Brewing By-Products: Source, Nature, and Handling in the Dawn of a Circular Economy Age
Abstract
1. Introduction
2. Overview of Brewing By-Products and Valorization Strategies
2.1. Brewer’s Spent Grain (BSG)
2.2. Spent Hops and Trub
2.2.1. Spent Hops
2.2.2. Trub
2.3. Brewer’s Spent Yeast (BSY)
2.4. Other Waste
2.4.1. Kieselguhr
2.4.2. Carbon Dioxide
2.4.3. Wastewater
2.4.4. Wastewater Sludge
2.4.5. Packaging Waste
3. Case Studies on the Valorization of Brewing By-Products
4. Practical Considerations for Valorizing Brewing By-Products in a Scale-Sensitive Approach
5. Preventive Strategies for By-Product Reduction in Brewing
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raihofer, L.; Zarnow, M.; Gastl, M.; Hutzler, M. A Short History of Beer Brewing. EMBO Rep. 2022, 23, e56355. [Google Scholar] [CrossRef] [PubMed]
- McGovern, P.E.; Zhang, J.; Tang, J.; Zhang, Z.; Hall, G.R.; Moreau, R.A.; Nuñez, A.; Butrym, E.D.; Richards, M.P.; Wang, C.; et al. Fermented Beverages of Pre- and Proto-Historic China. Proc. Natl. Acad. Sci. USA 2004, 101, 17593–17598. [Google Scholar] [CrossRef] [PubMed]
- Bamforth, C.W. Beer; Oxford University Press: New York, NY, USA, 2023; ISBN 0199996741. [Google Scholar]
- Statista Beer Production Worldwide from 1998 to 2023. 2024. Available online: https://www.statista.com/statistics/270275/worldwide-beer-production/ (accessed on 7 May 2025).
- Grand View Research. Beer Market Size, Share & Trends Analysis Report by Product (Ale, Lager, Stout), Packaging (Bottles, Cans), by Production (Macro Brewery, Micro Brewery, Craft Brewery), by Distribution Channel (On-Trade, Off-Trade), by Region, and Segment Forecasts, 2025–2030; Report ID: 978-1-68038-663-9; Grand View Research: San Francisco, CA, USA, 2024. [Google Scholar]
- Garavaglia, C.; Swinnen, J. Industry Concentration and the Entry of Craft Producers into the Global Beer Market. In New Developments in the Brewing Industry; Oxford University Press: Oxford, UK, 2020; pp. 216–234. [Google Scholar]
- Cabras, I.; Kogler, D.F.; Davies, R.B.; Higgins, D. Beer, Brewing, and Regional Studies. Reg. Stud. 2023, 57, 1905–1908. [Google Scholar] [CrossRef]
- Anderson, K. The Emergence of Lower-Alcohol Beverages: The Case of Beer. J. Wine Econ. 2023, 18, 66–86. [Google Scholar] [CrossRef]
- Kozłowski, R.; Dziedziński, M.; Stachowiak, B.; Kobus-Cisowska, J. Non- and Low-Alcoholic Beer–Popularity and Manufacturing Techniques [Pdf]. Acta Sci. Pol. Technol. Aliment. 2021, 20, 347–357. [Google Scholar] [CrossRef]
- Thesseling, F.A.; Bircham, P.W.; Mertens, S.; Voordeckers, K.; Verstrepen, K.J. A Hands-On Guide to Brewing and Analyzing Beer in the Laboratory. Curr. Protoc. Microbiol. 2019, 54, e91. [Google Scholar] [CrossRef]
- Goyal, A.; Shukla, G.; Mishra, S.; Mallik, S.; Singh, A.; Dubey, M. Beer Production by Fermentation Process: A Review. J. Microbiol. Biotechnol. Food Sci. 2023, 13, e9532. [Google Scholar] [CrossRef]
- Olajire, A.A. The Brewing Industry and Environmental Challenges. J. Clean. Prod. 2020, 256, 102817. [Google Scholar] [CrossRef]
- Noots, I.; Delcour, J.A.; Michiels, C.W. From Field Barley to Malt: Detection and Specification of Microbial Activity for Quality Aspects. Crit. Rev. Microbiol. 1999, 25, 121–153. [Google Scholar] [CrossRef]
- Laus, A.; Endres, F.; Hutzler, M.; Zarnkow, M.; Jacob, F. Isothermal Mashing of Barley Malt: New Insights into Wort Composition and Enzyme Temperature Ranges. Food Bioproc. Technol. 2022, 15, 2294–2312. [Google Scholar] [CrossRef]
- Gastl, M.; Kupetz, M.; Becker, T. Determination of Cytolytic Malt Modification—Part I: Influence of Variety Characteristics. J. Am. Soc. Brew. Chem. 2021, 79, 53–65. [Google Scholar] [CrossRef]
- Ledley, A.J.; Elias, R.J.; Cockburn, D.W. Evaluating the Role of Mashing in the Amino Acid Profiles of Worts Produced from Gluten-Free Malts. Beverages 2023, 9, 10. [Google Scholar] [CrossRef]
- Williaert, R.G.; Baron, G. V Wort Boiling Today-Boiling Systems with Low Thermal Stress in Combination with Volatile Stripping. Cerevisia 2001, 26, 217–230. [Google Scholar]
- Lisci, S.; Tronci, S.; Grosso, M.; Karring, H.; Hajrizaj, R.; Errico, M. Brewer’s Spent Grain: Its Value as Renewable Biomass and Its Possible Applications. Chem. Eng. Trans. 2022, 92, 259–264. [Google Scholar]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ Spent Grain: Generation, Characteristics and Potential Applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Aradwad, P.; Raut, S.; Abdelfattah, A.; Rauh, C.; Sturm, B. Brewer’s Spent Grain: Unveiling Innovative Applications in the Food and Packaging Industry. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70150. [Google Scholar] [CrossRef]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ Spent Grain: A Review with an Emphasis on Food and Health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Hejna, A.; Marć, M.; Kowalkowska-Zedler, D.; Pladzyk, A.; Barczewski, M. Insights into the Thermo-Mechanical Treatment of Brewers’ Spent Grain as a Potential Filler for Polymer Composites. Polymers 2021, 13, 879. [Google Scholar] [CrossRef]
- Dancker, P.; Glas, K.; Gastl, M. Potential Utilisation Methods for Brewer’s Spent Grain: A Review. Int. J. Food Sci. Technol. 2025, 60, vvae022. [Google Scholar] [CrossRef]
- Jaeger, A.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Brewer’s Spent Yeast (BSY), an Underutilized Brewing By-Product. Fermentation 2020, 6, 123. [Google Scholar] [CrossRef]
- Belardi, I.; De Francesco, G.; Alfeo, V.; Bravi, E.; Sileoni, V.; Marconi, O.; Marrocchi, A. Advances in the Valorization of Brewing By-Products. Food Chem. 2025, 465, 141882. [Google Scholar] [CrossRef] [PubMed]
- Umego, E.C.; Barry-Ryan, C. Review of the Valorization Initiatives of Brewing and Distilling By-Products. Crit. Rev. Food Sci. Nutr. 2024, 64, 8231–8247. [Google Scholar] [CrossRef] [PubMed]
- Maria, M.P.; Torres, N.H.; Nascimento, V.R.S.; Chagas, T.S.A.; Saratale, G.D.; Mulla, S.I.; Bharagava, R.N.; Cavalcanti, E.B.; Romanholo Ferreira, L.F. Current Advances in the Brewery Wastewater Treatment from Anaerobic Digestion for Biogas Production: A Systematic Review. Environ. Adv. 2023, 13, 100394. [Google Scholar] [CrossRef]
- Pasquet, P.-L.; Villain-Gambier, M.; Trébouet, D. By-Product Valorization as a Means for the Brewing Industry to Move toward a Circular Bioeconomy. Sustainability 2024, 16, 3472. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; Vinci, G.; Maddaloni, L.; Ruggeri, M.; Savastano, M. Application of Life Cycle Assessment in Beer Production: Systematic Review. Beverages 2024, 10, 86. [Google Scholar] [CrossRef]
- Milburn, T.; Guertin-Martín, F.A. Tapping into Environmental Harm in Brewing: An Exploration of Pollution and Waste in Beer Production. Crit. Criminol. 2020, 28, 407–423. [Google Scholar] [CrossRef]
- Thomas, K.R.; Rahman, P. Brewery Wastes. Strategies for Sustainability. A Review. Asp. Appl. Biol. 2006, 80, 147–153. [Google Scholar]
- Chattaraj, S.; Mitra, D.; Ganguly, A.; Thatoi, H.; Das Mohapatra, P.K. A Critical Review on the Biotechnological Potential of Brewers’ Waste: Challenges and Future Alternatives. Curr. Res. Microb. Sci. 2024, 6, 100228. [Google Scholar] [CrossRef]
- Kandasamy, S.; Dhandayuthapani, U.N.; Subramanian, V.; Palanisamy, J.; Shanmugam, M.K.; Dhakshanamoorthy, D.; Subramani, U.K.; Nagappan, S. Assessment of Brewery Wastewater as an Alternative Irrigation Source: Impacts on Soil Health and Nutrient Uptake by Maize in Tamil Nadu, India. BMC Agric. 2025, 1, 2. [Google Scholar] [CrossRef]
- Grand, T.; Jenkins, D.; Maskell, D.; Zhuang, S. Valorisation of Carbon Dioxide from Fermentation in Craft Brewing: Potential Technologies, Brewer Interviews, and Implication for a ‘Three-Level Valorisation System’. J. Am. Soc. Brew. Chem. 2025, 83, 248–259. [Google Scholar] [CrossRef]
- Assandri, D.; Giacomello, G.; Bianco, A.; Zara, G.; Budroni, M.; Pampuro, N. Environmental Sustainability of Brewers’ Spent Grains Composting: Effect of Turning Strategies and Mixtures Composition on Greenhouse Gas Emissions. Agronomy 2025, 15, 771. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current Options for the Valorization of Food Manufacturing Waste: A Review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef]
- Tan, E.C.D.; Lamers, P. Circular Bioeconomy Concepts—A Perspective. Front. Sustain. 2021, 2, 701509. [Google Scholar] [CrossRef]
- Ferraz, D.; Pyka, A. Circular Economy, Bioeconomy, and Sustainable Development Goals: A Systematic Literature Review. Environ. Sci. Pollut. Res. 2023, 2023, 1–22. [Google Scholar] [CrossRef]
- Gobbi, L.; Stanković, M.; Ruggeri, M.; Savastano, M. Craft Beer in Food Science: A Review and Conceptual Framework. Beverages 2024, 10, 91. [Google Scholar] [CrossRef]
- Villacreces, S.; Blanco, C.A.; Caballero, I. Developments and Characteristics of Craft Beer Production Processes. Food Biosci. 2022, 45, 101495. [Google Scholar] [CrossRef]
- Jesús Callejo, M.; Tesfaye, W.; Carmen González, M.; Morata, A. Craft Beers: Current Situation and Future Trends. In New Advances on Fermentation Processes; IntechOpen: London, UK, 2020. [Google Scholar][Green Version]
- Diniz, D.d.P.; Carvalho, M. Environmental Repercussions of Craft Beer Production in Northeast Brazil. Sustainability 2024, 16, 4566. [Google Scholar] [CrossRef]
- Mathias, T.R.d.S.; Alexandre, V.M.F.; Cammarota, M.C.; de Mello, P.P.M.; Sérvulo, E.F.C. Characterization and Determination of Brewer’s Solid Wastes Composition. J. Inst. Brew. 2015, 121, 400–404. [Google Scholar] [CrossRef]
- Mussatto, S.I. Biotechnological Potential of Brewing Industry By-Products. In Biotechnology for Agro-Industrial Residues Utilisation; Springer: Dordrecht, The Netherlands, 2009; pp. 313–326. [Google Scholar]
- Belardi, I.; Marrocchi, A.; Alfeo, V.; Sileoni, V.; De Francesco, G.; Paolantoni, M.; Marconi, O. Sequential Extraction and Attenuated Total Reflection–Fourier Transform Infrared Spectroscopy Monitoring in the Biorefining of Brewer’s Spent Grain. Molecules 2023, 28, 7992. [Google Scholar] [CrossRef] [PubMed]
- Luft, L.; Confortin, T.C.; Todero, I.; Ugalde, G.; Zabot, G.L.; Mazutti, M.A. Transformation of Residual Starch from Brewer’s Spent Grain into Fermentable Sugars Using Supercritical Technology. J. Supercrit. Fluids 2018, 140, 85–90. [Google Scholar] [CrossRef]
- Steiner, J.; Procopio, S.; Becker, T. Brewer’s Spent Grain: Source of Value-Added Polysaccharides for the Food Industry in Reference to the Health Claims. Eur. Food Res. Technol. 2015, 241, 303–315. [Google Scholar] [CrossRef]
- Rongpipi, S.; Ye, D.; Gomez, E.D.; Gomez, E.W. Progress and Opportunities in the Characterization of Cellulose—An Important Regulator of Cell Wall Growth and Mechanics. Front. Plant Sci. 2019, 9, 1894. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Bai, J.; Buccato, D.G.; Zhang, J.; He, Y.; Zhu, Y.; Yang, Z.; Xiao, X.; Daglia, M. Cereal-Derived Water-Unextractable Arabinoxylans: Structure Feature, Effects on Baking Products and Human Health. Foods 2024, 13, 2369. [Google Scholar] [CrossRef] [PubMed]
- Pérocheau Arnaud, S. Valorisation of Brewer’s Spent Grain: Lignocellulosic Fractionation and Its Potential for Polymer and Composite Material Applications. Chem. Afr. 2024, 7, 2989–3010. [Google Scholar] [CrossRef]
- Devnani, B.; Moran, G.C.; Grossmann, L. Extraction, Composition, Functionality, and Utilization of Brewer’s Spent Grain Protein in Food Formulations. Foods 2023, 12, 1543. [Google Scholar] [CrossRef]
- Ikram, S.; Huang, L.; Zhang, H.; Wang, J.; Yin, M. Composition and Nutrient Value Proposition of Brewers Spent Grain. J. Food Sci. 2017, 82, 2232–2242. [Google Scholar] [CrossRef]
