A Two-Step Cultivation Strategy for High Biomass Production and Lipid Accumulation of Raphidocelis subcapitata Immobilized in Alginate Gel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalga and Culture Medium
2.2. Immobilization of R. subcapitata Cells in Alginate Beads
2.3. Culture Conditions for Immobilized Microalgae
2.4. Determination of the Biomass of Immobilized Microalgae
2.5. Calculation of Growth Rate, Generation Time, and Biomass Productivity
2.6. Extraction of Total Lipid from Immobilized Microalgae
2.7. Calculation of Lipid Productivity
3. Results
3.1. The Effects of Nitrogen and Phosphorus Deficiency on Lipid Accumulation and Biomass Production in Immobilized R. subcapitata
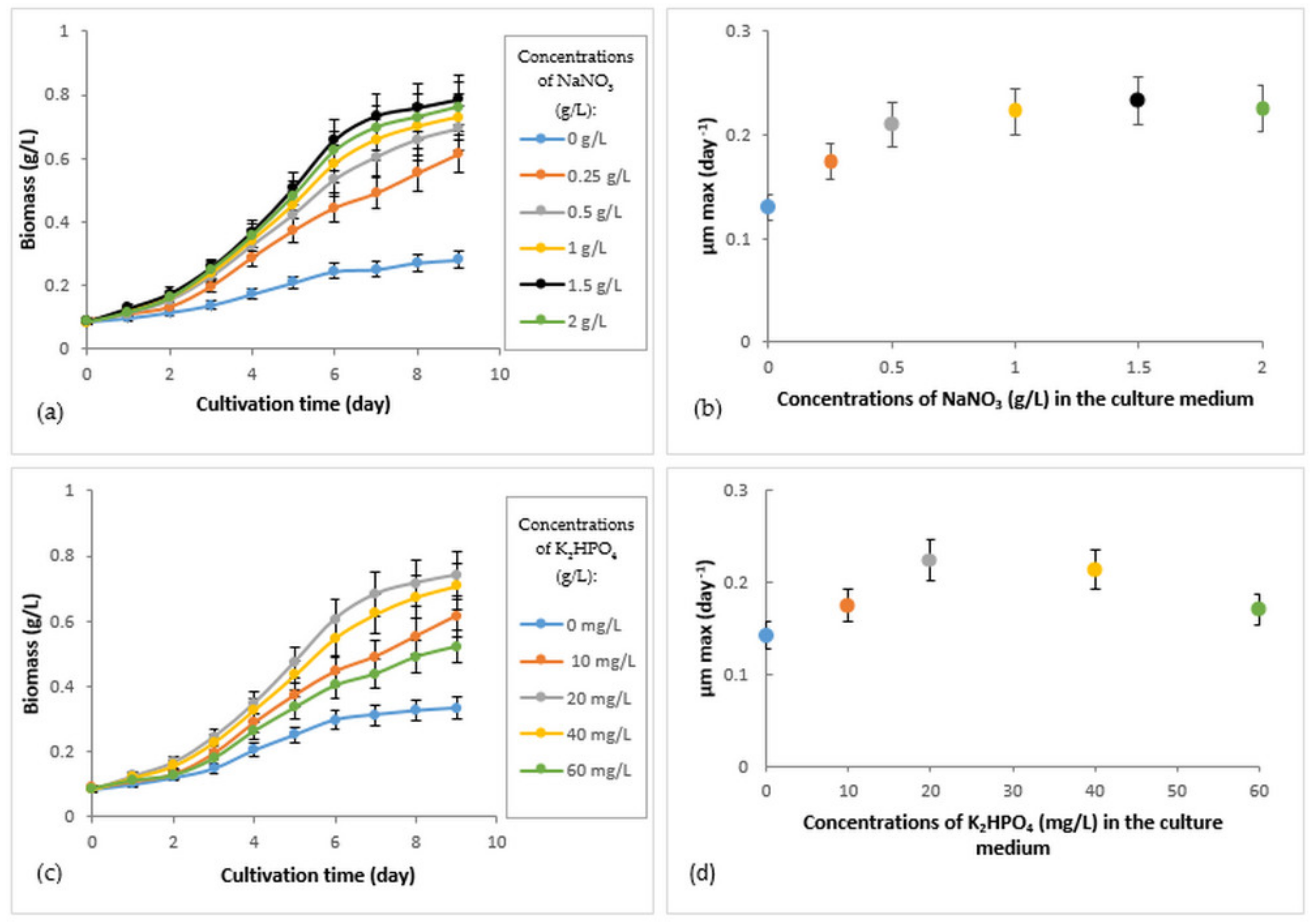
3.2. Growth of R. subcapitata Immobilized under Different Concentrations of Nitrogen and Phosphorus
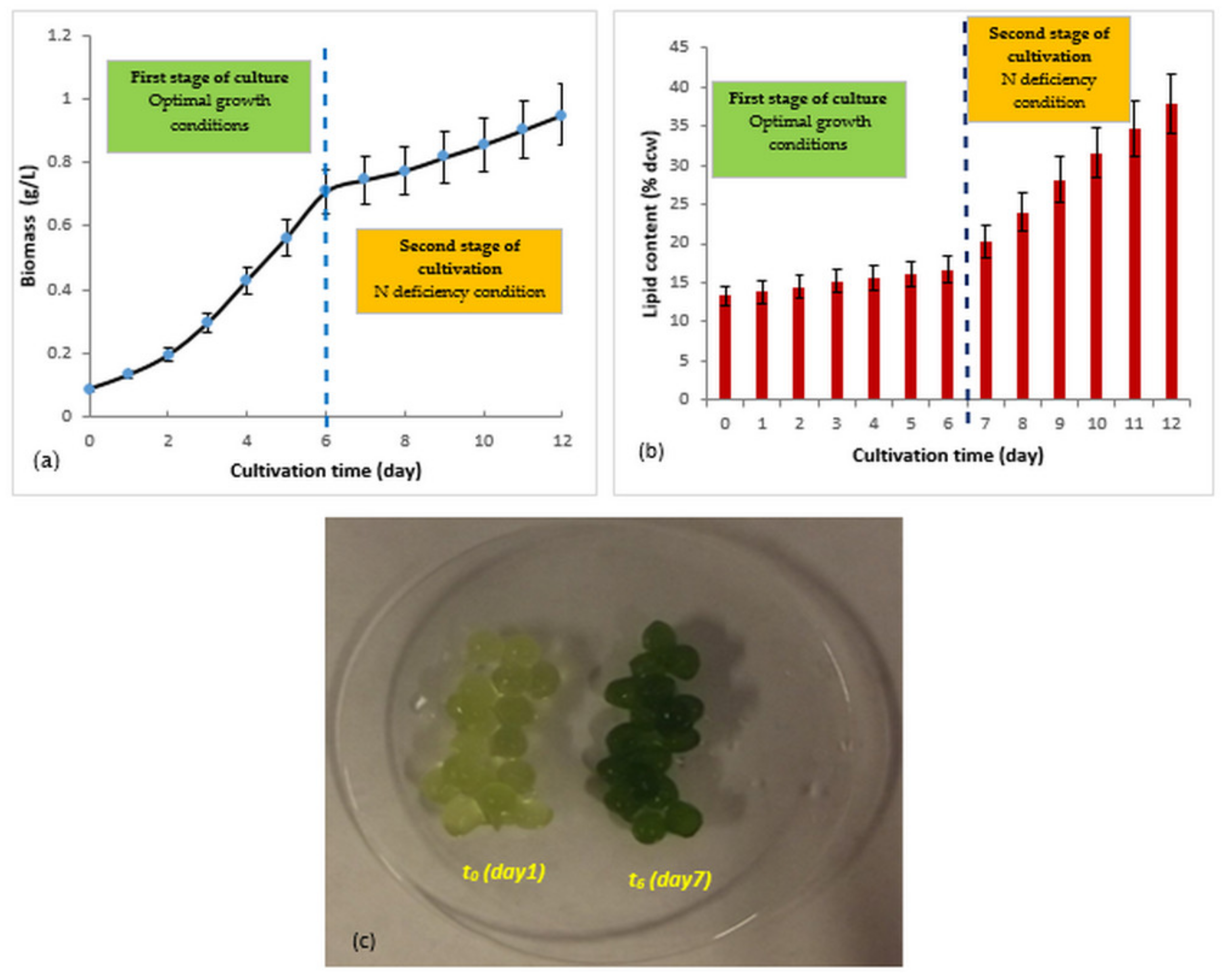
3.3. Two-Step Culture of Immobilized R. subcapitata
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bobin, J.-L.; Nifenecker, H.; Stéphan, C. 2. Les combustibles fossiles. In L’Énergie Dans le Monde; EDP Sciences: Les Ulis, France, 2021; pp. 21–36. [Google Scholar]
- Favennec, J.-P. L’avenir du pétrole. Available online: https://www.sciencespo.fr/ceri/sites/sciencespo.fr.ceri/files/art_jpf.pdf (accessed on 20 September 2021).
