Influence of Milling Conditions on Fecal Sludge-Based Biochar
Abstract
1. Introduction
2. Materials and Methods
Sample Preparation
3. Analyses and Characterization
3.1. Determination of Carbon and Nitrogen
3.2. Determination of Particle Size and Surface Morphology
3.3. Analysis of Functional Groups
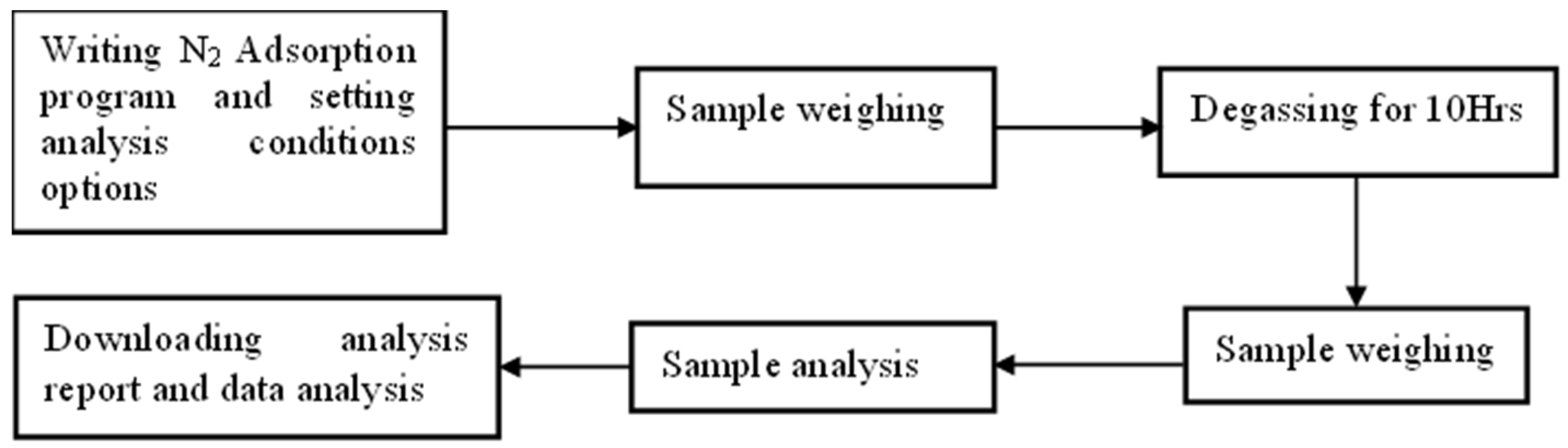
3.4. Measurement of Surface Area, Pore Volume, and Pore Size
3.5. Dye Adsorption
3.6. Statistical Analysis
4. Results and Discussions
4.1. Effect of BPR and Milling Time on Carbon and Nitrogen
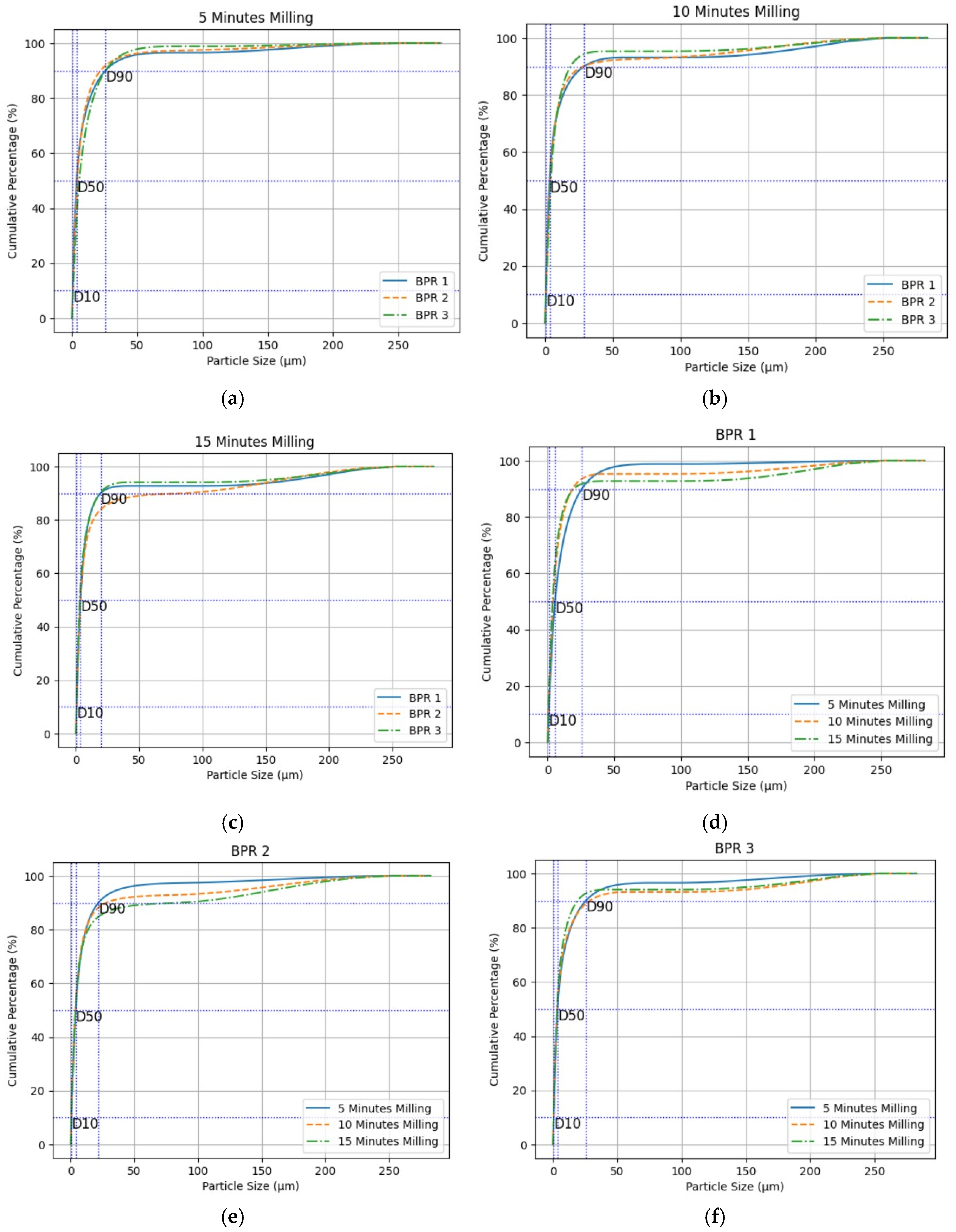
4.2. Particle Size Distribution
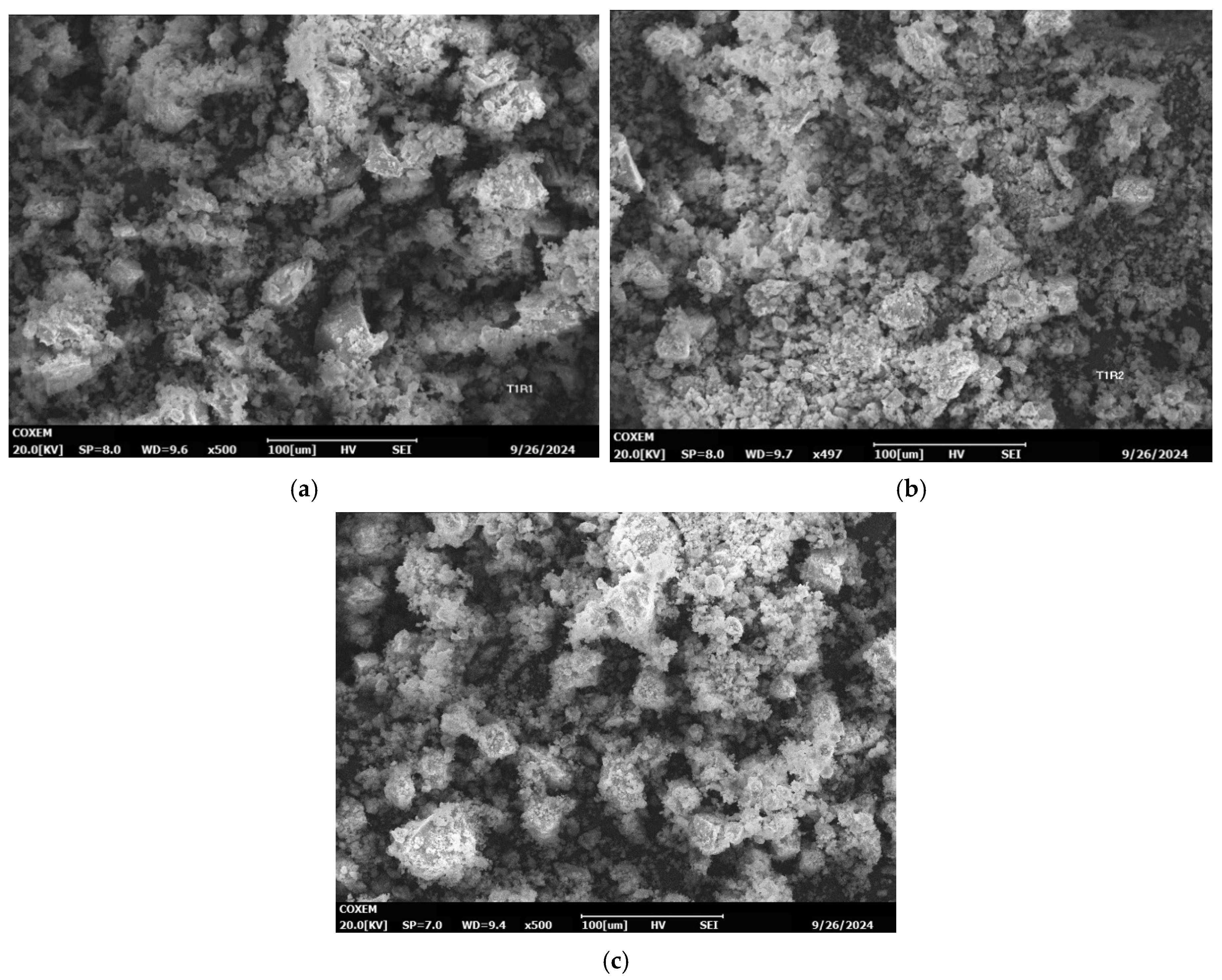
4.3. Surface Morphology
4.4. Functional Groups
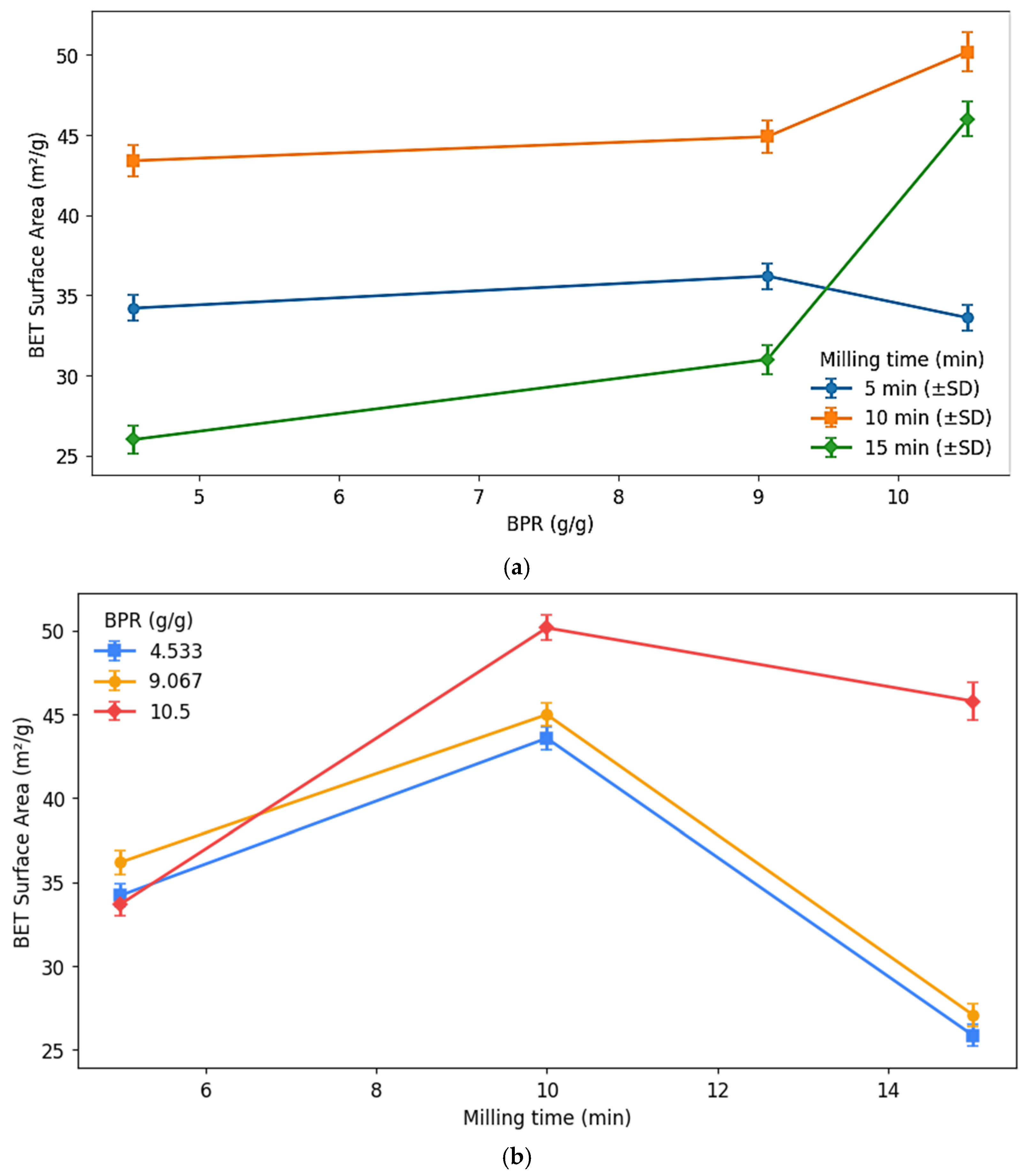
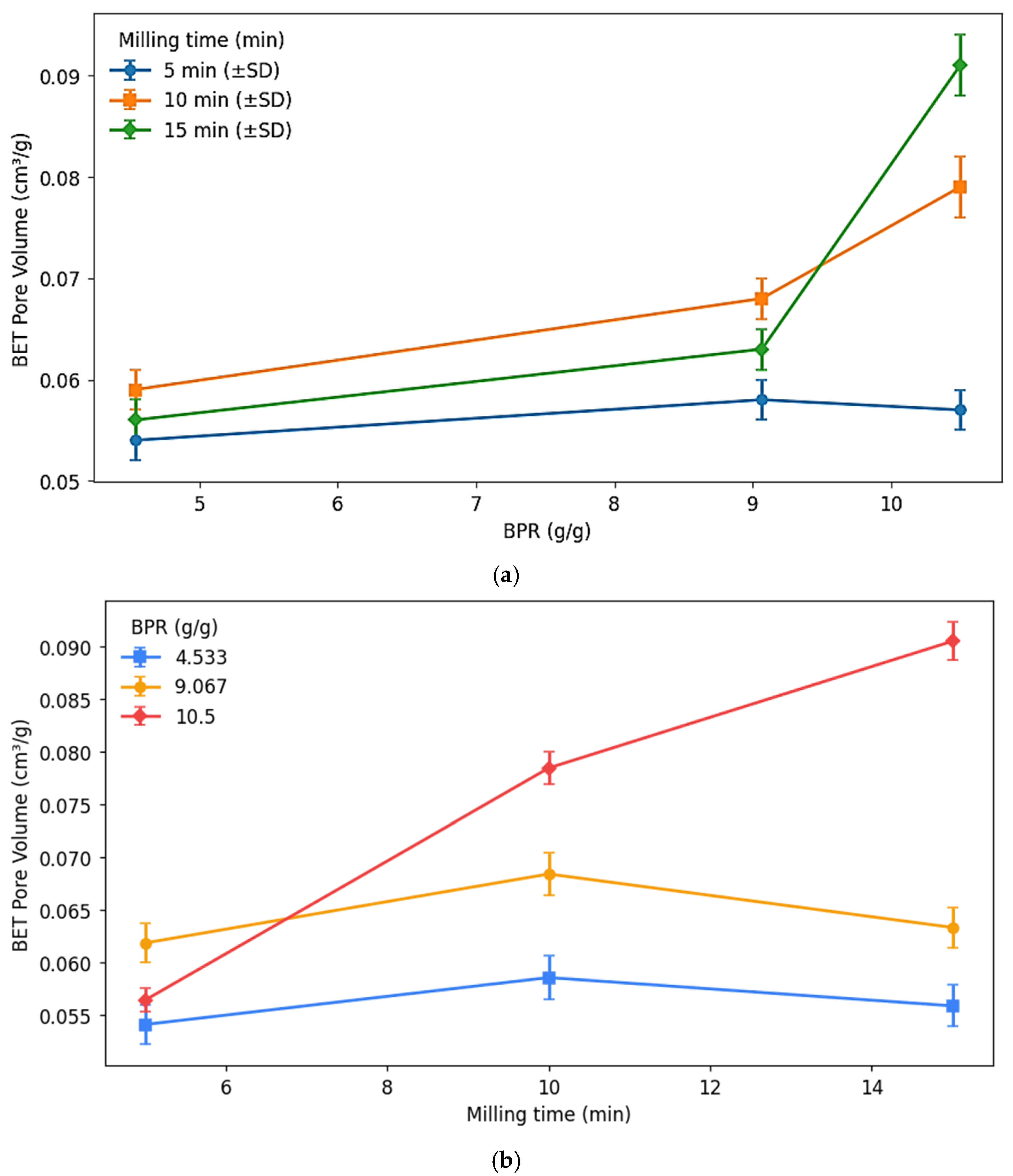
4.5. BET Surface Area and Pore Volume
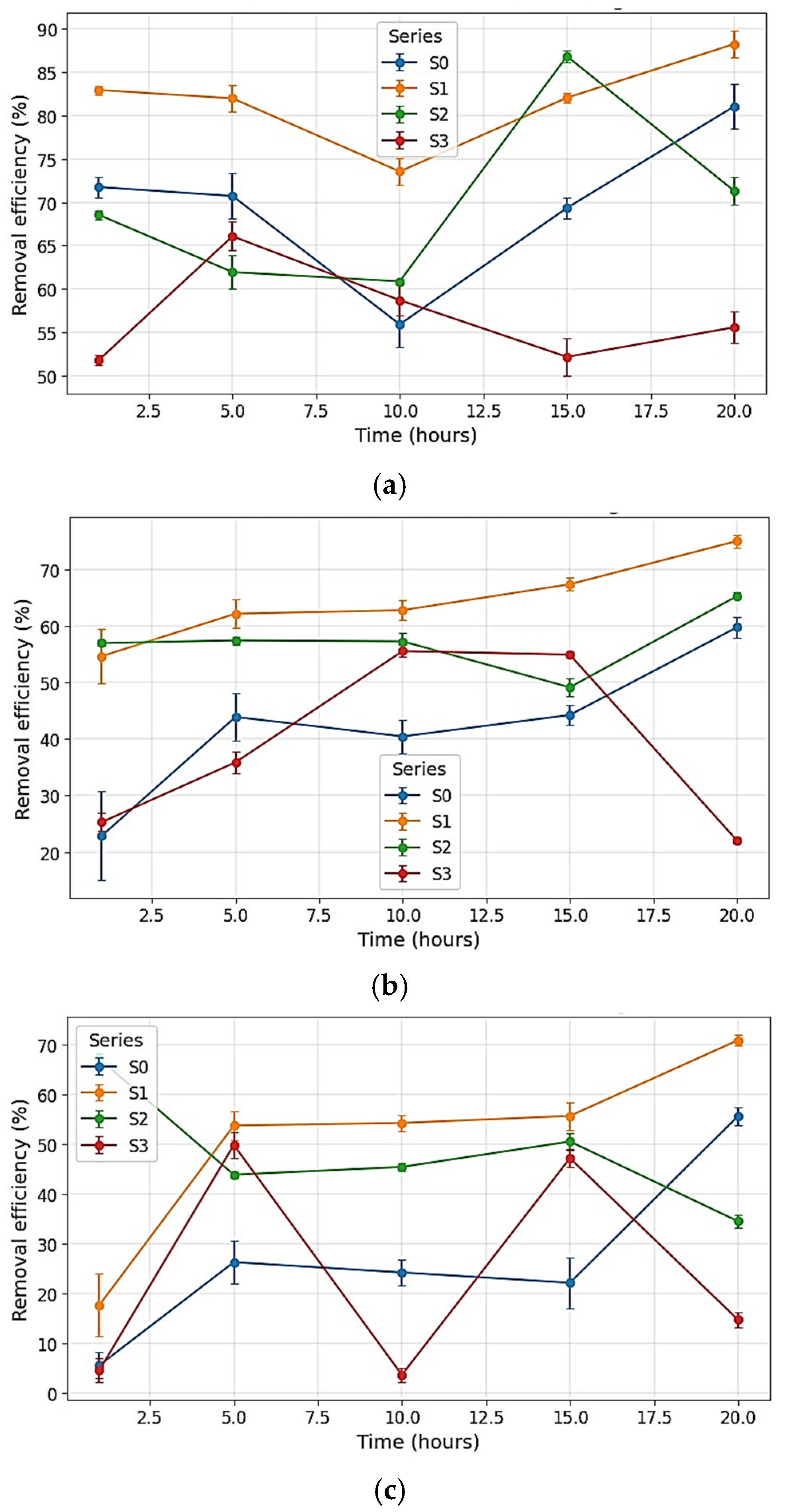
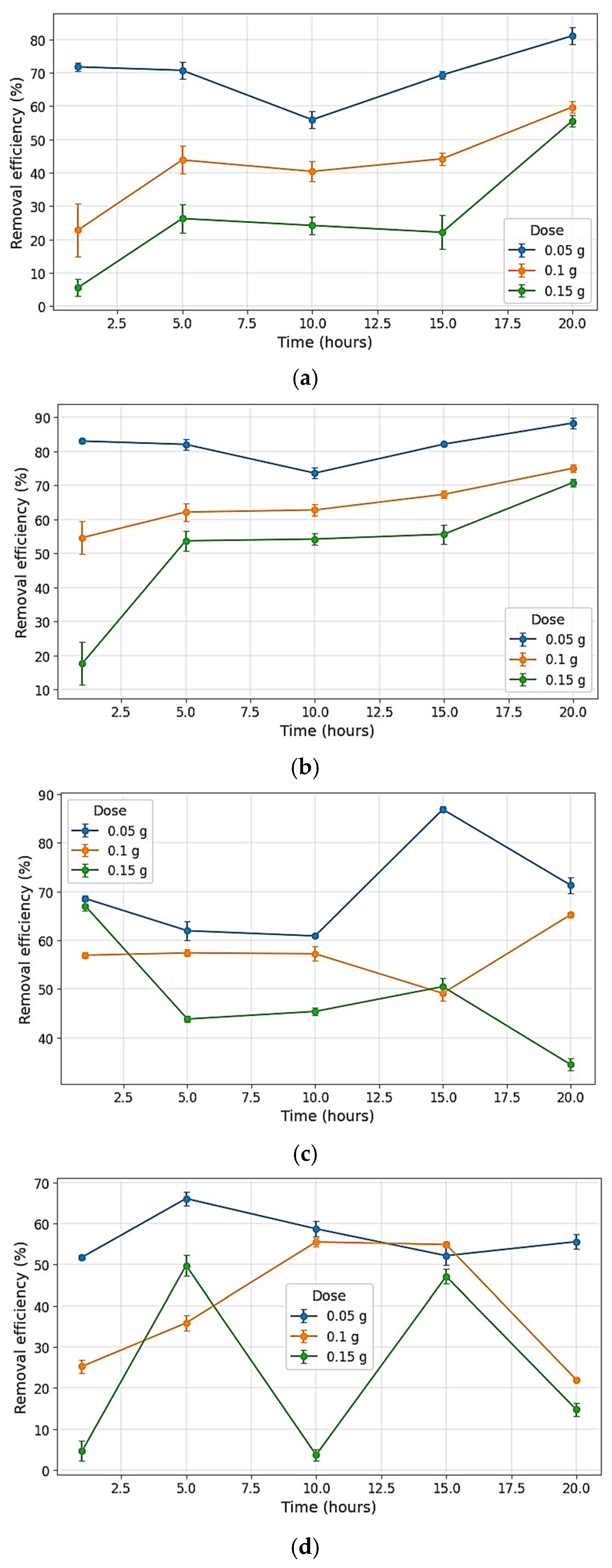
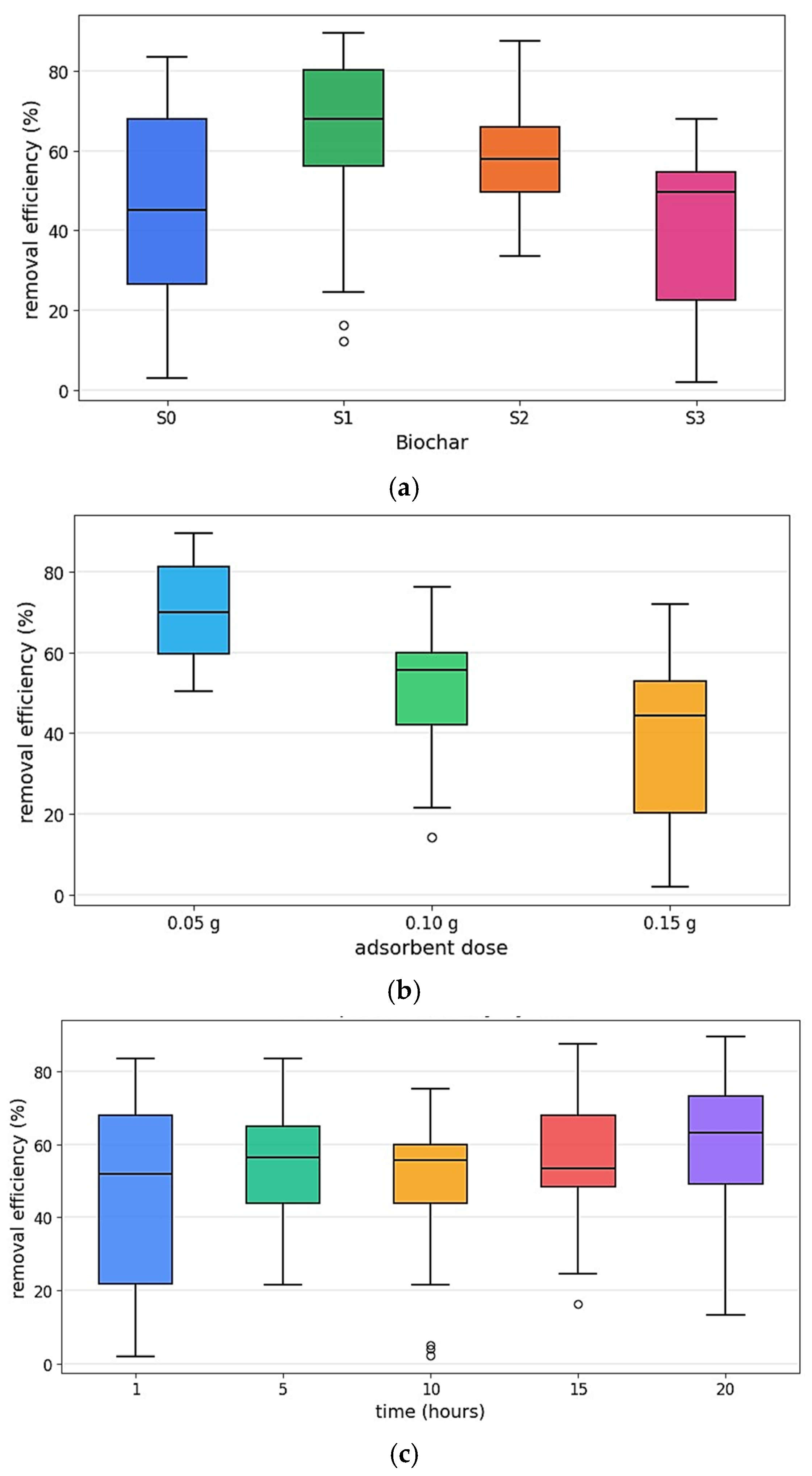
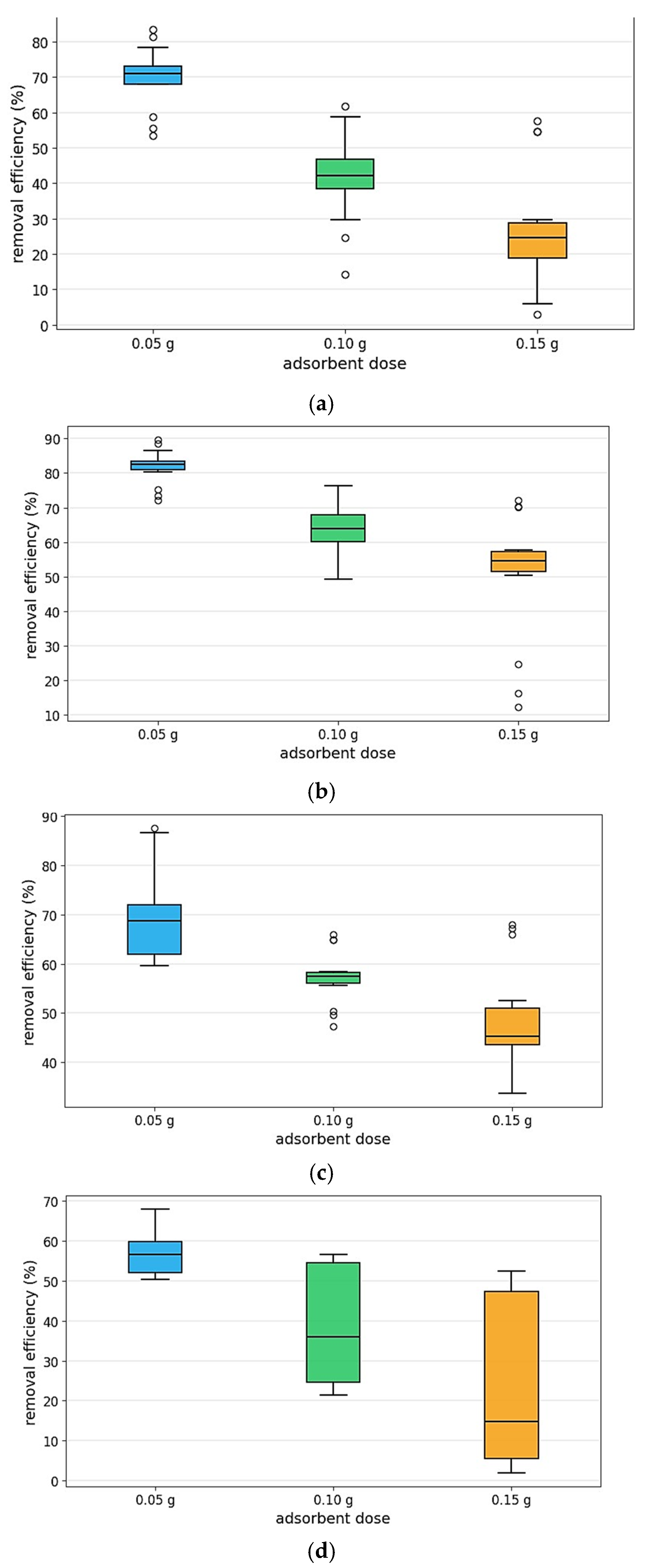
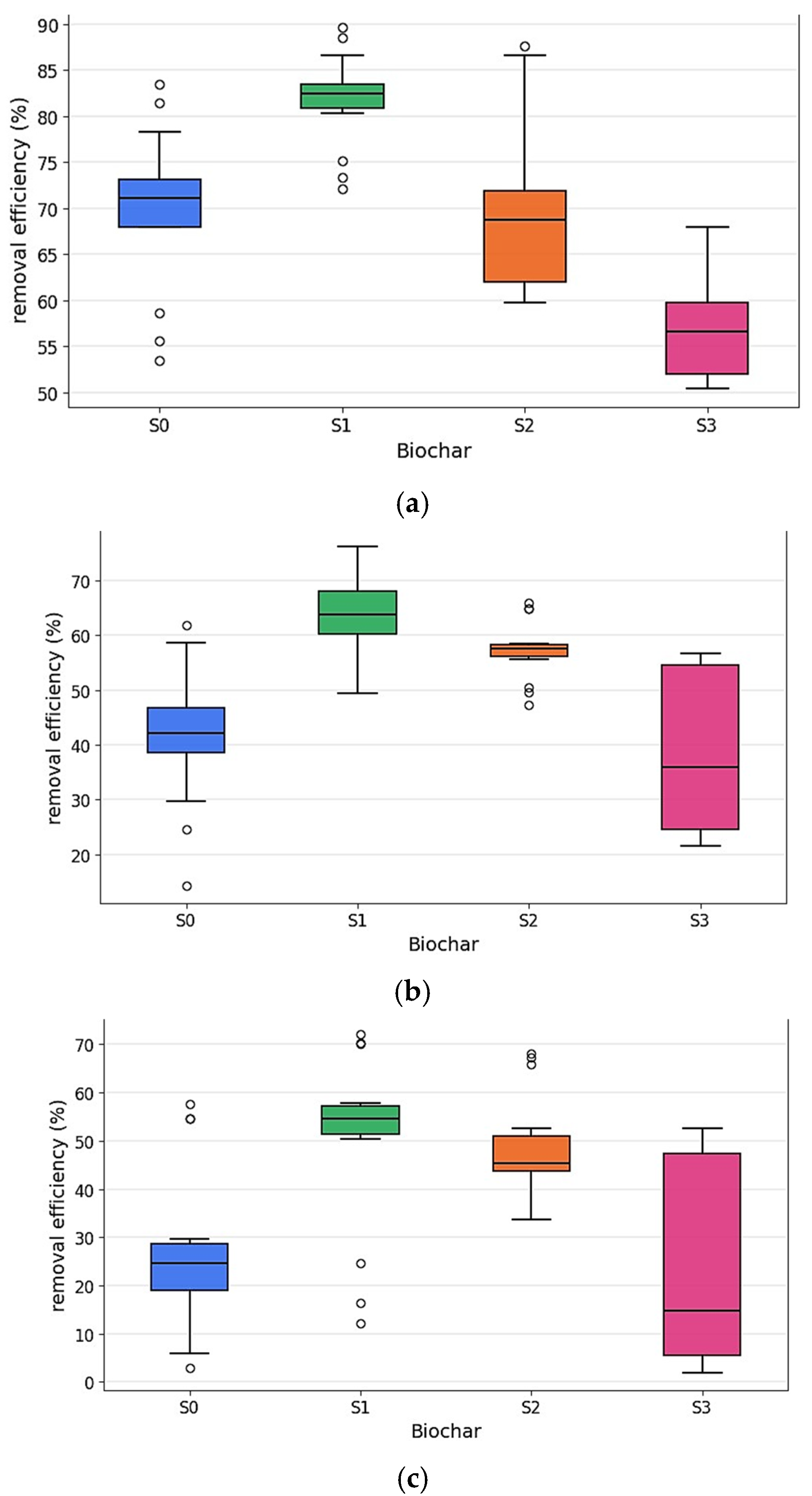
4.6. Methylene Blue Adsorption
5. Conclusions
6. Recommendations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khaydarov, R.; Khaydarov, R.; Gapurova, O. (Eds.) Application of carbon nanoparticles for water treatment. In Proceedings of the Water Treatment Technologies for the Removal of High-Toxicity Pollutants, Košice, Slovakia, 13–17 September 2008; Springer: Berlin/Heidelberg, Germany, 2010; pp. 253–258. [Google Scholar]
