Abstract
Microalgae have emerged as a valuable source of essential nutrients and bioactive compounds, such as proteins, polyphenols, and polysaccharides, which are critical for overall health. Recent research has demonstrated the therapeutic potential of microalgae in addressing a variety of health conditions, including inflammation, oxidative stress, Type 2 diabetes mellitus (T2DM), and neurological disorders. The aim of this paper is to investigate the chemical composition, nutritional value, and biological properties of microalgae. Relevant information was gathered through a comprehensive search of scientific databases, including PubMed, Science Direct, Google Scholar, and the Cochrane Library. Key microalgal strains such as Spirulina platensis, Chlorella vulgaris, Haematococcus pluvialis, and Dunaliella salina have shown notable health-promoting properties. For instance, Spirulina platensis is rich in proteins, vitamins, and polyunsaturated fatty acids, while Chlorella vulgaris offers significant levels of chlorophyll and carotenoids. Haematococcus pluvialis is recognized for its high astaxanthin content and Dunaliella salina for its beta-carotene content. These microalgae strains have demonstrated beneficial effects in managing type 2 diabetes mellitus, alleviating oxidative stress, and offering neuroprotective potential. This paper provides an overview of microalgae’s nutritional composition, their medicinal properties, and their promising role in treating chronic diseases, with a particular focus on their applications in antidiabetic and neuroprotective therapies.
1. Introduction
The history of plants encompasses the history of microalgae, dating back billions of years ago [1]. Algae can be broadly classified based on their pigmentation into red (Rhodophyta), brown (Phaeophyta), and green (Chlorophyta) algae. Additionally, they can be categorized by size as macroalgae and microalgae [2]. Macroalgae are large, multicellular organisms that can grow up to 60 m in length, whereas microalgae consist of a diverse group of microscopic unicellular organisms, ranging in size from as small as 0.2 μm. Microalgae include both prokaryotic and eukaryotic species and are predominantly found in freshwater and marine environments [3]. Microalgae are further classified based on various characteristics, including pigment composition, morphology (e.g., spherical, elliptic, rod-like, and fusiform cells), and the presence of structural features such as thorns, cilia, and flagella. Lastly, they are grouped by size into picoplankton (0.2–2 µm), nanoplankton (2–20 µm), and microplankton (20–200 µm) [4,5].
Microalgae are known for their rich nutritional composition and biologically active compounds, including proteins, polysaccharides, lipids, polyunsaturated fatty acids, vitamins, pigments, phycobiliproteins, and enzymes. These bioactive compounds exhibit various beneficial properties, such as antioxidant, antibacterial, antiviral, antitumor, regenerative, antihypertensive, and neuroprotective activities [6]. Based on these properties, dietary supplements derived from microalgae are becoming increasingly available in various forms, including tablets, powders, and beverages [7]. Moreover, recent studies have shown that biofuel production from this source is not cost-effective due to limited biomass productivity and the cost of commercial algae production, unless the process is coupled with commercialization of higher-value by-products [8]. Their functionality could be attributed to their ability to synthesize valuable compounds such as carotenoids, long-chain fatty acids, etc., with potential for food and pharmaceutical applications [9]. The most popular microalgae used worldwide as dietary supplements are the green algae (Chlorophyceae) Chlorella vulgaris, Haematococcus pluvialis, and Dunaliella salina, as well as the Cyanobacterium (also known as blue-green algae) Arthrospira platensis (commercially known as Spirulina) [10]. Microalgae are also used in cosmetic products, such as moisturizers and anti-aging creams, due to their beneficial properties [11].
Recently, microalgae have been used as an ingredient in the development of food products with health benefits, such as dietary supplements [12]. Some of these products take advantage of their micronutrient and macronutrient content compared to conventional alternatives [13]. In addition, protein supplementation from microalgae may be particularly beneficial for the elderly and individuals engaged in regular exercise, as it supports muscle repair, maintains immune function [14], and offers potential neuroprotective effects against neurodegenerative diseases (NDDs), including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis [15].
Although the mechanism of action remains unclear, many researchers associate the antioxidant activity of microalgae with their neuroprotective effects [5]. Another possible explanation is their ability to protect neuronal cells in the central nervous system (CNS) from injury, dysfunction, deterioration, and cell death [5]. Therefore, nutrition may play a crucial role in maintaining cognitive function and reducing the incidence of neurodegenerative and mental disorders [16,17]. There is growing evidence for the therapeutic effects of natural products from marine sources like microalgae, including their extracts and bioactive molecules, against free radical formation and thus for the prevention of NDDs caused by oxidative stress [5]. These algal metabolites, such as pro-inflammatory cytokines and eicosanoids, can exert effects through various processes such as regulating cellular activities, inhibiting two important signaling pathways, and modulating the activities of enzymes like cyclooxygenase-2 (COX2), nitric oxide synthase (NOS), and phospholipase A2 [18]. Bioactive molecules from some species, such as Spirulina, Chlorella, and Haematococus pluvialis, are known for their neuroprotective effects [18]. The specific causes of NDDs vary and can include genetic disorders, infections, lifestyle, or environmental health problems, including malnutrition [12]. Nutritional studies have shown that the intake of microalgae could play a neuroprotective role [18], due to their antioxidant and anti-inflammatory properties [5].
On the other hand, an in vitro study has shown that fucoxanthin, a brown xanthophyll isolated from golden algae, has an antioxidant effect 13 times higher than that of vitamin E (α-tocopherol) [19], which could be the main reason for the antidiabetic activity [20]. Phenols have a broad spectrum of biological activities, including antioxidant and antidiabetic effects [21]. Their effects on the microbiota short-chain fatty acids (SCFAs), which are produced during the fermentation of dietary fiber, are known to modulate neurotransmitters and inflation. Studies linking a polyphenol-rich Mediterranean diet high in polyphenols to a reduction in harmful bacteria and an increase in beneficial strains correlate with lower inflammation and better overall health [4,5].
This paper aims to provide a brief overview of the chemical and nutritional composition of microalgae, as well as their medicinal and biological properties and their impact on human health but also their role in a sustainable future. To find relevant information, a comprehensive search was performed across multiple scientific databases, including PubMed, ScienceDirect, Google Scholar, and the Cochrane Library.
2. Sustainability
Algae have high biomass productivity and are considered an excellent source of nutrients for food production. In addition, microalgae production requires limited resources such as water and land and is therefore considered sustainable for food production [22]. With increasing research and development and the introduction of new products being launched by global food manufacturers, the market has experienced significant growth. The use of biomass from various microphyte species and ingredients derived from microalgae has increased rapidly. They are now used in a variety of foods, mainly in baked goods, sauces, condiments, and others [8], but also in food supplements, pharmaceuticals, cosmetics, and health products (Figure 1) [3]. Sustainability is the main goal of the European Union according to the 2030 Agenda [22], and different types of biofuels can be produced from microalgae biomass depending on the raw materials used and the pretreatment before fermentation (Figure 2) [3].
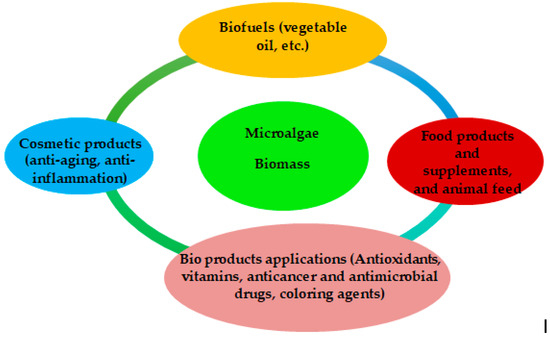
Figure 1.
Different uses of microalgae for a sustainable future [3].
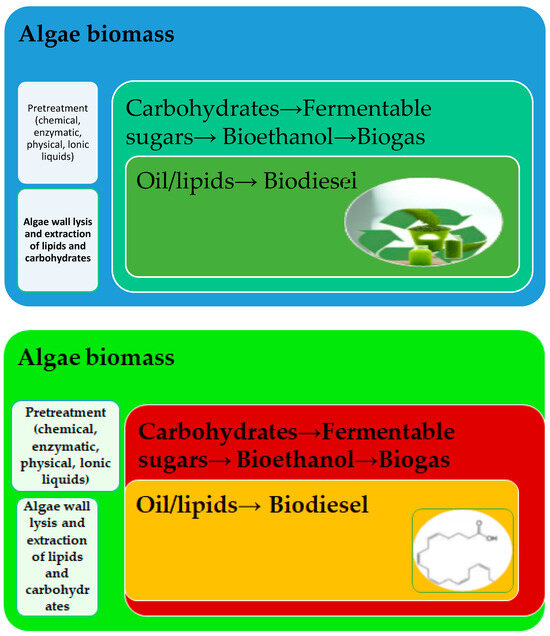
Figure 2.
Pretreatment of microalgae biomass for biofuel products [3].
Many types of algae, such as Spirulina, are widely consumed across the world [23], with Chilean consumers being among the first to recognize their nutritional value [23,24]. Edible algae are commonly available in various forms, including dietary supplements, tablets, and powders [23,25], though they can also be found in their raw form, particularly in regions such as Lake Texcoco in Spain [26]. The primary reason algae are considered a healthy dietary choice is their rich nutritional profile; however, achieving organoleptic acceptance among consumers remains a key challenge [25].
The United States of America is known to have a higher production rate worldwide [25], and the most well-known species at the market are Spirulina and Klamath and the Chlorophyceae species such as Chlorella [27] (Figure 3).
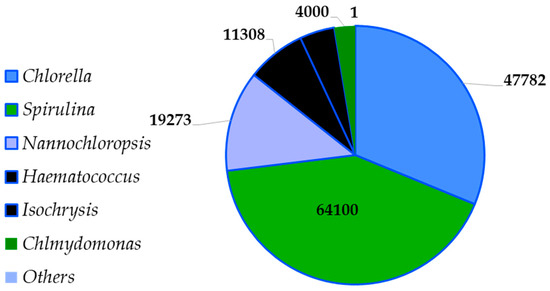
Figure 3.
Global microalgae market (2023) (www.fortunebusinessinsights.com accessed on 1 January 2025).
