Modified Hydrothermal Pretreatment Conditions Enhance Alcohol Solubility of Lignin from Wheat Straw Biorefining
Abstract
1. Introduction
2. Materials and Methods
2.1. Pretreatments in PARR Reactor
2.2. Enzymatic Saccharification
2.3. Washing of Solids After Enzymatic Saccharification
2.4. Analysis of Dissolved Monosaccharides
2.5. Determination of Structural Carbohydrates and Lignin
2.6. Ash Content Analysis
2.7. ATR-FTIR
2.8. Alcohol Solubility
2.9. Statistical Analysis
3. Results and Discussions
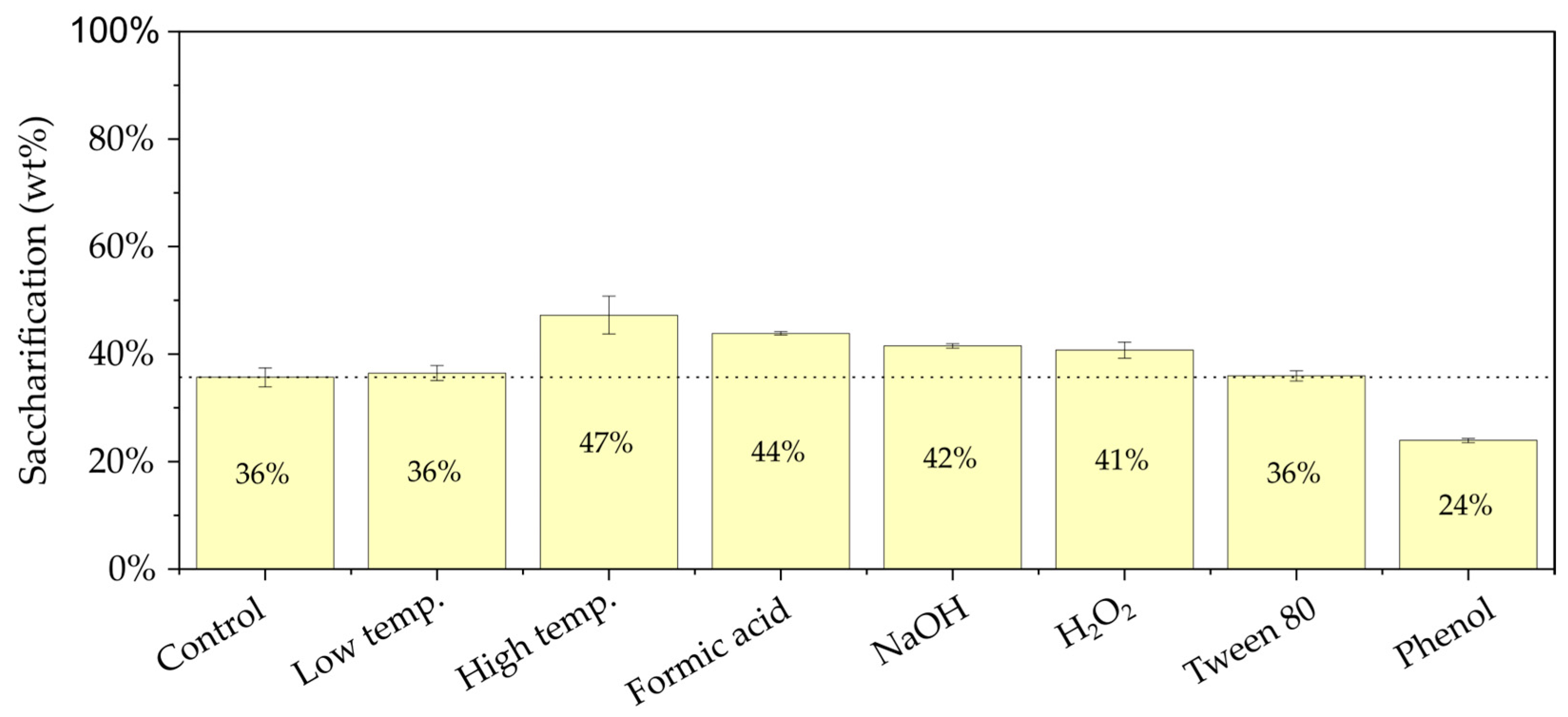
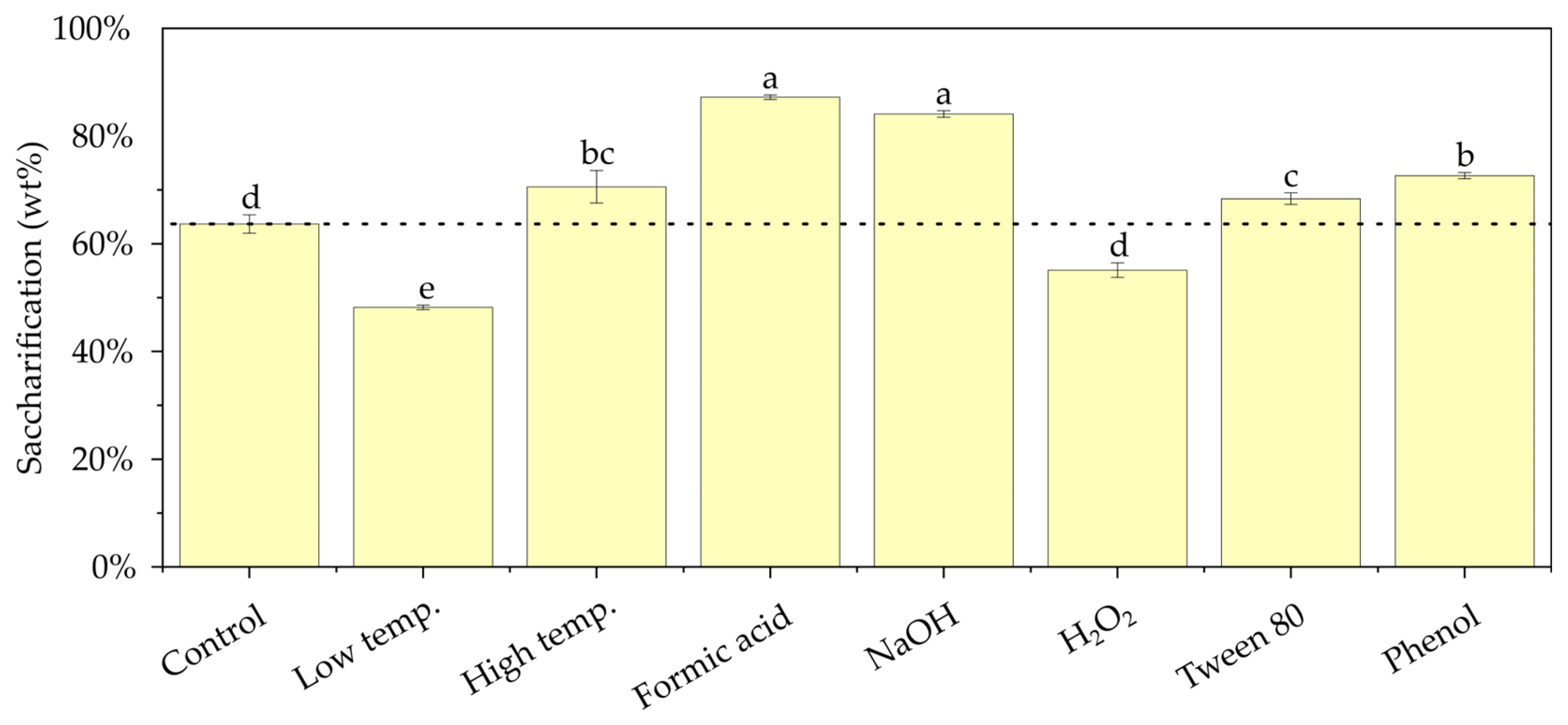
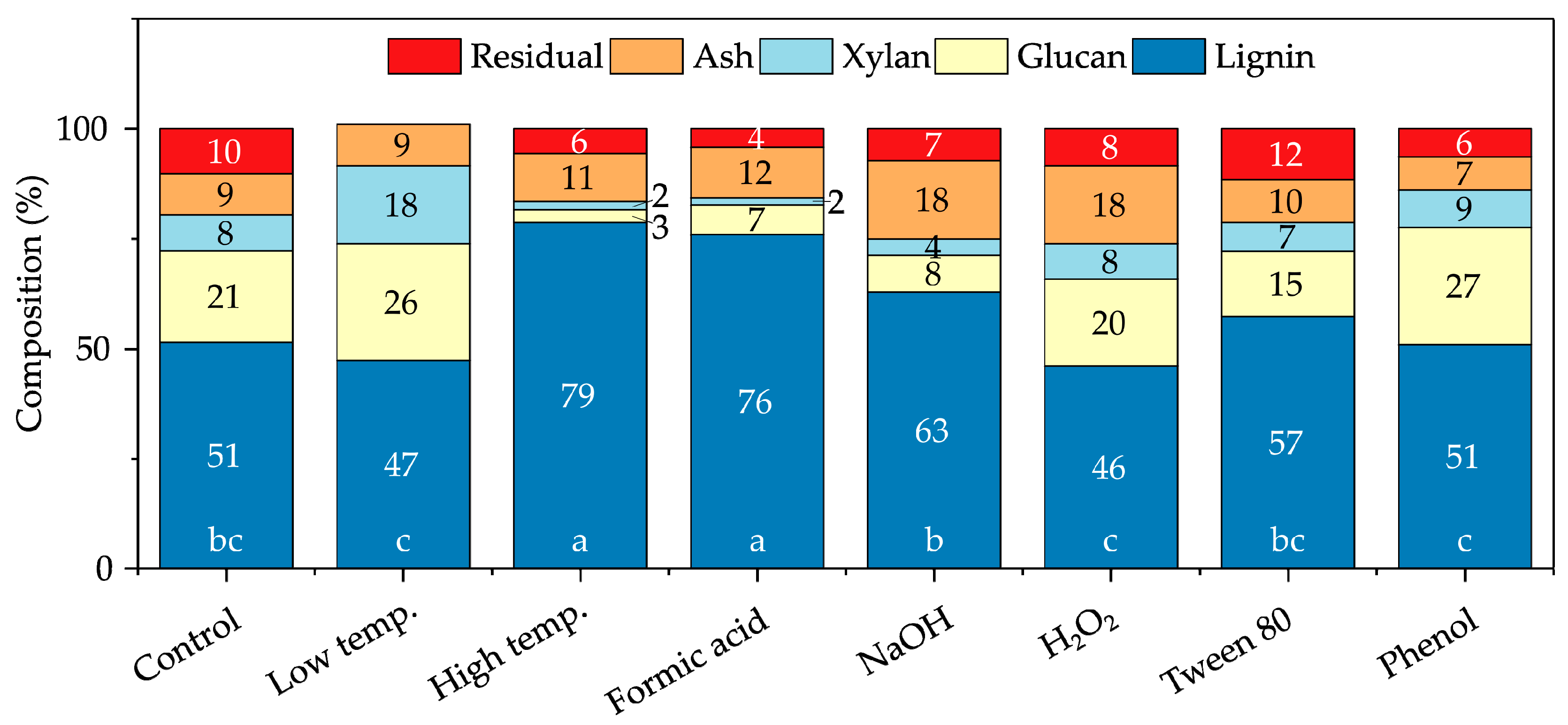
3.1. Effects of Pretreatment Modifications on Enzymatic Saccharification Yield
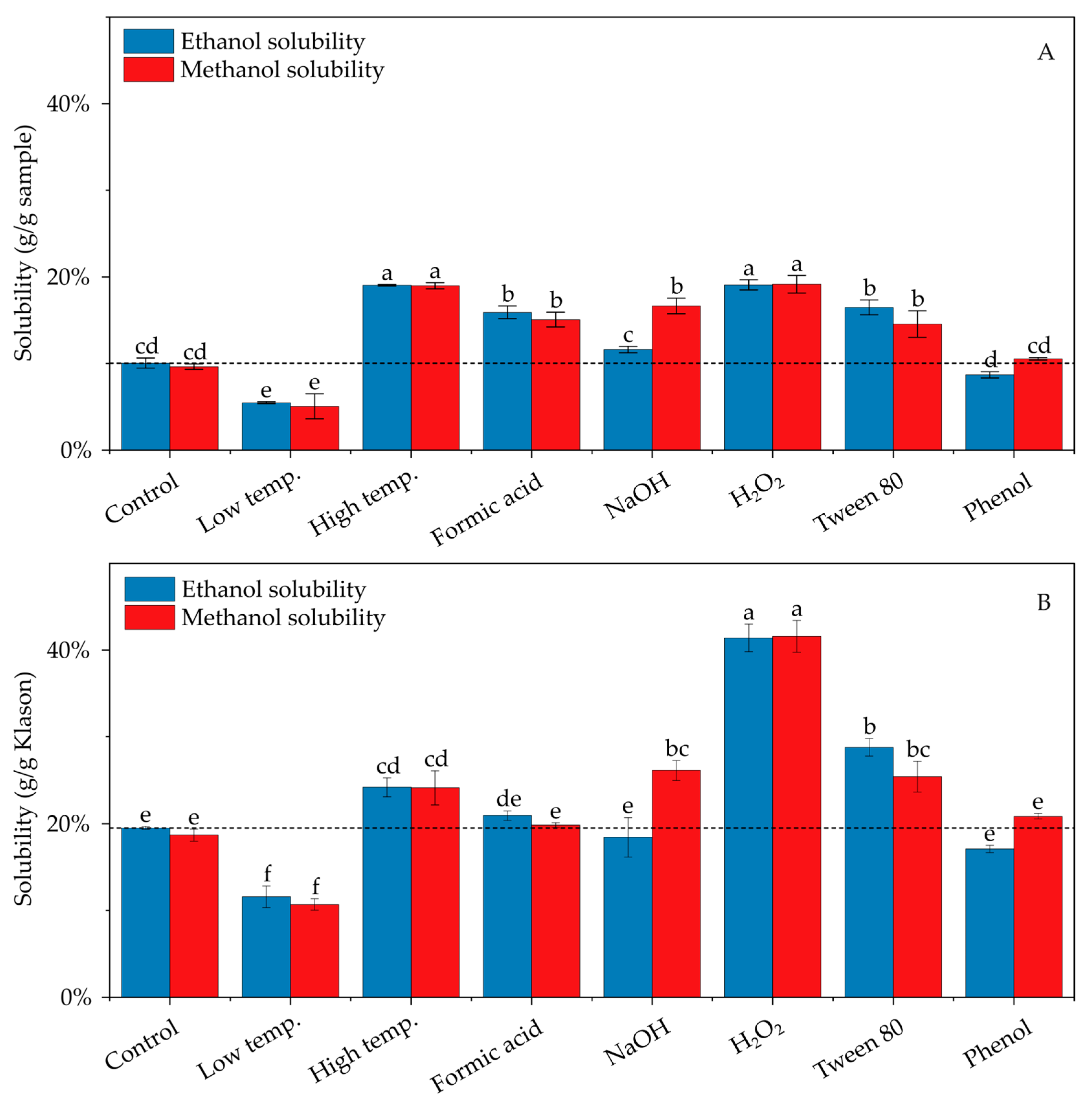
3.2. Effects of Pretreatment Modifications on Alcohol Solubility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALS | Asymmetric Least Squares Smoothing |
| ATR-FTIR | attenuated total reflectance-Fourier transform infrared |
| HPLC | High-Performance Liquid Chromatography |
| HTP | hydrothermal pretreatment |
| LCC | lignin–carbohydrate complex |
Appendix A
Appendix A.1. FTIR Spectra
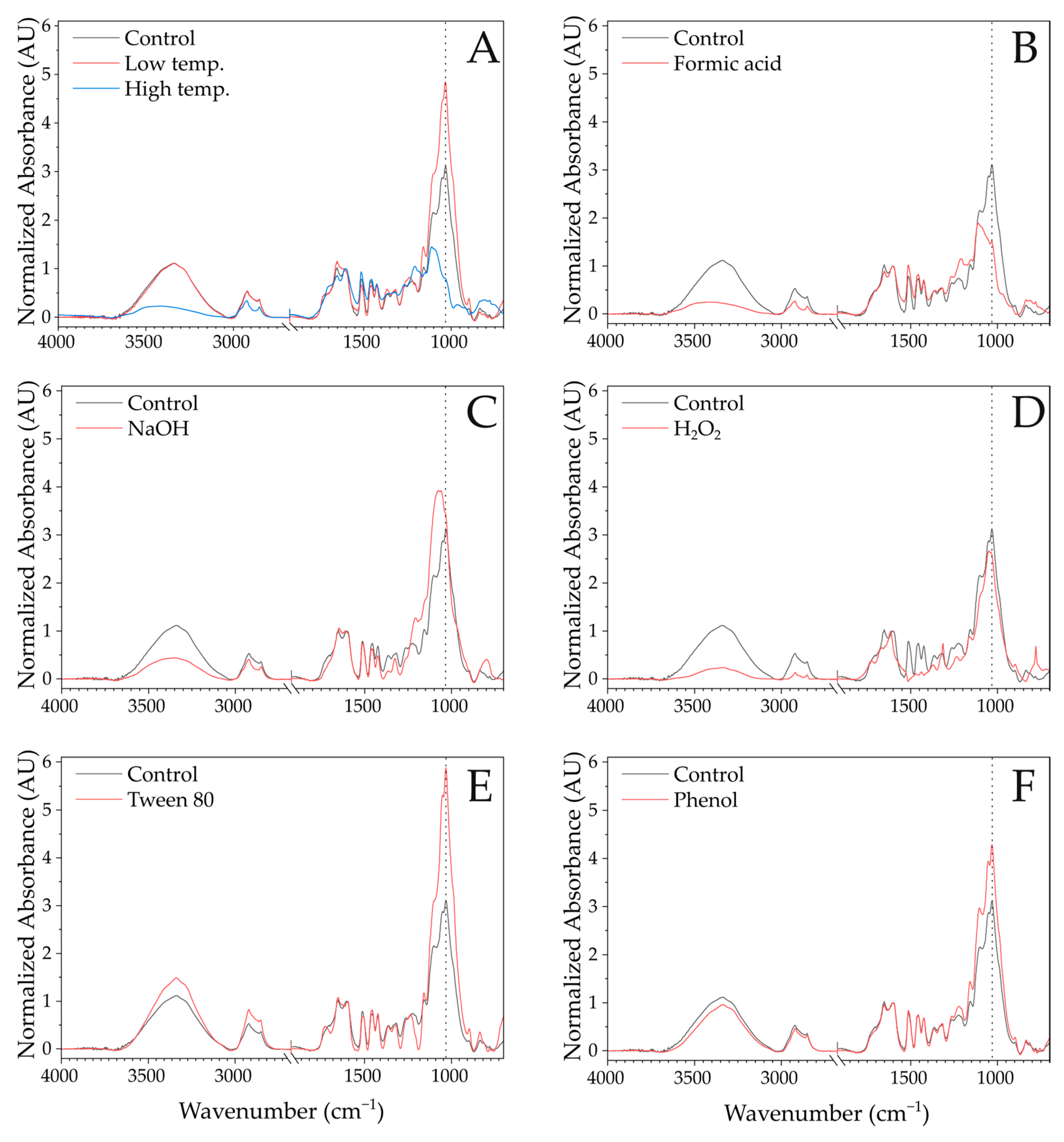
Appendix A.2. Enzymatic Saccharification Yields

Appendix A.3. Enzymatic Saccharification Yields of Glucan After 3 Days
References
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, O.Y. Lignin Composition. WO2022/117391, 9 June 2022. [Google Scholar]
- Simonsen, T.I.; Djajadi, D.T.; Montanvert, H.; Sgarzi, M.; Gigli, M.; Thomsen, S.T.; Cabrera Orozco, Y.; Crestini, C. Improving the production efficiency and sustainability of lignin-alcohol fuel processed at ambient temperature. Bioresour. Technol. 2024, 408, 131087. [Google Scholar] [CrossRef]
- Huo, L.; Lu, Y.; Ding, W.-L.; Wang, Y.; Li, X.; He, H. Recent advances in the preparation, properties, and applications of lignin-based hydrogels and adhesives. Green Chem. 2025, 27, 1895–1908. [Google Scholar] [CrossRef]
- Thybring, E.E.; Thomsen, S.T.; Ponzecchi, A.; Simonsen, T.I. Method for Impregnating Wood with a Solution Comprising Lignin or Derivatives Thereof. Patent WO2025031742, 13 February 2025. [Google Scholar]
