Valorization of Birch Biochar: An Efficient and Sustainable Solution for Lead Decontamination of Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar Characterization
2.1.1. Determination of Bulk Density
2.1.2. Determination of the Point of Zero Charge
2.1.3. Evaluation of Methylene Blue (MB) Uptake
2.1.4. Structural and Spectroscopic Analysis
2.2. Biochar Conditioning
2.3. Adsorption Experiments
2.3.1. Determination of Equilibrium Lead Concentrations by Potentiometric Method
2.3.2. Adsorption Isotherm Studies
2.3.3. Adsorption Kinetic Studies
2.3.4. Study of the Liquid-to-Solid Ratio Influence and Desorption
3. Results
3.1. Main Characteristics of Biochar
3.2. Methylene Blue Adsorption
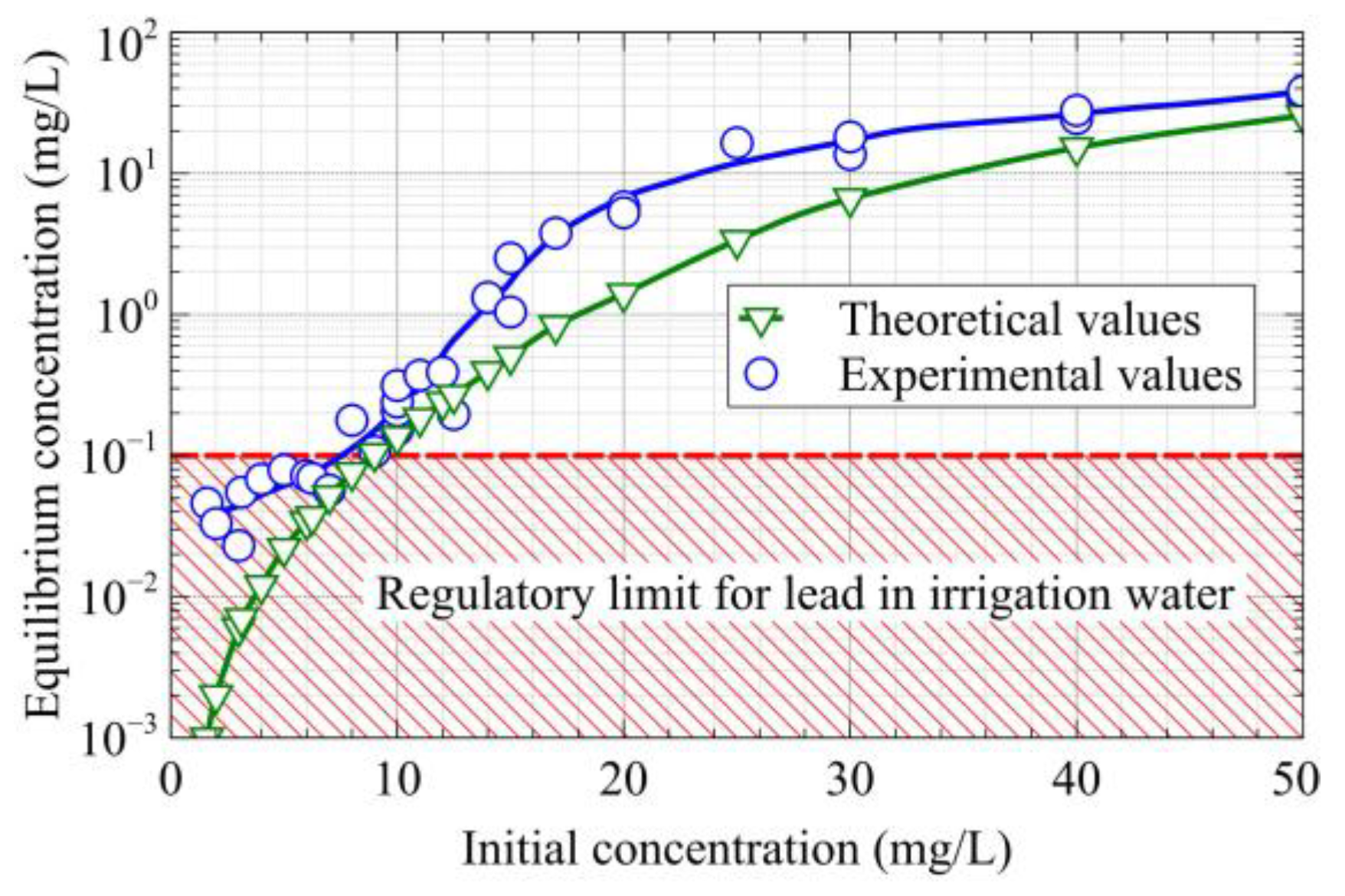
3.3. Adsorption Isotherm of Lead Ions
3.4. Kinetic Studies
3.5. Influence of Liquid-to-Solid Ratio and Desorption Studies
3.6. Microscopy and Elemental Analysis
4. Discussion
4.1. Sorption Properties and Adsorption Mechanisms
4.2. Adsorption Characteristics of Biochar with Respect to Lead
4.3. Structural Transformations, Surface Chemistry and Morphology
4.4. Discussion of Kinetic Studies
4.5. Influence of Liquid-to-Solid Ratio and Lead Desorption
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Cabral-Pinto, M.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Mustafa, F.S.; Omer, K.M.; Hama, S.; Hamarawf, R.F.; Rahman, K.O. Heavy metal pollution in the aquatic environment: Efficient and low-cost removal approaches to eliminate their toxicity: A review. RSC Adv. 2023, 13, 17595–17610. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; WHO Press: Geneva, Switzerland, 2011; Available online: https://www.who.int/publications/i/item/9789241548151 (accessed on 17 November 2025).
- Sanitary Rules and Norms SanPiN 1.2.3685-21 “Hygienic Standards and Requirements for Ensuring the Safety and (or) Harmlessness of Environmental Factors for Humans”. Decree of the Chief State Sanitary Doctor of the Russian Federation No. 2. (Note: This Is the Official Russian Standard Available from docs.cntd.ru. Access May Be Region-Dependent. The Authors Are Prepared to Provide Relevant Excerpts for Review Purposes Upon Request). 2021. Available online: https://docs.cntd.ru/document/573500115 (accessed on 2 November 2025).
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Asghar, N.; Hussain, A.; Nguyen, D.A.; Ali, S.; Hussain, I.; Junejo, A.; Ali, A. Advancement in nanomaterials for environmental pollutants remediation: A systematic review on bibliometrics analysis, material types, synthesis pathways, and related mechanisms. J. Nanobiotechnol. 2024, 22, 26. [Google Scholar] [CrossRef]
- Mehdi Sabzehmeidani, M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A.L. Carbon based materials: A review of adsorbents for inorganic and organic compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Kuoppamäki, K.; Hagner, M.; Valtanen, M.; Setälä, H. Using biochar to purify runoff in road verges of urbanised watersheds: A large-scale field lysimeter study. Watershed Ecol. Environ. 2019, 1, 15–25. [Google Scholar] [CrossRef]
