Organosolv Treatment/Polyphenol Extraction from Olive Leaves (Olea europaea L.) Using Glycerol and Glycerol-Based Deep Eutectic Solvents: Effect on Metabolite Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Deep Eutectic Solvent (DES) Synthesis
2.3. Olive Leaves (OLL)
2.4. Thermal Treatment/Extraction
2.5. Experimental Design and Response Surface Methodology
2.6. Extraction Efficiency Factor
2.7. Severity Factor (SF)
2.8. Determinations
2.9. Liquid Chromatography—Diode Array—Mass Spectrometry (LC—DAD—MS)
2.10. Statistical Treatments and Analyses
3. Results and Discussion
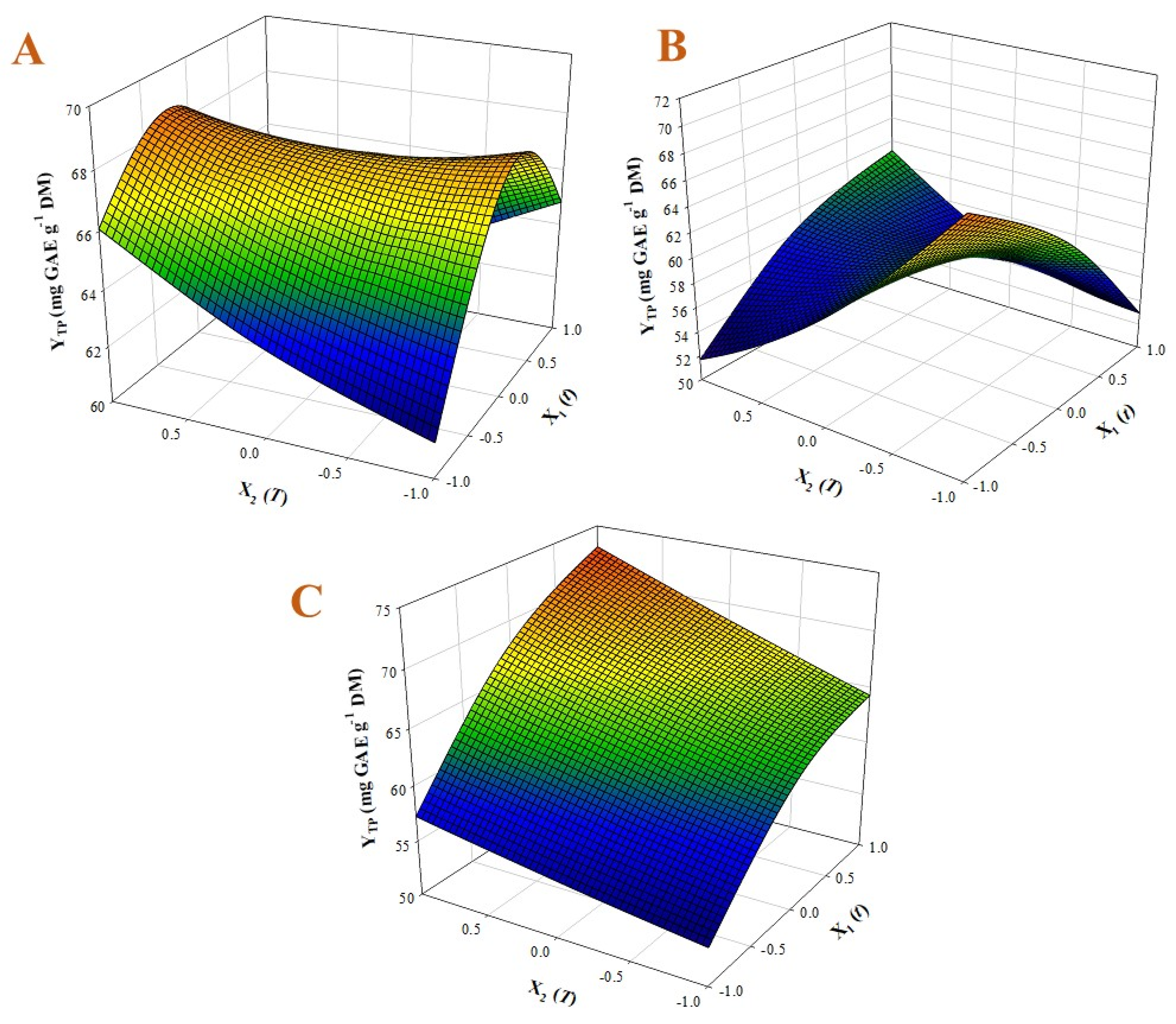
3.1. Process Modelling
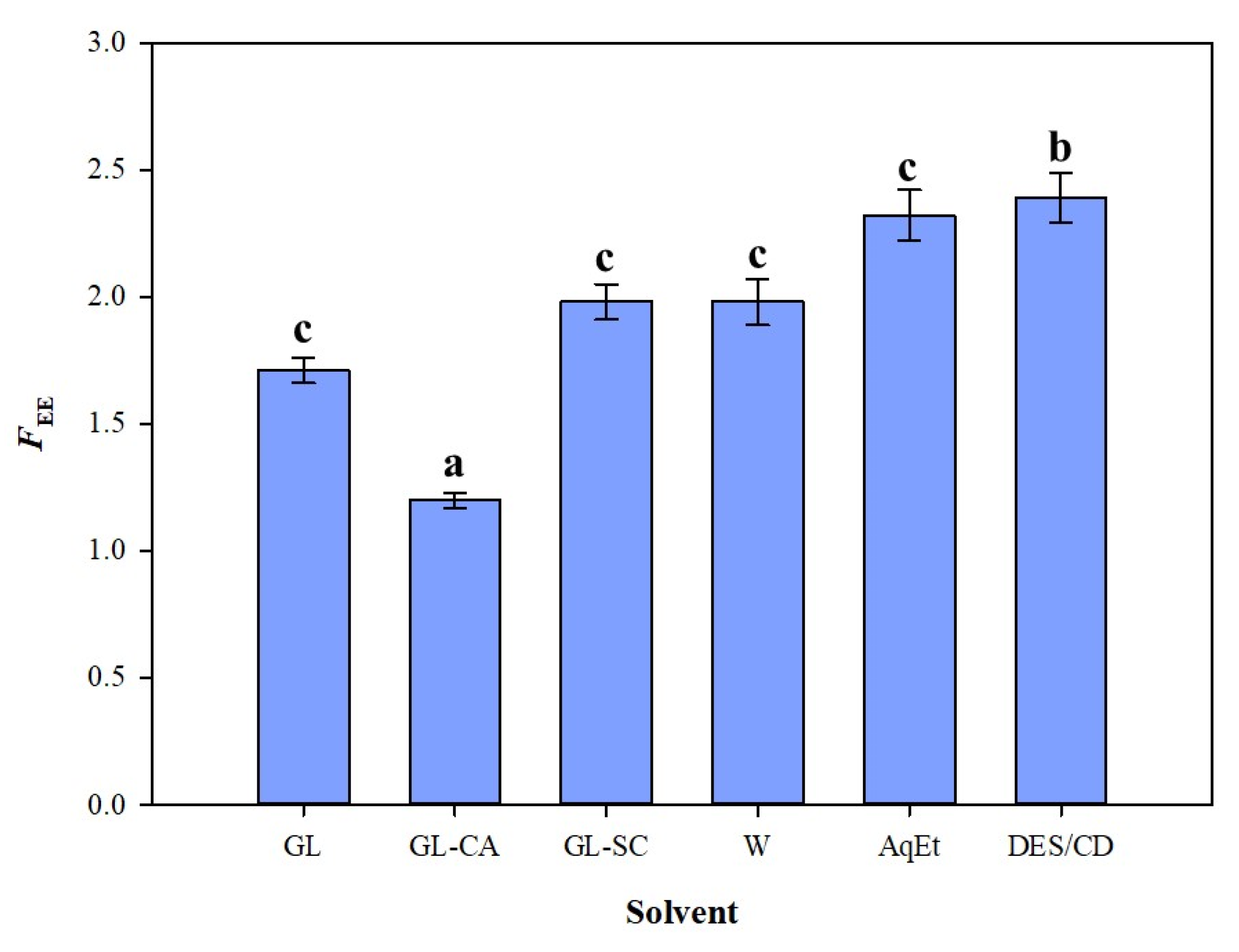
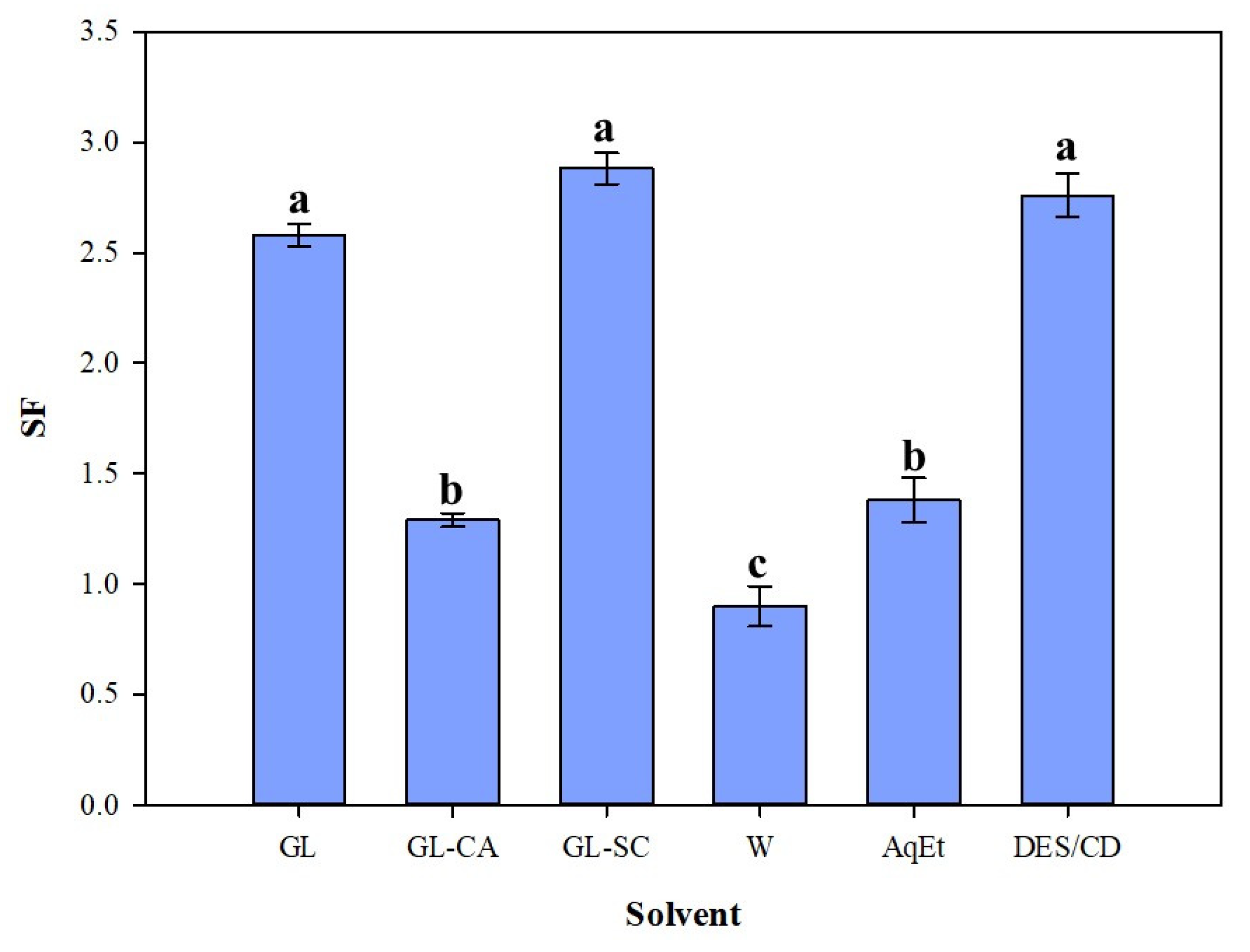
3.2. Process Severity and Efficiency
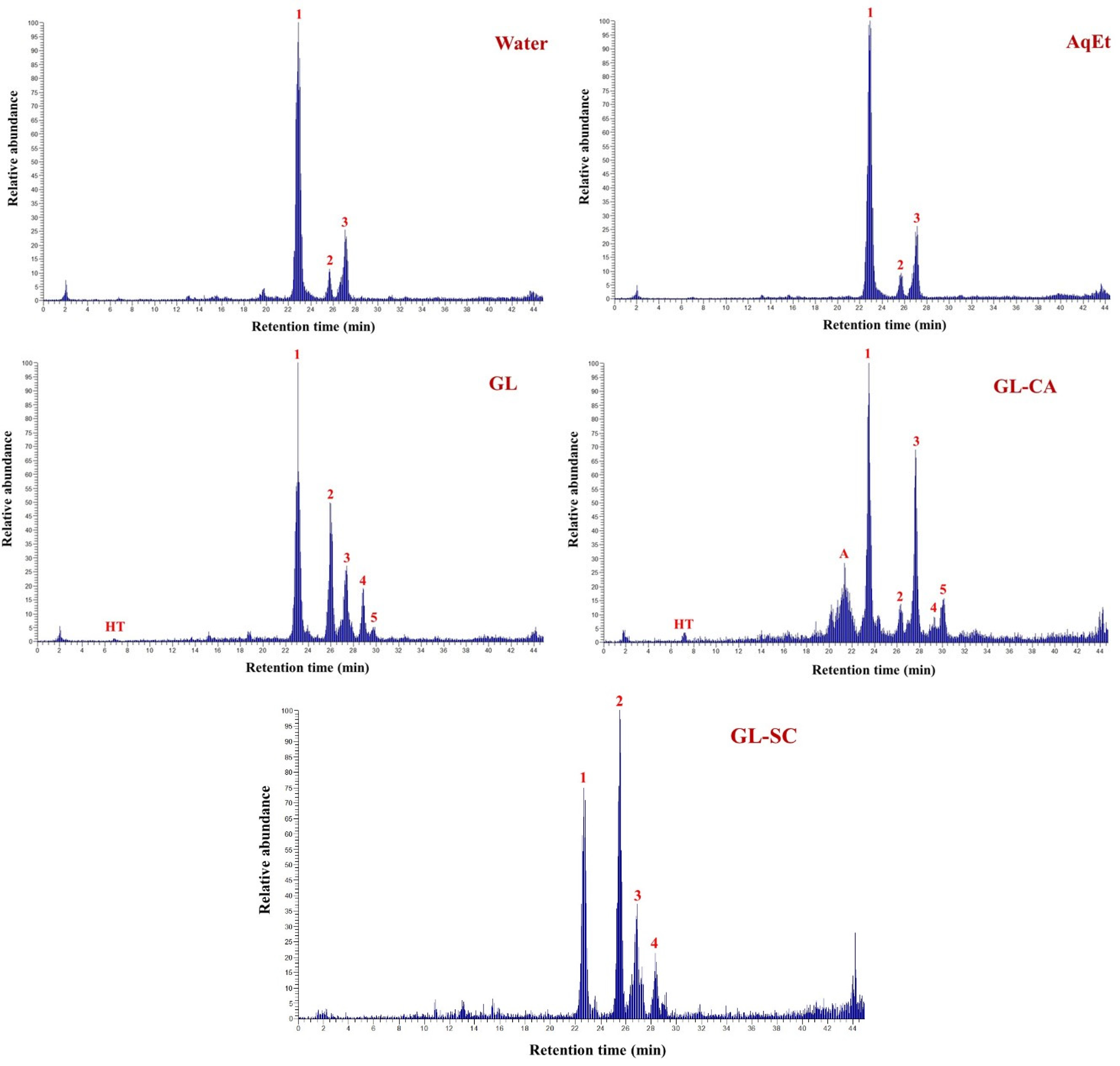
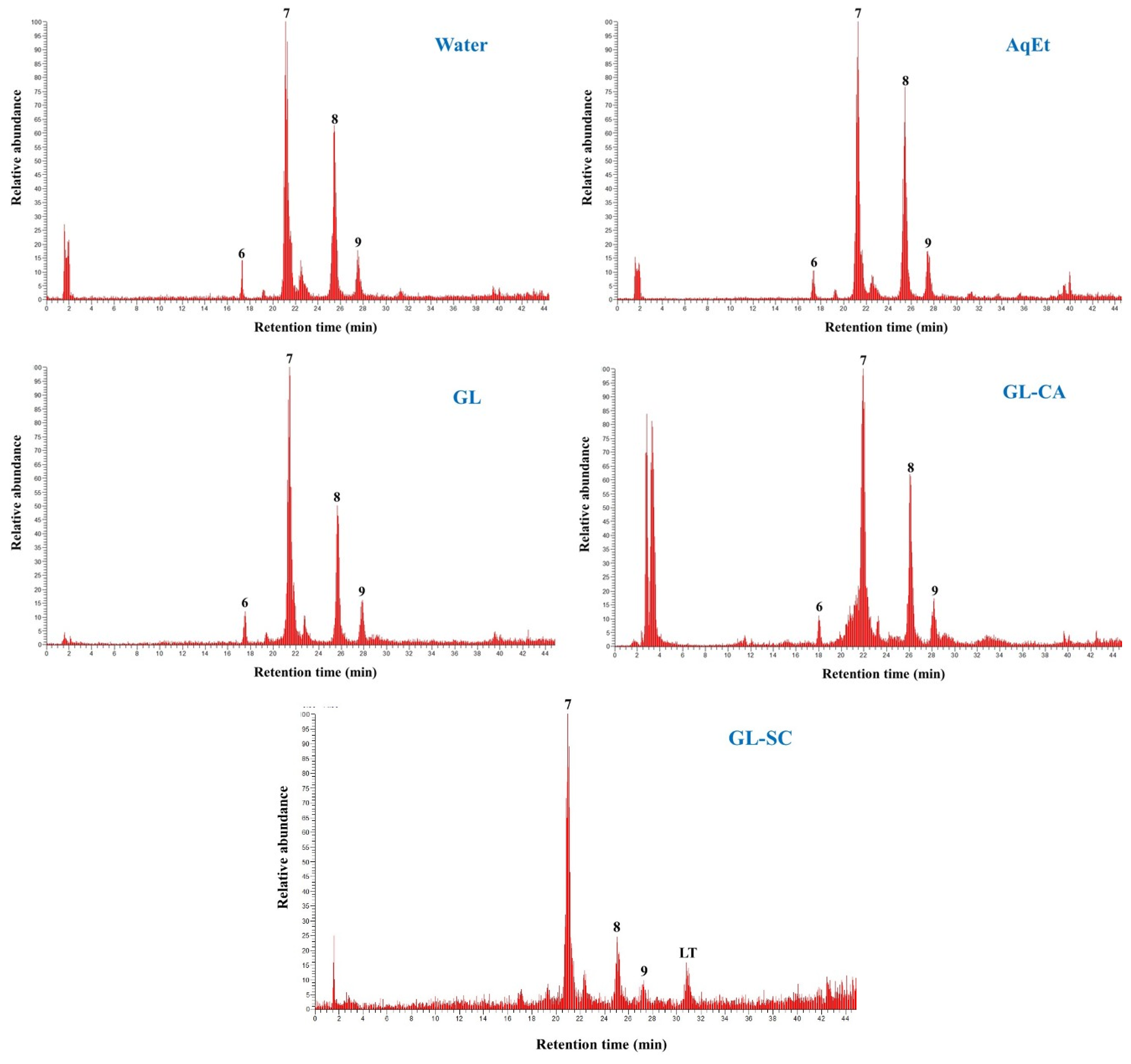
3.3. Polyphenolic Composition and Metabolite Stability
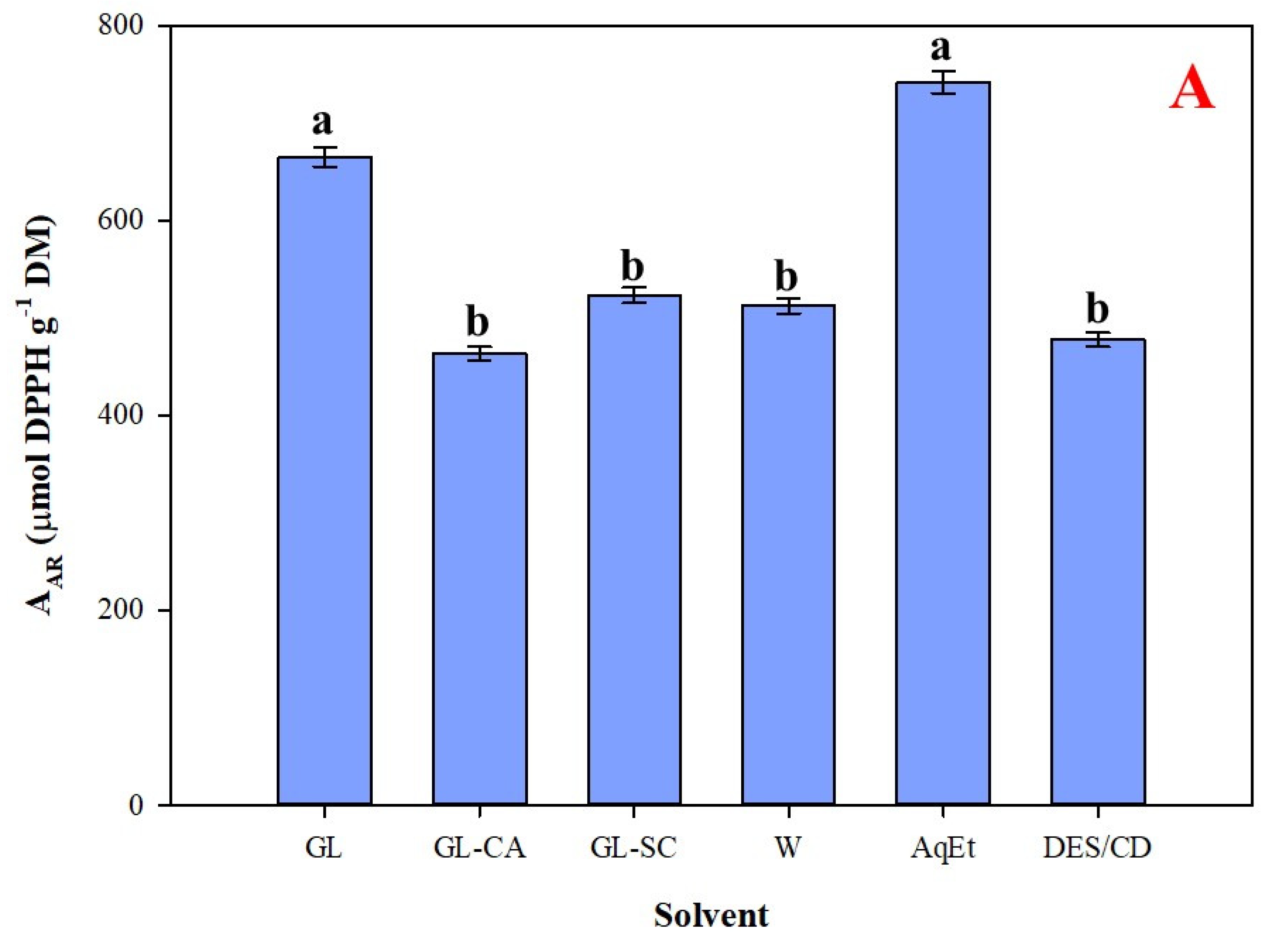
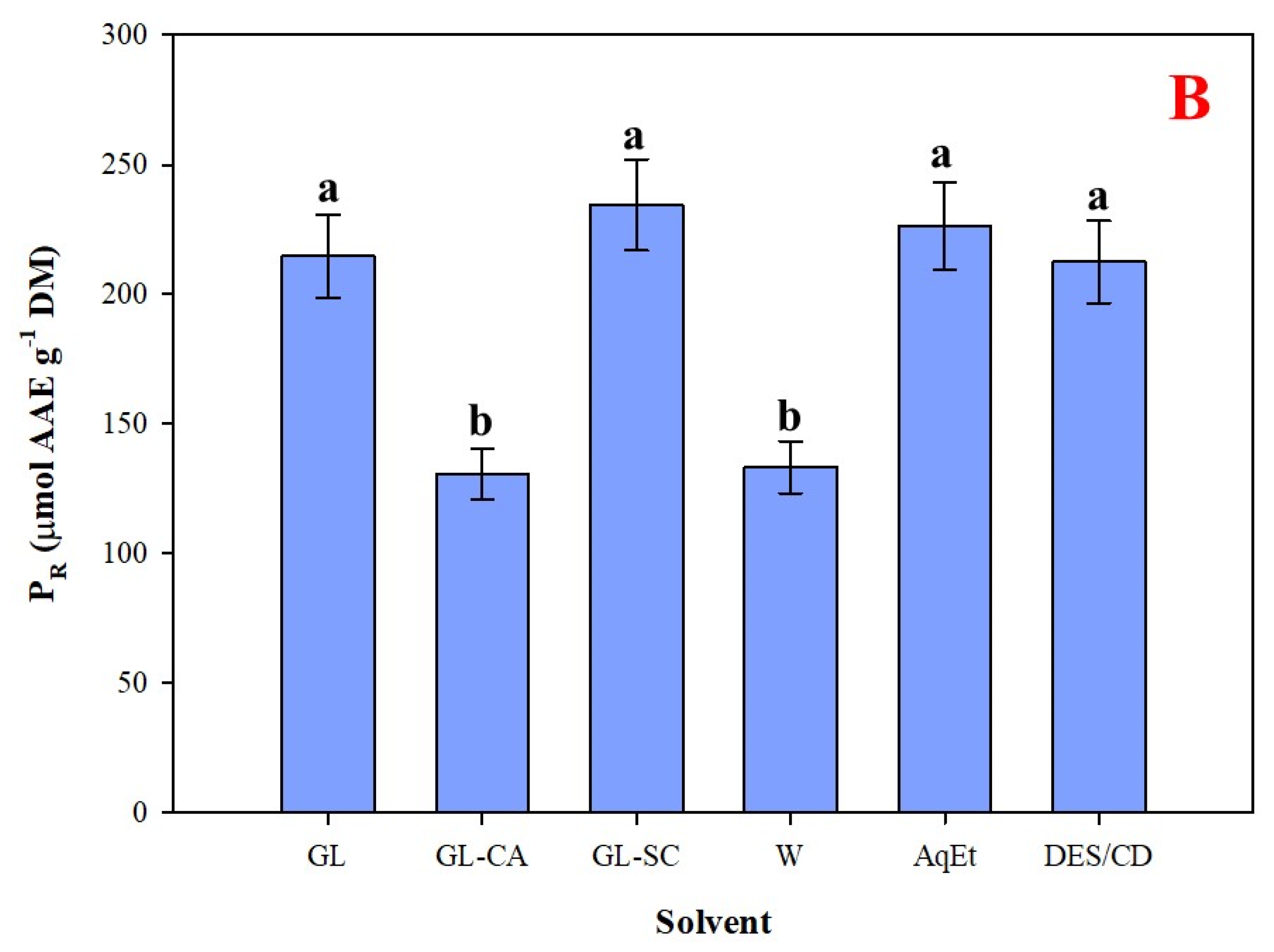
3.4. Efficiency Appraisal and Antioxidant Effects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zuin, V.G.; Ramin, L.Z. Green and sustainable separation of natural products from agro-industrial waste: Challenges, potentialities, and perspectives on emerging approaches. Chem. Chem. Technol. Waste Valor. 2018, 229–282. [Google Scholar] [CrossRef] [PubMed]
- Perino, S.; Chemat, F. Green process intensification techniques for bio-refinery. Curr. Op. Food Sci. 2019, 25, 8–13. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.; Lourenço-Lopes, C.; Prieto, M.; Simal-Gandara, J. Agriculture waste valorisation as a source of antioxidant phenolic compounds within a circular and sustainable bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Varatharajan, V.; Oh, W.Y.; Peng, H. Phenolic compounds in agri-food by-products, their bioavailability and health effects. J. Food Bioact. 2019, 5, 57–119. