Enhancing the Efficacy of the Subcritical Water-Based Alkali Lignin Depolymerization by Optimizing the Reaction Conditions and Using Heterogeneous Catalysts
Abstract
:1. Introduction
- Mechanism:
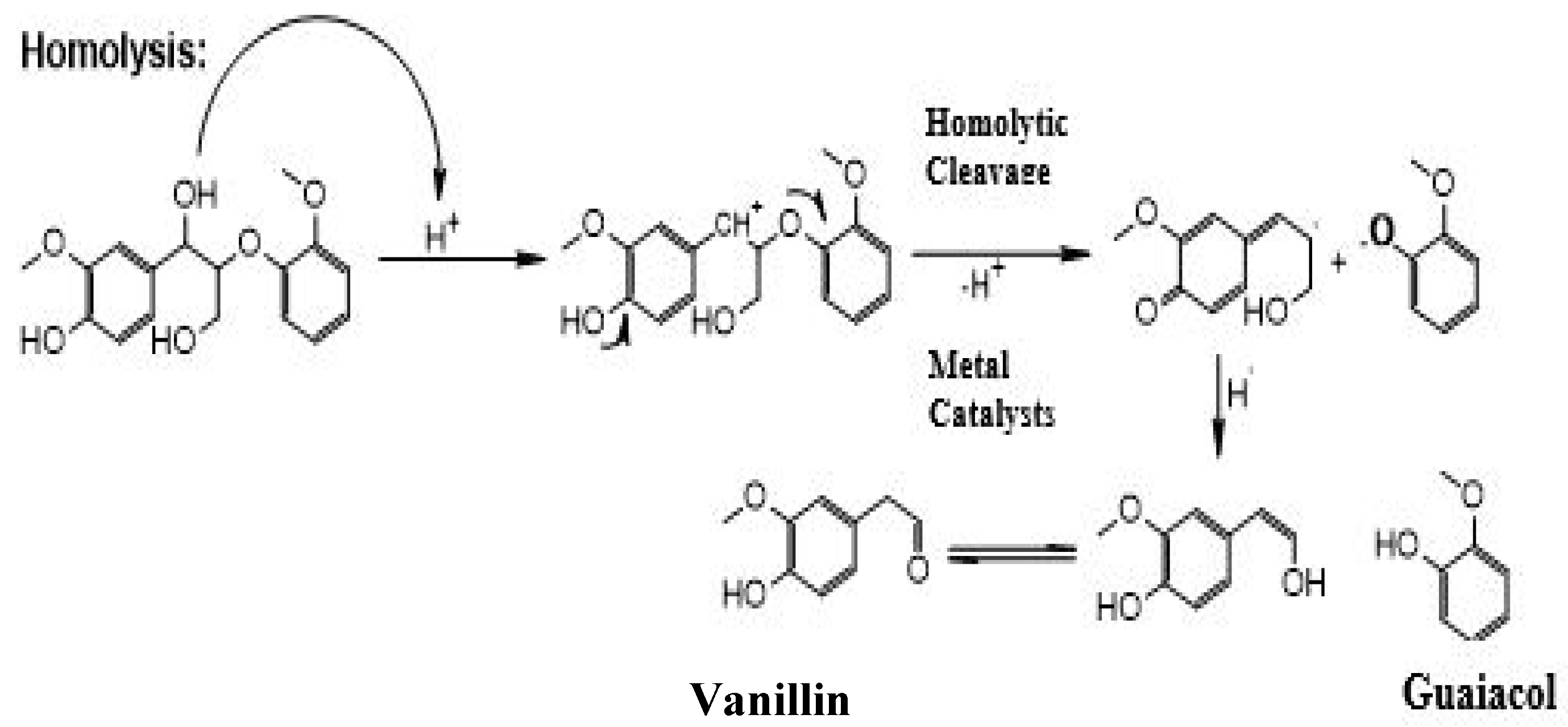
2. Materials and Methods
2.1. Materials and Catalysts
2.2. Brunauer–Emmett–Teller (BET) Analysis for Heterogeneous Catalysts
2.3. Catalytic Hydrothermal Depolymerization Reaction
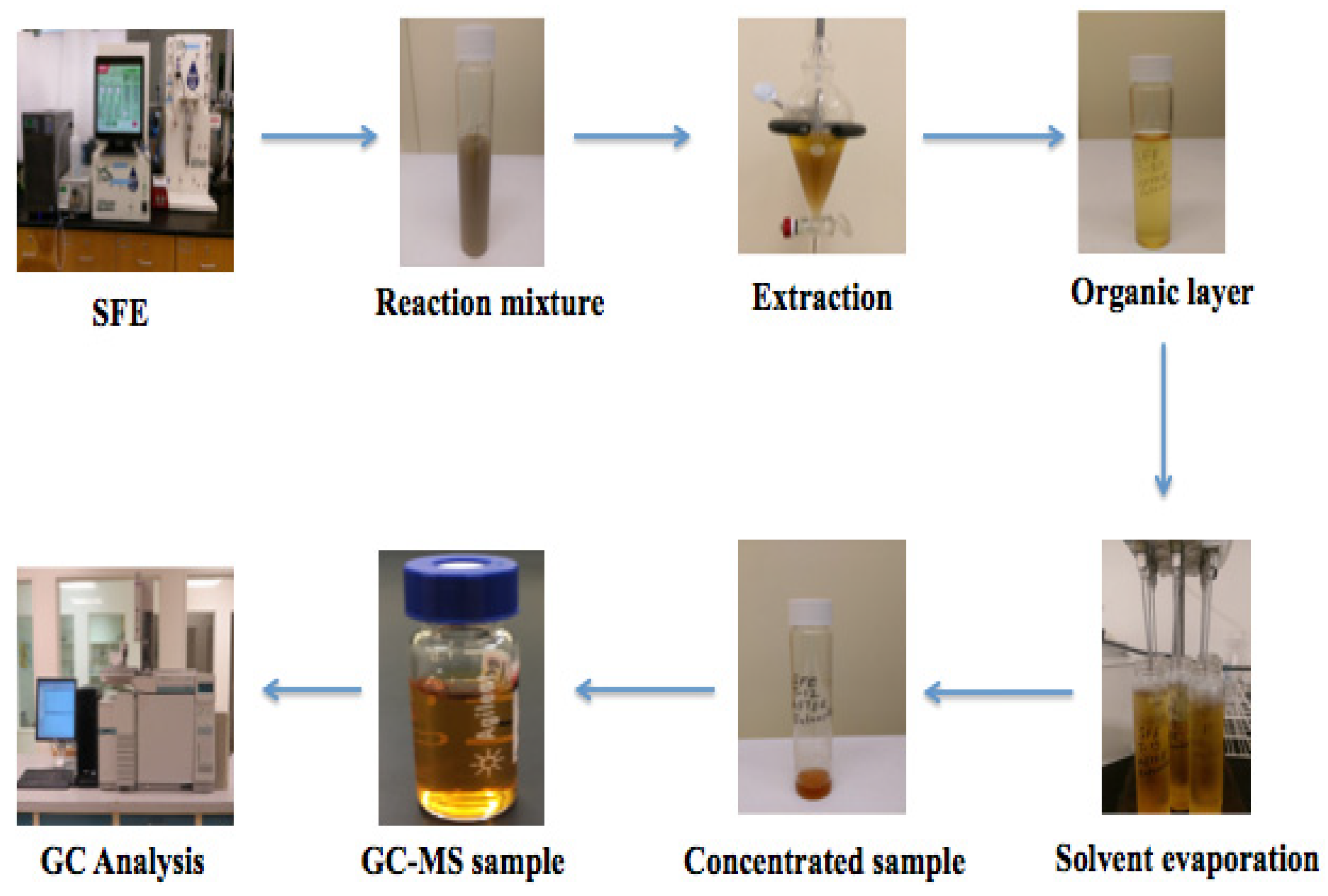
2.4. Extraction of Phenolic Monomers from Reaction Mixture
2.5. GC-MS Analysis
3. Results and Discussion
3.1. Product Analysis and Quantification
3.2. Effect of Reaction Time on Product Yield
3.3. Effect of Temperature on Product Yield
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roy, R.; Rahman, M.S.; Raynie, D.E. Recent Advances of Greener Pretreatment Technologies of Lignocellulose. Curr. Res. Green Sustain. Chem. 2020, 3, 100035. [Google Scholar] [CrossRef]
- Cheng, S.; Wilks, C.; Yuan, Z.; Leitch, M.; Xu, C.C. Hydrothermal degradation of alkali lignin to bio-phenolic compounds in sub/supercritical ethanol and water–ethanol co-solvent. Polym. Degrad. Stab. 2012, 97, 839–848. [Google Scholar] [CrossRef]
- Bridgewater, A.V. Biomass fast pyrolysis. Therm. Sci. 2004, 8, 21–50. [Google Scholar] [CrossRef]
- Zhou, M.; Sharma, B.K.; Li, J.; Zhao, J.; Xu, J.; Jiang, J. Catalytic valorization of lignin to liquid fuels over solid acid catalyst assisted by microwave heating. Fuel 2019, 239, 239–244. [Google Scholar] [CrossRef]
- Llevot, A.; Grau, E.; Carlotti, S.; Grelier, S.; Cramail, H. From Lignin-derived Aromatic Compounds to Novel Biobased Polymers. Macromol. Rapid Commun. 2016, 37, 9–28. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Tucker, M.; Ji, Y. Recent development in chemical depolymerization of lignin: A review. J. Appl. Chem. 2013, 2013, 838645. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.S.; Roy, R.; Jadhav, B.; Hossain, M.N.; Halim, M.A.; Raynie, D.E. Formulation, structure, and applications of therapeutic and amino acid-based deep eutectic solvents: An overview. J. Mol. Liq. 2020, 321, 114745. [Google Scholar] [CrossRef]