- Nasrollahzadeh, M.; Sajadi, S.M.; Sajjadi, M.; Issaabadi, Z. Applications of nanotechnology in daily life. Interface Sci. Technol. 2019, 28, 113–143. [Google Scholar]
- Rashidi, L.; Khosravi-Darani, K. The applications of nanotechnology in food industry. Crit. Rev. Food Sci. Nutr. 2011, 51, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Surendiran, A.; Sandhiya, S.; Pradhan, S.; Adithan, C. Novel applications of nanotechnology in medicine. Indian J. Med. Res. 2009, 130, 689–701. [Google Scholar]
- Song, Q.; Kong, F.; Liu, B.-F.; Song, X.; Ren, H.-Y. Biochar-based composites for removing chlorinated organic pollutants: Applications, mechanisms, and perspectives. Environ. Sci. Ecotechnol. 2024, 21, 100420. [Google Scholar] [CrossRef]
- Liang, L.; Xi, F.; Tan, W.; Meng, X.; Hu, B.; Wang, X. Review of organic and inorganic pollutants removal by biochar and biochar-based composites. Biochar 2021, 3, 255–281. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Ćwikła-Bundyra, W.; Bogusz, A.; Skwarek, E.; Ok, Y.S. Characterization of nanoparticles of biochars from different biomass. J. Anal. Appl. Pyrolysis 2016, 121, 165–172. [Google Scholar] [CrossRef]
- Wang, W.; Martin, J.C.; Fan, X.; Han, A.; Luo, Z.; Sun, L. Silica nanoparticles and frameworks from rice husk biomass. ACS Appl. Mater. Interfaces 2012, 4, 977–981. [Google Scholar] [CrossRef]