- Fritz, D. The Costs and Implications of Our Demand for Energy: A Comparative and Comprehensive Analysis of the Available Energy Resources. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3189719 (accessed on 20 September 2021).
- Le Treut, H. Changement climatique et gaz à effet de serre: Un problème ancien qui évolue de manière extrêmement rapide. Cites 2015, 63, 175–184. [Google Scholar] [CrossRef]
- Bonnefond, H.; Combe, C.; Cadoret, J.-P.; Sciandra, A.; Bernard, O. Potentiel des Microalgues; Lavoisier: Inria, France, 2020; Available online: https://hal.inria.fr/hal-02421830/document (accessed on 20 September 2021).
- Bougaran, G.; Saint-Jean, B. Microalgues: De petits végétaux aux grandes promesses! Biofutur 2014, 33, 28–31. [Google Scholar]
- Nafe Aziz, R.P.; Ibrahim, A.I.; Ahmed, A.I. Promising Applications for the Production of Biofuels Through Algae. Microb.Biotechnol. Appl. Agric. Environ. 2018, 1, 81–103. [Google Scholar]
- Suali, E.; Sarbatly, R. Conversion of microalgae to biofuel. Renew. Sustain. Energy Rev. 2012, 16, 4316–4342. [Google Scholar]
- Demirbas, A.; Demirbas, M.F. Algae Energy: Algae as a New Source of Biodiesel; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [PubMed]
- Doré-Deschênes, F. Utilisation des microalgues comme source d’énergie durable. Available online: https://savoirs.usherbrooke.ca/bitstream/handle/11143/7149/cufe_Deschenes_essai80.pdf?sequence=1 (accessed on 18 September 2021).
- Gouveia, L. Microalgae as a Feedstock for Biofuels. In Microalgae as a Feedstock for Biofuels; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–69. [Google Scholar]
- Khan, S.A.; Rashmi; Hussain, M.Z.; Prasad, S.; Banerjee, U.C. Prospects of biodiesel production from microalgae in India. Renew. Sustain. Energy Rev. 2009, 13, 2361–2372. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wu, N.; Lan, C.Q.; Dubois-Calero, N. Biocatalysts and bioreactor design. Biotechnol. Prog. 2008, 24, 815–820. [Google Scholar] [PubMed]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Cardozo, K.H.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P. Metabolites from algae with economical impact. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar]
- Jenck, J.; Lépine, O.; Legrand, J.; Dreno, P.; Grizeau, D.; Dupré, C. Valorisation industrielle des microalgues photosynthétiques. Available online: https://www.techniques-ingenieur.fr/base-documentaire/archives-th12/archives-plastiques-et-composites-tiaam/archive-1/valorisation-industrielle-des-microalgues-photosynthetiques-in201/perspectives-de-developpement-in201niv10003.html#3.1.1 (accessed on 17 September 2021).
- Taleb, A. Production de Biodiesel à Partir des Microalgues: Recherche des Souches Accumulatrices des Lipides et Optimisation des Conditions de Culture en Photobioréacteurs. Ph.D. Thesis, Angers Le Mans University, Nantes, France, 2015. [Google Scholar]
- Falkowski, P.G.; Raven, J.A. Aquatic Photosynthesis; Princeton University Press: Princeton, NJ, USA, 2013. [Google Scholar]
- Boileau, M.-È. Évaluation du potentiel d’utilisation d’une eau usée industrielle comme substrat de culture pour des microalgues d’eau douce dans une optique de production de biocarburants de 3e génération. Available online: https://savoirs.usherbrooke.ca/bitstream/handle/11143/6068/Boileau_Marie_Eve_MEnv_2015.pdf?sequence=3&isAllowed=y (accessed on 5 September 2021).