- Gigli, M.; Crestini, C. Fractionation of industrial lignins: Opportunities and challenges. Green Chem. 2020, 22, 4722–4746. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Lange, H.; Ago, M.; Crestini, C. Understanding lignin aggregation processes. A case study: Budesonide entrapment and stimuli controlled release from lignin nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 9342–9351. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sampedro, R.; Santos, J.I.; Fillat, Ú.; Wicklein, B.; Eugenio, M.E.; Ibarra, D. Characterization of lignins from Populus alba L. generated as by-products in different transformation processes: Kraft pulping, organosolv and acid hydrolysis. Int. J. Biol. Macromol. 2019, 126, 18–29. [Google Scholar] [CrossRef]
- Santos, J.I.; Fillat, Ú.; Martín-Sampedro, R.; Eugenio, M.E.; Negro, M.J.; Ballesteros, I.; Rodríguez, A.; Ibarra, D. Evaluation of lignins from side-streams generated in an olive tree pruning-based biorefinery: Bioethanol production and alkaline pulping. Int. J. Biol. Macromol. 2017, 105, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Wörmeyer, K.; Ingram, T.; Saake, B.; Brunner, G.; Smirnova, I. Comparison of different pretreatment methods for lignocellulosic materials. Part II: Influence of pretreatment on the properties of rye straw lignin. Bioresour. Technol. 2011, 102, 4157–4164. [Google Scholar] [CrossRef]
- Zhao, C.; Huang, J.; Yang, L.; Yue, F.; Lu, F. Revealing structural differences between alkaline and Kraft lignins by HSQC NMR. Ind. Eng. Chem. Res. 2019, 58, 5707–5714. [Google Scholar] [CrossRef]
- Simonsen, T.I. Lignin-Alcohol Dispersions—A Promising Marine Biofuel. Ph.D. Thesis, University of Copenhagen, Copenhagen, Denmark, 26 June 2024. [Google Scholar]
- Chiaramonti, D.; Prussi, M.; Ferrero, S.; Oriani, L.; Ottonello, P.; Torre, P.; Cherchi, F. Review of pretreatment processes for lignocellulosic ethanol production, and development of an innovative method. Biomass Bioenergy 2012, 46, 25–35. [Google Scholar] [CrossRef]