2.1. Chlorella
Chlorella is considered a hormone-balancing superfood due to its rich nutrient profile, which provides essential compounds required for hormone production [28], and is known for its potential benefits in supporting overall gut health [29]. In vivo and in vitro studies have shown that Chlorella’s extracts have antitumor and antioxidant properties [13]. Additionally, Chlorella may reduce postprandial glucose levels, which may increase the effects of antidiabetic medications [28,30]. Studies also suggest that the protein content in Chlorella is higher compared to other microalgae, as it contains 60% protein [18] and is a complete protein source, meaning it contains all nine essential amino acids. Another form of food supplements are species such as Chlorella sp., Arthrospira sp., and Aphanizomenon flos-aquae [31]. Another use is to produce healthy meals and food additives. Daily consumption of Chlorella as a supplement resulted in noticeable reduction in body fat percentage, serum total cholesterol, and fasting blood glucose levels over a 16-week trial [18].
2.2. Spirulina
Spirulina can grow in fresh or salt water and can be taken as a supplement, for instance, in tablet or powder form [28]. People consume Spirulina for its health benefits, as it is rich in several nutrients, including proteins, vitamins, minerals, and polyunsaturated fatty acids. Additionally, it possesses strong antioxidant properties, which contribute to its potential health-promoting effects [28,29]. It may help lower total cholesterol [32], low-density lipoproteins [32], or bad cholesterol [32] and triglycerides [33,34], while also increasing high-density lipoproteins [28]. Spirulina can also produce antigen-specific antibodies that help in the treatment of depression and attention deficit/hyperactivity disorder [13]. Spirulina is generally considered safe, even at high doses, up to 8 g per day. Additionally, individuals with phenylketonuria (PKU), a metabolic disorder, should avoid Spirulina due to its phenylalanine content [26,35]. Lastly, studies have shown that Spirulina can enhance the production of white blood cells and antibodies, which are crucial in fighting viruses and bacteria [27].
3. Chemical Composition and Bioactive Compounds of Microalgae
Many microalgae species have been shown to be rich in proteins, carbohydrates, lipids (Table 1) [1,7], and other bioactive substances such as polyunsaturated fatty acids (PUFA) [36]. Microalgae produce these metabolites via the mevalonate/non-mevalonate, shikimate, and polyketide pathways. In addition, microalgae are rich in vitamins A, B1, B2, B3, B7, B9, B5, C, and E, as well as minerals such as iodine, potassium, iron, magnesium, and calcium [10,36].
For algae to be considered as a potential new food source, their composition and nutrient content are a crucial factor [25]. Microalgae have high protein contents ranging from 11% to 71%, which makes them a valuable protein source [37]. For food use, the harvested and concentrated algal biomass must have a water content of less than 10% to be usable [9].
Spirulina contains many of the nutrients required by the human body [27]. In addition, Spirulina has a high iron content, about 28.5 mg/100 g, comparable to that from legumes and soybeans [38,39,40]. Although Spirulina does not contain vitamin B12, its impressive nutrient profile makes it a viable vegan or vegetarian choice [27].


Table 1.
Composition of different species of microalgae. Adapted from Barkia and Koyande [1,7].
Table 1.
Composition of different species of microalgae. Adapted from Barkia and Koyande [1,7].
| Species | % Protein (w/w) | % Carbohydrates (w/w) | % Fat (w/w) | References |
|---|---|---|---|---|
| Botryococcus braunii | 39–40 | 19–31 | 25–34 | [41] |
| Chaetoceros calcitrans | 36 | 27 | 15 | [42] |
| Chaetoceros gracilis | 12 | 4.7 | 7.2 | [43] |
| Chaetoceros muelleri | 59 | 10 | 31 | [44] |
| Chlamydomonas rheinhardii | 48 | 17 | 21 | [45] |
| Chlorella vulgaris | 51–58 | 12–17 | 14–22 | [46] |
| Chlorella pyrenoidosa | 57 | 26 | 2 | [42] |
| Diacronema vlkianum | 57 | 32 | 6 | [47] |
| Dunaliella primolecta | 12 | - | - | [48] |
| Dunaliela salina | 57 | 32 | 6 | [42] |
| Dunaliella sp. | 34.17 | 14.57 | 14.36 | [49] |
| Dunaliella tertiolecta | 11 | - | - | [50] |
| Euglena gracilis | 39–61 | 14–18 | 22–38 | [42] |
| Haematococcus pluvialis | 48 | 27 | 15 | [47] |
| Isochrysis galbana | 50–56 | 10–17 | 12–14 | [42] |
| Nannochloropsis granulata | 18–34 | 27–36 | 24–28 | [41] |
| Nannochloropsis sp. | 30 | 10 | 22 | [49] |
| Nitzschia closterium | 26 | 9.8 | 13 | [43] |
| Pavlova sp. | 24–29 | 6–9 | 9–14 | [43,46] |
| Phaeodactylum tricornutum | 34.8 | 16.8 | 16.1 | [41,43] |
| Porphyridium cruentum | 28–39 | 40–57 | 9–14 | [42] |
| Prymnesium parvum | 28–45 | 25–33 | 22–38 | [51] |
| Scenedesmus dimorphus | 8–18 | 21–52 | 16–40 | [42] |
| Scenedesmus obliquus | 50–56 | 10–17 | 12–14 | [45,52] |
| Scenedesmus quadricauda | 47 | 21–52 | 1.9 | [51] |
| Scenedesmus sp. | 31 | 28 | 15 | [49] |
| Schizochytrium sp. | - | - | 50–77 | [53] |
| Skeletonema costatum | 25 | 4.6 | 10 | [43] |
| Spirogyra sp. | 6–20 | 33–64 | 11–21 | [51] |
| Spirulina maxima | 60–71 | 13–16 | 6–7 | [42] |
| Spirulina platensis | 46–63 | 8–14 | 4–9 | [42] |
| Synechococcus sp. | 63 | 15 | 11 | [45] |
| Tetraselmis chuii | 31–46 | 25 | 12 | [41,43] |
| Tetraselmis maculata | 52 | 15 | 3 | [51] |
| Tetraselmis sp. | 36 | 24 | - | [54] |
| Thalassiosira pseudonana | 34 | 8.8 | 19 | [43] |
3.1. Proteins
Protein is an important part of the diet [55]. For the most health benefits, people can obtain protein from a variety of sources, such as fish, meat, soy, beans, and microalgae [56,57]. Species such as Cyanobacteria and Chlorella vulgaris contain over 60% protein on a dry weight basis [46,58] and are complete sources of protein, as they do not lack essential amino acids [25]. In addition, their amino acid profiles are balanced [55]. Despite the nutritional benefits, the taste of microalgae is still not widely accepted by consumers, and combined with the fact that some non-protein components can affect the color, it is still not widely used in the food industry [7]. On the other hand, algae biofuel could provide a renewable fuel source because up to 70% of their biomass is protein [25], without having a negative impact on the environment [25,59]. Microalgae have higher protein yield per unit compared to terrestrial crops [60,61].
Some microalgae have been reported to contain soluble proteins in their cytoplasm. In addition, chloroplast microalgae contain soluble proteins, central pyrenoids, and phycobiliproteins, although some microalgae such as Arthrospira plantesis, Chlamydomonas reinhardtii, and Neochloris cohaerens instead have thylakoid sacs surrounding the peripheral part of the cytoplasm associated with phycobilisomes [62]. In Chlorella vulgaris, Botryococcus braunii, and Scenedesmus obliquus, the essential amino acids cysteine and arginine make up around 44.7% of the total amino acid profile [6,63]. According to the WHO, not all people can cover the recommended dietary allowance (RDA) for protein that is 0.8 g of protein per kilogram of body weight [1,64].
Several microalgae can synthesize a high protein content, e.g., Spirulina platensis (60–65%) and Chlorella vulgaris (51–58%) of dry matter, and this is one of the main reasons to consider these organisms as a food source [9]. It must be taken into account that algae contain additional nitrogenous components, e.g., amines, glucosamides, nucleic acids, and cell wall materials [41]. For a correct determination of the N-to-P factor for algal protein content, a conversion factor of N × 4.78 was recommended [65]. Since protein is one of the most valuable algal constituents, four major parameters of protein quality are used to determine the appropriate nutritional value of algal protein, i.e., protein efficiency ratio (PER), biological value (BV), digestibility coefficient (DC) or true di-stability, and net protein utilization (NPU) [10]. There are a number of (mechanical or non-mechanical) methods for breaking down the algal cell wall to make the algal protein nutritionally usable [66]. The amino acid pattern of almost all algae is comparable to the requirements of the Food and Agriculture Organization (FAO), with minor deficiencies in the sulfur-containing amino acids methionine and cysteine [9,10]. Although some microalgae species have been reported to contain protein levels comparable to conventional sources such as milk, soybeans, eggs, and meat, the extraction of protein from microalgae offers several advantages in terms of nutritional value, efficiency, and productivity [1]. Furthermore, microalgae production has a significantly lower environmental impact. Specifically, 25% of the global water footprint is attributed to meat and dairy products [52], which is drastically higher compared to plant-based sources [67]. Additionally, microalgae production is considered eco-friendly, further reinforcing its potential as a sustainable protein source [1].
3.2. Fatty Acids
Many researchers have tried to evaluate the lipidemic content of microalgae species, and the results vary widely (1–70% of dry biomass) [31]. These results could be explained by describing the many growing factors of microalgae, such as nutrition, light intensity, temperature, and pH, that impact on their nutritional profile [68,69]. It has been shown that cultures in stationary phase double their content of neutral lipids and synthesize polysaccharides at the expense of proteins [7]. The most recent data concerning fatty acid composition in microalgae range from C12 to C24, often with monounsaturated and polyunsaturated fatty acids C16 and C18. This area needs further investigation because of the large differences in fatty acid profile (20–50% of the dry weight) [70]. As is shown in Table 1, Schizochytrium sp., Nannochloropsis sp., and Chlorella vulgaris are some of the species with a higher percentage of fats, and they are rich in arachidonic acid, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and palmitic acid [71]. The only known abundant, naturally occurring sources of ERA and DHA are marine algae and bacteria, highlighting their nutritional value [72,73]. However, freshwater species, e.g., Desmodesmus sp., have also been investigated as a source of long-chain omega-3 PUFA, EPA, and DHA acids [72,73]. Although, marine microalgae species were found to produce biomass with higher amounts of PUFA and could also be a healthy food choice for vegans or vegetarians due to their high lipid content (1–40%), which can be up to 85% of the dry weight under certain conditions [6,74]. Microalgae are a good source not only of the fatty acids that can be produced by the body, such as palmitic acid, but also of the essential ones such as omega-3 (ω-3), including α-linolenic acid (ALA, C18:3), eicosapentaenoic acid (EPA, C20:5), and docosahexaenoic acid (DHA, C22:6) and omega-6 (ω-6), including linoleic acid (LA, C18:2), ɣ-linolenic acid (GLA, C18:3), and arachidonic acid (ARA, C20:4) [11]. The proportion of PUFA varies greatly depending on the species. For example, C. cohnii microalgae contain only DHA and practically no other PUFA; N. oculata and P. tricornutum contain mainly EPA, while P. cruentum contains mainly ARA [9].
