriguez, Y.; Horton, J.K.; Wilson, S.H. Histone H3 Lysine 56 Acetylation Enhances AP Endonuclease 1-Mediated Repair of AP Sites in Nucleosome Core Particles. Biochemistry 2019, 58, 3646–3655. [Google Scholar] [CrossRef]
- Das, C.; Lucia, M.S.; Hansen, K.C.; Tyler, J.K. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature 2009, 459, 113–117. [Google Scholar] [CrossRef]
- Maas, N.L.; Miller, K.M.; DeFazio, L.G.; Toczyski, D.P. Cell cycle and checkpoint regulation of histone H3 K56 acetylation by Hst3 and Hst4. Mol. Cell 2006, 23, 109–119. [Google Scholar] [CrossRef]
- Miller, K.M.; Maas, N.L.; Toczyski, D.P. Taking it off: Regulation of H3 K56 acetylation by Hst3 and Hst4. Cell Cycle 2006, 5, 2561–2565. [Google Scholar] [CrossRef]
- Neumann, H.; Hancock, S.M.; Buning, R.; Routh, A.; Chapman, L.; Somers, J.; Owen-Hughes, T.; van Noort, J.; Rhodes, D.; Chin, J.W. A method for genetically installing site-specific acetylation in recombinant histones defines the effects of H3 K56 acetylation. Mol. Cell 2009, 36, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Ai, X.; Eugeni, E.E.; Zhang, L.; Carpenter, L.R.; Jelinek, M.A.; Freitas, M.A.; Parthun, M.R. Histone H4 lysine 91 acetylation a core domain modification associated with chromatin assembly. Mol. Cell 2005, 18, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-M.; Chen, H.Y.; Espino, P.S.; Davie, J.R. Phosphorylated serine 28 of histone H3 is associated with destabilized nucleosomes in transcribed chromatin. Nucleic Acids Res. 2007, 35, 6640–6647. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.R.; Deckard, C.E.; Zeng, Y.; Sczepanski, J.T. Acetylation of the histone H3 tail domain regulates base excision repair on higher-order chromatin structures. Sci. Rep. 2019, 9, 15972. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).