Recent Progress in Green Conversion of Biomass Alcohol to Chemicals via Aerobic Oxidation
Abstract
:1. Introduction
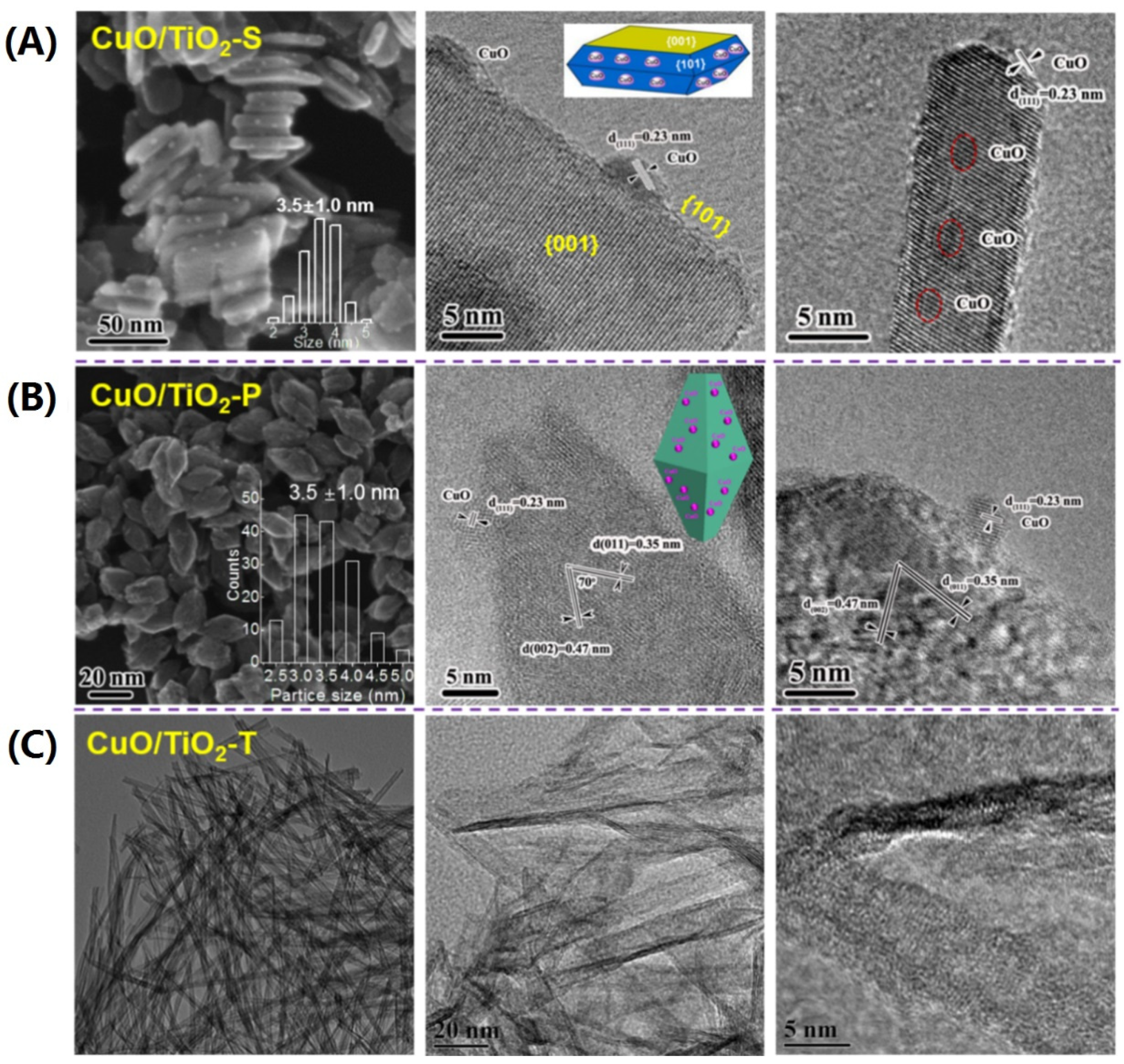
2. Methanol Conversion to Methyl Formate
3. Ethanol Conversion
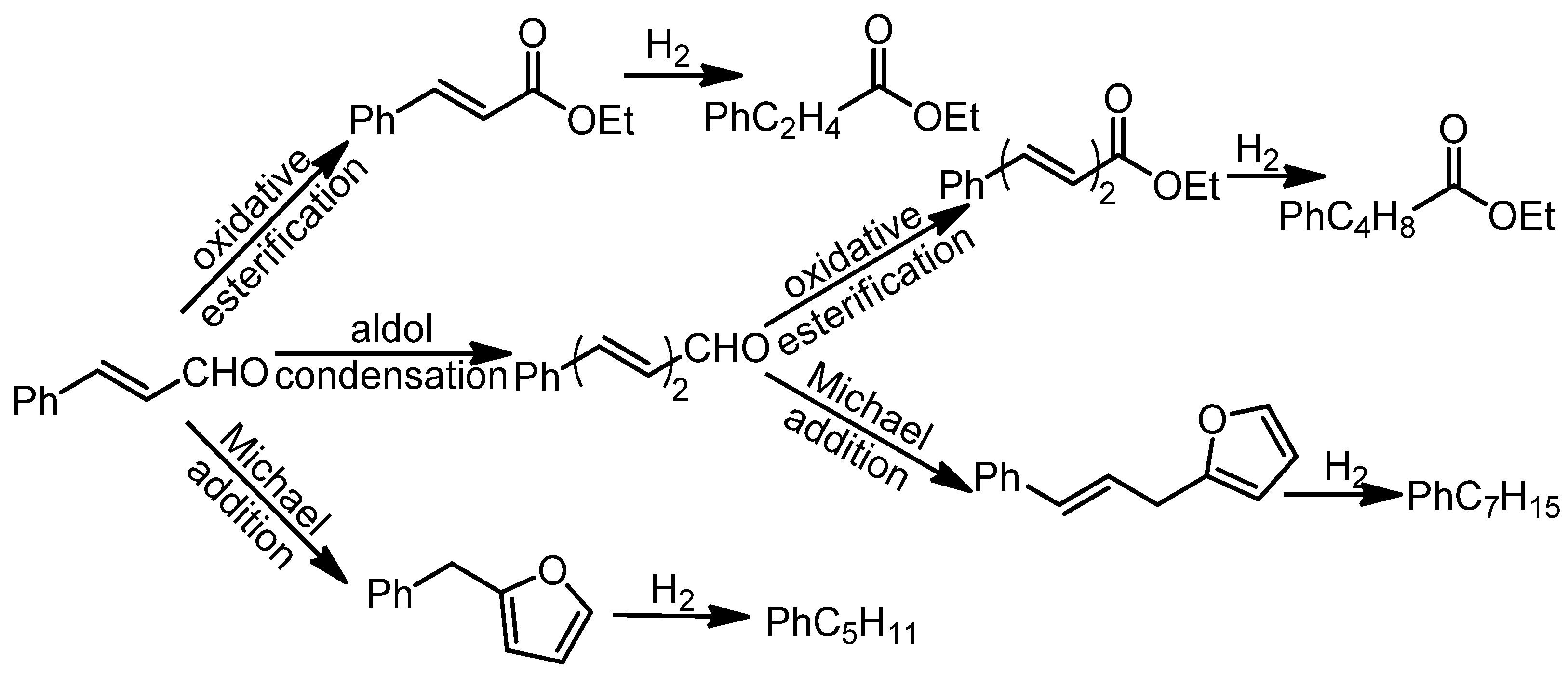
3.1. Hydrocarbons (Biofuels) via Thermal Reactions
3.2. Ethanal via Photocatalysis
4. Oxidation of D-Glucose to Gluconic Acid
5. Conclusions, Challenges, and Future Perspective
- (1)
- Three types of oxidations were achieved, photo-oxidation of methanol to methyl formate over CuOx/TiO2 nanocomposites, ethanol to hydrocarbons biofuels over Au/NiO, and glucose oxidation to gluconic acid catalyzed by Au/activated carbon.
- (2)
