A Review of the Sustainability, Chemical Composition, Bioactive Compounds, Antioxidant and Antidiabetic Activity, Neuroprotective Properties, and Health Benefits of Microalgae
Abstract
1. Introduction
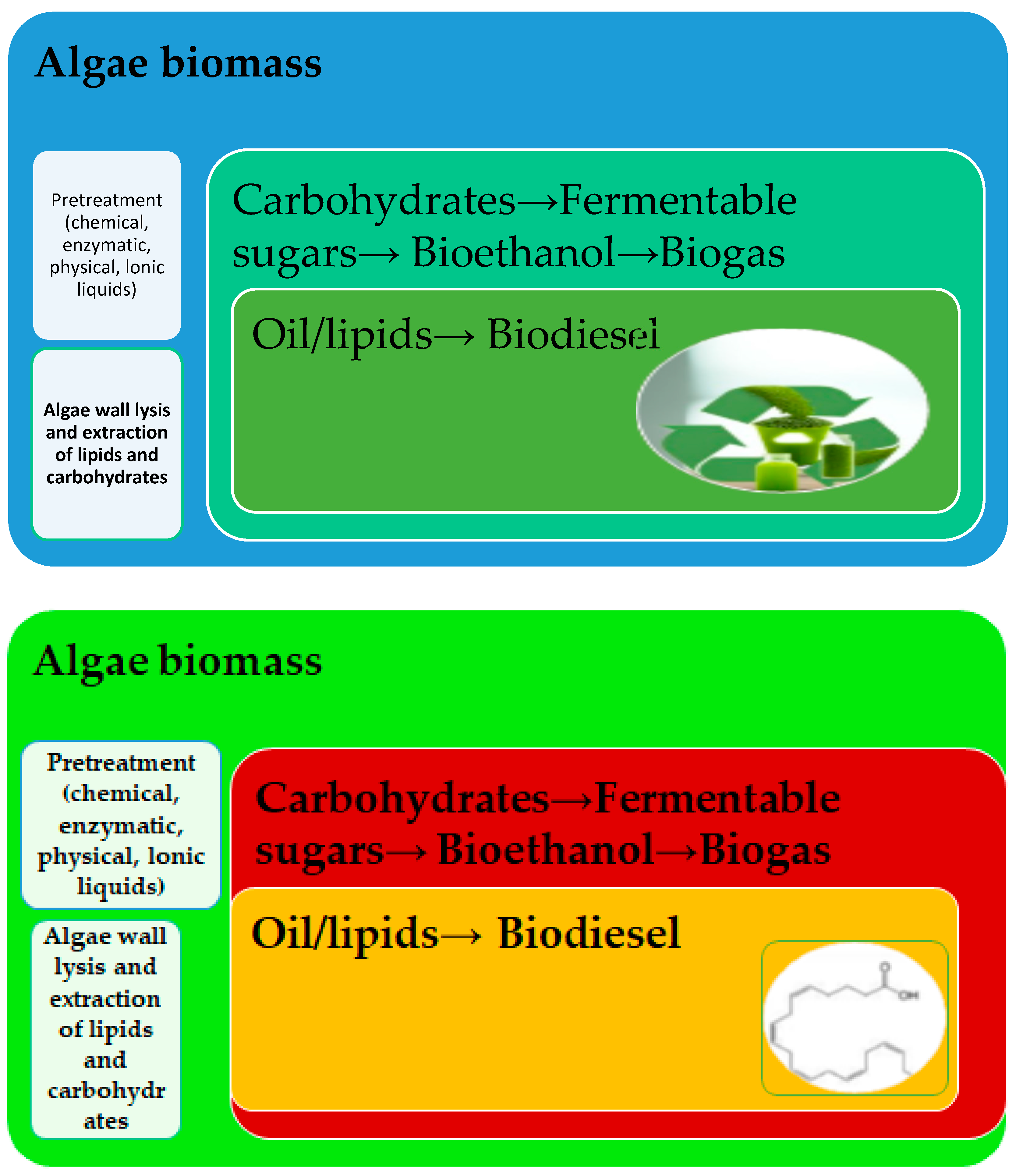
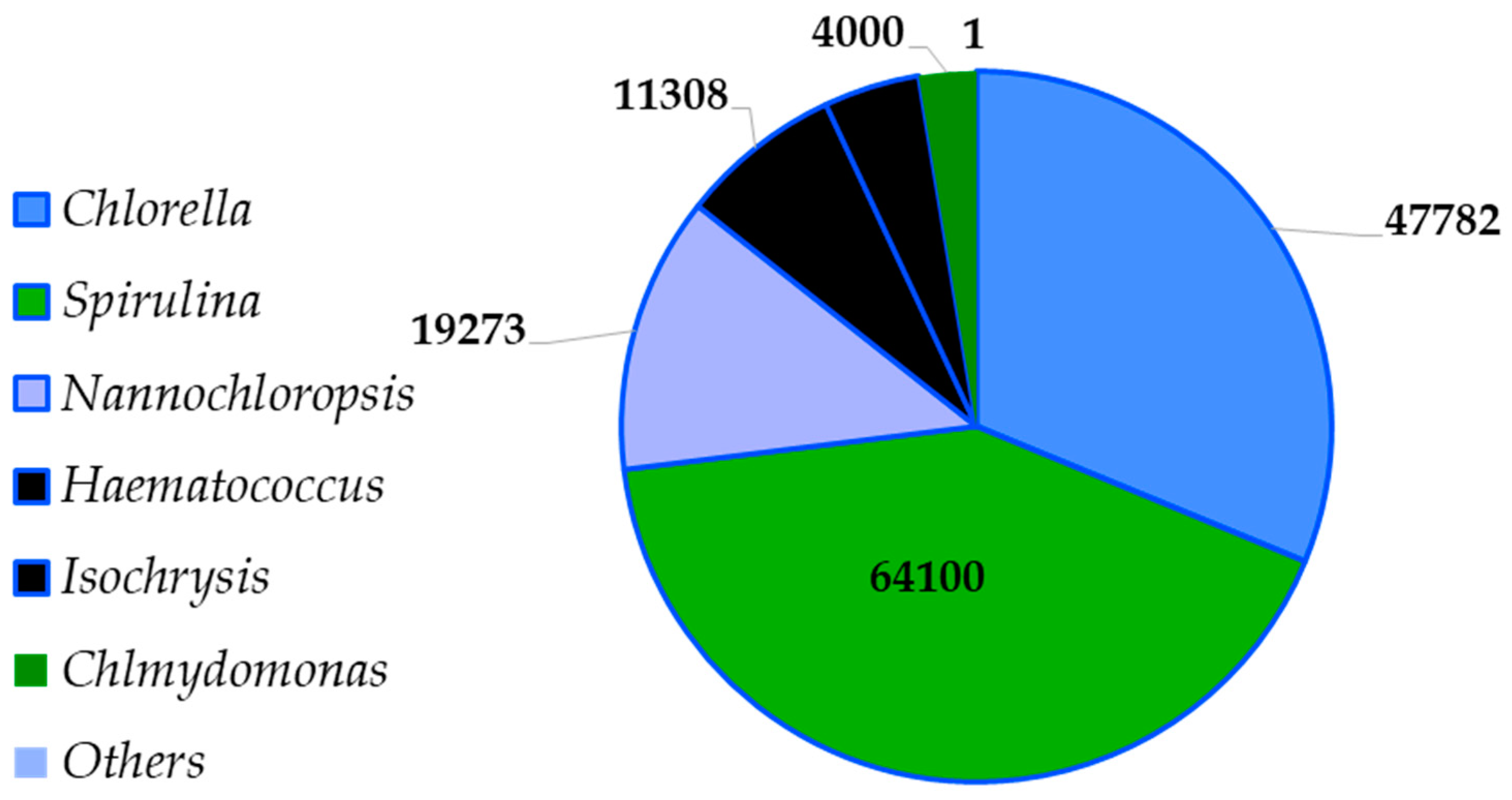
2. Sustainability
2.1. Chlorella
2.2. Spirulina
3. Chemical Composition and Bioactive Compounds of Microalgae
| Species | % Protein (w/w) | % Carbohydrates (w/w) | % Fat (w/w) | References |
|---|---|---|---|---|
| Botryococcus braunii | 39–40 | 19–31 | 25–34 | [41] |
| Chaetoceros calcitrans | 36 | 27 | 15 | [42] |
| Chaetoceros gracilis | 12 | 4.7 | 7.2 | [43] |
| Chaetoceros muelleri | 59 | 10 | 31 | [44] |
| Chlamydomonas rheinhardii | 48 | 17 | 21 | [45] |
| Chlorella vulgaris | 51–58 | 12–17 | 14–22 | [46] |
| Chlorella pyrenoidosa | 57 | 26 | 2 | [42] |
| Diacronema vlkianum | 57 | 32 | 6 | [47] |
| Dunaliella primolecta | 12 | - | - | [48] |
| Dunaliela salina | 57 | 32 | 6 | [42] |
| Dunaliella sp. | 34.17 | 14.57 | 14.36 | [49] |
| Dunaliella tertiolecta | 11 | - | - | [50] |
| Euglena gracilis | 39–61 | 14–18 | 22–38 | [42] |
| Haematococcus pluvialis | 48 | 27 | 15 | [47] |
| Isochrysis galbana | 50–56 | 10–17 | 12–14 | [42] |
| Nannochloropsis granulata | 18–34 | 27–36 | 24–28 | [41] |
| Nannochloropsis sp. | 30 | 10 | 22 | [49] |
| Nitzschia closterium | 26 | 9.8 | 13 | [43] |
| Pavlova sp. | 24–29 | 6–9 | 9–14 | [43,46] |
| Phaeodactylum tricornutum | 34.8 | 16.8 | 16.1 | [41,43] |
| Porphyridium cruentum | 28–39 | 40–57 | 9–14 | [42] |
| Prymnesium parvum | 28–45 | 25–33 | 22–38 | [51] |
| Scenedesmus dimorphus | 8–18 | 21–52 | 16–40 | [42] |
| Scenedesmus obliquus | 50–56 | 10–17 | 12–14 | [45,52] |
| Scenedesmus quadricauda | 47 | 21–52 | 1.9 | [51] |
| Scenedesmus sp. | 31 | 28 | 15 | [49] |
| Schizochytrium sp. | - | - | 50–77 | [53] |
| Skeletonema costatum | 25 | 4.6 | 10 | [43] |
| Spirogyra sp. | 6–20 | 33–64 | 11–21 | [51] |
| Spirulina maxima | 60–71 | 13–16 | 6–7 | [42] |
| Spirulina platensis | 46–63 | 8–14 | 4–9 | [42] |
| Synechococcus sp. | 63 | 15 | 11 | [45] |
| Tetraselmis chuii | 31–46 | 25 | 12 | [41,43] |
| Tetraselmis maculata | 52 | 15 | 3 | [51] |
| Tetraselmis sp. | 36 | 24 | - | [54] |
| Thalassiosira pseudonana | 34 | 8.8 | 19 | [43] |
3.1. Proteins
3.2. Fatty Acids
3.3. Carbohydrates and Polysaccharides
3.4. Pigments
3.5. Phenolic Compounds
4. Medicinal and Biological Properties
4.1. Antioxidant Activity
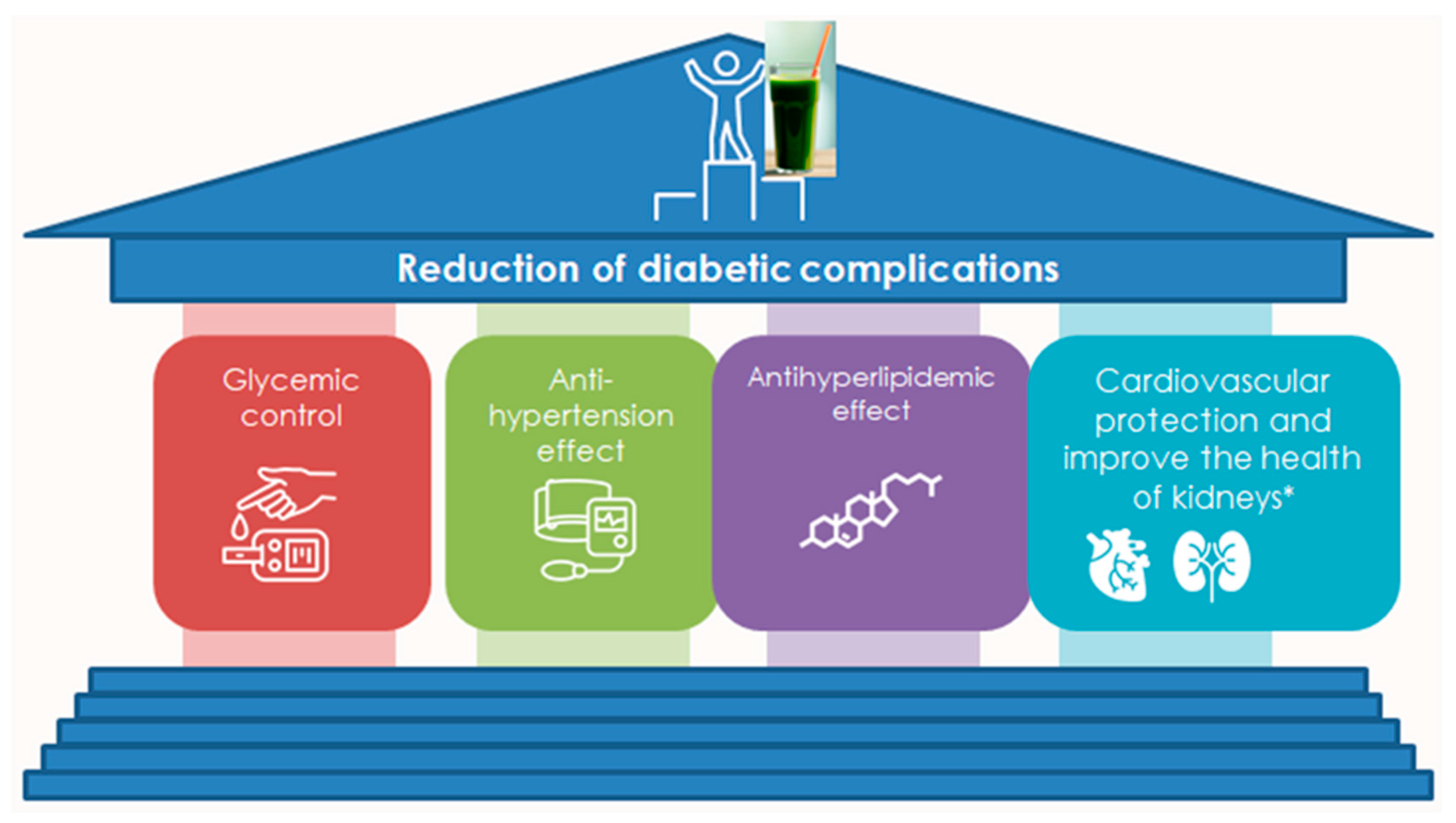
4.2. Antidiabetic Activity
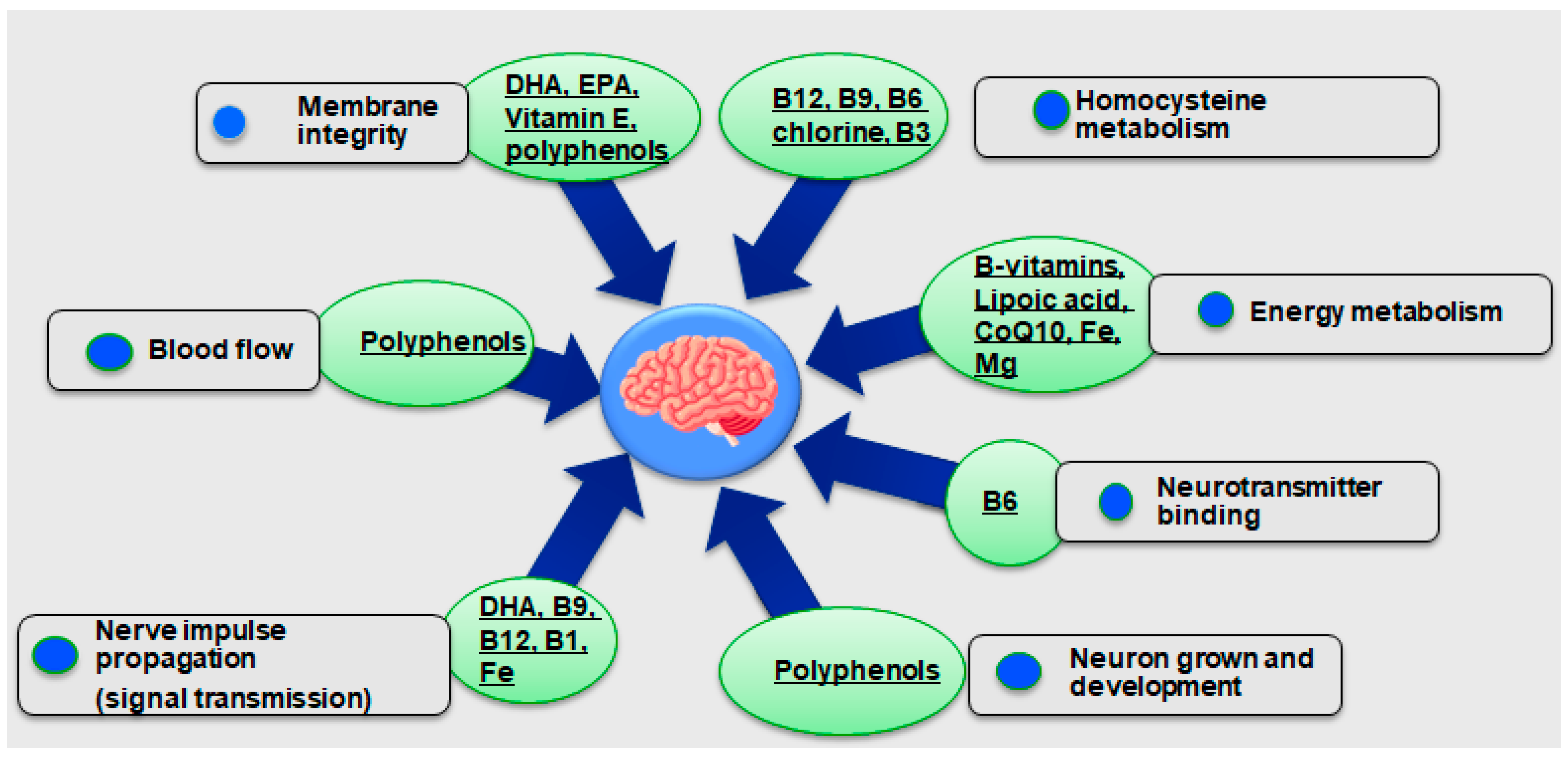
4.3. Neuroprotective Properties
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Khan, M.; Salman, M.; Ansari, J.; Bashir, U.; Malik, M.S.; Ikram, A. Joint external evaluation of IHR core capacities of the Islamic Republic of Pakistan 2016. Int. J. Infect. Dis. 2018, 73, 36–37. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Khavari, F.; Saidijam, M.; Taheri, M.; Nouri, F. Microalgae: Therapeutic potentials and applications. Mol. Biol. Rep. 2021, 48, 4757–4765. [Google Scholar] [CrossRef]
- Parameswari, R.P.; Lakshmi, T. Microalgae as a potential therapeutic drug candidate for neurodegenerative diseases. J. Biotechnol. 2022, 358, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A Promising Source of Valuable Bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products Towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.W.; Johnson, M.D.; Zhang, X.; Zemke, P.; Chen, W.; Hu, Q. A financial assessment of two alternative cultivation systems and their contributions to algae biofuel economic viability. Algal Res. 2014, 4, 96–104. [Google Scholar] [CrossRef]
- Matos, Â.P. The Impact of Microalgae in Food Science and Technology. J. Am. Oil Chem. Soc. 2017, 94, 1333–1350. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae for Human and Animal Nutrition. In Handbook of Microalgal Culture; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2013; pp. 461–503. [Google Scholar]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Gallego, R.; Valdés, A.; Suárez-Montenegro, Z.J.; Sánchez-Martínez, J.D.; Cifuentes, A.; Ibáñez, E.; Herrero, M. Anti-inflammatory and neuroprotective evaluation of diverse microalgae extracts enriched in carotenoids. Algal Res. 2022, 67, 102830. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef] [PubMed]