Lipids are essential for human nutrition, and research underscores the significant health benefits of consuming omega-3-rich foods and supplements. Auxenochlorella protothecoides stands out as it can accumulate up to 70% lipids in its dry biomass, making it a valuable source of fatty acids. This high lipid content makes Auxenochlorella protothecoides a promising supplement to promote the health benefits associated with omega-3 fatty acids. For example, a previous study found that individuals who consumed seafood one to four times per week had a reduced risk of heart disease-related mortality [75]. Omega-3 supplements have also been shown to effectively lower triglyceride levels, and additional research has even indicated that they may provide relief for individuals suffering from rheumatoid arthritis [76,77].
3.3. Carbohydrates and Polysaccharides
Carbohydrates are essential food nutrients that include sugars, starches, and dietary fiber [78]. The body uses carbohydrates to produce glucose (blood sugar) for energy. Carbohydrates are fast-acting and turn into energy as soon as they are ingested. This energy powers the brain and body. Microalgae are a good source of these compounds [7], and there is a growing interest in microalgal specific carbohydrates with potential therapeutic applications [11,79,80]. Notable among these is β-1,3-glucan, a natural soluble fiber that acts as an immunostimulant, antioxidant, and cholesterol-lowering agent and is accessible from the cultivation of Chlorella [11], Isochrysis galbana [79], and Euglena [81]. In contrast with processed foods, microalgae-based products are high in fibers and starches which have lower impact on blood sugar [9]. As in land plants, the major carbohydrates storage product of green algae is usually starch in the form of amylose or amylopectin [7]. These starches are polysaccharides in which the monomer, or fundamental unit, is glucose [7]. Diatoms (Bacillariophycae, Heterokontophyta) produce chrysolaminarin, a linear polymer of β(1,3)- and β(1,6)-linked glucose units [82,83]. Under severe nutrient limitation, cells can accumulate up to 80% of their dry weight as (1,3)-β-D-glucan during childhood [84]. Similarly, a strain of Tetraselmis suecica has been reported to accumulate between 11% and 47% of its dry weight as starch under nutrient-rich or -poor conditions [7]. Moreover, microalgae are suitable for use in skincare products. Upcycling cosmetics are a novel trend featuring cosmetic components derived from plant-based waste deemed too valuable to discard [85].
3.4. Pigments
Many natural products derived from microalgae have attracted particular attention due to their wide range of biological activities. Pigments as bioactive compounds from microalgae are related to light yield, CO2 fixation, protection of algal cells from damage due to excessive illumination, and, macroscopically, coloration or pigmentation of the algal culture. There are three main groups of pigments in microalgae: chlorophylls (greenish coloration), carotenoids (including carotenes, which provide orange coloration, while xanthophylls are yellow pigments), and phycobilins (red and blue coloration) [9].
Chlorophyll is registered and approved as a coloring additive (E140) and is mainly used in food pigmentation and in the food supplement industry. Famous “chefs” use chlorophyll for green coloring of food and beverages such as pasta, pesto, or absinthe [9,11]. Carotenoids are organic, lipophilic compounds produced by plants and algae. Their color is directly related to their structure and ranges from pale yellow to bright orange to deep red [86,87]. More than 1200 tons of β-carotene were produced in 2010 [10]; this pigment can be extracted from Dunaliella salina, a green alga that grows in open ponds under high salinity and light conditions [88]. Another commercially important carotenoid, astaxanthin, is synthesized by the freshwater green alga Haematococcus pluvialis [89], but canthaxanthin and lutein have also been found in Haematococcus pluvialis and in other species such as Chlorella zofingiensis and Arthrospira sp. [18]. Carotenoids contribute to the antioxidant capacity of some microalgae and also play an important role in the prevention of chronic diseases [18]. Finally, phycocyanin is a blue pigment found mainly in cyanobacteria, e.g., in the species Spirulina platensis and Aphanizomenon flos-aquae, while phycoerythrin is a pigment found mainly in red algae, e.g., in the species Porphyridium cruentum and Gracilaria gracilis, and is responsible for their characteristic red coloration [9,11,90].
3.5. Phenolic Compounds
Phenolic compounds are a class of compounds found in many plant foods [91] that include flavonoids, phenolic acids, lignans, and stilbenes. The higher the phenolic content, the higher the antioxidant properties of microalgal extracts [14,92,93]. Phloroglucinol and phenolic acids are phenolic compounds that have a role as algal metabolites [94]. Even though microalgae do not have the highest number of phenolic compounds compared with other plants [95], the antioxidant activity is directly related to their chemical structure such as the number of hydroxyl groups or their position in the aromatic cycle [14].
In addition, plant-derived polyphenols can reduce depression, inflammation, and oxidative stress [18]. Quercetin and epicatechin decrease atherosclerosis by augmenting nitric oxide activity and decreasing serological endothelin-1 levels [18]. Oboh et al. [96] reported that gallic acid and chlorogenic acid have a cholinesterase inhibitory effect and protect the brain from metal-induced lipid peroxidation. However, not all microalgae strains can synthesize phenolic compounds [18]. High-performance liquid chromatography (HPLC) analysis was often used for the determination of polyphenols in microalgae, and these analytic methods have shown that the phenolic content of the aqueous and methanolic extract of Spirulina platensis had the highest concentration of gallic acid and chlorogenic acid [97]. The same study revealed the presence of gallic acid, 4-hydroxybenzoic acid, and epigallocatechin in Chlorella pyrenoidosa, while catechin, epicatechin, and pyrocatechol were found in Spirulina platensis [97]. Except for gallic acid, ferulic acid and cinnamic acid were also evaluated in algal biomass from Nannochloropsis salina and Nannochloropsis limnetica [98]. Finally, many others phenolic acids (chlorogenic acid, gallic, ferulic, coumaric, vanillin, and cinnamic acids) and flavonoids (quercetin, genistein, kaempferol, pinostrobin, and galangin) highlight the value of Spirulina maxima [18].
4. Medicinal and Biological Properties
4.1. Antioxidant Activity
Free radicals are defined as atomic or molecular species with unpaired electrons in their atomic or molecular orbitals, which are produced constantly by different biochemical and physiological processes in living organisms [99]. Due to their unpaired electrons, free radicals are highly reactive species which, at high concentrations, can react with and cause damage to all biological macromolecules within cells. When free radicals are produced in excessive amounts, oxidative stress is caused, which is implicated in different pathological conditions such as diabetes, neurodegenerative diseases, and carcinogenesis [99]. For the treatment of diseases caused by free radicals, the intake of natural antioxidants either through the diet or as nutritional supplements has been suggested [100]. Currently, there has been significant research interest in the isolation of antioxidant compounds from microalgae [14,101,102]. It is noteworthy that our search in the “pubmed” database, using the keywords “microalgae” and “antioxidants”, yielded more than 1100 results. Microalgae, as versatile microorganisms, have emerged as a promising source of antioxidants due to their rapid growth, high productivity, and ability to synthesize diverse bioactive compounds under various environmental conditions [14,101,102,103]. The main antioxidant compounds identified in microalgae are carotenoids (e.g., β-carotene, lutein, and astaxanthin), phenolics (e.g., phloroglucinol, phenolic acids, and flavonoids), sulfated polysaccharides, proteins, and peptides (e.g., phycobiliproteins, glutathione, and mycosporine-like amino acids), vitamins (e.g., vitamin C and E), and polyunsaturated fatty acids (PUFAs), especially omega-3 fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [14]. For example, among the microalgae species, Spirulina platensis, Botryococcus braunii, Coelastrella striolata, Haematococcus pluvialis, and Dunaliella salina have been reported to contain carotenoids as antioxidants [104]. Microalgae species form the genera Skeletonema and Chaetoceros contained vitamin C and E [105,106], while Poterioochromonas malhamensis contained polysaccharides, such as β-1,3-glucan, with antioxidant properties [107]. Several microalgae species, such as Neochloris oleobundans, Chlamydomonas reinhardtii, and Wilmottia murravi, contain high percentages of antioxidant phenolic compounds [108,109]. Moreover, microalgae species such as Thalassiosira weissflogi and Crypthecodinium cohnii have been demonstrated to produce high amounts of omega-3 fatty acids [110,111].
A number of studies have shown that microalgae compounds are protected from oxidative stress through various mechanisms in cell culture or in vivo experiments. For example, a peptide from Isochrysis zhanjiangensis exhibited antioxidant activity in the SH-SY5Y neuroblastoma cell line by inducing the antioxidant pathway of the transcription nuclear factor erythroid 2-related factor 2 (Nrf2) and the expression of its target antioxidant enzymes heme oxygenase-1 (HO-1) and glutathione peroxidase (GPX) [112]. Again, in SH-SY5Y cells, astaxanthin, a major antioxidant compound of many microalgae, protected, at 20 μΜ, from oxidative stress-induced mitochondrial damage by activating Nrf2 pathway through the phosphoinositide 3-kinase/Akt (PI3K/Akt) signaling pathway [113]. In an experimental model of UVB-induced oxidative stress in human keratinocytes, Nannochloropsis oceanica extract inhibited cell death through enhancement of the antioxidant enzymes superoxide dismutase and catalase, as well as of the glutathione/thioredoxin-dependent systems [114]. Moreover, phycocyanin, a pigment found in Spirulina platensis, inhibited ultraviolet-B (UVB)-induced oxidative damage in primary skin cells, which was mediated through the protein kinase C (PKC) α/β II/Nrf2/OH-1 pathway [115]. Regarding in vivo experiments, El-Gendy et al. (2024) [116] have demonstrated that administration of Chlorella vulgaris and Spirulina platensis to rats protected them from thioacetamide-induced cardiotoxicity through reducing oxidative stress, as shown by the oxidative stress markers total antioxidant capacity (TAC) and malondialdehyde (MDA) measured in serum. In addition, Chlorella vulgaris supplementation improved the redox status in rabbits [117]. Specifically, Chlorella vulgaris supplementation decreased MDA and protein oxidation (i.e., protein carbonyls) and increased TAC, superoxide (SOD), and catalase (CAT) activities, reduced glutathione (GSH) levels, and upregulated the gpx1 gene expression in serum and liver and ovary tissues [117]. Thus, the previous and other studies suggest that microalgal antioxidants may be used as food additives or food supplements to prevent or to treat diseases caused by oxidative stress [101,102,116,118].
