Large Intra-Age Group Variation in Chromosome Abnormalities in Human Blastocysts
Abstract
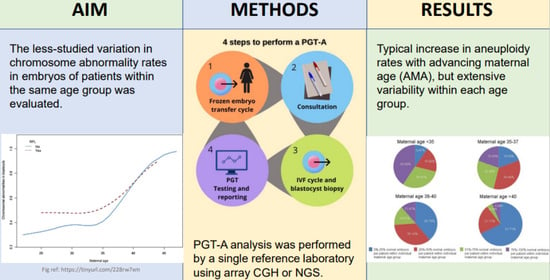
1. Introduction
2. Materials and Methods
Consent and IRB Approval
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hassold, T.; Hall, H.; Hunt, P. The origin of human aneuploidy: Where we have been. where we are going. Hum. Mol. Genet. 2007, 16, R203–R208. [Google Scholar] [CrossRef]
- Subramaniyam, S.; Pulijaal, V.R.; Mathew, S. Double and multiple chromosomal aneuploidies in spontaneous abortions: A single institutional experience. J. Hum. Reprod. Sci. 2014, 7, 262–268. [Google Scholar] [CrossRef]
- Munne, S.; Lee, A.; Rosenwaks, Z.; Grifo, J.; Cohen, J. Diagnosis of major chromosome aneuploidies in human preimplantation embryos. Hum. Reprod. 1993, 8, 2185–2191. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8150922 (accessed on 11 November 2021). [CrossRef]
- Munne, S.; Weier, H.U.; Stein, J.; Grifo, J.; Cohen, J. A fast and efficient method for simultaneous X and Y in situ hybridization of human blastomeres. J. Assist. Reprod. Genet. 1993, 10, 82–90. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8499685 (accessed on 11 November 2021). [CrossRef] [PubMed]
- Gutierrez-Mateo, C.; Colls, P.; Sanchez-Garcia, J.; Escudero, T.; Prates, R.; Ketterson, K.; Wells, D.; Munne, S. Validation of microarray comparative genomic hybridization for comprehensive chromosome analysis of embryos. Fertil. Steril. 2011, 95, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.S.; Gemelos, G.; Baner, J.; Ryan, A.; Cinnioglu, C.; Banjevic, M.; Ross, R.; Alper, M.; Barrett, B.; Frederick, J.; et al. Preclinical validation of a microarray method for full molecular karyotyping of blastomeres in a 24-h protocol. Hum. Reprod. 2010, 25, 1066–1075. [Google Scholar] [CrossRef]
- Kung, A.; Munne, S.; Bankowski, B.; Coates, A.; Wells, D. Validation of next-generation sequencing for comprehensive chromosome screening of embryos. Reprod. Biomed. Online 2015, 31, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Treff, N.R.; Fedick, A.; Tao, X.; Devkota, B.; Taylor, D.; Scott, R.T., Jr. Evaluation of targeted next-generation sequencing-based preimplantation genetic diagnosis of monogenic disease. Fertil. Steril. 2013, 99, 1377–1384.e6. [Google Scholar] [CrossRef] [PubMed]
- Treff, N.R.; Tao, X.; Ferry, K.M.; Su, J.; Taylor, D.; Scott, R.T., Jr. Development and validation of an accurate quantitative real-time polymerase chain reaction-based assay for human blastocyst comprehensive chromosomal aneuploidy screening. Fertil. Steril. 2012, 97, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.; Delhanty, J.D. Comprehensive chromosomal analysis of human preimplantation embryos using whole genome amplification and single cell comparative genomic hybridization. Mol. Hum. Reprod. 2000, 6, 1055–1062. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11044470 (accessed on 1 August 2016). [CrossRef]
