The Impact of Fibre Oxidation on the Preparation of Cellulose Nanocrystals (CNC)
Abstract
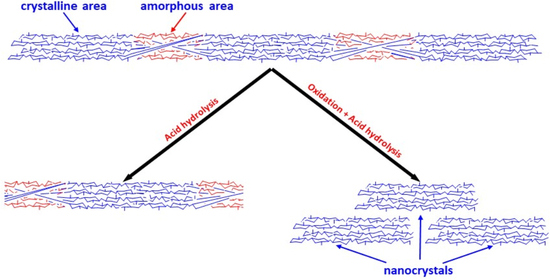
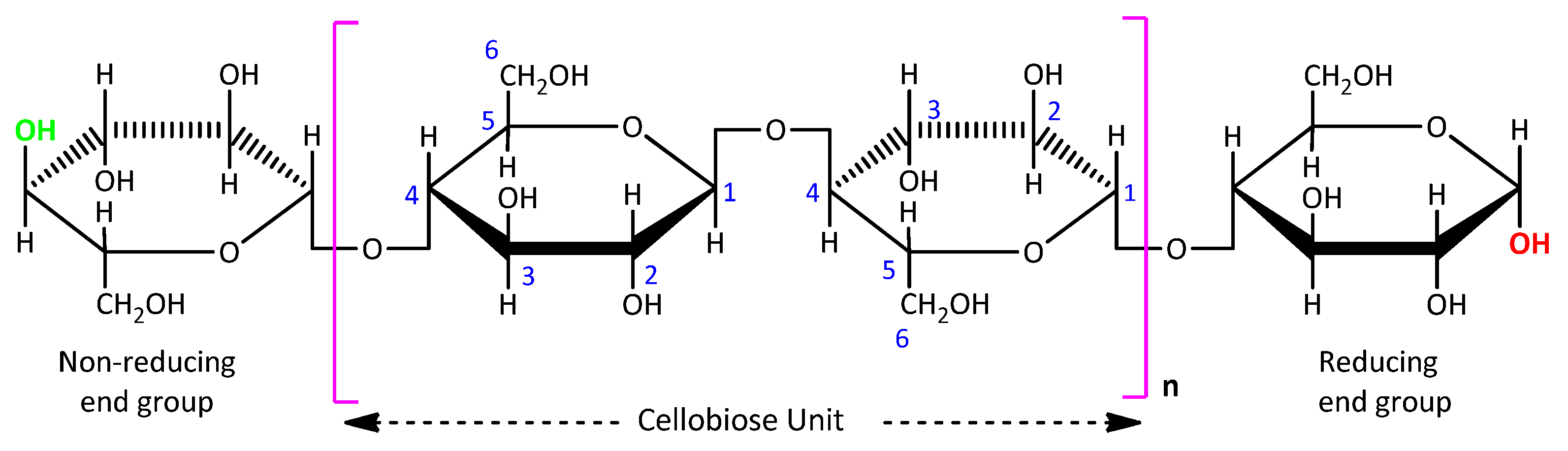
1. Introduction
2. Materials and Methods
2.1. Feedstock Furnish
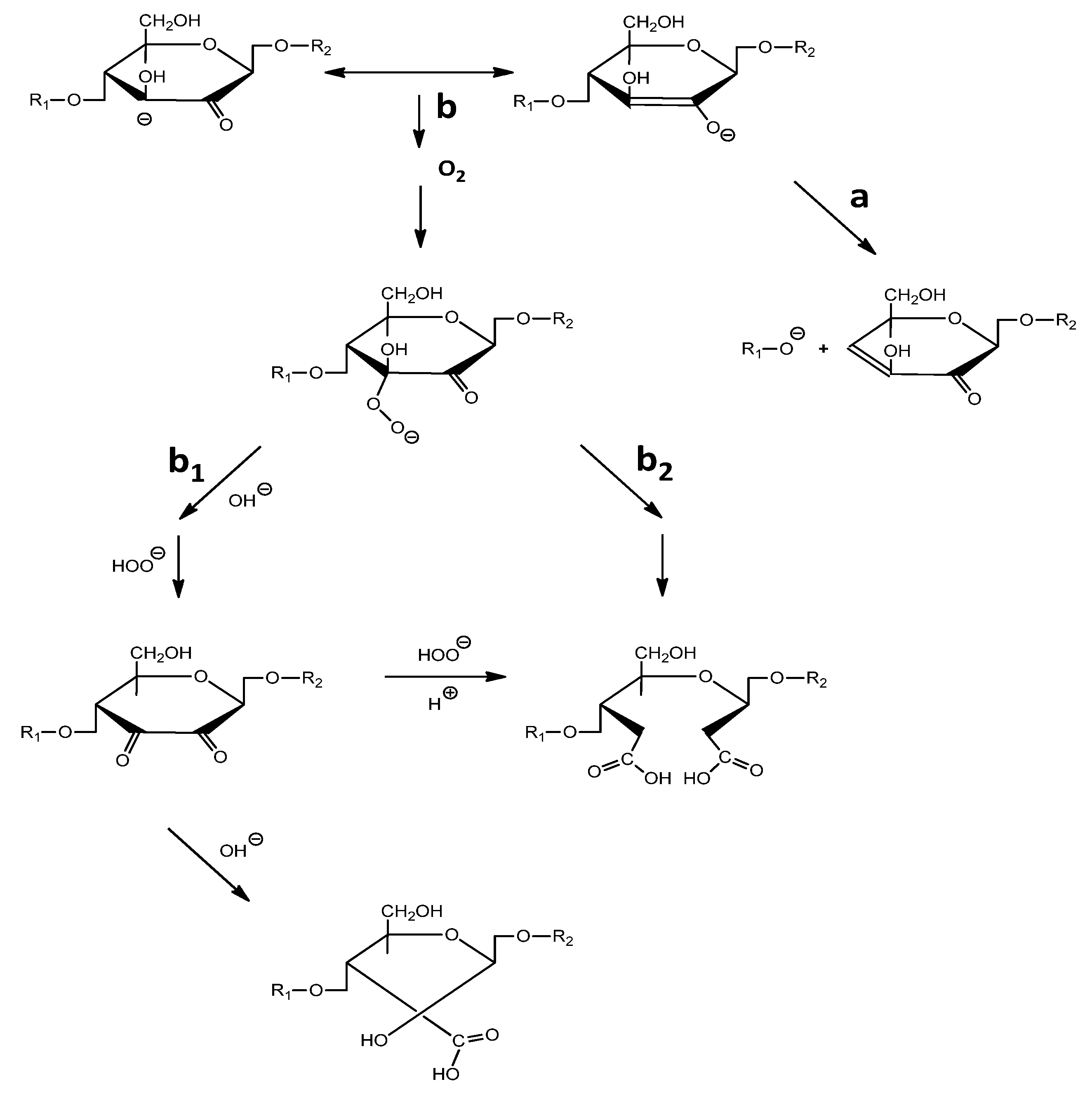
2.2. Oxidation Process
2.3. Standard InnoTech Alberta Lab-Scale CNC Production Process
2.4. CNC and CNC Reject Yield Calculations
2.5. Dynamic Light Scattering (DLS)
2.6. Zeta Potential (ZP) Measurements
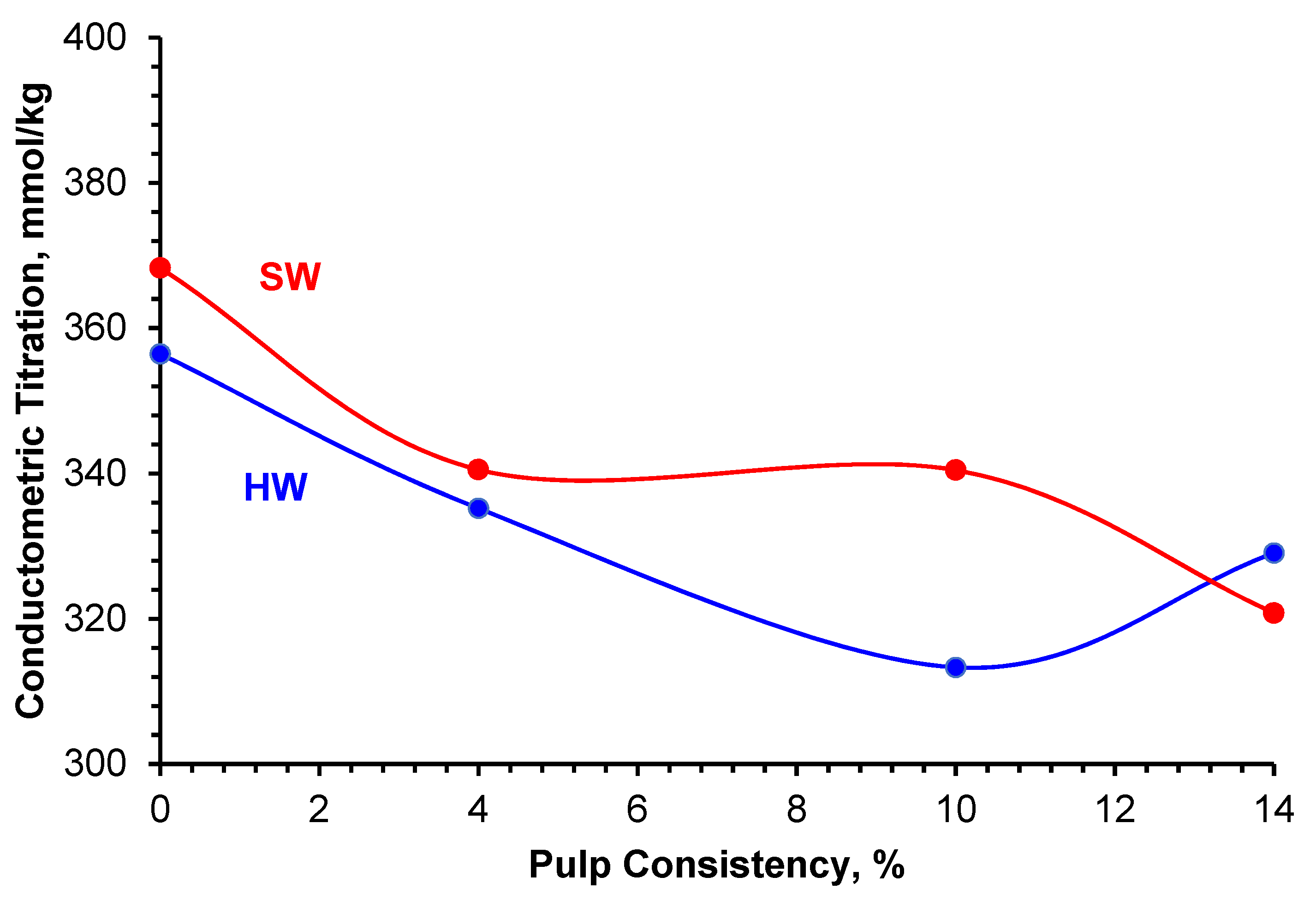
2.7. Conductometric Titration
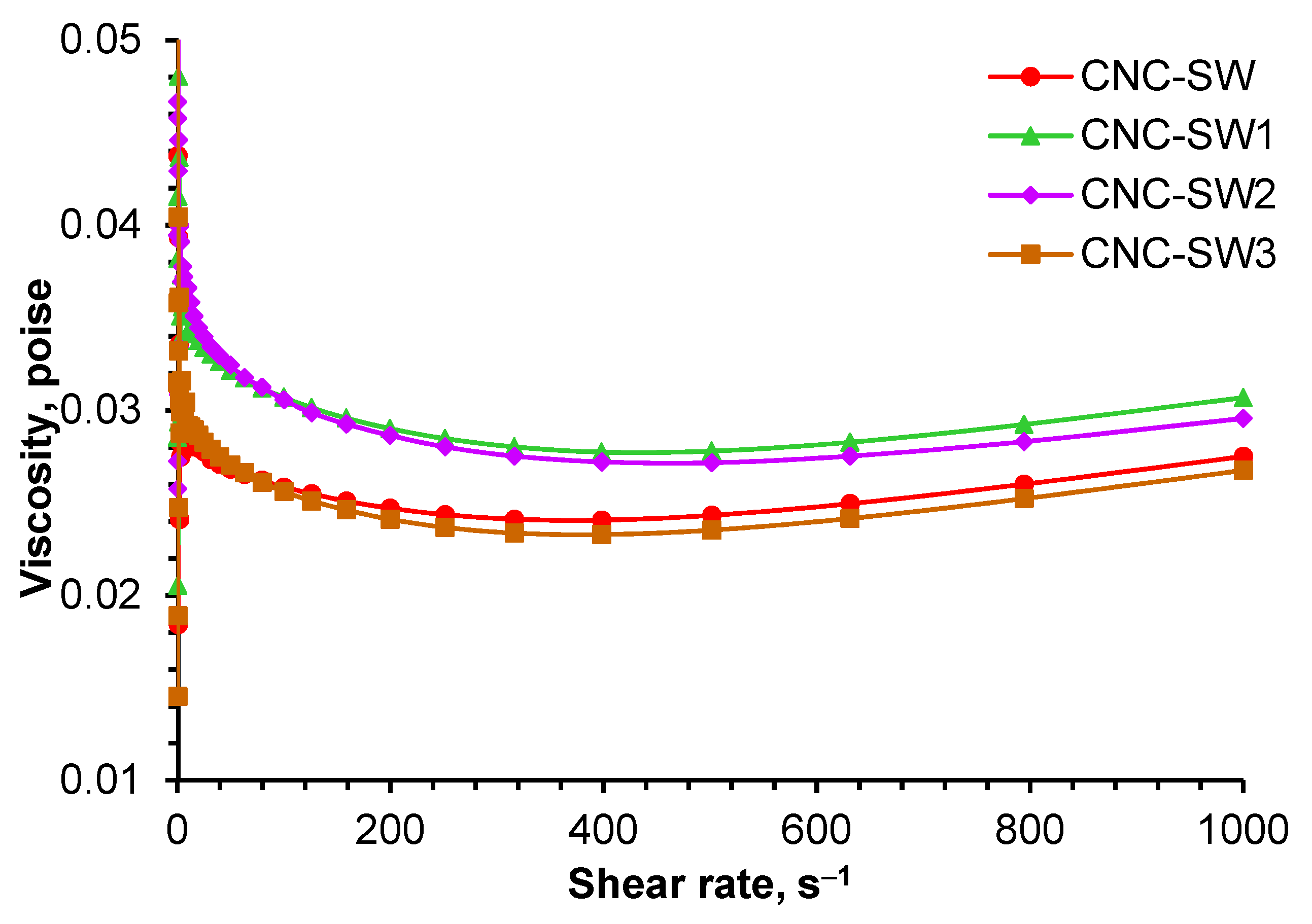
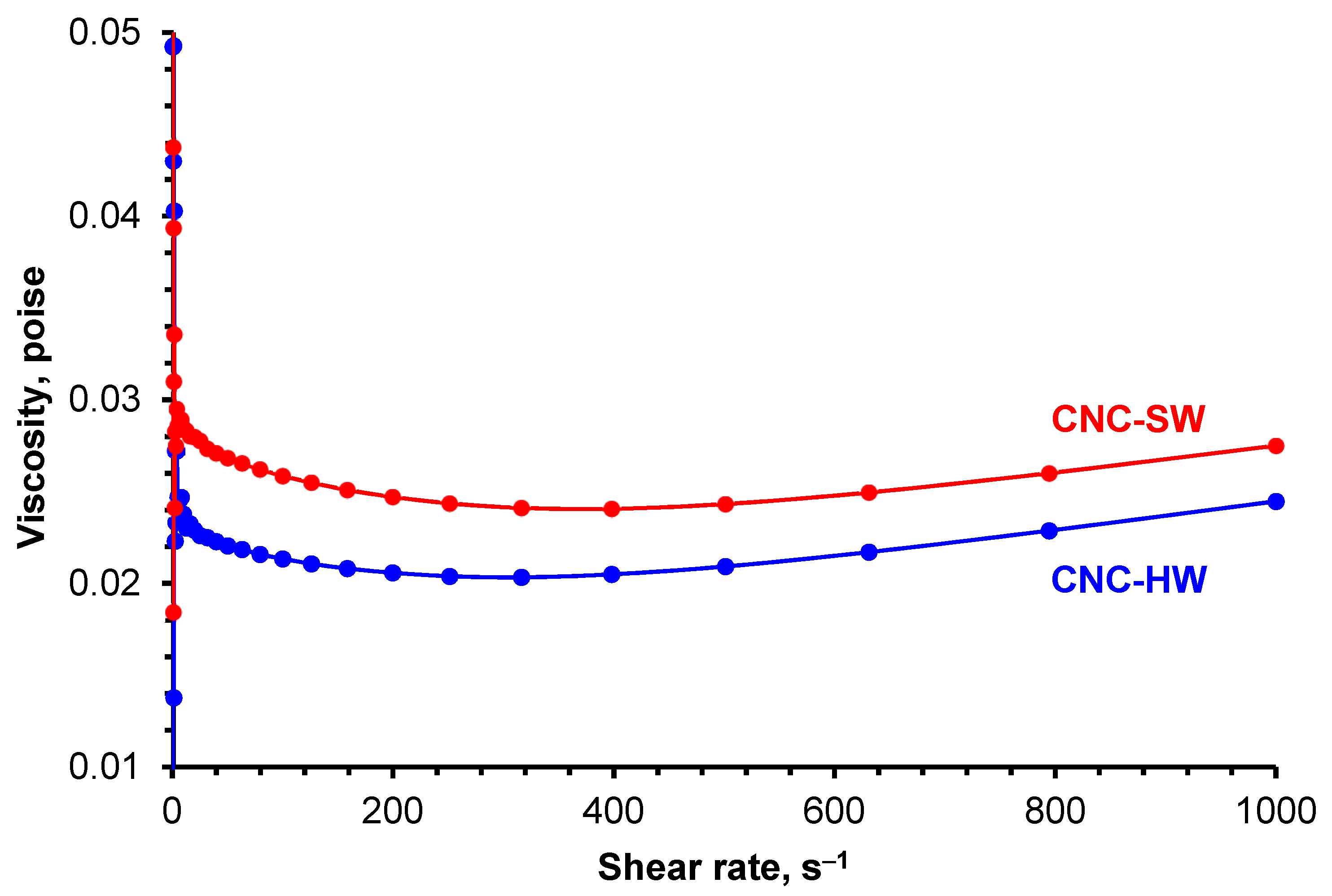
2.8. Rheological Measurements
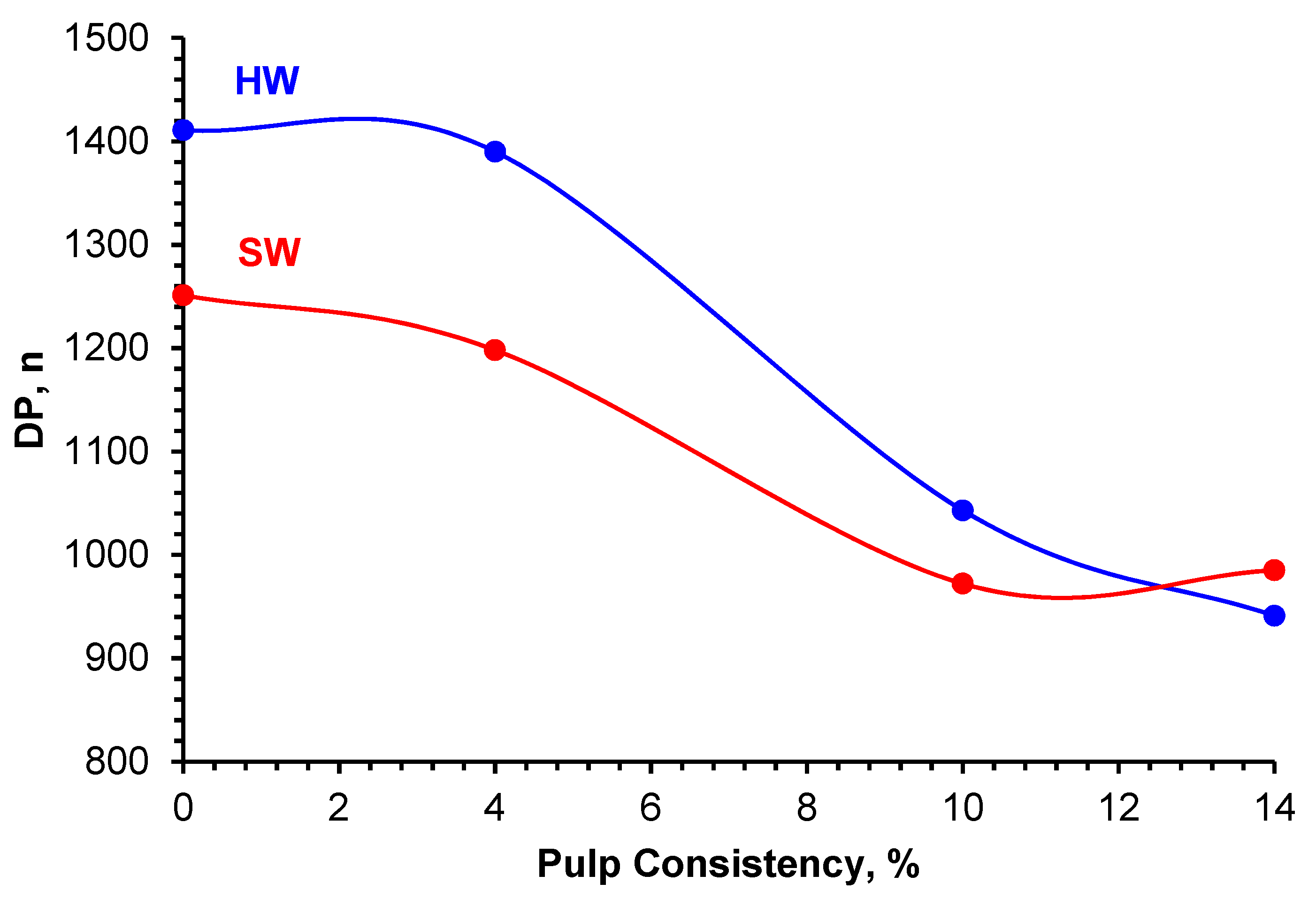
2.9. Pulp Viscosity Measurements
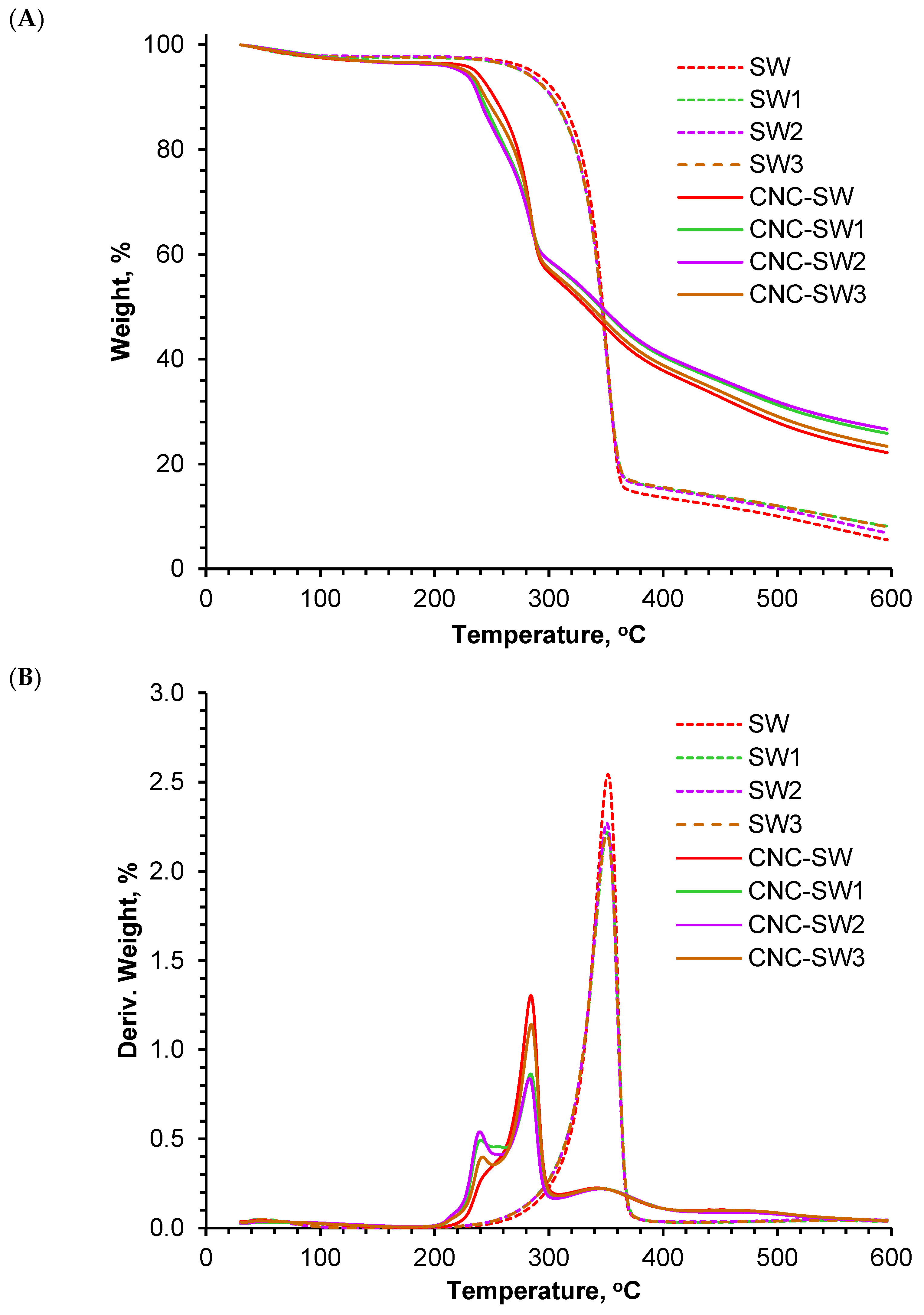
2.10. Thermogravimetric Analysis (TGA)
2.11. Scanning Electron Microscopy (SEM)
3. Results
3.1. Oxidation of SW and HW Kraft Pulps
3.2. CNC Preparation
3.3. Thermogravimetric Analysis (TGA)
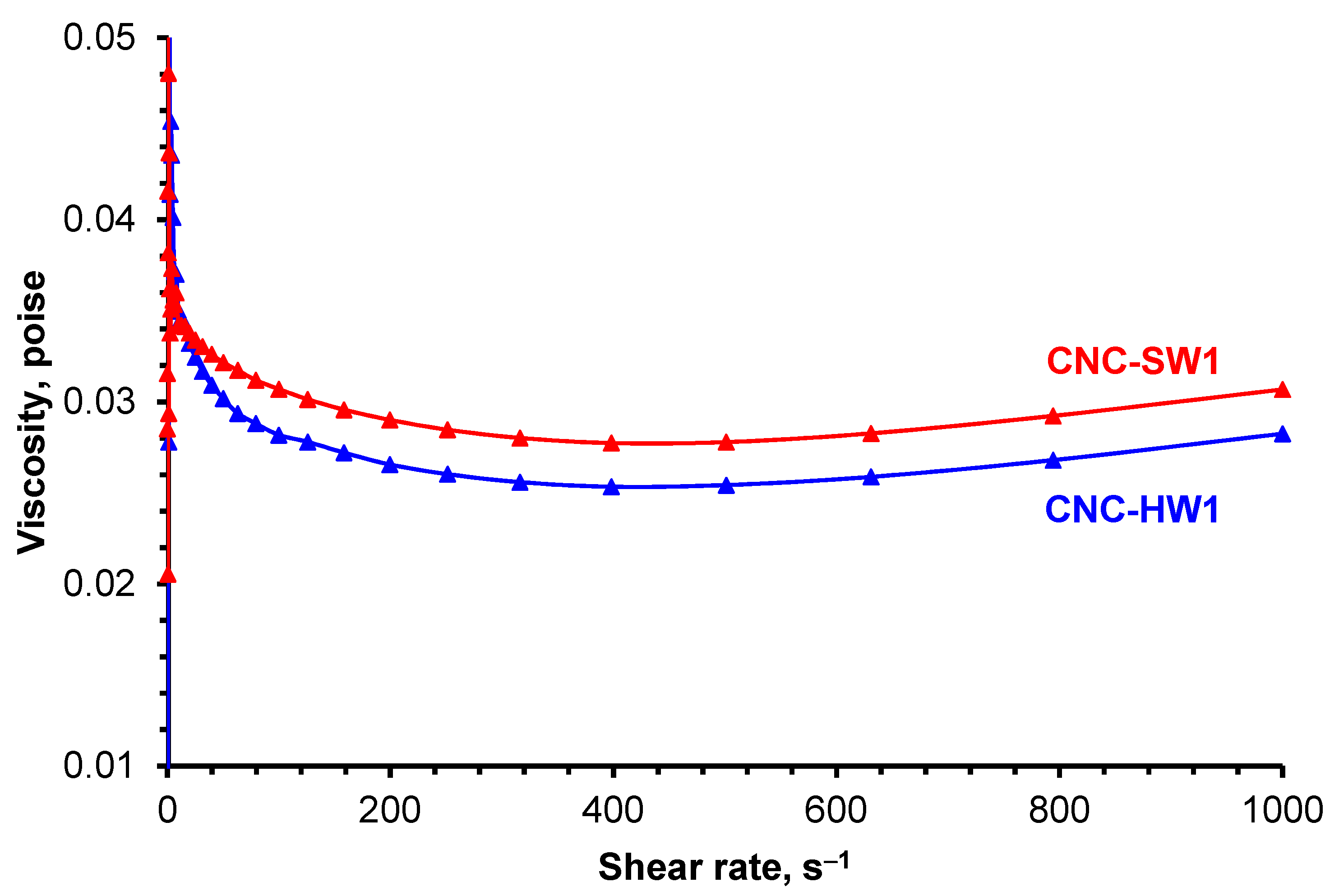
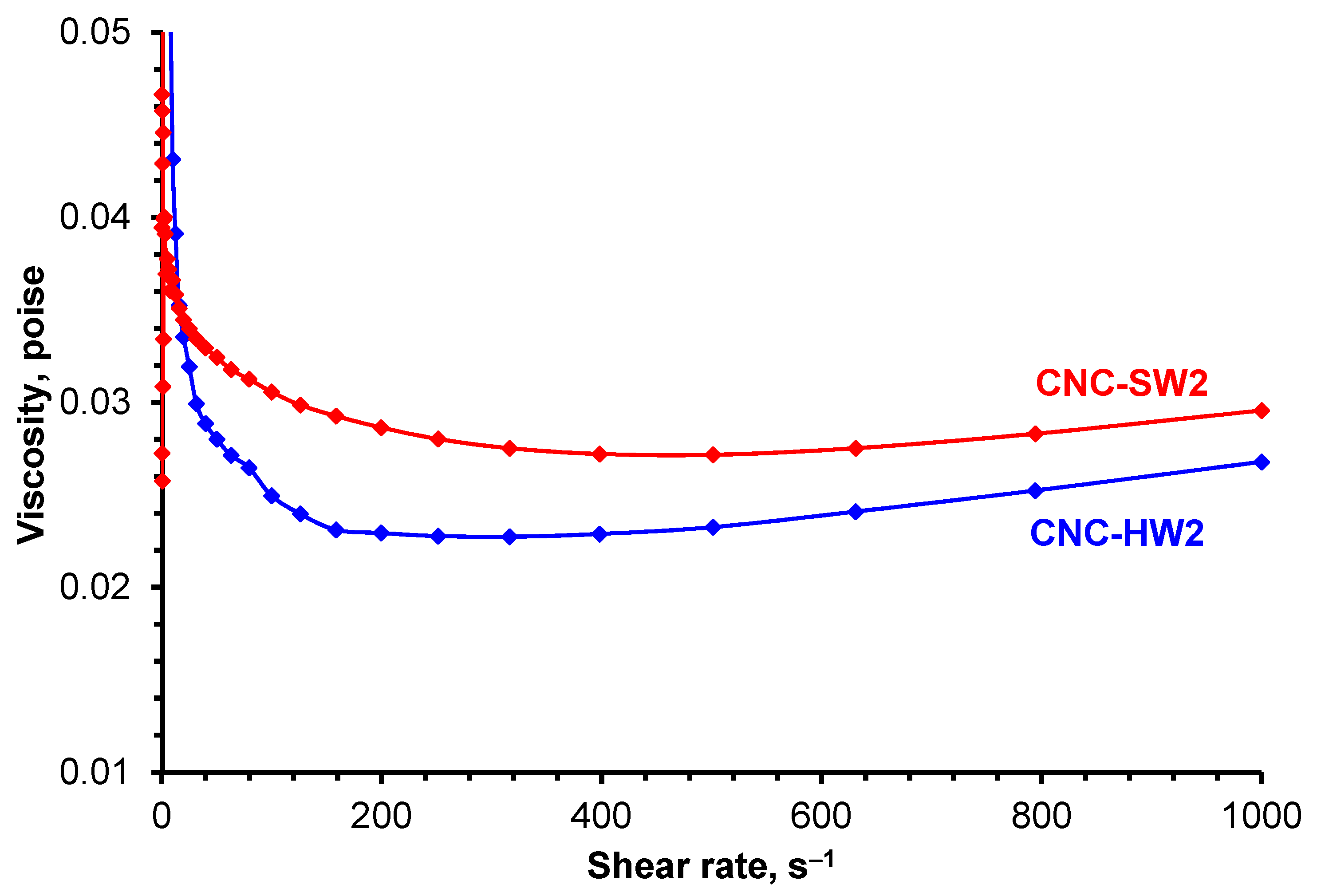
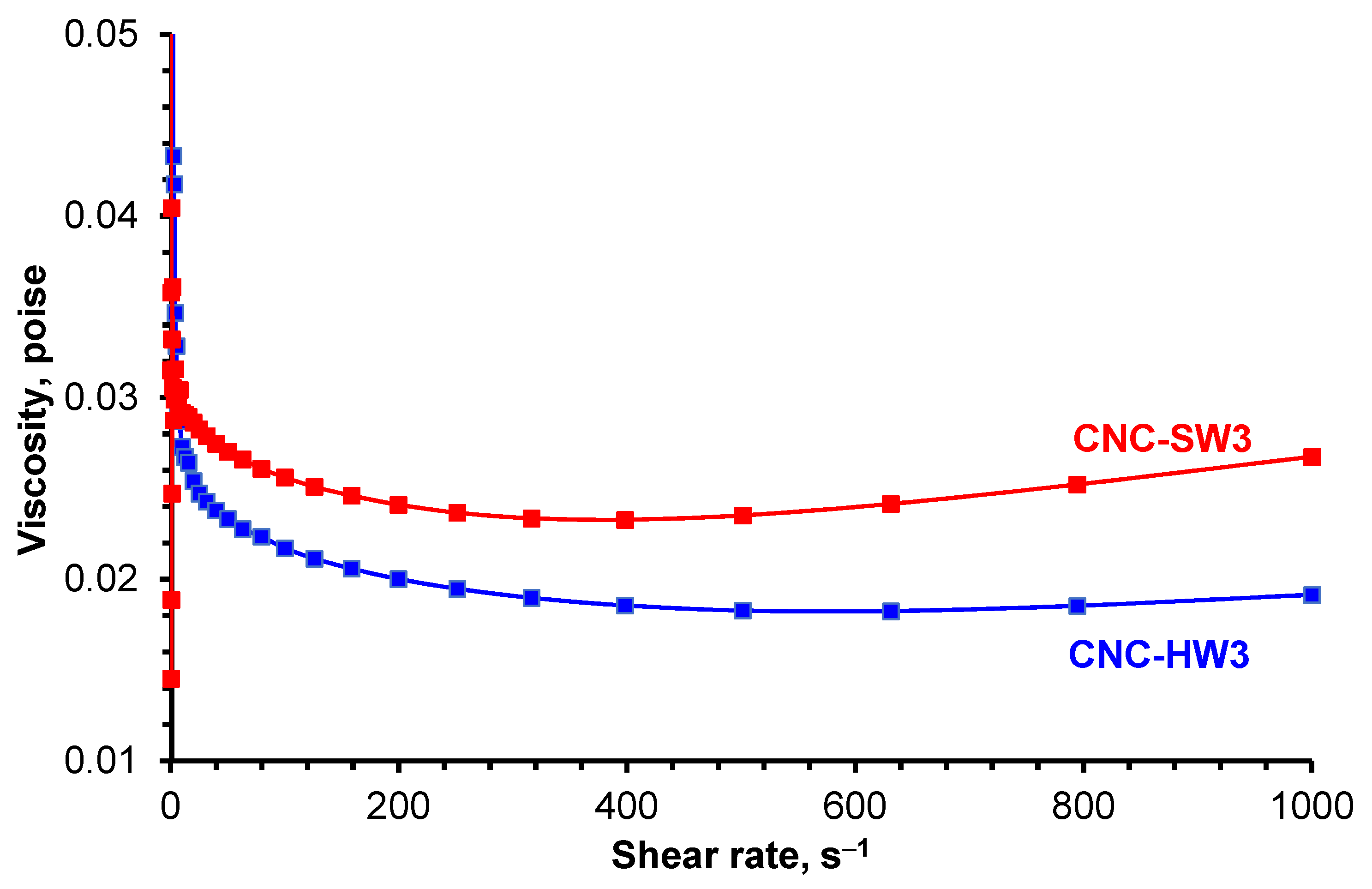
3.4. Rheological Measurements