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Schwember, A.R.; Parada, R.; Garcia, S.; Marostica Junior, M.R.; Franchin, M.; Regitano-d’Arce, M.A.B.; Shahidi, F. Opinion on the hurdles and potential health benefits in value-added use of plant food processing by-products as sources of phenolic compounds. Inter. J. Mol. Sci. 2018, 19, 3498. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Ravi, H.K. Towards petroleum-free with plant-based chemistry. Curr. Op. Green Sustain. Chem. 2021, 28, 100450. [Google Scholar] [CrossRef]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging green techniques for the extraction of antioxidants from agri-food by-products as promising ingredients for the food industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef]
- Espeso, J.; Isaza, A.; Lee, J.Y.; Sörensen, P.M.; Jurado, P.; Avena-Bustillos, R.d.J.; Olaizola, M.; Arboleya, J.C. Olive leaf waste management. Front. Sustain. Food Syst. 2021, 5, 162. [Google Scholar] [CrossRef]
- Najafi, E.; Castro, E.; Karimi, K. Biorefining for olive wastes management and efficient bioenergy production. Energy Convers. Manag. 2021, 244, 114467. [Google Scholar] [CrossRef]
- Requejo, A.; Peleteiro, S.; Garrote, G.; Rodríguez, A.; Jiménez, L. Biorefinery of olive pruning using various processes. Biores Technol. 2012, 111, 301–307. [Google Scholar] [CrossRef]
- Solarte-Toroa, J.C.; Romero-García, J.M.; López-Linares, J.C.; Ruiz Ramos, E.; Castro, E.; Cardona Alzate, C.A. Simulation approach through the biorefinery concept of the antioxidants, lignin and ethanol production using olive leaves as raw material. Chem. Eng. Trans. 2018, 70, 925–930. [Google Scholar]