- Jacob, R.H.; Shanab, S.M.; Shalaby, E.A. Algal biomass nanoparticles: Chemical characteristics, biological actions, and applications. Biomass Convers. Biorefin. 2021, 13, 11441–11455. [Google Scholar]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Rouissi, T.; Verma, M.; Surampalli, R.Y.; Valero, J.R. A green method for production of nanobiochar by ball milling-optimization and characterization. J. Clean. Prod. 2017, 164, 1394–1405. [Google Scholar] [CrossRef]
- Soares, O.; Rocha, R.; Gonçalves, A.; Figueiredo, J.; Órfão, J.; Pereira, M. Easy method to prepare N-doped carbon nanotubes by ball milling. Carbon 2015, 91, 114–121. [Google Scholar] [CrossRef]
- Fecht, H.; Hellstern, E.; Fu, Z.; Johnson, W. Nanocrystalline metals prepared by high-energy ball milling. Metall. Trans. A 1990, 21, 2333–2337. [Google Scholar] [CrossRef]
- Salah, N.; Habib, S.S.; Khan, Z.H.; Memic, A.; Azam, A.; Alarfaj, E.; Zahed, N.; Al-Hamedi, S. High-energy ball milling technique for ZnO nanoparticles as antibacterial material. Int. J. Nanomed. 2011, 6, 863–869. [Google Scholar] [CrossRef]
- Stolle, A.; Szuppa, T.; Leonhardt, S.E.; Ondruschka, B. Ball milling in organic synthesis: Solutions and challenges. Chem. Soc. Rev. 2011, 40, 2317–2329. [Google Scholar] [CrossRef]
- Bullen, H.A.; Garrett, S.J. TiO2 nanoparticle arrays prepared using a nanosphere lithography technique. Nano Lett. 2002, 2, 739–745. [Google Scholar] [CrossRef]