- Wen, T.-N.; Luthe, D.S. Biochemical Characterization of Rice Glutelin. Plant Physiol. 1985, 78, 172–177. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Lu, Y.; Fan, X. Structure and Characteristics of Lignin; Springer: Berlin/Heidelberg, Germany, 2020; pp. 17–75. [Google Scholar]
- Okonkwo, C.E.; Hussain, S.Z.; Onyeaka, H.; Adeyanju, A.A.; Nwonuma, C.O.; Bashir, A.A.; Farooq, A.; Zhou, C.; Shittu, T.D. Lignin Polyphenol: From Biomass to Innovative Food Applications, and Influence on Gut Microflora. Ind. Crops Prod. 2023, 206, 117696. [Google Scholar] [CrossRef]
- Youssefian, S.; Jakes, J.E.; Rahbar, N. Variation of Nanostructures, Molecular Interactions, and Anisotropic Elastic Moduli of Lignocellulosic Cell Walls with Moisture. Sci. Rep. 2017, 7, 2054. [Google Scholar] [CrossRef]
- Tarasov, D.; Leitch, M.; Fatehi, P. Lignin–Carbohydrate Complexes: Properties, Applications, Analyses, and Methods of Extraction: A Review. Biotechnol. Biofuels 2018, 11, 269. [Google Scholar] [CrossRef]
- Oyedeji, A.B.; Wu, J. Food-Based Uses of Brewers Spent Grains: Current Applications and Future Possibilities. Food Biosci. 2023, 54, 102774. [Google Scholar] [CrossRef]
- Saberian, H.; Ghandehari Yazdi, A.P.; Nejatian, M.; Bazsefidpar, N.; Mohammadian, A.H.; Rahmati, M.; Assadpour, E.; Jafari, S.M. Brewers’ Spent Grain as a Functional Ingredient in Bakery, Pasta, and Cereal-Based Products. Future Foods 2024, 10, 100479. [Google Scholar] [CrossRef]
- Stelick, A.; Sogari, G.; Rodolfi, M.; Dando, R.; Paciulli, M. Impact of Sustainability and Nutritional Messaging on Italian Consumers’ Purchase Intent of Cereal Bars Made with Brewery Spent Grains. J. Food Sci. 2021, 86, 531–539. [Google Scholar] [CrossRef]
- Mekonnen, W.E.; Augchew, E.D.; Terefe, Z.K. Evaluation of Proximate Composition, Physicochemical Properties, and Sensory Attributes of Instant Flour from Brewery Spent Grain, by Blending with Maize (Zea mays L.) and Germinated Chickpea (Cicer arietinum L.). Int. J. Food Sci. 2024, 2024, 2352758. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.F.; Abu-Ghannam, N. Antioxidant Capacity, Arabinoxylans Content and in Vitro Glycaemic Index of Cereal-Based Snacks Incorporated with Brewer’s Spent Grain. LWT Food Sci. Technol. 2014, 55, 269–277. [Google Scholar] [CrossRef]
- Naibaho, J.; Korzeniowska, M.; Sitanggang, A.B.; Lu, Y.; Julianti, E. Brewers’ Spent Grain as a Food Ingredient: Techno-Processing Properties, Nutrition, Acceptability, and Market. Trends Food Sci. Technol. 2024, 152, 104685. [Google Scholar] [CrossRef]
- Lao, E.J.; Dimoso, N.; Raymond, J.; Mbega, E.R. The Prebiotic Potential of Brewers’ Spent Grain on Livestock’s Health: A Review. Trop. Anim. Health Prod. 2020, 52, 461–472. [Google Scholar] [CrossRef]
- Rachwał, K.; Waśko, A.; Gustaw, K.; Polak-Berecka, M. Utilization of Brewery Wastes in Food Industry. PeerJ 2020, 8, e9427. [Google Scholar] [CrossRef]
- Borel, L.D.M.S.; Reis Filho, A.M.; Xavier, T.P.; Lira, T.S.; Barrozo, M.A.S. An Investigation on the Pyrolysis of the Main Residue of the Brewing Industry. Biomass Bioenergy 2020, 140, 105698. [Google Scholar] [CrossRef]
- Muhammed, A.A.; Thomas, K.; Bin-Hamed, U. Feasibility of Using Brewers Spent Grain as a Fertilizer in Agriculture. Int. J. Sci. Technol. 2015, 10, 23–31. [Google Scholar] [CrossRef]
- Bianco, A.; Melito, S.; Garau, M.; Giannini, V.; Zara, G.; Assandri, D.; Oufensou, S.; Coronas, R.; Pampuro, N.; Budroni, M. The Potential Use of Brewers’ Spent Grain-Based Substrates as Horticultural Bio-Fertilizers. Front. Sustain. Food Syst. 2024, 8, 1404914. [Google Scholar] [CrossRef]
- Assandri, D.; Pampuro, N.; Zara, G.; Cavallo, E.; Budroni, M. Suitability of Composting Process for the Disposal and Valorization of Brewer’s Spent Grain. Agriculture 2020, 11, 2. [Google Scholar] [CrossRef]
- Ibarruri, J.; Cebrián, M.; Hernández, I. Solid State Fermentation of Brewer’s Spent Grain Using Rhizopus Sp. to Enhance Nutrition Value. Waste Biomass Valorization 2019, 10, 3687–3700. [Google Scholar] [CrossRef]
- Gupta, S.; Jaiswal, A.K.; Abu-Ghannam, N. Optimization of Fermentation Conditions for the Utilization of Brewing Waste to Develop a Nutraceutical Rich Liquid Product. Ind. Crops Prod. 2013, 44, 272–282. [Google Scholar] [CrossRef]
- Koirala, P.; Maina, N.H.; Nihtilä, H.; Katina, K.; Coda, R. Brewers’ Spent Grain as Substrate for Dextran Biosynthesis by Leuconostoc Pseudomesenteroides DSM20193 and Weissella Confusa A16. Microb. Cell Fact. 2021, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Terfa, R.D.; Patel, P.N.; Kim, H.D.; Gacura, M.D.; Vanderlaan, G.; Chen, L.; Ji, X.; Piovesan, D. Harnessing Brewery Spent Grain for Polyhydroxyalkanoate Production. Macromol 2024, 4, 448–461. [Google Scholar] [CrossRef]
- Parafati, L.; Proetto, I.; Palmeri, R.; Pesce, F.; Fallico, B.; Restuccia, C. Reuse of Brewer’s Spent Grain (BSG) for the Induction of Wickerhamomyces Anomalus BS91 β-Glucosidase with Bioflavoring Potential. Fermentation 2024, 10, 472. [Google Scholar] [CrossRef]
- Plessas, S.; Trantallidi, M.; Bekatarou, A.; Kanellaki, M.; Nigam, P.; Koutinas, A. Immobilization of Kefir and Lactobacillus Casei on Brewery Spent Grains for Use in Sourdough Wheat Bread Making. Food Chem. 2007, 105, 187–194. [Google Scholar] [CrossRef]
- Dragone, G.; Mussatto, S.I.; Almeida e Silva, J.B. High Gravity Brewing by Continuous Process Using Immobilised Yeast: Effect of Wort Original Gravity on Fermentation Performance. J. Inst. Brew. 2007, 113, 391–398. [Google Scholar] [CrossRef]
- Almeida, C.; Brányik, T.; Moradas-Ferreira, P.; Teixeira, J. Continuous Production of Pectinase by Immobilized Yeast Cells on Spent Grains. J. Biosci. Bioeng. 2003, 96, 513–518. [Google Scholar] [CrossRef]
- Rocha, C.; Gonçalves, M.P.; Teixeira, J.A. Immobilization of Trypsin on Spent Grains for Whey Protein Hydrolysis. Process Biochem. 2011, 46, 505–511. [Google Scholar] [CrossRef]
- Ferraz, E.; Coroado, J.; Gamelas, J.; Silva, J.; Rocha, F.; Velosa, A. Spent Brewery Grains for Improvement of Thermal Insulation of Ceramic Bricks. J. Mater. Civ. Eng. 2013, 25, 1638–1646. [Google Scholar] [CrossRef]
- Pedro Silva, J.; Sousa, S.; Rodrigues, J.; Antunes, H.; Porter, J.J.; Gonçalves, I.; Ferreira-Dias, S. Adsorption of Acid Orange 7 Dye in Aqueous Solutions by Spent Brewery Grains. Sep. Purif. Technol. 2004, 40, 309–315. [Google Scholar] [CrossRef]
- Rossi, L.; Wechsler, L.; Peltzer, M.A.; Ciannamea, E.M.; Ruseckaite, R.A.; Stefani, P.M. Sustainable Particleboards Based on Brewer’s Spent Grains. Polymers 2023, 16, 59. [Google Scholar] [CrossRef]
- Sousa, S.C.; Silva, J.P.; Ramos, A.M.; Simoes, R. Pulping and Papermaking Potential of Brewery Spent Grain. Cell. Chem. Technol. 2007, 41, 183–191. [Google Scholar]
- Silva, N.C.; Santos, A.O.; Duarte, C.R.; Barrozo, M.A.S. Refractance Window Drying as an Alternative Method for Brewer’s Spent Grain Preservation. Appl. Biosci. 2024, 3, 71–86. [Google Scholar] [CrossRef]
- Aliyu, S.; Bala, M. Brewer’s Spent Grain: A Review of Its Potentials and Applications. Afr. J. Biotechnol. 2011, 10, 324–331. [Google Scholar]
- Sterczyńska, M.; Zdaniewicz, M.; Wolny-Koładka, K. Rheological and Microbiological Characteristics of Hops and Hot Trub Particles Formed during Beer Production. Molecules 2021, 26, 681. [Google Scholar] [CrossRef]
- Lech, M.; Labus, K. The Methods of Brewers’ Spent Grain Treatment towards the Recovery of Valuable Ingredients Contained Therein and Comprehensive Management of Its Residues. Chem. Eng. Res. Des. 2022, 183, 494–511. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C. Recent Advances in Biotechnological Valorization of Brewers’ Spent Grain. Food Sci. Biotechnol. 2021, 30, 341–353. [Google Scholar] [CrossRef]
- Báez, J.; Fernández-Fernández, A.M.; Briozzo, F.; Díaz, S.; Dorgans, A.; Tajam, V.; Medrano, A. Effect of Enzymatic Hydrolysis of Brewer’s Spent Grain on Bioactivity, Techno-Functional Properties, and Nutritional Value When Added to a Bread Formulation. Biol. Life Sci. Forum 2021, 6, 100. [Google Scholar]
- Verni, M.; Pontonio, E.; Krona, A.; Jacob, S.; Pinto, D.; Rinaldi, F.; Verardo, V.; Díaz-de-Cerio, E.; Coda, R.; Rizzello, C.G. Bioprocessing of Brewers’ Spent Grain Enhances Its Antioxidant Activity: Characterization of Phenolic Compounds and Bioactive Peptides. Front. Microbiol. 2020, 11, 1831. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Barrutia, M.B.; del Castillo, M.D.; Arcia, P.; Cozzano, S. Feasibility of Extruded Brewer’s Spent Grain as a Food Ingredient for a Healthy, Safe, and Sustainable Human Diet. Foods 2022, 11, 1403. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Sanches, M.A.; Stochi, V.A.L.; Borges-Machado, A.L.; Augusto, P.E.D.; Polachini, T.C.; Telis-Romero, J. Valorization of Brewer’s Spent Grains (BSG) through Alkaline Hydrogen Peroxide Processing: Effect on Composition, Structure and Rheological Properties. Food Bioprod. Process. 2024, 147, 239–250. [Google Scholar] [CrossRef]
- Amezcua-Allieri, M.A.; Sánchez Durán, T.; Aburto, J. Study of Chemical and Enzymatic Hydrolysis of Cellulosic Material to Obtain Fermentable Sugars. J. Chem. 2017, 2017, 5680105. [Google Scholar] [CrossRef]
- Gomez-Contreras, P.A.; Obando, C.; Freitas, P.A.V.d.; Martin-Perez, L.; Chiralt, A.; Gonzalez-Martinez, C. Applying Subcritical Water Extraction to Obtain Bioactive Compounds and Cellulose Fibers from Brewer Spent Grains. Molecules 2024, 29, 4897. [Google Scholar] [CrossRef]
- Ansari, M.M.; Heo, Y.; Do, K.; Ghosh, M.; Son, Y.-O. Nanocellulose Derived from Agricultural Biowaste By-Products–Sustainable Synthesis, Biocompatibility, Biomedical Applications, and Future Perspectives: A Review. Carbohydr. Polym. Technol. Appl. 2024, 8, 100529. [Google Scholar] [CrossRef]
- Jin, T.; Xing, X.; Xie, Y.; Sun, Y.; Bian, S.; Liu, L.; Chen, G.; Wang, X.; Yu, X.; Su, Y. Evaluation of Preparation and Detoxification of Hemicellulose Hydrolysate for Improved Xylitol Production from Quinoa Straw. Int. J. Mol. Sci. 2022, 24, 516. [Google Scholar] [CrossRef]
- Dulie, N.W.; Woldeyes, B.; Demsash, H.D.; Jabasingh, A.S. An Insight into the Valorization of Hemicellulose Fraction of Biomass into Furfural: Catalytic Conversion and Product Separation. Waste Biomass Valorization 2021, 12, 531–552. [Google Scholar] [CrossRef]
- Jana, U.K.; Kango, N.; Pletschke, B. Hemicellulose-Derived Oligosaccharides: Emerging Prebiotics in Disease Alleviation. Front. Nutr. 2021, 8, 670817. [Google Scholar] [CrossRef]
- Antoun, K.; Tabib, M.; Salameh, S.J.; Koubaa, M.; Ziegler-Devin, I.; Brosse, N.; Khelfa, A. Isolation and Structural Characterization of Natural Deep Eutectic Solvent Lignin from Brewer’s Spent Grains. Polymers 2024, 16, 2791. [Google Scholar] [CrossRef] [PubMed]
- Sadeghifar, H.; Ragauskas, A.J. Lignin as a Natural Antioxidant: Chemistry and Applications. Macromol. 2025, 5, 5. [Google Scholar] [CrossRef]
- Parchami, M.; Agnihotri, S.; Taherzadeh, M.J. Aqueous Ethanol Organosolv Process for the Valorization of Brewer’s Spent Grain (BSG). Bioresour. Technol. 2022, 362, 127764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qiu, X.; Wang, C.; Zhong, L.; Fu, F.; Zhu, J.; Zhang, Z.; Qin, Y.; Yang, D.; Xu, C.C. Lignin Derived Carbon Materials: Current Status and Future Trends. Carbon Res. 2022, 1, 14. [Google Scholar] [CrossRef]
- Ávila, M.I.; Alonso-Doncel, M.M.; Cueto, J.; Briones, L.; Gómez-Pozuelo, G.; Escola, J.M.; Serrano, D.P.; Peral, A.; Botas, J.A. Production of High Value-Added Phenolic Compounds through Lignin Catalytic Pyrolysis over Ion-Exchanged Hierarchical ZSM-5 and Beta Zeolites. Catal. Today 2025, 456, 115343. [Google Scholar] [CrossRef]