- Clodoveo, M.L.; Crupi, P.; Annunziato, A.; Corbo, F. Innovative extraction technologies for development of functional ingredients based on polyphenols from olive leaves. Foods 2022, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Sarker, T.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K.; Meda, V.; Naik, S. Hydrothermal pretreatment technologies for lignocellulosic biomass: A review of steam explosion and subcritical water hydrolysis. Chemosphere 2021, 284, 131372. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Lin, R.; Lam, C.H.; Wu, H.; Tsui, T.-H.; Yu, Y. Recent advances and challenges of inter-disciplinary biomass valorization by integrating hydrothermal and biological techniques. Renew. Sustain. Energy Rev. 2021, 135, 110370. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.-J. Lignocellulosic biomass pretreatment by deep eutectic solvents on lignin extraction and saccharification enhancement: A review. Bioresour. Technol. 2021, 339, 125587. [Google Scholar] [CrossRef] [PubMed]
- Wei Kit Chin, D.; Lim, S.; Pang, Y.L.; Lam, M.K. Fundamental review of organosolv pretreatment and its challenges in emerging consolidated bioprocessing. Biofuels Bioprod. Biorefin. 2020, 14, 808–829. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Villaflores, O.B.; Ordono, E.E.; Caparanga, A.R. Effects of acidified aqueous glycerol and glycerol carbonate pretreatment of rice husk on the enzymatic digestibility, structural characteristics, and bioethanol production. Bioresour. Technol. 2017, 228, 264–271. [Google Scholar] [CrossRef]
- Kurtulbaş, E.; Pekel, A.G.; Bilgin, M.; Makris, D.P.; Şahin, S. Citric acid-based deep eutectic solvent for the anthocyanin recovery from Hibiscus sabdariffa through microwave-assisted extraction. Biomass Convers. Biorefin. 2022, 12, 351–360. [Google Scholar] [CrossRef]
- Grigorakis, S.; Halahlah, A.; Makris, D.P. Batch stirred-tank green extraction of Salvia fruticosa Mill. polyphenols using newly designed citrate-based deep eutectic solvents and ultrasonication pretreatment. Appl. Sci. 2020, 10, 4774. [Google Scholar] [CrossRef]