- Kim, M.; Osone, S.; Kim, T.; Higashi, H.; Seto, T. Synthesis of nanoparticles by laser ablation: A review. KONA Powder Part. J. 2017, 34, 80–90. [Google Scholar] [CrossRef]
- Semaltianos, N. Nanoparticles by laser ablation. Crit. Rev. Solid State Mater. Sci. 2010, 35, 105–124. [Google Scholar] [CrossRef]
- Romano, L.; Kagias, M.; Vila-Comamala, J.; Jefimovs, K.; Tseng, L.-T.; Guzenko, V.A.; Stampanoni, M. Metal assisted chemical etching of silicon in the gas phase: A nanofabrication platform for X-ray optics. Nanoscale Horiz. 2020, 5, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Salavati-Niasari, M.; Javidi, J.; Dadkhah, M. Ball milling synthesis of silica nanoparticle from rice husk ash for drug delivery application. Comb. Chem. High Throughput Screen. 2013, 16, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Lolkema, B.; Mawioo, P.; Hooijmans, C.M.; Dupont, C. Systematic characterization of faecal sludge from various sources for its use as a solid fuel. Biomass Convers. Biorefin. 2023, 15, 2779–2789. [Google Scholar] [CrossRef]
- Muspratt, A.M.; Nakato, T.; Niwagaba, C.; Dione, H.; Kang, J.; Stupin, L.; Regulinski, J.; Mbéguéré, M.; Strande, L. Fuel potential of faecal sludge: Calorific value results from Uganda, Ghana and Senegal. J. Water Sanit. Hyg. Dev. 2014, 4, 223–230. [Google Scholar] [CrossRef]