- Gosselink, R.J.; Teunissen, W.; Van Dam, J.E.; De Jong, E.; Gellerstedt, G.; Scott, E.L.; Sanders, J.P. Lignin depolymerisation in supercritical carbon dioxide/acetone/water fluid for the production of aromatic chemicals. Bioresour. Technol. 2012, 106, 173–177. [Google Scholar] [CrossRef]
- Stärk, K.; Taccardi, N.; Bösmann, A.; Wasserscheid, P. Oxidative depolymerization of lignin in ionic liquids. ChemSusChem 2010, 3, 719–723. [Google Scholar] [CrossRef]
- Jiang, G.; Nowakowski, D.J.; Bridgwater, A.V. Effect of the temperature on the composition of lignin pyrolysis products. Energy Fuels 2010, 24, 4470–4475. [Google Scholar] [CrossRef]
- Meier, D.; Faix, O. Pyrolysis-gas chromatography-mass spectrometry. In Methods in Lignin Chemistry; Springer: Berlin/Heidelberg, Germany, 1992; pp. 177–199. [Google Scholar]
- Jha, S.; Okolie, J.A.; Nanda, S.; Dalai, A.K. A review of biomass resources and thermochemical conversion technologies. Chem. Eng. Technol. 2022, 45, 791–799. [Google Scholar] [CrossRef]
- Ye, K.; Liu, Y.; Wu, S.; Zhuang, J. A review for lignin valorization: Challenges and perspectives in catalytic hydrogenolysis. Ind. Crops Prod. 2021, 172, 114008. [Google Scholar] [CrossRef]
- Zhao, M.; Hu, J.; Lu, P.; Wu, S.; Liu, C.; Sun, Y. Efficient hydrodeoxygenation of lignin-derived bio-oil ro hydrocarbon fuels over bifunctional RuCoWx/NC catalyst. Fuel 2022, 326, 125020. [Google Scholar] [CrossRef]
- Suziana, N.N.; Asikin-Mijan, N.; Zulkarnain, Z.; Taufiq-Yap, Y.H. Catalytic hydrothermal liquefaction of empty fruit bunch in subcrititcal water over bimetallic modified zeolite. Chem. Eng. Res. Des. 2022, 183, 250–262. [Google Scholar] [CrossRef]
- Zhong, S.; Zhang, B.; Liu, C.; Aldeen, A.S.; Mwenya, S.; Zhang, H. A minireview on catalytic fast co-pyrolysis of lignocellulosic biomass for bio-oil upgrading via enhancing monocyclic aromatics. J. Anal. Appl. Pyrolysis 2022, 164, 105544. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Li, J.; Yan, B.; Tao, J.; Cheng, Z.; Chen, G. In-situ hydrodeoyxgenation of lignin via hydrothermal liquefaction with water splitting metals: Comparison between autocatalytic and non-autocatalytic processes. Int. J. Hydrogen Energy 2022, 47, 7252–7262. [Google Scholar] [CrossRef]
- Jiang, W.; Cao, J.-P.; Yang, Z.; Xie, J.-X.; Zhao, L.; Zhu, C.; Zhang, C.; Zhao, X.-Y.; Zhao, Y.-P.; Zhang, J.-L. Hydrodeoxygenation of lignin and its model compounds to hydrocarbon fuels over a bifunctional Ga-doped HZSM-5 supported metal Ru catalyst. Appl. Catal. A Gen. 2022, 633, 118516. [Google Scholar] [CrossRef]
