Selecting Catalysts for Pyrolysis of Lignocellulosic Biomass
Abstract
:1. Introduction
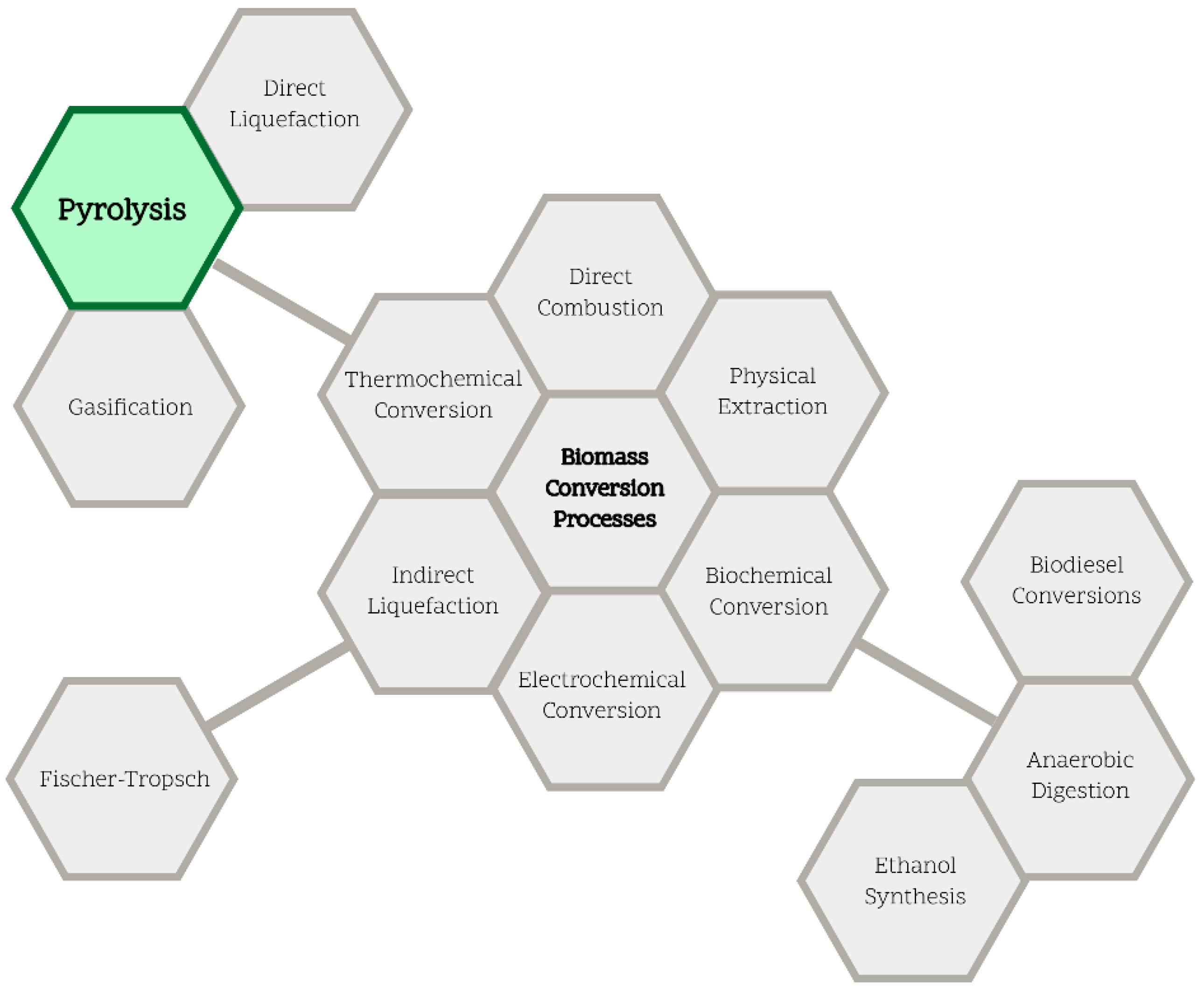
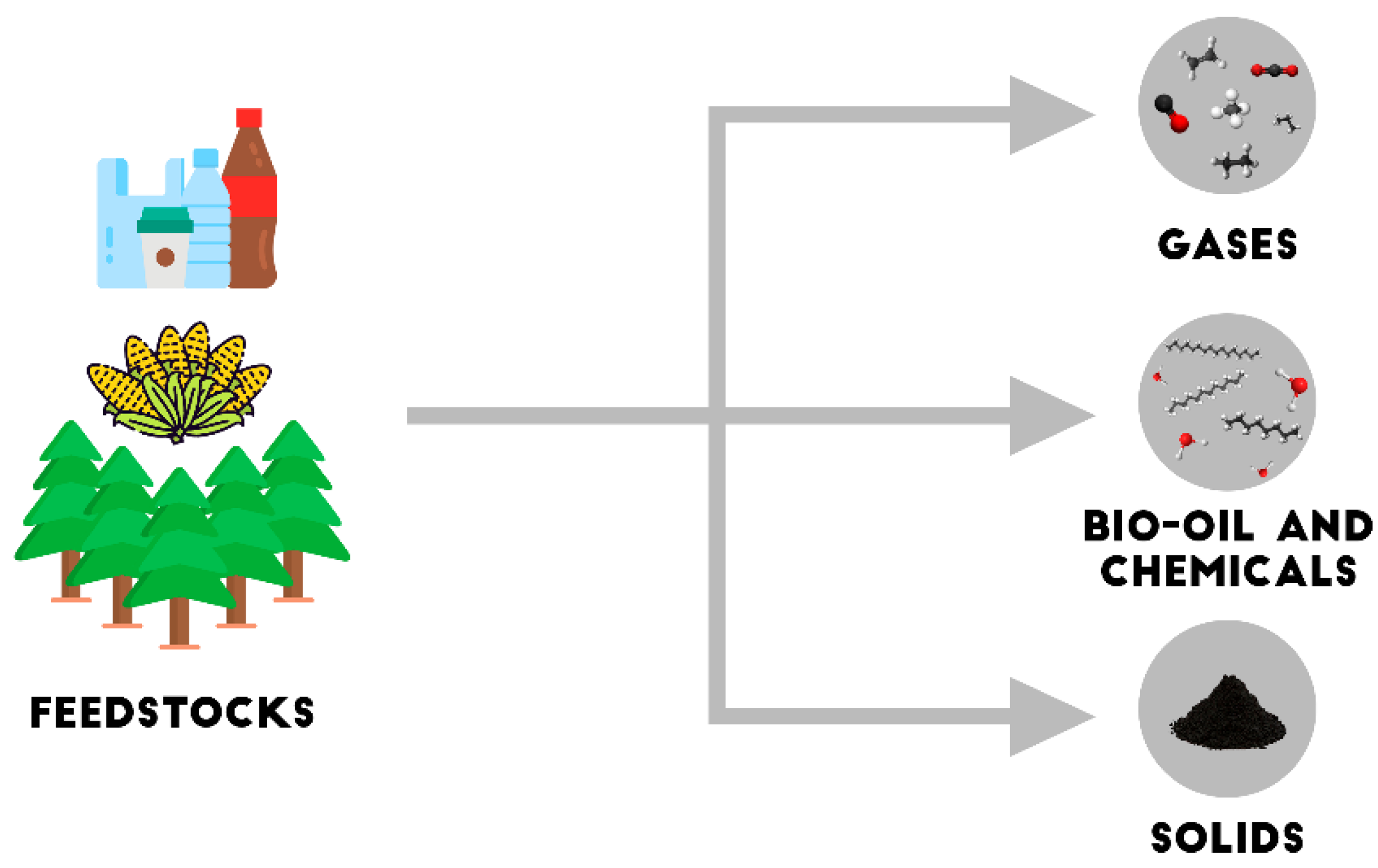
2. Biomass Pyrolysis
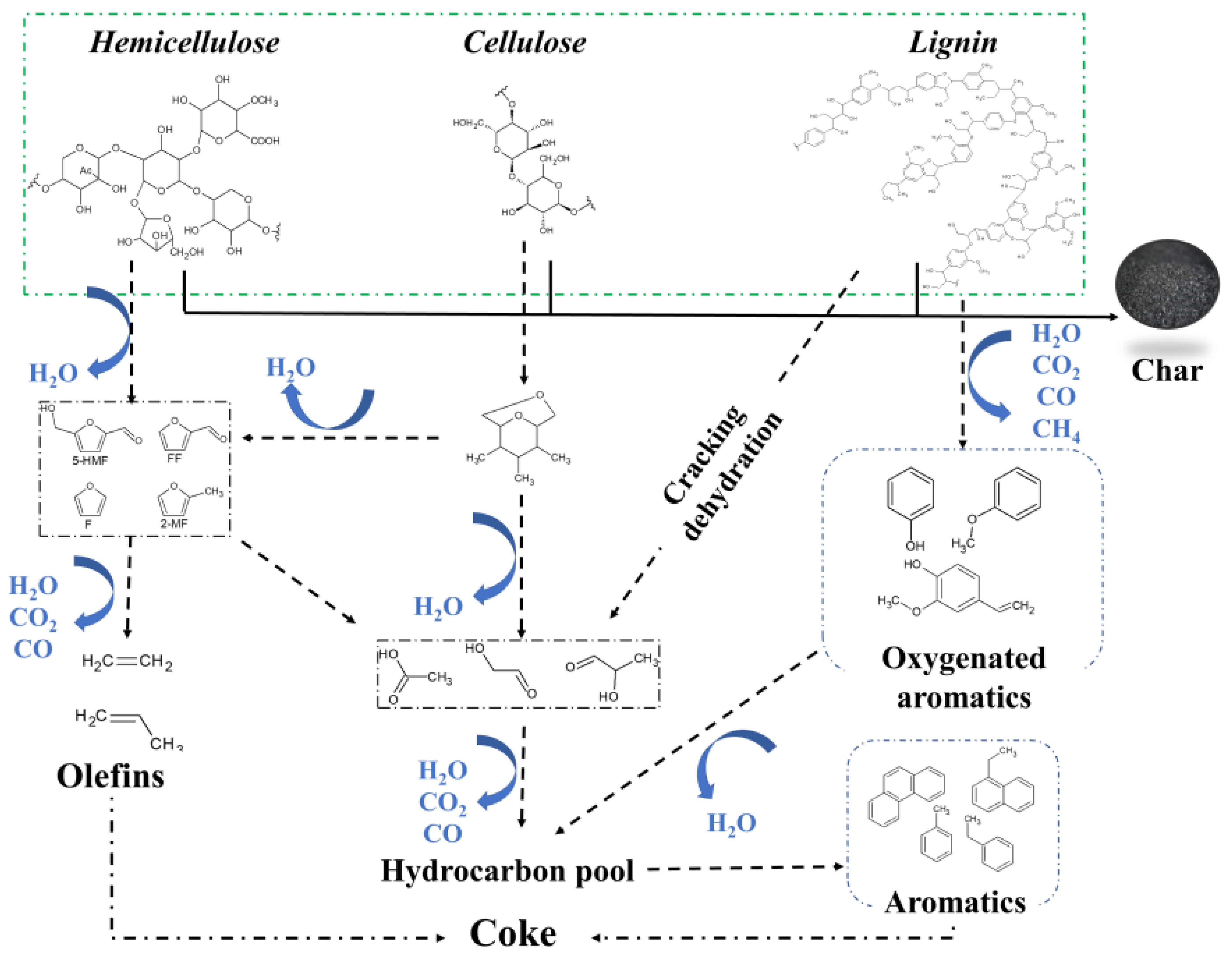
3. Reaction Pathway during Biomass Pyrolysis
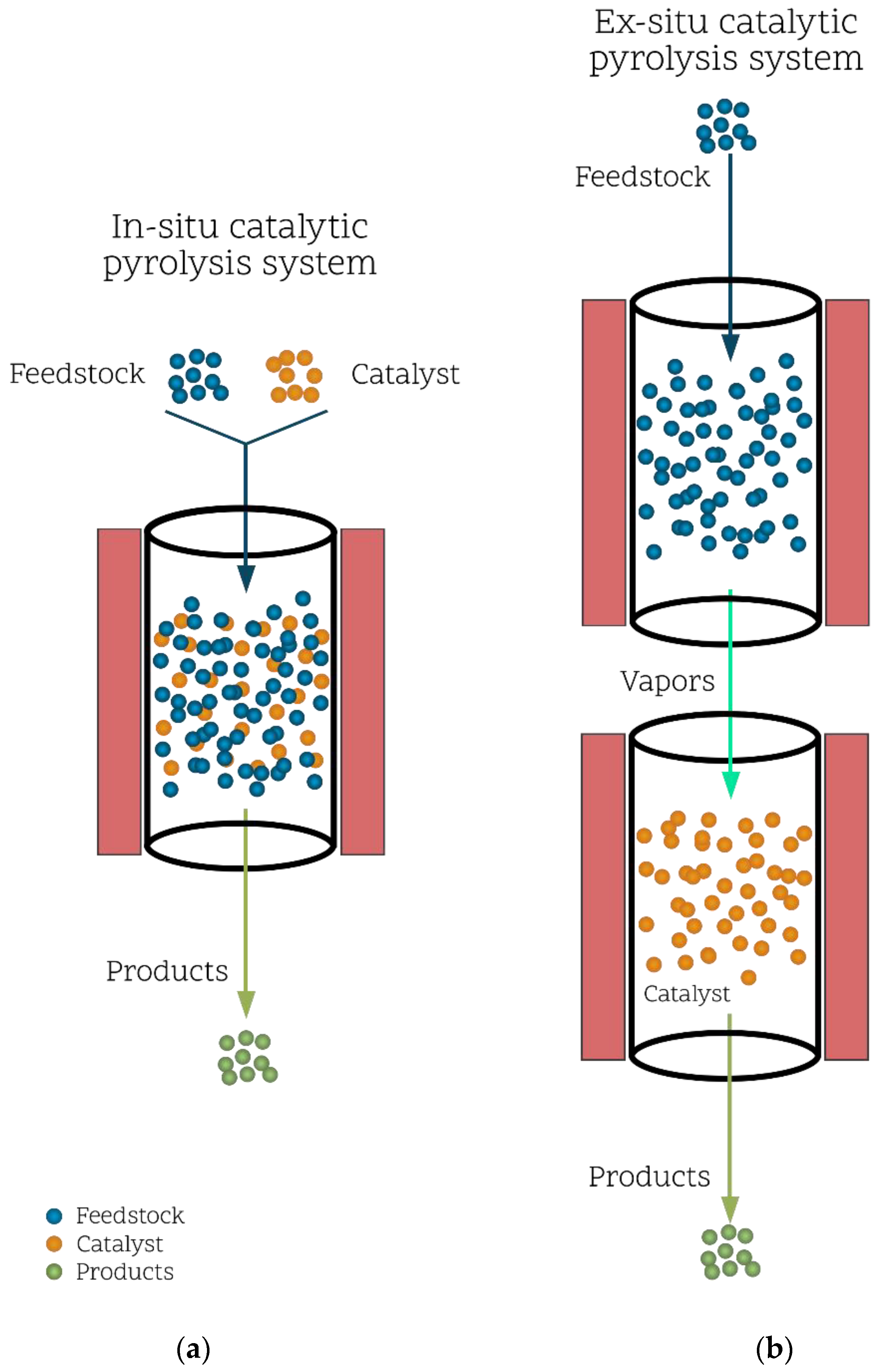
4. Catalytic Pyrolysis of Biomass
5. Properties of the Catalysts for Biomass Pyrolysis
5.1. Specific Surface Area and Porosity
5.1.1. Oxides and Other Microporous Solids
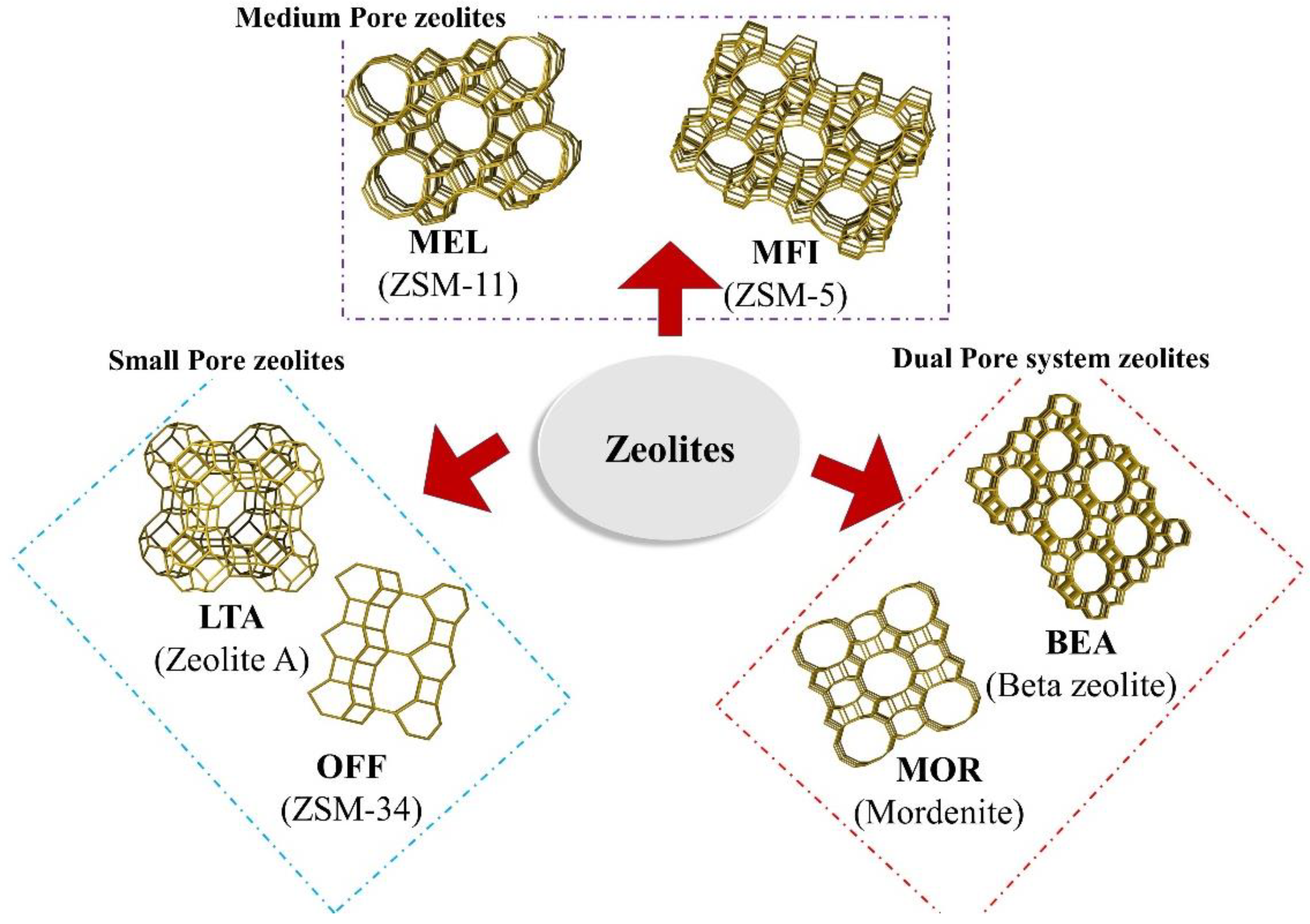
5.1.2. Zeolites and Mesoporous Materials
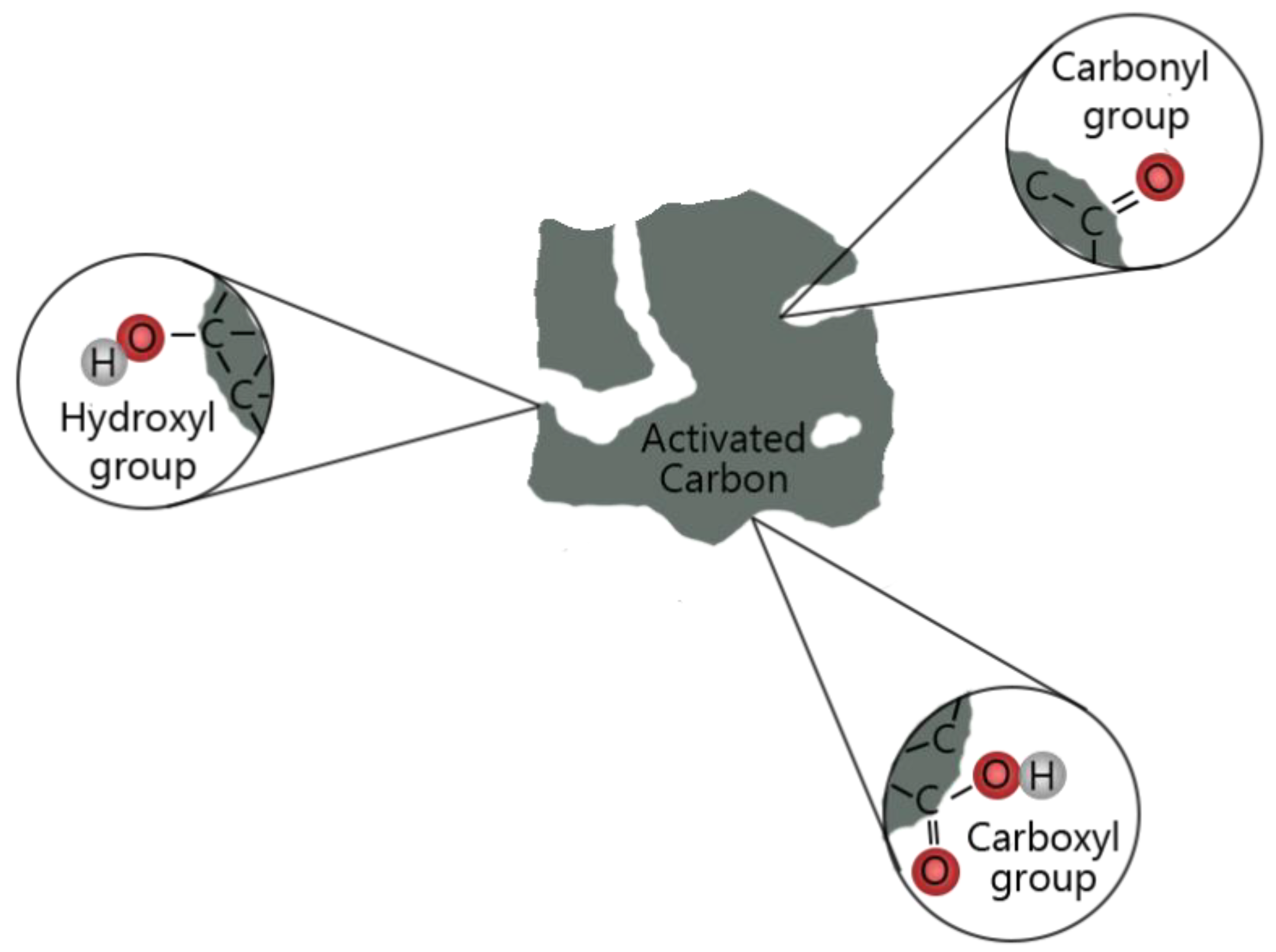
5.1.3. Carbonaceous Materials
5.2. Acidic and Basic Sites
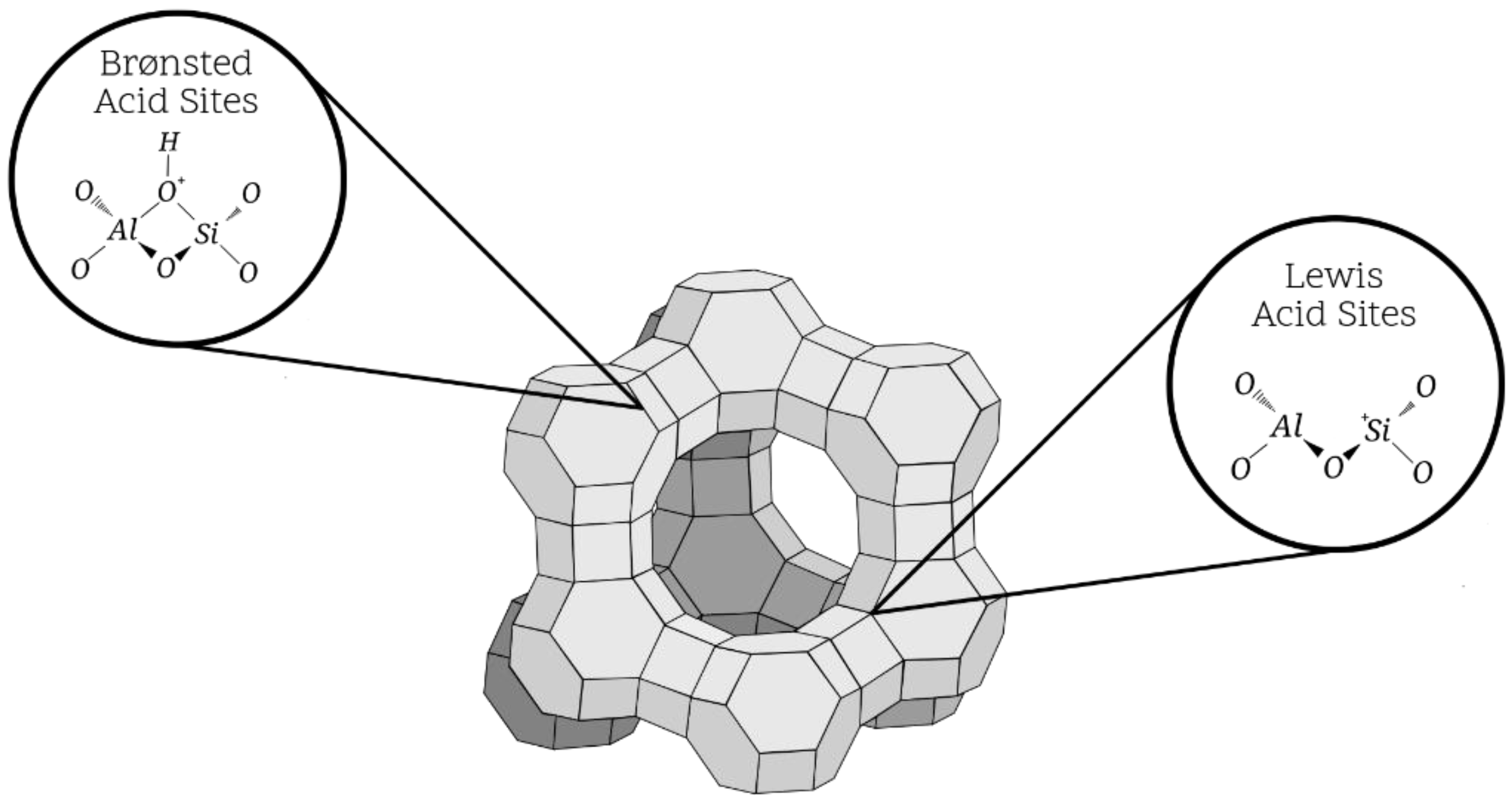
5.2.1. Acidic and Basic Zeolites
5.2.2. Acidic and Basic Metal Oxide Catalysts
5.2.3. Metal Salts Catalysts
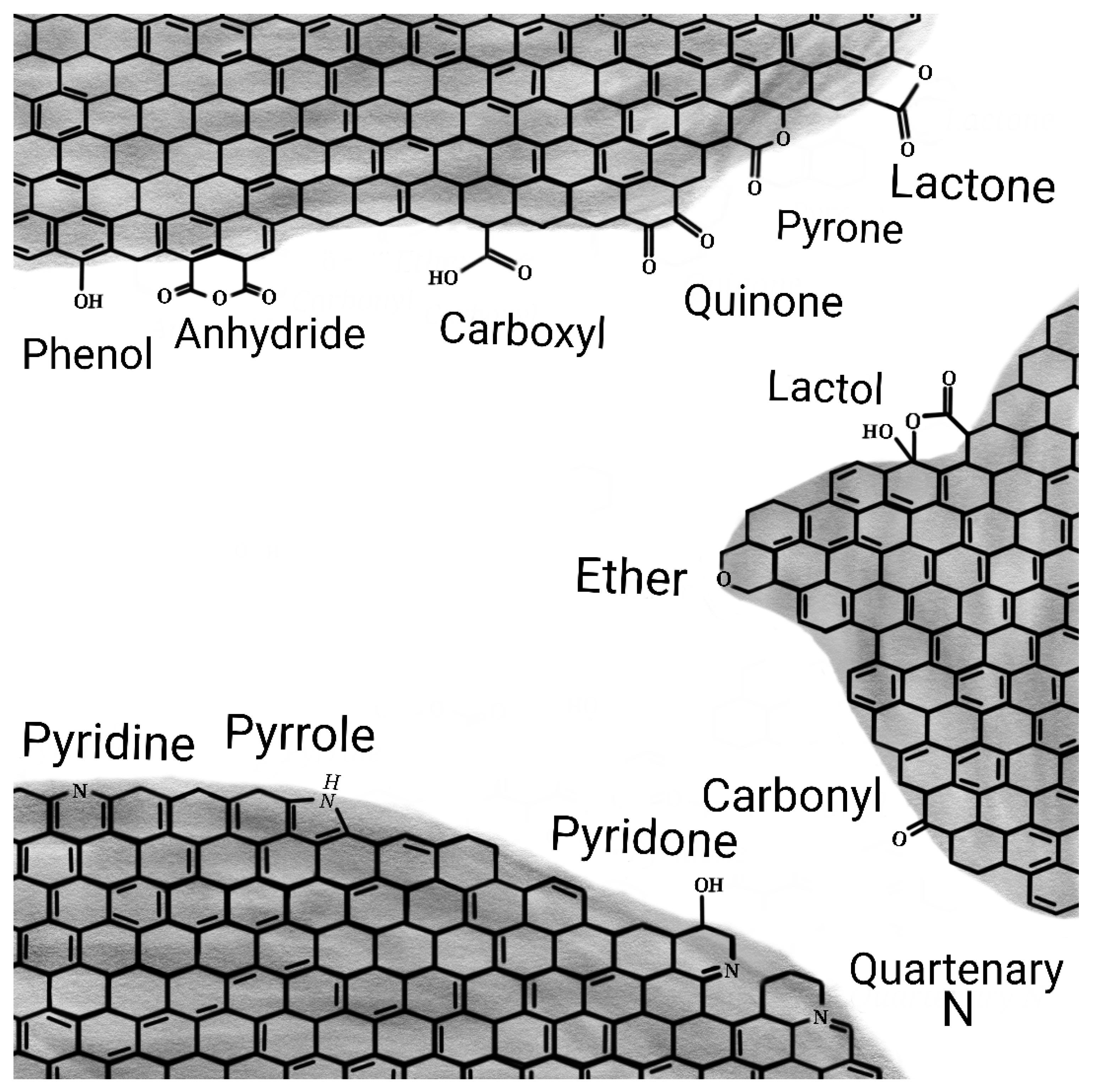
5.2.4. Acidic and Basic Carbonaceous Catalysts
5.3. Redox Properties of the Catalysts
5.3.1. Zeolites-Based Catalysts
| Metal Loading | Zeolite Framework | Feedstock | Operational Conditions | Ref. |
|---|---|---|---|---|
| Ga | HZSM-5 | Cotton-stalk | T: 723–1123 K t: 20 s hr: 20 K/ms | [143] |
| Fe, La, Cu, Mg, Al and Ce | ZSM-5 | Cellulose | T: 873 K p(N2): 300 mL/min t: 30 min | [144] |
| Zn | HZSM-5 | Milled wood Lignin | T: 923 K t: 20 s hr: 20 K/ms | [145] |
| none | HY, USY | |||
| none | ZSM-5/H-Beta | Wood polymer composites | T: 773–873 K t: 20 K/min | [53] |
| none | Y-zeolite | Coal | T: 973 °C t: 10 K/ms hr: 15 s | [146] |
| none | HY-zeolite | Baiyinhua lignite | T: 873 K p(N2): 300 mL/min t: 30 min | [147] |
| none | HY-zeolite | Waste Engine Oil | T: 773 K p(N2): 80 mL/min | [148] |
| Ni | ZSM-5 | Lignin | T: 723 K p(N2): 97 cm3/min t: <5 s | [149] |