- Saarela, T.; Lafdani, E.K.; Laurén, A.; Pumpanen, J.; Palviainen, M. Biochar as adsorbent in purification of clear-cut forest runoff water: Adsorption rate and adsorption capacity. Biochar 2020, 2, 227–237. [Google Scholar] [CrossRef]
- Baltrėnas, P.; Baltrėnaitė, E.; Kleiza, J.; Švedienė, J. A biochar-based medium in the biofiltration system: Removal efficiency, microorganism propagation, and the medium penetration modeling. J. Air Waste Manag. Assoc. 2016, 66, 673–686. [Google Scholar] [CrossRef]
- Ruseva, A.; Minnikova, T.; Kolesnikov, S.; Kushnareva, E.; Desyatnikov, M.; Daoud, R. Assessment of the ecological state and health of oil-contaminated Luvic Phaeozems Albic, Gleyic Albeluvisols, and Greyic Phaeozems after remediation by biochar. Sains Tanah J. Soil Sci. Agroclimatol. 2025, 22, 107–118. [Google Scholar] [CrossRef]
- Dubovik, M.; Warsta, L.; Tamm, O.; Wendling, L.; Rinta-Hiiro, V.; Koivusalo, H. Processes influencing stormwater purification by roadside biochar-amended sand filter in cold climate conditions. Boreal Environ. Res. 2024, 29, 17–34. [Google Scholar] [CrossRef]
- Koivusalo, H.; Dubovik, M.; Wendling, L.; Assmuth, E.; Sillanpää, N.; Kokkonen, T. Performance of sand and mixed sand–biochar filters for treatment of road runoff quantity and quality. Water 2023, 15, 1631. [Google Scholar] [CrossRef]
- Cederlund, H.; Börjesson, E.; Lundberg, D.; Stenström, J. Adsorption of pesticides with different chemical properties to a wood biochar treated with heat and iron. Water Air Soil Pollut. 2016, 227, 203. [Google Scholar] [CrossRef]
- Bovsun, M.A.; Castaldi, S.; Nesterova, O.V.; Semal, V.A.; Sakara, N.A.; Brikmans, A.V.; Khokhlova, A.I.; Karpenko, T.Y. Effect of Biochar on Soil CO2 Fluxes from Agricultural Field Experiments in Russian Far East. Agronomy 2021, 11, 1559. [Google Scholar] [CrossRef]
- Nesterova, O.; Bovsun, M.; Semal, V.; Brikmans, A.; Sakara, N. Positive experience in the application of soil- and carbon-saving agricultural technologies with the introduction of biochar in the conditions of the Russian Far East. E3S Web Conf. 2024, 498, 03011. [Google Scholar] [CrossRef]
- Nesterova, O.; Semal, V.; Bovsun, M.; Brikmans, A.; Sakara, N.; Tarasova, T. Effects of biochar and fertilizer amendments on soil acidity and soil organic carbon content on Luvic Anthrosols in Russian Far East. E3S Web Conf. 2023, 371, 06013. [Google Scholar] [CrossRef]
- Doszhanova, A.; Ospanbayev, Z.; Sembayeva, A.; Bekbauov, M.; Kazkeyev, D.; Myrzabek, K.; Duisenbekova, O. Effectiveness of biochar on degraded irrigated soils in Southeast Kazakhstan. Casp. J. Environ. Sci. 2025, 23, 1–11. [Google Scholar] [CrossRef]
- Bovsun, M.; Nesterova, O.; Semal, V.; Khokhlova, A.; Sakara, N. Changes in the composition and properties of biochar after one-year application. E3S Web Conf. 2020, 217, 10009. [Google Scholar] [CrossRef]
- Kinnunen, N.; Laurén, A.A.; Pumpanen, J.; Nieminen, T.M.; Palviainen, M. Biochar capacity to mitigate acidity and adsorb metals—Laboratory tests for acid sulfate soil drainage water. Water Air Soil Pollut. 2021, 232, 464. [Google Scholar] [CrossRef]
