Abstract
Rare earth elements (REEs) possess unique physical and chemical properties that render them indispensable in various industries, including electronics, energy production and storage, hybrid and electric vehicles, metallurgy, and petro-chemical processing. The criticality of REE underscores the need to enhance the efficiency of primary resource extraction and promote circularity through increased recycling from secondary sources. This paper provides a brief overview of REE recovery from secondary sources, particularly waste from electronic and electric equipment (WEEE). The discussion encompasses direct reuse of magnets, short-loop recycling (direct recycling), hydro- and pyrometallurgical processes, highlighting microwave (MW) technology. Original results are presented, focusing on the recovery of neodymium (Nd) from permanent magnet scraps from hard disk drives (HDD-PC) using microwave-assisted liquid metal extraction (LME) with magnesium (Mg) as the extractant. The subsequent separation of Nd from the Mg-Nd alloy via vacuum Mg distillation that is reused in the process is described. The experimental study demonstrates that the LME process, conducted in a microwave furnace, is a viable method for recovering Nd from permanent magnet scraps, which are essential for reducing the environmental impact of REE extraction and promoting a circular economy. By separating Nd from the alloy through vacuum distillation (450–550 mmHg), at temperatures of 850–900 °C for 8 h, a Nd sponge with a content of 95–98 wt.% Nd was obtained. The extracted content of Nd in the Mg alloy increases with increasing temperature and holding time. It was found that ≈ 97% of the Nd in the scrap was extracted from 2 to 5 mm crushed scrap at 800 °C for 8 h, using a LiF-LiCl-MgF2 protecting flux in a furnace Ar atmosphere.
1. Introduction
Rare earth elements (REEs) refer to a group of elements in the periodic table, including scandium (Sc), yttrium (Y), and the lanthanides: lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), and europium (Eu). Scandium and yttrium are considered rare earth elements due to their consistent association with the lanthanides and similar chemical properties [1,2,3,4,5]. Due to their reactivity and chemical similarity, efficient separation processes for REEs were not developed until the 20th century [6]. The permanent magnet industry is heavily reliant on NdFeB magnets, which are the primary application of several REEs, including Nd, Dy, Pr, Gd, and Tb [3,4,5,6,7].
The global supply of certain REEs is expected to face challenges in the coming decades, particularly Dy, Nd, and Tb. In 2022, magnets were the top application for REEs, followed at a notable distance by catalysts and polishing powders (Figure 1). Demand for REEs (especially Pr, Nd, Tb, and Dy, the most used elements for magnet manufacturing) is projected to surge due to growth in industries like electric vehicles, wind turbines, and robotics [8,9,10].
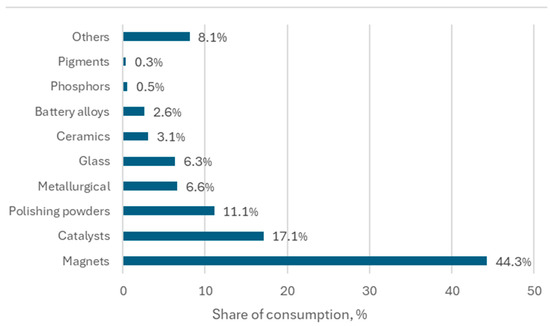
Figure 1.
Distribution of rare earth element consumption worldwide in 2022, by end use. Source: https://www.statista.com [9].
Researchers are exploring alternative solutions, including recovering REEs from end-of-life NdFeB magnets [3]. NdFeB magnets, developed in the 1980s by General Motors and Sumitomo Special Metals, are made from an Nd, Fe, and B alloy forming the Nd₂Fe₁₄B tetragonal crystalline structure [11,12]. They excel as permanent magnets due to their high magnetic moments, strong ferromagnetic bonds between Nd and Fe, and significant magneto-crystalline anisotropy [12,13]. With the highest energy product (BHmax) among permanent magnets (200–400 kJm−3 vs. 30–40 kJm−3 for ferrite magnets), NdFeB magnets contain 27–31 wt.% REEs, including Nd, Pr, Dy, Tb, Gd, or Ho, and may include additives like Co, Zr, Mo, or Nb, depending on their application [14,15,16].