- Richardson, B.; Orcutt, D.; Schwertner, H.; Martinez, C.L.; Wickline, H.E. Effects of nitrogen limitation on the growth and composition of unicellular algae in continuous culture. Appl. Environ. Microbiol. 1969, 18, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Khozin-Goldberg, I.; Cohen, Z. The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus. Phytochemistry 2006, 67, 696–701. [Google Scholar] [CrossRef]
- Roopnarain, A.; Gray, V.; Sym, S. Phosphorus limitation and starvation effects on cell growth and lipid accumulation in Isochrysis galbana U4 for biodiesel production. Bioresour. Technol. 2014, 156, 408–411. [Google Scholar] [CrossRef]
- Mandal, S.; Mallick, N. Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl. Microbiol. Biotechnol. 2009, 84, 281–291. [Google Scholar]
- Xin, L.; Hong-Ying, H.; Ke, G.; Ying-Xue, S. Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour. Technol. 2010, 101, 5494–5500. [Google Scholar] [CrossRef]
- Huntley, M.E.; Redalje, D.G. CO2 mitigation and renewable oil from photosynthetic microbes: A new appraisal. Mitig. Adapt. Strateg. Glob. Chang. 2007, 12, 573–608. [Google Scholar]
- Feng, P.; Deng, Z.; Fan, L.; Hu, Z. Lipid accumulation and growth characteristics of Chlorella zofingiensis under different nitrate and phosphate concentrations. J. Biosci. Bioeng. 2012, 114, 405–410. [Google Scholar]
- Massart, A.; Aubry, É.; Hantson, A.-L. Étude de stratégies de culture de Dunaliella tertiolecta combinant haute densité cellulaire et accumulation de lipides en vue de produire du biodiesel. BASE 2010, 14, 567–572. [Google Scholar]
- Lee, J.-Y.; Yoo, C.; Jun, S.-Y.; Ahn, C.-Y.; Oh, H.-M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef] [PubMed]
- Sander, K.; Murthy, G.S. Life cycle analysis of algae biodiesel. Int. J. Life Cycle Assess. 2010, 15, 704–714. [Google Scholar] [CrossRef]
- Dulieu, C.; Poncelet, D.; Neufeld, R.J. Encapsulation and immobilization techniques. Birkhauser/Springer 1999, 1, 1–17. [Google Scholar]
- Lam, M.K.; Lee, K.T. Immobilization as a feasible method to simplify the separation of microalgae from water for biodiesel production. Chem. Eng. J. 2012, 191, 263–268. [Google Scholar] [CrossRef]
- Benasla, A.; Hausler, R. Growth and production of lipids in Raphidocelis subcapitata immobilized in sodium alginate beads. Energies 2020, 13, 506. [Google Scholar] [CrossRef] [Green Version]
- Environnement et changement climatique Canada. Méthode d’essai biologique: Essai d’inhibition de la croissance d’une algue d’eau douce. Available online: https://www.canada.ca/fr/environnement-changement-climatique/services/recherche-faune-science-paysage/publications-methodes-essai-biologique/inhibition-croissance-macroscopique-dulcicole-algue-eau-douce.html#toc23 (accessed on 10 September 2021).