- Chakroun, D.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Enhanced-performance extraction of olive (Olea europaea) leaf polyphenols using L-lactic acid/ammonium acetate deep eutectic solvent combined with β-cyclodextrin: Screening, optimisation, temperature effects and stability. Biomass Convers. Bioref. 2021, 11, 1125–1136. [Google Scholar] [CrossRef]
- Morsli, F.; Grigorakis, S.; Halahlah, A.; Poulianiti, K.P.; Makris, D.P. Appraisal of the combined effect of time and temperature on the total polyphenol yield in batch stirred-tank extraction of medicinal and aromatic plants: The extraction efficiency factor. J. Appl. Res. Med. Arom. Plants 2021, 25, 100340. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Kaltsa, O.; Bozinou, E.; Lalas, S.; Makris, D.P. Saffron processing wastes as a bioresource of high-value added compounds: Development of a green extraction process for polyphenol recovery using a natural deep eutectic solvent. Antioxidants 2019, 8, 586. [Google Scholar] [CrossRef] [PubMed]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Phil. Trans. R. Soc. London. Ser. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar]
- Ruiz, H.A.; Galbe, M.; Garrote, G.; Ramirez-Gutierrez, D.M.; Ximenes, E.; Sun, S.-N.; Lachos-Perez, D.; Rodríguez-Jasso, R.M.; Sun, R.-C.; Yang, B. Severity factor kinetic model as a strategic parameter of hydrothermal processing (steam explosion and liquid hot water) for biomass fractionation under biorefinery concept. Bioresour. Technol. 2021, 342, 125961. [Google Scholar] [CrossRef]
- Paleologou, I.; Vasiliou, A.; Grigorakis, S.; Makris, D.P. Optimisation of a green ultrasound-assisted extraction process for potato peel (Solanum tuberosum) polyphenols using bio-solvents and response surface methodology. Biomass Convers. Biorefin. 2016, 6, 289–299. [Google Scholar] [CrossRef]
- Xu, H.; Peng, J.; Kong, Y.; Liu, Y.; Su, Z.; Li, B.; Song, X.; Liu, S.; Tian, W. Key process parameters for deep eutectic solvents pretreatment of lignocellulosic biomass materials: A review. Bioresour. Technol. 2020, 310, 123416. [Google Scholar] [CrossRef]