Minerals, metals, and materials are essential for modern technologies, with global demand rising as new technologies emerge. The 2023 criticality assessment highlights REEs as critical elements due to their economic importance to the EU and supply risks [17]. The circular economy promotes economic growth while protecting the environment by reducing waste and resource consumption through the 3Rs: reduce, reuse, and recycle. Unlike the linear economy, it treats waste as a resource, including coal combustion products (fly ash, bottom ash, incinerator ash), industrial byproducts (slags, dross, phosphogypsum, red mud), and electronic waste (batteries, hard drives, cell phones, speakers) [18]. Industries relying on CRMs can benefit from circular practices, such as increasing secondary material use and reducing raw material dependence, thus lowering environmental impact and waste [19,20]. Recycling offers significant benefits over processing natural ores and concentrates, mainly due to its energy efficiency and selectivity. NdFeB magnets are particularly valuable as a secondary resource, as they contain a high percentage (around 20%) of REEs, including Nd, Dy, and smaller amounts of other REEs like Pr. Notably, 20–25% of the world’s REE production is used to manufacture NdFeB magnets [21]. Recycling NdFeB magnets is a promising solution to address the global REE supply gap. These magnets contain a significant amount of REEs, making them a valuable secondary resource. Recycling can reduce dependence on primary production, mitigate supply risks, and offer energy efficiency and selectivity benefits. Overall, REE recycling is challenging, but necessary. Scientists struggle with small amounts in end-products and difficulties in collection, extraction, and recovery. There are three types of recycling: direct recycling of scrap/residues, urban mining of end-of-life products, and recycling of industrial wastes [22]. Until 2016, only around 1% of the REEs was recycled from scrap [23]. In 2019, the Platform For Accelerating The Circular Economy (PACE) and the E-waste Coalition reported that 50 million tonnes of e-waste are produced annually, with only 20% recycled. Without action, this could rise to 120 million tonnes by 2050 [24].
Designing electronic devices from a recyclability perspective and developing efficient methods for recovering REEs from electronic waste are crucial steps towards sustainability and circular economy. In his 2023 book chapter, Hassan states that there are several key methods for REE recycling as shown in Table 1 [25]. By adopting one of these methods or a mix of them, we can reduce the need for primary REE production, minimize environmental impacts, and promote a more circular economy [25]. Blet et al. [25] described a novel combined hydro- and pyrometallurgical process to recover REE and many other elements from spent NdFeB magnets (Dy, Nd, Pr) and NiMH batteries (La, Dy). The process was recently developed by the French Alternative Energies and Atomic Energy Commission (France) and has three major steps: a step for physico-chemical treatment of magnets, a solvent extraction step (for recovery and REE separation) using a selective extractant and a third step (molten chloride salt electrolysis) to isolate pure Dy metal. In 2023, He et al. published an article in which they reviewed recent development and challenges of the combined process in recycling spent LIBs [26]. Also in 2023, Holzer et al. proposed a three-stage combined process flow: a hydromechanical process (to open and disintegrate the cells), a flotation process, and a pyrometallurgical treatment [27]. Xiao et al. combined electrodeposition with high-temperature crystallization to recover REEs and iron from NdFeB waste [16].

Table 1.
REE recycling methods [28,29,30,31].
In their 2017 review, Yang et al. [31] highlighted several technologies with varying levels of readiness for rare earth element (REE) recycling: hydrogen decrepitation, chemical vapor transport, liquid metal extraction, hydrometallurgical processing and pyrometallurgical slag. Most of these methods are mainly designed for processing pre-consumer scrap, which is generally clean, consistent, and rich in rare earth elements (REEs), unlike post-consumer scrap. As of 2023, the most challenging aspect of recycling small REE magnets, like almost any end-of-life (EoL) products, is preprocessing, which involves classification and dismantling. NdFeB magnets, depending on their condition, can be reused or recycled through methods like hydrogen decrepitation, waste-to-REE, waste-to-alloy, or magnet-to-magnet (MtM) approaches. Magnets’ properties such as heterogenity, oxidation, brittleness, and magnetic forces complicate extraction, while dismantling and adhesive removal techniques significantly impact efficiency. Proper preprocessing is essential to reduce contamination [31,32]. To this end, automated options have been proposed for the fastest possible disassembly and with the lowest possible degree of contamination. Burkhardt et al. [33], Ormerod et al. [34], Simon et al. [35], and Li et al. [36] wrote comprehensive articles regarding automated or semi-automated disassembly systems for preprocessing EoL products with magnet content. Projects such as SUSMAGPRO [37] and VALOMAG [34,38] lay the groundwork for understanding the requirements in developing automated systems for the disassembly and recycling of magnet-containing equipment. Waste Management, Inc, Umicore, H.C. Starck, and DuPont are some of the major companies that deal with WEEE recycling to recover the elements of interest in a way that is as sustainable as possible from an ecological and financial point of view. In 2016, Oak Ridge National Laboratory (ORNL), managed by UT-Battelle for DOE’s Office of Science, partnered with Momentum Technologies to develop a process for recovering rare earth magnets from spent hard disk drives (HDDs) [39]. More literary reviews can, for example, be found in Hansjosten et al.’s 2023 article [40].