However, the promising potential of microalgae for their use as a significant source of antioxidant compounds faces several challenges. The production costs remain high due to the need for controlled environmental conditions and nutrient inputs. Innovations in open-pond systems and the use of wastewater as a growth medium are being investigated to reduce costs [119]. In addition, current extraction methods should be improved to yield optimal concentrations of antioxidant compounds from microalgae. For this purpose, methods like supercritical CO2 extraction and ultrasound-assisted extraction are being optimized for higher efficiency and sustainability [120,121]. Furthermore, stability and effective delivery of microalgal antioxidants in end-use applications are crucial. Nanotechnology and polymer-based encapsulation are promising approaches to address this issue [122,123]. It should also be ensured that the antioxidant product does not have any safety risk for human health [124], since some microalgae produce phytotoxins [125]. Future strategies to address the above challenges include genetic engineering to enhance metabolite production, optimizing cultivation systems (e.g., photobioreactors), and developing advanced extraction and encapsulation techniques. Furthermore, collaboration between academia and industry will play a pivotal role in scaling up production and commercialization.
4.2. Antidiabetic Activity
The main reason for the protection against the complications of type 2 diabetes mellitus (T2DM) [20] is the higher content of polyphenols, vitamin E, and α-tocopherol [126], which can regulate glucose levels by many mechanisms of action [127]. Even though the mechanism has been described (Figure 4 and Figure 5) [21], the optimal dosage for different species remains unclear in terms of their beneficial effects on patients with type 2 diabetes mellitus. However, for Spirulina, which has been the most extensively studied due to its significant antidiabetic activity [19,128], the recommended dosage ranges from 250 mg/kg to 2 g daily [127,129]. Another mechanism of action that explains the antidiabetic effect is the antioxidant [14,130]. Oxidative stress is associated with the development and pathogenesis of diabetes mellitus [19]. Cells are predisposed to free radical attack due to their high lipid content, low levels of antioxidant enzymes, and the inability of their neurons to synthesize glutathione [4,5]. Overproduction of reactive oxygen species (ROS) in cells can lead to free radical attack on PUFAs at the cell membrane, which in turn can trigger lipid peroxidation. Lipid peroxidation products (4-hydroxynonenal and malondialdehyde) are very toxic to neurons and can cause neuronal death [18]. Chlorella vulgaris extract alleviated cell death triggered by oxidative stress [19]. Spirulina and Chlorella contain almost 40 known antioxidant compounds [131]. Also, Synechococcus sp. FACHB283, Chlamydomonas nivalis, and Nostoc ellipsosporum CCAP 1453/17 are excellent sources of antioxidants [14,130]. The antioxidant activity of the individual microalgae strains was associated with their carotenoid and phenolic content (coumaric, gallic, and caffeic acids) [98] and shows antioxidant protection in rat homogenates [132]. Phenolic extracts from S. maxima also scavenge hydroxyl radicals and inhibit lipid peroxidation [18]. In conclusion, Spirulina platensis microalgae showed promise in managing diabetes progression by (1) reducing blood glucose levels effectively, (2) improving renal function and antioxidant defense, and (3) demonstrating significant diabetic benefits [14,19,128,130,132].
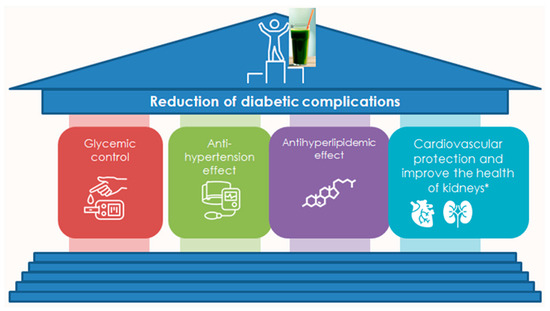
Figure 4.
The impact of Spirulina’s and Chlorella’s water extracts on the reduction of diabetic complications based on their nutritional profile and findings of previous studies (in vivo and in vitro) [19,20,127,128,129,130,131,132].
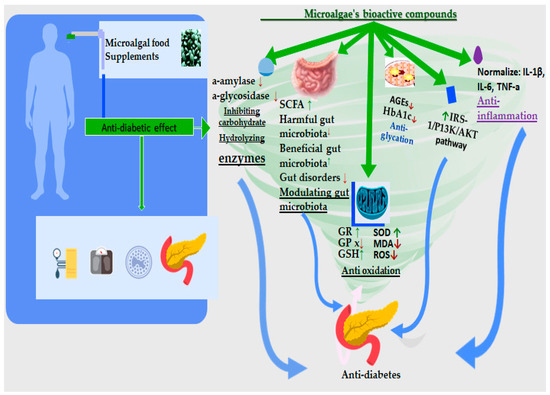
Figure 5.
Microalgal food supplements’ impact on T2DM and the antidiabetic mechanism of action of microalgae’s bioactive compounds [19,20,21].
4.3. Neuroprotective Properties
The potential neuroprotective effects of carotenoid-enriched extracts from various microalgal extracts (Haematococcus pluvialis, Porphyridium cru-entum, Nannochloropsis oceanica, and Tisochrysis lutea) and their anti-inflammatory properties were evaluated in the THP-1 cell model [12]. Anticholinergic activities of the microalgae extracts were found as well, with the exception of the N. oceanica microalga [15]. On the other hand, all microalgae extracts showed a partial anti-inflammatory effect through the attenuation of LPS-induced inflammation in THP-1 cells [12].
Numerous studies have reported the anti-inflammatory [133,134], antioxidant, anticancer [14,130], and antidiabetic effects of microalgae and their bioactive metabolites. In particular, the antidiabetic effect was demonstrated with a daily dose of 2 g Spirulina over 4 months [127]. In addition, a serving of 1 g of Spirulina contributed to the improvement of lung function in patients in combination with or without medication [135]. In addition, microalgae exhibit biological properties such as antimicrobial [136], antiviral, and immunomodulatory properties by influencing the production of cytokines, chemokines, and other inflammatory mediators, the production of antibodies by B lymphocytes, and the proliferation of T lymphocytes, as well as improving the lipid profile and redox capacity [12]. Based on the published results of previous clinical and preclinical studies, it can be assumed that microalgae and their bioactive compounds have the potential to be valued as a natural resource with novel bioactive molecules with remarkable neuroprotective properties [12] (Figure 6).
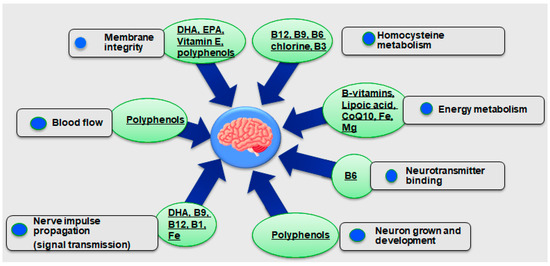
Figure 6.
The association between microalgae’s nutrients/bioactive compounds and brain health [5,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123].
There is growing evidence that microalgae-derived bioactive compounds and extracts can improve cholinergic function and antioxidant status and prevent memory impairment and neuronal damage in the Alzheimer’s disease (AD) brain. Their nutrients, such as carotenoids and isoflavones, could be explored for nutraceuticals and drugs to treat Alzheimer’s disease. However, the neuroprotective effects of microalgae-derived compounds and extracts in animal and clinical studies on Alzheimer’s disease have not yet been sufficiently explored. The neuroprotective activities of some microalgae strains are mediated by carotenoids, PUFAs, phenolic compounds, and sterols [18].
The extract of Spirulina maxima was tested against trimethyltin chloride-induced neurotoxicity in HT-22 hippocampal neuronal cells. Spirulina maxima had the ability to modulate immune factions with a dose of 50–100 μg/mL [137]. In addition, the results showed that Aurantiochytrium extract increased the proliferation rate and differentiation of neural stem cells and neural progenitor cells [138]. While Spirulina is a food supplement rich in essential nutrients, phycocyanin has specific therapeutic qualities related in particular to its antioxidant and anti-inflammatory properties [139]. In another study, the researchers included at the mechanism of action against PC12 of the microalgae Chlorella sp., the activation of caspase 3 in apoptotic cells that also had several nonapoptotic functions such as involving in tissue differentiation, regeneration, and neural development [131]. The available literature shows that the neuroprotective potential of microalgae was exerted on in vitro neuronal cell lines through their antioxidant and anti-inflammatory properties [12].
The cause of Parkinson’s disease is still not known, but some research shows that some factors may help protect against it. These factors include nutritional aspects such as a Spirulina diet, which in rats exhibited enhanced neurogenesis and decreased microglia activation as assessed by MHC-II expression [140]. Another study focused on another mechanism of action from Spirulina fusiform by improving locomotor activity and muscle coordination, increasing antioxidant levels while decreasing levels of the oxidative stress marker lipid peroxide [141], and upregulating BDNF expression, thereby improving cognitive abilities [142,143]. The results showed the suppressive effect of Nannochloropsis oceanica against neuro-inflammation, oxidative stress, and amyloidogenesis with improved memory function [12,144]. The prolongation of transit and escape latency in mice administered Spirulina maxima underpins the cognitive-enhancing effect of the microalgae [137]. The neuroprotective effect of SM was associated with increased phosphorylation of ERK and p-CREB and an increase in BDNF levels [145]. In conclusion, current neuroprotectors such as Spirulina cannot reverse existing damage, but they can protect against further nerve damage and slow down any degeneration of the central nervous system [17].