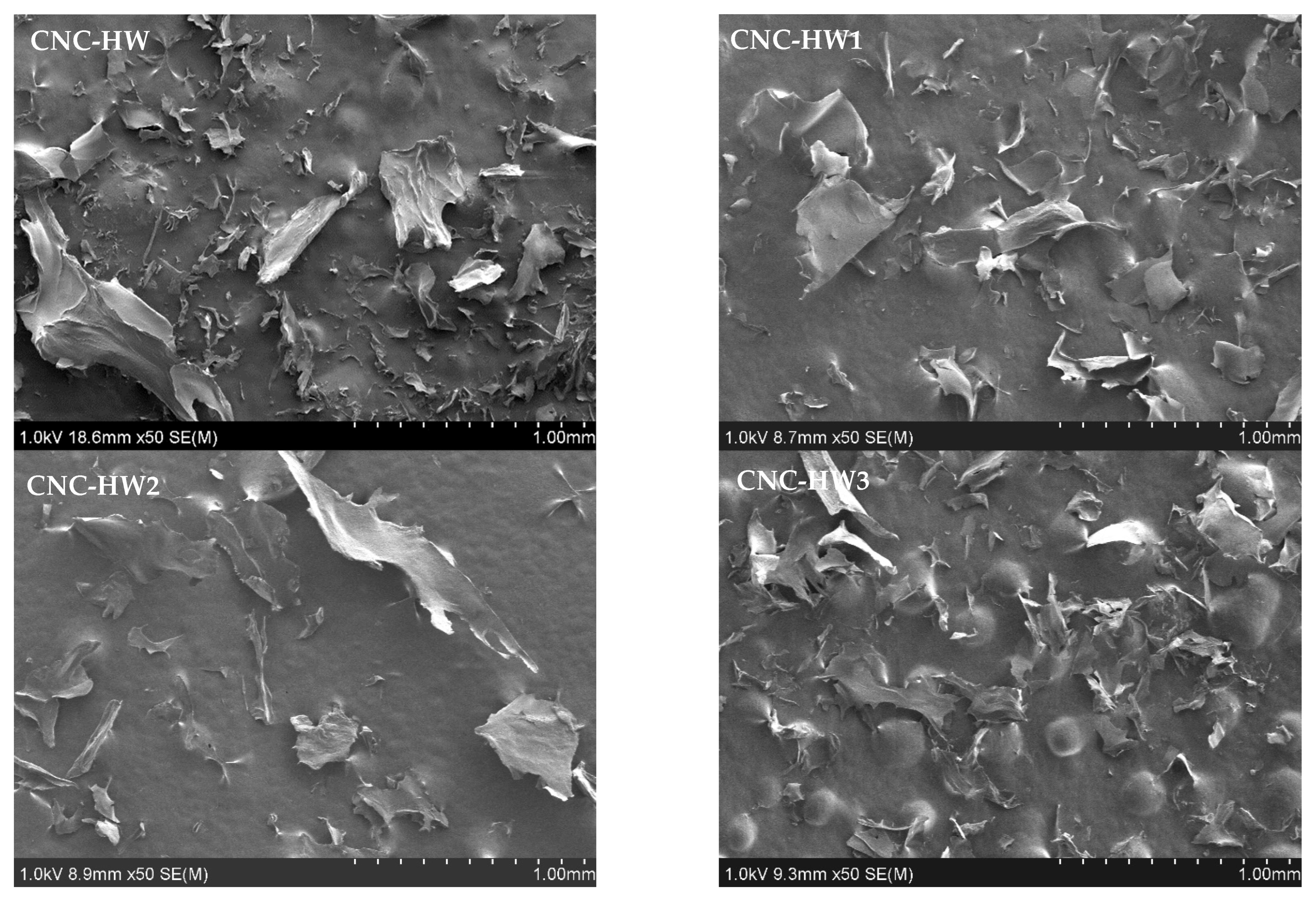
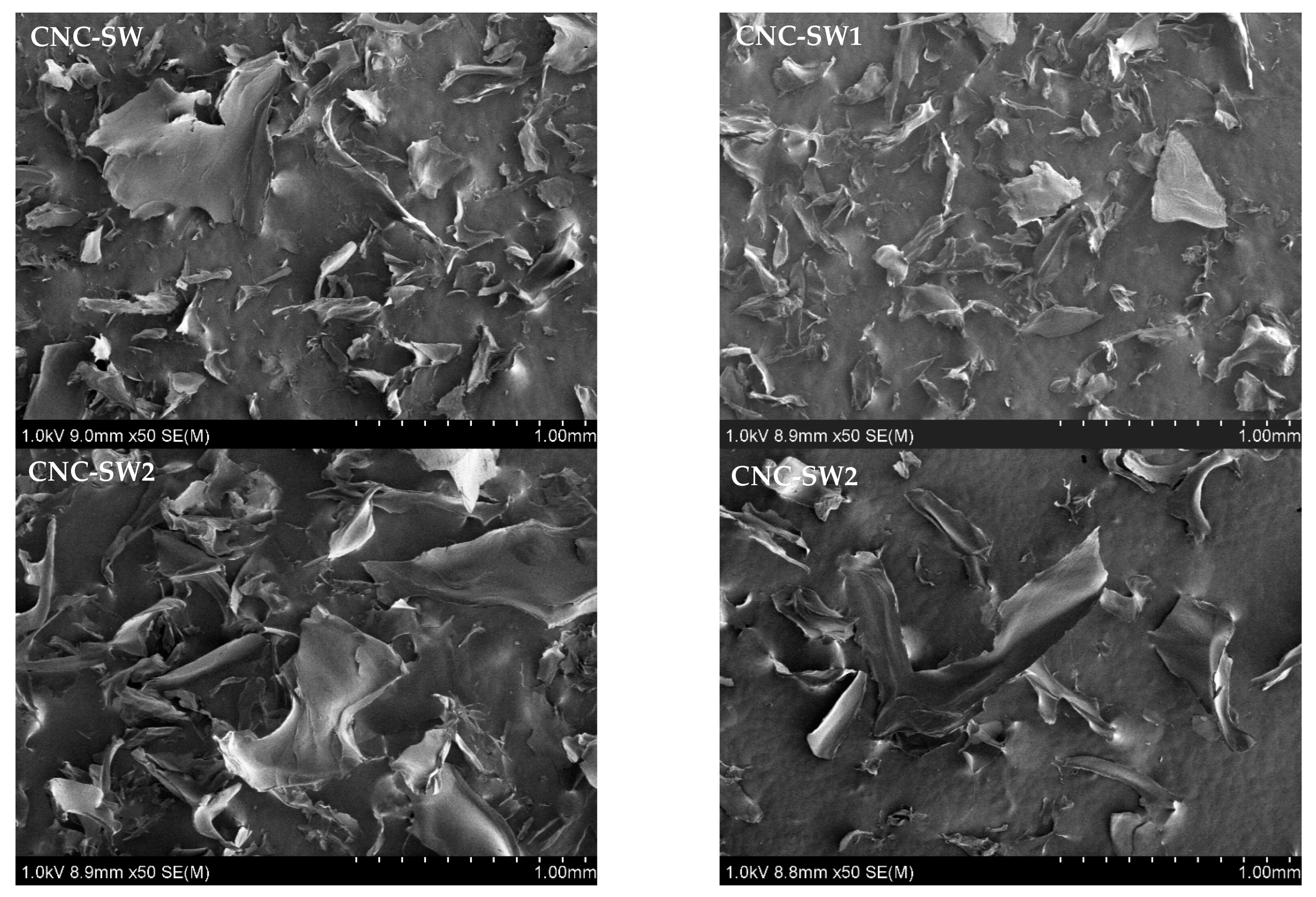
3.5. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Souza Lima, M.M.; Borsali, R. Rodlike cellulose microcrystals: Structure, properties, and applications. Macromol. Rapid Commun. 2004, 25, 771–787. [Google Scholar] [CrossRef]
- Huang, J.; Chang, P.R.; Dufresne, A. Polysaccharide Nanocrystals: Current Status and Prospects in Material Science, Polysaccharide-Based Nanocrystals: Chemistry and Applications, 1st ed.; Chemical Industry Press: Beijing, China; Wiley-VCH Verlag GmbH & Co. KGaA: Berlin, Germany, 2015; pp. 1–13. [Google Scholar]
- Oksman, K.; Aitomaki, Y.; Mathew, A.P.; Siqueira, G.; Zhou, Q.; Butylina, S.; Tanpichai, S.; Zhou, X.; Hooshmand, S. Review of the recent developments in cellulose nanocomposite processing. Compos. Part A 2016, 83, 2–18. [Google Scholar] [CrossRef]
- Dufresne, A. Cellulose nanomaterials as green nanoreinforcements for polymer nanocomposites. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170040. [Google Scholar] [CrossRef] [PubMed]