- Coulombier, N.; Jauffrais, T.; Lebouvier, N. Antioxidant Compounds from Microalgae: A Review. Mar. Drugs 2021, 19, 549. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Castagna, D.A.; Fortinguerra, S.; Buriani, A.; Scapagnini, G.; Willcox, D.C. Spirulina Microalgae and Brain Health: A Scoping Review of Experimental and Clinical Evidence. Mar. Drugs 2021, 19, 293. [Google Scholar] [CrossRef] [PubMed]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Therapeutic Potentials of Microalgae in the Treatment of Alzheimer’s Disease. Molecules 2017, 22, 480. [Google Scholar] [CrossRef] [PubMed]
- Abo-Shady, A.M.; Gheda, S.F.; Ismail, G.A.; Cotas, J.; Pereira, L.; Abdel-Karim, O.H. Antioxidant and antidiabetic activity of algae. Life 2023, 13, 460. [Google Scholar] [CrossRef]
- Tamel Selvan, K.; Goon, J.A.; Makpol, S.; Tan, J.K. Therapeutic potentials of microalgae and their bioactive compounds on diabetes mellitus. Mar. Drugs 2023, 21, 462. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, J.; Xiong, R.-G.; Wu, S.-X.; Xu, X.-Y.; Tang, G.-Y.; Huang, S.-Y.; Zhou, D.-D.; Li, H.; Feng, Y. Effects and mechanisms of anti-diabetic dietary natural products: An updated review. Food Funct. 2024, 15, 1758–1778. [Google Scholar] [CrossRef] [PubMed]
- Show, P.L. Global market and economic analysis of microalgae technology: Status and perspectives. Bioresour. Technol. 2022, 357, 127329. [Google Scholar]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Dillehay, T.; Ramírez, C.; Pino, M.; Collins, M.; Rossen, J.; Pino-Navarro, J. Monte Verde: Seaweed, Food, Medicine, and the Peopling of South America. Science 2008, 320, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.A.; Hasan, M. A Review on Culture, Production and Use of Spirulina as Food for Humans and Feed for Domestic Animals and Fish. FAO Fish. Aquac. Circ. 2008, 57, 171–180. [Google Scholar]
- Nicoletti, M. Microalgae Nutraceuticals. Foods 2016, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Bishop, W.; Zubeck, H. Evaluation of Microalgae for use as Nutraceuticals and Nutritional Supplements. J. Nutr. Food Sci. 2012, 2, 147. [Google Scholar] [CrossRef]
- de Morais, M.G.; Vaz Bda, S.; de Morais, E.G.; Costa, J.A. Biologically Active Metabolites Synthesized by Microalgae. BioMed Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef] [PubMed]
- de Mello-Sampayo, C.; Paterna, A.; Polizzi, A.; Duarte, D.; Batista, I.; Pinto, R.; Gonçalves, P.; Raymundo, A.; Batista, A.P.; Gouveia, L.; et al. Evaluation of Marine Microalga Diacronema vlkianum Biomass Fatty Acid Assimilation in Wistar Rats. Molecules 2017, 22, 1097. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, L.; Marques, A.; Sousa, J.; Moura, P.; Bandarra, N. Microalgae–source of natural bioactive molecules as functional ingredients. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 21–37. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine Bioactives as Functional Food Ingredients: Potential to Reduce the Incidence of Chronic Diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine Bioactive Compounds and Their Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Campanella, L.; Russo, M.V.; Avino, P. Free and total amino acid composition in blue-green algae. Ann. Chim. 2002, 92, 343–352. [Google Scholar] [PubMed]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef]
- Matos, Â.P.; Feller, R.; Moecke, E.H.S.; de Oliveira, J.V.; Junior, A.F.; Derner, R.B.; Sant’Anna, E.S. Chemical Characterization of Six Microalgae with Potential Utility for Food Application. J. Am. Oil Chem. Soc. 2016, 93, 963–972. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Vareltzis, P.; Gortzi, O. A Systematic Review of the Twelve Most Popular Bean Varieties, Highlighting Their Potential as Functional Foods Based on the Health Benefits Derived from Their Nutritional Profiles, Focused on Non-Communicable Diseases. Appl. Sci. 2024, 14, 10215. [Google Scholar] [CrossRef]
- Watanabe, F.; Katsura, H.; Takenaka, S.; Fujita, T.; Abe, K.; Tamura, Y.; Nakatsuka, T.; Nakano, Y. Pseudovitamin B12 Is the Predominant Cobamide of an Algal Health Food, Spirulina Tablets. J. Agric. Food Chem. 1999, 47, 4736–4741. [Google Scholar] [CrossRef]
- Watanabe, F. Vitamin B12 Sources and Bioavailability. Exp. Biol. Med. 2007, 232, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J. Appl. Phycol. 2015, 27, 1109–1119. [Google Scholar] [CrossRef]