- Santos, M.M.D.; Moreno-Garrido, I.; Gonçalves, F.; Soares, A.M.; Ribeiro, R. An in situ bioassay for estuarine environments using the microalga Phaeodactylum tricornutum. Environ. Toxicol. Chem. 2002, 21, 567–574. [Google Scholar] [CrossRef]
- Benasla, A.; Hausler, R. Optimisation of growth of Raphidocelis subcapitata immobilised for biofuel production: Influence of alginate and CaCl2 concentrations on growth. Environments 2018, 5, 60. [Google Scholar] [CrossRef] [Green Version]
- Voo, W.-P.; Lee, B.-B.; Idris, A.; Islam, A.; Tey, B.-T.; Chan, E.-S. Production of ultra-high concentration calcium alginate beads with prolonged dissolution profile. RSC Adv. 2015, 5, 36687–36695. [Google Scholar] [CrossRef]
- Del Río, E.; Armendáriz, A.; García-Gómez, E.; García-González, M.; Guerrero, M.G. Continuous culture methodology for the screening of microalgae for oil. J. Biotechnol. 2015, 195, 103–107. [Google Scholar] [CrossRef] [Green Version]
- Richmond, A. Handbook of Microalgal Culture: Biotechnology and Applied Phycology; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Clément-Larosière, B. Etude de la Croissance de Chlorella Vulgaris en Photobioréacteur Batch et Continu, en Présence de Concentrations Élevées de CO2. Ph.D. Thesis, Ecole centrale de Paris, Châtenay-Malabry, France, 2012. Available online: https://tel.archives-ouvertes.fr/tel-00697006/document (accessed on 19 September 2021).
- Gonçalves, A.L.; Pires, J.C.; Simões, M. Biotechnological potential of Synechocystis salina co-cultures with selected microalgae and cyanobacteria: Nutrients removal, biomass and lipid production. Bioresour. Technol. 2016, 200, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Van Vooren, G.; Le Grand, F.; Legrand, J.; Cuiné, S.; Peltier, G.; Pruvost, J. Investigation of fatty acids accumulation in Nannochloropsis oculata for biodiesel application. Bioresour. Technol. 2012, 124, 421–432. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.M.; Mumtaz, A.S.; Russell, M.; Grogger, M.; Veverka, D.; Hallenbeck, P.C. Isolation and Characterization of Microalgae from Diverse Pakistani Habitats: Exploring Third-Generation Biofuel Potential. Energies 2019, 12, 2660. [Google Scholar] [CrossRef] [Green Version]