- The titania supports copper oxide clusters with different morphology of nanosheets, nanospindles, and nanotubes have been designed to investigate the catalytic performance in the photo-oxidation reaction of the method.
- (3)
- The active-site identification and creation in the aerobic oxidation were exemplified. The CuOx/TiO2{101} interface was identified in the photo-oxidation of methanol, and only the metallic Au0 clusters were clarified to be active-site for the glucose oxidation.
- (4)
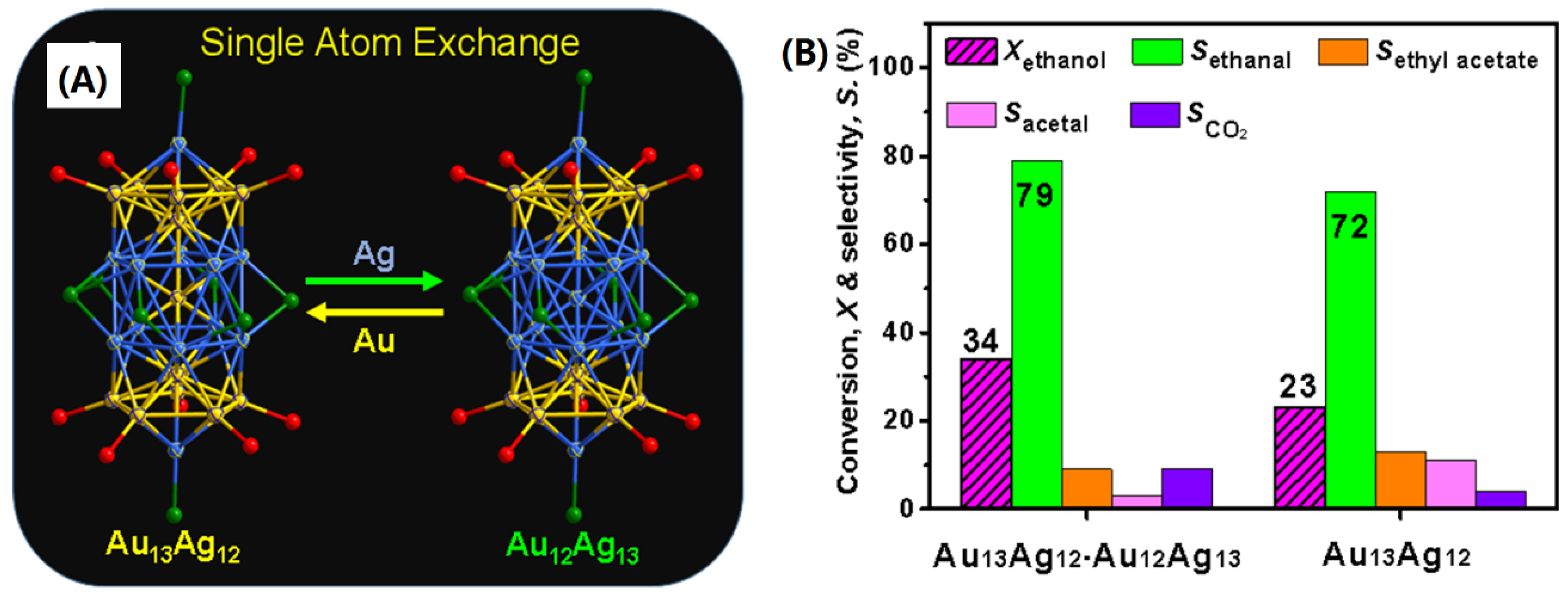
- It has been observed that the single-atom-exchanging in the metal clusters largely affects the catalytic activity instead of product selectivity. However, the detailed mechanism is still unknown.