- Kaltsa, O.; Grigorakis, S.; Lakka, A.; Bozinou, E.; Lalas, S.; Makris, D.P. Green valorization of olive leaves to produce polyphenol-enriched extracts using an environmentally benign deep eutectic solvent. AgriEngineering 2020, 2, 226–239. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Grigorakis, S.; Lalas, S.; Makris, D.P. Stability effects of methyl β-cyclodextrin on Olea europaea leaf extracts in a natural deep eutectic solvent. Eur. Food Res. Technol. 2018, 244, 1783–1792. [Google Scholar] [CrossRef]
- Karageorgou, I.; Grigorakis, S.; Lalas, S.; Makris, D.P. Effect of 2-hydroxypropyl β-cyclodextrin on the stability of polyphenolic compounds from Moringa oleifera Lam leaf extracts in a natural low-transition temperature mixture. Nova Biotech. Chim. 2018, 17, 29–37. [Google Scholar] [CrossRef][Green Version]
- Grigorakis, S.; Halahlah, A.; Makris, D.P. Stability of Salvia fruticosa Mill. polyphenols and antioxidant activity in a citrate-based natural deep eutectic solvent. Nova Biotech. Chim. 2020, 19, 200–207. [Google Scholar] [CrossRef]
- Romero-García, J.M.; Lama-Muñoz, A.; Rodríguez-Gutiérrez, G.; Moya, M.; Ruiz, E.; Fernández-Bolaños, J.; Castro, E. Obtaining sugars and natural antioxidants from olive leaves by steam-explosion. Food Chem. 2016, 210, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Dedousi, M.; Mamoudaki, V.; Grigorakis, S.; Makris, D.P. Ultrasound-assisted extraction of polyphenolic antioxidants from olive (Olea europaea) leaves using a novel glycerol/sodium-potassium tartrate low-transition temperature mixture (LTTM). Environments 2017, 4, 31. [Google Scholar] [CrossRef]