- Wells, D.; Kaur, K.; Grifo, J.; Glassner, M.; Taylor, J.C.; Fragouli, E.; Munne, S. Clinical utilisation of a rapid low-pass whole genome sequencing technique for the diagnosis of aneuploidy in human embryos prior to implantation. J. Med. Genet. 2014, 51, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Wilton, L.; Williamson, R.; McBain, J.; Edgar, D.; Voullaire, L. Birth of a healthy infant after preimplantation confirmation of euploidy by comparative genomic hybridization. N. Engl. J. Med. 2001, 345, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- McArthur, S.J.; Leigh, D.; Marshall, J.T.; de Boer, K.A.; Jansen, R.P. Pregnancies and live births after trophectoderm biopsy and preimplantation genetic testing of human blastocysts. Fertil. Steril. 2005, 84, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Schoolcraft, W.B.; Fragouli, E.; Stevens, J.; Munne, S.; Katz-Jaffe, M.G.; Wells, D. Clinical application of comprehensive chromosomal screening at the blastocyst stage. Fertil. Steril. 2010, 94, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Sermon, K.; Capalbo, A.; Cohen, J.; Coonen, E.; De Rycke, M.; De Vos, A.; Delhanty, J.; Fiorentino, F.; Gleicher, N.; Griesinger, G.; et al. The why, the how and the when of PGS 2.0: Current practices and expert opinions of fertility specialists, molecular biologists, and embryologists. MHR Basic Sci. Reprod. Med. 2016, 22, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Ata, B.; Kaplan, B.; Danzer, H.; Glassner, M.; Opsahl, M.; Tan, S.L.; Munne, S. Array CGH analysis shows that aneuploidy is not related to the number of embryos generated. Reprod. Biomed. Online 2012, 24, 614–620. [Google Scholar] [CrossRef]
- Franasiak, J.M.; Forman, E.J.; Hong, K.H.; Werner, M.D.; Upham, K.M.; Treff, N.R.; Scott, R.T., Jr. The nature of aneuploidy with increasing age of the female partner: A review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil. Steril. 2014, 101, 656–663.e1. [Google Scholar] [CrossRef]
- Harton, G.L.; Munne, S.; Surrey, M.; Grifo, J.; Kaplan, B.; McCulloh, D.H.; Griffin, D.K.; Wells, D.; PGD Practitioners Group. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil. Steril. 2013, 100, 1695–1703. [Google Scholar] [CrossRef]
- Munne, S.; Alikani, M.; Tomkin, G.; Grifo, J.; Cohen, J. Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities. Fertil. Steril. 1995, 64, 382–391. Available online: http://www.ncbi.nlm.nih.gov/pubmed/7615118 (accessed on 11 November 2021). [CrossRef] [PubMed]
- Munne, S.; Chen, S.; Colls, P.; Garrisi, J.; Zheng, X.; Cekleniak, N.; Lenzi, M.; Hughes, P.; Fischer, J.; Garrisi, M.; et al. Maternal age, morphology, development and chromosome abnormalities in over 6000 cleavage-stage embryos. Reprod. Biomed. Online 2007, 14, 628–634. Available online: http://www.ncbi.nlm.nih.gov/pubmed/17509208 (accessed on 1 August 2016). [CrossRef]