- Chandana, N.; Rao, B. Evaluating the physicochemical, nutrient, and pathogenic characteristics of fecal sludge for fertilizer application: Case from vadgaon maval, Maharashtra, India. J. Environ. Eng. 2021, 147, 04021003. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Z.; Huang, J.; Gao, B.; Zhao, L.; Qiu, H.; Cao, X. Sorption of reactive red by biochars ball milled in different atmospheres: Co-effect of surface morphology and functional groups. Chem. Eng. J. 2021, 413, 127468. [Google Scholar] [CrossRef]
- Mi, L.; Liu, Y.; Huang, Q.; Zhao, L.; Qin, X.; Sun, Y.; Li, B. Enhanced adsorption and regulation mechanism of ball milling-modified biochar on Cd and atrazine in soil. Environ. Sci. Pollut. Res. 2025, 32, 5091–5105. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Jung, G.-B. Effects of pyrolysis and ball-milling on the physicochemical and rhodamine b removal characteristics of rice-bran-derived biochar. Appl. Sci. 2023, 13, 4288. [Google Scholar] [CrossRef]
- Lopez-Tenllado, F.J.; Motta, I.L.; Hill, J.M. Modification of biochar with high-energy ball milling: Development of porosity and surface acid functional groups. Bioresour. Technol. Rep. 2021, 15, 100704. [Google Scholar] [CrossRef]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Huang, H.; Tang, J. Effects of ball milling on the physicochemical and sorptive properties of biochar: Experimental observations and governing mechanisms. Environ. Pollut. 2018, 233, 54–63. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; You, S.; Igalavithana, A.D.; Xia, Y.; Bhatnagar, A.; Gupta, S.; Kua, H.W.; Kim, S.; Kwon, J.-H.; Tsang, D.C. Biochar-based adsorbents for carbon dioxide capture: A critical review. Renew. Sustain. Energy Rev. 2020, 119, 109582. [Google Scholar] [CrossRef]