- Fan, D.; Xie, X.-a.; Li, Y.; Li, L.; Sun, J. Comparative study about catalytic liquefaction of alkali lignin to aromatics by HZSM-5 in sub-and supercritical ethanol. J. Renew. Sustain. Energy 2018, 10, 013106. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Xu, D.; Zhang, X.; Ye, N.; Chen, F.; Chen, S. Preparation and characteristics of bio-oil from the marine brown alga Sargassum patens C. Agardh. Bioresour. Technol. 2012, 104, 737–742. [Google Scholar] [CrossRef]
- Pandey, M.P.; Kim, C.S. Lignin depolymerization and conversion: A review of thermochemical methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- Rajappagowda, R.; Numan-Al-Mobin, A.M.; Yao, B.; Cook, R.D.; Smirnova, A. Toward selective lignin liquefaction: Synergistic effect of hetero-and homogeneous catalysis in sub-and supercritical fluids. Energy Fuels 2017, 31, 578–586. [Google Scholar] [CrossRef]
- Perego, C.; Bianchi, D. Biomass upgrading through acid–base catalysis. Chem. Eng. J. 2010, 161, 314–322. [Google Scholar] [CrossRef]
- Carr, A.G.; Mammucari, R.; Foster, N. A review of subcritical water as a solvent and its utilisation for the processing of hydrophobic organic compounds. Chem. Eng. J. 2011, 172, 1–17. [Google Scholar] [CrossRef]
- Holliday, R.L.; King, J.W.; List, G.R. Hydrolysis of vegetable oils in sub-and supercritical water. Ind. Eng. Chem. Res. 1997, 36, 932–935. [Google Scholar] [CrossRef]
- Heger, K.; Uematsu, M.; Franck, E. The static dielectric constant of water at high pressures and temperatures to 500 MPa and 550 C. Ber. Bunsenges. Phys. Chem. 1980, 84, 758–762. [Google Scholar] [CrossRef]
- Kruse, A.; Dinjus, E. Hot compressed water as reaction medium and reactant: Properties and synthesis reactions. J. Supercrit. Fluids 2007, 39, 362–380. [Google Scholar] [CrossRef]
- Krammer, P.; Vogel, H. Hydrolysis of esters in subcritical and supercritical water. J. Supercrit. Fluids 2000, 16, 189–206. [Google Scholar] [CrossRef]
- Nagel, E.; Zhang, C. Hydrothermal Decomposition of a Lignin Dimer under Neutral and Basic Conditions: A Mechanism Study. Ind. Eng. Chem. Res. 2019, 58, 18866–18880. [Google Scholar] [CrossRef]
