Kinetic Study of Pd-Promoting Effect on Cu/ZnO/Al2O3 Catalyst for Glycerol Hydrogenolysis to Produce 1,2-Propanediol at Low Hydrogen Pressure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Characterizations
2.3. Catalytic Activity Test
3. Results and Discussion
3.1. Temperature Programmed Reduction
3.2. Surface Physicochemical Properties of CZA Catalyst and Its Supported Pd Catalyst
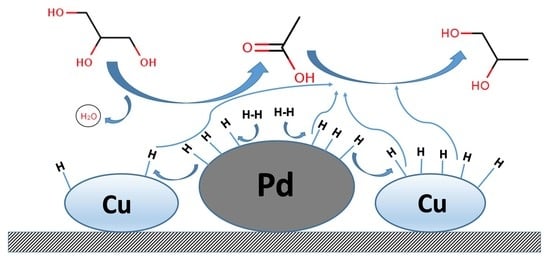
3.3. The Promoting Effect of Pd on the Acetol Hydrogenation Activity of the Cu/ZnO/Al2O3 Catalyst
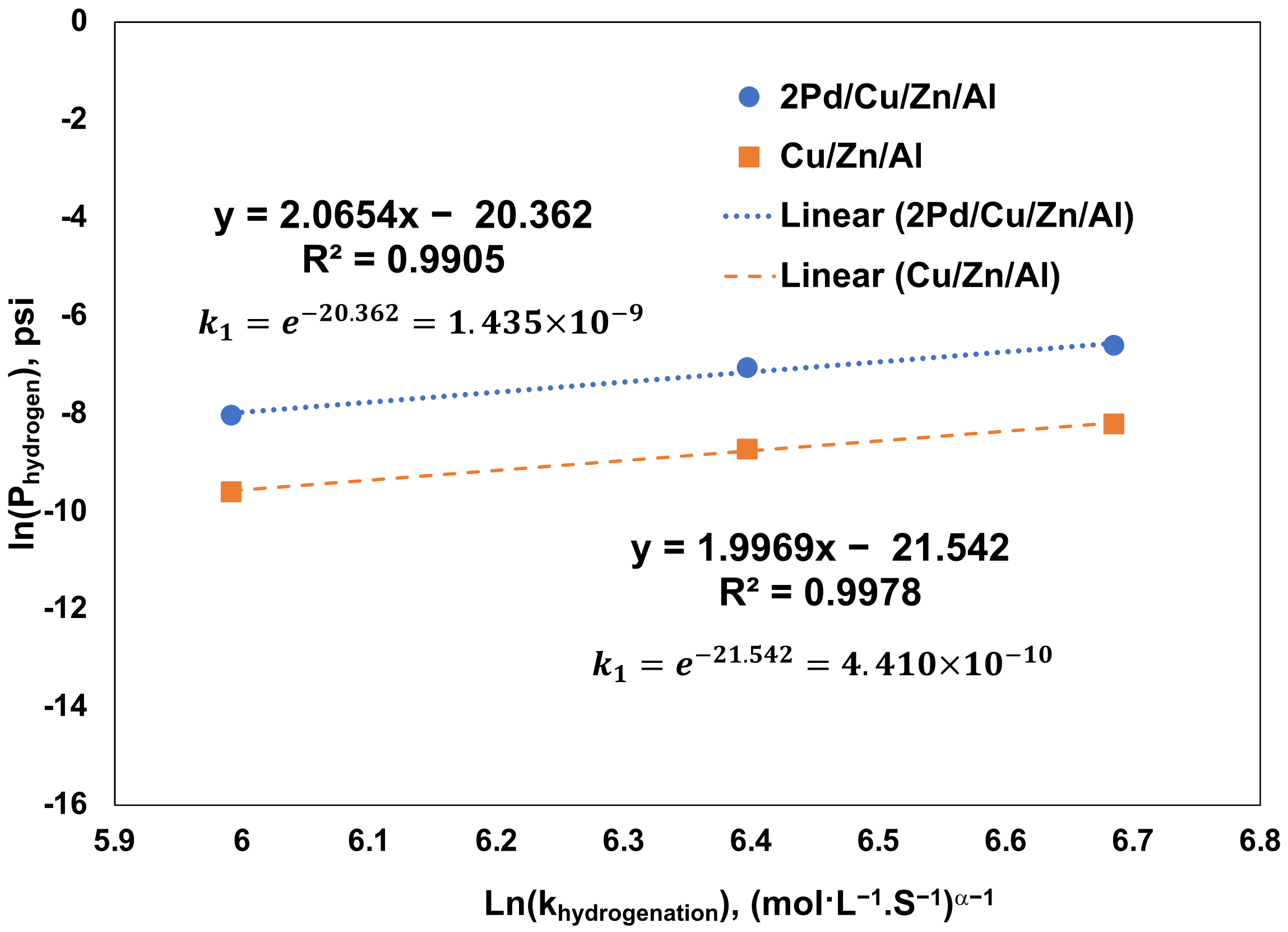
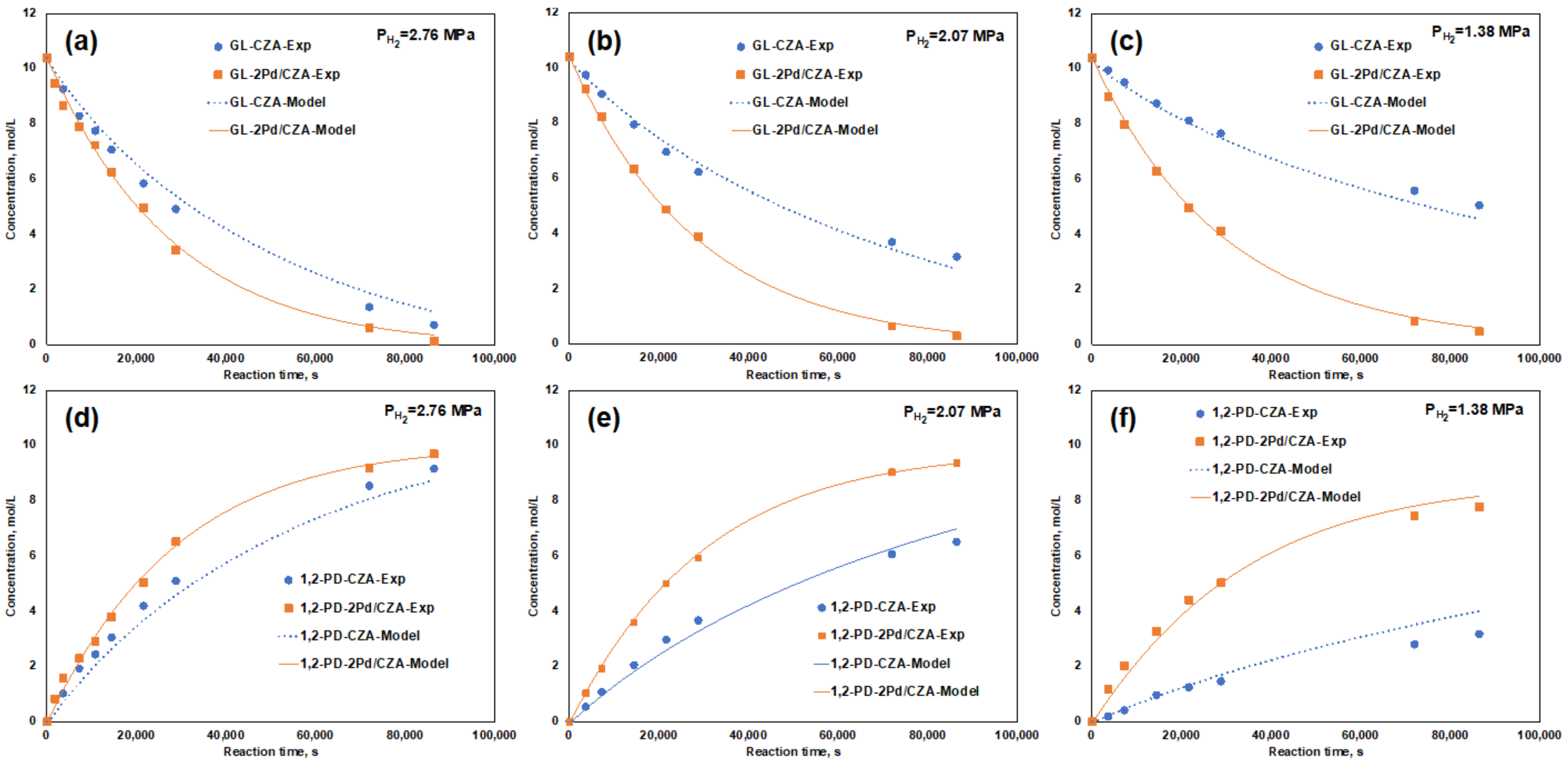
3.4. Promoting Effect of Pd on the Glycerol Hydrogenolysis Activity of the Cu/ZnO/Al2O3 Catalyst
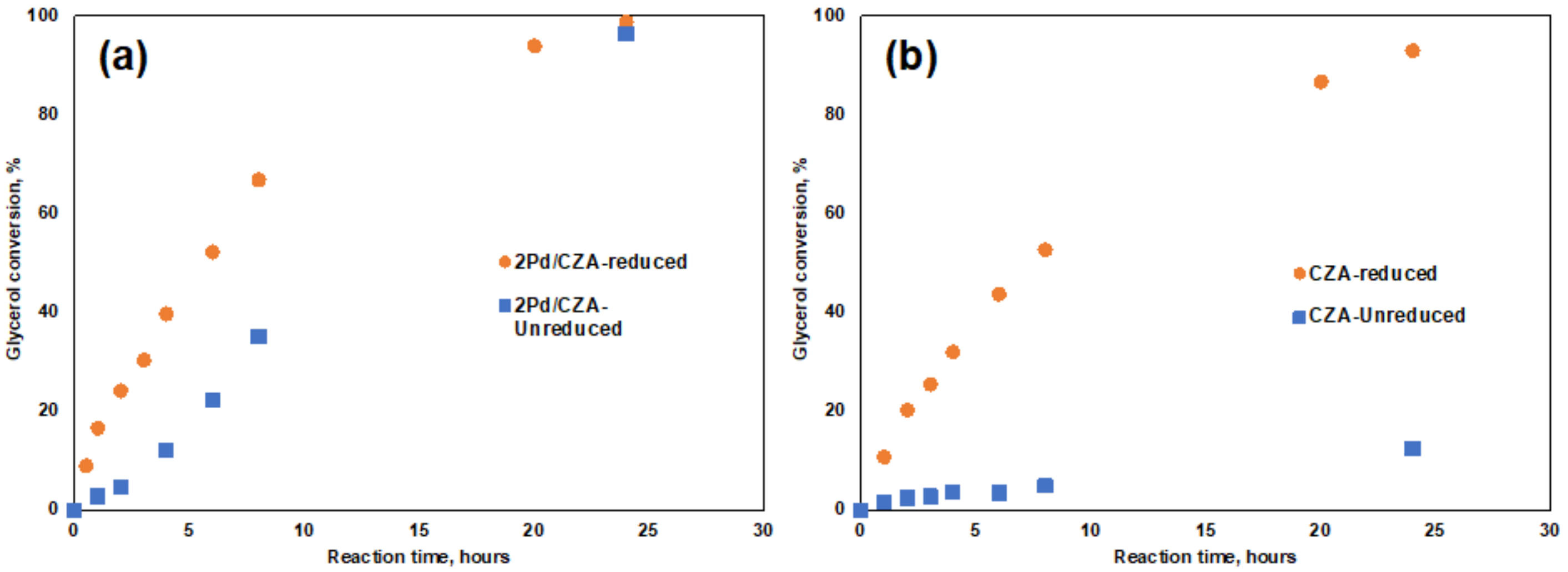
3.5. Activities of the Unreduced Catalysts with Pd and without Pd Promoted for Glycerol Hydrogenolysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, J.; Sarma, A.K.; Jha, M.K.; Gera, P. Valorisation of crude glycerol to value-added products: Perspectives of process technology, economics and environmental issues. Biotechnol. Rep. 2020, 27, e00487. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pasupulety, N.; Gunda, K.; Rempel, G.L.; Ng, F.T.T. Glycerol Hydrogenolysis to 1,2-Propanediol by Cu/ZnO/Al2O3 Catalysts. Top. Catal. 2014, 57, 1454–1462. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, X.; Rempel, L.G.; Ng, T.T.F. The Promoting Effect of Ni on Glycerol Hydrogenolysis to 1,2-Propanediol with In Situ Hydrogen from Methanol Steam Reforming Using a Cu/ZnO/Al2O3 Catalyst. Catalysts 2019, 9, 412. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Mai, C.T.Q.; Ng, F.T.T. Glycerol Hydrogenolysis with In Situ Hydrogen Produced via Methanol Steam Reforming: The Promoting Effect of Pd on a Cu/ZnO/Al2O3 Catalyst. Catalysts 2021, 11, 110. [Google Scholar] [CrossRef]