- Weng, C.; Peng, X.; Han, Y. Depolymerization and Conversion of Lignin to Value-Added Bioproducts by Microbial and Enzymatic Catalysis. Biotechnol. Biofuels 2021, 14, 84. [Google Scholar] [CrossRef]
- Martín-García, B.; Tylewicz, U.; Verardo, V.; Pasini, F.; Gómez-Caravaca, A.M.; Caboni, M.F.; Dalla Rosa, M. Pulsed Electric Field (PEF) as Pre-Treatment to Improve the Phenolic Compounds Recovery from Brewers’ Spent Grains. Innov. Food Sci. Emerg. Technol. 2020, 64, 102402. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; González-Paramás, A.M.; Oludemi, T.; Ayuda-Durán, B.; González-Manzano, S. Plant Phenolics as Functional Food Ingredients. Adv. Food Nutr. Res. 2019, 90, 183–257. [Google Scholar] [CrossRef]
- Caleja, C.; Ribeiro, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Phenolic Compounds as Nutraceuticals or Functional Food Ingredients. Curr. Pharm. Des. 2017, 23, 2787–2806. [Google Scholar] [CrossRef]
- de Oliveira, I.; Santos-Buelga, C.; Aquino, Y.; Barros, L.; Heleno, S.A. New Frontiers in the Exploration of Phenolic Compounds and Other Bioactives as Natural Preservatives. Food Biosci. 2025, 68, 106571. [Google Scholar] [CrossRef]
- Iadecola, R.; Ciccoritti, R.; Ceccantoni, B.; Bellincontro, A.; Amoriello, T. Optimization of Phenolic Compound Extraction from Brewers’ Spent Grain Using Ultrasound Technologies Coupled with Response Surface Methodology. Sustainability 2022, 14, 3309. [Google Scholar] [CrossRef]
- Bharadvaja, N.; Gautam, S.; Singh, H. Natural Polyphenols: A Promising Bioactive Compounds for Skin Care and Cosmetics. Mol. Biol. Rep. 2023, 50, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Herbst, G.; Hamerski, F.; Errico, M.; Corazza, M.L. Pressurized Liquid Extraction of Brewer’s Spent Grain: Kinetics and Crude Extracts Characterization. J. Ind. Eng. Chem. 2021, 102, 370–383. [Google Scholar] [CrossRef]
- Jantason, N.; Suphantharika, M.; Wipatanawin, A.; Chansong, S.; Payongsri, P. Valorization of Spent Grains from Beer Production through β-Glucan Extraction. Foods 2024, 13, 440. [Google Scholar] [CrossRef]
- Gautério, G.V.; Silvério, S.I.D.C.; Egea, M.B.; Lemes, A.C. β-Glucan from Brewer’s Spent Yeast as a Techno-Functional Food Ingredient. Front. Food Sci. Technol. 2022, 2, 1074505. [Google Scholar] [CrossRef]
- Mebrek, S.; Djeghim, H.; Mehdi, Y.; Meghezzi, A.; Anwar, S.; Ali Awadh, N.A.; Benali, M. Antioxidant, Anti-Cholinesterase, Anti-α-Glucosidase and Prebiotic Properties of Beta-Glucan Extracted from Algerian Barley. Int. J. Phytomed. 2018, 10, 58. [Google Scholar] [CrossRef]
- Šokarda Slavić, M.; Kojić, M.; Margetić, A.; Ristović, M.; Pavlović, M.; Nikolić, S.; Vujčić, Z. Improvement of Nutritional and Bioactive Properties of Barley Β-glucan-based Food Products Using Bacillus Subtilis 168 Endo-β-1,3-1,4-glucanase. Int. J. Food Sci. Technol. 2023, 58, 6825–6835. [Google Scholar] [CrossRef]
- Chen, L.; Cui, C.; Wang, Z.; Che, F.; Chen, Z.; Feng, S. Structural Characterization and Antioxidant Activity of β-Glucans from Highland Barley Obtained with Ultrasonic–Microwave-Assisted Extraction. Molecules 2024, 29, 684. [Google Scholar] [CrossRef]
- Peltzer, M.; Delgado, J.F.; Salvay, A.G.; Wagner, J.R. β-Glucan, a Promising Polysaccharide for Bio-Based Films Developments for Food Contact Materials and Medical Applications. Curr. Org. Chem. 2018, 22, 1249–1254. [Google Scholar] [CrossRef]
- Avramia, I.; Amariei, S. A Comparative Study on the Development of Bioactive Films Based on β-Glucan from Spent Brewer’s Yeast and Pomegranate, Bilberry, or Cranberry Juices. Appl. Sci. 2023, 13, 2807. [Google Scholar] [CrossRef]
- Junttila, M.H. Extraction of Brewers’ Spent Grain in near Subcritical Conditions: A Method to Obtain High Protein Contents Extracts. J. Agric. Food Res. 2022, 10, 100378. [Google Scholar] [CrossRef]
- Naibaho, J.; Korzeniowska, M. Brewers’ Spent Grain in Food Systems: Processing and Final Products Quality as a Function of Fiber Modification Treatment. J. Food Sci. 2021, 86, 1532–1551. [Google Scholar] [CrossRef] [PubMed]
- Bazsefidpar, N.; Ahmadi Gavlighi, H.; Ghandehari Yazdi, A.P.; Jafari, S.M. Optimization of Protein Extraction from Brewer’s Spent Grain and Production of Bioactive Peptides. Biomass Convers. Biorefin. 2024, 14, 17455–17465. [Google Scholar] [CrossRef]
- Naibaho, J.; Jonuzi, E.; Butula, N.; Korzeniowska, M.; Föste, M.; Sinamo, K.N.; Chodaczek, G.; Yang, B. Fortification of Milk-Based Yogurt with Protein Hydrolysates from Brewers’ Spent Grain: Evaluation on Microstructural Properties, Lactic Acid Bacteria Profile, Lactic Acid Forming Capability and Its Physical Behavior. Curr. Res. Food Sci. 2022, 5, 1955–1964. [Google Scholar] [CrossRef]
- Naibaho, J.; Korzeniowska, M.; Wojdyło, A.; Muchdatul Ayunda, H.; Foste, M.; Yang, B. Techno-Functional Properties of Protein from Protease-Treated Brewers’ Spent Grain (BSG) and Investigation of Antioxidant Activity of Extracted Proteins and BSG Residues. J. Cereal Sci. 2022, 107, 103524. [Google Scholar] [CrossRef]
- Roy, S.; Rathod, G.; Amamcharla, J. Foaming Capacity and Stability. In Plant-Based Proteins. Methods and Protocols in Food Science; Li, Y., Ed.; Humana: New York, NY, USA, 2025; pp. 315–322. [Google Scholar]
- da Silva, A.M.M.; Almeida, F.S.; da Silva, M.F.; Goldbeck, R.; Sato, A.C.K. How Do PH and Temperature Influence Extraction Yield, Physicochemical, Functional, and Rheological Characteristics of Brewer Spent Grain Protein Concentrates? Food Bioprod. Process. 2023, 139, 34–45. [Google Scholar] [CrossRef]
- Bazsefidpar, N.; Ghandehari Yazdi, A.P.; Karimi, A.; Yahyavi, M.; Amini, M.; Ahmadi Gavlighi, H.; Simal-Gandara, J. Brewers Spent Grain Protein Hydrolysate as a Functional Ingredient for Muffins: Antioxidant, Antidiabetic, and Sensory Evaluation. Food Chem. 2024, 435, 137565. [Google Scholar] [CrossRef]
- Kashtoh, H.; Baek, K.-H. Recent Updates on Phytoconstituent Alpha-Glucosidase Inhibitors: An Approach towards the Treatment of Type Two Diabetes. Plants 2022, 11, 2722. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.-B.; Shim, J.-H.; Abd El-Aty, A.M. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides From Food and By-Products: A Review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef]
- Chin, Y.L.; Keppler, J.K.; Dinani, S.T.; Chen, W.N.; Boom, R. Brewers’ Spent Grain Proteins: The Extraction Method Determines the Functional Properties. Innov. Food Sci. Emerg. Technol. 2024, 94, 103666. [Google Scholar] [CrossRef]
- Sanna, A.; Li, S.; Linforth, R.; Smart, K.A.; Andrésen, J.M. Bio-Oil and Bio-Char from Low Temperature Pyrolysis of Spent Grains Using Activated Alumina. Bioresour. Technol. 2011, 102, 10695–10703. [Google Scholar] [CrossRef] [PubMed]
- Bieniek, A.; Jerzak, W.; Sieradzka, M.; Mika, Ł.; Sztekler, K.; Magdziarz, A. Intermediate Pyrolysis of Brewer’s Spent Grain: Impact of Gas Atmosphere. Energies 2022, 15, 2491. [Google Scholar] [CrossRef]
- Coronado, M.A.; Montero, G.; Montes, D.G.; Valdez-Salas, B.; Ayala, J.R.; García, C.; Carrillo, M.; León, J.A.; Moreno, A. Physicochemical Characterization and SEM-EDX Analysis of Brewer’s Spent Grain from the Craft Brewery Industry. Sustainability 2020, 12, 7744. [Google Scholar] [CrossRef]
- Hwang, I.H.; Nakajima, D.; Matsuto, T.; Sugimoto, T. Improving the Quality of Waste-Derived Char by Removing Ash. Waste Manag. 2008, 28, 424–434. [Google Scholar] [CrossRef]
- Kebede, N.G. Effects of Brewery Spent Grain Ash on Lime-Stabilized Clayey Soil. Int. J. Pavement Res. Technol. 2024, 17, 1072–1078. [Google Scholar] [CrossRef]
- Silva, F.C.; Cruz, N.C.; Tarelho, L.A.C.; Rodrigues, S.M. Use of Biomass Ash-Based Materials as Soil Fertilisers: Critical Review of the Existing Regulatory Framework. J. Clean. Prod. 2019, 214, 112–124. [Google Scholar] [CrossRef]
- Alonso-Riaño, P.; Melgosa, R.; Trigueros, E.; Illera, A.E.; Beltrán, S.; Sanz, M.T. Valorization of Brewer’s Spent Grain by Consecutive Supercritical Carbon Dioxide Extraction and Enzymatic Hydrolysis. Food Chem. 2022, 396, 133493. [Google Scholar] [CrossRef]
- Moirangthem, K.; Koirala, P.; Maina, H.N.; Rai, D.K.; Coda, R. Impact of Pre-Treatments and Bioprocessing on the Carbohydrate and Polyphenol Profile of Brewers’ Spent Grain. Food Bioprod. Process. 2024, 148, 62–71. [Google Scholar] [CrossRef]
- Pan, J.; Li, C.; Liu, J.; Jiao, Z.; Zhang, Q.; Lv, Z.; Yang, W.; Chen, D.; Liu, H. Polysaccharide-Based Packaging Coatings and Films with Phenolic Compounds in Preservation of Fruits and Vegetables—A Review. Foods 2024, 13, 3896. [Google Scholar] [CrossRef]
- González-García, E.; Marina, M.L.; García, M.C. Impact of the Use of Pressurized Liquids on the Extraction and Functionality of Proteins and Bioactives from Brewer’s Spent Grain. Food Chem. 2021, 359, 129874. [Google Scholar] [CrossRef]
- Wang, B.; Pham, L.B.; Adhikari, B. Complexation and Conjugation between Phenolic Compounds and Proteins: Mechanisms, Characterisation and Applications as Novel Encapsulants. Sustain. Food Technol. 2024, 2, 1206–1227. [Google Scholar] [CrossRef]
- Vieira, E.; Rocha, M.A.M.; Coelho, E.; Pinho, O.; Saraiva, J.A.; Ferreira, I.M.P.L.V.O.; Coimbra, M.A. Valuation of Brewer’s Spent Grain Using a Fully Recyclable Integrated Process for Extraction of Proteins and Arabinoxylans. Ind. Crops Prod. 2014, 52, 136–143. [Google Scholar] [CrossRef]
- Parchami, M.; Ferreira, J.A.; Taherzadeh, M.J. Starch and Protein Recovery from Brewer’s Spent Grain Using Hydrothermal Pretreatment and Their Conversion to Edible Filamentous Fungi – A Brewery Biorefinery Concept. Bioresour. Technol. 2021, 337, 125409. [Google Scholar] [CrossRef] [PubMed]
- Maini Rekdal, V.; Villalobos-Escobedo, J.M.; Rodriguez-Valeron, N.; Olaizola Garcia, M.; Prado Vásquez, D.; Rosales, A.; Sörensen, P.M.; Baidoo, E.E.K.; Calheiros de Carvalho, A.; Riley, R.; et al. Neurospora Intermedia from a Traditional Fermented Food Enables Waste-to-Food Conversion. Nat. Microbiol. 2024, 9, 2666–2683. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, M.; Mierzwa-Hersztek, M.; Gondek, K.; Wolny-Koładka, K.; Zdaniewicz, M.; Jarosz, R. Biological Activity of Composts Obtained from Hop Waste Generated during the Brewing. Biomass Convers. Biorefin. 2022, 12, 1271–1279. [Google Scholar] [CrossRef]
- Grudniewska, A.; Pastyrczyk, N. New Insight for Spent Hops Utilization: Simultaneous Extraction of Protein and Xanthohumol Using Deep Eutectic Solvents. Biomass Convers. Biorefin. 2023, 13, 14975–14986. [Google Scholar] [CrossRef]
- Kopeć, M.; Mierzwa-Hersztek, M.; Gondek, K.; Wolny-Koładka, K.; Zdaniewicz, M.; Suder, A. The Application Potential of Hop Sediments from Beer Production for Composting. Sustainability 2021, 13, 6409. [Google Scholar] [CrossRef]
- Fărcaş, A.C.; Socaci, S.A.; Mudura, E.; Dulf, F.V.; Vodnar, D.C.; Tofană, M.; Salanță, L.C. Exploitation of Brewing Industry Wastes to Produce Functional Ingredients. In Brewing Technology; InTechOpen: London, UK, 2017. [Google Scholar]
- Bartmańska, A.; Wałecka-Zacharska, E.; Tronina, T.; Popłoński, J.; Sordon, S.; Brzezowska, E.; Bania, J.; Huszcza, E. Antimicrobial Properties of Spent Hops Extracts, Flavonoids Isolated Therefrom, and Their Derivatives. Molecules 2018, 23, 2059. [Google Scholar] [CrossRef]
- Belal, A.; Elballal, M.S.; Al-Karmalawy, A.A.; Hassan, A.H.E.; Roh, E.J.; Ghoneim, M.M.; Ali, M.A.M.; Obaidullah, A.J.; Alotaibi, J.M.; Shaaban, S.; et al. Exploring the Sedative Properties of Natural Molecules from Hop Cones (Humulus lupulus) as Promising Natural Anxiolytics through GABA Receptors and the Human Serotonin Transporter. Front. Chem. 2024, 12, 1425485. [Google Scholar] [CrossRef]