- Skripkina, T.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Sorption of methylene blue for studying the specific surface properties of biomass carbohydrates. Coatings 2020, 10, 1115. [Google Scholar] [CrossRef]
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Methylene blue adsorption by algal biomass based materials: Biosorbents characterization and process behaviour. J. Hazard. Mater. 2007, 147, 120–132. [Google Scholar] [CrossRef]
- Yener, N.; Biçer, C.; Önal, M.; Sarıkaya, Y. Simultaneous determination of cation exchange capacity and surface area of acid activated bentonite powders by methylene blue sorption. Appl. Surf. Sci. 2012, 258, 2534–2539. [Google Scholar] [CrossRef]
- Ortiz-Anaya, I.; Nishina, Y. Refined surface area determination of graphene oxide using methylene blue as a probe molecule: A comparative approach. Bull. Chem. Soc. Jpn. 2024, 97, uoae118. [Google Scholar] [CrossRef]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Dalmieda, J.; Kruse, P. Metal Cation Detection in Drinking Water. Sensors 2019, 19, 5134. [Google Scholar] [CrossRef]
- Kipling, J.J.; Wilson, R.B. Adsorption of methylene blue in the determination of surface areas. J. Appl. Chem. 1960, 10, 109–113. [Google Scholar] [CrossRef]
- Yukselen, Y.; Kaya, A. Suitability of the methylene blue test for surface area, cation exchange capacity and swell potential determination of clayey soils. Eng. Geol. 2008, 102, 38–45. [Google Scholar] [CrossRef]
- Sun, L.; Sun, C.; Sun, X. Screening highly selective ionophores for heavy metal ion-selective electrodes and potentiometric sensors. Electrochim. Acta 2016, 220, 690–698. [Google Scholar] [CrossRef]
- Radu, A.; Diamond, D. Ion-selective electrodes in trace level analysis of heavy metals: Potentiometry for the XXI century. In Comprehensive Analytical Chemistry; Alegret, S., Merkoçi, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 49, pp. 25–52. [Google Scholar] [CrossRef]
- Tang, X.; Wang, P.-Y.; Buchter, G. Ion-Selective Electrodes for Detection of Lead (II) in Drinking Water: A Mini-Review. Environments 2018, 5, 95. [Google Scholar] [CrossRef]
- De Marco, R.; Clarke, G.; Pejcic, B. Ion-Selective Electrode Potentiometry in Environmental Analysis. Electroanalysis 2007, 19, 1987–2001. [Google Scholar] [CrossRef]
- Kadum, A.H.K.; Badin, D.A.; Rybakova, S.O.; Burakova, I.V.; Burakov, A.E.; Dyachkova, T.P.; Tkachev, A.G. Effect of carbonization temperature on the physicochemical and sorption properties of coals from plant biomass for removing dyes from solutions. Inorg. Mater. Appl. Res. 2025, 16, 1394–1403. [Google Scholar] [CrossRef]
- Itodo, A.U.; Abdulrahman, F.W.; Hassan, L.G.; Maigandi, S.A.; Itodo, H.U. Application of methylene blue and iodine adsorption in the measurement of specific surface area by four acid and salt treated activated carbons. N. Y. Sci. J. 2010, 3, 25–33. [Google Scholar]
- Hegyesi, N.; Vad, R.T.; Pukánszky, B. Determination of the specific surface area of layered silicates by methylene blue adsorption: The role of structure, pH and layer charge. Appl. Clay Sci. 2017, 146, 50–55. [Google Scholar] [CrossRef]