| Fe | ZSM-5 | Wood sawdust | T: 553 K p(N2): 30 mL/min hr: 10 K/min | [150] |
| Sr, Ni, Cu, Ag and Fe | Y-zeolite | Waste cooking oil | T: 823–1023 K t: 30 s hr: 10 K/min | [151] |
5.3.2. Oxide-Based Catalysts
6. Catalyst to Biomass Ratio
7. Co-Pyrolysis of Biomass and Hydrogen Donors
8. Guidelines for Selecting Catalysts for Pyrolysis of Lignocellulosic Biomass
- The acidic sites facilitate the breaking of C-C and C-O bonds, and then are able to catalyze the majority of reactions that occur during the pyrolysis of lignocellulosic biomass, such as cracking, aromatization, dehydration, decarboxylation, decarbonylation, oligomerization, polymerization/depolymerization, ketylation and H-transfer reactions.
- The metallic sites act cooperatively with the acidic sites through a bifunctional mechanism, and also promote hydrogenation/dehydrogenation reactions.
- Acidic zeolites and hierarchical zeolites are the most studied catalysts for lignocellulosic biomass pyrolysis because of intrinsic acidity and large pores. In addition, the pores can be tailored to favor the formation of the desired products by shape selectivity. Due to these properties, they are the best option by which to obtain monoaromatics, such as the high-value benzene, toluene and xylenes (BTX), without polyaromatics and coke formation. However, large pores can also allow the production of undesirable polyaromatics, which, in turn, can be avoided by the metal. Therefore, the pores have to be modulated. The use of a metal is usually beneficial in such cases.
- Catalysts based on acidic metal oxides usually lead to the production of aromatics, anhydrosugars and furans, in addition to gasses and solids, during pyrolysis of lignocellulosic biomass; however, the kind and distribution of products depend on the metal oxide.