- Milledge, J.J. Commercial application of microalgae other than as biofuels: A brief review. Rev. Environ. Sci. Bio Technol. 2011, 10, 31–41. [Google Scholar] [CrossRef]
- Brown, M.R. The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 1991, 145, 79–99. [Google Scholar] [CrossRef]
- Velasco, L.; Carrera, S.; Barros, J. Isolation, culture and evaluation of Chaetoceros muelleri from the Caribbean as food for the native scallops, Argopecten nucleus and Nodipecten nodosus. Lat. Am. J. Aquat. Res. 2016, 44, 557–568. [Google Scholar] [CrossRef]
- Christaki, E.; Florou-Paneri, P.; Bonos, E. Microalgae: A novel ingredient in nutrition. Int. J. Food Sci. Nutr. 2011, 62, 794–799. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Slocombe, S.P.; Ross, M.; Thomas, N.; McNeill, S.; Stanley, M.S. A rapid and general method for measurement of protein in micro-algal biomass. Bioresour. Technol. 2013, 129, 51–57. [Google Scholar] [CrossRef]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional Evaluation of Australian Microalgae as Potential Human Health Supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef]
- Barbarino, E.; Lourenço, S.O. An evaluation of methods for extraction and quantification of protein from marine macro- and microalgae. J. Appl. Phycol. 2005, 17, 447–460. [Google Scholar] [CrossRef]
- van Krimpen, M.M.; Bikker, P.; van der Meer, I.M.; van der Peet-Schwering, C.M.C.; Vereijken, J.M. Cultivation, Processing and Nutritional Aspects for Pigs and Poultry of European Protein Sources as Alternatives for Imported Soybean Products; Wageningen UR Livestock Research: Lelystad, The Netherlands, 2013. [Google Scholar]
- Wallace, J.S. Increasing agricultural water use efficiency to meet future food production. Agric. Ecosyst. Environ. 2000, 82, 105–119. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Schwenzfeier, A.; Wierenga, P.A.; Gruppen, H. Isolation and characterization of soluble protein from the green microalgae Tetraselmis sp. Bioresour. Technol. 2011, 102, 9121–9127. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.l.B.; Laurens, L.M.L. Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energy Environ. Sci. 2010, 3, 554–590. [Google Scholar]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef]
- Graziani, G.; Schiavo, S.; Nicolai, M.A.; Buono, S.; Fogliano, V.; Pinto, G.; Pollio, A. Microalgae as human food: Chemical and nutritional characteristics of the thermo-acidophilic microalga Galdieria sulphuraria. Food Funct. 2013, 4, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Innovative Natural Functional Ingredients from Microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar] [CrossRef]
- Chae, S.R.; Hwang, E.J.; Shin, H.S. Single cell protein production of Euglena gracilis and carbon dioxide fixation in an innovative photo-bioreactor. Bioresour. Technol. 2006, 97, 322–329. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Joy, J.M.; Lowery, R.P.; Wilson, J.M.; Purpura, M.; Souza, E.O.; Wilson, S.; Kalman, D.S.; Dudeck, J.E.; Jager, R. The effects of 8 weeks of whey or rice protein supplementation on body composition and exercise performance. Nutr. J. 2013, 12, 86. [Google Scholar] [CrossRef]
- Safi, C.; Ursu, A.V.; Laroche, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Aqueous extraction of proteins from microalgae: Effect of different cell disruption methods. Algal Res. 2014, 3, 61–65. [Google Scholar] [CrossRef]
- Templeton, D.W.; Laurens, L.M.L. Nitrogen-to-protein conversion factors revisited for applications of microalgal biomass conversion to food, feed and fuel. Algal Res. 2015, 11, 359–367. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Madsen, M. 14-Algal proteins. In Handbook of Food Proteins; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Sawston, UK, 2011; pp. 353–394. [Google Scholar]
- Lourenço, S.O.; Barbarino, E.; Lavín, L.; Lanfer Marquez, U.M.; Aidar, E. Distribution of intracellular nitrogen in marine microalgae: Calculation of new nitrogen-to-protein conversion factors. Eur. J. Phycol. 2004, 39, 17–32. [Google Scholar] [CrossRef]
- Günerken, E.; D’Hondt, E.; Eppink, M.H.M.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.; Pimentel, M. Sustainability of meat-based and plant-based diets and the environment1. Am. J. Clin. Nutr. 2003, 78, 660S–663S. [Google Scholar] [CrossRef]