- Mariano, M.; Kissi, N.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J. Polym. Sci. 2014, 52, 791–806. [Google Scholar] [CrossRef]
- Marchessault, R.H.; Morehead, F.F.; Koch, M.J. Some hydrodynamic properties of neutral suspensions of cellulose crystallites as related to size and shape. J. Colloid Sci. 1961, 16, 327–344. [Google Scholar] [CrossRef]
- Candanedo, S.B.; Roman, M.; Gray, D.G. Effect of conditions on the properties and behavior of wood cellulose nanocrystal suspension. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Ranby, B.G.; Banderet, A.; Sillén, L.G. Aqueous colloidal solutions of cellulose micelles. Acta Chem. Scand. 1949, 3, 649–650. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 36, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A. Nanocellulose: From Nature to High-Performance Tailored Materials; Walter de Gruyter GmbH & Co KG: Berlin, Germany; Boston, MA, USA, 2012. [Google Scholar]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.; Mazeau, K. Conformations, structures and morphologies of celluloses. In Polysaccharides: Structural Diversity and Functional Versatility; Dimitriu, S., Ed.; Marcel Dekker: New York, NY, USA, 2004; pp. 41–68. [Google Scholar]
- Sarkanen, K.V.; Islam, A.; Anderson, C.D. Ozonation. In Methods in Lignin Chemistry; Lin, S.Y., Dence, C.W., Eds.; Springer: Berlin, Germany, 1992; pp. 387–406. [Google Scholar]
- Sjöström, E. Behavior of Pulp Polysaccharides during Oxygen-Alkali Delignification. In Chemistry of Delignification with Oxygen, Ozone, and Peroxide; The University of Tokyo: Tokyo, Japan, 1980; pp. 61–77. [Google Scholar]
- Gratzl, J.S. Reaction of Polysaccharides and Lignins in Bleaching with Oxygen and Related Species; Tappi Oxygen Delignification Symposium Notes; Tappi Press: Atlanta, GA, USA, 1990; pp. 1–8. [Google Scholar]
- Randriamanantena, T.; Razafindramisa, F.L.; Ramanantsizehena, G.; Bernes, A.; Lacabane, C. Thermal behaviour of three woods of Madagascar by thermogravimetric analysis in inert atmosphere. In Proceedings of the Fourth High-Energy Physics International Conference, Antananarivo, Madagascar, 21–28 August 2009. [Google Scholar]