- Silva, K.F.C.e.; Strieder, M.M.; Pinto, M.B.C.; Rostagno, M.A.; Hubinger, M.D. Processing Strategies for Extraction and Concentration of Bitter Acids and Polyphenols from Brewing By-Products: A Comprehensive Review. Processes 2023, 11, 921. [Google Scholar] [CrossRef]
- Schrickel, F.; Bilge, D.; Pahl, R.; Rettberg, N. Valorization of Spent Hops from Dry Hopping for Bittering of Pilsener Style Beer. J. Am. Soc. Brew. Chem. 2025, 83, 17–29. [Google Scholar] [CrossRef]
- Böhm, W.; Stegmann, R.; Gulbis, O.; Henle, T. Amino Acids and Glycation Compounds in Hot Trub Formed during Wort Boiling. Eur. Food Res. Technol. 2023, 249, 119–131. [Google Scholar] [CrossRef]
- Kühbeck, F.; Schütz, M.; Thiele, F.; Krottenthaler, M.; Back, W. Influence of Lauter Turbidity and Hot Trub on Wort Composition, Fermentation, and Beer Quality. J. Am. Soc. Brew. Chem. 2006, 64, 16–28. [Google Scholar] [CrossRef]
- Varga, Á.; Márki, E. Microfiltration: A Novel Technology for Removal of Trub from Hopped Wort. J. Food Process Eng. 2019, 42, e13200. [Google Scholar] [CrossRef]
- Senna Ferreira Costa, F.; Roquete Amparo, T.; Brandão Seibert, J.; Silveira, B.M.; Gomes da Silva, R.; Inocêncio Pereira, D.; Gontijo Garcia Barbosa, R.; dos Santos, O.D.H.; Brandão, G.C.; de Medeiros Teixeira, L.F.; et al. Reuse of Hot Trub as an Active Ingredient with Antioxidant and Antimicrobial Potential. Waste Biomass Valorization 2021, 12, 2037–2047. [Google Scholar] [CrossRef]
- Lewis, M.J.; Bamforth, C.W. Essays in Brewing Science; Springer: Boston, MA, USA, 2007; ISBN 978-0-387-33010-5. [Google Scholar]
- Santos, L.G.; Martins, V.G. Functional, Thermal, Bioactive and Antihypertensive Properties of Hot Trub Derived from Brewing Waste as an Alternative Source of Protein. Food Hydrocoll. 2024, 146, 109292. [Google Scholar] [CrossRef]
- Techakriengkrai, I.; Paterson, A.; Taidi, B.; Piggott, J.R. Relationships of Sensory Bitterness in Lager Beers to Iso-α-Acid Contents. J. Inst. Brew. 2004, 110, 51–56. [Google Scholar] [CrossRef]
- Habschied, K.; Košir, I.J.; Krstanović, V.; Kumrić, G.; Mastanjević, K. Beer Polyphenols—Bitterness, Astringency, and Off-Flavors. Beverages 2021, 7, 38. [Google Scholar] [CrossRef]
- Saraiva, B.R.; Anjo, F.A.; Vital, A.C.P.; da Silva, L.H.M.; Ogawa, C.Y.L.; Sato, F.; Coimbra, L.B.; Matumoto-Pintro, P.T. Waste from Brewing (Trub) as a Source of Protein for the Food Industry. Int. J. Food Sci. Technol. 2019, 54, 1247–1255. [Google Scholar] [CrossRef]
- Russ, W.; Meyer-Pittroff, R. The Use of Phenolic Protein Precipitates (Trub) from Beer Production in Animal Feed. Monatsschrift Fur Brauwiss. 2003, 56, 84–88. [Google Scholar]
- Saraiva, B.R.; Zancheta, J.C.; Sversut Gibin, M.; Anjo, F.A.; Lazzari, A.; Machado Filho, E.R.; Sato, F.; Matumoto-Pintro, P. Brewing By-Product Valorisation: Trub Debittered for Nutritional and Quality Improvement of Pasta. Int. J. Food Sci. Nutr. 2022, 73, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.F.C.e.; Feltre, G.; Zandonadi, F.S.; Rabelo, R.S.; Sussulini, A.; Hubinger, M.D. Unlocking Hot Trub’s Potential: A Simple Method for Extracting Bitter Acids and Xanthohumol. J. Sci. Food Agric. 2024, 104, 5381–5390. [Google Scholar] [CrossRef] [PubMed]
- Wolny-Koładka, K.; Malinowski, M.; Zdaniewicz, M. Energy-Related and Microbiological Evaluation of the Effects of Bulking Agents on the Brewery Hot Trub Biodrying. Food Bioprod. Process. 2021, 127, 398–407. [Google Scholar] [CrossRef]
- Tesio, A.Y.; Gómez-Cámer, J.L.; Morales, J.; Caballero, A. Simple and Sustainable Preparation of Nonactivated Porous Carbon from Brewing Waste for High-Performance Lithium–Sulfur Batteries. ChemSusChem 2020, 13, 3439–3446. [Google Scholar] [CrossRef]
- Mathias, T.R.D.S.; Fernandes de Aguiar, P.; de Almeida e Silva, J.B.; Moretzsohn de Mello, P.P.; Camporese Servulo, E.F. Brewery Wastes Reuse for Protease Production by Lactic Acid Bacteria Fermentation. Food Technol. Biotechnol. 2017, 55, 218–224. [Google Scholar] [CrossRef]
- Nazareth, T.C.; Zanutto, C.P.; Tripathi, L.; Juma, A.; Maass, D.; de Souza, A.A.U.; de Arruda Guelli Ulson de Souza, S.M.; Banat, I.M. The Use of Low-Cost Brewery Waste Product for the Production of Surfactin as a Natural Microbial Biocide. Biotechnol. Rep. 2020, 28, e00537. [Google Scholar] [CrossRef]
- Kühbeck, F.; Müller, M.; Back, W.; Kurz, T.; Krottenthaler, M. Effect of Hot Trub and Particle Addition on Fermentation Performance of Saccharomyces Cerevisiae. Enzym. Microb. Technol. 2007, 41, 711–720. [Google Scholar] [CrossRef]
- Eßlinger, H.M.; Narziß, L. Beer. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Gandolpho, B.; Almeida, A.; Gandolpho, G.; Freitas, D.; Gasparini, O.; Machado, M.; Barreto, P. Optimization Of Brewing Waste’s (Trub) Phenolic Compounds Extraction By Ultrasound Assisted Using Response Surface Methodology. Quim. Nova 2021, 44, 478–483. [Google Scholar] [CrossRef]
- Soceanu, A.; Dobrinas, S.; Popescu, V.; Buzatu, A.; Sirbu, A. Sustainable Strategies for the Recovery and Valorization of Brewery By-Products—A Multidisciplinary Approach. Sustainability 2023, 16, 220. [Google Scholar] [CrossRef]
- Grudniewska, A.; Popłoński, J. Simple and Green Method for the Extraction of Xanthohumol from Spent Hops Using Deep Eutectic Solvents. Sep. Purif. Technol. 2020, 250, 117196. [Google Scholar] [CrossRef]
- Santos, B.; Sousa, M.J. Review Hops- Bioactive Compounds and Their Applications. World J. Pharm. Pharm. Sci. 2022, 12, 1854–1886. [Google Scholar]
- Legette, L.; Ma, L.; Reed, R.L.; Miranda, C.L.; Christensen, J.M.; Rodriguez-Proteau, R.; Stevens, J.F. Pharmacokinetics of Xanthohumol and Metabolites in Rats after Oral and Intravenous Administration. Mol. Nutr. Food Res. 2012, 56, 466–474. [Google Scholar] [CrossRef]
- Betancur, M.; López, J.; Salazar, F. Antimicrobial Activity of Compounds from Hop (Humulus lupulus L.) Following. Supercritical Fluid Extraction: An Overview. Chil. J. Agric. Res. 2023, 83, 499–509. [Google Scholar] [CrossRef]
- Erzinger, G.S.; Lopes, P.C.; del Ciampo, L.F.; Zimath, S.C.; Vicente, D.; Martins de Albuquerque, F.; Prates, R.C. Bioactive Compounds of Hops Resulting from the Discarding of the Beer Industry in the Control of Pathogenic Bacteria. In Natural Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2021; pp. 41–55. [Google Scholar]
- Santos, L.G.; Gomes, B.M.; da Silva Noda, K.; Martins, V.G. Trub Brewery Waste as a Novel and Sustainable Protein Source for the Development of Active Packaging Materials. Int. J. Biol. Macromol. 2025, 313, 144328. [Google Scholar] [CrossRef]
- van Zadelhoff, A.; de Bruijn, W.J.C.; Sanders, M.G.; O’Sullivan, T.; Vincken, J.-P. Barley-Derived Beer Brewing by-Products Contain a High Diversity of Hydroxycinnamoylagmatines and Their Dimers. Food Chem. 2024, 453, 139586. [Google Scholar] [CrossRef] [PubMed]
- Hamany Djande, C.Y.; Dubery, I.A. Hordatines, Dimerised Hydroxycinnamoylagmatine Conjugates of Barley (Hordeum vulgare L.): An Appraisal of the Biosynthesis, Chemistry, Identification and Bioactivities. Phytochem. Rev. 2025, 24, 303–320. [Google Scholar] [CrossRef]
- Ashburn, B.E.; Brennan, M.W.; Bryant, R.W., Jr. Animal Food Product and Method and Apparatus Therefor. US Patent Application No. 2024/0373877 A1, 14 November 2024. [Google Scholar]
- Podpora, B.; Świderski, F.; Sadowska, A.; Rakowska, R.; Wasiak-Zys, G. Spent Brewer’s Yeast Extracts as a New Component of Functional Food. Czech J. Food Sci. 2016, 34, 554–563. [Google Scholar] [CrossRef]
- Avramia, I.; Amariei, S. Spent Brewer’s Yeast as a Source of Insoluble β-Glucans. Int. J. Mol. Sci. 2021, 22, 825. [Google Scholar] [CrossRef] [PubMed]
- Horn, P.A.; Zeni, A.L.B.; Cavichioli, N.; Winter, E.; Batista, K.Z.S.; Vitali, L.; de Almeida, E.A. Chemical Profile of Craft Brewer’s Spent Yeast and Its Antioxidant and Antiproliferative Activities. Eur. Food Res. Technol. 2023, 249, 2001–2015. [Google Scholar] [CrossRef]
- Schlabitz, C.; Kuhn, D.; Grambusch, I.M.; Pedralli, L.; Jacobs, W.; Neutzling Lehn, D.; Volken de Souza, C.F. Reuse of Residual Brewer’s Yeast: Valorization of Industrial Waste as a Source of Nutrients for Dairy Cattle. Waste Biomass Valorization 2024, 15, 5487–5499. [Google Scholar] [CrossRef]
- Ciobanu, L.T.; Constantinescu-Aruxandei, D.; Farcasanu, I.C.; Oancea, F. Spent Brewer’s Yeast Lysis Enables a Best Out of Waste Approach in the Beer Industry. Int. J. Mol. Sci. 2024, 25, 12655. [Google Scholar] [CrossRef]
- Marson, G.V.; de Castro, R.J.S.; Belleville, M.-P.; Hubinger, M.D. Spent Brewer’s Yeast as a Source of High Added Value Molecules: A Systematic Review on Its Characteristics, Processing and Potential Applications. World J. Microbiol. Biotechnol. 2020, 36, 95. [Google Scholar] [CrossRef] [PubMed]
- León-González, M.E.; Gómez-Mejía, E.; Rosales-Conrado, N.; Madrid-Albarrán, Y. Residual Brewing Yeast as a Source of Polyphenols: Extraction, Identification and Quantification by Chromatographic and Chemometric Tools. Food Chem. 2018, 267, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Covert, V.L.; Farzad, R.; Li, M.; Thompson-Witrick, K.A.; MacIntosh, A.J. Spent Brewer’s Yeast as an Alternative Ingredient in Aquafeed. J. Am. Soc. Brew. Chem. 2025, 83, 1–16. [Google Scholar] [CrossRef]
- Zeko-Pivač, A.; Habschied, K.; Kulisic, B.; Barkow, I.; Tišma, M. Valorization of Spent Brewer’s Yeast for the Production of High-Value Products, Materials, and Biofuels and Environmental Application. Fermentation 2023, 9, 208. [Google Scholar] [CrossRef]
- Vieira, E.; Brandão, T.; Ferreira, I.M.P.L.V.O. Evaluation of Brewer’s Spent Yeast To Produce Flavor Enhancer Nucleotides: Influence of Serial Repitching. J. Agric. Food Chem. 2013, 61, 8724–8729. [Google Scholar] [CrossRef]
- Ferreira, I.M.P.L.V.O.; Pinho, O.; Vieira, E.; Tavarela, J.G. Brewer’s Saccharomyces Yeast Biomass: Characteristics and Potential Applications. Trends Food Sci. Technol. 2010, 21, 77–84. [Google Scholar] [CrossRef]
- Kerby, C.; Vriesekoop, F. An Overview of the Utilisation of Brewery By-Products as Generated by British Craft Breweries. Beverages 2017, 3, 24. [Google Scholar] [CrossRef]
- Neira, K.; Jeison, D. Anaerobic Co-Digestion of Surplus Yeast and Wastewater to Increase Energy Recovery in Breweries. Water Sci. Technol. 2010, 61, 1129–1135. [Google Scholar] [CrossRef]
- Jacob, F.F.; Striegel, L.; Rychlik, M.; Hutzler, M.; Methner, F.-J. Spent Yeast from Brewing Processes: A Biodiverse Starting Material for Yeast Extract Production. Fermentation 2019, 5, 51. [Google Scholar] [CrossRef]
- Manurung, A.R.; Koentjoro, M.P.; Isdiantoni; Ekawati, I.; Alami, N.H.; Prasetyo, E.N. Enzymatic Conversion of Brewer’s Spent Yeast as Raw Material for Glutamic Acid Production. AIP Conf. Proc. 2021, 2330, 070012. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, K.; Liu, Z.; Wei, P.; Ying, H.; Chang, H. Succinic Acid Production by Actinobacillus Succinogenes Using Spent Brewer’s Yeast Hydrolysate as a Nitrogen Source. Appl. Biochem. Biotechnol. 2010, 160, 244–254. [Google Scholar] [CrossRef]
- Estrada-García, J.; Hernández-Aguilar, E.; Gutiérrez-Casiano, N.; Méndez-Contreras, J.M. Monitoring of Rheological Parameters in the Anaerobic Fermentation Process to Obtain Bioethanol from Craft Brewer’s Spent Grain Using Brewer’s Spent Yeast and Saccharomyces Cerevisiae S-04. Biomass Convers. Biorefin. 2025, 15, 15585–15602. [Google Scholar] [CrossRef]
- Jaeger, A.; Nyhan, L.; Sahin, A.W.; Zannini, E.; Arendt, E.K. Lactic Acid Fermentation as a Valorising Agent for Brewer’s Spent Yeast—Improving the Sensory Quality and Nutritional Potential. Fermentation 2024, 10, 54. [Google Scholar] [CrossRef]