- Guschina, I.A.; Harwood, J.L. Lipids and lipid metabolism in eukaryotic algae. Prog. Lipid Res. 2006, 45, 160–186. [Google Scholar] [CrossRef] [PubMed]
- Richmond, A. Biological Principles of Mass Cultivation of Photoautotrophic Microalgae. In Handbook of Microalgal Culture; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2013; pp. 169–204. [Google Scholar]
- Bellou, S.; Baeshen, M.N.; Elazzazy, A.M.; Aggeli, D.; Sayegh, F.; Aggelis, G. Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol. Adv. 2014, 32, 1476–1493. [Google Scholar] [CrossRef] [PubMed]
- Villarruel-López, A.; Ascencio, F.; Nuño, K. Microalgae, a Potential Natural Functional Food Source—A Review. Pol. J. Food Nutr. Sci. 2017, 67, 251–263. [Google Scholar] [CrossRef]
- Sprague, M.; Betancor, M.B.; Tocher, D.R. Microbial and genetically engineered oils as replacements for fish oil in aquaculture feeds. Biotechnol. Lett. 2017, 39, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.F.; Ríos Pinto, L.F.; Maciel Filho, R.; Fregolente, L.V. A review on lipid production from microalgae: Association between cultivation using waste streams and fatty acid profiles. Renew. Sustain. Energy Rev. 2019, 109, 448–466. [Google Scholar] [CrossRef]
- Tasić, M.B.; Pinto, L.S.; Klein, B.C.; Velijkovic, V.B.; Filho, R.M. Botryococcus braunii for biodiesel production. Renew. Sustain. Energy Rev. 2016, 64, 260–270. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Harrison, S.T.L. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 2009, 21, 493–507. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Manuell, A.L.; Beligni, M.V.; Elder, J.H.; Siefker, D.T.; Tran, M.; Weber, A.; McDonald, T.L.; Mayfield, S.P. Robust expression of a bioactive mammalian protein in Chlamydomonas chloroplast. Plant Biotechnol. J. 2007, 5, 402–412. [Google Scholar] [CrossRef]
- Arad, S.; Levy-Ontman, O. Red microalgal cell-wall polysaccharides: Biotechnological aspects. Curr. Opin. Biotechnol. 2010, 21, 358–364. [Google Scholar] [CrossRef]
- Sadovskaya, I.; Souissi, A.; Souissi, S.; Grard, T.; Lencel, P.; Greene, C.M.; Duin, S.; Dmitrenok, S.; Chizhov, A.O.; Shashkov, A.S.; et al. Chemical structure and biological activity of a highly branched (1→3,1→6)-β-d-glucan from Isochrysis galbana. Carbohydr. Polym. 2014, 111, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Turu, I.C.; Turkcan-Kayhan, C.; Kazan, A.; Yildiz-Ozturk, E.; Akgol, S.; Yesil-Celiktas, O. Synthesis and characterization of cryogel structures for isolation of EPSs from Botryococcus braunii. Carbohydr. Polym. 2016, 150, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Shibakami, M.; Tsubouchi, G.; Sohma, M.; Hayashi, M. Synthesis of nanofiber-formable carboxymethylated Euglena-derived β-1,3-glucan. Carbohydr. Polym. 2016, 152, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Busi, M.V.; Barchiesi, J.; Martín, M.; Gomez-Casati, D.F. Starch metabolism in green algae. Starch-Stärke 2014, 66, 28–40. [Google Scholar] [CrossRef]
- Gügi, B.; Le Costaouec, T.; Burel, C.; Lerouge, P.; Helbert, W.; Bardor, M. Diatom-Specific Oligosaccharide and Polysaccharide Structures Help to Unravel Biosynthetic Capabilities in Diatoms. Mar. Drugs 2015, 13, 5993–6018. [Google Scholar] [CrossRef] [PubMed]
- Dismukes, G.C.; Carrieri, D.; Bennette, N.; Ananyev, G.M.; Posewitz, M.C. Aquatic phototrophs: Efficient alternatives to land-based crops for biofuels. Curr. Opin. Biotechnol. 2008, 19, 235–240. [Google Scholar] [CrossRef]
- Raposo, M.F.d.J.; De Morais, R.M.S.C.; de Morais, A.M.M.B. Bioactivity and Applications of Sulphated Polysaccharides from Marine Microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S. Carotenoids in Algae: Distributions, Biosyntheses and Functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Coesel, S.N.; Baumgartner, A.C.; Teles, L.M.; Ramos, A.A.; Henriques, N.M.; Cancela, L.; Varela, J.C.S. Nutrient Limitation is the Main Regulatory Factor for Carotenoid Accumulation and for Psy and Pds Steady State Transcript Levels in Dunaliella salina (Chlorophyta) Exposed to High Light and Salt Stress. Mar. Biotechnol. 2008, 10, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Aremu, A.O.; Neményi, M.; Stirk, W.A.; Ördög, V.; van Staden, J. Manipulation of nitrogen levels and mode of cultivation are viable methods to improve the lipid, fatty acids, phytochemical content, and bioactivities in Chlorella minutissima. J. Phycol. 2015, 51, 659–669. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Coulombier, N.; Nicolau, E.; Le Déan, L.; Antheaume, C.; Jauffrais, T.; Lebouvier, N. Impact of Light Intensity on Antioxidant Activity of Tropical Microalgae. Mar. Drugs 2020, 18, 122. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar]
- Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Olasehinde, T.A.; Oyeleye, S.I.; Boligon, A.A.; Athayde, M.L. Phenolic Extract from Moringa oleifera Leaves Inhibits Key Enzymes Linked to Erectile Dysfunction and Oxidative Stress in Rats’ Penile Tissues. Biochem. Res. Int. 2015, 2015, 175950. [Google Scholar] [CrossRef]
- Machu, L.; Misurcova, L.; Vavra Ambrozova, J.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic Content and Antioxidant Capacity in Algal Food Products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Safafar, H.; Van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive oxygen species (ROS), oxygen radicals and antioxidants: Where are we now, where is the field going and where should we go? Biochem. Biophys. Res. Commun. 2022, 633, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Giurranna, E.; Nencini, F.; Bettiol, A.; Borghi, S.; Argento, F.R.; Emmi, G.; Silvestri, E.; Taddei, N.; Fiorillo, C.; Becatti, M. Dietary Antioxidants and Natural Compounds in Preventing Thrombosis and Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 11457. [Google Scholar] [CrossRef]
- Mansour, F.B.; Guermazi, W.; Chamkha, M.; Bellassoued, K.; Salah, H.B.; Harrath, A.H.; Aldahmash, W.; Rahman, M.A.; Ayadi, H. Bioactive Potential of the Sulfated Exopolysaccharides from the Brown Microalga Halamphora sp.: Antioxidant, Antimicrobial, and Antiapoptotic Profiles. Anal. Sci. Adv. 2024, 5, e202400030. [Google Scholar] [CrossRef]
- Morais, A.M.; Kumla, D.; Martins, V.F.; Alves, A.; Gales, L.; Silva, A.M.; Costa, M.; Mistry, S.; Kijjoa, A.; Morais, R.M. Monoterpene Hydroxy Lactones Isolated from Thalassiosira sp. Microalga and Their Antibacterial and Antioxidant Activities. Molecules 2024, 29, 5175. [Google Scholar] [CrossRef] [PubMed]
- Vignaud, J.; Loiseau, C.; Hérault, J.; Mayer, C.; Côme, M.; Martin, I.; Ulmann, L. Microalgae produce antioxidant molecules with potential preventive effects on mitochondrial functions and skeletal muscular oxidative stress. Antioxidants 2023, 12, 1050. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Kozani, S.; Kozani, S.; Pierre, G.; Michaud, P.; Delattre, C. Marine bacteria versus microalgae: Who is the best for biotechnological production of bioactive compounds with antioxidant properties and other biological applications? Mar. Drugs 2019, 18, 28. [Google Scholar] [CrossRef]
- Del Mondo, A.; Smerilli, A.; Sané, E.; Sansone, C.; Brunet, C. Challenging microalgal vitamins for human health. Microb. Cell Factories 2020, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, C.A.; Senhorinho, G.N.; Laamanen, C.A.; Scott, J.A. Microalgae as an alternative to oil crops for edible oils and animal feed. Algal Res. 2022, 64, 102663. [Google Scholar] [CrossRef]
- Ma, M.; Li, Y.; Chen, J.; Wang, F.; Yuan, L.; Li, Y.; Zhang, B.; Ye, D.; Han, D.; Jin, H. High-cell-density cultivation of the flagellate alga Poterioochromonas malhamensis for biomanufacturing the water-soluble β-1,3-glucan with multiple biological activities. Bioresour. Technol. 2021, 337, 125447. [Google Scholar] [CrossRef]
- Almendinger, M.; Saalfrank, F.; Rohn, S.; Kurth, E.; Springer, M.; Pleissner, D. Characterization of selected microalgae and cyanobacteria as sources of compounds with antioxidant capacity. Algal Res. 2021, 53, 102168. [Google Scholar] [CrossRef]
- Faraloni, C.; Di Lorenzo, T.; Bonetti, A. Impact of light stress on the synthesis of both antioxidants polyphenols and carotenoids, as fast photoprotective response in Chlamydomonas reinhardtii: New prospective for biotechnological potential of this microalga. Symmetry 2021, 13, 2220. [Google Scholar] [CrossRef]
- Katayama, T.; Rahman, N.A.; Khatoon, H.; Kasan, N.A.; Nagao, N.; Yamada, Y.; Takahashi, K.; Furuya, K.; Abd Wahid, M.E.; Yusoff, F.M. Bioprospecting of tropical microalgae for high-value products: N-3 polyunsaturated fatty acids and carotenoids. Aquac. Rep. 2022, 27, 101406. [Google Scholar] [CrossRef]