- Ping-Sheng, L.; Qiang, C.; Xiang, L.; Bo, Y.; Shi-Shan, W.; Jian, S.; Si-Cong, L. Grafting of zwitterion from cellulose membranes via ATRP for improving blood compatibility. Biomacromolecules 2009, 10, 2809–2816. [Google Scholar]
- Jie, Y.; Qunxing, X.; Xuefei, Z.; Hailiang, Z. Temperature-induced chiral nematic phase changes of suspensions of poly (N,N-dimethylaminoethyl methacrylate)-grafted cellulose nanocrystals. Cellulose 2009, 16, 989–997. [Google Scholar]


| Sample Name | Solid Content, % | α-Cellulose, % | Acid Insoluble Lignin, % | Ash, % | Acetone Extractive, % |
|---|---|---|---|---|---|
| SW | 97.30 | 84.4 | 3.87 | 0.50 | 0.069 |
| HW | 96.87 | 92.5 | 1.83 | 0.56 | 0.356 |
| Sample | Pulp, g OD | NaOH, % | Consistency, % | Temp., °C | O2, psi | Time, min |
|---|---|---|---|---|---|---|
| HW1 | 100 | 1.0 | 4 | 85 | 80 | 50 |
| HW2 | 100 | 2.0 | 10 | 105 | 90 | 60 |
| HW3 | 100 | 3.0 | 14 | 115 | 100 | 90 |
| SW1 | 100 | 1.0 | 4 | 85 | 80 | 50 |
| SW2 | 100 | 2.0 | 10 | 105 | 90 | 60 |
| SW3 | 100 | 3.0 | 14 | 115 | 100 | 90 |
| Sample | Consistency, % | DP, n |
|---|---|---|
| HW Control | 0 | 1411 |
| HW1 | 4 | 1390 |
| HW2 | 10 | 1043 |
| HW3 | 14 | 941 |
| SW Control | 0 | 1251 |
| SW1 | 4 | 1198 |