- Konstantinidis, M.; Prates, R.; Goodall, N.-N.; Fischer, J.; Tecson, V.; Lemma, T.; Chu, B.; Jordan, A.; Armenti, E.; Wells, D.; et al. Live births following Karyomapping of human blastocysts: Experience from clinical application of the method. Reprod. Biomed. Online 2015, 31, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Kuliev, A.; Verlinsky, Y. Meiotic and mitotic nondisjunction: Lessons from preimplantation genetic diagnosis. Hum. Reprod. Update 2004, 10, 401–407. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rabinowitz, M.; Ryan, A.; Gemelos, G.; Hill, M.; Baner, J.; Cinnioglu, C.; Banjevic, M.; Potter, D.; Petrov, D.A.; Demko, Z. Origins and rates of aneuploidy in human blastomeres. Fertil. Steril. 2012, 97, 395–401. [Google Scholar] [CrossRef]
- Treff, N.R.; Su, J.; Tao, X.; Miller, K.A.; Levy, B.; Scott, R.T., Jr. A novel single-cell DNA fingerprinting method successfully distinguishes sibling human embryos. Fertil. Steril. 2010, 94, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Bielanska, M.; Tan, S.L.; Ao, A. High rate of mixoploidy among human blastocysts cultured in vitro. Fertil. Steril. 2002, 78, 1248–1253. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12477520 (accessed on 11 November 2021). [CrossRef]
- Campbell, I.M.; Yuan, B.; Robberecht, C.; Pfundt, R.; Szafranski, P.; McEntagart, M.E.; Nagamani, S.C.S.; Erez, A.; Bartnik, M.; Wiśniowiecka-Kowalnik, B.; et al. Parental somatic mosaicism is underrecognized and influences recurrence risk of genomic disorders. Am. J. Hum. Genet. 2014, 95, 173–182. [Google Scholar] [CrossRef]
- Fragouli, E.; Alfarawati, S.; Spath, K.; Wells, D. Morphological and cytogenetic assessment of cleavage and blastocyst stage embryos. Mol. Hum. Reprod. 2014, 20, 117–126. [Google Scholar] [CrossRef]
- Magli, M.C.; Gianaroli, L.; Ferraretti, A.P.; Lappi, M.; Ruberti, A.; Farfalli, V. Embryo morphology and development are dependent on the chromosomal complement. Fertil. Steril. 2007, 87, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Munne, S.; Ary, J.; Zouves, C.; Escudero, T.; Barnes, F.; Cinioglu, C.; Ary, B.; Cohen, J. Wide range of chromosome abnormalities in the embryos of young egg donors. Reprod. Biomed. Online 2006, 12, 340–346. Available online: http://www.ncbi.nlm.nih.gov/pubmed/16569324 (accessed on 11 November 2021). [CrossRef]
- Munne, S.; Grifo, J.; Wells, D. Mosaicism: “survival of the fittest” versus “no embryo left behind”. Fertil. Steril. 2016, 105, 1146–1149. [Google Scholar] [CrossRef] [PubMed]
- Sandalinas, M.; Sadowy, S.; Alikani, M.; Calderon, G.; Cohen, J.; Munne, S. Developmental ability of chromosomally abnormal human embryos to develop to the blastocyst stage. Hum. Reprod. 2001, 16, 1954–1958. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11527904 (accessed on 11 November 2021). [CrossRef] [PubMed]