- (5)
- By the ATR-IR spectra method, we clearly mapped out the whole conversion pathway and worked out the controversy in this photocatalysis.
- (1)
- (2)
- In the future, sincere efforts will be put forward to develop more biomass conversion systems.
- (3)
- DFT studies with in situ characterizations must be advanced to establish plausible reaction mechanisms.
- (4)
- Attempts must be made to increase the reaction scales to meet the industrial needs, especially the photo-oxidation reactions [72].
- (5)
- Alloy metal nanoparticle catalysts need to be exploited in the biomass conversions, as the electronic property can be well modified to tailor their catalytic performances.
- (6)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lee, J.S.; Kim, J.; Kim, Y. Methyl formate as a new building block in C1 chemistry. Appl. Catal. 1990, 57, 1–30. [Google Scholar] [CrossRef]
- Nwosu, U.; Wang, A.; Palma, B.; Zhao, H.; Khan, M.A.; Kibria, M.; Hu, J. Selective biomass photoreforming for valuable chemicals and fuels: A critical review. Renew. Sustain. Energy Rev. 2021, 148, 111266. [Google Scholar] [CrossRef]
- Guo, Y.L.; Lu, G.Z.; Mo, X.H.; Wang, Y.S. Vapor phase dehydrogenation of methanol to methyl formate in the catalytic membrane reactor with Cu/SiO2/ceramic composite membrane. Catal. Lett. 2005, 99, 105–108. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Chen, W.; Xu, W.W.; Han, Z.-K.; Waheed, A.; Ye, Z.; Li, G.; Baiker, A. Continuous Di-methyl Carbonate Synthesis from CO2 and Methanol over BixCe1-xOδ Monoliths: Effect of Bismuth Doping on Population of Oxygen Vacancies, Activity, and Reaction Pathway. Nano Res. 2022, 15, 1366–1374. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Q.; Ye, Z.; Li, Y.; Yang, Y.; Pu, H.; Li, G. Monolithic ZnxCe1−xO2 catalysts for catalytic synthesis of dimethyl carbonate from CO2 and methanol. New J. Chem. 2020, 44, 12522–12530. [Google Scholar] [CrossRef]
- Tatibouët, J. Methanol oxidation as a catalytic surface probe. Appl. Catal. A Gen. 1997, 148, 213–252. [Google Scholar] [CrossRef]
- Forzatti, P.; Tronconi, E.; Elmi, A.S.; Busca, G. Methanol oxidation over vanadia-based catalysts. Appl. Catal. A Gen. 1997, 157, 387–408. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Qin, Z.; Tian, S.; Ye, Z.; Ye, L.; Abroshan, H.; Li, G. TixCe1−xO2 Nanocomposites: A Monolithic Catalyst for Direct Conversion of Carbon Dioxide and Methanol to Dimethyl Carbonate. Green Chem. 2019, 21, 4642–4649. [Google Scholar] [CrossRef]
- Kou, H.; Lu, C.H.; Wang, J.; Chen, Y.K.; Xu, Z.Z.; Varma, R.S. Selectivity Enhancement in Heteroge-neous Photocatalytic Transformations. Chem. Rev. 2017, 117, 1445–1514. [Google Scholar] [CrossRef] [Green Version]
- Badwal, S.P.S.; Giddey, S.; Kulkarni, A. Direct ethanol fuel cells for transport and stationary applications—A comprehensive review. Appl. Energy 2015, 145, 80–103. [Google Scholar] [CrossRef]