- Yukselen, Y.; Kaya, A. Comparison of Methods for Determining Specific Surface Area of Soils. J. Geotech. Geoenviron. Eng. 2006, 132, 931–936. [Google Scholar] [CrossRef]
- Maltsev, A.A.; Bibikov, S.B.; Kalinichenko, V.N.; Gudkov, M.V.; Melnikov, V.P.; Varfolomeev, S.D. Determining the Specific Surface Area of Carbon Electrode Materials for Electrodes of Supercapacitors via the Adsorption of Methylene Blue Dye. Russian J. Phys. Chem. A 2018, 92, 772–777. [Google Scholar] [CrossRef]
- Barton, S.S. The adsorption of methylene active carbon. Carbon 1987, 25, 343–350. [Google Scholar] [CrossRef]
- Chen, L.; Batchelor-McAuley, C.; Rasche, B.; Johnston, C.; Hindle, N.; Compton, R.G. Surface area measurements of graphene and graphene oxide samples: Dopamine adsorption as a complement or alternative to methylene blue? Appl. Mater. Today 2020, 18, 100506. [Google Scholar] [CrossRef]
- Nunes, C.A.; Guerreiro, M.C. Estimation of surface area and pore volume of activated carbons by methylene blue and iodine numbers. Quím. Nova 2011, 34, 472–476. [Google Scholar] [CrossRef]
- Hoslett, J.; Ghazal, H.; Mohamad, N.; Jouhara, H. Removal of methylene blue from aqueous solutions by biochar prepared from the pyrolysis of mixed municipal discarded material. Sci. Total Environ. 2020, 714, 136832. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Li, J.; Ma, D.; Han, R. Characterization of bio-char from pyrolysis of wheat straw and its evaluation on methylene blue adsorption. Desalin. Water Treat. 2012, 46, 115–123. [Google Scholar] [CrossRef]
- Chen, J.; Tang, C.; Li, X.; Sun, J.; Liu, Y.; Huang, W.; Wang, A.; Lu, Y. Preparation and Modification of Rape Straw Biochar and Its Adsorption Characteristics for Methylene Blue in Water. Water 2022, 14, 3761. [Google Scholar] [CrossRef]
- Mahdi, Z.; Hanandeh, A.E.; Yu, Q. Influence of Pyrolysis Conditions on Surface Characteristics and Methylene Blue Adsorption of Biochar Derived from Date Seed Biomass. Waste Biomass Valor. 2017, 8, 2061–2073. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K.; Phan, C. Adsorptive removal of methylene blue (MB) dye from aqueous solution by biochar prepared from Eucalyptus sheathiana bark: Kinetics, equilibrium, mechanism, thermodynamics and process design. Desalin. Water Treat. 2016, 57, 28964–28980. [Google Scholar] [CrossRef]
- Ge, Q.; Li, P.; Liu, M.; Xiao, G.-M.; Xiao, Z.-Q.; Mao, J.-W.; Gai, X.-K. Removal of methylene blue by porous biochar obtained by KOH activation from bamboo biochar. Bioresour. Bioprocess. 2023, 10, 51. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Wang, L.; Tuo, Y.; Yan, S.; Ma, J.; Zhang, X.; Shen, Y.; Guo, H.; Han, L. Experimental and DFT insights into the adsorption mechanism of methylene blue by alkali-modified corn straw biochar. RSC Adv. 2024, 14, 1854–1865. [Google Scholar] [CrossRef]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; FAO Irrigation and Drainage Paper 29 Rev. 1; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985; Available online: https://www.fao.org/3/t0234e/t0234e00.htm (accessed on 17 November 2025).