- Gong, X.; Tian, W.; Wang, L.; Bai, J.; Qiao, K.; Zhao, J. Biological Regeneration of Brewery Spent Diatomite and Its Reuse in Basic Dye and Chromium (III) Ions Removal. Process Saf. Environ. Prot. 2019, 128, 353–361. [Google Scholar] [CrossRef]
- Huaccallo Aguilar, Y.; Ferreira da Silva, A.P.; Diaz de Tuesta, J.L.; Lima, O.; Teixeira Gomes, H. Geopolymers from Spent Diatomaceous Earth/Activated Carbon: Synthesis and Application on Winery Wastewater Treatment. SSRN J. 2022. [Google Scholar] [CrossRef]
- Ferraz, E.; Coroado, J.; Silva, J.; Gomes, C.; Rocha, F. Manufacture of Ceramic Bricks Using Recycled Brewing Spent Kieselguhr. Mater. Manuf. Process. 2011, 26, 1319–1329. [Google Scholar] [CrossRef]
- Haile, D. Manufacturing of Tiles from Kieselguhr Sludge /Diatomaceous Earth/. In Advances of Science and Technology. ICAST 2021. Lecture Notes of the Institute for Computer Sciences, Social Informatics and Telecommunications Engineering; Berihun, M.L., Ed.; Springer Nature: Berlin/Heidelberg, Germany, 2022; Volume 411, pp. 106–114. [Google Scholar]
- Kipsanai, J.J.; Wambua, P.M.; Namango, S.S.; Amziane, S. A Review on the Incorporation of Diatomaceous Earth as a Geopolymer-Based Concrete Building Resource. Materials 2022, 15, 7130. [Google Scholar] [CrossRef]
- Rawalgaonkar, D.; Zhang, Y.; Walker, S.; Kirchman, P.; Zhang, Q.; Ergas, S.J. Recovery of Energy and Carbon Dioxide from Craft Brewery Wastes for Onsite Use. Fermentation 2023, 9, 831. [Google Scholar] [CrossRef]
- Silkina, A.; Emran, M.A.; Turner, S.; Tang, K.W. Using Microalgae to Convert Brewery Carbon Gas Emissions into Valuable Bioproducts. Energies 2024, 17, 6125. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, A.; Palai, T.; Aeshala, L.M. Electrochemical CO2 Reduction to Value-Added Chemicals. In From Waste to Wealth; Arya, R.K., Verros, G.D., Verma, O.P., Hussain, C.M., Eds.; Springer Nature: Singapore, 2024; pp. 525–545. [Google Scholar]
- Velvizhi, G.; Sarkar, O.; Rovira-Alsina, L.; Puig, S.; Mohan, S.V. Conversion of Carbon Dioxide to Value Added Products through Anaerobic Fermentation and Electro Fermentation: A Comparative Approach. Int. J. Hydrogen Energy 2022, 47, 15442–15455. [Google Scholar] [CrossRef]
- Chen, G.; Wang, R.; Sun, M.; Chen, J.; Iyobosa, E.; Zhao, J. Carbon Dioxide Reduction to High–Value Chemicals in Microbial Electrosynthesis System: Biological Conversion and Regulation Strategies. Chemosphere 2023, 344, 140251. [Google Scholar] [CrossRef] [PubMed]
- Werkneh, A.A.; Beyene, H.D.; Osunkunle, A.A. Recent Advances in Brewery Wastewater Treatment; Approaches for Water Reuse and Energy Recovery: A Review. Environ. Sustain. 2019, 2, 199–209. [Google Scholar] [CrossRef]
- Brito, A.G.; Peixoto, J.; Oliveira, J.M.; Oliveira, J.A.; Costa, C.; Nogueira, R.; Rodrigues, A. Brewery and Winery Wastewater Treatment: Some Focal Points of Design and Operation. In Utilization of By-Products and Treatment of Waste in the Food Industry; Oreopoulou, V., Russ, W., Eds.; Springer: New York, NY, USA, 2007; pp. 109–131. [Google Scholar][Green Version]
- Smetana, G.; Grosser, A. The Application of an Upflow Anaerobic Sludge Blanket Reactor in the Treatment of Brewery and Dairy Wastewater: A Critical Review. Energies 2024, 17, 1504. [Google Scholar] [CrossRef]
- Li, Q.; Feng, R.; Li, Y. Energy Assessment and Inner Microbial Community Analysis of Internal Circulation (IC) Reactor for Bio-Hydrogen Production Using Brewery Wastewater. Bioenergy Res. 2022, 15, 2122–2131. [Google Scholar] [CrossRef]
- Mutsvene, B.; Chetty, M.; Kumari, S.; Bux, F. Biohydrogen Production from Brewery Wastewater in an Anaerobic Baffled Reactor. A Preliminary Techno-Economic Evaluation. S. Afr. J. Chem. Eng. 2023, 43, 9–23. [Google Scholar] [CrossRef]
- Su, H.; Wang, K.; Lian, J.; Wang, L.; He, Y.; Li, M.; Han, D.; Hu, Q. Advanced Treatment and Resource Recovery of Brewery Wastewater by Co-Cultivation of Filamentous Microalga Tribonema Aequale and Autochthonous Bacteria. J. Environ. Manag. 2023, 348, 119285. [Google Scholar] [CrossRef]
- Yang, P.; Li, X.; Leng, L. Microbial Dynamics with the Introduction of Brewery Waste in a Long-term Chain Elongation Process for Caproate Production. J. Chem. Technol. Biotechnol. 2023, 98, 2283–2294. [Google Scholar] [CrossRef]
- Tamang, P.; Banerjee, R.; Köster, S.; Nogueira, R. Comparative Study of Polyhydroxyalkanoates Production from Acidified and Anaerobically Treated Brewery Wastewater Using Enriched Mixed Microbial Culture. J. Environ. Sci. 2019, 78, 137–146. [Google Scholar] [CrossRef]
- Barati, B.; Li, Y.; Gusev, S.; Rousseau, D.P.L.; Van Hulle, S.W.H. Unlocking the Potential of Brewery Wastewater: Sustainable Cultivation of Arthrospira Platensis for Biomass and Phycocyanin Production. J. Water Process Eng. 2025, 70, 107107. [Google Scholar] [CrossRef]
- Achilleos, P.; Roberts, K.R.; Williams, I.D. Struvite Precipitation within Wastewater Treatment: A Problem or a Circular Economy Opportunity? Heliyon 2022, 8, e09862. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.; Dastidar, M.G.; Sreekrishnan, T.R. Bioleaching of Heavy Metals from Sewage Sludge: A Review. J. Environ. Manag. 2009, 90, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Strezov, V.; Yin Chan, K.; Nelson, P.F. Agronomic Properties of Wastewater Sludge Biochar and Bioavailability of Metals in Production of Cherry Tomato (Lycopersicon esculentum). Chemosphere 2010, 78, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Tsadik, Y.K.G.; Hailu, A.M.; Asfaw, S.L.; Mekonnen, Y.S. The Effect of Brewery Sludge Biochar on Immobilization of Bio-Available Cadmium and Growth of Brassica Carinata. Heliyon 2020, 6, e05573. [Google Scholar] [CrossRef] [PubMed]
- Kanagachandran, K.; Jayaratne, R. Utilization Potential of Brewery Waste Water Sludge as an Organic Fertilizer. J. Inst. Brew. 2006, 112, 92–96. [Google Scholar] [CrossRef]
- Christian, C.I.; Prince, C.O.; Peace, E.O. Impact of Brewery Wastewater Sludge on Microbiological Quality of Agricultural Soil. J. Ecobiotechnol. 1970, 13–17. [Google Scholar] [CrossRef]
- Alayu, E.; Leta, S. Brewery Sludge Quality, Agronomic Importance and Its Short-Term Residual Effect on Soil Properties. Int. J. Environ. Sci. Technol. 2020, 17, 2337–2348. [Google Scholar] [CrossRef]
- Demeke, M.; Gabbiye, N. Organic Biofertilizer from Brewery Wastewater Sludges via Aerobic Composting Process. In Advances of Science and Technology. ICAST 2019. Lecture Notes of the Institute for Computer Sciences, Social Informatics and Telecommunications Engineering; Habtu, N., Ayele, D., Fanta, S., Admasu, B., Bitew, M., Eds.; Springer: Cham, Switzerland, 2020; Volume 308, pp. 3–15. [Google Scholar]
- Agler, M.T.; Aydinkaya, Z.; Cummings, T.A.; Beers, A.R.; Angenent, L.T. Anaerobic Digestion of Brewery Primary Sludge to Enhance Bioenergy Generation: A Comparison between Low- and High-Rate Solids Treatment and Different Temperatures. Bioresour. Technol. 2010, 101, 5842–5851. [Google Scholar] [CrossRef]
- Edunjobi, T.D.; Agbede, O.O.; Aworanti, O.A.; Adebayo, A.O.; Agarry, S.E.; Ogunkunle, O.; Laseinde, O.T. Enhanced Anaerobic Digestion of Brewers’ Spent Grain: Effect of Inoculum, Poultry Manure Application and Iron (III) Chloride Supplementation on Biogas Production and Its Kinetics. Biomass Convers. Biorefin. 2024, 14, 29561–29577. [Google Scholar] [CrossRef]
- Teshome, B.; Assefa, B.; Angassa, K. Production of Composite Briquette Fuel from Brewery Wastewater Sludge and Spent Grains. Int. J. Biomater. 2024, 2024, 1710628. [Google Scholar] [CrossRef]
- Cimini, A.; Moresi, M. Circular Economy in the Brewing Chain. Ital. J. Food Sci. 2021, 33, 47–69. [Google Scholar] [CrossRef]
- Wojnarowska, M.; Muradin, M.; Paiano, A.; Ingrao, C. Recycled Glass Bottles for Craft-Beer Packaging: How to Make Them Sustainable? An Environmental Impact Assessment from the Combined Accounting of Cullet Content and Transport Distance. Resources 2025, 14, 23. [Google Scholar] [CrossRef]
- Larson, J. Brewery Effluents, Emissions, and Sustainability*. In Handbook of Brewing; Stewart, G., Russell, I., Anstruther, A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 723–753. [Google Scholar]
- Bristogianni, T.; Oikonomopoulou, F. Glass Up-Casting: A Review on the Current Challenges in Glass Recycling and a Novel Approach for Recycling “as-Is” Glass Waste into Volumetric Glass Components. Glass Struct. Eng. 2023, 8, 255–302. [Google Scholar] [CrossRef]
- Xin, Y.; Kurmus, H.; Mohajerani, A.; Dallol, Y.; Lao, Y.; Robert, D.; Pramanik, B.; Tran, P. Recycling Crushed Waste Beer Bottle Glass in Fired Clay Bricks. Buildings 2021, 11, 483. [Google Scholar] [CrossRef]
- Ahmed, K.S.; Rana, L.R. Fresh and Hardened Properties of Concrete Containing Recycled Waste Glass: A Review. J. Build. Eng. 2023, 70, 106327. [Google Scholar] [CrossRef]
- Henao Rios, L.M.; Hoyos Triviño, A.F.; Villaquirán-Caicedo, M.A.; de Gutiérrez, R.M. Effect of the Use of Waste Glass (as Precursor, and Alkali Activator) in the Manufacture of Geopolymer Rendering Mortars and Architectural Tiles. Constr. Build. Mater. 2023, 363, 129760. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Y.; Jin, Y.; Li, Z.; Sun, S.; Xu, H.; Wu, C.; Zhang, D. Solid State Recycling of Used Aluminum Alloy Beverage Cans by Thermomechanical Consolidation. Heat Treat. Surf. Eng. 2022, 4, 90–98. [Google Scholar] [CrossRef]
- Bulei, C.; Todor, M.; Heput, T.; Kiss, I. Recovering Aluminium for Recycling in Reusable Backyard Foundry That Melts Aluminium Cans. IOP Conf. Ser. Mater. Sci. Eng. 2018, 416, 012099. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, K.; Saunders, T.; Wang, G.; Wesling, K.; Liu, J.; Busfield, J.; Bilotti, E.; Yan, H. Low Cost Small Scale Recycling Aluminium Cans for Energy Conservation and Environmental Sustainability. Environ. Technol. 2025, 1–8. [Google Scholar] [CrossRef]
- Bonato, S.V.; Augusto de Jesus Pacheco, D.; Schwengber ten Caten, C.; Caro, D. The Missing Link of Circularity in Small Breweries’ Value Chains: Unveiling Strategies for Waste Management and Biomass Valorization. J. Clean. Prod. 2022, 336, 130275. [Google Scholar] [CrossRef]
- Joshi, G.; Chauhan, S.S. Synthesis and Characterization of Cellulose Nanofibers from Waste Paper and Their Utilization in Wood Adhesion. Polym. Compos. 2024, 45, 9103–9118. [Google Scholar] [CrossRef]
- Couret, L.; Irle, M.; Belloncle, C.; Cathala, B. Extraction and Characterization of Cellulose Nanocrystals from Post-Consumer Wood Fiberboard Waste. Cellulose 2017, 24, 2125–2137. [Google Scholar] [CrossRef]
- Das, P.P.; Kalyani, P.; Kumar, R.; Khandelwal, M. Cellulose-Based Natural Nanofibers for Fresh Produce Packaging: Current Status, Sustainability and Future Outlook. Sustain. Food Technol. 2023, 1, 528–544. [Google Scholar] [CrossRef]
- Partridge, M. Packaging: Historical Perspectives and Packaging Technology. In Handbook of Brewing; Stewart, G., Russell, I., Anstruther, A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 529–546. [Google Scholar]
- Morgan, D.R.; Styles, D.; Thomas Lane, E. Packaging Choice and Coordinated Distribution Logistics to Reduce the Environmental Footprint of Small-Scale Beer Value Chains. J. Environ. Manag. 2022, 307, 114591. [Google Scholar] [CrossRef]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef]
- Arroyave, A.; Cui, S.; Lopez, J.C.; Kocen, A.L.; LaPointe, A.M.; Delferro, M.; Coates, G.W. Catalytic Chemical Recycling of Post-Consumer Polyethylene. J. Am. Chem. Soc. 2022, 144, 23280–23285. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Appleyard, D. Cutting Plastics from Brewing; First Key, Consultants to the Brewing and Beverage Industry. 2021. Available online: https://firstkey.com/cutting-plastics-from-brewing/ (accessed on 5 August 2025).