- Guo, S.; Li, Y.; Wang, Y.; Wang, L.; Sun, Y.; Liu, L. Recent advances in biochar-based adsorbents for CO2 capture. Carbon Capture Sci. Technol. 2022, 4, 100059. [Google Scholar] [CrossRef]
- Ajala, O.A.; Akinnawo, S.O.; Bamisaye, A.; Adedipe, D.T.; Adesina, M.O.; Okon-Akan, O.A.; Adebusuyi, T.A.; Ojedokun, A.T.; Adegoke, K.A.; Bello, O.S. Adsorptive removal of antibiotic pollutants from wastewater using biomass/biochar-based adsorbents. RSC Adv. 2023, 13, 4678–4712. [Google Scholar] [CrossRef] [PubMed]
- Brassard, P.; Godbout, S.; Lévesque, V.; Palacios, J.H.; Raghavan, V.; Ahmed, A.; Hogue, R.; Jeanne, T.; Verma, M. Biochar for soil amendment. In Char and Carbon Materials Derived from Biomass; Elsevier: Amsterdam, The Netherlands, 2019; pp. 109–146. [Google Scholar]
- Adhikari, S.; Timms, W.; Mahmud, M.P. Optimising water holding capacity and hydrophobicity of biochar for soil amendment–A review. Sci. Total Environ. 2022, 851, 158043. [Google Scholar] [CrossRef] [PubMed]
- Anto, S.; Sudhakar, M.; Ahamed, T.S.; Samuel, M.S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Activation strategies for biochar to use as an efficient catalyst in various applications. Fuel 2021, 285, 119205. [Google Scholar] [CrossRef]