- Larsen, J.; Østergaard Petersen, M.; Thirup, L.; Wen Li, H.; Krogh Iversen, F. The IBUS Process—Lignocellulosic Bioethanol Close to a Commercial Reality. Chem. Eng. Technol. 2008, 31, 765–772. [Google Scholar] [CrossRef]
- Tomás-Pejó, E.; Alvira, P.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for lignocellulose-to-bioethanol conversion. In Biofuels; Elsevier: Amsterdam, The Netherlands, 2011; pp. 149–176. [Google Scholar]
- Pu, Y.; Hu, F.; Huang, F.; Ragauskas, A.J. Lignin Structural Alterations in Thermochemical Pretreatments with Limited Delignification. Bioenergy Res. 2015, 8, 992–1003. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Rahikainen, J.; Leskinen, T.; Pihlajaniemi, V.; Mattinen, M.L.; Lange, H.; Crestini, C.; Österberg, M.O. Structural changes of lignin in biorefinery pretreatments and consequences to enzyme-lignin interactions. Nord. Pulp Pap. Res. J. 2017, 32, 550–571. [Google Scholar] [CrossRef]
- Djajadi, D.T.; Hansen, A.R.; Jensen, A.; Thygesen, L.G.; Pinelo, M.; Meyer, A.S.; Jørgensen, H. Surface properties correlate to the digestibility of hydrothermally pretreated lignocellulosic Poaceae biomass feedstocks. Biotechnol. Biofuels 2017, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Silveira, R.L.; Stoyanov, S.R.; Gusarov, S.; Skaf, M.S.; Kovalenko, A. Supramolecular interactions in secondary plant cell walls: Effect of lignin chemical composition revealed with the molecular theory of solvation. J. Phys. Chem. Lett. 2015, 6, 206–211. [Google Scholar] [CrossRef]
- Ralph, J.; Bunzel, M.; Marita, J.M.; Hatfield, R.D.; Lu, F.; Kim, H.; Schatz, P.F.; Grabber, J.H.; Steinhart, H. Peroxidase-dependent cross-linking reactions of p-hydroxycinnamates in plant cell walls. Phytochem. Rev. 2004, 3, 79–96. [Google Scholar] [CrossRef]
- Zhao, Y.; Shakeel, U.; Saif Ur Rehman, M.; Li, H.; Xu, X.; Xu, J. Lignin-carbohydrate complexes (LCCs) and its role in biorefinery. J. Clean Prod. 2020, 253, 120076. [Google Scholar] [CrossRef]