- Activated carbons are normally used to catalyze the production of levoglucosenone and phenols. The activity and selectivity of such catalysts are modulated by functional groups on the surface.
- Alkali and alkaline earth metal oxides selectively catalyze the formation of phenolic compounds, ketones and furans, but also produce high-quality bio-oil. On the other hand, transition metal oxides are the most suitable to produce phenolic compounds because of their basic properties. The balance between acidic and basic sites affects the distribution of products obtained thanks to the formation of other compounds, such as alcohols, furans, ketones and phenolics.
- Basic zeolites (modified with a basic component) are also active and selective in biomass pyrolysis producing high quality bio-oil, due to a decrease in oxygenates and acidic compounds, and a small amount of coke. They are usually more active than acidic zeolite in deoxygenation.
- The catalyst-to-biomass ratio is a critical process variable for catalyst performance during biomass pyrolysis, the optimal ratio being found between 0.3 and 0.7. However, this range may change according to the catalyst, variable processes and biomass.
- The efficiency of pyrolysis of lignocellulosic biomass can be largely improved by adding hydrogen donors to the process (co-pyrolysis), such as plastics, ethanol and methanol. Several plastics have been studied, including low- (LDPE) and high (HDPE)-density polyethylene, polyethylene terephthalate (PET), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC) and plastic residues. The latter is particularly attractive for environmental reasons.