- On Approval of the Rules for Cold Water Supply and Sanitation and on Amendments to Certain Acts of the Government of the Russian Federation. Decree of the Government of the Russian Federation No. 644 (29 July 2013, as Amended 28 November 2023). (Note: This Is the Official Russian Standard Available from docs.cntd.ru. Access May Be Region-Dependent. The Authors Are Prepared to Provide Relevant Excerpts for Review Purposes Upon Request). Available online: http://pravo.gov.ru/proxy/ips/?docbody=&prevDoc=102420837&backlink=1&&nd=102159947 (accessed on 17 November 2025). (In Russian)
- Mass Concentration of Lead in Waters. Measurement Procedure by Photometric Method with Hexaoxacycloazochrom. Guidance Document RD 52.24.448-2009. Available online: https://files.stroyinf.ru/Data2/1/4293750/4293750952.pdf (accessed on 17 November 2025). (In Russian).
- Ding, W.; Dong, X.; Ime, I.M.; Gao, B.; Ma, L.Q. Pyrolytic temperatures impact lead sorption mechanisms by bagasse biochars. Chemosphere 2014, 105, 68–74. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Zhao, M.; Dai, Y.; Zhang, M.; Feng, C.; Qin, B.; Zhang, W.; Zhao, N.; Li, Y.; Ni, Z.; Xu, Z.; et al. Mechanisms of Pb and/or Zn adsorption by different biochars: Biochar characteristics, stability, and binding energies. Sci. Total Environ. 2020, 717, 136894. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Y.; Jin, F.; McMillan, O.; Al-Tabbaa, A. Qualitative and quantitative characterisation of adsorption mechanisms of lead on four biochars. Sci. Total Environ. 2017, 609, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Li, N.; Tao, J.; Yan, B.; Cui, X.; Chen, G. Adsorption of Lead from Aqueous Solution by Biochar: A Review. Clean Technol. 2022, 4, 629–652. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Chatla, A.; Zakaria, Y.; Kochkodan, V.; Shanableh, A.; Laoui, T.; Atieh, M.A. Palm leaves based biochar: Advanced material characterization and heavy metal adsorption study. Biomass Conv. Bioref. 2024, 14, 14811–14830. [Google Scholar] [CrossRef]
- Ye, H.; Yu, K.; Li, B.; Guo, J. Study on adsorption properties and mechanism of sodium hydroxide–modified ball-milled biochar to dislodge lead(II) and MB from water. Biomass Conv. Bioref. 2024, 14, 15989–16003. [Google Scholar] [CrossRef]
- Rabiee Abyaneh, M.; Nabi Bidhendi, G.; Daryabeigi Zand, A. Pb(ΙΙ), Cd(ΙΙ), and Mn(ΙΙ) adsorption onto pruning-derived biochar: Physicochemical characterization, modeling and application in real landfill leachate. Sci. Rep. 2024, 14, 3426. [Google Scholar] [CrossRef]
- Chi, T.; Zuo, J.; Liu, F. Performance and mechanism for cadmium and lead adsorption from water and soil by corn straw biochar. Front. Environ. Sci. Eng. 2017, 11, 15. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, Z.; Feng, Q.; Yao, D.; Yu, J.; Wang, D.; Lv, S.; Liu, Y.; Zhou, N.; Zhong, M.-E. Effect of pyrolysis condition on the adsorption mechanism of lead, cadmium and copper on tobacco stem biochar. J. Clean. Prod. 2018, 187, 996–1005. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, H.; Ren, J.; Deng, L.; Che, D. Effect of inherent alkali and alkaline earth metals in biochar on adsorption of Pb2+ in aqueous solution: Different roles of Na/Mg/K/Ca. Sep. Purif. Technol. 2025, 354, 128766. [Google Scholar] [CrossRef]