- Wyman, C.E. Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 978-0470972021. [Google Scholar]
- Jensen, A.; Cabrera, Y.; Hsieh, C.-W.; Nielsen, J.; Ralph, J.; Felby, C. 2D NMR characterization of wheat straw residual lignin after dilute acid pretreatment with different severities. Holzforschung 2017, 71, 461–469. [Google Scholar] [CrossRef]
- Das, P.; Stoffel, R.B.; Area, M.C.; Ragauskas, A.J. Effects of one-step alkaline and two-step alkaline/dilute acid and alkaline/steam explosion pretreatments on the structure of isolated pine lignin. Biomass Bioenergy 2019, 120, 350–358. [Google Scholar] [CrossRef]
- Li, M.; Si, S.; Hao, B.; Zha, Y.; Wan, C.; Hong, S.; Kang, Y.; Jia, J.; Zhang, J.; Li, M.; et al. Mild alkali-pretreatment effectively extracts guaiacyl-rich lignin for high lignocellulose digestibility coupled with largely diminishing yeast fermentation inhibitors in Miscanthus. Bioresour. Technol. 2014, 169, 447–454. [Google Scholar] [CrossRef]
- Min, D.; Wei, L.; Zhao, T.; Li, M.; Jia, Z.; Wan, G.; Zhang, Q.; Qin, C.; Wang, S. Combination of hydrothermal pretreatment and sodium hydroxide post-treatment applied on wheat straw for enhancing its enzymatic hydrolysis. Cellulose 2018, 25, 1197–1206. [Google Scholar] [CrossRef]
- Pandey, A.K.; Negi, S. Impact of surfactant assisted acid and alkali pretreatment on lignocellulosic structure of pine foliage and optimization of its saccharification parameters using response surface methodology. Bioresour. Technol. 2015, 192, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Martín, C.; Klinke, H.B.; Thomsen, A.B. Wet oxidation as a pretreatment method for enhancing the enzymatic convertibility of sugarcane bagasse. Enzyme Microb. Technol. 2007, 40, 426–432. [Google Scholar] [CrossRef]
- Abu-Omar, M.M.; Barta, K.; Beckham, G.T.; Luterbacher, J.S.; Ralph, J.; Rinaldi, R.; Romań-Leshkov, Y.; Samec, J.S.M.; Sels, B.F.; Wang, F. Guidelines for performing lignin-first biorefining. Energy Environ. Sci. 2021, 14, 262–292. [Google Scholar] [CrossRef]