- Obtaining target products from biomass pyrolysis requires the careful planning of catalyst properties combined with the process variables according to the biomass to be used. Co-pyrolysis of plastic residues is attractive as an economic and eco-friendly route, providing clean fuels and chemicals, as well as decreasing plastic waste around the world.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| AH | aromatic hydrocarbon |
| Al-MCM-41 | Mobil Composition of Matter-forty-one impregnated with aluminum |
| Al-Fe-MCM-41 | Mobil Composition of Matter-forty-one impregnated with aluminum and iron |
| ASU-7 | Arizona State University (seven) ([(DMA)2(H2O)2 [Ge20O40] where DMA = dimethylamine) |
| ASV | Arizona Seven, (group, [(DMA)2(H2O)2 [Ge20O40] where DMA = dimethylamine) |
| AZ | Hierarchical HZSM-5 obtained by conventional desilication |
| AZM | micro-mesoporous composite catalyst produced by alkali treatment |
| BEA | Zeolite Beta polymorph A, (group Na7[Al7Si57O128]) |
| BTEX | benzene, toluene, ethylbenzene, and xylenes |
| BTX | benzene, toluene, and xylenes |
| CO2-TPD | carbon dioxide temperature-programmed desorption |
| Cu-MCM-41 | Mobil Composition of Matter-forty-one impregnated with cupper |
| FCC | Fluid Catalytic Cracking |
| Fe-MCM-41 | Mobil Composition of Matter-forty-one impregnated with iron |
| HC | hydrocarbons |
| HDO | hydrodeoxygenation |
| HDPE | high density polyethylene |
| hr | heating rate |
| ISV | Instituto de Tecnologia Quimica Valencia (seven) (group, Si64O128) |
| ITQ-7 | Instituto de Tecnologia Quimica Valencia (seven) (Si64O128) |
| ITE | Instituto de Tecnologia Quimica Valencia (three) (group, Si64O128) |
| ITQ-3 | Instituto de Tecnologia Quimica Valencia (three) (Si64O128) |
| LDPE | low density polyethylene |
| LTA | Linde Type A, (group, [Na12(H2O)278]8[Al12Si12O48]8) |
| MAH | monoaromatic hydrocarbon |
| MCM-41 | Mobil Composition of Matter-forty-one, SiO2 |
| MDF | medium density fiber |
| MEL | Mobil—eleven (group, Nax(H2O)16[AlxSi96-xO192] (x < 16)) |
| MFI | Mobil—five (group, Nax(H2O)16[AlxSi96-xO192] (x < 27)) |
| MOR | Mordenite, (group, Na8(H2O)24[Al8Si40O96]) |
| MWW | Mobil Twenty-two (group, [H+2.4Na+3.1Al0.4B5.1Si66.5O144]) |
| MCM-22 | Mobil Composittion of Matter (Twenty-two) ([H+2.4Na+3.1Al0.4B5.1Si66.5O144]) |
| N-compounds | nitrogen content compounds |
| NH3-TPD | ammonia temperature-programmed desorption |
| NiMo/AZM | Micro-mesoporous hierarchical zeolite impregnated with nickel and molybdenum |
| Ni/beta zeolite | Beta zeolite impregnated with nickel |
| Ni/ZSM-5 | Zeolite Socony Mobil—five impregnated with nickel |
| O-compounds | oxygen content compounds |
| OFF=ZSM-34 | Offretite, (group, (Ca, Mg)1.5K(H2O)14[Al4Si14O36]) |
| PAH | polyaromatic hydrocarbon |
| PET | polyethylene terephthalate |
| PP | polypropylene |
| PS | polystyrene |
| PVC | polyvinyl chloride |
| Py-GC/MS | Pyrolysis-gas chromatography-mass spectrometry |
| SBA-15 | Santa Barbara Amorphous-fifteen, SiO2 |
| SSY | Standard Sixty (group, [B0.75Si26.25O54]) |
| SSZ-60 | Standard Oil Synthetic Zeolite—sixty ([B0.75Si26.25O54]) |
| t | residence time |
| T | temperature |
| ZSM-11MEL | Zeolite Socony Mobil—eleven, Nax(H2O)16[AlxSi96-xO192] (x < 16) |
| ZSM-34=OFF | Zeolite Socony Mobil—thirty-four, (Ca, Mg)1.5K(H2O)14[Al4Si14O36] |
| ZSM-5=MFI | Zeolite Socony Mobil—five, Nax(H2O)16[AlxSi96-xO192] (x < 27) |
| Z@M | core-shell (ZSM-5 and MCM-41) hierarchical zeolite |
References
- Ayhan, D. Biorefineries—For Biomass Upgrading Facilities; Springer: London, UK, 2010. [Google Scholar] [CrossRef]
- Zhang, L.; Bao, Z. Catalytic Pyrolysis of Biomass and Polymer Wastes. Catalysts 2018, 8, 659. [Google Scholar] [CrossRef] [Green Version]
- Basu, P. Biomass Gasification and Pyrolysis: Practical Design and Theory; Elsevier: Burlington, UK, 2010; pp. 1–365. [Google Scholar] [CrossRef]
- Yan, K.; Li, H. State of the Art and Perspectives in Catalytic Conversion Mechanism of Biomass to Bio-aromatics. Energy Fuels 2021, 35, 45–62. [Google Scholar] [CrossRef]
- Serio, M.A.; Kroo, E.; Wójtowicz, M.A. Biomass pyrolysis for distributed energy generation. Am. Chem. Soc. Fuel Chem. 2003, 48, 584–589. [Google Scholar]
- Rangel, M.C.; Carvalho, M.d.S.; Mayer, F.M.; Saboia, G.; de Andrade, A.M.; de Oliveira, A.P.S.; dos Santos, P.L. Improving Fast Pyrolysis by Tailoring High-Quality Products Using Catalysts. In Advances in Chemistry Research, 1st ed.; Taylor, J., Ed.; Nova Publishers: New York, NY, USA, 2022; Volume 75, pp. 119–169. [Google Scholar]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Ong, H.C.; Chen, W.; Farooq, A.; Gan, Y.Y.; Lee, K.T.; Ashokkumar, V. Catalytic thermochemical conversion of biomass for biofuel production: A comprehensive review. Renew. Sustain. Energy Rev. 2019, 113, 109266. [Google Scholar] [CrossRef]
- Lyu, G.; Wu, S.; Zhang, H. Estimation and comparison of bio-oil components from different pyrolysis conditions. Front. Energy Res. 2015, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-S.; Choi, G.-G. Pyrolysis of lignocellulosic biomass for biochemical production. In Waste Biorefinery: Potential and Perspectives; Elsevier: Amsterdam, The Netherlands, 2018; pp. 323–348. [Google Scholar] [CrossRef]
- Guilhaume, N.; Schuurman, Y.; Geantet, G. The role of catalysis in the valorization of woody biomass fast pyrolysis liquids: Overview and contribution of IRCELYON. Catal. Today 2021, 373, 5–23. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, Y.; Wang, H.; Li, H.; Han, X.; Zeng, Y.; Xu, C.C. A review of bio-oil upgrading by catalytic hydrotreatment: Advances, challenges and prospects. Mol. Catal. 2021, 504, 111438. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, J.; Liu, Y.; Li, S.; Chu, H. Research progress in the preparation of high-quality liquid fuels and chemicals by catalytic pyrolysis of biomass: A review. Energy Convers. Manag. 2022, 261, 115647. [Google Scholar] [CrossRef]
- Morgan, H.M.; Bu, Q.; Liang, J.; Liu, Y.; Mao, H.; Shi, A.; Lei, H.; Ruan, R. A review of catalytic microwave pyrolysis of lignocellulosic biomass for value-added fuel and chemicals. Bioresour. Technol. 2017, 230, 112–121. [Google Scholar] [CrossRef]
- Carlson, T.R.; Tompsett, G.A.; Conner, W.C.; Huber, G.W. Aromatic production from catalytic fast pyrolysis of biomass-derived feedstocks. Top. Catal. 2009, 52, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Akbarzadeh, A.; Chong, L.; Du, J.; Tahir, N.; Awasthi, M.K. Catalytic pyrolysis of lignocellulosic biomass for bio-oil production: A review. Chemosphere 2022, 297, 134181. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Kumar, S.; Kumar, J.; Bhaskar, T. A detailed assessment of pyrolysis kinetics of invasive lignocellulosic biomasses (Prosopis juliflora and Lantana camara) by thermogravimetric analysis. Bioresour. Technol. 2021, 312, 124060. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Mehariya, S.; Kumar, A.; Singh, E.; Yang, J.; Kumar, S.; Li, H.; Awasthi, M.K. Apple orchard waste recycling and valorization of valuable product-A review. Bioengineered 2021, 12, 476–495. [Google Scholar] [CrossRef]
- Ansari, K.B.; Arora, J.S.; Chew, J.W.; Dauenhauer, P.J.; Mushrif, S.H. Fast Pyrolysis of Cellulose, Hemicellulose, and Lignin: Effect of Operating Temperature on Bio-oil Yield and Composition and Insights into the Intrinsic Pyrolysis Chemistry. Ind. Eng. Chem. Res. 2019, 58, 15838–15852. [Google Scholar] [CrossRef]
- Jae, J.; Coolman, R.; Mountziaris, T.; Huber, G.W. Catalytic pyrolysis of lignocellulosic biomass in a process development unit with continual catalyst addition and removal. Chem. Eng. Sci. 2014, 108, 33–46. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Z.; Ding, K.; Deng, A.; Hao, N.; Meng, X.; Ben, H.; Ruan, R.; Ragauskas, A.J. Catalytic fast pyrolysis of bamboo sawdust via a two-step bench scale bubbling fluidized bed/fixed bed reactor: Study on synergistic effect of alkali metals oxides and HZSM-5. Energy Convers. Manag. 2018, 176, 287–298. [Google Scholar] [CrossRef]
- Leng, L.; Yang, L.; Chen, J.; Leng, S.; Li, H.; Li, H.; Yuan, X.; Zhou, W.; Huang, H. A review on pyrolysis of protein-rich biomass: Nitrogen transformation. Bioresour. Technol. 2020, 315, 123801. [Google Scholar] [CrossRef]
- Rjeily, M.A.; Gennequin, C.; Pron, H.; Abi-Aad, E.; Randrianalisoa, J.H. Pyrolysis-catalytic upgrading of bio-oil and pyrolysis-catalytic steam reforming of biogas: A review. Environ. Chem. Lett. 2021, 19, 2825–2872. [Google Scholar] [CrossRef]
- Duan, Y.; Pandey, A.; Zhang, Z.; Awasthi, M.K.; Bhatia, S.K.; Taherzadeh, M.J. Organic solid waste biorefinery: Sustainable strategy for emerging circular bioeconomy in China. Ind. Crops Prod. 2020, 153, 112568. [Google Scholar] [CrossRef]
- Singh, E.; Kumar, A.; Mishra, R.; You, S.; Singh, L.; Kumar, S.; Kumar, R. Pyrolysis of waste biomass and plastics for production of biochar and its use for removal of heavy metals from aqueous solution. Bioresour. Technol. 2021, 320, 124278. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, Y.; Dai, L.; Yu, Z.; Yang, Q.; Yang, S.; Jiang, D.; Ma, Z.; Wu, Q.; Zhang, B.; et al. Co-pyrolysis of biomass and soapstock in a downdraft reactor using a novel ZSM-5/SiC composite catalyst. Bioresour. Technol. 2019, 279, 202–208. [Google Scholar] [CrossRef]
- Tan, Y.L.; Abdullah, A.Z.; Hameed, B.H. Product distribution of the thermal and catalytic fast pyrolysis of karanja (Pongamia pinnata) fruit hulls over a reusable silica-alumina catalyst. Fuel 2019, 245, 89–95. [Google Scholar] [CrossRef]
- Yung, M.M.; Starace, A.K.; Griffin, M.B.; Wells, J.D.; Patalano, R.E.; Smith, K.R.; Schaidle, J.A. Restoring ZSM-5 performance for catalytic fast pyrolysis of biomass: Effect of regeneration temperature. Catal. Today 2019, 323, 76–85. [Google Scholar] [CrossRef]
- Jerzak, W.; Gao, N.; Kalemba-Rec, I.; Magdziarz, A. Catalytic intermediate pyrolysis of post-extraction rapeseed meal by reusing ZSM-5 and Zeolite Y catalyst. Catal. Today 2022, 404, 63–77. [Google Scholar] [CrossRef]
- Panahi, H.K.S.; Tabatabaei, M.; Aghbashlo, M.; Dehhaghi, M.; Rehan, M.; Nizami, A.-S. Recent updates on the production and upgrading of bio-crude oil from microalgae. Bioresour. Technol. Rep. 2019, 7, 100216. [Google Scholar] [CrossRef]