5. Discussion
After the first outbreak of COVID-19, mental health became a global priority due to the increase in mental disorders. There is an urgent need for cost-effective, scalable interventions to improve and maintain people’s mental wellbeing. Diabetes mellitus is a comorbidity that is usually associated with poor mental health [146]. Managing type 2 diabetes brings particular challenges as nutritional needs change with age. A well-planned diet not only helps control blood glucose levels but also promotes overall health, energy, and quality of life [147]. On the other hand, the climate crisis calls for the use of alternative energy sources [148]. Microalgae are high in lean protein, which supports muscle mass, which can decrease with age, and also helps stabilize blood sugar levels. In addition, microalgae fatty acids contribute positively to heart health, which is particularly important for the treatment of diabetics [147]. Some adults may benefit from special dietary supplements, especially if they have problems absorbing certain nutrients. This need combined with the large amount of scientific data on the medicinal effects of microalgae motivated the team of the University of Thessaly to conduct a bibliographic review and thorough research to investigate such a cultured food as an alternative protein source and to improve the health benefits according to their mechanism of action in different internal body organs.
The effect of a wide range of subcritical and supercritical conditions on the extraction of carotenoids from D. salina and their effect on the inhibition of AChE activity has been studied [16]. To this end, the extracts were chemically characterized, and a mathematical model was created to predict their AChE inhibitory activity. One of the potential targets for the bioactivity of these ingredients is the inhibition of acetylcholinesterase (AChE) activity, as a significant decrease in acetylcholine levels has been demonstrated in the hippocampus and cerebral cortex of Alzheimer’s patients [16]. Consequently, AChE inhibitors may not only cause an increase in acetylcholine levels in the brain but also reduce and prevent the formation of β-amyloid plaques, thus protecting neurons from neurodegeneration [17]. Previous studies found that D. salina extracts obtained with compressed CO2 can be used as a natural, safe, and sustainable alternative for future nutraceuticals or functional foods for the prevention of multifactorial neurological diseases such as Alzheimer’s disease [149].
Furthermore, a great number of phosphatidylcholines, triacylglycerols, and fatty acids significantly increased, while several phosphatidylglycerols decreased. This fact, along with the possible role exerted by carotenoids and other minor compounds on the cell membrane and on the chemical structure of the neurotoxic agents, might explain the observed antidiabetic effect (Figure 4 and Figure 5) [21] and neuroprotective effect (Figure 6) [12].
Moreover, the effects of the microalga Aurantiochytrium sp. on glucose regulation biomarkers have been studied in human beings as well [127]. The supplementation of Spirulina successfully reduced glucose blood levels [127]. The exact mechanism of the neuroprotective activities that are performed by microalgae is not fully understood. The protective activities that are derived from some microalgal strains are mediated by DHA, carotenoids, polyunsaturated fatty acids, phenolic compounds [19,128], sterols [150], and vitamin E [126] and can inhibit α-amylase and a-glycosidase activity [20] but also enhance insulin action by reducing HbA1c and AGEs [127]. Their mechanism also includes exerting antioxidants through increasing GSH, CAT, and GPx, reducing ROS, and modulating the gut microbiota by increasing SCFA anti-inflammatory properties (normalize IL-6, IL-ιβ, TNFa) [21] (Figure 7).

Figure 7.
Microalgae’s possible mechanism of action and their impact on overall health [5,6,7,8,9,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123].
The previous results highlight the necessity of using such plant-based nutrient products as an alternative medicine solution [127]. Furthermore, many age groups, for different reasons, could benefit from algae’s protein and lipidemic content (Table 1).
The functional properties of microalgae are indisputable, but in terms of legislation there are still some issues [9]. Food safety at the international level is governed by the Codex Alimentarius Commission (CAC), which was created by the Food and Agriculture Organization (FAO) and the World Health Organization (WHO). The Codex Alimentarius is a collection of internationally recognized standards, codes of practice, guidelines, and other recommendations relating to food production and food safety [9]. When it comes to food additives and novel foods, including nutraceuticals and functional foods, regulations may vary across countries. For instance, in the USA, the FDA is primarily responsible for regulating new food ingredients, while in the EU, the European Food Safety Authority (EFSA) regulates food additives, novel foods, and genetically modified organisms [9,90].
Generally Recognized as Safe (GRAS) is a status given by the Food and Drug Administration to any substance or chemical that is considered safe for human consumption [151]. There are only a few microalgae that have GRAS status as recognized by the FDA, including Arthrospira platensis, Chlamydomonas reinhard-tii, Auxenochlorella protothecoides, Chlorella vulgaris, Dunaliella bardawil, and Euglena gracilis [25].
In the European Union (EU), the European Food Safety Authority (EFSA) oversees food safety regulations for both human food and animal feed. Taking a “precautionary principle”, foods consumed within the EU prior to May 1997 are considered safe, and any other food, excluding Genetically Modified Organisms (GMOs), are labelled as “novel food” and must undergo a safety assessment by EFSA before being marketed [152]. New microalgae products entering the market must obtain this authorization, demonstrating that they do not pose any safety risks for human health, especially since some microalgae are known to produce phytotoxins [153,154]. However, an increasing number of microalgae species or microalgae extracts are now approved for human consumption as food or food ingredients in Europe [14] (Figure 3).
6. Conclusions
Microalgae have emerged as a potential source of bioactive compounds with applications in nutrition and health. Their antioxidant, antidiabetic, and neuroprotective properties suggest their possible use in functional foods and nutraceuticals. The bioactive components found in microalgae, including carotenoids, polyphenols, polysaccharides, vitamins, and polyunsaturated fatty acids, have demonstrated protective effects against oxidative stress, inflammation, and metabolic disorders in various studies.
The growing prevalence of chronic diseases such as diabetes and neurodegenerative conditions underscores the need for alternative dietary strategies and therapeutic agents. Research findings indicate that microalgae-derived compounds may play a role in modulating oxidative stress, improving insulin sensitivity, and supporting neuronal health. However, further clinical research is required to establish their efficacy, optimal dosages, and mechanisms of action in human populations.
Despite their potential, several challenges remain regarding the large-scale application of microalgae-based products, including production costs, extraction efficiency, bioavailability, and regulatory constraints. Advances in cultivation techniques and extraction methods may contribute to improving the commercial feasibility of microalgae-based functional foods and nutraceuticals. Moreover, ensuring compliance with regulatory frameworks and conducting thorough safety assessments will be essential for their integration into mainstream food and healthcare markets.
Future studies should focus on investigating the specific bioactive compounds found in microalgae, particularly their antidiabetic and neuroprotective properties. Research should aim to identify and isolate key metabolites such as carotenoids, polyphenols, polyunsaturated fatty acids, and other bioactive components to better understand their individual contributions to health outcomes. Studies could further explore the mechanisms by which these compounds exert their effects, particularly in relation to the pathological mechanisms underlying type 2 diabetes mellitus (T2DM) and Alzheimer’s disease.
Author Contributions
Conceptualization, O.G. and D.S.; methodology, O.G., D.S., A.K. and M.D.; software, D.S. and M.D.; validation, O.G., D.S. and M.D.; formal analysis, D.S. and M.D.; investigation, D.S., M.D. and A.K.; resources, D.S. and M.D.; data curation, D.S. and M.D.; writing—original draft preparation, D.S. and M.D.; writing—review and editing, O.G., D.S., A.K. and M.D.; visualization, O.G. and D.S.; supervision, O.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Khan, M.; Salman, M.; Ansari, J.; Bashir, U.; Malik, M.S.; Ikram, A. Joint external evaluation of IHR core capacities of the Islamic Republic of Pakistan 2016. Int. J. Infect. Dis. 2018, 73, 36–37. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Khavari, F.; Saidijam, M.; Taheri, M.; Nouri, F. Microalgae: Therapeutic potentials and applications. Mol. Biol. Rep. 2021, 48, 4757–4765. [Google Scholar] [CrossRef]
- Parameswari, R.P.; Lakshmi, T. Microalgae as a potential therapeutic drug candidate for neurodegenerative diseases. J. Biotechnol. 2022, 358, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A Promising Source of Valuable Bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products Towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.W.; Johnson, M.D.; Zhang, X.; Zemke, P.; Chen, W.; Hu, Q. A financial assessment of two alternative cultivation systems and their contributions to algae biofuel economic viability. Algal Res. 2014, 4, 96–104. [Google Scholar] [CrossRef]