- van Ryneveld, E.; Mahomed, A.S.; van Heerden, P.S.; Friedrich, H.B. Direct Hydrogenolysis of Highly Concentrated Glycerol Solutions over Supported Ru, Pd and Pt Catalyst Systems. Catal. Lett. 2011, 141, 958–967. [Google Scholar] [CrossRef]
- Chiu, C.; Tekeei, A.; Ronco, J.M.; Banks, M.; Suppes, G.J. Reducing Byproduct Formation during Conversion of Glycerol to Propylene Glycol. Ind. Eng. Chem. Res. 2008, 47, 6878–6884. [Google Scholar] [CrossRef]
- Kim, N.D.; Park, J.R.; Park, D.S.; Kwak, B.K.; Yi, J. Promoter effect of Pd in CuCr2O4 catalysts on the hydrogenolysis of glycerol to 1,2-propanediol. Green Chem. 2012, 14, 2638–2646. [Google Scholar] [CrossRef]
- Shan, J.; Liu, H.; Lu, K.; Zhu, S.; Li, J.; Wang, J.; Fan, W. Identification of the dehydration active sites in glycerol hydrogenolysis to 1,2-propanediol over Cu/SiO2 catalysts. J. Catal. 2020, 383, 13–23. [Google Scholar] [CrossRef]
- Sharma, R.V.; Kumar, P.; Dalai, A.K. Selective hydrogenolysis of glycerol to propylene glycol by using Cu:Zn:Cr:Zr mixed metal oxides catalyst. Appl. Catal. A 2014, 477, 147–156. [Google Scholar] [CrossRef]
- Liu, Y. Catalytic Glycerol Hydrogenolysis to Produce 1,2-Propanediol with Molecular Hydrogen and In Situ Hydrogen Produced from Steam Reforming. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2014. [Google Scholar]
- Kumar, P.; Shah, A.K.; Lee, J.; Park, Y.H.; Štangar, U.L. Selective Hydrogenolysis of Glycerol over Bifunctional Copper–Magnesium-Supported Catalysts for Propanediol Synthesis. Ind. Eng. Chem. Res. 2020, 59, 6506–6516. [Google Scholar] [CrossRef]
- Cao, X.; Mirjalili, A.; Wheeler, J.; Xie, W.; Jang, B.W. Investigation of the preparation methodologies of Pd-Cu single atom alloy catalysts for selective hydrogenation of acetylene. Front. Chem. Sci. Eng. 2015, 9, 442–449. [Google Scholar] [CrossRef]