- Carlson, T.R.; Vispute, T.P.; Huber, G.W. Green gasoline by catalytic fast pyrolysis of solid biomass derived compounds. Chemsuschem 2008, 1, 397–400. [Google Scholar] [CrossRef] [Green Version]
- Banks, S.W.; Bridgwater, A.V. Catalytic fast pyrolysis for improved liquid quality. In Handbook of Biofuels Production, 2nd ed.; Luque, R., Lin, C.S.K., Eds.; Woodhead Publishing: New York, NY, USA, 2016; pp. 391–429. [Google Scholar]
- French, R.; Czernik, S. Catalytic pyrolysis of biomass for biofuels production. Fuel Process. Technol. 2010, 91, 25–32. [Google Scholar] [CrossRef]
- Diebold, J.P. A Review of the Chemical and Physical Mechanisms of the Storage Stability of Fast Pyrolysis Bio-Oils; National Renewable Energy Laboratory: Golden, CO, USA, 1999. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, M.; Oliveira, A.P.; Mayer, F.; Virgens, C.; Rangel, M.D.C. Thermokinetic and thermodynamic parameters for catalytic pyrolysis of medium density fiber over Ni/beta zeolite. Catal. Res. 2022, 2, 1–20. [Google Scholar] [CrossRef]
- Mayer, F.M.; de Oliveira, A.P.S.; Junior, D.L.d.O.; Agustini, B.C.; da Silva, G.A.; Tanabe, E.H.; Ruiz, D.; Rangel, M.D.C.; Zini, C.A. Influence of Nickel Modified Beta Zeolite in the Production of BTEX During Analytical Pyrolysis of Medium-Density Fiberboard (MDF). Waste Biomass Valorization 2022, 13, 1717–1729. [Google Scholar] [CrossRef]
- Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Review on the catalytic effects of alkali and alkaline earth metals (AAEMs) including sodium, potassium, calcium and magnesium on the pyrolysis of lignocellulosic biomass and on the co-pyrolysis of coal with biomass. J. Anal. Appl. Pyrolysis 2022, 163, 105479. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Li, Q.; Wang, Y.; Liu, Q.; Wei, T.; Dong, D.; Salavati, S.; Gholizadeh, M.; Hu, X. Catalytic pyrolysis of poplar wood over transition metal oxides: Correlation of catalytic behaviors with physicochemical properties of the oxides. Biomass Bioenergy 2019, 124, 125–141. [Google Scholar] [CrossRef]
- Dickerson, T.; Soria, J. Catalytic fast pyrolysis: A review. Energy 2013, 6, 514–538. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.L.S.; Marchetti, S.G.; Júnior, A.D.C.F.; Silva, T.D.F.; Assaf, J.M.; Rangel, M.D.C. Effect of gadolinium on the catalytic properties of iron oxides for WGSR. Catal. Today 2013, 213, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, L.S.; Martins, A.R.; Reyes, P.; Oportus, M.; Albonoz, A.; Vicentini, V.; Rangel, M.D.C. Preparation and characterization of Ru/MgO-Al2O3 catalysts for methane steam reforming. Catal. Today 2009, 142, 52–60. [Google Scholar] [CrossRef]
- Querino, P.; Bispo, J.R.C.; Rangel, M.C. The effect of cerium on the properties of Pt/ZrO2 catalysts in the WGSR. Catal. Today 2005, 107–108, 920–925. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Kalogiannis, K.G. In-situ upgrading of biomass pyrolysis vapors: Catalyst screening on a fixed bed reactor. Bioresour. Technol. 2011, 102, 8261–8267. [Google Scholar] [CrossRef]
- Mochizuki, T.; Atong, D.; Chen, S.-Y.; Toba, M.; Yoshimura, Y. Effect of SiO2 pore size on catalytic fast pyrolysis of Jatropha residues by using pyrolyzer-GC/MS. Catal. Commun. 2013, 36, 1–4. [Google Scholar] [CrossRef]
- Lv, Y.; Qian, X.; Tu, B.; Zhao, D. Generalized synthesis of core–shell structured nano-zeolite@ordered mesoporous silica composites. Catal. Today 2013, 204, 2–7. [Google Scholar] [CrossRef]
- Hassan, A.F.; Alafid, F.; Hrdina, R. Preparation of melamine formaldehyde/nanozeolite Y composite based on nanosilica extracted from rice husks by sol–gel method: Adsorption of lead (II) ion. J. Sol.-Gel Sci. Technol. 2020, 95, 211–222. [Google Scholar] [CrossRef]
- Wan, Z.; Li, G.K.; Wang, C.; Yang, H.; Zhang, D. Relating coke formation and characteristics to deactivation of ZSM-5 zeolite in methanol to gasoline conversion. Appl. Catal. A Gen. 2018, 549, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Gao, L.; Yang, Y.; Chen, G.; Guo, S.; Omran, M.; Chen, J.; Ruan, R. Study on thermochemical characteristics properties and pyrolysis kinetics of the mixtures of waste corn stalk and pyrolusite. Bioresour. Technol. 2021, 324, 124660. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Z.; Wang, Q. Evaluation of zeolite catalyst on product distribution and synergy during wood-plastic composite catalytic pyrolysis. Energy 2019, 189, 116174. [Google Scholar] [CrossRef]
- Lazaridis, P.A.; Fotopoulos, A.P.; Karakoulia, S.A.; Triantafyllidis, K.S. Catalytic fast pyrolysis of kraft lignin with conventional, mesoporous and nanosized ZSM-5 zeolite for the production of alkyl-phenols and aromatics. Front. Chem. 2018, 6, 295. [Google Scholar] [CrossRef] [Green Version]
- Hassan, N.S.; Jalil, A.A.; Hitam, C.N.C.; Vo, D.V.N.; Nabgan, W. Biofuels and renewable chemicals production by catalytic pyrolysis of cellulose: A review. Environ. Chem. Lett. 2020, 18, 1625–1648. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Su, L.; Zhang, Y.; Wang, Y.; Zhang, H. The role of shape selectivity in catalytic fast pyrolysis of lignin with zeolite catalysts. Appl. Catal. A Gen. 2012, 447–448, 115–123. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Jeong, J.; Ryu, S.; Lee, H.W.; Jung, J.S.; Siddiqui, M.Z.; Jung, S.-C.; Jeon, J.-K.; Jae, J.; Park, Y.-K. Catalytic pyrolysis of wood polymer composites over hierarchical mesoporous zeolites. Energy Convers. Manag. 2019, 195, 727–737. [Google Scholar] [CrossRef]
- Soltanian, S.; Lee, C.L.; Lam, S.S. A review on the role of hierarchical zeolites in the production of transportation fuels through catalytic fast pyrolysis of biomass. Biofuel Res. J. 2020, 7, 1217–1234. [Google Scholar] [CrossRef]
- Qian, M.; Zhao, Y.; Huo, E.; Wang, C.; Zhang, X.; Lin, X.; Wang, L.; Kong, X.; Ruan, R.; Lei, H. Improving catalytic production of aromatic hydrocarbons with a mesoporous ZSM-5 modified with nanocellulose as a green template. J. Anal. Appl. Pyrolysis 2022, 166, 105624. [Google Scholar] [CrossRef]
- Casoni, A.I.; Nievas, M.L.; Moyano, E.L.; Álvarez, M.; Diez, A.; Dennehy, M.; Volpe, M.A. Catalytic pyrolysis of cellulose using MCM-41 type catalysts. Appl. Catal. A Gen. 2016, 514, 235–240. [Google Scholar] [CrossRef]
- Veloso, C.M.; Rangel, M.C. Preparação de Carbonos Porosos por Moldagem Seqüencial. Química Nova 2009, 32, 2133–2141. [Google Scholar] [CrossRef] [Green Version]
- Hagemann, N.; Spokas, K.; Schmidt, H.-P.; Kägi, R.; Böhler, M.A.; Bucheli, T.D. Activated carbon, biochar and charcoal: Linkages and synergies across pyrogenic carbon’s ABCs. Water 2018, 10, 182. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, L.A.; Borges, S.M.S.; Paulino, P.N.; Fraga, M.A.; de Oliva, S.T.; Marchetti, S.G.; Rangel, M.D.C. Methylene blue oxidation over iron oxide supported on activated carbon derived from peanut hulls. Catal. Today 2017, 289, 237–248. [Google Scholar] [CrossRef]
- Lima, S.B.; Borges, S.M.S.; Rangel, M.D.C.; Marchetti, S.G. Effect of iron content on the catalytic properties of activated carbon-supported magnetite derived from biomass. J. Braz. Chem. Soc. 2013, 24, 344–354. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Z.; Chen, W.; Chen, Y.; Wang, X.; Chen, H. Role of porous structure and active O-containing groups of activated biochar catalyst during biomass catalytic pyrolysis. Energy 2020, 210, 118646. [Google Scholar] [CrossRef]
- Dai, L.; Zeng, Z.; Tian, X.; Jiang, L.; Yu, Z.; Wu, Q.; Wang, Y.; Liu, Y.; Ruan, R. Microwave-assisted catalytic pyrolysis of torrefied corn cob for phenol-rich bio-oil production over Fe modified bio-char catalyst. J. Anal. Appl. Pyrolysis 2019, 143, 104691. [Google Scholar] [CrossRef]
- Liu, S.; Wu, G.; Gao, Y.; Li, B.; Feng, Y.; Zhou, J.; Hu, X.; Huang, Y.; Zhang, S.; Zhang, H. Understanding the catalytic upgrading of bio-oil from pine pyrolysis over CO2-activated biochar. Renew. Energy 2021, 174, 538–546. [Google Scholar] [CrossRef]
- Huo, E.; Duan, D.; Lei, H.; Liu, C.; Zhang, Y.; Wu, J.; Zhao, Y.; Huang, Z.; Qian, M.; Zhang, Q.; et al. Phenols production from Douglas fir catalytic pyrolysis with MgO and biomass-derived activated carbon catalysts. Energy 2020, 199, 117459. [Google Scholar] [CrossRef]
- Han, T.; Ding, S.; Yang, W.; Jönsson, P. Catalytic pyrolysis of lignin using low-cost materials with different acidities and textural properties as catalysts. Chem. Eng. J. 2019, 373, 846–856. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids (Technical Report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.; Chen, S.S.; Tsang, D.C.; Wang, L.; Xiong, X.; Zhang, S.; Ok, Y.S.; Kwon, E.E.; Song, H.; et al. Production of 5-hydroxymethylfurfural from starch-rich food waste catalyzed by sulfonated biochar. Bioresour. Technol. 2018, 252, 76–82. [Google Scholar] [CrossRef]
- Mahajan, A.; Gupta, P. Carbon-based solid acids: A review. Environ. Chem. Lett. 2020, 18, 299–314. [Google Scholar] [CrossRef]
- Chen, S.S.; Maneerung, T.; Tsang, D.C.; Ok, Y.S.; Wang, C.-H. Valorization of biomass to hydroxymethylfurfural, levulinic acid, and fatty acid methyl ester by heterogeneous catalysts. Chem. Eng. J. 2017, 328, 246–273. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Wang, L.; Wei, Y.; Zhu, L.; Liu, Y.; Liang, J.; Tang, J. Renewable phenols production by catalytic microwave pyrolysis of Douglas fir sawdust pellets with activated carbon catalysts. Bioresour. Technol. 2013, 142, 546–552. [Google Scholar] [CrossRef]
- Mamaeva, A.; Tahmasebi, A.; Tian, L.; Yu, J. Microwave-assisted catalytic pyrolysis of lignocellulosic biomass for production of phenolic-rich bio-oil. Bioresour. Technol. 2016, 211, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Omoriyekomwan, J.E.; Tahmasebi, A.; Yu, J. Production of phenol-rich bio-oil during catalytic fixed-bed and microwave pyrolysis of palm kernel shell. Bioresour. Technol. 2016, 207, 188–196. [Google Scholar] [CrossRef]
- Ye, X.-N.; Lu, Q.; Wang, X.; Guo, H.-Q.; Cui, M.-S.; Dong, C.-Q.; Yang, Y.-P. Catalytic Fast Pyrolysis of Cellulose and Biomass to Selectively Produce Levoglucosenone Using Activated Carbon Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 10815–10825. [Google Scholar] [CrossRef]