- Chalima, A.; Boukouvalas, C.; Oikonomopoulou, V.; Topakas, E. Optimizing the production of docosahexaenoic fatty acid by Crypthecodinium cohnii and reduction in process cost by using a dark fermentation effluent. Chem. Eng. J. Adv. 2022, 11, 100345. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, L.; Zheng, H.; Huang, H.; Qian, Z.J. Microalgae Octapeptide IIAVEAGC Alleviates Oxidative Stress and Neurotoxicity in 6-OHDA-Induced SH-SY5Y Cells by Regulating the Nrf2/HO-1and Jak2/Stat3 Pathways. Chem. Biodivers. 2024, 21, e202301509. [Google Scholar] [CrossRef]
- Brasil, F.B.; Bertolini Gobbo, R.C.; Souza de Almeida, F.J.; Luckachaki, M.D.; Dall’oglio, E.L.; de Oliviera, M.R. The signaling pathway PI3K/Akt/Nrf2/HO-1 plays a role in the mitochondrial protection promoted by astaxanthin in the SH-SY5Y cells exposed to hydrogen peroxide. Neurochem. Int. 2021, 146, 105024. [Google Scholar] [CrossRef]
- Stasiewicz, A.; Conde, T.; Gęgotek, A.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Prevention of UVB induced metabolic changes in epidermal cells by lipid extract from microalgae Nannochloropsis oceanica. Int. J. Mol. Sci. 2023, 24, 11302. [Google Scholar] [CrossRef]
- Kim, K.M.; Lee, J.Y.; Im, A.-R.; Chae, S. Phycocyanin protects against UVB-induced apoptosis through the PKC α/βII-Nrf-2/HO-1 dependent pathway in human primary skin cells. Molecules 2018, 23, 478. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, H.F.; Khalifa, H.K.; Omran, A.; Korany, R.M.; Selim, S.; Hussein, E.; Alhotan, R.A.; Ayyoub, A.; Masoud, S.R. Unveiling the Potential of Silymarin, Spirulina platensis, and Chlorella vulgaris towards Cardiotoxicity via Modulating Antioxidant Activity, Inflammation, and Apoptosis in Rats. Life 2024, 14, 1289. [Google Scholar] [CrossRef] [PubMed]
- Sikiru, A.B.; Arangasamy, A.; Alemede, I.; Guvvala, P.; Egena, S.; Ippala, J.; Bhatta, R. Chlorella vulgaris supplementation effects on performances, oxidative stress and antioxidant genes expression in liver and ovaries of New Zealand White rabbits. Heliyon 2019, 5, e02470. [Google Scholar] [CrossRef] [PubMed]
- Bulut, O.; Köse, I.E.; Sönmez, Ç.; Öktem, H.A. Antioxidant activity of Micractinium sp. (Chlorophyta) extracts against H2O2 induced oxidative stress in human breast adenocarcinoma cells. Sci. Rep. 2024, 14, 27593. [Google Scholar] [CrossRef] [PubMed]
- Alavianghavanini, A.; Moheimani, N.R.; Bahri, P.A. Process design and economic analysis for the production of microalgae from anaerobic digestates in open raceway ponds. Sci. Total Environ. 2024, 923, 171554. [Google Scholar] [CrossRef]
- Rangel Pinto, J.D.; Guerrero, J.L.; Rivera, L.; Parada-Pinilla, M.P.; Cala, M.P.; López, G.; González Barrios, A.F. Predicting the microalgae lipid profile obtained by supercritical fluid extraction using a machine learning model. Front. Chem. 2024, 12, 1480887. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, W.; Li, X.; Chen, X.; Wang, Y.; Huang, G.; Wang, J.; Jia, Z. Enzyme-Assisted Ultrasonic Extraction and Antioxidant Activities of Polysaccharides from Schizochytrium limacinum Meal. Foods 2024, 13, 880. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mafukidze, D.; Zheng, Y. Microalgae aggregation induced by thermoresponsive polymers. Bioresour. Technol. 2025, 415, 131650. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Perumal, K.; Sumathi, Y.; Singhania, R.R.; Chen, C.-W.; Dong, C.-D.; Patel, A.K. Nano-Enabled Microalgae Bioremediation: Advances in Sustainable Pollutant Removal and Value-Addition. Environ. Res. 2024, 263, 120011. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA); Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; et al. Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA J. 2016, 14, e04594. [Google Scholar]