- Boucher, M.B.; Zugic, B.; Cladaras, G.; Kammert, J.; Marcinkowski, M.D.; Lawton, T.J.; Sykes, E.C.; Flytzani-Stephanopoulos, M. Single atom alloy surface analogs in Pd0.18Cu15 nanoparticles for selective hydrogenation reactions. Phys. Chem. Chem. Phys. 2013, 15, 12187–12196. [Google Scholar] [CrossRef]
- Hu, B.; Yin, Y.; Liu, G.; Chen, S.; Hong, X.; Tsang, S.C.E. Hydrogen spillover enabled active Cu sites for methanol synthesis from CO2 hydrogenation over Pd doped CuZn catalysts. J. Catal. 2018, 359, 17–26. [Google Scholar] [CrossRef]
- Ma, L.; Lv, C.; Wang, G. A DFT study and micro-kinetic analysis of acetylene selective hydrogenation on Pd-doped Cu(111) surfaces. Appl. Surf. Sci. 2017, 410, 154–165. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.; Trenary, M. Propyne Hydrogenation over a Pd/Cu(111) Single-Atom Alloy Studied Using Ambient Pressure Infrared Spectroscopy. ACS Catal. 2020, 10, 9716–9724. [Google Scholar] [CrossRef]
- Liu, Y.; He, Y.; Zhou, D.; Feng, J.; Li, D. Catalytic performance of Pd-promoted Cu hydrotalcite-derived catalysts in partial hydrogenation of acetylene: Effect of Pd-Cu alloy formation. Catal. Sci. Technol. 2016, 6, 3027–3037. [Google Scholar] [CrossRef]
- Kyriakou, G.; Boucher, M.B.; Jewell, A.D.; Lewis, E.A.; Lawton, T.J.; Baber, A.E.; Tierney, H.L.; Flytzani-Stephanopoulos, M.; Sykes, E.C. Isolated Metal Atom Geometries as a Strategy for Selective Heterogeneous Hydrogenations. Science 2012, 335, 1209–1212. [Google Scholar] [CrossRef]
- Tierney, H.L.; Baber, A.E.; Kitchin, J.R.; Sykes, E.C. Hydrogen Dissociation and Spillover on Individual Isolated Palladium Atoms. Phys. Rev. Lett. 2009, 103, 246102. [Google Scholar] [CrossRef]