- Chen, W.; Li, K.; Xia, M.; Yang, H.; Chen, Y.; Chen, X.; Che, Q.; Chen, H. Catalytic deoxygenation co-pyrolysis of bamboo wastes and microalgae with biochar catalyst. Energy 2018, 157, 472–482. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, H.; Yang, Z.; Qian, K.; Villota, E. Renewable high-purity mono-phenol production from catalytic microwave-induced pyrolysis of cellulose over biomass-derived activated carbon catalyst. ACS Sustain. Chem. Eng. 2018, 6, 5349–5357. [Google Scholar] [CrossRef]
- Chen, W.; Fang, Y.; Li, K.; Chen, Z.; Xia, M.; Gong, M.; Chen, Y.; Yang, H.; Tu, X.; Chen, H. Bamboo wastes catalytic pyrolysis with N-doped biochar catalyst for phenols products. Appl. Energy 2020, 260, 114242. [Google Scholar] [CrossRef]
- Nejati, B.; Adami, P.; Bozorg, A.; Tavasoli, A.; Mirzahosseini, A.H. Catalytic pyrolysis and bio-products upgrading derived from Chlorella vulgaris over its biochar and activated biochar-supported Fe catalysts. J. Anal. Appl. Pyrolysis 2020, 152, 104799. [Google Scholar] [CrossRef]
- Mgbemere, H.E.; Ekpe, I.C.; Lawai, G.I. Zeolite Synthesis, Characterization and Application Areas: A Review. Int. Res. J. Environ. Sci. 2017, 6, 45–59. [Google Scholar]
- Rahman, M.M.; Liu, R.; Cai, J. Catalytic fast pyrolysis of biomass over zeolites for high quality bio-oil—A review. Fuel Process. Technol. 2018, 180, 32–46. [Google Scholar] [CrossRef]
- Nippes, R.P.; Macruz, P.D.; Molina, L.C.A.; Scaliante, M.H.N.O. Hydroxychloroquine Adsorption in Aqueous Medium Using Clinoptilolite Zeolite. Water Air Soil Pollut. 2022, 233, 287. [Google Scholar] [CrossRef] [PubMed]
- Usman, M. Recent Progress of SAPO-34 Zeolite Membranes for CO2 Separation: A Review. Membranes 2022, 12, 507. [Google Scholar] [CrossRef]
- Yoon, J.S.; Lee, T.; Choi, J.-W.; Suh, D.J.; Lee, K.; Ha, J.-M.; Choi, J. Layered MWW zeolite-supported Rh catalysts for the hydrodeoxygenation of lignin model compounds. Catal. Today 2017, 293–294, 142–150. [Google Scholar] [CrossRef]
- Hita, I.; Cordero-Lanzac, T.; García-Mateos, F.J.; Azkoiti, M.J.; Rodríguez-Mirasol, J.; Cordero, T.; Bilbao, J. Enhanced production of phenolics and aromatics from raw bio-oil using HZSM-5 zeolite additives for PtPd/C and NiW/C catalysts. Appl. Catal. B Environ. 2019, 259, 128112. [Google Scholar] [CrossRef]
- Vayssilov, G.N.; Rösch, N. Influence of alkali and alkaline earth cations on the Brønsted acidity of zeolites. J. Phys. Chem. B 2001, 105, 4277–4284. [Google Scholar] [CrossRef]
- Moreno, E.; Rajagopal, K. Desafios da acidez na catálise em estado sólido. Quím. Nova 2009, 32, 538–543. [Google Scholar] [CrossRef] [Green Version]
- Aho, A.; Salmi, T.; Murzin, D.Y. Catalytic Pyrolysis of Lignocellulosic Biomass. In Production of Bio-Fuels and Bio-Chemicals; Kostas, S.T., Angelos, A.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 137–159. [Google Scholar] [CrossRef]
- Ani, F.N. Utilization of bioresources as fuels and energy generation. In Electric Renewable Energy Systems; Elsevier: Amsterdam, The Netherlands, 2016; pp. 140–155. [Google Scholar] [CrossRef]
- Dabros, T.M.; Stummann, M.Z.; Høj, M.; Jensen, P.A.; Grunwaldt, J.-D.; Gabrielsen, J.; Mortensen, P.M.; Jensen, A.D. Transportation fuels from biomass fast pyrolysis, catalytic hydrodeoxygenation, and catalytic fast hydropyrolysis. Prog. Energy Combust. Sci. 2018, 68, 268–309. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Liu, Q.; Zhang, Q.; Chen, L.; Ma, L. A review of conversion of lignocellulose biomass to liquid transport fuels by integrated refining strategies. Fuel Process. Technol. 2020, 208, 106485. [Google Scholar] [CrossRef]
- Mayer, F.M.; Teixeira, C.M.; Pacheco, J.G.A.; de Souza, C.T.; Bauer, D.D.V.; Caramão, E.B.; Espíndola, J.D.S.; Trierweiler, J.O.; Lopez, O.W.P.; Zini, C.A. Characterization of analytical fast pyrolysis vapors of medium-density fiberboard (MDF) using metal-modified HZSM-5. J. Anal. Appl. Pyrolysis 2018, 136, 87–95. [Google Scholar] [CrossRef]
- Jin, T.; Wang, H.; Peng, J.; Wu, Y.; Huang, Z.; Tian, X.; Ding, M. Catalytic pyrolysis of lignin with metal-modified HZSM-5 as catalysts for monocyclic aromatic hydrocarbons production. Fuel Process. Technol. 2022, 230, 107201. [Google Scholar] [CrossRef]
- Kantarelis, E.; Javed, R.; Stefanidis, S.; Psarras, A.; Iliopoulou, E.; Lappas, A. Engineering the Catalytic Properties of HZSM5 by Cobalt Modification and Post-synthetic Hierarchical Porosity Development. Top. Catal. 2019, 62, 773–785. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 1–23. [Google Scholar] [CrossRef]
- Escola, J.; Aguado, J.; Serrano, D.; García, A.; Peral, A.; Briones, L.; Calvo, R.; Fernandez, E. Catalytic hydroreforming of the polyethylene thermal cracking oil over Ni supported hierarchical zeolites and mesostructured aluminosilicates. Appl. Catal. B Environ. 2011, 106, 405–415. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Liu, Y.; Ruan, R.; Duan, D.; Zhao, Y.; Yu, Z.; Jiang, L. Catalytic fast pyrolysis of torrefied corn cob to aromatic hydrocarbons over Ni-modified hierarchical ZSM-5 catalyst. Bioresour. Technol. 2019, 272, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Hernando, H.; Moreno, I.; Fermoso, J.; Ochoa-Hernández, C.; Pizarro, P.; Coronado, J.M.; Čejka, J.; Serrano, D.P. Biomass catalytic fast pyrolysis over hierarchical ZSM-5 and Beta zeolites modified with Mg and Zn oxides. Biomass Convers. Biorefinery 2017, 7, 289–304. [Google Scholar] [CrossRef]
- Ryu, H.W.; Lee, H.W.; Jae, J.; Park, Y.-K. Catalytic pyrolysis of lignin for the production of aromatic hydrocarbons: Effect of magnesium oxide catalyst. Energy 2019, 179, 669–675. [Google Scholar] [CrossRef]
- Hernando, H.; Jiménez-Sánchez, S.; Fermoso, J.; Pizarro, P.; Coronado, J.M.; Serrano, D.P. Assessing biomass catalytic pyrolysis in terms of deoxygenation pathways and energy yields for the efficient production of advanced biofuels. Catal. Sci. Technol. 2016, 6, 2829–2843. [Google Scholar] [CrossRef]
- Ding, K.; Zhong, Z.; Wang, J.; Zhang, B.; Fan, L.; Liu, S.; Wang, Y.; Liu, Y.; Zhong, D.; Chen, P.; et al. Improving hydrocarbon yield from catalytic fast co-pyrolysis of hemicellulose and plastic in the dual-catalyst bed of CaO and HZSM-5. Bioresour. Technol. 2018, 261, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, H.; Karim, A.M.; Sun, J.; Wang, Y. Catalytic fast pyrolysis of lignocellulosic biomass. Chem. Soc. Rev. 2014, 43, 7594–7623. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, Z.-X.; Xie, W.-L.; Liu, J.; Li, Y.; Zhang, W.-M.; Fu, H.; Lu, Q. Advances on the fast pyrolysis of biomass for the selective preparation of phenolic compounds. Fuel Process. Technol. 2022, 237, 107465. [Google Scholar] [CrossRef]
- Sharifzadeh, M.; Sadeqzadeh, M.; Guo, M.; Borhani, T.N.; Konda, N.M.; Garcia, M.C.; Wang, L.; Hallett, J.; Shah, N. The multi-scale challenges of biomass fast pyrolysis and bio-oil upgrading: Review of the state of art and future research directions. Prog. Energy Combust. Sci. 2019, 71, 1–80. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Yang, H.; Wang, X.; Che, Q.; Chen, W.; Chen, H. Catalytic fast pyrolysis of biomass: Selective deoxygenation to balance the quality and yield of bio-oil. Bioresour. Technol. 2019, 273, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Strezov, V.; Lovell, E.; Kan, T.; Weldekidan, H.; He, J.; Jahan, S.; Dastjerdi, B.; Scott, J. Enhanced bio-oil deoxygenation activity by Cu/zeolite and Ni/zeolite catalysts in combined in-situ and ex-situ biomass pyrolysis. J. Anal. Appl. Pyrolysis 2019, 140, 148–160. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, J.; Yuan, H.; Chen, Y. Catalytic fast pyrolysis of waste mixed cloth for the production of value-added chemicals. Waste Manag. 2021, 127, 141–146. [Google Scholar] [CrossRef]
- Chong, Y.Y.; Thangalazhy-Gopakumar, S.; Ng, H.K.; Lee, L.Y.; Gan, S. Effect of oxide catalysts on the properties of bio-oil from in-situ catalytic pyrolysis of palm empty fruit bunch fiber. J. Environ. Manag. 2019, 247, 38–45. [Google Scholar] [CrossRef]
- Chireshe, F.; Collard, F.-X.; Görgens, J.F. Production of an upgraded bio-oil with minimal water content by catalytic pyrolysis: Optimisation and comparison of Cao and MgO performances. J. Anal. Appl. Pyrolysis 2020, 146, 104751. [Google Scholar] [CrossRef]
- Gupta, J.; Papadikis, K.; Konysheva, E.Y.; Lin, Y.; Kozhevnikov, I.V.; Li, J. CaO catalyst for multi-route conversion of oakwood biomass to value-added chemicals and fuel precursors in fast pyrolysis. Appl. Catal. B Environ. 2021, 285, 119858. [Google Scholar] [CrossRef]
- Raymundo, L.M.; Mullen, C.A.; Boateng, A.A.; DeSisto, W.J.; Trierweiler, J.O. Production of partially deoxygenated pyrolysis oil from switchgrass via Ca(OH)2, CaO, and Ca(COOH)2 cofeeding. Energy Fuels 2020, 34, 12616–12625. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Z.; Zhang, Z.; Sun, J.; Wang, Q.; Pittman, C.U. Catalytic fast pyrolysis of a wood-plastic composite with metal oxides as catalysts. Waste Manag. 2018, 79, 38–47. [Google Scholar] [CrossRef]
- Shao, S.; Liu, C.; Xiang, X.; Li, X.; Zhang, H.; Xiao, R.; Cai, Y. In situ catalytic fast pyrolysis over CeO2 catalyst: Impact of biomass source, pyrolysis temperature and metal ion. Renew. Energy 2021, 177, 1372–1381. [Google Scholar] [CrossRef]
- Li, Y.; Hu, B.; Fu, H.; Zhang, Z.-X.; Guo, Z.-T.; Zhou, G.-Z.; Zhu, L.-J.; Liu, J.; Lu, Q. Fast pyrolysis of bagasse catalyzed by mixed alkaline-earth metal oxides for the selective production of 4-vinylphenol. J. Anal. Appl. Pyrolysis 2022, 164, 105531. [Google Scholar] [CrossRef]
- Veses, A.; Aznar, M.; Martínez, I.; Martínez, J.; López, J.; Navarro, M.; Callén, M.; Murillo, R.; García, T. Catalytic pyrolysis of wood biomass in an auger reactor using calcium-based catalysts. Bioresour. Technol. 2014, 162, 250–258. [Google Scholar] [CrossRef]
- Locatel, W.d.R.; Laurenti, D.; Schuurman, Y.; Guilhaume, N. Ex-situ catalytic upgrading of pyrolysis vapors using mixed metal oxides. J. Anal. Appl. Pyrolysis 2021, 158, 105241. [Google Scholar] [CrossRef]