- Clingman, A. Member Highlight: Sierra Nevada Brewing Co Leading the Way for Sustainability and Energy Efficiency. Available online: https://www.energync.org/blog/member-highlight-sierra-nevada-brewing-co-leading-the-way-for-sustainability-and-energy-efficiency/ (accessed on 5 August 2025).
- Solar United Neighbors Brews from the Sun Competitor Sierra Nevada Brewing Co. Available online: https://Www.Brewsfromthesun.Org/Breweries/Sierra-Nevada-Brewing-Co/ (accessed on 5 August 2025).
- Heineken Heineken France Launches Project Circle. 2024. Available online: https://www.theheinekencompany.com/newsroom/heineken-france-launches-project-circle/ (accessed on 6 August 2025).
- Heineken Projet Life FWFB. 2024. Available online: https://www.heinekenfrance.fr/nos-actions/projet-circle/ (accessed on 6 August 2025).
- Nyhan, L.; Sahin, A.W.; Schmitz, H.H.; Siegel, J.B.; Arendt, E.K. Brewers’ Spent Grain: An Unprecedented Opportunity to Develop Sustainable Plant-Based Nutrition Ingredients Addressing Global Malnutrition Challenges. J. Agric. Food Chem. 2023, 71, 10543–10564. [Google Scholar] [CrossRef]
- Chryssolouris, D.; Itten, R.; Regula, K.; Stucki, M. Life Cycle Sustainability Assessment of a Biorefinery for the Valorisation of Brewer’s Spent Grain. 11th International Conference on Life Cycle Management (LCM). 2023. Available online: https://digitalcollection.zhaw.ch/handle/11475/30535 (accessed on 6 August 2025).
- Fondazione Messina Life Restart—Reuse of BEer SpenT Grain foR BioplasTics. 2022. Available online: https://fdcmessina.org/life-restart/?l_lang=en (accessed on 6 August 2025).
- Aimplas POLYMEER Kicks off to Transform Brewers’ Waste into High-Value Bioplastics. 2024. Available online: https://www.aimplas.net/blog/polymeer-kicks-off-transform-brewers-waste-high-value-bioplastics (accessed on 6 August 2025).
- Mussatto, S.I. Brewer’s Spent Grain: A Valuable Feedstock for Industrial Applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef]
- Bolwig, S.; Mark, M.S.; Happel, M.K.; Brekke, A. Beyond Animal Feed? In From Waste to Value; Routledge: London, UK, 2019; pp. 107–126. [Google Scholar]
- Chetrariu, A.; Dabija, A. Spent Grain: A Functional Ingredient for Food Applications. Foods 2023, 12, 1533. [Google Scholar] [CrossRef]
- Sturm, B.; Hugenschmidt, S.; Joyce, S.; Hofacker, W.; Roskilly, A.P. Opportunities and Barriers for Efficient Energy Use in a Medium-Sized Brewery. Appl. Therm. Eng. 2013, 53, 397–404. [Google Scholar] [CrossRef]
- Kalayu, G. Serial Re-Pitching: Its Effect on Yeast Physiology, Fermentation Performance, and Product Quality. Ann. Microbiol. 2019, 69, 787–796. [Google Scholar] [CrossRef]
- Carvalheira, M.; Amorim, C.L.; Oliveira, A.C.; Guarda, E.C.; Costa, E.; Ribau Teixeira, M.; Castro, P.M.L.; Duque, A.F.; Reis, M.A.M. Valorization of Brewery Waste through Polyhydroxyalkanoates Production Supported by a Metabolic Specialized Microbiome. Life 2022, 12, 1347. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Howell, J.A.; Field, R.W.; England, R.; Bird, M.R.; O’Shaughnessy, C.L.; MeKechinie, M.T. Beer Clarification by Microfiltration—Product Quality Control and Fractionation of Particles and Macromolecules. J. Memb. Sci. 2001, 194, 185–196. [Google Scholar] [CrossRef]
- Gan, Q. Beer Clarification by Cross-Flow Microfiltration—Effect of Surface Hydrodynamics and Reversed Membrane Morphology. Chem. Eng. Process. Process Intensif. 2001, 40, 413–419. [Google Scholar] [CrossRef]
- Serviss, M.T.; Van Hout, D.; Britton, S.J.; MacIntosh, A.J. Brewing for the Future: Balancing Tradition and Sustainability. J. Am. Soc. Brew. Chem. 2025, 1–19. [Google Scholar] [CrossRef]
- Muster-Slawitsch, B.; Brunner, C. Intensified Brewing Systems. In Intensification of Biobased Processes; The Royal Society of Chemistry: London, UK, 2018; pp. 430–461. [Google Scholar]
- Strobl, M. Continuous Beer Production. In New Advances on Fermentation Processes; IntechOpen: London, UK, 2020. [Google Scholar]
- Ambrosi, A.; Cardozo, N.S.M.; Tessaro, I.C. Membrane Separation Processes for the Beer Industry: A Review and State of the Art. Food Bioproc. Technol. 2014, 7, 921–936. [Google Scholar] [CrossRef]
- Shopska, V.; Dzhivoderova-Zarcheva, M.; Kostov, G. Continuous Primary Beer Fermentation with Yeast Immobilized in Alginate–Chitosan Microcapsules with a Liquid Core. Beverages 2024, 10, 87. [Google Scholar] [CrossRef]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Carrier-Free, Continuous Primary Beer Fermentation. J. Inst. Brew. 2014, 120, 500–506. [Google Scholar] [CrossRef]
- Rocha dos Santos Mathias, T.; Moreira Menezes, L.; Camporese Sérvulo, E.F. Effect of Maize as Adjunct and the Mashing Proteolytic Step on the Brewer Wort Composition. Beverages 2019, 5, 65. [Google Scholar] [CrossRef]
- Kühbeck, F.; Dickel, T.; Krottenthaler, M.; Back, W.; Mitzscherling, M.; Delgado, A.; Becker, T. Effects of Mashing Parameters on Mash β-Glucan, FAN and Soluble Extract Levels. J. Inst. Brew. 2005, 111, 316–327. [Google Scholar] [CrossRef]
- Durand, G.A.; Corazza, M.L.; Blanco, A.M.; Corazza, F.C. Dynamic Optimization of the Mashing Process. Food Control 2009, 20, 1127–1140. [Google Scholar] [CrossRef]
- Silva, D.; Brányik, T.; Dragone, G.; Vicente, A.; Teixeira, J.; Almeida e Silva, J. High Gravity Batch and Continuous Processes for Beer Production: Evaluation of Fermentation Performance and Beer Quality. Chem. Pap. 2008, 62, 34–41. [Google Scholar] [CrossRef]
- Puligundla, P.; Smogrovicova, D.; Mok, C.; Obulam, V.S.R. Recent Developments in High Gravity Beer-Brewing. Innov. Food Sci. Emerg. Technol. 2020, 64, 102399. [Google Scholar] [CrossRef]
- Stewart, G.G. High-Gravity Brewing and Distilling—Past Experiences and Future Prospects. J. Am. Soc. Brew. Chem. 2010, 68, 1–9. [Google Scholar] [CrossRef]
- Rögener, F. Filtration Technology for Beer and Beer Yeast Treatment. IOP Conf. Ser. Earth. Environ. Sci. 2021, 941, 012016. [Google Scholar] [CrossRef]
- Pinguli, L.; Malollari, I.; Lici, L. Optimization of Cleaning Process in Breweries: An Important Tool in Efficient Use of Water and Minimization of Discharges. Int. J. Adv. Res. 2017, 5, 123–135. [Google Scholar]
- NX Filtration Sustainable Solutions in Beer Breweries through Membrane Filtration. Available online: https://nxfiltration.com/knowledge-base/publications/sustainable-solutions-in-beer-breweries-through-membrane-filtration/ (accessed on 7 August 2025).
- Cimini, A.; Moresi, M. Beer Clarification by Novel Ceramic Hollow-Fiber Membranes: Effect of Pore Size on Product Quality. J. Food Sci. 2016, 81, E2521–E2528. [Google Scholar] [CrossRef]
- Li, H. Application of Centrifugation and Soilless Filtration Technologies in the Beer Production Process and Empirical Research on Their Impact on Beer Quality. BIO Web Conf. 2023, 72, 01014. [Google Scholar] [CrossRef]
- Mosher, M.; Trantham, K. Clarification and Filtration. In Brewing Science: A Multidisciplinary Approach; Springer: Cham, Switzerland, 2021; pp. 353–374. [Google Scholar]
- GEA GEA Centrifuges in Breweries. Available online: https://cdn.gea.com/-/media/migratedfromtridion/stories/centifuges-in-breweries-gea-62541.pdf?rev=d16c142941b84fc198be7db08c55380d (accessed on 6 August 2025).
- Cimini, A.; Moresi, M. Innovative Rough Beer Conditioning Process Free from Diatomaceous Earth and Polyvinylpolypyrrolidone. Foods 2020, 9, 1228. [Google Scholar] [CrossRef]
- Cadar, O.; Vagner, I.; Miu, I.; Scurtu, D.; Senila, M. Preparation, Characterization, and Performance of Natural Zeolites as Alternative Materials for Beer Filtration. Materials 2023, 16, 1914. [Google Scholar] [CrossRef]
- Carvalho, G.; Leite, A.C.; Leal, R.; Pereira, R. The Role of Emergent Processing Technologies in Beer Production. Beverages 2023, 9, 7. [Google Scholar] [CrossRef]
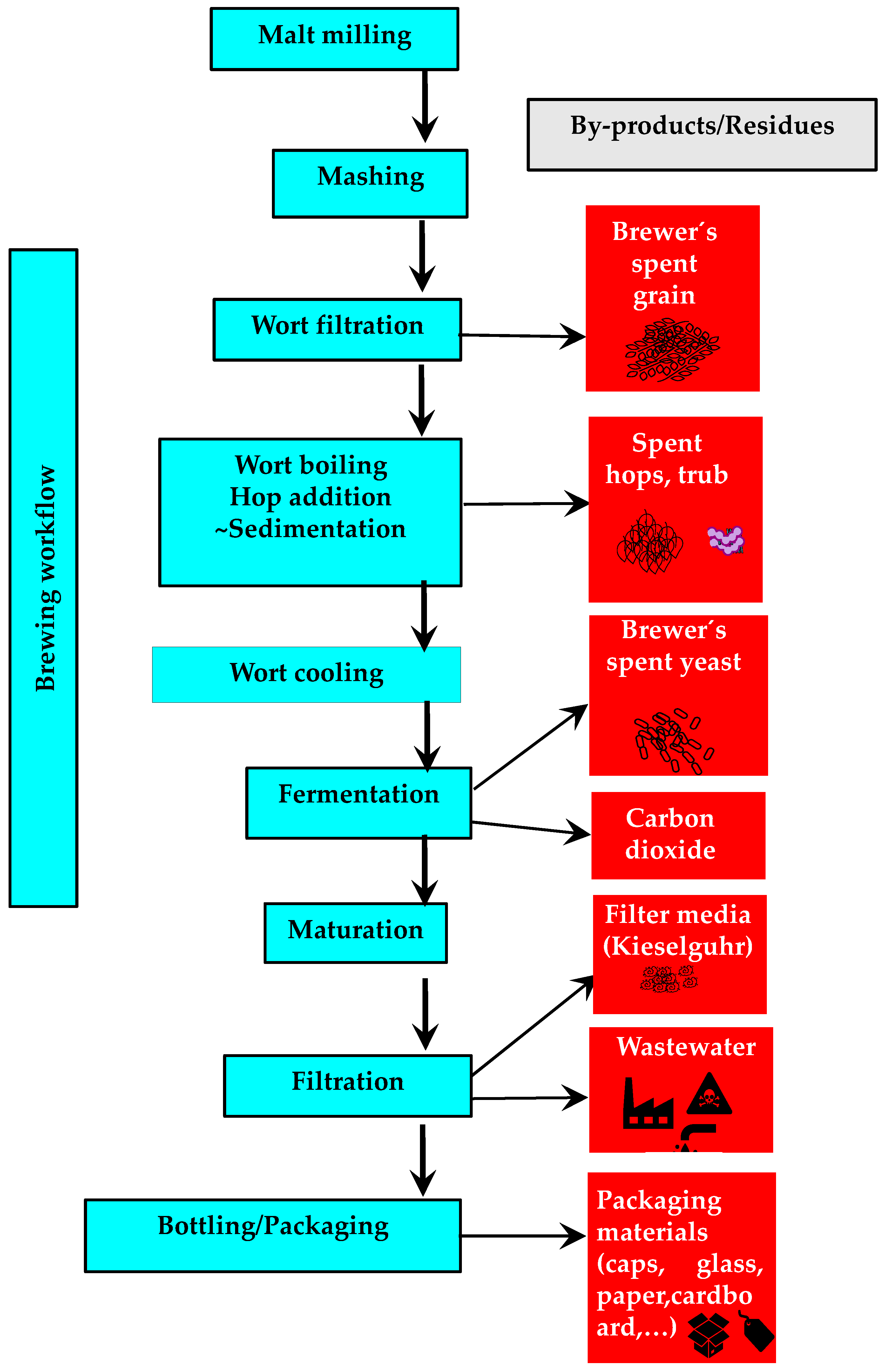
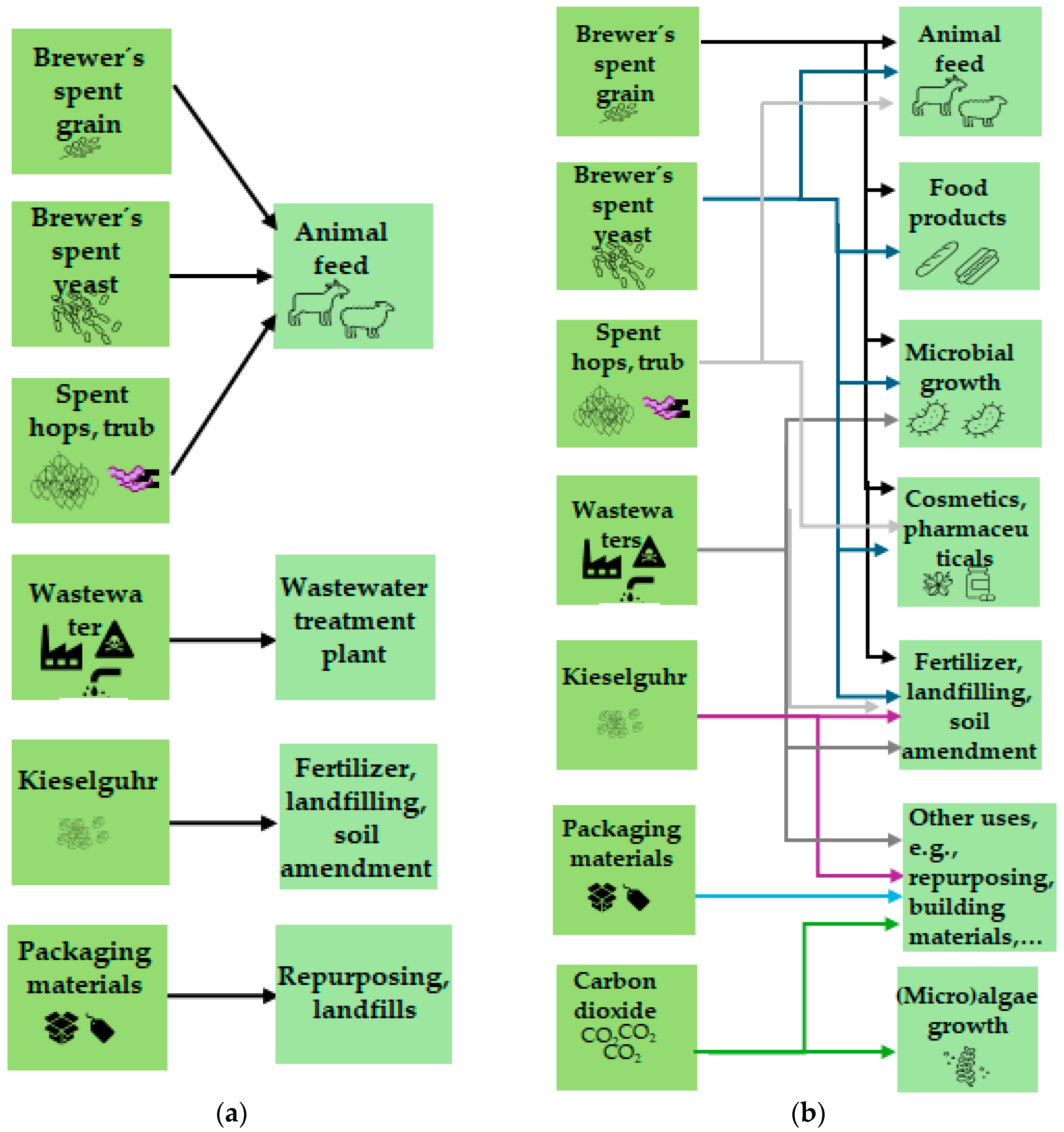
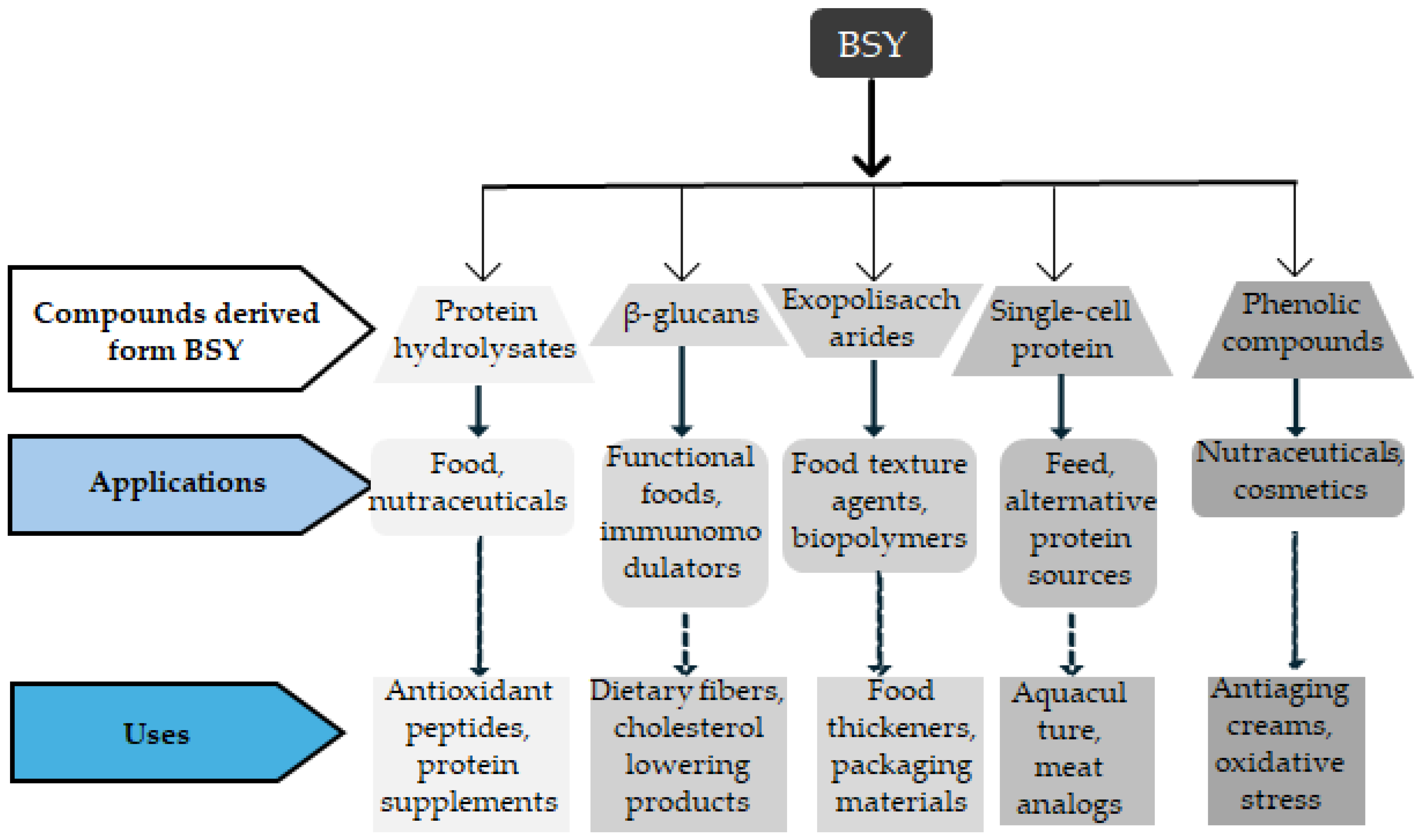
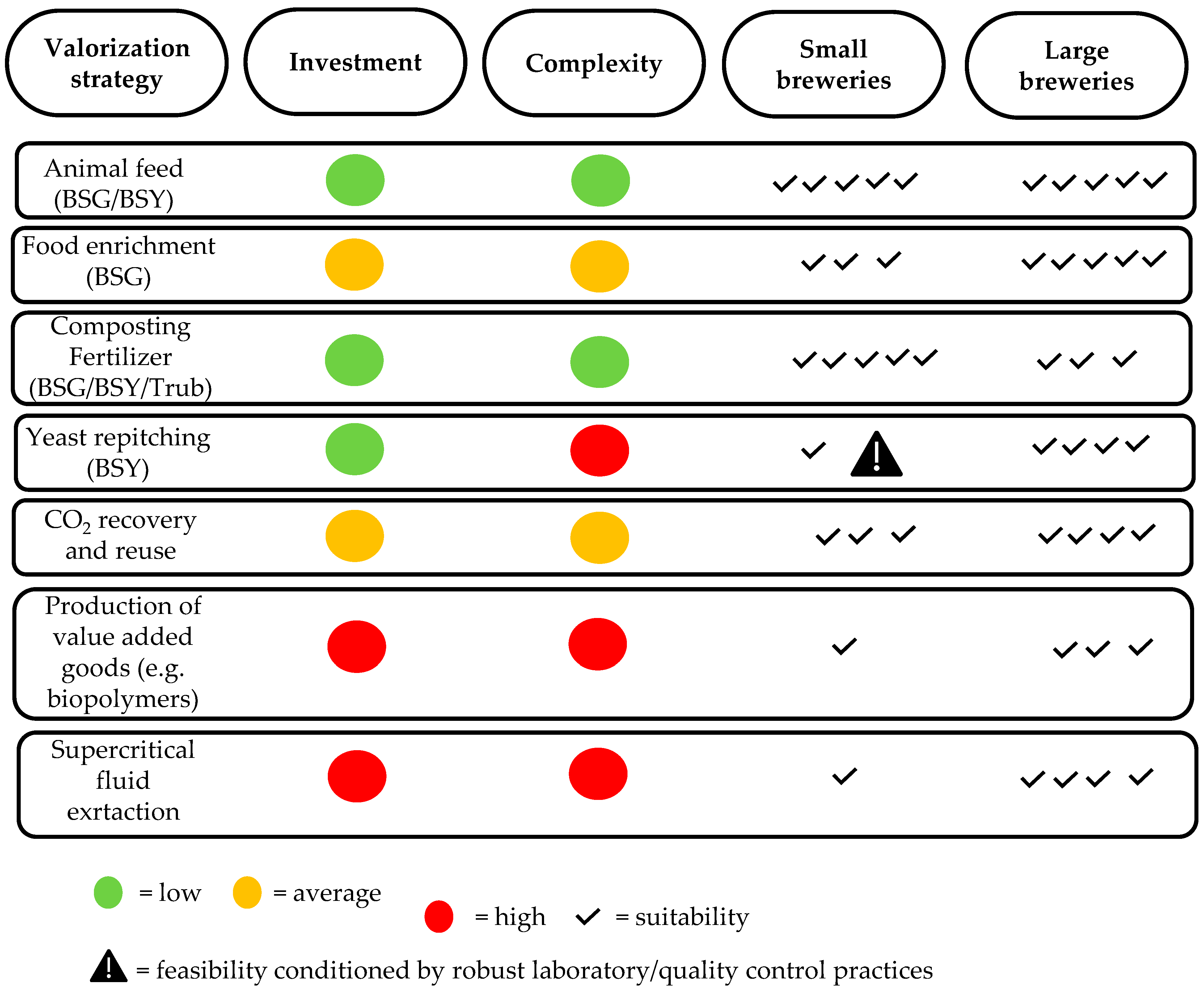
| Stage | Comments | References |
|---|---|---|
| Malting | Barley (mostly) grains are soaked in water, germinated and dried, so that the sprouted grain acquires the intended color and flavor. Hydrolases, e.g., amylolytic and proteolytic enzymes that will be used to obtain fermentable sugars and peptides/amino acids, are produced. | [13] |
| Mashing | Malt is mixed with hot water to activate enzymes (amylases, glucanases, proteases) and facilitate the dissolution and breakdown of malt components, producing a wort rich in fermentable sugars and essential nutrients. Maximizing amylase activity is critical, as wort typically contains high concentrations of fermentable sugars (~90–100 g/L) compared to amino acids (~1–2 g/L), as advised for proper yeast metabolism. | [14,15,16] |