| SW2 | 10 | 972 |
| SW3 | 14 | 985 |
| Sample | Yield, % | |
|---|---|---|
| CNC | Reject | |
| CNC-HW Control | 13.4 | 3.1 |
| CNC-HW1 | 18.9 | 7.4 |
| CNC-HW2 | 20.4 | 5.1 |
| CNC-HW3 | 21.4 | 5.1 |
| CNC-SW Control | 17.3 | 4.7 |
| CNC-SW1 | 19.1 | 3.2 |
| CNC-SW2 | 19.9 | 3.2 |
| CNC-SW3 | 23.1 | 1.6 |
| Sample | DLS | Zeta Pot., mV | |
|---|---|---|---|
| dav, nm | PDI | ||
| CNC-HW Control | 116.8 ± 4.4 | 0.397 ± 0.017 | −45.4 ± 1.3 |
| CNC-HW1 | 137.8 ± 4.3 | 0.453 ± 0.017 | −45.4 ± 0.8 |
| CNC-HW2 | 116.4 ± 10.4/383.1 ± 5.7 | 0.723 ± 0.030 | −52.2 ± 0.2 |
| CNC-HW3 | 130.8 ± 6.4 | 0.436 ± 0.008 | −46.3 ± 2.4 |
| CNC-SW Control | 102.4 ± 22.6 | 0.424 ± 0.026 | −43.5 ± 1.2 |
| CNC-SW1 | 120.8 ± 7.3 | 0.465 ± 0.016 | −46.2 ± 0.4 |
| CNC-SW2 | 112.7 ± 6.3 | 0.437 ± 0.008 | −54.3 ± 0.1 |
| CNC-SW3 | 173.6 ± 3.5 | 0.570 ± 0.037 | −51.7 ± 0.8 |
| Sample | Conductometric Titration, mmol/kg |
|---|---|
| CNC-HW Control | 356.47 |
| CNC-HW1 | 335.20 |
| CNC-HW2 | 313.30 |
| CNC-HW3 | 329.05 |
| CNC-SW Control | 368.30 |
| CNC-SW1 | 340.52 |
| CNC-SW2 | 340.45 |
| CNC-SW3 | 320.78 |