- Delhanty, J.D.A.; Griffin, D.K.; Handyside, A.H.; Harper, J.; Pieters, M.H.E.C.; Winston, R.M.L. Detection of aneuploidy and chromosomal mosaicism in human embryos during preimplantation sex determination by fluorescent in-situ hybridisation. Hum. Mol. Genet. 1993, 2, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.K.; Handyside, A.H.; Penketh, R.J.A.; Winston, R.M.L.; Delhanty, J.D.A. Fluorescent in-situ hybridisation to interphase nuclei of human preimplantation embryos with X and Y specific probes. Hum. Reprod. 1991, 6, 101–105. [Google Scholar] [CrossRef]
- Munne, S.; Weier, H.U.; Grifo, J.; Cohen, J. Chromosome mosaicism in human embryos. Biol. Reprod. 1994, 51, 373–379. Available online: http://www.ncbi.nlm.nih.gov/pubmed/7803609 (accessed on 11 November 2021). [CrossRef] [PubMed]
- Fragouli, E.; Alfarawati, S.; Spath, K.; Tarozzi, N.; Borini, A.; Wells, D. The developmental potential of mosaic embryos. Fertil. Steril. 2017, 104, e96. [Google Scholar] [CrossRef][Green Version]
- Greco, E.; Biricik, A.; Cotarelo, R.P.; Iammarone, E.; Rubino, P.; Tesarik, J.; Fiorentino, F.; Minasi, M.G. Successful implantation and live birth of a healthy boy after triple biopsy and double vitrification of oocyte-embryo-blastocyst. SpringerPlus 2015, 4, 22. [Google Scholar] [CrossRef]
- Grifo, J.; Colls, P.; Ribustello, L.; Escudero, T.; Liu, E.; Munne, S. Why do array-CGH (ACGH) euploid embryos miscarry? Reanalysis by NGS reveals undetected abnormalities which would have prevented 56% of the miscarriages. Fertil. Steril. 2017, 104, e14. [Google Scholar] [CrossRef][Green Version]
- Munne, S.; Large, M.; Ribustello, L.; Blazek, J.; Gouw, F.; Grifo, J.; Haddad, G.; Chang, W.; Grunert, G.M.; Huang, A.; et al. PGS analysis of over 33,000 blastocysts using high resolution Next Generation Sequencing (HRNGS) of over 33,000 blastocysts using high resolution Next Generation Sequencing (HRNGS). Fertil. Steril. 2016, 106, e18–e19. [Google Scholar] [CrossRef]
- Fiorentino, F.; Spizzichino, L.; Bono, S.; Biricik, A.; Kokkali, G.; Rienzi, L.; Ubaldi, F.M.; Iammarrone, E.; Gordon, A.; Pantos, K. PGD for reciprocal and Robertsonian translocations using array comparative genomic hybridization. Hum. Reprod. 2011, 26, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Munne, S.; Magli, C.; Adler, A.; Wright, G.; de Boer, K.; Mortimer, D.; Tucker, M.; Cohen, J.; Gianaroli, L. Treatment-related chromosome abnormalities in human embryos. Hum. Reprod. 1997, 12, 780–784. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9159442 (accessed on 11 November 2021). [CrossRef] [PubMed]
- Munne, S.; Alikani, M. Culture-induced chromosome abnormalities: The canary in the mine. Reprod. Biomed. Online 2011, 22, 506–508. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Munne, S.; Alikani, M.; Barritt, J.; Hesla, J.; Kaplan, B.; Alper, M.; McCulloh, D. Egg donor aneuploidy rates significantly differ between fertility centers. Fertil. Steril. 2014, 102, e121–e122. [Google Scholar] [CrossRef]