- Vichaphund, S.; Sricharoenchaikul, V.; Aton, D. Industrial waste derived CaO-based catalysts for upgrading volatiles during pyrolysis of Jatropha residues. J. Anal. Appl. Pyrolysis 2017, 124, 568–575. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, B.; Chen, J.; Hong, W.; Zhang, Y.; Liu, H. Comparative investigation on the effects of formate salt additives on pyrolysis characteristics of corn straw. J. Anal. Appl. Pyrolysis 2022, 162, 105450. [Google Scholar] [CrossRef]
- Xia, S.; Yang, H.; Lei, S.; Lu, W.; Cai, N.; Xiao, H.; Chen, Y.; Chen, H. Iron salt catalytic pyrolysis of biomass: Influence of iron salt type. Energy 2023, 262, 125415. [Google Scholar] [CrossRef]
- Eibner, S.; Broust, F.; Blin, J.; Julbe, A. Catalytic effect of metal nitrate salts during pyrolysis of impregnated biomass. J. Anal. Appl. Pyrolysis 2015, 113, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, W.S.; Cunha, I.F.; Pereira, M.S.; Ataíde, C.H. Thermal decomposition profile and product selectivity of analytical pyrolysis of sweet sorghum bagasse. Effect of addition of inorganic salts. Ind. Crops Prod. 2015, 74, 372–380. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Zhou, N.; Dai, L.; Deng, W.; Liu, C.; Cheng, Y.; Liu, Y.; Cobb, K.; Chen, P.; et al. Applications of calcium oxide-based catalysts in biomass pyrolysis/gasification—A review. J. Clean. Prod. 2021, 291, 125826. [Google Scholar] [CrossRef]
- Cao, Z.; Niu, J.; Gu, Y.; Zhang, R.; Liu, Y.; Luo, L. Catalytic pyrolysis of rice straw: Screening of various metal salts, metal basic oxides, acidic metal oxide and zeolite catalyst on products yield and characterization. J. Clean. Prod. 2020, 269, 122079. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Chen, L.; Zhao, B.; Yang, S.; Xie, X. Effects of Fe contents on fast pyrolysis of biomass with Fe/CaO catalysts. J. Anal. Appl. Pyrolysis 2016, 119, 133–138. [Google Scholar] [CrossRef]
- Rahman, M.; Nishu; Sarker, M.; Chai, M.; Li, C.; Liu, R.; Cai, J. Potentiality of combined catalyst for high quality bio-oil production from catalytic pyrolysis of pinewood using an analytical Py-GC/MS and fixed bed reactor. J. Energy Inst. 2020, 93, 1737–1746. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, X.; Guo, H.; Wei, T.; Dong, D.; Hu, G.; Hu, S.; Xiang, J.; Liu, Q.; Wang, Y. Pyrolysis of poplar, cellulose and lignin: Effects of acidity and alkalinity of the metal oxide catalysts. J. Anal. Appl. Pyrolysis 2018, 134, 590–605. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Gu, J.; Yuan, H.; Chen, Y. Insight into the role of varied acid-base sites on fast pyrolysis kinetics and mechanism of cellulose. Waste Manag. 2021, 135, 140–149. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Yang, H.; Chen, W.; Wang, X.; Chen, H. Fast pyrolysis of cotton stalk biomass using calcium oxide. Bioresour. Technol. 2017, 233, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lyu, G.; Zhang, A.; Li, X.; Xie, J. Effects of ferric chloride pretreatment and surfactants on the sugar production from sugarcane bagasse. Bioresour. Technol. 2018, 265, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Loow, Y.-L.; Wu, T.Y.; Lim, Y.S.; Tan, K.A.; Siow, L.F.; Jahim, J.M.; Mohammad, A.W. Improvement of xylose recovery from the stalks of oil palm fronds using inorganic salt and oxidative agent. Energy Convers. Manag. 2017, 138, 248–260. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A.A. review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Ania, C.O. Surface chemistry of activated carbons and its characterization. Interface Sci. Technol. 2006, 7, 159–229. [Google Scholar]
- Salame, I.I.; Bandosz, T.J. Role of surface chemistry in adsorption of phenol on activated carbons. J. Colloid Interface Sci. 2003, 264, 307–312. [Google Scholar] [CrossRef]
- Pradhan, B.K.; Sandle, N.K. Effect of different oxidizing agent treatments on the surface properties of activated carbons. Carbon 1999, 37, 1323–1332. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Yu, M.J.; Ko, C.H.; Jeon, J.-K.; Jae, J.; Park, S.H.; Jung, S.-C.; Park, Y.-K. Catalytic Hydrodeoxygenation of Bio-oil Model Compounds over Pt/HY Catalyst. Sci. Rep. 2016, 6, 28765. [Google Scholar] [CrossRef]
- Vichaphund, S.; Aht-Ong, D.; Sricharoenchaikul, V.; Atong, D. Production of aromatic compounds from catalytic fast pyrolysis of Jatropha residues using metal/HZSM-5 prepared by ion-exchange and impregnation methods. Renew. Energy 2015, 79, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Stummann, M.Z.; Elevera, E.; Hansen, A.B.; Hansen, L.P.; Beato, P.; Davidsen, B.; Wiwel, P.; Gabrielsen, J.; Jensen, P.A.; Jensen, A.D.; et al. Catalytic hydropyrolysis of biomass using supported CoMo catalysts—Effect of metal loading and support acidity. Fuel 2020, 264, 116867. [Google Scholar] [CrossRef]
- Li, P.; Li, D.; Yang, H.; Wang, X.; Chen, H. Effects of Fe-, Zr-, and Co-Modified Zeolites and Pretreatments on Catalytic Upgrading of Biomass Fast Pyrolysis Vapors. Energy Fuels 2016, 30, 3004–3013. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Liu, X.; Zhu, S.; Hu, L.; Zhang, Q. Upgrading of bio-oil from catalytic pyrolysis of pretreated rice husk over Fe-modified ZSM-5 zeolite catalyst. Fuel Process. Technol. 2018, 175, 17–25. [Google Scholar] [CrossRef]
- Dai, G.; Wang, S.; Zou, Q.; Huang, S. Improvement of aromatics production from catalytic pyrolysis of cellulose over metal-modified hierarchical HZSM-5. Fuel Process. Technol. 2018, 179, 319–323. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V.; Lovell, E.; Kan, T.; Weldekidan, H.; He, J.; Dastjerdi, B.; Scott, J. Bio-oil upgrading with catalytic pyrolysis of biomass using Copper/zeolite-Nickel/zeolite and Copper-Nickel/zeolite catalysts. Bioresour. Technol. 2019, 279, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Strezov, V.; Kan, T.; Weldekidan, H.; He, J.; Jahan, S. Investigating the Effect of Mono- And Bimetallic/Zeolite Catalysts on Hydrocarbon Production during Bio-oil Upgrading from Ex Situ Pyrolysis of Biomass. Energy Fuels 2019, 34, 389–400. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Li, D.; Liu, C.; Lu, Y.; Lin, X.; Zheng, Z. Activity and selectivity of Ni–Cu bimetallic zeolites catalysts on biomass conversion for bio-aromatic and bio-phenols. J. Energy Inst. 2021, 97, 57–72. [Google Scholar] [CrossRef]
- Xue, S.; Luo, Z.; Zhou, Q.; Sun, H.; Du, L. Regulation mechanism of three key parameters on catalytic characterization of molybdenum modified bimetallic micro-mesoporous catalysts during catalytic fast pyrolysis of enzymatic hydrolysis lignin. Bioresour. Technol. 2021, 337, 125396. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Hu, Z.; Huang, M.; Peng, H.; Zhang, W.; Chen, D.; Ma, Z. Valorisation of cotton stalk toward bio-aromatics: Effect of wet torrefaction deoxygenation and deminerization pretreatment on catalytic fast pyrolysis using Ga modified hierarchical zeolite. Fuel 2022, 330, 125571. [Google Scholar] [CrossRef]
- Shao, J.; Jiang, H.; Yang, M.; Xiao, J.; Yang, H.; Chen, Y.; Zhang, S.; Chen, H. Catalytic fast pyrolysis of cellulose over different metal-modified ZSM-5 zeolites for light olefins. J. Anal. Appl. Pyrolysis 2022, 166, 105628. [Google Scholar] [CrossRef]
- Huang, M.; Xu, J.; Ma, Z.; Yang, Y.; Zhou, B.; Wu, C.; Ye, J.; Zhao, C.; Liu, X.; Chen, D.; et al. Bio-BTX production from the shape selective catalytic fast pyrolysis of lignin using different zeolite catalysts: Relevance between the chemical structure and the yield of bio-BTX. Fuel Process. Technol. 2021, 216, 106792. [Google Scholar] [CrossRef]
- Lv, P.; Yan, L.; Liu, Y.; Wang, M.; Bao, W.; Li, F. Catalytic conversion of coal pyrolysis vapors to light aromatics over hierarchical Y-type zeolites. J. Energy Inst. 2020, 93, 1354–1363. [Google Scholar] [CrossRef]
- Wei, B.; Jin, L.; Wang, D.; Shi, H.; Hu, H. Catalytic upgrading of lignite pyrolysis volatiles over modified HY zeolites. Fuel 2020, 259, 116234. [Google Scholar] [CrossRef]
- Osei, G.K.; Nzihou, A.; Yaya, A.; Minh, D.P.; Onwona-Agyeman, B. Catalytic Pyrolysis of Waste Engine Oil over Y Zeolite Synthesized from Natural Clay. Waste Biomass Valorization 2021, 12, 4157–4170. [Google Scholar] [CrossRef]
- Paysepar, H.; Rao, K.T.V.; Yuan, Z.; Shui, H.; Xu, C. Improving activity of ZSM-5 zeolite catalyst for the production of monomeric aromatics/phenolics from hydrolysis lignin via catalytic fast pyrolysis. Appl. Catal. A Gen. 2018, 563, 154–162. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Chen, L.; Zhao, B.; Yang, S.; Xie, X. Comparison of catalytic fast pyrolysis of biomass to aromatic hydrocarbons over ZSM-5 and Fe/ZSM-5 catalysts. J. Anal. Appl. Pyrolysis 2016, 121, 342–346. [Google Scholar] [CrossRef]
- Dada, T.K.; Islam, A.; Duan, A.X.; Antunes, E. Catalytic co-pyrolysis of ironbark and waste cooking oil using X-strontium/Y-zeolite (X = Ni, Cu, Zn, Ag, and Fe). J. Energy Inst. 2022, 104, 89–97. [Google Scholar] [CrossRef]
- Robinson, A.M.; Hensley, J.E.; Medlin, J.W. Bifunctional Catalysts for Upgrading of Biomass-Derived Oxygenates: A Review. Catalysis 2022, 6, 5026–5043. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, L.; Chen, L.; Xie, X.; Zhao, B.; Si, H.; Meng, G. Comparison of catalytic upgrading of biomass fast pyrolysis vapors over CaO and Fe(III)/CaO catalysts. J. Anal. Appl. Pyrolysis 2014, 108, 35–40. [Google Scholar] [CrossRef]
- Kantarelis, E.; Yang, W.; Blasiak, W. Effects of Silica-Supported Nickel and Vanadium on Liquid Products of Catalytic Steam Pyrolysis of Biomass. Energy Fuels 2014, 28, 591–599. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Krishna, B.B.; Bhaskar, T.; Perkins, G. Advances in the thermo-chemical production of hydrogen from biomass and residual wastes: Summary of recent techno-economic analyses. Bioresour. Technol. 2020, 299, 122557. [Google Scholar] [CrossRef]