Recycling is hindered by economic factors, as it remains cheaper to purchase REE master alloys or newly produced magnets derived from primary resources than from recycled ones [31,41]. To overcome the disadvantages of conventional melting, advanced melting technologies such as microwave melting, dielectric melting, infrared melting, electron beam melting, plasma melting, etc., have been developed during the last decades, for specific requirements and applications (special alloys, high purity/quality, low batches, etc.) [42].
Microwaves are electromagnetic waves (1–1000 mm) that convert wave energy into heat upon interacting with materials. Microwave processing, influenced by a material’s dielectric and magnetic properties, has been used for synthesis, drying, sintering, and advanced material processing over the past 65 years. Mishra et al. classify it into three categories: low-temperature (<500 °C) for food and polymers, moderate-temperature (500–1000 °C) for ceramics and powders, and high-temperature (>1000 °C) for dense ceramics and composites [43]. Unlike conventional heating, which transfers heat externally, microwave heating generates rapid and uniform heat throughout a material’s volume (Figure 2) [43,44].
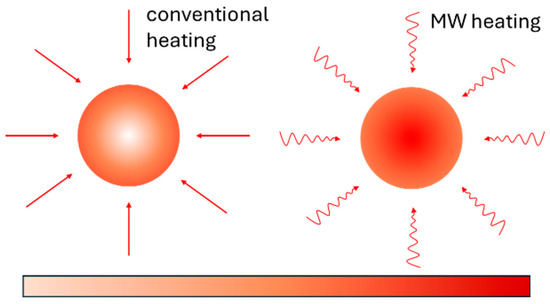
Figure 2.
Microwave heating (MW) compared to standard heating. Source: Materials and Manufacturing Processes, 2015, 30, 1–29, [44].
Microwave heating has gained attention for its many advantages, including selective, contactless heating, rapid heating rates, reduced processing times and energy consumption, smaller equipment size, and lower environmental impact. It also enhances diffusion processes and reduces sintering temperatures, improving material properties beyond what conventional methods achieve [45,46].
Microwave heating shows promise in recovering rare earth elements (REEs) from NdFeB magnets by selectively breaking down their structure, often with chemical agents. Microwave-assisted reduction uses carbon-based agents to separate metallic iron from rare earth oxides, offering greater energy efficiency and environmental benefits. It can also melt or decompose NdFeB for recycling, preserving magnetic properties, or oxidize NdFeB to extract REEs via hydrometallurgical methods [45,47,48].
Liquid metal extraction (LME) was developed to overcome the limitations of hydrometallurgical and electroslag refining methods, offering a versatile process for separating transition metals like Fe, Co, and Ni from various waste types with minimal byproducts. The LME process operates similarly to conventional liquid–liquid solvent extraction, but replaces the liquid solvent with a liquid metal solvent. The process uses a liquid metal solvent, such as magnesium or silver, to selectively dissolve rare earth elements (REEs) and form a Me-REE alloy. For neodymium recovery from Nd-Fe-B magnets, crushed magnets are immersed in molten Mg at a moderate temperature, where Nd dissolves, and Fe-B solid particles are separated [49]; Nd is then recovered after Mg distillation. This extraction process gives magnesium the ability to be reused indefinitely, and the remaining Fe-B is recycled. This pair of methods reduces waste and recycles Mg, but requires quite high temperatures, is energy-intensive, and takes 24–72 h to complete. Also, it is ineffective for oxidized NdFeB waste; for this, silver can be used as an extractant. Despite these drawbacks, LME offers a more sustainable alternative to traditional extraction methods [50,51,52].