- Munne, S.; Held, K.R.; Magli, C.M.; Ata, B.; Wells, D.; Fragouli, E.; Baukloh, V.; Fischer, R.; Gianaroli, L. Intra-age, intercenter, and intercycle differences in chromosome abnormalities in oocytes. Fertil. Steril. 2012, 97, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, A.S.; Thornhill, A.R.; Ottolini, C.S.; Gordon, A.; Brown, A.P.C.; Taylor, J.; Bennett, K.; Handyside, A.; Griffin, D.K. Array comparative genomic hybridisation on first polar bodies suggests that non-disjunction is not the predominant mechanism leading to aneuploidy in humans. J. Med. Genet. 2011, 48, 433–437. Available online: http://jmg.bmj.com/content/48/7/433.abstract (accessed on 11 November 2021). [CrossRef] [PubMed]
- Fiorentino, F.; Biricik, A.; Bono, S.; Spizzichino, L.; Cotroneo, E.; Cottone, G.; Kokocinski, F.; Michel, C.E. Development and validation of a next-generation sequencing-based protocol for 24-chromosome aneuploidy screening of embryos. Fertil. Steril. 2014, 101, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Colls, P.; Escudero, T.; Fischer, J.; Cekleniak, N.A.; Ben-Ozer, S.; Meyer, B.; Damien, M.; Grifo, J.A.; Hershlag, A.; Munne, S. Validation of array comparative genome hybridization for diagnosis of translocations in preimplantation human embryos. Reprod. Biomed. Online 2012, 24, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Troude, P.; Guibert, J.; Bouyer, J.; de La Rochebrochard, E. Medical factors associated with early IVF discontinuation. Reprod. BioMed. Online 2017, 28, 321–329. [Google Scholar] [CrossRef]
- Forman, E.J.; Hong, K.H.; Ferry, K.M.; Tao, X.; Taylor, D.; Levy, B.; Treff, N.R.; Scott, R.T., Jr. In-vitro fertilization with single euploid blastocyst transfer: A randomized controlled trial. Fertil. Steril. 2017, 100, 100–107.e1. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.; Bellver, J.; Rodrigoa, L.; Castillón, G.; Guillén, A.; Vidal, C.; Giles, J.; Ferrando, M.; Cabanillas, S.; Remohí, J.; et al. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: A randomized controlled study. Fertil. Steril. 2017, 107, 1122–1129. [Google Scholar] [CrossRef]
- Scott, R.T., Jr.; Upham, K.M.; Forman, E.J.; Hong, K.H.; Scott, K.L.; Taylor, D.; Tao, X.; Treff, N.R. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: A randomized controlled trial. Fertil. Steril. 2017, 100, 697–703. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, J.; Collins, G.S.; Salem, S.A.; Liu, X.; Lyle, S.S.; Peck, A.C.; Sills, E.S.; Salem, R.D. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: Results from a randomized pilot study. Mol. Cytogenet. 2012, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Katz-Jaffe, M.G.; Surrey, E.S.; Minjarez, D.A.; Gustofson, R.L.; Stevens, J.M.; Schoolcraft, W.B. Association of abnormal ovarian reserve parameters with a higher incidence of aneuploid blastocysts. Obstet. Gynecol. 2013, 121, 71–77. [Google Scholar] [CrossRef]
- Miyamoto, T.; Hasuike, S.; Yogev, L.; Maduro, M.R.; Ishikawa, M.; Westphal, H.; Lamb, D.J. Azoospermia in patients heterozygous for a mutation in SYCP3. Lancet 2003, 362, 1714–1719. [Google Scholar] [CrossRef]