- Fu, Q.; Luo, Y. Active Sites of Pd-Doped Flat and Stepped Cu(111) Surfaces for H2 Dissociation in Heterogeneous Catalytic Hydrogenation. ACS Catal. 2013, 3, 1245–1252. [Google Scholar] [CrossRef]
- Xia, S.; Yuan, Z.; Wang, L.; Chen, P.; Hou, Z. Hydrogenolysis of glycerol on bimetallic Pd-Cu/solid-base catalysts prepared via layered double hydroxides precursors. Appl. Catal. A 2011, 403, 173–182. [Google Scholar] [CrossRef]
- Ardila, A.N.; Sánchez-Castillo, M.A.; Zepeda, T.A.; Villa, A.L.; Fuentes, G.A. Glycerol hydrodeoxygenation to 1,2-propanediol catalyzed by CuPd/TiO2-Na. Appl. Catal. B 2017, 219, 658–671. [Google Scholar] [CrossRef]
- Ardila A, A.N.; Arriola-Villaseñor, E.; Fuentes, G.A. Nature and Distribution of Cu and Pd Species in CuPd/TiO2–Na Bimetallic Catalysts for Glycerol Hydrodeoxygenation. ACS Omega 2020, 5, 19497–19505. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Roy, D.; Subramaniam, B.; Chaudhari, R.V. Kinetic Modeling of Aqueous-Phase Glycerol Hydrogenolysis in a Batch Slurry Reactor. Ind. Eng. Chem. Res. 2010, 49, 10826–10835. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Eggenhuisen, T.M.; Munnik, P.; de Jongh, P.E.; de Jong, K.P.; Lemonidou, A.A. Synthesis and performance of highly dispersed Cu/SiO2 catalysts for the hydrogenolysis of glycerol. Appl. Catal. B 2014, 145, 108–119. [Google Scholar] [CrossRef]
- Gabrysch, T.; Muhler, M.; Peng, B. The kinetics of glycerol hydrodeoxygenation to 1,2-propanediol over Cu/ZrO2 in the aqueous phase. Appl. Catal. A 2019, 576, 47–53. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.; Zeng, T.; Hong, W.; Cheng, Z.; Yuan, W. Kinetics of Hydrogenolysis of Glycerol to Propylene Glycol over Cu-ZnO-Al2O3 Catalysts. Chin. J. Chem. Eng. 2010, 18, 384–390. [Google Scholar] [CrossRef]