- Shao, C.; Li, J.; Dai, Y. The removal mechanism of lead from wastewater on pharmaceutical sludge-based biochar. Desalination Water Treat. 2024, 319, 100499. [Google Scholar] [CrossRef]
- Somba, B.E.; Napitupulu, M.; Walanda, D.K.; Anshary, A.; Talo, W.S. Optimizing the performance of sunflower (Helianthus annuus L.) seed shell-derived biochar for lead ion adsorption. IJDNE 2024, 19, 259–265. [Google Scholar] [CrossRef]
- Rohman, G.A.N.; Aziz, M.A.; Nawaz, A.; Elgzoly, M.A.; Hossain, M.M.; Razzak, S.A. High-performance biochar from Chlorella pyrenoidosa algal biomass for heavy metals removal in wastewater. Sep. Purif. Technol. 2024, 341, 126870. [Google Scholar] [CrossRef]
- Singh, J.; Bhattu, M.; Verma, M.; Bechelany, M.; Brar, S.K.; Jadeja, R. Sustainable valorization of rice straw into biochar and carbon dots using a novel one-pot approach for dual applications in detection and removal of lead ions. Nanomaterials 2025, 15, 66. [Google Scholar] [CrossRef]
- Vaičiukynienė, D.; Alaburdaitė, R.; Nizevičienė, D.; Tamošaitis, G. Sorption properties of Pb2+ ions from water by alkali activated slag/biochar composites. Biomass Conv. Bioref. 2025, 15, 5075–5087. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, R. Comparison of characteristics of twenty-one types of biochar and their ability to remove multi-heavy metals and methylene blue in solution. Fuel Process. Technol. 2017, 160, 55–63. [Google Scholar] [CrossRef]
- Komkiene, J.; Baltrenaite, E. Biochar as adsorbent for removal of heavy metal ions [Cadmium(II), Copper(II), Lead(II), Zinc(II)] from aqueous phase. Int. J. Environ. Sci. Technol. 2016, 13, 471–482. [Google Scholar] [CrossRef]
- Drobniak, A.; Mastalerz, M.; Knauth, W.; Ardakani, O.; Dos Santos, T.; De Faria, V.; Congo, T.; Hackley, P.; Hatcherian, J.; Hower, J.; et al. Atlas of Microscopic Images of Biochar: Using Reflected Light Microscopy in Biochar Characterization. Int. J. Environ. Sci. 2024, 6. [Google Scholar] [CrossRef]
- Gąsior, D.; Tic, W.J. Application of the Biochar-Based Technologies as the Way of Realization of the Sustainable Development Strategy. Econ. Environ. Stud. 2017, 17, 597–611. [Google Scholar] [CrossRef]
- Al-Tabbal, J.; Al-Zboon, K.K.; Qrunfleh, I.M. Biochar: Potential eco-friendly waste for mitigation of the stress impacts of irrigation with brackish water on beans. Irrig. Drain. 2025. [Google Scholar] [CrossRef]
- Sharma, P.; Abhilasha; Abhishek, K.; Bhattacharya, S.; Sengupta, S.; Seth, C.S. Removal of lead in water by potassium hydroxide-activated biochar developed from Syzygium cumini stem. Discov. Chem. Eng. 2024, 4, 17. [Google Scholar] [CrossRef]
- Kieush, L.; Koveria, A.; Huynh, T.-P.; Zuber, J.; Vogt, C.; Smått, J.-H. Comprehensive microstructural evaluation of biochar, biocoke and anthracite with respect to the CO2 reactivity. Results Eng. 2025, 27, 105690. [Google Scholar] [CrossRef]