- Gong, C.; Bryant, N.; Meng, X.; Bhagia, S.; Pu, Y.; Xin, D.; Bender Koch, C.; Felby, C.; Thygesen, L.G.; Ragauskas, A.; et al. Double bonus: Surfactant-assisted biomass pelleting benefits both the pelleting process and subsequent enzymatic saccharification of the pretreated pellets. Green Chem. 2021, 23, 1050–1061. [Google Scholar] [CrossRef]
- Cao, S.; Aita, G.M. Enzymatic hydrolysis and ethanol yields of combined surfactant and dilute ammonia treated sugarcane bagasse. Bioresour. Technol. 2013, 131, 357–364. [Google Scholar] [CrossRef] [PubMed]
- da Costa Nogueira, C.; de Araújo Padilha, C.E.; de Sá Leitão, A.L.; Rocha, P.M.; de Macedo, G.R.; dos Santos, E.S. Enhancing enzymatic hydrolysis of green coconut fiber—Pretreatment assisted by tween 80 and water effect on the post-washing. Ind. Crops Prod. 2018, 112, 734–740. [Google Scholar] [CrossRef]
- Qing, Q.; Yang, B.; Wyman, C.E. Impact of surfactants on pretreatment of corn stover. Bioresour. Technol. 2010, 101, 5941–5951. [Google Scholar] [CrossRef]
- Shen, X.-J.; Wang, B.; Huang, P.-L.; Wen, J.-L.; Sun, R.-C. Effects of aluminum chloride-catalyzed hydrothermal pretreatment on the structural characteristics of lignin and enzymatic hydrolysis. Bioresour. Technol. 2016, 206, 57–64. [Google Scholar] [CrossRef]
- Yan, X.; Cheng, J.-R.; Wang, Y.-T.; Zhu, M.-J. Enhanced lignin removal and enzymolysis efficiency of grass waste by hydrogen peroxide synergized dilute alkali pretreatment. Bioresour. Technol. 2020, 301, 122756. [Google Scholar] [CrossRef]
- Ahlbom, A.; Maschietti, M.; Nielsen, R.; Hasani, M.; Theliander, H. Using guaiacol as a capping agent in the hydrothermal depolymerisation of kraft lignin. Nord. Pulp Pap. Res. J. 2023, 38, 619–631. [Google Scholar] [CrossRef]
- Ahlbom, A.; Maschietti, M.; Nielsen, R.; Hasani, M.; Theliander, H. On the hydrothermal depolymerisation of kraft lignin using glycerol as a capping agent. Holzforschung 2023, 77, 159–169. [Google Scholar] [CrossRef]
- Moure, A.; Garrote, G.; Domínguez, H. Effect of Hydrothermal Pretreatment on Lignin and Antioxidant Activity. In Hydrothermal Processing in Biorefineries; Springer International Publishing: Cham, Switzerland, 2017; pp. 5–43. [Google Scholar]