- Yin, C.; Liu, S.; Qin, Z.; Zhang, Y.; Li, G.; Zhao, Z. Butterfly-Like Tetranuclear Copper(I) Clusters for Efficient Alkyne Homocoupling Reactions. Eur. J. Inorg. Chem. 2020, 2021, 392–397. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, Z.; Ping, G.; Liu, S.; Xu, H.; Li, G. Alkynyl- and Phosphine-Ligated Quaternary Au2Ag2 Clusters Featuring an Alkynyl-AuAg Motif for Multicomponent Coupling. RSC Adv. 2020, 10, 21650–21655. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, A.V.; Thayumanavan, S.; Huber, G.W. C-C Bond Formation Reactions for Bio-mass-Derived Molecules. ChemSusChem 2010, 3, 1158–1161. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Qin, Z.; Xu, H.; Li, G. Heterogeneous Cross-Coupling over Gold Nanoclusters. Nanomaterials 2019, 9, 838. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Tong, X.; Liu, J.; Xue, S. A smart catalyst system for the valorization of renewable furfural in aliphatic alcohols. Catal. Sci. Technol. 2015, 6, 1214–1221. [Google Scholar] [CrossRef]
- Ning, L.; Liao, S.; Cui, H.; Yu, L.; Tong, X. Selective Conversion of Renewable Furfural with Ethanol to Produce Furan-2-acrolein Mediated by Pt@MOF-5. ACS Sustain. Chem. Eng. 2017, 6, 135–142. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, G. A Critical Review on Carbon-Carbon Coupling over Ultra-Small Gold Nanoclusters. Acta Phys.-Chim. Sin. 2017, 33, 1297–1309. [Google Scholar] [CrossRef]
- Cavani, F.; Albonetti, S.; Basile, F.; Gandini, A. Chemicals and Fuels from Bio-Based Building Blocks; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Wang, F.; Wen, Z.; Qin, Z.; Fang, Q.; Ge, Q.; Li, Z.; Sun, J.; Li, G. Manganese cluster induce the control synthesis of RHO- and CHA-type silicoaluminaphosphates for dimethylether to light olefin conversion. Fuel 2019, 244, 104–109. [Google Scholar] [CrossRef]
- Delidovich, I.; Hausoul, P.J.C.; Deng, L.; Pfützenreuter, R.; Rose, M.; Palkovits, R. Alternative Monomers Based on Lignocellulose and Their Use for Polymer Production. Chem. Rev. 2015, 116, 1540–1599. [Google Scholar] [CrossRef]
- Wen, Z.; Li, Z.; Ge, Q.; Zhou, Y.; Sun, J.; Abroshan, H.; Li, G. Robust nickel cluster@Mes-HZSM-5 composite nanostructure with enhanced catalytic activity in the DTG reaction. J. Catal. 2018, 363, 26–33. [Google Scholar] [CrossRef]
- Ping, G.; Zheng, K.; Fang, Q.; Li, G. Composite Nanostructure of Manganese Cluster and CHA-Type Sili-coaluminaphosphates: Enhanced Catalytic Performance in Dimethylether to Light Olefins Conversion. Nanomaterials 2021, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Biella, S.; Prati, L.; Rossi, M. Selective Oxidation of D-Glucose on Gold Catalyst. J. Catal. 2002, 206, 242–247. [Google Scholar] [CrossRef]
- Anastassiadis, S.; Morgunov, I.G. Recent Pat. Biotechnology 2007, 1, 167–180. [Google Scholar]
- Hanson, A.D.; Roje, S. One-carbon metabolism in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 119–137. [Google Scholar] [CrossRef]
- Liu, J.; Zhan, E.; Cai, W.; Li, J.; Shen, W. Methanol Selective Oxidation to Methyl Formate over ReOx/CeO2 Catalysts. Catal. Lett. 2007, 120, 274–280. [Google Scholar] [CrossRef]
- Kaiser, D.; Beckmann, L.; Walter, J.; Bertau, M. Conversion of Green Methanol to Methyl Formate. Catalysts 2021, 11, 869. [Google Scholar] [CrossRef]
- Shi, Q.; Wei, X.; Raza, A.; Li, G. Recent Advances in Aerobic Photo-Oxidation of Methanol to Valuable Chemicals. ChemCatChem 2021, 13, 3381–3395. [Google Scholar] [CrossRef]