- Amit, T.; Roy, R.; Raynie, D.E. Thermal and structural characterization of two commercially available technical lignins for potential depolymerization by hydrothermal liquefaction. Curr. Res. Green Sustain. Chem. 2021, 4, 100106. [Google Scholar] [CrossRef]
- Scherdel, C.; Reichenauer, G.; Wiener, M. Relationship between pore volumes and surface areas derived from the evaluation of N2-sorption data by DR-, BET-and t-plot. Microporous Mesoporous Mater. 2010, 132, 572–575. [Google Scholar] [CrossRef]
- Meier, D.; Oasmaa, A.; Peacocke, G. Properties of fast pyrolysis liquids: Status of test methods. In Developments in Thermochemical Biomass Conversion; Springer: Berlin/Heidelberg, Germany, 1997; pp. 391–408. [Google Scholar]
- Roy, R.; Jadhav, B.; Rahman, M.S.; Raynie, D.E. Characterization of residue from catalytic hydrothermal depolymerization of lignin. Curr. Res. Green Sustain. Chem. 2021, 4, 100052. [Google Scholar] [CrossRef]
- Meier, D.; Ante, R.; Faix, O. Catalytic hydropyrolysis of lignin: Influence of reaction conditions on the formation and composition of liquid products. Bioresour. Technol. 1992, 40, 171–177. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Wang, H.; Ma, Q.; Li, S.; Chang, H.-M.; Jameel, H. Liquefaction of kraft lignin by hydrocracking with simultaneous use of a novel dual acid-base catalyst and a hydrogenation catalyst. Bioresour. Technol. 2017, 243, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Kristianto, I.; Limarta, S.O.; Lee, H.; Ha, J.-M.; Suh, D.J.; Jae, J. Effective depolymerization of concentrated acid hydrolysis lignin using a carbon-supported ruthenium catalyst in ethanol/formic acid media. Bioresour. Technol. 2017, 234, 424–431. [Google Scholar] [CrossRef]


| Catalyst | SBET (m2/g) |
|---|---|
| 5% V/Zeolite | 374.53 ± 10.49 |
| 1.7% V/ZrO2 (Sulfate) | 440.15 ± 1.27 |
| 1.7% V/ZrO2 (Neutral) | 11.81 ± 1.33 |
| Ni-Graphene | 615.40 ± 13.60 |
| Ni-Zn | 0.81 ± 0.20 |
| 5% V/Ni-Graphene | 281.96 ± 6.05 |
| 1.7% V/Zeolite | 353.50 ± 3.64 |
| No | Catalyst | Total Yield (mg/g of Lignin) |
|---|---|---|
| 1 | 5% V/Zeolite | 33.28 ± 0.44 |
| 2 | 1.7% V/ZrO2 (Sulfate) | 34.46 ± 0.22 |
| 3 | 1.7% V/ZrO2 (Neutral) | 24.97 ± 0.36 |
| 4 | Ni-Graphene | 41.16 ± 0.27 |
| 5 | Ni-Zinc | 24.57 ± 0.14 |