- Matos, Â.P. The Impact of Microalgae in Food Science and Technology. J. Am. Oil Chem. Soc. 2017, 94, 1333–1350. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae for Human and Animal Nutrition. In Handbook of Microalgal Culture; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2013; pp. 461–503. [Google Scholar]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Gallego, R.; Valdés, A.; Suárez-Montenegro, Z.J.; Sánchez-Martínez, J.D.; Cifuentes, A.; Ibáñez, E.; Herrero, M. Anti-inflammatory and neuroprotective evaluation of diverse microalgae extracts enriched in carotenoids. Algal Res. 2022, 67, 102830. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef] [PubMed]
- Coulombier, N.; Jauffrais, T.; Lebouvier, N. Antioxidant Compounds from Microalgae: A Review. Mar. Drugs 2021, 19, 549. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Castagna, D.A.; Fortinguerra, S.; Buriani, A.; Scapagnini, G.; Willcox, D.C. Spirulina Microalgae and Brain Health: A Scoping Review of Experimental and Clinical Evidence. Mar. Drugs 2021, 19, 293. [Google Scholar] [CrossRef] [PubMed]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Therapeutic Potentials of Microalgae in the Treatment of Alzheimer’s Disease. Molecules 2017, 22, 480. [Google Scholar] [CrossRef] [PubMed]
- Abo-Shady, A.M.; Gheda, S.F.; Ismail, G.A.; Cotas, J.; Pereira, L.; Abdel-Karim, O.H. Antioxidant and antidiabetic activity of algae. Life 2023, 13, 460. [Google Scholar] [CrossRef]
- Tamel Selvan, K.; Goon, J.A.; Makpol, S.; Tan, J.K. Therapeutic potentials of microalgae and their bioactive compounds on diabetes mellitus. Mar. Drugs 2023, 21, 462. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, J.; Xiong, R.-G.; Wu, S.-X.; Xu, X.-Y.; Tang, G.-Y.; Huang, S.-Y.; Zhou, D.-D.; Li, H.; Feng, Y. Effects and mechanisms of anti-diabetic dietary natural products: An updated review. Food Funct. 2024, 15, 1758–1778. [Google Scholar] [CrossRef] [PubMed]
- Show, P.L. Global market and economic analysis of microalgae technology: Status and perspectives. Bioresour. Technol. 2022, 357, 127329. [Google Scholar]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Dillehay, T.; Ramírez, C.; Pino, M.; Collins, M.; Rossen, J.; Pino-Navarro, J. Monte Verde: Seaweed, Food, Medicine, and the Peopling of South America. Science 2008, 320, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.A.; Hasan, M. A Review on Culture, Production and Use of Spirulina as Food for Humans and Feed for Domestic Animals and Fish. FAO Fish. Aquac. Circ. 2008, 57, 171–180. [Google Scholar]
- Nicoletti, M. Microalgae Nutraceuticals. Foods 2016, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Bishop, W.; Zubeck, H. Evaluation of Microalgae for use as Nutraceuticals and Nutritional Supplements. J. Nutr. Food Sci. 2012, 2, 147. [Google Scholar] [CrossRef]
- de Morais, M.G.; Vaz Bda, S.; de Morais, E.G.; Costa, J.A. Biologically Active Metabolites Synthesized by Microalgae. BioMed Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef] [PubMed]
- de Mello-Sampayo, C.; Paterna, A.; Polizzi, A.; Duarte, D.; Batista, I.; Pinto, R.; Gonçalves, P.; Raymundo, A.; Batista, A.P.; Gouveia, L.; et al. Evaluation of Marine Microalga Diacronema vlkianum Biomass Fatty Acid Assimilation in Wistar Rats. Molecules 2017, 22, 1097. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, L.; Marques, A.; Sousa, J.; Moura, P.; Bandarra, N. Microalgae–source of natural bioactive molecules as functional ingredients. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 21–37. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine Bioactives as Functional Food Ingredients: Potential to Reduce the Incidence of Chronic Diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine Bioactive Compounds and Their Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Campanella, L.; Russo, M.V.; Avino, P. Free and total amino acid composition in blue-green algae. Ann. Chim. 2002, 92, 343–352. [Google Scholar] [PubMed]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef]
- Matos, Â.P.; Feller, R.; Moecke, E.H.S.; de Oliveira, J.V.; Junior, A.F.; Derner, R.B.; Sant’Anna, E.S. Chemical Characterization of Six Microalgae with Potential Utility for Food Application. J. Am. Oil Chem. Soc. 2016, 93, 963–972. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Vareltzis, P.; Gortzi, O. A Systematic Review of the Twelve Most Popular Bean Varieties, Highlighting Their Potential as Functional Foods Based on the Health Benefits Derived from Their Nutritional Profiles, Focused on Non-Communicable Diseases. Appl. Sci. 2024, 14, 10215. [Google Scholar] [CrossRef]
- Watanabe, F.; Katsura, H.; Takenaka, S.; Fujita, T.; Abe, K.; Tamura, Y.; Nakatsuka, T.; Nakano, Y. Pseudovitamin B12 Is the Predominant Cobamide of an Algal Health Food, Spirulina Tablets. J. Agric. Food Chem. 1999, 47, 4736–4741. [Google Scholar] [CrossRef]
- Watanabe, F. Vitamin B12 Sources and Bioavailability. Exp. Biol. Med. 2007, 232, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J. Appl. Phycol. 2015, 27, 1109–1119. [Google Scholar] [CrossRef]
- Milledge, J.J. Commercial application of microalgae other than as biofuels: A brief review. Rev. Environ. Sci. Bio Technol. 2011, 10, 31–41. [Google Scholar] [CrossRef]
- Brown, M.R. The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 1991, 145, 79–99. [Google Scholar] [CrossRef]
- Velasco, L.; Carrera, S.; Barros, J. Isolation, culture and evaluation of Chaetoceros muelleri from the Caribbean as food for the native scallops, Argopecten nucleus and Nodipecten nodosus. Lat. Am. J. Aquat. Res. 2016, 44, 557–568. [Google Scholar] [CrossRef]
- Christaki, E.; Florou-Paneri, P.; Bonos, E. Microalgae: A novel ingredient in nutrition. Int. J. Food Sci. Nutr. 2011, 62, 794–799. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Slocombe, S.P.; Ross, M.; Thomas, N.; McNeill, S.; Stanley, M.S. A rapid and general method for measurement of protein in micro-algal biomass. Bioresour. Technol. 2013, 129, 51–57. [Google Scholar] [CrossRef]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional Evaluation of Australian Microalgae as Potential Human Health Supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef]
- Barbarino, E.; Lourenço, S.O. An evaluation of methods for extraction and quantification of protein from marine macro- and microalgae. J. Appl. Phycol. 2005, 17, 447–460. [Google Scholar] [CrossRef]
- van Krimpen, M.M.; Bikker, P.; van der Meer, I.M.; van der Peet-Schwering, C.M.C.; Vereijken, J.M. Cultivation, Processing and Nutritional Aspects for Pigs and Poultry of European Protein Sources as Alternatives for Imported Soybean Products; Wageningen UR Livestock Research: Lelystad, The Netherlands, 2013. [Google Scholar]
- Wallace, J.S. Increasing agricultural water use efficiency to meet future food production. Agric. Ecosyst. Environ. 2000, 82, 105–119. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Schwenzfeier, A.; Wierenga, P.A.; Gruppen, H. Isolation and characterization of soluble protein from the green microalgae Tetraselmis sp. Bioresour. Technol. 2011, 102, 9121–9127. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.l.B.; Laurens, L.M.L. Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energy Environ. Sci. 2010, 3, 554–590. [Google Scholar]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef]
- Graziani, G.; Schiavo, S.; Nicolai, M.A.; Buono, S.; Fogliano, V.; Pinto, G.; Pollio, A. Microalgae as human food: Chemical and nutritional characteristics of the thermo-acidophilic microalga Galdieria sulphuraria. Food Funct. 2013, 4, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Innovative Natural Functional Ingredients from Microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar] [CrossRef]
- Chae, S.R.; Hwang, E.J.; Shin, H.S. Single cell protein production of Euglena gracilis and carbon dioxide fixation in an innovative photo-bioreactor. Bioresour. Technol. 2006, 97, 322–329. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Joy, J.M.; Lowery, R.P.; Wilson, J.M.; Purpura, M.; Souza, E.O.; Wilson, S.; Kalman, D.S.; Dudeck, J.E.; Jager, R. The effects of 8 weeks of whey or rice protein supplementation on body composition and exercise performance. Nutr. J. 2013, 12, 86. [Google Scholar] [CrossRef]
- Safi, C.; Ursu, A.V.; Laroche, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Aqueous extraction of proteins from microalgae: Effect of different cell disruption methods. Algal Res. 2014, 3, 61–65. [Google Scholar] [CrossRef]
- Templeton, D.W.; Laurens, L.M.L. Nitrogen-to-protein conversion factors revisited for applications of microalgal biomass conversion to food, feed and fuel. Algal Res. 2015, 11, 359–367. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Madsen, M. 14-Algal proteins. In Handbook of Food Proteins; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Sawston, UK, 2011; pp. 353–394. [Google Scholar]
- Lourenço, S.O.; Barbarino, E.; Lavín, L.; Lanfer Marquez, U.M.; Aidar, E. Distribution of intracellular nitrogen in marine microalgae: Calculation of new nitrogen-to-protein conversion factors. Eur. J. Phycol. 2004, 39, 17–32. [Google Scholar] [CrossRef]
- Günerken, E.; D’Hondt, E.; Eppink, M.H.M.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.; Pimentel, M. Sustainability of meat-based and plant-based diets and the environment1. Am. J. Clin. Nutr. 2003, 78, 660S–663S. [Google Scholar] [CrossRef]
- Guschina, I.A.; Harwood, J.L. Lipids and lipid metabolism in eukaryotic algae. Prog. Lipid Res. 2006, 45, 160–186. [Google Scholar] [CrossRef] [PubMed]
- Richmond, A. Biological Principles of Mass Cultivation of Photoautotrophic Microalgae. In Handbook of Microalgal Culture; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2013; pp. 169–204. [Google Scholar]