- Apostolakis, A.; Grigorakis, S.; Makris, D.P. Optimisation and comparative kinetics study of polyphenol extraction from olive leaves (Olea europaea) using heated water/glycerol mixtures. Separ. Purif. Technol. 2014, 128, 89–95. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Grigorakis, S.; Lalas, S.; Makris, D.P. Methyl β-cyclodextrin as a booster for the extraction for Olea europaea leaf polyphenols with a bio-based deep eutectic solvent. Biomass Convers. Biorefin. 2018, 8, 345–355. [Google Scholar] [CrossRef]
- Blasi, F.; Urbani, E.; Simonetti, M.S.; Chiesi, C.; Cossignani, L. Seasonal variations in antioxidant compounds of Olea europaea leaves collected from different Italian cultivars. J. Appl. Bot. Food Qual. 2016, 89. [Google Scholar]
- Guebebia, S.; Othman, K.B.; Yahia, Y.; Romdhane, M.; Elfalleh, W.; Hannachi, H. Effect of genotype and extraction method on polyphenols content, phenolic acids, and flavonoids of olive leaves (Olea europaea L. subsp. europaea). Inter. J. Plant Based Pharm. 2022, 2, 17–24. [Google Scholar]
- Goulas, V.; Papoti, V.T.; Exarchou, V.; Tsimidou, M.Z.; Gerothanassis, I.P. Contribution of flavonoids to the overall radical scavenging activity of olive (Olea europaea L.) leaf polar extracts. J. Agric. Food Chem. 2010, 58, 3303–3308. [Google Scholar] [CrossRef]
- Xie, P.-J.; Huang, L.-X.; Zhang, C.-H.; Zhang, Y.-L. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure–activity relationships. J. Funct. Foods 2015, 16, 460–471. [Google Scholar] [CrossRef]
- Abou Samra, M.; Chedea, V.S.; Economou, A.; Calokerinos, A.; Kefalas, P. Antioxidant/prooxidant properties of model phenolic compounds: Part I. Studies on equimolar mixtures by chemiluminescence and cyclic voltammetry. Food Chem. 2011, 125, 622–629. [Google Scholar] [CrossRef]
- Choueiri, L.; Chedea, V.S.; Calokerinos, A.; Kefalas, P. Antioxidant/pro-oxidant properties of model phenolic compounds. Part II: Studies on mixtures of polyphenols at different molar ratios by chemiluminescence and LC–MS. Food Chem. 2012, 133, 1039–1044. [Google Scholar] [CrossRef]