| Boiling | Hops are added and wort is boiled, resulting in relevant chemical and physical changes. Boiling serves to extract and convert hop compounds for bitterness, sterilize the wort, inactivate enzymes, coagulate proteins, and enhance flavor, aroma, and color through Maillard reactions. Water evaporation concentrates the wort, while unwanted volatile compounds, e.g., dimethyl sulfide, are removed. Prior to fermentation, wort undergoes clarification and cooling. | [10,17] |
| Fermentation | Yeasts primarily convert sugars into ethanol and carbon dioxide. Besides alcohol production, fermentation generates a wide range of secondary metabolites, e.g., isoamyl acetate (banana aroma), ethyl acetate (solvent-like), and ethyl hexanoate (pineapple), that shape the flavor and aroma of beer. The specific profile of these compounds depends on the yeast strain used. | [3,10] |
| Bottling/Packaging | After fermentation/maturation, beer is filtered (to remove the remaining yeast), carbonated (to adjust the final dissolved carbon dioxide level), pasteurized (to eliminate harmful bacteria) and packed (in bottles, cans, or kegs for distribution and consumption). | [11,12] |
| Environmental Impact | Comments | References |
|---|---|---|
| Water pollution | Brewery wastewater often depicts high concentrations of organic matter, nutrients, and various chemicals. Unless adequately treated prior to discharge, this effluent significantly increases the biochemical oxygen demand (BOD) in receiving water bodies, leading to oxygen depletion and eutrophication. These processes can result in harmful algal blooms, oxygen deficits, and subsequent negative impacts on aquatic ecosystems. | [30,31,32,33] |
| Soil contamination | Inadequate disposal of solid by-products may lead to soil nutrient overload or imbalances. Runoff from such sites may introduce excessive nutrients and disturb local fauna and flora. | [30,31] |
| Greenhouse Gas Emissions | Besides the direct emission of CO2 from alcoholic fermentation, inadequate landfilling of by-products such as BSG trub and spent hops leads to anaerobic decomposition and ultimately the generation of methane, which has higher global warming potential than carbon dioxide. | [23,34,35] |
| Food Product | BSG Incorporation (%) | Key Advantages | Challenges | References |
|---|---|---|---|---|
| Cereal-based products, e.g., biscuits, bread, cookies, muffins, pasta) | Optimal level of BSG * bread and biscuits: <10 cookies: <25 muffins: <20 pasta: <12 | High fiber, protein and antioxidants content, with potential overall health benefits, e.g., antidiabetic, anti-inflammatory, and antithrombotic features. | Variability of raw material composition compromises standardization; strong BSG flavor and aroma, overall acceptability. | [60] |
| Cereal bars | ~12 | Perceived as more natural; sustainability and nutrition information increase purchase intent; similar acceptable price range to control. | Lower sensory and hedonic ratings; relies on external info (e.g., sustainability) to enhance consumer interest; lower optimal price point. | [61] |
| Instant flours | 10 to 20 | Nutritionally comparable to commercial products; potential to reduce malnutrition; good protein source. | Poor sensory traits due to high fiber (20%); microbial instability; require further safety and quality research. | [62] |
| Snacks and breadsticks | 20 to 40 | BSG increases antioxidant capacity and fiber content and reduces glycemic index (GI). | Heat loss of nutrients; limited GI reduction in extrudates; formulation challenges at high BSG levels, e.g., unwanted polysaccharide–protein complexes. | [63] |
| BSG Component | Deconstruction Method | Valorization Pathways |
|---|---|---|
| Cellulose and hemicellulose | Alkaline processing with hydrogen peroxide (AHP) effectively removed proteins and lignin from BSG, increasing the relative content of cellulose and hemicellulose. Higher AHP concentrations and longer treatment times enhanced removal efficiency, aiding in (hemi)cellulose recovery for further use [92]. | Enzymatic or chemical hydrolysis of the polysaccharide to yield fermentable sugars towards the production of goods, e.g., ethanol, biodiesel, biopolymers, enzymes [88,93]. |
| Subcritical water extraction (SWE) of defatted BSG enabled temperature-controlled fractionation, yielding cellulose-rich residues, alongside phenolic- and protein-rich extracts. At 150 °C, cellulose recovery was highest, although yields (20–25%) and purity (42–71%) remained limited even after H2O2 bleaching. SWE offers a sustainable method for cellulose recovery while also retrieving bioactive phenolic co-products [94]. | Through mechanical or chemical treatments, cellulose can be processed into nanocellulose, which finds applications in advanced biocomposites, packaging, and biomedical devices, due to its high strength and renewability [95]. | |
| Hydrolysis of hemicellulose yields pentose sugars (e.g., xylose) for fermentation to ethanol, 1-butanol or xylitol, or dehydration to furfural (a precursor for bioplastics and platform chemicals) [96,97]. | ||
| Partial hydrolysis of hemicelluloses can yield oligosaccharides with prebiotic features that may enhance gut health when added to functional foods [98]. | ||
| Lignin | Combining acidic natural deep eutectic solvents DES, namely choline chloride–lactic acid (ChCl-La), with microwave-assisted extraction enabled efficient lignin recovery from BSG. Lignin with 79% purity and strong antioxidant activity (IC50 * ≈ 0.022 mg/mL) was obtained under optimal conditions of irradiation (150 °C, 15 min). Lignin structural integrity was preserved, and low carbohydrate contamination was observed, suggesting a promising approach for high-quality lignin extraction [99]. | Given its polyphenolic structure, lignin displays antioxidant activity which can be used by incorporating extracted lignin into polymeric composites and coatings [100]. |
| BSG was fractionated using ethanol organosolv pretreatment. Under optimal conditions (180 °C, 120 min of incubation and 50% ethanol concentration), lignin was recovered with 95% purity and 58% yield. Moreover, the process enabled separation of cellulose and hemicellulose [101]. | Lignin can be used to obtain carbon-based materials with application in energy storage, novel catalysts and environmental remediation [102]. | |
| Lignin can be used as feedstock for the chemical or biological production of valuable phenolic compounds [103] and other bioproducts [104], respectively. | ||
| Phenolic compounds | Pulsed Electric Field (PEF) was used as a pre-treatment to enhance ethanol/water (4:1, v/v) leaching of phenolic compounds from BSG. Optimized PEF conditions (2.5 kV/cm, 50 Hz, 14.5 s) increased total free and bound phenolic content by 2.7-fold and 1.7-fold, respectively, compared to untreated samples [105]. | Phenolic compounds from BSG offer antioxidant and antimicrobial benefits, supporting their use in functional foods and nutraceuticals. Their incorporation is enhanced through techniques like encapsulation to improve stability, bioavailability, and effectiveness. Standardization of extracts is key to consistent application in health-promoting products [106,107,108] |
| Optimized ultrasound-assisted extraction (UAE) using response surface methodology efficiently recovered phenolic compounds from BSG, achieving a 156% higher yield than conventional methods. Under optimal conditions (80 °C, 50 min, 65:35 ethanol/water) extracts rich in ferulic, vanillic, and p-coumaric acids with strong antioxidant activity were obtained [109]. | Phenolic compounds in skincare and cosmetics offer antioxidant, antiaging, and protective benefits. They help treat skin issues like inflammation and pigmentation and support natural skin defenses. Although effective, they act slower than common synthetic-derived cosmetics and need more research for optimized use in humans [110]. | |
| Pressurized liquid extraction (PLE) ** of propane pressed defatted BSG using water, ethanol, and their mixtures at varying temperatures and flow rates (10 MPa) achieved up to 20.1 (w/w) yield. Optimal conditions (120 °C, ethanol/H2O 0.5, 2 mL/min) favored recovery of total phenolic (2.130 g GAE ***/100 g), flavonoids (0.778 g CE ****/100 g), and antioxidant activity (9.944 mmol TE/100 g). Water and ethanol/H2O/H2O mixtures outperformed Soxhlet in extracting bioactives [111]. | ||
| Polysaccharides | Hot water extraction of β-glucan from BSG yielded highest concentration and purity at 60 °C for 90 min, followed by ethanol precipitation. Extracted β-glucan displayed water-holding capacity (6.82 g/g) and outperformed oat β-glucan and gum arabic in viscosity and emulsion stability [112]. | β-glucan can be used as a stabilizer and viscosity enhancer in various food products, e.g., in bakery, dairy, fruit juices and meat products, albeit its effectiveness is concentration dependent and must thus be tailored to each application. It can also be used as a fat replacer, e.g., in mayonnaises, with nutritional and sensory benefits [112,113], and play a prebiotic role [114]. Antioxidant activity depicted by β-glucan can potentially be used in (functional) foods, including upon partial depolymerization [115] and cosmetics [114,116], and incorporated in bioactive biofilms to be used as packaging materials in food and pharma [117,118]. |