- Shi, Q.; Ping, G.; Wang, X.; Xu, H.; Li, J.; Cui, J.; Abroshan, H.; Ding, H.; Li, G. CuO/TiO2 heterojunction composites: An efficient photocatalyst for selective oxidation of methanol to methyl formate. J. Mater. Chem. A 2019, 7, 2253–2260. [Google Scholar] [CrossRef]
- Wittstock, A.; Zielasek, V.; Biener, J.; Friend, C.M.; Baumer, M. Nanoporous Gold Catalysts for Selective Gas-Phase Oxidative Coupling of Methanol at Low Temperature. Science 2010, 327, 319–322. [Google Scholar] [CrossRef] [Green Version]
- Kominami, H.; Sugahara, H.; Hashimoto, K. Photocatalytic selective oxidation of methanol to methyl formate in gas phase over titanium(IV) oxide in a flow-type reactor. Catal. Commun. 2010, 11, 426–429. [Google Scholar] [CrossRef]
- Liu, J.; Han, C.; Yang, X.; Gao, G.; Shi, Q.; Tong, M.; Liang, X.; Li, C. Methyl formate synthesis from methanol on titania supported copper catalyst under UV irradiation at ambient condition: Performance and mechanism. J. Catal. 2016, 333, 162–170. [Google Scholar] [CrossRef]
- Russier, V.; Badiali, J. Calculation of the electronic work function of Cu and Ag from an extended jel-lium model. Phys. Rev. B 1989, 39, 13193. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, S.H.; Fang, Q.H.; Abroshan, H.D.; Kim, H.; Haruta, M.; Li, G. Gold-Palladium Nanoalloys Supported by Graphene Oxide and Lamellar TiO2 for Direct Synthesis of Hydrogen Per-oxide. ACS Appl. Mater. Interfaces 2018, 10, 40599–40607. [Google Scholar] [CrossRef] [PubMed]
- Kollhoff, F.; Schneider, J.; Li, G.; Barkaoui, S.; Shen, W.; Berger, T.; Diwald, O.; Libuda, J. Anchoring of carboxyl-functionalized porphyrins on MgO, TiO2, and Co3O4 nanoparticles. Phys. Chem. Chem. Phys. 2018, 20, 24858–24868. [Google Scholar] [CrossRef]
- Shao, B.; Zhao, W.; Miao, S.; Huang, J.; Wang, L.; Li, G.; Shen, W. Facet-dependent anchoring of gold nanoparticles on TiO2 for CO oxidation. Chin. J. Catal. 2019, 40, 1534–1539. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.; Wang, M.; Zhang, C.; Luo, N.; Wang, Y.; Abroshan, H.; Li, G.; Wang, F. Visible Light Gold Nanocluster Photocatalyst: Selective Aerobic Oxidation of Amines to Imines. ACS Catal. 2017, 7, 3632–3638. [Google Scholar] [CrossRef]
- Shi, Q.Q.; Qin, Z.X.; Yu, C.L.; Waheed, A.; Xu, H.; Gao, Y.; Abroshan, H.; Li, G. Experimental and mechanistic understanding of photo-oxidation of methanol catalyzed by CuO/TiO2-spindle nano-composite: Oxygen vacancy engineering. Nano Res. 2020, 13, 939–946. [Google Scholar] [CrossRef]
- Zhang, S.; Gong, X.; Shi, Q.; Ping, G.; Xu, H.; Waleed, A.; Li, G. CuO Nanoparticle-Decorated TiO2-Nanotube Heterojunctions for Direct Synthesis of Methyl Formate via Photo-Oxidation of Methanol. ACS Omega 2020, 5, 15942–15948. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, Y.; Guo, S.; Han, Z.-K.; Ta, N.; Li, G.; Baiker, A. NO reduction with CO over CuOx/CeO2 Nanocomposites: Influence of Oxygen Vacancies and Lattice Strain. Catal. Sci. Technol. 2021, 11, 6543–6552. [Google Scholar] [CrossRef]