| Process Variables | Codes | Coded Variable Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| t (min) | X1 | 10 | 30 | 50 |
| T (°C) | X2 | 110 | 125 | 140 |
| Design Point | Independent Variables | Response (YTP, mg GAE g−1 dw) | ||||||
|---|---|---|---|---|---|---|---|---|
| t (min) (X1) | T (°C) (X2) | GL | GL-CA | GL-SC | ||||
| Measured | Predicted | Measured | Predicted | Measured | Predicted | |||
| 1 | 10 (−1) | 110 (−1) | 61.10 | 61.24 | 70.29 | 69.35 | 54.82 | 53.42 |
| 2 | 10 (−1) | 140 (1) | 65.94 | 66.07 | 51.34 | 51.71 | 57.02 | 57.25 |
| 3 | 50 (1) | 110 (−1) | 64.57 | 64.81 | 53.14 | 52.96 | 64.42 | 64.25 |
| 4 | 50 (1) | 140 (1) | 60.53 | 60.76 | 60.00 | 61.12 | 71.61 | 73.07 |
| 5 | 10 (−1) | 125 (0) | 63.40 | 63.13 | 57.97 | 58.55 | 54.01 | 55.18 |
| 6 | 50 (1) | 125 (0) | 62.73 | 62.26 | 56.02 | 55.06 | 69.80 | 68.51 |
| 7 | 30 (0) | 110 (−1) | 68.28 | 67.91 | 62.12 | 63.25 | 60.04 | 61.57 |
| 8 | 30 (0) | 140 (1) | 68.67 | 68.30 | 60.05 | 58.51 | 69.59 | 67.90 |
| 9 | 30 (0) | 125 (0) | 67.05 | 67.58 | 58.67 | 58.90 | 64.25 | 64.58 |
| 10 | 30 (0) | 125 (0) | 67.11 | 67.58 | 58.48 | 58.90 | 66.38 | 64.58 |
| 11 | 30 (0) | 125 (0) | 68.08 | 67.58 | 59.18 | 58.90 | 63.00 | 64.58 |
| Solvent | 2nd Order Polynomial Equations | R2 | p |
|---|---|---|---|
| GL | 67.58 − 2.22X1X2 − 4.89X12 | 0.98 | 0.0002 |
| GL-CA | 58.90 − 1.74X1 − 2.37X2 + 6.45X1X2 − 2.09X12 + 1.98X22 | 0.97 | 0.0008 |
| GL-SC | 64.58 + 6.66X1 + 3.16X2 | 0.95 | 0.0029 |
| Solvent | Maximum Predicted Response (mg GAE g−1 DM) | Optimal Conditions | Indices | ||
|---|---|---|---|---|---|
| t (min) | T (°C) | FEE | SF | ||
| GL | 68.66 ± 1.02 | 25 | 140 | 1.71 | 2.58 |
| GL-CA | 69.35 ± 2.78 | 10 | 110 | 1.20 | 1.29 |
| GL-SC | 73.07 ± 4.39 | 50 | 140 | 1.98 | 2.88 |
| Peak | Rt (min) | UV-Vis | [M + H]+ | Other Ions | Tentative Identity |
|---|---|---|---|---|---|
| HT | 6.79 | 238, 280 | 137 | - | Hydroxytyrosol |