- Özyürek, Ö.; van Heiningen, A. Formic acid reinforced autohydrolysis of wheat straw for high yield production of monosugars and minimal lignin precipitation. Ind. Crops Prod. 2018, 112, 320–326. [Google Scholar] [CrossRef]
- Thomsen, M.H.; Thygesen, A.; Jørgensen, H.; Larsen, J.; Christensen, B.H.; Thomsen, A.B. Preliminary results on optimization of pilot scale pretreatment of wheat straw used in coproduction of bioethanol and electricity. Appl. Biochem. Biotechnol. 2006, 130, 448–460. [Google Scholar] [CrossRef]
- Zhang, H.; Lopez, P.C.; Holland, C.; Lunde, A.; Ambye-Jensen, M.; Felby, C.; Thomsen, S.T. The multi-feedstock biorefinery—Assessing the compatibility of alternative feedstocks in a 2G wheat straw biorefinery process. GCB Bioenergy 2018, 10, 946–959. [Google Scholar] [CrossRef]
- Thomsen, S.T.; Weiss, N.D.; Zhang, H.; Felby, C. Water retention value predicts biomass recalcitrance for pretreated biomass: Biomass water interactions vary based on pretreatment chemistry and reflect composition. Cellulose 2021, 28, 317–330. [Google Scholar] [CrossRef]
- Sluiter, J.B.; Ruiz, R.O.; Scarlata, C.J.; Sluiter, A.D.; Templeton, D.W. Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods. J. Agric. Food Chem. 2010, 58, 9043–9053. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, U.P. An Overview of Raman Spectroscopy as Applied to Lignocellulosic Materials. In Advances in Lignocellulosics Characterization; TAPPI Press: Atlanta, GA, USA, 1999; pp. 201–225. [Google Scholar]
- Larsen, K.L.; Barsberg, S. Theoretical and Raman Spectroscopic Studies of Phenolic Lignin Model Monomers. J. Phys. Chem. B 2010, 114, 8009–8021. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, L.; Niu, Z.; Wu, R.; Wang, G. Effect of hydrothermal pretreatment of corn stover with pH adjustment on properties of pulp and hydrolysate. BioResources 2020, 15, 6826–6839. [Google Scholar] [CrossRef]