- Shifrin, N.S.; Chisholm, S.W. Phytoplankton Lipids: Interspecific differences and effets of nitrate, silicate and light-dark cycles 1. J. Phycol. 1981, 17, 374–384. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Bhola, V.; Desikan, R.; Santosh, S.K.; Subburamu, K.; Sanniyasi, E.; Bux, F. Effects of parameters affecting biomass yield and thermal behaviour of Chlorella vulgaris. J. Biosci. Bioeng. 2011, 111, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Golueke, C.G.; Oswald, W.J.; Gee, H.K. Effect of nitrogen additives on algal yield. J. Water Pollut. Control. Fed. 1967, 39, 823–834. [Google Scholar]
- Go, S.; Lee, S.-J.; Jeong, G.-T.; Kim, S.-K. Factors affecting the growth and the oil accumulation of marine microalgae, Tetraselmis suecica. Bioprocess Biosyst. Eng. 2012, 35, 145–150. [Google Scholar] [CrossRef]
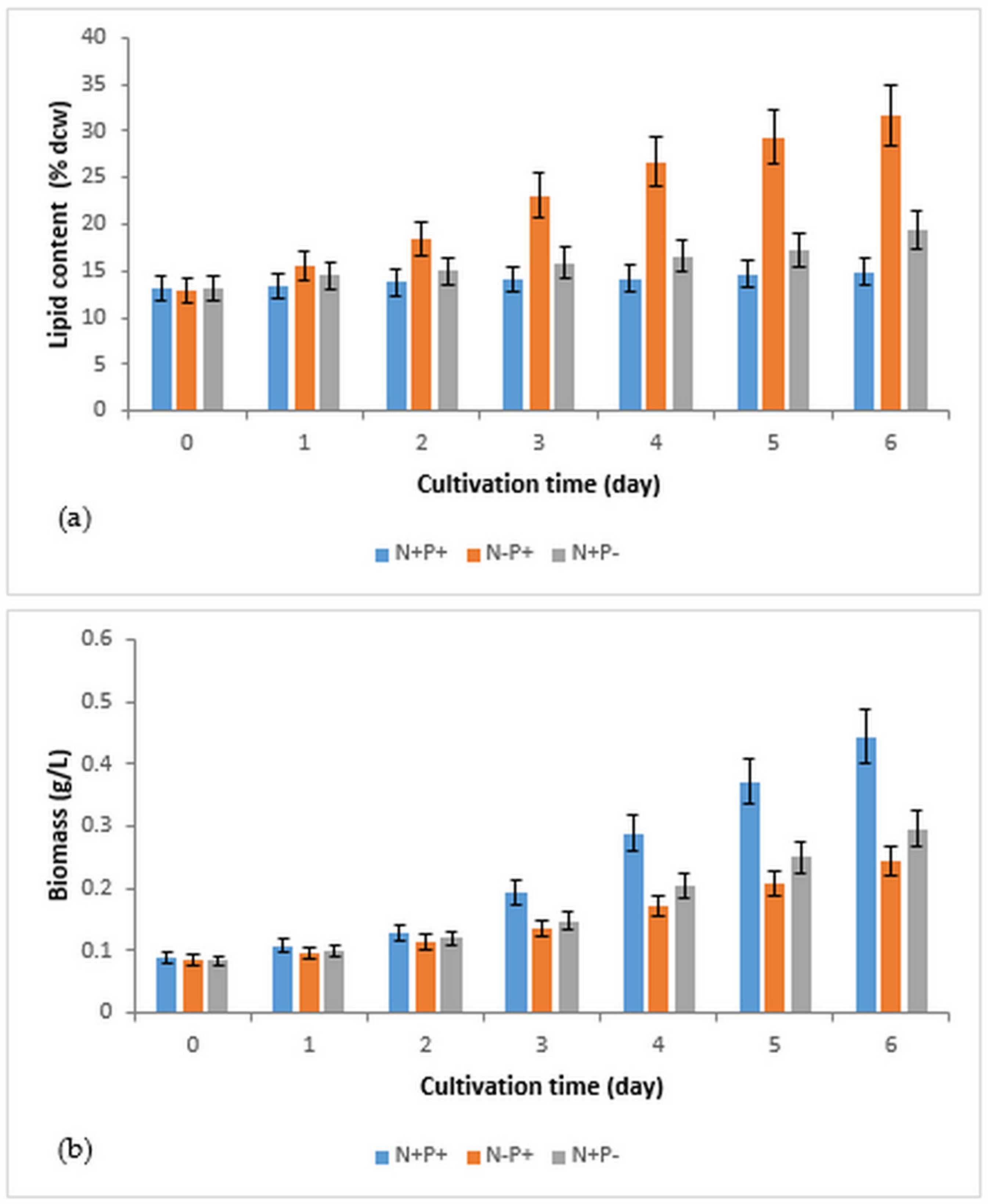
| Conditions of Culture | Lipid Content (% dcw) | Biomass (g/L) | Biomass Productivity (mg/L/day) | Lipid Productivity (mg/L/day) |
|---|---|---|---|---|
| N+P+ (control) | 14.9 ± 1.5 | 0.444 ± 0.044 | 59.3 ± 5.9 | 9.1 ± 0.9 |
| N−P+ | 31.7 ± 3.2 | 0.244 ± 0.024 | 26.5 ± 2.6 | 11.1 ± 1.1 |
| N+P− | 19.4 ± 1.9 | 0.296 ± 0.029 | 35.3 ± 3.5 | 7.7 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benasla, A.; Hausler, R. A Two-Step Cultivation Strategy for High Biomass Production and Lipid Accumulation of Raphidocelis subcapitata Immobilized in Alginate Gel. Biomass 2021, 1, 94-104. https://doi.org/10.3390/biomass1020007
Benasla A, Hausler R. A Two-Step Cultivation Strategy for High Biomass Production and Lipid Accumulation of Raphidocelis subcapitata Immobilized in Alginate Gel. Biomass. 2021; 1(2):94-104. https://doi.org/10.3390/biomass1020007
Chicago/Turabian StyleBenasla, Amel, and Robert Hausler. 2021. "A Two-Step Cultivation Strategy for High Biomass Production and Lipid Accumulation of Raphidocelis subcapitata Immobilized in Alginate Gel" Biomass 1, no. 2: 94-104. https://doi.org/10.3390/biomass1020007
APA StyleBenasla, A., & Hausler, R. (2021). A Two-Step Cultivation Strategy for High Biomass Production and Lipid Accumulation of Raphidocelis subcapitata Immobilized in Alginate Gel. Biomass, 1(2), 94-104. https://doi.org/10.3390/biomass1020007