- Bellou, S.; Baeshen, M.N.; Elazzazy, A.M.; Aggeli, D.; Sayegh, F.; Aggelis, G. Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol. Adv. 2014, 32, 1476–1493. [Google Scholar] [CrossRef] [PubMed]
- Villarruel-López, A.; Ascencio, F.; Nuño, K. Microalgae, a Potential Natural Functional Food Source—A Review. Pol. J. Food Nutr. Sci. 2017, 67, 251–263. [Google Scholar] [CrossRef]
- Sprague, M.; Betancor, M.B.; Tocher, D.R. Microbial and genetically engineered oils as replacements for fish oil in aquaculture feeds. Biotechnol. Lett. 2017, 39, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.F.; Ríos Pinto, L.F.; Maciel Filho, R.; Fregolente, L.V. A review on lipid production from microalgae: Association between cultivation using waste streams and fatty acid profiles. Renew. Sustain. Energy Rev. 2019, 109, 448–466. [Google Scholar] [CrossRef]
- Tasić, M.B.; Pinto, L.S.; Klein, B.C.; Velijkovic, V.B.; Filho, R.M. Botryococcus braunii for biodiesel production. Renew. Sustain. Energy Rev. 2016, 64, 260–270. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Harrison, S.T.L. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 2009, 21, 493–507. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Manuell, A.L.; Beligni, M.V.; Elder, J.H.; Siefker, D.T.; Tran, M.; Weber, A.; McDonald, T.L.; Mayfield, S.P. Robust expression of a bioactive mammalian protein in Chlamydomonas chloroplast. Plant Biotechnol. J. 2007, 5, 402–412. [Google Scholar] [CrossRef]
- Arad, S.; Levy-Ontman, O. Red microalgal cell-wall polysaccharides: Biotechnological aspects. Curr. Opin. Biotechnol. 2010, 21, 358–364. [Google Scholar] [CrossRef]
- Sadovskaya, I.; Souissi, A.; Souissi, S.; Grard, T.; Lencel, P.; Greene, C.M.; Duin, S.; Dmitrenok, S.; Chizhov, A.O.; Shashkov, A.S.; et al. Chemical structure and biological activity of a highly branched (1→3,1→6)-β-d-glucan from Isochrysis galbana. Carbohydr. Polym. 2014, 111, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Turu, I.C.; Turkcan-Kayhan, C.; Kazan, A.; Yildiz-Ozturk, E.; Akgol, S.; Yesil-Celiktas, O. Synthesis and characterization of cryogel structures for isolation of EPSs from Botryococcus braunii. Carbohydr. Polym. 2016, 150, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Shibakami, M.; Tsubouchi, G.; Sohma, M.; Hayashi, M. Synthesis of nanofiber-formable carboxymethylated Euglena-derived β-1,3-glucan. Carbohydr. Polym. 2016, 152, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Busi, M.V.; Barchiesi, J.; Martín, M.; Gomez-Casati, D.F. Starch metabolism in green algae. Starch-Stärke 2014, 66, 28–40. [Google Scholar] [CrossRef]
- Gügi, B.; Le Costaouec, T.; Burel, C.; Lerouge, P.; Helbert, W.; Bardor, M. Diatom-Specific Oligosaccharide and Polysaccharide Structures Help to Unravel Biosynthetic Capabilities in Diatoms. Mar. Drugs 2015, 13, 5993–6018. [Google Scholar] [CrossRef] [PubMed]
- Dismukes, G.C.; Carrieri, D.; Bennette, N.; Ananyev, G.M.; Posewitz, M.C. Aquatic phototrophs: Efficient alternatives to land-based crops for biofuels. Curr. Opin. Biotechnol. 2008, 19, 235–240. [Google Scholar] [CrossRef]
- Raposo, M.F.d.J.; De Morais, R.M.S.C.; de Morais, A.M.M.B. Bioactivity and Applications of Sulphated Polysaccharides from Marine Microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S. Carotenoids in Algae: Distributions, Biosyntheses and Functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Coesel, S.N.; Baumgartner, A.C.; Teles, L.M.; Ramos, A.A.; Henriques, N.M.; Cancela, L.; Varela, J.C.S. Nutrient Limitation is the Main Regulatory Factor for Carotenoid Accumulation and for Psy and Pds Steady State Transcript Levels in Dunaliella salina (Chlorophyta) Exposed to High Light and Salt Stress. Mar. Biotechnol. 2008, 10, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Aremu, A.O.; Neményi, M.; Stirk, W.A.; Ördög, V.; van Staden, J. Manipulation of nitrogen levels and mode of cultivation are viable methods to improve the lipid, fatty acids, phytochemical content, and bioactivities in Chlorella minutissima. J. Phycol. 2015, 51, 659–669. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Coulombier, N.; Nicolau, E.; Le Déan, L.; Antheaume, C.; Jauffrais, T.; Lebouvier, N. Impact of Light Intensity on Antioxidant Activity of Tropical Microalgae. Mar. Drugs 2020, 18, 122. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar]
- Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Olasehinde, T.A.; Oyeleye, S.I.; Boligon, A.A.; Athayde, M.L. Phenolic Extract from Moringa oleifera Leaves Inhibits Key Enzymes Linked to Erectile Dysfunction and Oxidative Stress in Rats’ Penile Tissues. Biochem. Res. Int. 2015, 2015, 175950. [Google Scholar] [CrossRef]
- Machu, L.; Misurcova, L.; Vavra Ambrozova, J.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic Content and Antioxidant Capacity in Algal Food Products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Safafar, H.; Van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive oxygen species (ROS), oxygen radicals and antioxidants: Where are we now, where is the field going and where should we go? Biochem. Biophys. Res. Commun. 2022, 633, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Giurranna, E.; Nencini, F.; Bettiol, A.; Borghi, S.; Argento, F.R.; Emmi, G.; Silvestri, E.; Taddei, N.; Fiorillo, C.; Becatti, M. Dietary Antioxidants and Natural Compounds in Preventing Thrombosis and Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 11457. [Google Scholar] [CrossRef]
- Mansour, F.B.; Guermazi, W.; Chamkha, M.; Bellassoued, K.; Salah, H.B.; Harrath, A.H.; Aldahmash, W.; Rahman, M.A.; Ayadi, H. Bioactive Potential of the Sulfated Exopolysaccharides from the Brown Microalga Halamphora sp.: Antioxidant, Antimicrobial, and Antiapoptotic Profiles. Anal. Sci. Adv. 2024, 5, e202400030. [Google Scholar] [CrossRef]
- Morais, A.M.; Kumla, D.; Martins, V.F.; Alves, A.; Gales, L.; Silva, A.M.; Costa, M.; Mistry, S.; Kijjoa, A.; Morais, R.M. Monoterpene Hydroxy Lactones Isolated from Thalassiosira sp. Microalga and Their Antibacterial and Antioxidant Activities. Molecules 2024, 29, 5175. [Google Scholar] [CrossRef] [PubMed]
- Vignaud, J.; Loiseau, C.; Hérault, J.; Mayer, C.; Côme, M.; Martin, I.; Ulmann, L. Microalgae produce antioxidant molecules with potential preventive effects on mitochondrial functions and skeletal muscular oxidative stress. Antioxidants 2023, 12, 1050. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Kozani, S.; Kozani, S.; Pierre, G.; Michaud, P.; Delattre, C. Marine bacteria versus microalgae: Who is the best for biotechnological production of bioactive compounds with antioxidant properties and other biological applications? Mar. Drugs 2019, 18, 28. [Google Scholar] [CrossRef]
- Del Mondo, A.; Smerilli, A.; Sané, E.; Sansone, C.; Brunet, C. Challenging microalgal vitamins for human health. Microb. Cell Factories 2020, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, C.A.; Senhorinho, G.N.; Laamanen, C.A.; Scott, J.A. Microalgae as an alternative to oil crops for edible oils and animal feed. Algal Res. 2022, 64, 102663. [Google Scholar] [CrossRef]
- Ma, M.; Li, Y.; Chen, J.; Wang, F.; Yuan, L.; Li, Y.; Zhang, B.; Ye, D.; Han, D.; Jin, H. High-cell-density cultivation of the flagellate alga Poterioochromonas malhamensis for biomanufacturing the water-soluble β-1,3-glucan with multiple biological activities. Bioresour. Technol. 2021, 337, 125447. [Google Scholar] [CrossRef]
- Almendinger, M.; Saalfrank, F.; Rohn, S.; Kurth, E.; Springer, M.; Pleissner, D. Characterization of selected microalgae and cyanobacteria as sources of compounds with antioxidant capacity. Algal Res. 2021, 53, 102168. [Google Scholar] [CrossRef]
- Faraloni, C.; Di Lorenzo, T.; Bonetti, A. Impact of light stress on the synthesis of both antioxidants polyphenols and carotenoids, as fast photoprotective response in Chlamydomonas reinhardtii: New prospective for biotechnological potential of this microalga. Symmetry 2021, 13, 2220. [Google Scholar] [CrossRef]
- Katayama, T.; Rahman, N.A.; Khatoon, H.; Kasan, N.A.; Nagao, N.; Yamada, Y.; Takahashi, K.; Furuya, K.; Abd Wahid, M.E.; Yusoff, F.M. Bioprospecting of tropical microalgae for high-value products: N-3 polyunsaturated fatty acids and carotenoids. Aquac. Rep. 2022, 27, 101406. [Google Scholar] [CrossRef]
- Chalima, A.; Boukouvalas, C.; Oikonomopoulou, V.; Topakas, E. Optimizing the production of docosahexaenoic fatty acid by Crypthecodinium cohnii and reduction in process cost by using a dark fermentation effluent. Chem. Eng. J. Adv. 2022, 11, 100345. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, L.; Zheng, H.; Huang, H.; Qian, Z.J. Microalgae Octapeptide IIAVEAGC Alleviates Oxidative Stress and Neurotoxicity in 6-OHDA-Induced SH-SY5Y Cells by Regulating the Nrf2/HO-1and Jak2/Stat3 Pathways. Chem. Biodivers. 2024, 21, e202301509. [Google Scholar] [CrossRef]
- Brasil, F.B.; Bertolini Gobbo, R.C.; Souza de Almeida, F.J.; Luckachaki, M.D.; Dall’oglio, E.L.; de Oliviera, M.R. The signaling pathway PI3K/Akt/Nrf2/HO-1 plays a role in the mitochondrial protection promoted by astaxanthin in the SH-SY5Y cells exposed to hydrogen peroxide. Neurochem. Int. 2021, 146, 105024. [Google Scholar] [CrossRef]
- Stasiewicz, A.; Conde, T.; Gęgotek, A.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Prevention of UVB induced metabolic changes in epidermal cells by lipid extract from microalgae Nannochloropsis oceanica. Int. J. Mol. Sci. 2023, 24, 11302. [Google Scholar] [CrossRef]
- Kim, K.M.; Lee, J.Y.; Im, A.-R.; Chae, S. Phycocyanin protects against UVB-induced apoptosis through the PKC α/βII-Nrf-2/HO-1 dependent pathway in human primary skin cells. Molecules 2018, 23, 478. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, H.F.; Khalifa, H.K.; Omran, A.; Korany, R.M.; Selim, S.; Hussein, E.; Alhotan, R.A.; Ayyoub, A.; Masoud, S.R. Unveiling the Potential of Silymarin, Spirulina platensis, and Chlorella vulgaris towards Cardiotoxicity via Modulating Antioxidant Activity, Inflammation, and Apoptosis in Rats. Life 2024, 14, 1289. [Google Scholar] [CrossRef] [PubMed]