- Bannister, L.A.; Pezza, R.J.; Donaldson, J.R.; de Rooij, D.G.; Schimenti, K.J.; Camerini-Otero, R.D.; Schimenti, J.C. A dominant, recombination-defective allele of Dmc1 causing male-specific sterility. PLoS Biol. 2007, 5, e105. [Google Scholar] [CrossRef]
- Cherry, S.M.; Adelman, C.A.; Theunissen, J.W.; Hassold, T.J.; Hunt, P.A.; Petrini, J.H. The Mre11 complex influences DNA repair, synapsis, and crossing over in murine meiosis. Curr. Biol. 2007, 17, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.E.; Schrump, S.E.; Cherry, J.P.; Hassold, T.J.; Hunt, P.A. Near-human aneuploidy levels in female mice with homeologous chromosomes. Curr. Biol. 2006, 16, R579–R580. [Google Scholar] [CrossRef]
- Kuznetsov, S.; Pellegrini, M.; Shuda, K.; Fernandez-Capetillo, O.; Liu, Y.; Martin, B.K.; Burkett, S.; Southon, E.; Pati, D.; Tessarollo, L.; et al. RAD51C deficiency in mice results in early prophase I arrest in males and sister chromatid separation at metaphase II in females. J. Cell Biol. 2007, 176, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Matzuk, M.M. Deconstructing mammalian reproduction: Using knockouts to define fertility pathways. Reproduction 2006, 131, 207–219. [Google Scholar] [CrossRef][Green Version]
- Singh, P.; Schimenti, J.C. The genetics of human infertility by functional interrogation of SNPs in mice. Proc. Natl. Acad. Sci. USA 2015, 112, 10431–10436. [Google Scholar] [CrossRef] [PubMed]
- Munné, S.; Alikani, M.; Ribustello, L.; Colls, P.; Martínez-Ortiz, P.A.; Referring Physician Group; McCulloh, D.H. Euploidy rates in donor egg cycles significantly differ between fertility centers. Human Reprod. 2017, 32, 743–749. [Google Scholar] [CrossRef]
- Tan, C.Y.T.; Mahbub, S.B.; Campbell, J.M.; Habibalahi, A.; Campugan, C.A.; Rose, R.D.; Chow, D.J.X.; Mustafa, S.; Goldys, E.M.; Dunning, K.R. Non-invasive, label-free optical analysis to detect aneuploidy within the inner cell mass of the preimplantation embryo. Hum. Reprod. 2021, deab233. [Google Scholar] [CrossRef] [PubMed]
- Victor, A.R.; Griffin, D.K.; Brake, A.J.; Tyndall, J.C.; Murphy, A.E.; Lepkowsky, L.T.; Lal, A.; Zouves, C.G.; Barnes, F.L.; McCoy, R.C.; et al. Assessment of aneuploidy concordance between clinical trophectoderm biopsy and blastocyst. Hum. Reprod. 2019, 34, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Coorens, T.H.H.; Oliver, T.R.W.; Sanghvi, R.; Sovio, U.; Cook, E.; Vento-Tormo, R.; Haniffa, M.; Young, M.D.; Rahbari, R.; Sebire, N.; et al. Inherent mosaicism and extensive mutation of human placentas. Nature 2021, 592, 80–85. [Google Scholar] [CrossRef]
- Desmyttere, S.; De Schepper, J.; Nekkebroeck, J.; De Vos, A.; De Rycke, M.; Staessen, C.; Liebaers, I.; Bonduelle, M. Two-year auxological and medical outcome of singletons born after embryo biopsy applied in preimplantation genetic diagnosis or preimplantation genetic screening. Hum. Reprod. 2009, 24, 470–476. [Google Scholar] [CrossRef] [PubMed]



| Age | Min. (%) | 1st Quartile | Median | Mean | 3rd Quartile | Max. (%) | IQR | n (Cycles) |