| Sample | Consistency, % | M.C. at 150 °C, % | T5%, °C | T50%, °C | Tmax, °C | Residue at 600 °C, % | TiD, °C |
|---|---|---|---|---|---|---|---|
| 1 | |||||||
| HW Control | 0 | 3.4 | 273.6 | 340.7 | 344.8 | 6.8 | 275.0 |
| HW1 | 4 | 3.1 | 272.0 | 344.4 | 348.8 | 6.3 | 275.0 |
| HW2 | 10 | 3.0 | 276.8 | 346.0 | 350.4 | 4.2 | 275.0 |
| HW3 | 14 | 3.0 | 281.6 | 343.2 | 348.0 | 2.7 | 275.0 |
| SW Control | 0 | 3.5 | 271.2 | 347.0 | 352.0 | 5.5 | 275.0 |
| SW1 | 4 | 3.5 | 264.8 | 346.2 | 351.0 | 8.1 | 275.0 |
| SW2 | 10 | 3.4 | 263.2 | 346.4 | 350.4 | 6.8 | 275.0 |
| SW3 | 14 | 3.4 | 264.0 | 346.5 | 350.4 | 8.0 | 275.0 |
| Sample | M.C. at 150 °C, % | T5%, °C | T50%, °C | Tmax, °C | Residue at 600 °C, % | TiD, °C | |
|---|---|---|---|---|---|---|---|
| 1 | 2 | ||||||
| CNC-HW Control | 3.7 | 225.6 | 336.0 | 244.0 | 284.8 | 29.5 | 220.0 |
| CNC-HW1 | 3.8 | 241.4 | 318.6 | n/a | 285.6 | 20.6 | 235.0 |
| CNC-HW2 | 4.3 | 239.0 | 338.6 | 259.2 | 285.1 | 23.8 | 235.0 |
| CNC-HW3 | 3.8 | 247.5 | 315.0 | n/a | 286.4 | 27.2 | 240.0 |
| CNC-SW Control | 4.5 | 217.6 | 329.0 | n/a | 284.8 | 21.9 | 220.0 |