- Li, W.L.; Liu, C.; Abroshan, H.D.; Ge, Q.J.; Yang, X.J.; Xu, H.Y.; Li, G. Catalytic CO Oxidation Using Bimetallic MxAu25-x Clusters: A Combined Experimental and Computational Study on Doping Effects. J. Phys. Chem. C 2016, 120, 10261–10267. [Google Scholar] [CrossRef]
- Han, C.; Yang, X.; Gao, G.; Wang, J.; Lu, H.; Liu, J.; Tong, M.; Liang, X. Selective oxidation of methanol to methyl formate on catalysts of Au–Ag alloy nanoparticles supported on titania under UV irradiation. Green Chem. 2014, 16, 3603–3615. [Google Scholar] [CrossRef]
- Liang, X.Y.; Yang, X.Z.; Gao, G.J.; Li, C.F.; Li, Y.Y.; Zhang, W.D.; Chen, X.T.; Zhang, Y.B.; Zhang, B.B.; Lei, Y.Q.; et al. Performance and mechanism of CuO/CuZnAl hydrotalcites-ZnO for photo-catalytic selective oxidation of gaseous methanol to methyl formate at ambient temperature. J. Catal. 2016, 339, 68–76. [Google Scholar] [CrossRef]
- Lisowski, P.; Colmenares, J.C.; Łomot, D.; Chernyayeva, O.; Lisovytskiy, D. Preparation by sonopho-todeposition method of bimetallic photocatalysts Pd-Cu/TiO2 for sustainable gaseous selective oxidation of methanol to methyl formate. J. Mol. Catal. A Chem. 2016, 411, 247–256. [Google Scholar] [CrossRef]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Oumer, A.; Hasan, M.; Baheta, A.T.; Mamat, R.; Abdullah, A. Bio-based liquid fuels as a source of renewable energy: A review. Renew. Sustain. Energy Rev. 2018, 88, 82–98. [Google Scholar] [CrossRef]
- Zhao, C.; Brück, T.; Lercher, J.A. Catalytic deoxygenation of microalgae oil to green hydrocarbons. Green Chem. 2013, 15, 1720–1739. [Google Scholar] [CrossRef]
- Bridgwater, A. Renewable fuels and chemicals by thermal processing of biomass. Chem. Eng. J. 2003, 91, 87–102. [Google Scholar] [CrossRef]
- Huber, G.W.; Chheda, J.N.; Barrett, C.J.; Dumesic, J.A. Production of Liquid Alkanes by Aque-ous-Phase Processing of Biomass-Derived Carbohydrates. Science 2005, 308, 1446–1450. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Wang, R.; Li, G. Non-metallic gold nanoclusters for oxygen activation and aerobic oxidation. Chin. Chem. Lett. 2018, 29, 687–693. [Google Scholar] [CrossRef]
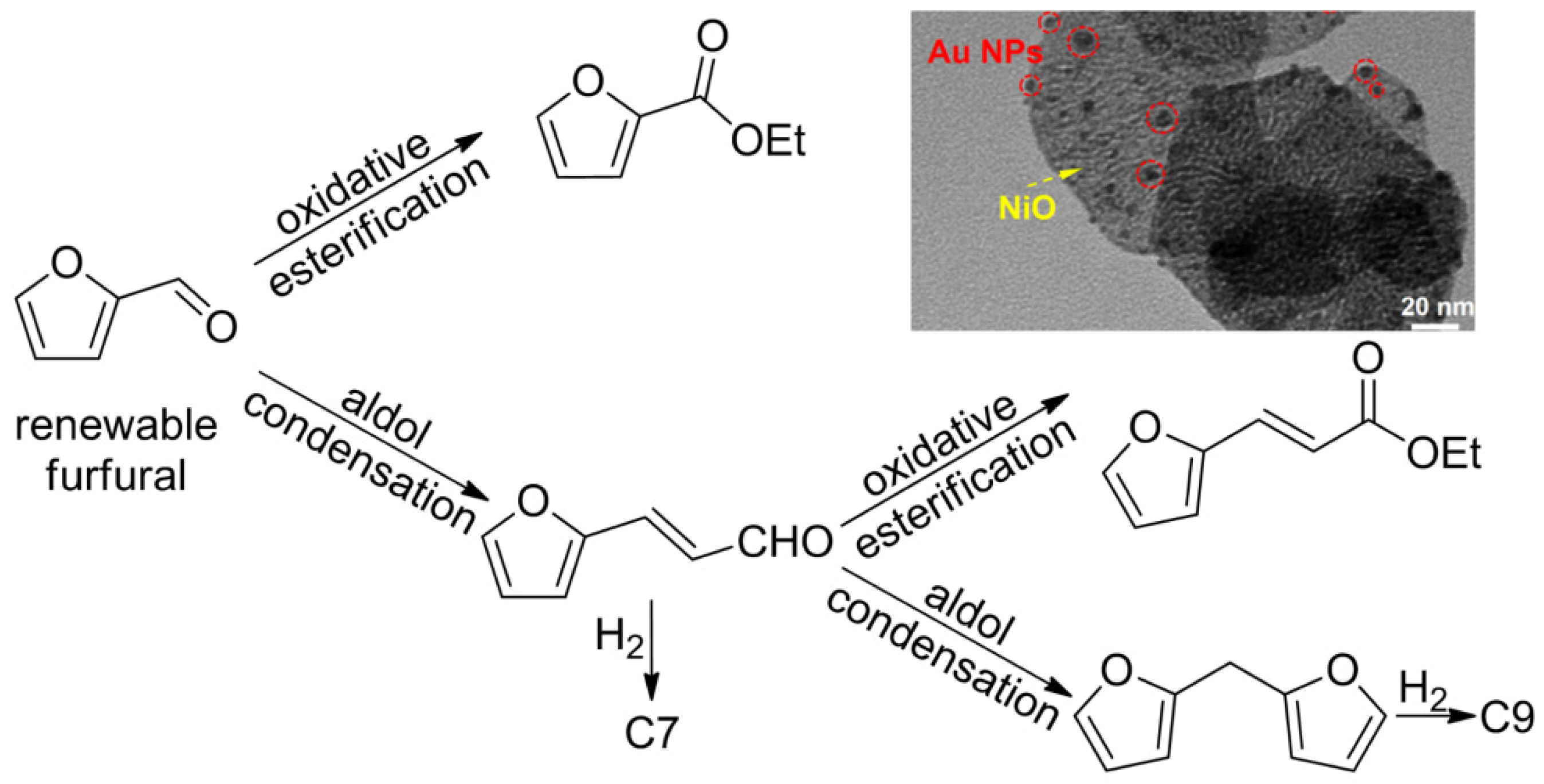
- Li, G.; Jiao, W.; Sun, Z.; Zhao, Y.; Shi, Z.; Yan, Y.; Feng, L.; Zhang, Y.; Tang, Y. A Scalable Upgrading of Concentrated Furfural in Ethanol: Combining Meerwein–Ponndorf–Verley Reduction with in Situ Cross Aldol Condensation. ACS Sustain. Chem. Eng. 2018, 6, 4316–4320. [Google Scholar] [CrossRef]
- Fang, Q.; Qin, Z.; Shi, Y.; Liu, F.; Barkaoui, S.; Abroshan, H.; Li, G. Au/NiO Composite: A Catalyst for One-Pot Cascade Conversion of Furfural. ACS Appl. Energy Mater. 2019, 2, 2654–2661. [Google Scholar] [CrossRef]
- Shi, Y.; Tian, S.; Shi, Q.; Waheed, A.; Cao, Y.; Li, G. Cascade aldol condensation of an aldehyde via the aerobic oxidation of ethanol over an Au/NiO composite. Nanoscale Adv. 2019, 1, 3654–3659. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Qin, Z.; Sharma, S.; Li, G. Recent Progress in Heterogeneous Catalysis by Atomically and Structurally Precise Metal Nanoclusters. Chem. Rec. 2021, 21, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Zhang, J.; Zhao, D.; Yang, Y.; Li, Z.; Li, G. Motif-mediated Au25(SPh)5(PPh3)10X2 nanorods with conjugated electron delocalization. Nano Res. 2018, 12, 501–507. [Google Scholar] [CrossRef]