- McKenzie, C.H.; Bates, S.S.; Martin, J.L.; Haigh, N.; Howland, K.L.; Lewis, N.I.; Locke, A.; Peña, A.; Poulin, M.; Rochon, A.; et al. Three decades of Canadian marine harmful algal events: Phytoplankton and phycotoxins of concern to human and ecosystem health. Harmful Algae 2021, 102, 101852. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulou, M.; Vareltzis, P.; Floros, S.; Androutsos, O.; Bargiota, A.; Gortzi, O. Development of a Functional Acceptable Diabetic and Plant-Based Snack Bar Using Mushroom (Coprinus comatus) Powder. Foods 2023, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, F.; Amerizadeh, A.; Behshood, P.; Moradi, S.; Asgary, S. Effect of microalgae Arthrospira on biomarkers of glycemic control and glucose metabolism: A systematic review and meta-analysis. Curr. Probl. Cardiol. 2022, 47, 100942. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Ali, M.S.; Madkour, F.F.; Elgendy, H. Oral spirulina platensis attenuates hyperglycemia and exhibits antinociceptive effect in streptozotocin-induced diabetic neuropathy rat model. J. Pain Res. 2020, 13, 2289–2296. [Google Scholar] [CrossRef]
- Lympaki, F.; Giannoglou, M.; Magriplis, E.; Bothou, D.L.; Andreou, V.; Dimitriadis, G.D.; Markou, G.; Zampelas, A.; Theodorou, G.; Katsaros, G.; et al. Short-term effects of spirulina consumption on glycemic responses and blood pressure in healthy young adults: Results from two randomized clinical trials. Metabolites 2022, 12, 1180. [Google Scholar] [CrossRef] [PubMed]
- Nazih, H.; Bard, J.-M. Microalgae in human health: Interest as a functional food. In Microalgae in Health and Disease Prevention; Academic Press: Cambridge, UK, 2018; pp. 211–226. [Google Scholar]
- Vahdati, S.N.; Lashkari, A.; Navasatli, S.A.; Ardestani, S.K.; Safavi, M. Butylated hydroxyl-toluene, 2,4-Di-tert-butylphenol, and phytol of Chlorella sp. protect the PC12 cell line against H2O2-induced neurotoxicity. Biomed. Pharmacother. 2022, 145, 112415. [Google Scholar] [CrossRef]
- Piovan, A.; Battaglia, J.; Filippini, R.; Dalla Costa, V.; Facci, L.; Argentini, C.; Pagetta, A.; Giusti, P.; Zusso, M. Pre-and early post-treatment with Arthrospira platensis (Spirulina) extract impedes lipopolysaccharide-triggered neuroinflammation in microglia. Front. Pharmacol. 2021, 12, 724993. [Google Scholar] [CrossRef] [PubMed]
- Tabarzad, M.; Atabaki, V.; Hosseinabadi, T. Anti-inflammatory activity of bioactive compounds from microalgae and cyanobacteria by focusing on the mechanisms of action. Mol. Biol. Rep. 2020, 47, 6193–6205. [Google Scholar] [CrossRef] [PubMed]
- Choo, W.-T.; Teoh, M.-L.; Phang, S.-M.; Convey, P.; Yap, W.-H.; Goh, B.-H.; Beardall, J. Microalgae as potential anti-inflammatory natural product against human inflammatory skin diseases. Front. Pharmacol. 2020, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Unadkat, K.; Parikh, P.; Chandrasekhar, K. Algae mediated pesticides bioremediation: Mechanisms, approaches, limitations, and prospects for future research. In Pesticides Bioremediation; Springer: Berlin/Heidelberg, Germany, 2022; pp. 353–380. [Google Scholar]
- Alsenani, F.; Tupally, K.R.; Chua, E.T.; Eltanahy, E.; Alsufyani, H.; Parekh, H.S.; Schenk, M. Evaluation of microalgae and cyanobacteria as potential sources of antimicrobial compounds. Saudi Pharm. J. 2020, 28, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.-J.; Kim, K.-J.; Song, J.-H.; Choi, J.; Lee, H.Y.; Kang, D.-H.; Heo, H.J.; Lee, B.-Y. Spirulina maxima extract ameliorates learning and memory impairments via inhibiting GSK-3β phosphorylation induced by intracerebroventricular injection of amyloid-β 1–42 in mice. Int. J. Mol. Sci. 2017, 18, 2401. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Othman, M.B.; Ferdousi, F.; Yoshida, M.; Watanabe, M.; Tominaga, K.; Isoda, H. Modulation of the neurotransmitter systems through the anti-inflammatory and antidepressant-like effects of squalene from Aurantiochytrium sp. PLoS ONE 2019, 14, e0218923. [Google Scholar] [CrossRef] [PubMed]
- Min, S.K.; Park, J.S.; Luo, L.; Kwon, Y.S.; Lee, H.C.; Shim, H.J.; Kim, I.-D.; Lee, J.-K.; Shin, H.S. Assessment of C-phycocyanin effect on astrocytes-mediated neuroprotection against oxidative brain injury using 2D and 3D astrocyte tissue model. Sci. Rep. 2015, 5, 14418. [Google Scholar] [CrossRef] [PubMed]
- Pabon, M.M.; Jernberg, J.N.; Morganti, J.; Contreras, J.; Hudson, C.E.; Klein, R.L.; Bickford, C. A Spirulina-Enhanced diet provides neuroprotection in an α-synuclein model of Parkinson’s disease. PLoS ONE 2012, 7, e45256. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyaya, I.; Gupta, S.; Mohammed, A.; Mushtaq, N.; Chauhan, S.; Ghosh, S. Neuroprotective effect of Spirulina fusiform and amantadine in the 6-OHDA induced Parkinsonism in rats. BMC Complement. Altern. Med. 2015, 15, 296. [Google Scholar]