- Rajkhowa, T.; Marin, G.B.; Thybaut, J.W. A comprehensive kinetic model for Cu catalyzed liquid phase glycerol hydrogenolysis. Appl. Catal. B 2017, 205, 469–480. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, M.; Rempel, G.L.; Ng, F.T.T. Glycerol Hydrogenolysis to Produce 1,2-Propanediol in Absence of Molecular Hydrogen Using a Pd Promoted Cu/MgO/Al2O3 catalyst. Catalysts 2021, 11, 1299. [Google Scholar] [CrossRef]
- Yfanti, V.L.; Ipsakis, D.; Lemonidou, A.A. Kinetic study of liquid phase glycerol hydrodeoxygenation under inert conditions over a Cu-based catalyst. React. Chem. Eng. 2018, 3, 559–571. [Google Scholar] [CrossRef]
- Iwasa, N.; Mayanagi, T.; Nomura, W.; Arai, M.; Takezawa, N. Effect of Zn addition to supported Pd catalysts in the steam reforming of methanol. Appl. Catal. A 2003, 248, 153–160. [Google Scholar] [CrossRef]
- Cai, F.; Jin, F.; Hao, J.; Xiao, G. Selective hydrogenolysis of glycerol to 1,2-propanediol on Nb-modified Pd-Zr-Al catalysts. Catal. Commun. 2019, 131, 105801. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Ma, L.; He, D. Glycerol hydrogenolysis to propanediols over supported Pd-Re catalysts. RSC Adv. 2014, 4, 5503–5512. [Google Scholar] [CrossRef]
- Ma, T.; Yun, Z.; Xu, W.; Chen, L.; Li, L.; Ding, J.; Shao, R. Pd-H3PW12O40/Zr-MCM-41: An efficient catalyst for the sustainable dehydration of glycerol to acrolein. Chem. Eng. J. 2016, 294, 343–352. [Google Scholar] [CrossRef]
- Patankar, S.C.; Yadav, G.D. Cascade Engineered Synthesis of γ-Valerolactone, 1,4-Pentanediol, and 2-Methyltetrahydrofuran from Levulinic Acid Using Pd–Cu/ZrO2 Catalyst in Water as Solvent. ACS Sustain. Chem. Eng. 2015, 3, 2619–2630. [Google Scholar] [CrossRef]