- Majee, P.; Periyavaram, S.R.; Uppala, L.; Reddy, P.H.P. Physicochemical and energy characteristics of biochar and hydrochar derived from cotton stalks: A comparative study. Bioenerg. Res. 2025, 18, 20. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Kamal, K.H.; El-Sakhawy, M.; Hassan, E.B.; Kamel, S. Highly efficient removal of lead (II) and methylene blue using oxidized biochar decorated with MnO2. J. Water Process Eng. 2025, 72, 107478. [Google Scholar] [CrossRef]
- Yang, Z.; Hou, J.; Wu, J.; Miao, L. The effect of carbonization temperature on the capacity and mechanisms of Pb(II) adsorption by microalgae residue-derived biochar. Ecotoxicol. Environ. Saf. 2021, 225, 112750. [Google Scholar] [CrossRef] [PubMed]













| Parameter | Value |
|---|---|
| Fraction (mm) | 0.3–0.5 |
| Bulk weight (g/mL) | 0.32 ± 0.01 |
| Ash content (%) | 9.27% ± 1.98% |
| Zero charge point | 6.97 |
| Equation | Parameter | Value |
|---|---|---|
| Freundlich | Kf | 2.68 ± 0.29 |
| nF | 5.89 ± 1.08 | |
| Langmuir | Kl | 0.82 ± 0.22 |
| qm | 5.18 ± 0.22 | |
| Sips | Klf | 0.75 ± 0.21 |
| qm | 5.70 ± 0.76 | |
| nS | 1.56 ± 0.63 |
| Equation | Parameter | Value |
|---|---|---|
| Freundlich | Kf | 9.94 ± 0.49 |
| nF | 7.71 ± 1.10 | |
| Langmuir | Kl | 9.32 ± 1.30 |
| qm | 14.21 ± 0.43 | |
| Sips | Klf | 16.40 ± 8.23 |
| qm | 13.94 ± 0.44 | |
| nS | 0.81 ± 0.13 |
| Model | Parameter | Kinetic Experiment No. 1 | Kinetic Experiment No. 2 |
|---|---|---|---|
| PFO | qe | 4.39 ± 0.03 | 14.3 ± 0.1 |
| k1 | 0.0018 ± 0.0001 | 0.0015 ± 0.0001 | |
| R2 | 0.99457 | 0.99542 | |
| PSO | qe | 4.97 ± 0.03 | 17.1 ± 0.2 |
| k2 | 0.00052 ± 0.00002 | 0.00010 ± 0.00001 | |
| R2 | 0.99838 | 0.99543 | |
| Elovich | a | 0.038 ± 0.001 | 0.046 ± 0.001 |
| β | 1.089 ± 0.006 | 0.232 ± 0.002 | |
| R2 | 0.99984 | 0.99943 |
| Dependent Variable | Equation | Parameter a | Parameter b | R2 |
|---|---|---|---|---|
| qe | 0.046 ± 0.007 | 0.716 ± 0.110 | 0.979 | |
| PF | (4.41 ± 0.70) × 105 | −1.418 ± 0.229 | 0.986 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egorin, A.M.; Novikova, S.A.; Priymak, I.D.; Privar, Y.O.; Brikmans, A.V.; Shlyk, D.K.; Gilev, A.M.; Nesterova, O.V. Valorization of Birch Biochar: An Efficient and Sustainable Solution for Lead Decontamination of Water. Biomass 2025, 5, 75. https://doi.org/10.3390/biomass5040075
Egorin AM, Novikova SA, Priymak ID, Privar YO, Brikmans AV, Shlyk DK, Gilev AM, Nesterova OV. Valorization of Birch Biochar: An Efficient and Sustainable Solution for Lead Decontamination of Water. Biomass. 2025; 5(4):75. https://doi.org/10.3390/biomass5040075
Chicago/Turabian StyleEgorin, Andrei M., Svetlana A. Novikova, Igor D. Priymak, Yulia O. Privar, Anastasia V. Brikmans, Daria Kh. Shlyk, Andrei M. Gilev, and Olga V. Nesterova. 2025. "Valorization of Birch Biochar: An Efficient and Sustainable Solution for Lead Decontamination of Water" Biomass 5, no. 4: 75. https://doi.org/10.3390/biomass5040075
APA StyleEgorin, A. M., Novikova, S. A., Priymak, I. D., Privar, Y. O., Brikmans, A. V., Shlyk, D. K., Gilev, A. M., & Nesterova, O. V. (2025). Valorization of Birch Biochar: An Efficient and Sustainable Solution for Lead Decontamination of Water. Biomass, 5(4), 75. https://doi.org/10.3390/biomass5040075