- Hwang, J.-H.; Lee, I.-T.; Jeng, K.-C.; Wang, M.-F.; Hou, R.C.-W.; Wu, S.-M.; Chan, Y.-C. Spirulina prevents memory dysfunction, reduces oxidative stress damage and augments antioxidant activity in senescence-accelerated mice. J. Nutr. Sci. Vitaminol. 2011, 57, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.-J.; Seo, Y.-J.; Choi, J.; Lee, H.Y.; Kang, D.-H.; Kim, K.-J.; Lee, B.-Y. Spirulina maxima extract prevents neurotoxicity via promoting activation of BDNF/CREB signaling pathways in neuronal cells and mice. Molecules 2017, 22, 1363. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Hwang, C.J.; Lee, H.P.; Kim, H.S.; Han, S.-B.; Hong, J.T. Inhibitory effect of ethanol extract of Nannochloropsis oceanica on lipopolysaccharide-induced neuroinflammation, oxidative stress, amyloidogenesis and memory impairment. Oncotarget 2017, 8, 45517. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Jang, H.M.; Kan, E. Microalgal biomass and lipid production on dairy effluent using a novel microalga, Chlorella sp. isolated from dairy wastewater. Biotechnol. Bioprocess Eng. 2018, 23, 333–340. [Google Scholar] [CrossRef]
- Moradian, S.; Teufel, M.; Jahre, L.; Musche, V.; Fink, M.; Dinse, H.; Schweda, A.; Weismüller, B.; Dörrie, N.; Tan, S. Mental health burden of patients with diabetes before and after the initial outbreak of COVID-19: Predictors of mental health impairment. BMC Public Health 2021, 21, 2068. [Google Scholar] [CrossRef] [PubMed]
- Gortzi, O.; Dimopoulou, M.; Androutsos, O.; Vraka, A.; Gousia, H.; Bargiota, A. Effectiveness of a Nutrition Education Program for Patients with Type 2 Diabetes Mellitus. Appl. Sci. 2024, 14, 2114. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Kolonas, A.; Mourtakos, S.; Androutsos, O.; Gortzi, O. Nutritional Composition and Biological Properties of Sixteen Edible Mushroom Species. Appl. Sci. 2022, 12, 8074. [Google Scholar] [CrossRef]
- Bueno, M.; Gallego, R.; Chourio, A.M.; Ibáñez, E.; Herrero, M.; Saldaña, M.D. Green ultra-high pressure extraction of bioactive compounds from Haematococcus pluvialis and Porphyridium cruentum microalgae. Innov. Food Sci. Emerg. Technol. 2020, 66, 102532. [Google Scholar] [CrossRef]
- Yu, Z.; Hong, Y.; Xie, K.; Fan, Q. Research progresses on the physiological and pharmacological benefits of microalgae-derived biomolecules. Foods 2022, 11, 2806. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. Generally Recognized as Safe (GRAS). 2023. Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 17 October 2023).
- EFSA. Guidance on the preparation and submission of an application for authorisation of a novel food in the context of Regulation (EU) 2015/22831 (Revision 1) 2. EFSA J. 2021, 19, e06555. [Google Scholar]
- Lefebvre, K.A.; Robertson, A. Domoic acid and human exposure risks: A review. Toxicon 2010, 56, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.D.; Vasconcelos, V. Legal Aspects of Microalgae in the European Food Sector. Foods 2024, 13, 124. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimopoulou, M.; Kolonas, A.; Stagos, D.; Gortzi, O. A Review of the Sustainability, Chemical Composition, Bioactive Compounds, Antioxidant and Antidiabetic Activity, Neuroprotective Properties, and Health Benefits of Microalgae. Biomass 2025, 5, 11. https://doi.org/10.3390/biomass5010011
Dimopoulou M, Kolonas A, Stagos D, Gortzi O. A Review of the Sustainability, Chemical Composition, Bioactive Compounds, Antioxidant and Antidiabetic Activity, Neuroprotective Properties, and Health Benefits of Microalgae. Biomass. 2025; 5(1):11. https://doi.org/10.3390/biomass5010011
Chicago/Turabian StyleDimopoulou, Maria, Alexandros Kolonas, Dimitris Stagos, and Olga Gortzi. 2025. "A Review of the Sustainability, Chemical Composition, Bioactive Compounds, Antioxidant and Antidiabetic Activity, Neuroprotective Properties, and Health Benefits of Microalgae" Biomass 5, no. 1: 11. https://doi.org/10.3390/biomass5010011
APA StyleDimopoulou, M., Kolonas, A., Stagos, D., & Gortzi, O. (2025). A Review of the Sustainability, Chemical Composition, Bioactive Compounds, Antioxidant and Antidiabetic Activity, Neuroprotective Properties, and Health Benefits of Microalgae. Biomass, 5(1), 11. https://doi.org/10.3390/biomass5010011