|---|---|---|---|---|---|---|---|---|
| Donor | 0 | 16.67 | 30.77 | 32.64 | 46.15 | 100 | 29.48 | 1538 |
| 17–25 | 0 | 21.43 | 40 | 38.01 | 50 | 100 | 28.57 | 85 |
| 26 | 0 | 17.05 | 33.33 | 36.25 | 50 | 100 | 32.95 | 78 |
| 27 | 0 | 20 | 37.5 | 38.72 | 50 | 100 | 30 | 101 |
| 28 | 0 | 14.29 | 28.57 | 31.96 | 50 | 100 | 35.71 | 192 |
| 29 | 0 | 20 | 33.33 | 36.58 | 50 | 100 | 30 | 251 |
| 30 | 0 | 17.8 | 33.33 | 36.05 | 50 | 100 | 32.2 | 336 |
| 31 | 0 | 16.67 | 33.33 | 36.27 | 50 | 100 | 33.33 | 461 |
| 32 | 0 | 25 | 40 | 41.96 | 60 | 100 | 35 | 529 |
| 33 | 0 | 20 | 37.5 | 39.62 | 55.56 | 100 | 35.56 | 669 |
| 34 | 0 | 25 | 40 | 42.11 | 60 | 100 | 35 | 680 |
| 35 | 0 | 25 | 45.8 | 44.91 | 62.5 | 100 | 37.5 | 816 |
| 36 | 0 | 26.58 | 50 | 46.22 | 66.67 | 100 | 40.09 | 896 |
| 37 | 0 | 33.33 | 50 | 50.93 | 70.72 | 100 | 37.39 | 939 |
| 38 | 0 | 37.5 | 57.14 | 56.81 | 77.78 | 100 | 40.28 | 1043 |
| 39 | 0 | 40 | 62.5 | 59.96 | 83.33 | 100 | 43.33 | 1095 |
| 40 | 0 | 50 | 75 | 68.09 | 100 | 100 | 50 | 1044 |
| 41 | 0 | 57.14 | 80 | 74.01 | 100 | 100 | 42.86 | 938 |
| 42 | 0 | 66.67 | 93.33 | 78.14 | 100 | 100 | 33.33 | 811 |
| 43 | 0 | 75 | 100 | 84.75 | 100 | 100 | 25 | 613 |
| 44 | 0 | 80 | 100 | 87.29 | 100 | 100 | 20 | 358 |
| 45 | 0 | 75 | 100 | 83.5 | 100 | 100 | 25 | 182 |
| a | ||||||||||||||||
| Cohort Size | EGD | <30 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | >42 |
| 1 to 3 | 59.24% | 59.42% | 64.88% | 64.58% | 60.52% | 58.58% | 56.97% | 50.45% | 49.85% | 48.00% | 43.85% | 38.91% | 30.70% | 25.45% | 21.29% | 13.33% |
| 4 to 6 | 64.57% | 61.47% | 58.25% | 59.12% | 53.88% | 58.15% | 53.73% | 53.81% | 52.17% | 46.88% | 39.52% | 38.93% | 30.98% | 26.25% | 20.20% | 15.08% |
| 7 to 9 | 66.31% | 62.56% | 60.42% | 61.04% | 56.24% | 56.96% | 57.29% | 53.50% | 52.89% | 45.77% | 42.91% | 38.33% | 28.10% | 22.90% | 18.28% | 12.71% |
| 10 to 12 | 67.12% | 63.94% | 65.67% | 63.62% | 56.63% | 60.13% | 54.86% | 59.37% | 53.93% | 49.62% | 40.96% | 38.67% | 30.92% | 22.42% | 24.76% | 20.39% |
| 13 to 15 | 68.00% | 63.01% | 66.82% | 65.30% | 58.12% | 60.10% | 61.25% | 52.92% | 53.35% | 56.00% | 43.44% | 41.56% | 35.22% | 29.78% | 25.37% | 24.44% |
| >16 | 67.26% | 64.46% | 66.33% | 70.88% | 60.25% | 60.23% | 61.29% | 59.06% | 57.41% | 48.42% | 44.68% | 43.55% | 38.71% | 29.41% | 17.91% | 11.11% |
| Total | 17,032 | 4662 | 2228 | 2873 | 3199 | 3993 | 3817 | 4523 | 4786 | 4785 | 5144 | 5109 | 4624 | 4036 | 3246 | 4471 |
| b | ||||||||||||||||
| Cohort Size | EGD | 21–31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45+ |
| 1 | 63.64% | 60.00% | 48.57% | 57.14% | 68.18% | 45.71% | 54.79% | 53.75% | 40.00% | 43.48% | 36.43% | 28.69% | 25.16% | 15.52% | 9.64% | 15.48% |
| 2 | 61.93% | 63.30% | 60.00% | 64.66% | 52.24% | 50.51% | 45.10% | 46.85% | 42.04% | 36.25% | 30.66% | 25.15% | 21.23% | 13.54% | 12.21% | 13.04% |