- Yfanti, V.L.; Vasiliadou, E.S.; Lemonidou, A.A. Glycerol hydro-deoxygenation aided by in situ H2 generation via methanol aqueous phase reforming over a Cu-ZnO-Al2O3 catalyst. Catal. Sci. Technol. 2016, 6, 5415–5426. [Google Scholar] [CrossRef]
- Yfanti, V.L.; Lemonidou, A.A. Mechanistic study of liquid phase glycerol hydrodeoxygenation with in-situ generated hydrogen. J. Catal. 2018, 368, 98–111. [Google Scholar] [CrossRef]
- Pandhare, N.N.; Pudi, S.M.; Mondal, S.; Pareta, K.; Kumar, M.; Biswas, P. Development of Kinetic Model for Hydrogenolysis of Glycerol over Cu/MgO Catalyst in a Slurry Reactor. Ind. Eng. Chem. Res. 2018, 57, 101–110. [Google Scholar] [CrossRef]
- Mondal, S.; Malviya, H.; Biswas, P. Kinetic modelling for the hydrogenolysis of bio-glycerol in the presence of a highly selective Cu–Ni–Al2O3 catalyst in a slurry reactor. React. Chem. Eng. 2019, 4, 595–609. [Google Scholar] [CrossRef]
- Chiu, C.; Dasari, M.A.; Suppes, G.J.; Sutterlin, W.R. Dehydration of glycerol to acetol via catalytic reactive distillation. AIChE J. 2006, 52, 3543–3548. [Google Scholar] [CrossRef]
- Jiang, T.; Huai, Q.; Geng, T.; Ying, W.; Xiao, T.; Cao, F. Catalytic performance of Pd-Ni bimetallic catalyst for glycerol hydrogenolysis. Biomass Bioenergy 2015, 78, 71–79. [Google Scholar] [CrossRef]
- Sato, S.; Akiyama, M.; Takahashi, R.; Hara, T.; Inui, K.; Yokota, M. Vapor-phase reaction of polyols over copper catalysts. Appl. Catal. A 2008, 347, 186–191. [Google Scholar] [CrossRef]
- Hou, M.; Jiang, H.; Liu, Y.; Chen, R. Role of initial water content in glycerol hydrogenolysis to 1,2-propanediol over Cu-ZnO catalyst. React. Kinet. Mech. Catal. 2017, 122, 1129–1143. [Google Scholar] [CrossRef]
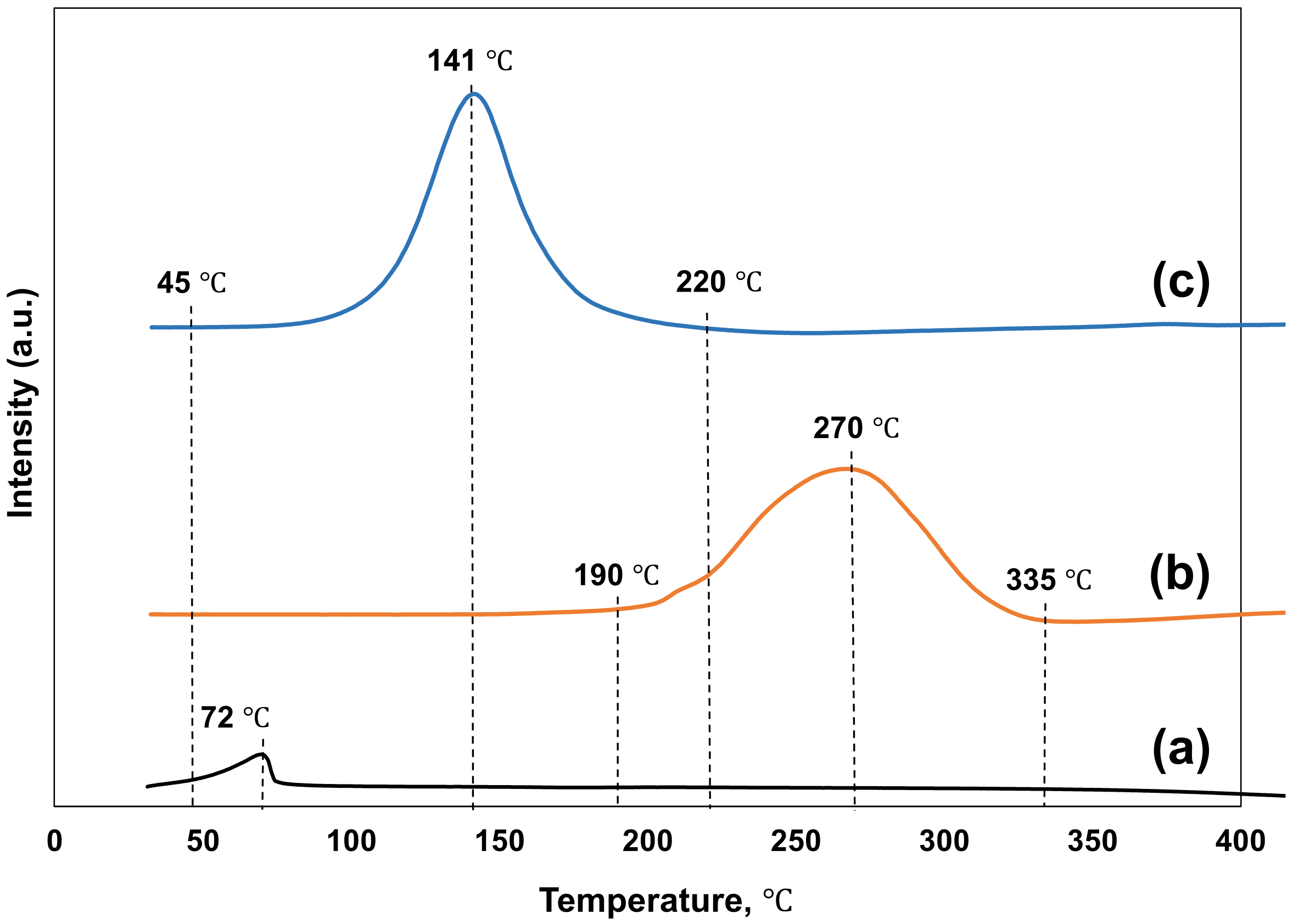
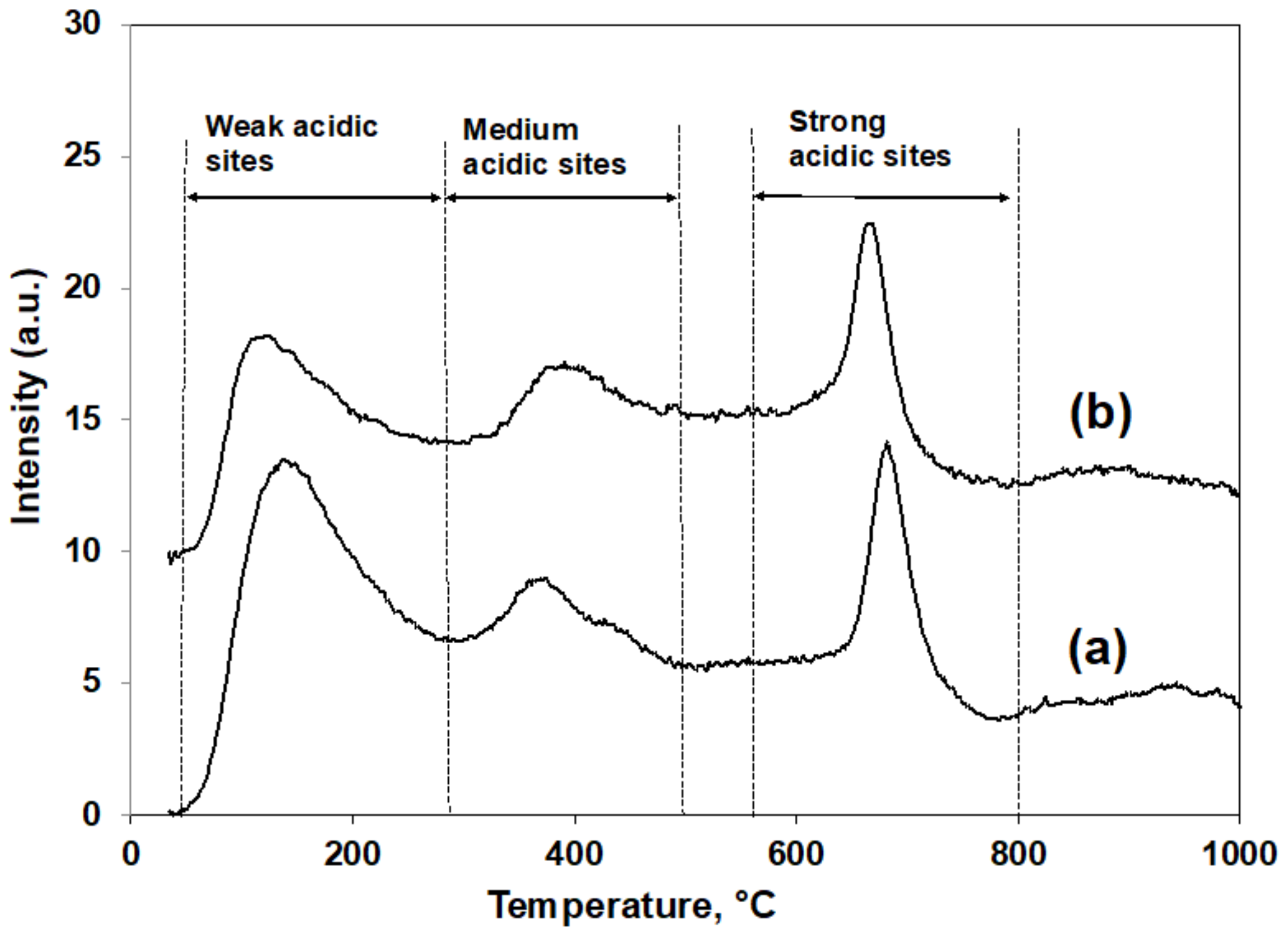