- Rasmussen, H.; Sørensen, H.R.; Meyer, A.S. Formation of degradation compounds from lignocellulosic biomass in the biorefinery: Sugar reaction mechanisms. Carbohydr. Res. 2014, 385, 45–57. [Google Scholar] [CrossRef]
- Thomsen, S.T.; Jensen, M.; Schmidt, J.E. Production of 2nd generation bioethanol from lucerne—Optimization of hydrothermal pretreatment. BioResources 2012, 7, 1582–1593. [Google Scholar] [CrossRef]
- Trajano, H.L.; Engle, N.L.; Foston, M.; Ragauskas, A.J.; Tschaplinski, T.J.; Wyman, C.E. The fate of lignin during hydrothermal pretreatment. Biotechnol. Biofuels 2013, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Wiley, J.H.; Atalla, R.H. Band assignments in the Raman spectra of celluloses. Carbohydr. Res. 1987, 160, 113–129. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Fiţigău, F.I.; Gosselink, R.J.A.; Frissen, A.E.; Stoutjesdijk, J.; Peter, F. Fractionation of five technical lignins by selective extraction in green solvents and characterisation of isolated fractions. Ind. Crops Prod. 2014, 62, 481–490. [Google Scholar] [CrossRef]
- Gosselink, R.J.A.; van Dam, J.E.G.; de Jong, E.; Scott, E.L.; Sanders, J.P.M.; Li, J.; Gellerstedt, G. Fractionation, analysis, and PCA modeling of properties of four technical lignins for prediction of their application potential in binders. Holzforschung 2010, 64, 193–200. [Google Scholar] [CrossRef]
- Mörck, R.; Reimann, A.; Kringstad, K.P. Fractionation of Kraft Lignin by Successive Extraction with Organic Solvents. III. Fractionation of Kraft Lignin from Birch. Holzforschung 1988, 42, 111–116. [Google Scholar] [CrossRef]
- Thring, R.W.; Vanderlaan, M.N.; Griffin, S.L. Fractionation of Alcell® Lignin by Sequential Solvent Extraction. III. Fractionation of Kraft Lignin from Birch. J. Wood Chem. Technol. 1996, 16, 139–154. [Google Scholar] [CrossRef]
- Wang, K.; Xu, F.; Sun, R. Molecular characteristics of Kraft-AQ pulping lignin fractionated by sequential organic solvent extraction. Int. J. Mol. Sci. 2010, 11, 2988–3001. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Li, M.; Wyman, C.E.; Cai, C.M.; Ragauskas, A.J. Fast fractionation of technical lignins by organic cosolvents. ACS Sustain. Chem. Eng. 2018, 6, 6064–6072. [Google Scholar] [CrossRef]