- Harindintwali, J.D.; He, C.; Xiang, L.; Dou, Q.; Liu, Y.; Wang, M.; Wen, X.; Fu, Y.; Islam, M.U.; Chang, S.X. Effects of ball milling on biochar adsorption of contaminants in water: A meta-analysis. Sci. Total Environ. 2023, 882, 163643. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, N.; Hu, X. Effects of wet and dry ball milling on the physicochemical properties of sawdust derived-biochar. Instrum. Sci. Technol. 2020, 48, 287–300. [Google Scholar] [CrossRef]
- Tan, X.-F.; Zhu, S.-S.; Wang, R.-P.; Chen, Y.-D.; Show, P.-L.; Zhang, F.-F.; Ho, S.-H. Role of biochar surface characteristics in the adsorption of aromatic compounds: Pore structure and functional groups. Chin. Chem. Lett. 2021, 32, 2939–2946. [Google Scholar] [CrossRef]
- Liu, X.; Yin, H.; Liu, H.; Cai, Y.; Qi, X.; Dang, Z. Multicomponent adsorption of heavy metals onto biogenic hydroxyapatite: Surface functional groups and inorganic mineral facilitating stable adsorption of Pb (II). J. Hazard. Mater. 2023, 443, 130167. [Google Scholar] [CrossRef]
- Mardis, K.L.; Brune, B.J.; Vishwanath, P.; Giorgis, B.; Payne, G.F.; Gilson, M.K. Intramolecular versus intermolecular hydrogen bonding in the adsorption of aromatic alcohols onto an acrylic ester sorbent. J. Phys. Chem. B 2000, 104, 4735–4744. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Johir, M.A.H.; Sun, L.; Asadullah, M.; Belhaj, D. Sorption of hydrophobic organic contaminants on functionalized biochar: Protagonist role of π-π electron-donor-acceptor interactions and hydrogen bonds. J. Hazard. Mater. 2018, 360, 270–278. [Google Scholar] [CrossRef]