| Catalysts | Number of Acidic Sites (mmolNH3/g-cat) | Total Acidic Sites (mmolNH3/g-cat) | BET Surface Area 1 (m2/g-cat) | CuO Particle Size 2 (nm) |
|---|---|---|---|---|
| CZA | 0.225 (50 °C~290 °C) | 0.320 | 86.6 | 7.4 |
| 0.020 (290 °C~500 °C) | ||||
| 0.075 (590 °C~800 °C) | ||||
| 2Pd/CZA | 0.141 (50 °C~290 °C) | 0.222 | 84.6 | 7.6 |
| 0.026 (290 °C~590 °C) | ||||
| 0.065 (590 °C~800 °C) |
| Equations of Reaction Rates | |
|---|---|
| (1) | |
| (2) | |
| (3) | |
| Equations of Material Balances | |
| (4) | |
| (5) | |
| (6) | |
| [i]: molar concentration of each compound (mol·L−1) ri: rate of the reaction (mol·L−1·s−1) | |
| Catalysts | Hydrogen Pressure | Acetol Conversion | Selectivity, % | Kinetic Parameters | ||
|---|---|---|---|---|---|---|
| MPa | % | 1,2-PD | Others | k1 | α | |
| CZA | 2.76 | 81.0 | 52.9 | 47.1 | 6.818 × 10−5 | 0.83 |
| 4.14 | 94.3 | 69.2 | 30.8 | 1.616 × 10−4 | ||
| 5.52 | 100.0 | 77.9 | 22.1 | 2.704 × 10−4 | ||
| 2Pd/CZA | 2.76 | 99.4 | 82.0 | 18.0 | 3.287 × 10−4 | 1.00 |
| 4.14 | 100.0 | 87.8 | 12.2 | 8.516 × 10−4 | ||
| 5.52 | 100.0 | 92.0 | 8.0 | 1.357 × 10−3 | ||
| Equations of Reaction Rates | |
|---|---|
| (9) | |
| (10) | |
| (11) | |
| (12) | |
| (13) | |
| Equations of Material Balances | |
| (14) | |
| (15) | |
| (16) | |
| (17) | |
| (18) | |
| [i]: molar concentration of each compound(mol·L−1) ri: rate of the reaction (mol·L−1·s−1) | |
| Catalysts | Hydrogen Pressure MPa | Glycerol Conversion % | Selectivity, % | ||||
|---|---|---|---|---|---|---|---|
| 1,2-PD | Acetol | EG | Propanol | Others | |||
| CZA | 2.76 | 93.0 | 94.8 | 0.4 | 4.5 | 0.3 | 0.0 |
| 2.07 | 69.4 | 89.7 | 1.8 | 3.0 | 1.1 | 4.5 | |
| 1.38 | 51.3 | 58.1 | 3.9 | 1.9 | 1.5 | 34.7 | |
| 2Pd/CZA | 2.76 | 98.9 | 94.0 | 0.3 | 5.1 | 0.5 | 0.0 |
| 2.07 | 97.2 | 93.3 | 0.6 | 4.0 | 0.0 | 2.1 | |
| 1.38 | 95.3 | 78.0 | 1.5 | 2.5 | 0.9 | 17.1 | |