- Kadla, J.; Chang, H. The Reactions of Peroxides with Lignin and Lignin Model Compounds. In Oxidative Delignification Chemistry—Fundamentals and Catalysis; Argyropoulos, D.S., Ed.; ACS Publications: Washington, DC, USA, 2001; pp. 108–128. [Google Scholar]
- Werhan, H.; Mir, J.M.; Voitl, T.; Rudolf von Rohr, P. Acidic oxidation of kraft lignin into aromatic monomers catalyzed by transition metal salts. Holzforschung 2011, 65, 703–709. [Google Scholar] [CrossRef]
| Name | Temperature | Additive | Effect |
|---|---|---|---|
| Control | 190 °C | - | Control |
| Low temp. | 170 °C | - | Reduced temperature |
| High temp. | 210 °C | - | Increased temperature |
| Formic acid [38] | 190 °C | 0.1 M Formic acid | Acid |
| NaOH | 190 °C | 1% (w/v) NaOH | Alkaline |
| H2O2 [39] | 190 °C | 5% (w/w) H2O2 | Oxidant |
| Tween 80 [32] | 190 °C | 3% (w/w) Tween 80 * | Surfactant |
| Phenol | 190 °C | 1% (w/v) Phenol | Capping agent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonsen, T.I.; Djajadi, D.T.; Thomsen, S.T. Modified Hydrothermal Pretreatment Conditions Enhance Alcohol Solubility of Lignin from Wheat Straw Biorefining. Biomass 2025, 5, 23. https://doi.org/10.3390/biomass5020023
Simonsen TI, Djajadi DT, Thomsen ST. Modified Hydrothermal Pretreatment Conditions Enhance Alcohol Solubility of Lignin from Wheat Straw Biorefining. Biomass. 2025; 5(2):23. https://doi.org/10.3390/biomass5020023
Chicago/Turabian StyleSimonsen, Tor Ivan, Demi Tristan Djajadi, and Sune Tjalfe Thomsen. 2025. "Modified Hydrothermal Pretreatment Conditions Enhance Alcohol Solubility of Lignin from Wheat Straw Biorefining" Biomass 5, no. 2: 23. https://doi.org/10.3390/biomass5020023
APA StyleSimonsen, T. I., Djajadi, D. T., & Thomsen, S. T. (2025). Modified Hydrothermal Pretreatment Conditions Enhance Alcohol Solubility of Lignin from Wheat Straw Biorefining. Biomass, 5(2), 23. https://doi.org/10.3390/biomass5020023