| Proteins | Proteins were extracted from BSG using PLE with water under near-subcritical conditions (<100 °C, ~1.0 × 107 Pa) and varying NaOH concentrations (0.01 to 0.1 M). Protein yields ranged from 21.3% to 65.3% (dry basis), with the highest yield (66%) from H2SO4-pretreated BSG. Peak protein purity (69.7%) was achieved at 40 °C, 60 min, 0.05 N NaOH. Mild alkaline extraction proved efficient for enriching protein with minimal chemical input [119]. | Upon treatment with proteases, proteins retrieved from BSG depicted high oil-holding capacity, foaming formation capability, and foaming stability [120], as well as high solubility, water absorption, and emulsifying capacity [121], and dense, soft, and stable microstructure with consistent texture during food storage [122]. Overall, these techno-functional features suggest potential for improving food formulations [123,124]. |
| One-stage alkaline extraction of BSG yielded up to 87% protein at pH 11, 60 °C, and 1:17 g/mL solid/solvent ratio. Mild conditions (pH 8) produced protein concentrates with higher purity (>40%), better solubility, and emulsifying properties, while harsher conditions (pH 11–12, 80 °C) enhanced gelling behavior but lowered solubility and protein content (~30%). Temperature and pH of extraction pH strongly influenced both yield and functional properties [125]. | BSG protein hydrolysates produced upon protease treatment have been shown to depict high antioxidant activity, alongside α-amylase and α-glucosidase inhibition [121,126]; the latter enables slow carbohydrate digestion, ultimately reducing postprandial blood glucose spikes [127]. These features highlight the potential of BSG proteins as sources of bioactive peptides that can be used to develop functional foods and nutraceuticals [128]. | |
| Proteins were extracted from BSG using alkali (pH 12), ethanol (55% with 2-mercaptoethanol), and enzymatic methods. Alkaline extraction produced glutelin-rich, partially unfolded proteins with high water holding capacity (2.5–4.0 g/g) and gelation potential. Ethanol extraction yielded hordein-rich, aggregated proteins with gel-forming ability. Enzymatic extraction yielded soluble peptides (<10 kDa) with high emulsifying (83 m2/g) and antioxidant activity but no gelation. Protein structure, solubility, and functionality varied markedly with the extraction method, impacting future applications [129]. | ||
| Ashes | Upon pyrolysis to produce bio-oil and biochar [130,131], ashes, typically rich in Ca, P, K, and S, among other minerals [132], can be recovered by sieving [133]. | Ashes can be used as soil stabilizer [134] or fertilizer [135]. |
| Phenolics and sugars | Supercritical CO2 (sc-CO2) extraction of BSG enables the recovery of phenolic-rich oil and enhanced enzymatic hydrolysis. High pressure and temperature improved phenolic yield and antioxidant capacity. Moreover, sc-CO2 extraction eased the assess of enzymes to polysaccharides and concomitantly led to a 20% increase in sugar release as compared to non-treated BSG [136]. | Develop prebiotic dietary fiber blends, where the oligosaccharide fraction selectively stimulates beneficial gut bacteria (e.g., Bifidobacterium, Lactobacillus), while phenolic compounds convey radical-scavenging and anti-inflammatory properties, without compromising rheology or sensory profile [53,137]. Create active edible films and coatings that are mechanically robust, hinder oxygen diffusion, and release antioxidants over time, extending shelf life of packaged foods [138]. |
| Proteins and bioactive (phenolic) compounds | PSE was used to extract proteins and bioactives from BSG. Under optimal conditions, namely 4.7% ethanol, 155 °C, and 10 min, PSE yielded 36% more protein than ultrasound-assisted alkaline extraction (UAE). A higher ethanol concentration (35%) and extended extraction time (17 min) maximized phenolic content. PSE extracts depicted a higher antioxidant capacity and angiotensin-converting enzyme and cholesterol esterase inhibition ability than UAE. The higher bioactivity of PSE extracts compared to UAE was attributed to a greater proportion of hydrophobic peptides [139]. | Protein and polyphenol conjugates can be used as green emulsifiers and delivery vehicles for lipophilic or sensitive biomolecules. These complexes improve emulsion stability (low droplet coalescence), enhance oxidative stability (low peroxide formation) and enable the controlled release of encapsulated nutraceuticals in foods [140]. The protein fraction from BSG, together with bound phenolic compounds, yields an ingredient with a simultaneous increase in protein, fiber and antioxidant content. Its incorporation of food formulations, e.g., bread, cookies, snacks, may increase the content in protein and total phenolics, improving radical-scavenging capacity without adversely affecting dough handling or final texture [53]. |
| Proteins and polysaccharides | Integrated process developed for sequential extraction of proteins (79–85%) and arabinoxylans (AX, 62–86%) from BSG using increasing concentrations of NaOH or KOH (0.1 to 4.0 M) at room temperature (24 h, 1:2 w/v BSG: alkali). Proteins were selectively precipitated by acidifying extracts to pH 3 with saturated citric acid (food-grade, recyclable); AX were recovered by further HCl acidification (pH < 2) and ethanol precipitation (70% v/v). The method avoids dialysis, reduces process time, and allows recovery of high-purity protein fractions and AX with minimized salt coprecipitation. Recycled solvents were used, stressing the environmental and economic advantages of the method [141]. | Polysaccharides and proteins retrieved from BSG can be used to foster the cultivation of edible fungi, e.g., Neurospora intermedia, within a biorefinery concept [142,143]. |
| Hot Trub Component | Deconstruction Method | Valorization Pathway |
|---|---|---|
| Phenolic compounds | UAE with ethanol as extractor, upon optimization of key operational parameters [150,170] | Use of extracts as food-preservative or nutraceutical ingredients [66,171] |
| A DES, composed of choline chloride and propylene glycol (1:2 mol/mol), was used to retrieve xanthohumol [172] | Use in drug design, given anti-inflammatory, antimicrobial, antioxidant, antihypertension and anticancer activities of spent hops major bioactives, e.g., α- and β-acids, essential-oil terpenes and phenolic compounds [155,157,173,174,175,176] | |
| Proteins | Hydrothermal pre-treatment at 25 °C for 20 min followed by dying [157] | Production of biofilms for the development of active packaging materials [177]. |
| Valorization Route | Comments | References |
|---|---|---|
| Dark fermentation | Anaerobic baffled reactors were used to produce biohydrogen and volatile fatty acids, FAs (e.g., acetate, butyrate, caproate). | [213] |
| Mixed cultures | The microalga Tribonema aequale was co-cultured with native bacteria to effectively remove major nutrients. The treated water met discharge standards, and the resulting biomass contained valuable compounds, e.g., chrysolaminarin, palmitoleic acid, and eicosapentaenoic acid, with potential commercial applications. | [214] |
| Chain elongation to produce caproate without compromising waste management. | [215] | |
| Polyhydroxyalkanoates were successfully produced with similar yields from anaerobically treated and acidified streams, namely under acetate pulse feeding. Favors production of bioplastics. | [216] | |
| Microalgae cultivation | Arthrospira platensis was effectively used for nutrient recovery, CO2 capture, and production of pigments, fatty acids, and biogas. | [217] |
| Nutrient recovery | Controlled struvite recovery using a crystallization reactor proved economically viable. Fertilizer shortages are addressed, and eutrophication is mitigated. | [218] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, P.C.B.; Silva, J. Brewing By-Products: Source, Nature, and Handling in the Dawn of a Circular Economy Age. Biomass 2025, 5, 49. https://doi.org/10.3390/biomass5030049
Fernandes PCB, Silva J. Brewing By-Products: Source, Nature, and Handling in the Dawn of a Circular Economy Age. Biomass. 2025; 5(3):49. https://doi.org/10.3390/biomass5030049
Chicago/Turabian StyleFernandes, Pedro C. B., and Joaquim Silva. 2025. "Brewing By-Products: Source, Nature, and Handling in the Dawn of a Circular Economy Age" Biomass 5, no. 3: 49. https://doi.org/10.3390/biomass5030049
APA StyleFernandes, P. C. B., & Silva, J. (2025). Brewing By-Products: Source, Nature, and Handling in the Dawn of a Circular Economy Age. Biomass, 5(3), 49. https://doi.org/10.3390/biomass5030049