| 3 | 57.43% | 62.33% | 64.29% | 55.00% | 57.41% | 50.91% | 51.42% | 46.96% | 45.53% | 39.53% | 29.31% | 24.84% | 19.73% | 13.57% | 12.28% | 14.29% |
| >3 | 66.53% | 62.39% | 56.05% | 58.49% | 56.11% | 54.72% | 53.11% | 47.66% | 41.33% | 38.92% | 30.74% | 24.96% | 20.17% | 15.12% | 15.29% | 15.23% |
| a | ||||||||||||||||
| Cohort Size | EGD | <30 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | >42 |
| 1 to 3 | 58.59% | 47.92% | 40.54% | 46.09% | 48.28% | 40.00% | 43.69% | 43.25% | 42.86% | 32.26% | 30.03% | 28.81% | 25.00% | 21.91% | 12.28% | 11.69% |
| 4 to 6 | 53.72% | 48.40% | 52.03% | 54.10% | 49.75% | 42.63% | 44.20% | 45.83% | 38.46% | 32.77% | 35.53% | 30.54% | 23.46% | 17.09% | 14.38% | 12.64% |
| 7 to 9 | 57.09% | 47.45% | 53.69% | 49.75% | 43.60% | 45.53% | 42.09% | 43.81% | 39.36% | 37.76% | 36.48% | 27.29% | 24.74% | 19.48% | 12.72% | 11.48% |
| 10 to 12 | 60.42% | 52.38% | 49.48% | 43.75% | 42.46% | 44.95% | 38.67% | 45.56% | 40.52% | 37.50% | 34.97% | 25.91% | 26.58% | 15.79% | 6.58% | 13.79% |
| 13 to 15 | 64.11% | 52.98% | 34.29% | 61.87% | 32.80% | 37.76% | 36.14% | 46.94% | 49.70% | 25.77% | 26.61% | 24.03% | 19.20% | 13.89% | 7.94% | 9.41% |
| >16 | 62.84% | 59.26% | 33.33% | 43.40% | 39.56% | 47.78% | 44.25% | 30.36% | 41.03% | 36.72% | 23.97% | 24.09% | 17.56% | 20.29% | 8.33% | 7.27% |
| Total | 6090 | 1822 | 862 | 1270 | 1615 | 1436 | 1680 | 1959 | 1913 | 1898 | 1964 | 2026 | 1893 | 1570 | 1149 | 1386 |
| b | ||||||||||||||||
| Cohort Size | EGD | 21–31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45+ |
| 1 | 40.91% | 38.19% | 53.33% | 37.50% | 31.58% | 33.33% | 36.11% | 33.33% | 21.95% | 28.57% | 17.74% | 19.05% | 15.52% | 11.11% | 8.82% | 11.11% |
| 2 | 50.00% | 41.35% | 56.52% | 28.85% | 40.22% | 37.93% | 38.30% | 32.00% | 24.60% | 36.96% | 25.27% | 26.71% | 13.11% | 6.67% | 12.00% | 6.90% |
| 3 | 60.45% | 46.24% | 39.58% | 40.15% | 46.94% | 46.30% | 41.83% | 27.32% | 32.28% | 20.29% | 23.50% | 17.95% | 11.90% | 13.33% | 12.00% | 5.88% |
| >3 | 59.98% | 50.81% | 43.96% | 44.36% | 42.22% | 44.80% | 40.31% | 35.96% | 33.68% | 28.35% | 22.74% | 17.56% | 11.20% | 10.68% | 12.62% | 12.10% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawarkar, S.; Griffin, D.K.; Ribustello, L.; Munné, S. Large Intra-Age Group Variation in Chromosome Abnormalities in Human Blastocysts. DNA 2021, 1, 91-104. https://doi.org/10.3390/dna1020010
Sawarkar S, Griffin DK, Ribustello L, Munné S. Large Intra-Age Group Variation in Chromosome Abnormalities in Human Blastocysts. DNA. 2021; 1(2):91-104. https://doi.org/10.3390/dna1020010
Chicago/Turabian StyleSawarkar, Sarthak, Darren K. Griffin, Lia Ribustello, and Santiago Munné. 2021. "Large Intra-Age Group Variation in Chromosome Abnormalities in Human Blastocysts" DNA 1, no. 2: 91-104. https://doi.org/10.3390/dna1020010
APA StyleSawarkar, S., Griffin, D. K., Ribustello, L., & Munné, S. (2021). Large Intra-Age Group Variation in Chromosome Abnormalities in Human Blastocysts. DNA, 1(2), 91-104. https://doi.org/10.3390/dna1020010