- Fermoso, J.; Hernando, H.; Jana, P.; Moreno, I.; Přech, J.; Ochoa-Hernández, C.; Pizarro, P.; Coronado, J.M.; Čejka, J.; Serrano, D.P. Lamellar and pillared ZSM-5 zeolites modified with MgO and ZnO for catalytic fast-pyrolysis of eucalyptus woodchips. Catal. Today 2016, 277, 171–181. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Fan, L.; Zhou, N.; Tian, G.; Zhu, X.; Cheng, Y.; Wang, Y.; Liu, Y.; Chen, P.; et al. Bio-oil production from sequential two-step catalytic fast microwave-assisted biomass pyrolysis. Fuel 2017, 196, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, S.R.; Uemura, Y.; Yusup, S.B. Catalytic pyrolysis of paddy husk in a drop type pyrolyzer for bio-oil production: The role of temperature and catalyst. J. Anal. Appl. Pyrolysis 2014, 106, 57–62. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, H.; Wu, H.; Wang, M.; Cheng, D. Catalytic pyrolysis of rice husk by mixing with zinc oxide: Characterization of bio-oil and its rheological behavior. Fuel Process. Technol. 2013, 106, 385–391. [Google Scholar] [CrossRef]
- Veses, A.; Aznar, M.; López, J.; Callén, M.; Murillo, R.; García, T. Production of upgraded bio-oils by biomass catalytic pyrolysis in an auger reactor using low cost materials. Fuel 2015, 141, 17–22. [Google Scholar] [CrossRef]
- Dai, L.; Fan, L.; Duan, D.; Ruan, R.; Wang, Y.; Liu, Y.; Zhou, Y.; Yu, Z.; Liu, Y.; Jiang, L. Production of hydrocarbon-rich bio-oil from soapstock via fast microwave-assisted catalytic pyrolysis. J. Anal. Appl. Pyrolysis 2017, 125, 356–362. [Google Scholar] [CrossRef]
- Abnisa, F.; Alaba, P. Recovery of liquid fuel from fossil-based solid wastes via pyrolysis technique: A review. J. Environ. Chem. Eng. 2021, 9, 106593. [Google Scholar] [CrossRef]
- Zheng, Y.; Tao, L.; Yang, X.; Huang, Y.; Liu, C.; Zheng, Z. Study of the thermal behavior, kinetics, and product characterization of biomass and low-density polyethylene co-pyrolysis by thermogravimetric analysis and pyrolysis-GC/MS. J. Anal. Appl. Pyrolysis 2018, 133, 185–197. [Google Scholar] [CrossRef]
- Yang, J.; Rizkiana, J.; Widayatno, W.B.; Karnjanakom, S.; Kaewpanha, M.; Hao, X.; Abudula, A.; Guan, G. Fast co-pyrolysis of low density polyethylene and biomass residue for oil production. Energy Convers. Manag. 2016, 120, 422–429. [Google Scholar] [CrossRef]
- Rahman, H.; Bhoi, P.R.; Saha, A.; Patil, V.; Adhikari, S. Thermo-catalytic co-pyrolysis of biomass and high-density polyethylene for improving the yield and quality of pyrolysis liquid. Energy 2021, 225, 120231. [Google Scholar] [CrossRef]
- Hassan, H.; Hameed, B.; Lim, J. Co-pyrolysis of sugarcane bagasse and waste high-density polyethylene: Synergistic effect and product distributions. Energy 2020, 191, 116545. [Google Scholar] [CrossRef]
- Izzatie, N.; Basha, M.H.; Uemura, Y.; Hashim, M.S.M.; Amin, N.A.M.; Hamid, M.F. Co-pyrolysis of rice straw and Polyethylene Terephtalate (PET) using a fixed bed drop type pyrolyzer. J. Phys. Conf. Ser. 2017, 908, 012073. [Google Scholar] [CrossRef] [Green Version]
- Çepelioğullar, Ö.; Pütün, A. A pyrolysis study for the thermal and kinetic characteristic of an agricultural waste with two different plastic wastes. Waste Manag. Res. 2014, 32, 971–979. [Google Scholar] [CrossRef]
- Abnisa, F.; Daud, W.W.; Ramalingam, S.; Azemi, M.N.B.M.; Sahu, J. Co-pyrolysis of palm shell and polystyrene waste mixtures to synthesis liquid fuel. Fuel 2013, 108, 311–318. [Google Scholar] [CrossRef]
- Stančin, H.; Šafář, M.; Růžičková, J.; Mikulčić, H.; Raclavská, H.; Wang, X.; Duić, N. Co-pyrolysis and synergistic effect analysis of biomass sawdust and polystyrene mixtures for production of high-quality bio-oils. Process Saf. Environ. Prot. 2021, 145, 1–11. [Google Scholar] [CrossRef]
- Yuan, R.; Yafei, S. Catalytic pyrolysis of biomass-plastic wastes in the presence of MgO and MgCO3 for hydrocarbon-rich oils production. Bioresour. Technol. 2019, 293, 122076. [Google Scholar] [CrossRef] [PubMed]
- López-Urionabarrenechea, A.; de Marco, I. Catalytic stepwise pyrolysis of packaging plastic waste. J. Anal. Appl. Pyrolysis 2012, 96, 54–62. [Google Scholar] [CrossRef]
| Feedstock | Catalyst | Pyrolysis System | Operational Conditions * | Main Products | Ref. |
|---|---|---|---|---|---|
| Douglas Fir sawdust pellet | Commercial acid-washed steam-activated carbon | Microwave-assisted reactor (power 1 kW, frequency 2450 MHz) | 673 K | Phenols | [70] |
| 8 min | |||||
| 1:3 | |||||
| NA ** | |||||
| Peanut shell | Activated carbon | Microwave-assisted reactor (power 2 kW, frequency 2450 MHz) | 573–873 K | Phenols | [71] |
| 50 min | |||||
| Pine sawdust | 8:1 and 8:2 | ||||
| N2 400 mL·min−1 | |||||
| Palm kernel shell | Activated carbon | Fixed-bed reactor | 673–873 K | Phenols | [72] |
| 40 min | |||||
| 3:0.5 and 3:1 | |||||
| N2 1000 mL·min−1 | |||||
| Microwave-assisted reactor (power 2 kW, frequency 2450 MHz) | 673–873 K | ||||
| NA ** | |||||
| 10:1 and 10:2 | |||||
| N2 400 mL·min−1 | |||||
| Sugar cane bagasse | H3PO4-activated carbon | Py-GC/MS | 523–773 K | Levoglucosenone | [73] |
| H2O-activated carbon | 20 s | ||||
| Poplar wood | CO2-activated carbon | 1:0, 10:1, 5:1, 3:1, 2:1, 1:1 and 1:2 | |||
| Pine wood | ZnCl2-activated carbon | NA ** | |||
| Bamboo wastes | Biochar | Fixed-bed reactor | 873 K | Aromatic Hydrocarbons | [74] |
| 10 min | |||||
| Spirulina platensis | 2:1 | Phenols | |||
| Ar 200 mL·min−1 | |||||
| Corn sotver | H3PO4-activated carbon | Fixed-bed reactor | 702–843 K | Phenols | [75] |
| 8 min | |||||
| NA ** | Syngas | ||||
| N2 100 mL·min−1 | |||||
| Fe-supported biochar | Microwave-assisted reactor (power 1 kW, frequency 2450 MHz) | 773 K | Phenols | [62] | |
| Rice husk | 20 min | ||||
| Corn cob | 1:1 (ex-situ) | ||||
| Under vacuum | |||||
| Bamboo waste | N–doped biochar | Fixed-bed reactor | 873 K | Phenols | [76] |
| 30 min | |||||
| 2:1 | |||||
| Ar 200 mL·min−1 | |||||
| Douglas Fir | H3PO4-activated carbon impregnated with MgO | Fixed-bed reactor | 673–873 K | Phenols | [64] |
| 15 min | Furans | ||||
| 1:0, 2:1, 1:1 and 1:2 | Aldehydes | ||||
| N2 60 mL·min−1 | Ketones | ||||
| Bamboo waste | KOH-activated biochar | Fixed-bed reactor | 873 K | Aromatic Hydrocarbons | [61] |
| K2CO3-activated biochar | 30 min | ||||
| KHCO3-activated biochar | 2:1 (ex-situ) | Phenols | |||
| CH3COOK-activated biochar | Ar 200 mL·min−1 | ||||
| Chlorella vulgaris | Acid-washed biochar | Dual-bed reactor | 723–1123 K | Hydrocarbons | [77] |
| KOH-activated carbon | 30 min | Phenols | |||
| Fe acid-washed biochar | 2:1 (ex-situ) | Acids | |||
| Fe KOH-activated carbon | Ar 30 mL·min−1 | Alcohols | |||
| Pine sawdust | CO2-activated carbon | Fixed-bed reactor | 873 K | Phenols | [63] |
| 10 min | |||||
| 2:5 (ex-situ) | |||||
| NA ** |
| Catalysts | Biomass | Reactor | Main Results | Ref. | |
|---|---|---|---|---|---|
| Metallic oxides | CaO and HZSM-5 | Waste mixed cloth | Py-GC/MS | CaO increased ketones, aliphatic HC and aromatics. | [105] |
| CaO | Cotton stalk | Fixed-bed reactor | Increased furans. Decreased carboxylic acids. | [101] | |
| CaO, MgO and ZnO | Palm empty fruit bunches | Fixed-bed reactor | CaO promoted deacidification. MgO decreased levoglucosan. Catalysts increased water in the bio-oil. | [106] | |
| CaO and MgO | Forest residues | Batch tubular reactor | CaO showed better deoxygenation power than MgO, and increased H2 content and CO2 absorption. | [107] | |
| CaO | Oakwood | Py-GC/MS | Increased ketones and light phenols. Decreased carboxylic acids, furans, and heavy phenols. | [108] | |
| CaO, Ca(OH)2 and Ca(COOH)2 | Switchgrass | Fluidized bed reactor | Decreased acetic acid and levoglucosan. Increased phenols and HC. | [109] | |
| CoO, Cr2O3, CuO, Fe2O3, Mn2O3, NiO, TiO2, V2O5 and CeO2 | Poplar wood | Fixed-bed reactor | Catalysts promoted alcohol, furans, ketones, acetic acid and phenolic compounds, except Fe2O3. | [38] | |
| ZnO, CaO, Fe2O3 and MgO | Poplar wood-polypropylene composite | Py-GC/MS | CaO eliminated carboxylic acids and phenols, while slightly increasing cyclopentanones and alkenes. | [110] | |
| Al2O3, CaO, MgO, CuO, Fe2O3, NiO, ZnO, ZrO2, TiO2, HZSM-5 and MCM-41 | Cotton stalk | Fixed-bed reactor | Al2O3, CaO and NiO showed the best balance between bio-oil yield and deoxygenation. | [111] | |
| Mixed metal oxide of BaMg, BaCa, and CaMg | Bagasse | Py-GC/MS | BaMg-MMO showed a yield maximal of 7.3 wt% and selectivity of 44.4% to 4-VP. | [112] | |
| CaO and CaO/MgO | Wood | Auger reactor pilot plant | Decreased acidity and oxygen content. Increased pH and calorific value | [113] | |
| NbxWyOz, NbxAlyOz, NbxMnyOz, and HZSM-5 | Beech wood | Fixed-bed reactor | NbxMnyOz performed similarly to HZSM-5. Reduced O/C fraction from 0.34 to 0.15 and 0.17 with HZSM-5 and NbxMnyOz, respectively. | [114] | |
| Cao, Fe/CaO and Ni/CaO | Jatropha residues | Py-GC/MS | Eliminated carboxylic acids. Decreased N and O-compounds (except ketones, esters and aldehydes). Ni/CaO was the best catalyst for aliphatic HC production | [115] | |
| Metallic salts | HCOOK, Ni(HCOO)2, and Zn(HCOO)2 | Corn straw | Fixed-bed reactor | Increased phenols and ketones. Decreased carboxylic acids and esters. | [116] |
| Fe(NO3)3, Fe2(SO4)3, FeCl2, and FeCl3 | Bamboo | Fixed-bed reactor | FeCl2 and FeCl3 produced a bio-oil rich in ketones. Fe2(SO4)3 produced a bio-oil rich in acids. | [117] | |
| Ce, Mn, Fe, Co, Ni, Cu and Zn nitrate salts | Eucalyptus | Fixed-bed reactor | Increased CO2 yield and gas phase. Increased anhydrosugars (Zn > Co > Mn > Ni > Ce > Cu > catalyst-free) | [118] | |
| MgCl2 and ZnCl2 | Sweet sorghum bagasse | Py-GC/MS | All catalysts modified the biomass degradation profile and increased solid waste. ZnCl2 increased furfural. | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangel, M.d.C.; Mayer, F.M.; Carvalho, M.d.S.; Saboia, G.; de Andrade, A.M. Selecting Catalysts for Pyrolysis of Lignocellulosic Biomass. Biomass 2023, 3, 31-63. https://doi.org/10.3390/biomass3010003
Rangel MdC, Mayer FM, Carvalho MdS, Saboia G, de Andrade AM. Selecting Catalysts for Pyrolysis of Lignocellulosic Biomass. Biomass. 2023; 3(1):31-63. https://doi.org/10.3390/biomass3010003
Chicago/Turabian StyleRangel, Maria do Carmo, Francieli Martins Mayer, Mateus da Silva Carvalho, Giovanni Saboia, and Arthur Motta de Andrade. 2023. "Selecting Catalysts for Pyrolysis of Lignocellulosic Biomass" Biomass 3, no. 1: 31-63. https://doi.org/10.3390/biomass3010003
APA StyleRangel, M. d. C., Mayer, F. M., Carvalho, M. d. S., Saboia, G., & de Andrade, A. M. (2023). Selecting Catalysts for Pyrolysis of Lignocellulosic Biomass. Biomass, 3(1), 31-63. https://doi.org/10.3390/biomass3010003