- Qin, Z.; Sharma, S.; Wan, C.-Q.; Malola, S.; Xu, W.-W.; Häkkinen, H.; Li, G. A Homoleptic Alkynyl-Ligated [Au13Ag16L24]3- Cluster as a Catalytically Active Eight-Electron Superatom. Angew. Chem. Int. Ed. 2020, 59, 970–975. [Google Scholar]
- Qin, Z.; Zhang, J.; Wan, C.; Liu, S.; Abroshan, H.; Jin, R.; Li, G. Atomically precise nanoclusters with reversible isomeric transformation for rotary nanomotors. Nat. Commun. 2020, 11, 1–6. [Google Scholar] [CrossRef]
- Qin, Z.; Hu, S.; Han, W.; Li, Z.; Xu, W.W.; Zhang, J.; Li, G. Tailoring optical and photocatalytic properties by single-Ag-atom exchange in Au13Ag12(PPh3)10Cl8 nanoclusters. Nano Res. 2021, 15, 2971–2976. [Google Scholar] [CrossRef]
- Zhu, H.; Yuan, X.; Yao, Q.; Xie, J. Shining Photocatalysis by Gold-based Nanomaterials. Nano Energy 2021, 88, 106306. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, Z.; Cui, C.; Luo, Z.; Yang, B.; Jiang, Y.; Lai, C.; Wang, Z.; Wang, X.; Fang, X.; et al. In-Situ Generation and Global Property Profiling of Metal nanoclusters by Ultraviolet Laser Dissociation-Mass Spectrometry. Sci. China Chem. 2022, 65. [Google Scholar] [CrossRef]
- Qin, Z.; Zhao, D.; Zhao, L.; Xiao, Q.; Wu, T.; Zhang, J.; Wan, C.; Li, G. Tailoring the Stability, Photocatalysis and Photoluminescence Properties of Au11 Nanocluster via Doping Engineering. Nanoscale Adv. 2019, 1, 2529–2536. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Huang, Y.; Pan, X.; Han, B.; Su, Y.; Jiang, Q.; Li, M.; Tang, H.; Li, G.; Qiao, B. Size-dependent strong metal-support interaction in TiO2 supported Au nanocatalysts. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Kinoshita, N.; Okatsu, H.; Akita, T.; Takei, T.; Haruta, M. Influence of the support and the size of gold clusters on catalytic activity for glucose oxidation. Angew. Chem. Int. Ed. 2008, 47, 9265–9268. [Google Scholar] [CrossRef] [PubMed]
- Perovic, M.; Tarakina, N.V.; Hofmann, J.P.; Oschatz, M. Influence of Local Environments in Pores of Different Size on the Catalytic Liquid-Phase Oxidation of d-Glucose by Au Nanoparticles Supported on Nanoporous Carbon. ACS Appl. Nano Mater. 2020, 3, 7695–7703. [Google Scholar] [CrossRef]
- Zhang, H.; Toshima, N. Glucose oxidation using Au-containing bimetallic and trimetallic nanoparticles. Catal. Sci. Technol. 2013, 3, 268–278. [Google Scholar] [CrossRef]
- Cattaneo, S.; Stucchi, M.; Villa, A.; Prati, L. Gold Catalysts for the Selective Oxidation of Biomass-Derived Products. ChemCatChem 2018, 11, 309–323. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, J.; Huang, J.; Zhang, C.; Hong, F.; Zhou, Y.; Li, G.; Haruta, M. Efficient Aerobic Oxidation of Glucose to Gluconic Acid over Activated Carbon-Supported Gold Clusters. ChemSusChem 2017, 10, 1976–1980. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Huang, J.; Liu, C.; Hong, F.; Zheng, K.; Li, G. Size dependence of gold clusters with precise numbers of atoms in aerobic oxidation of d-glucose. Nanoscale 2017, 9, 16879–16886. [Google Scholar] [CrossRef]