- Abbas, F.M.; Al Ahmad, Z.A.; Elgezouly, R.O.E.; Abdelrahman, A.E. Characterization of Pores Size, Surface area and Fractal Di-mensions of Activated Carbon Powder Prepared from Date Palm Leaves. Mol. Biol. 2022, 11, 94544. [Google Scholar]
- Peterson, S.C.; Jackson, M.A.; Kim, S.; Palmquist, D.E. Increasing biochar surface area: Optimization of ball milling parameters. Powder Technol. 2012, 228, 115–120. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basika, E.; Komakech, A.J.; Kizito, S.S.; Lee, R.D.; Schwarzböck, T. Influence of Milling Conditions on Fecal Sludge-Based Biochar. Biomass 2025, 5, 74. https://doi.org/10.3390/biomass5040074
Basika E, Komakech AJ, Kizito SS, Lee RD, Schwarzböck T. Influence of Milling Conditions on Fecal Sludge-Based Biochar. Biomass. 2025; 5(4):74. https://doi.org/10.3390/biomass5040074
Chicago/Turabian StyleBasika, Elisa, Allan J. Komakech, Simon S. Kizito, Richard D. Lee, and Therese Schwarzböck. 2025. "Influence of Milling Conditions on Fecal Sludge-Based Biochar" Biomass 5, no. 4: 74. https://doi.org/10.3390/biomass5040074
APA StyleBasika, E., Komakech, A. J., Kizito, S. S., Lee, R. D., & Schwarzböck, T. (2025). Influence of Milling Conditions on Fecal Sludge-Based Biochar. Biomass, 5(4), 74. https://doi.org/10.3390/biomass5040074