| CNC-SW1 | 4.0 | 216.8 | 343.1 | 240.0 | 284.0 | 25.7 | 205.0 |
| CNC-SW2 | 4.2 | 211.2 | 343.2 | 239.2 | 283.2 | 26.4 | 205.0 |
| CNC-SW3 | 4.4 | 212.0 | 333.6 | 242.4 | 284.8 | 23.1 | 210.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahvazi, B.; Danumah, C.; Ngo, T.-D.; Zhu, Z.; Lorenz, H. The Impact of Fibre Oxidation on the Preparation of Cellulose Nanocrystals (CNC). Biomass 2022, 2, 316-333. https://doi.org/10.3390/biomass2040021
Ahvazi B, Danumah C, Ngo T-D, Zhu Z, Lorenz H. The Impact of Fibre Oxidation on the Preparation of Cellulose Nanocrystals (CNC). Biomass. 2022; 2(4):316-333. https://doi.org/10.3390/biomass2040021
Chicago/Turabian StyleAhvazi, Behzad, Christophe Danumah, Tri-Dung Ngo, Zhengxiang Zhu, and Heather Lorenz. 2022. "The Impact of Fibre Oxidation on the Preparation of Cellulose Nanocrystals (CNC)" Biomass 2, no. 4: 316-333. https://doi.org/10.3390/biomass2040021
APA StyleAhvazi, B., Danumah, C., Ngo, T.-D., Zhu, Z., & Lorenz, H. (2022). The Impact of Fibre Oxidation on the Preparation of Cellulose Nanocrystals (CNC). Biomass, 2(4), 316-333. https://doi.org/10.3390/biomass2040021






_Ngo.png)