- Guo, S.; Fang, Q.; Li, Z.; Zhang, J.; Zhang, J.; Li, G. Efficient base-free direct oxidation of glucose to gluconic acid over TiO2-supported gold clusters. Nanoscale 2018, 11, 1326–1334. [Google Scholar] [CrossRef]
- Almada, C.C.; Kazachenko, A.; Fongarland, P.; Perez, D.D.S.; Kuznetsov, B.; Djakovitch, L. Supported-Metal Catalysts in Upgrading Lignin to Aromatics by Oxidative Depolymerization. Catalysts 2021, 11, 467. [Google Scholar] [CrossRef]
- Raza, A.; Zhang, X.; Ali, S.; Cao, C.; Rafi, A.A.; Li, G. Photoelectrochemical Energy Conversion over 2D Materials. Photochem 2022, 2, 272–298. [Google Scholar] [CrossRef]
- Roman, K.; Barwicki, J.; Hryniewicz, M.; Szadkowska, D.; Szadkowski, J. Production of Electricity and Heat from Biomass Wastes Using a Converted Aircraft Turbine AI-20. Processes 2021, 9, 364. [Google Scholar] [CrossRef]
- Cao, Y.; Su, Y.; Xu, L.; Yang, X.; Han, Z.; Cao, R.; Li, G. Ionic liquids modified oxygen vacancy-rich amorphous FeNi hydroxide nanoclusters on carbon-based materials as an efficient electrocatalyst for electrochemical water oxidation. J. Energy Chem. 2022, 71, 167–173. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, X.; Liu, X.; Xu, L.; Liu, B.; Zhang, J.; Xu, H.; Han, Z.; Li, G. In-situ exfoliation and assembly of 2D/2D g-C3N4/TiO2(B) hierarchical microflower: Enhanced photo-oxidation of benzyl alcohol under visible light. Carbon 2022, 196, 401–409. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Q.; Liu, X.; Li, J.; Xu, H.; Ding, H.; Li, G. Facile assembly of InVO4/TiO2 heterojunction for enhanced photo-oxidation of benzyl alcohol. Nanomaterials 2022, 12, 1544. [Google Scholar] [CrossRef]
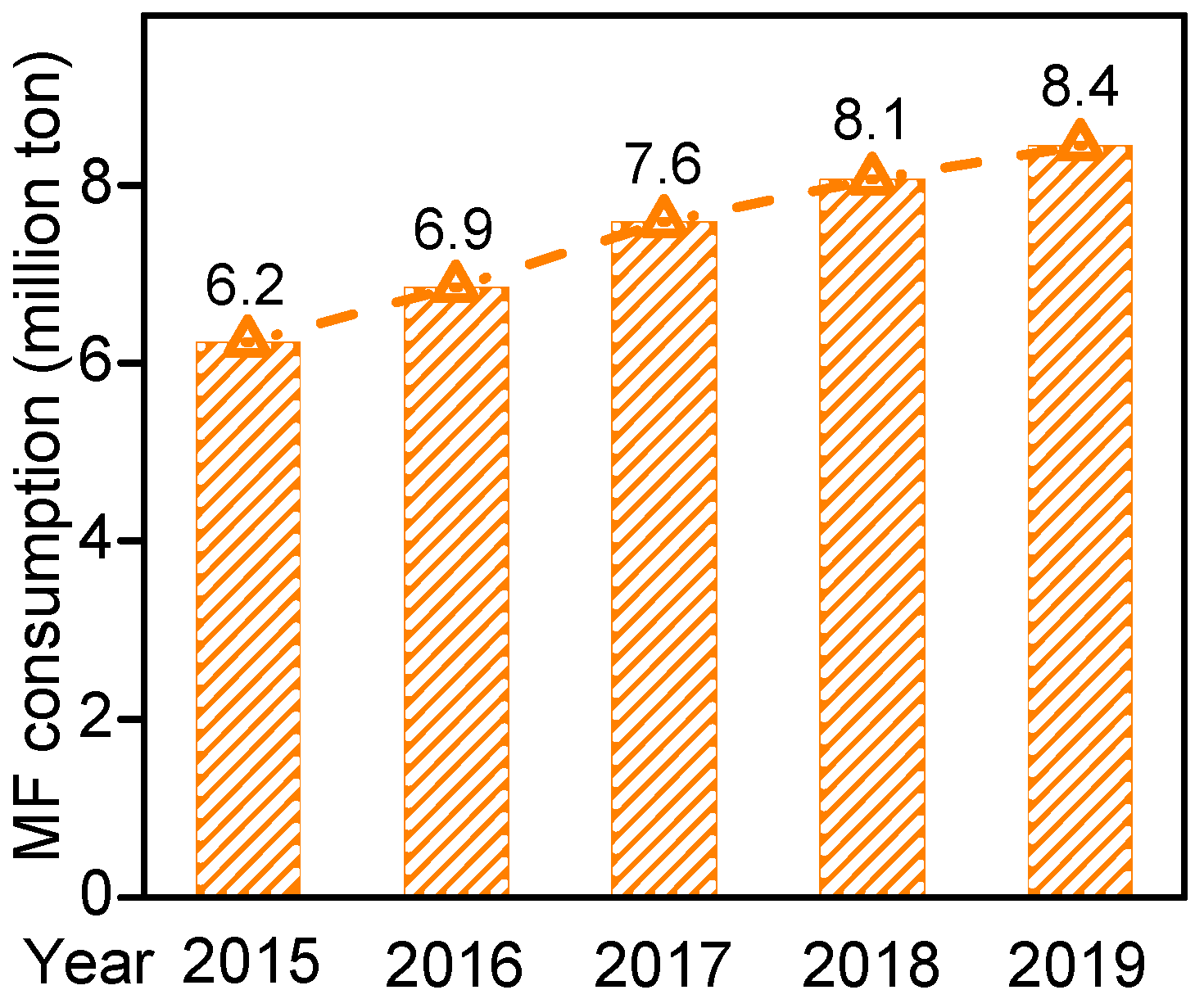



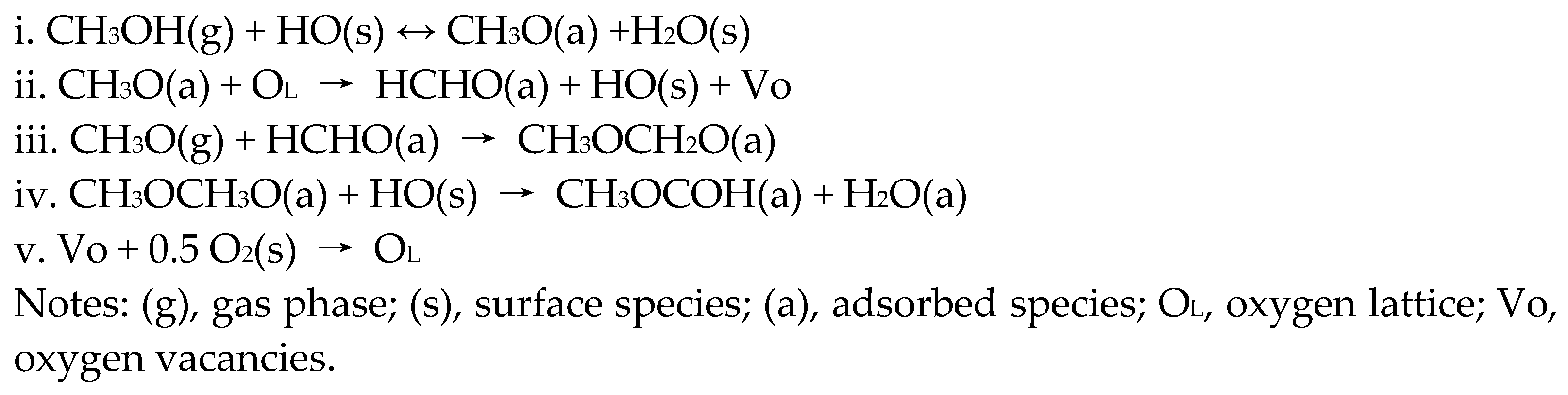

| Photocatalysts | CH3OH Conversion | Methyl Formate Selectivity | Methyl Formate Formation Rate (mmolCH3OH gcat.−1 h−1) | Refs. |
|---|---|---|---|---|
| A-TiO2 | 10% | 91% | 1.5 | [30] |
| P25 | 27% | 56% | 1.8 | [42] |
| Ag/TiO2 | 75% | 80% | 7.3 | [42] |
| Au/TiO2 | 65% | 75% | 5.9 | [42] |
| Cu/TiO2 | 65% | 55% | 4.4 | [32] |
| CuO/CuZnAl | 80% | 60% | 5.8 | [43] |
| Pd–Cu/TiO2-P90 | 53% | 80% | 5.7 | [44] |
| CuO/TiO2-S | 95% | 84% | 10.8 | [29] |
| CuO/TiO2-P | 97% | 83% | 10.5 | [38] |
| CuO/TiO2-T | 93% | 90% | 22.9 | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Cao, C.; Li, G. Recent Progress in Green Conversion of Biomass Alcohol to Chemicals via Aerobic Oxidation. Biomass 2022, 2, 103-115. https://doi.org/10.3390/biomass2020007
Zhang Y, Cao C, Li G. Recent Progress in Green Conversion of Biomass Alcohol to Chemicals via Aerobic Oxidation. Biomass. 2022; 2(2):103-115. https://doi.org/10.3390/biomass2020007
Chicago/Turabian StyleZhang, Yifei, Changhai Cao, and Gao Li. 2022. "Recent Progress in Green Conversion of Biomass Alcohol to Chemicals via Aerobic Oxidation" Biomass 2, no. 2: 103-115. https://doi.org/10.3390/biomass2020007
APA StyleZhang, Y., Cao, C., & Li, G. (2022). Recent Progress in Green Conversion of Biomass Alcohol to Chemicals via Aerobic Oxidation. Biomass, 2(2), 103-115. https://doi.org/10.3390/biomass2020007