| 6 | 5% V/Ni-Graphene | 31.21 ± 0.16 |
| 7 | 1.7% V/Zeolite | 30.90 ± 0.40 |
| 8 | No catalyst | 24.06 ± 0.27 |
| Yield (mg/g of Lignin) | |||
|---|---|---|---|
| Catalyst | 5 min | 10 min | 15 min |
| Ni-Graphene | 11.89 ± 0.14 | 41.16 ± 0.27 | 16.95 ± 0.11 |
| 5% V/Zeolite | 13.90 ± 0.07 | 33.28 ± 0.44 | 17.51 ± 0.12 |
| 1.7% V/ZrO2 (Sulfate) | 13.36 ± 0.11 | 34.46 ± 0.22 | 26.96 ± 0.26 |
| Yield (mg/g of Lignin) | ||
|---|---|---|
| Catalyst | 200 °C | 240 °C |
| Ni-Graphene | 17.90 ± 0.19 | 41.16 ± 0.27 |
| 5% V/Zeolite | 18.03 ± 0.13 | 33.28 ± 0.44 |
| 1.7% V/ZrO2 (Sulfate) | 18.72 ± 0.11 | 34.46 ± 0.22 |
| Phenolic Monomer | Yield (mg Monomer/g Alkali Lignin) | Std. Dev. |
|---|---|---|
| Guaiacol | 3.70 | 0.16 |
| Vanillin | 9.59 | 0.34 |
| Isoeugenol | 3.26 | 0.15 |
| Acetovanillone | 2.95 | 0.06 |
| Guaiacylacetone | 6.38 | 0.26 |
| Homovanillic acid | 14.96 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadhav, B.; Roy, R.; Rahman, M.S.; Amit, T.A.; Subedi, S.; Hummel, M.; Gu, Z.; Raynie, D.E. Enhancing the Efficacy of the Subcritical Water-Based Alkali Lignin Depolymerization by Optimizing the Reaction Conditions and Using Heterogeneous Catalysts. Biomass 2022, 2, 178-187. https://doi.org/10.3390/biomass2030011
Jadhav B, Roy R, Rahman MS, Amit TA, Subedi S, Hummel M, Gu Z, Raynie DE. Enhancing the Efficacy of the Subcritical Water-Based Alkali Lignin Depolymerization by Optimizing the Reaction Conditions and Using Heterogeneous Catalysts. Biomass. 2022; 2(3):178-187. https://doi.org/10.3390/biomass2030011
Chicago/Turabian StyleJadhav, Balawanthrao, Ranen Roy, Md Sajjadur Rahman, Tanvir A. Amit, Shiksha Subedi, Matthew Hummel, Zhengrong Gu, and Douglas E. Raynie. 2022. "Enhancing the Efficacy of the Subcritical Water-Based Alkali Lignin Depolymerization by Optimizing the Reaction Conditions and Using Heterogeneous Catalysts" Biomass 2, no. 3: 178-187. https://doi.org/10.3390/biomass2030011
APA StyleJadhav, B., Roy, R., Rahman, M. S., Amit, T. A., Subedi, S., Hummel, M., Gu, Z., & Raynie, D. E. (2022). Enhancing the Efficacy of the Subcritical Water-Based Alkali Lignin Depolymerization by Optimizing the Reaction Conditions and Using Heterogeneous Catalysts. Biomass, 2(3), 178-187. https://doi.org/10.3390/biomass2030011