| 6 | 17.53 | 244, 340 | 611 | 287, 377, 449 | Luteolin rutinoside |
| A | 21.34 | 240, 280 | 433 | 137 | Hydroxytyrosol derivative |
| 7 | 21.45 | 254, 352 | 449 | 287 | Luteolin 7-O-glucoside |
| 8 | 25.76 | 248, 266, 344 | 449 | 287 | Luteolin glucoside |
| 9 | 27.93 | 266, 344 | 449 | 287 | Luteolin glucoside |
| 1 | 22.85 | 246, 280 | 541 | 563, 361, 137 | Oleuropein |
| 2 | 25.94 | 242, 280 | 541 | 563, 361, 137 | Oleuropein derivative |
| 3 | 27.32 | 242, 280 | 541 | 563, 379, 361, 137 | Oleuropein derivative |
| 4 | 28.88 | 242, 280 | - | 563, 379, 361, 137 | Oleuropein derivative |
| 5 | 29.54 | 242, 280 | 475 | 361, 137 | Hydroxytyrosol derivative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houasni, A.; Grigorakis, S.; Kellil, A.; Makris, D.P. Organosolv Treatment/Polyphenol Extraction from Olive Leaves (Olea europaea L.) Using Glycerol and Glycerol-Based Deep Eutectic Solvents: Effect on Metabolite Stability. Biomass 2022, 2, 46-61. https://doi.org/10.3390/biomass2010004
Houasni A, Grigorakis S, Kellil A, Makris DP. Organosolv Treatment/Polyphenol Extraction from Olive Leaves (Olea europaea L.) Using Glycerol and Glycerol-Based Deep Eutectic Solvents: Effect on Metabolite Stability. Biomass. 2022; 2(1):46-61. https://doi.org/10.3390/biomass2010004
Chicago/Turabian StyleHouasni, Abdelhak, Spyros Grigorakis, Abdessamie Kellil, and Dimitris P. Makris. 2022. "Organosolv Treatment/Polyphenol Extraction from Olive Leaves (Olea europaea L.) Using Glycerol and Glycerol-Based Deep Eutectic Solvents: Effect on Metabolite Stability" Biomass 2, no. 1: 46-61. https://doi.org/10.3390/biomass2010004
APA StyleHouasni, A., Grigorakis, S., Kellil, A., & Makris, D. P. (2022). Organosolv Treatment/Polyphenol Extraction from Olive Leaves (Olea europaea L.) Using Glycerol and Glycerol-Based Deep Eutectic Solvents: Effect on Metabolite Stability. Biomass, 2(1), 46-61. https://doi.org/10.3390/biomass2010004