- Sikiru, A.B.; Arangasamy, A.; Alemede, I.; Guvvala, P.; Egena, S.; Ippala, J.; Bhatta, R. Chlorella vulgaris supplementation effects on performances, oxidative stress and antioxidant genes expression in liver and ovaries of New Zealand White rabbits. Heliyon 2019, 5, e02470. [Google Scholar] [CrossRef] [PubMed]
- Bulut, O.; Köse, I.E.; Sönmez, Ç.; Öktem, H.A. Antioxidant activity of Micractinium sp. (Chlorophyta) extracts against H2O2 induced oxidative stress in human breast adenocarcinoma cells. Sci. Rep. 2024, 14, 27593. [Google Scholar] [CrossRef] [PubMed]
- Alavianghavanini, A.; Moheimani, N.R.; Bahri, P.A. Process design and economic analysis for the production of microalgae from anaerobic digestates in open raceway ponds. Sci. Total Environ. 2024, 923, 171554. [Google Scholar] [CrossRef]
- Rangel Pinto, J.D.; Guerrero, J.L.; Rivera, L.; Parada-Pinilla, M.P.; Cala, M.P.; López, G.; González Barrios, A.F. Predicting the microalgae lipid profile obtained by supercritical fluid extraction using a machine learning model. Front. Chem. 2024, 12, 1480887. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, W.; Li, X.; Chen, X.; Wang, Y.; Huang, G.; Wang, J.; Jia, Z. Enzyme-Assisted Ultrasonic Extraction and Antioxidant Activities of Polysaccharides from Schizochytrium limacinum Meal. Foods 2024, 13, 880. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mafukidze, D.; Zheng, Y. Microalgae aggregation induced by thermoresponsive polymers. Bioresour. Technol. 2025, 415, 131650. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Perumal, K.; Sumathi, Y.; Singhania, R.R.; Chen, C.-W.; Dong, C.-D.; Patel, A.K. Nano-Enabled Microalgae Bioremediation: Advances in Sustainable Pollutant Removal and Value-Addition. Environ. Res. 2024, 263, 120011. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA); Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; et al. Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA J. 2016, 14, e04594. [Google Scholar]
- McKenzie, C.H.; Bates, S.S.; Martin, J.L.; Haigh, N.; Howland, K.L.; Lewis, N.I.; Locke, A.; Peña, A.; Poulin, M.; Rochon, A.; et al. Three decades of Canadian marine harmful algal events: Phytoplankton and phycotoxins of concern to human and ecosystem health. Harmful Algae 2021, 102, 101852. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulou, M.; Vareltzis, P.; Floros, S.; Androutsos, O.; Bargiota, A.; Gortzi, O. Development of a Functional Acceptable Diabetic and Plant-Based Snack Bar Using Mushroom (Coprinus comatus) Powder. Foods 2023, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, F.; Amerizadeh, A.; Behshood, P.; Moradi, S.; Asgary, S. Effect of microalgae Arthrospira on biomarkers of glycemic control and glucose metabolism: A systematic review and meta-analysis. Curr. Probl. Cardiol. 2022, 47, 100942. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Ali, M.S.; Madkour, F.F.; Elgendy, H. Oral spirulina platensis attenuates hyperglycemia and exhibits antinociceptive effect in streptozotocin-induced diabetic neuropathy rat model. J. Pain Res. 2020, 13, 2289–2296. [Google Scholar] [CrossRef]
- Lympaki, F.; Giannoglou, M.; Magriplis, E.; Bothou, D.L.; Andreou, V.; Dimitriadis, G.D.; Markou, G.; Zampelas, A.; Theodorou, G.; Katsaros, G.; et al. Short-term effects of spirulina consumption on glycemic responses and blood pressure in healthy young adults: Results from two randomized clinical trials. Metabolites 2022, 12, 1180. [Google Scholar] [CrossRef] [PubMed]
- Nazih, H.; Bard, J.-M. Microalgae in human health: Interest as a functional food. In Microalgae in Health and Disease Prevention; Academic Press: Cambridge, UK, 2018; pp. 211–226. [Google Scholar]
- Vahdati, S.N.; Lashkari, A.; Navasatli, S.A.; Ardestani, S.K.; Safavi, M. Butylated hydroxyl-toluene, 2,4-Di-tert-butylphenol, and phytol of Chlorella sp. protect the PC12 cell line against H2O2-induced neurotoxicity. Biomed. Pharmacother. 2022, 145, 112415. [Google Scholar] [CrossRef]
- Piovan, A.; Battaglia, J.; Filippini, R.; Dalla Costa, V.; Facci, L.; Argentini, C.; Pagetta, A.; Giusti, P.; Zusso, M. Pre-and early post-treatment with Arthrospira platensis (Spirulina) extract impedes lipopolysaccharide-triggered neuroinflammation in microglia. Front. Pharmacol. 2021, 12, 724993. [Google Scholar] [CrossRef] [PubMed]
- Tabarzad, M.; Atabaki, V.; Hosseinabadi, T. Anti-inflammatory activity of bioactive compounds from microalgae and cyanobacteria by focusing on the mechanisms of action. Mol. Biol. Rep. 2020, 47, 6193–6205. [Google Scholar] [CrossRef] [PubMed]
- Choo, W.-T.; Teoh, M.-L.; Phang, S.-M.; Convey, P.; Yap, W.-H.; Goh, B.-H.; Beardall, J. Microalgae as potential anti-inflammatory natural product against human inflammatory skin diseases. Front. Pharmacol. 2020, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Unadkat, K.; Parikh, P.; Chandrasekhar, K. Algae mediated pesticides bioremediation: Mechanisms, approaches, limitations, and prospects for future research. In Pesticides Bioremediation; Springer: Berlin/Heidelberg, Germany, 2022; pp. 353–380. [Google Scholar]
- Alsenani, F.; Tupally, K.R.; Chua, E.T.; Eltanahy, E.; Alsufyani, H.; Parekh, H.S.; Schenk, M. Evaluation of microalgae and cyanobacteria as potential sources of antimicrobial compounds. Saudi Pharm. J. 2020, 28, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.-J.; Kim, K.-J.; Song, J.-H.; Choi, J.; Lee, H.Y.; Kang, D.-H.; Heo, H.J.; Lee, B.-Y. Spirulina maxima extract ameliorates learning and memory impairments via inhibiting GSK-3β phosphorylation induced by intracerebroventricular injection of amyloid-β 1–42 in mice. Int. J. Mol. Sci. 2017, 18, 2401. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Othman, M.B.; Ferdousi, F.; Yoshida, M.; Watanabe, M.; Tominaga, K.; Isoda, H. Modulation of the neurotransmitter systems through the anti-inflammatory and antidepressant-like effects of squalene from Aurantiochytrium sp. PLoS ONE 2019, 14, e0218923. [Google Scholar] [CrossRef] [PubMed]
- Min, S.K.; Park, J.S.; Luo, L.; Kwon, Y.S.; Lee, H.C.; Shim, H.J.; Kim, I.-D.; Lee, J.-K.; Shin, H.S. Assessment of C-phycocyanin effect on astrocytes-mediated neuroprotection against oxidative brain injury using 2D and 3D astrocyte tissue model. Sci. Rep. 2015, 5, 14418. [Google Scholar] [CrossRef] [PubMed]
- Pabon, M.M.; Jernberg, J.N.; Morganti, J.; Contreras, J.; Hudson, C.E.; Klein, R.L.; Bickford, C. A Spirulina-Enhanced diet provides neuroprotection in an α-synuclein model of Parkinson’s disease. PLoS ONE 2012, 7, e45256. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyaya, I.; Gupta, S.; Mohammed, A.; Mushtaq, N.; Chauhan, S.; Ghosh, S. Neuroprotective effect of Spirulina fusiform and amantadine in the 6-OHDA induced Parkinsonism in rats. BMC Complement. Altern. Med. 2015, 15, 296. [Google Scholar]
- Hwang, J.-H.; Lee, I.-T.; Jeng, K.-C.; Wang, M.-F.; Hou, R.C.-W.; Wu, S.-M.; Chan, Y.-C. Spirulina prevents memory dysfunction, reduces oxidative stress damage and augments antioxidant activity in senescence-accelerated mice. J. Nutr. Sci. Vitaminol. 2011, 57, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.-J.; Seo, Y.-J.; Choi, J.; Lee, H.Y.; Kang, D.-H.; Kim, K.-J.; Lee, B.-Y. Spirulina maxima extract prevents neurotoxicity via promoting activation of BDNF/CREB signaling pathways in neuronal cells and mice. Molecules 2017, 22, 1363. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Hwang, C.J.; Lee, H.P.; Kim, H.S.; Han, S.-B.; Hong, J.T. Inhibitory effect of ethanol extract of Nannochloropsis oceanica on lipopolysaccharide-induced neuroinflammation, oxidative stress, amyloidogenesis and memory impairment. Oncotarget 2017, 8, 45517. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Jang, H.M.; Kan, E. Microalgal biomass and lipid production on dairy effluent using a novel microalga, Chlorella sp. isolated from dairy wastewater. Biotechnol. Bioprocess Eng. 2018, 23, 333–340. [Google Scholar] [CrossRef]
- Moradian, S.; Teufel, M.; Jahre, L.; Musche, V.; Fink, M.; Dinse, H.; Schweda, A.; Weismüller, B.; Dörrie, N.; Tan, S. Mental health burden of patients with diabetes before and after the initial outbreak of COVID-19: Predictors of mental health impairment. BMC Public Health 2021, 21, 2068. [Google Scholar] [CrossRef] [PubMed]
- Gortzi, O.; Dimopoulou, M.; Androutsos, O.; Vraka, A.; Gousia, H.; Bargiota, A. Effectiveness of a Nutrition Education Program for Patients with Type 2 Diabetes Mellitus. Appl. Sci. 2024, 14, 2114. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Kolonas, A.; Mourtakos, S.; Androutsos, O.; Gortzi, O. Nutritional Composition and Biological Properties of Sixteen Edible Mushroom Species. Appl. Sci. 2022, 12, 8074. [Google Scholar] [CrossRef]
- Bueno, M.; Gallego, R.; Chourio, A.M.; Ibáñez, E.; Herrero, M.; Saldaña, M.D. Green ultra-high pressure extraction of bioactive compounds from Haematococcus pluvialis and Porphyridium cruentum microalgae. Innov. Food Sci. Emerg. Technol. 2020, 66, 102532. [Google Scholar] [CrossRef]
- Yu, Z.; Hong, Y.; Xie, K.; Fan, Q. Research progresses on the physiological and pharmacological benefits of microalgae-derived biomolecules. Foods 2022, 11, 2806. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. Generally Recognized as Safe (GRAS). 2023. Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 17 October 2023).
- EFSA. Guidance on the preparation and submission of an application for authorisation of a novel food in the context of Regulation (EU) 2015/22831 (Revision 1) 2. EFSA J. 2021, 19, e06555. [Google Scholar]
- Lefebvre, K.A.; Robertson, A. Domoic acid and human exposure risks: A review. Toxicon 2010, 56, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.D.; Vasconcelos, V. Legal Aspects of Microalgae in the European Food Sector. Foods 2024, 13, 124. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).