| Catalysts | γ | α | β | ε | δ | μ | ω | k1 | k−1 | k2 | k4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CZA | 0.80 | 0.83 | 2.00 | 1.68 | 3.52 | 2.87 | 2.17 | 5.661 × 10−5 | 3.273 × 10−7 | 6.464 × 10−9 | 7.781 × 10−14 |
| 2Pd/CZA | 0.85 | 1.00 | 2.07 | 0.62 | 2.34 | 1.92 | 0.70 | 4.815 × 10−5 | 1.918 × 10−7 | 5.232 × 10−8 | 5.085 × 10−9 |
| Catalysts | Glycerol Conversion % | Selectivity, % | ||||
|---|---|---|---|---|---|---|
| 1,2-PD | Acetol | EG | Propanol | Others | ||
| CZA reduced | 93.0 | 94.8 | 0.4 | 4.5 | 0.3 | 0.0 |
| CZA unreduced | 12.6 | 93.4 | 2.3 | 0.0 | 4.3 | 0.0 |
| 2Pd/CZA reduced | 98.9 | 94.0 | 0.3 | 5.1 | 0.5 | 0.0 |
| 2Pd/CZA unreduced | 96.4 | 93.5 | 0.4 | 4.5 | 0.9 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Rempel, G.L.; Ng, F.T.T. Kinetic Study of Pd-Promoting Effect on Cu/ZnO/Al2O3 Catalyst for Glycerol Hydrogenolysis to Produce 1,2-Propanediol at Low Hydrogen Pressure. Biomass 2022, 2, 27-45. https://doi.org/10.3390/biomass2010003
Liu Y, Rempel GL, Ng FTT. Kinetic Study of Pd-Promoting Effect on Cu/ZnO/Al2O3 Catalyst for Glycerol Hydrogenolysis to Produce 1,2-Propanediol at Low Hydrogen Pressure. Biomass. 2022; 2(1):27-45. https://doi.org/10.3390/biomass2010003
Chicago/Turabian StyleLiu, Yuanqing, Garry L. Rempel, and Flora T. T. Ng. 2022. "Kinetic Study of Pd-Promoting Effect on Cu/ZnO/Al2O3 Catalyst for Glycerol Hydrogenolysis to Produce 1,2-Propanediol at Low Hydrogen Pressure" Biomass 2, no. 1: 27-45. https://doi.org/10.3390/biomass2010003
APA StyleLiu, Y., Rempel, G. L., & Ng, F. T. T. (2022). Kinetic Study of Pd-Promoting Effect on Cu/ZnO/Al2O3 Catalyst for Glycerol Hydrogenolysis to Produce 1,2-Propanediol at Low Hydrogen Pressure. Biomass, 2(1), 27-45. https://doi.org/10.3390/biomass2010003