Rasheed et al. [53] studied the extracting agents used for liquid metal extraction of REE and concluded that magnesium is the only extraction agent with which pure Nd and Dy metals have been recovered.
The literature regarding the use of LME for the recovery of REE from waste containing rare metals is quite succinct. However, the results of these studies are promising (over 90% Nd recovery). Xu et al. [49] were the first to report that during the recovery of Nd from NdFeB magnets using LME, Mg reacts with Nd, but not with the other two elements. Also, Mg has several other characteristics that recommend it as a good extracting agent for neodymium: weak chemical affinity with iron; easy elimination and transportation of Mg through the gas phase (high vapor pressure over 800 °C–0.045 atm at 800 °C); the ability of being recirculated (its melting point is 922 K (649 °C) and recovered and reused by condensing as a pure solid. The research conducted by Chae et al. and Kim et al. further extended the knowledge on LME of REE from NdFeB.; Okabe et al. and Takeda et al. demonstrated this process on a lab scale [54,55,56].
For example, in comparison to NdFeB waste, NiMH battery recycling has already reached maturity. It is necessary that the magnet recycling field also reaches that milestone [22]. First, it must be efficient in terms of yield, inexpensive, and, at the same time, environment-friendly.
Recycling waste, in this case Nd-based permanent magnets, is critical for reducing environmental impact and securing a sustainable supply of the valuable materials. Nd is present in HDD magnets in significant concentrations, but the other REEs should also be recovered if possible. Nam et al. experimented with extraction of REEs by LME in an induction furnace [56]. They discovered that the Dy2Fe17 phase significantly hinders dysprosium (Dy) extraction from Nd-Dy-Fe-B magnets during liquid magnesium (Mg) processing. Initially, Dy extraction is dominated by the decomposition of the Dy2Fe17 phase (3–24 h), followed by the reaction of Dy2O3 with Mg. Despite these obstacles, a high Dy extraction rate of 93% was achieved after 48 h. The study highlights that controlling the Dy2Fe17 phase is crucial for improving Dy recycling efficiency [56].
As demand for rare earth elements increases, effective recycling methods are essential to reduce dependence on mining and minimize environmental damage. The complexity of magnet composition presents challenges, but developing energy-efficient, cost-effective, and scalable recycling processes is key to supporting a circular economy and meeting the growing demand for technologies like electric vehicles and renewable energy systems.
In the present work, a high-throughput recycling route is proposed, employing microwave technology in liquid metal extraction, with magnesium as the extracting agent, followed by vacuum distillation to further separate rare earth elements (REEs) from magnesium–REE alloys. Several experiments were performed to study the influence of the process parameters over the efficiency of the process.
2. Materials and Methods
The hard disk drives (HDDs) used in the experiments were sourced from various types of desktop PCs and notebooks. These drives were from different manufacturers, countries, and production years, and had varying storage capacities. The magnets used in this experiment were from the voice coil motor (VCM). The amount of magnets collected and used in this stage was approx. 3.8 kg.
Quantities used in the experiments were measured with an analytical balance. Total metal content in the REE magnet waste was determined by ICP Spectrometry (SPECTROFLAME—ICP model P produced by SPECTRO—Analytical Instruments (Kleve, Germany)). Results of the chemical characterization of permanent magnet samples are presented in Table 2.

Table 2.
Chemical analysis of the permanent magnet samples used in the experiments [wt.%].
An electric furnace (Vulcan, Romania) was used to demagnetize the EoL magnets and a specially designed microwave heated furnace was used for LME and Mg distillation. The design of the MW furnace, used for LME and Mg distillation, is shown in Figure 3. The furnace has 5 magnetrons and the thermal filter (for capturing and neutralizing toxic gases) has 3 magnetrons; each one of them has a power of 850 W. The maximum melt temperature of the MW furnace is 1400 °C. Mg is recovered with a distillation vertical retort (Figure 4).
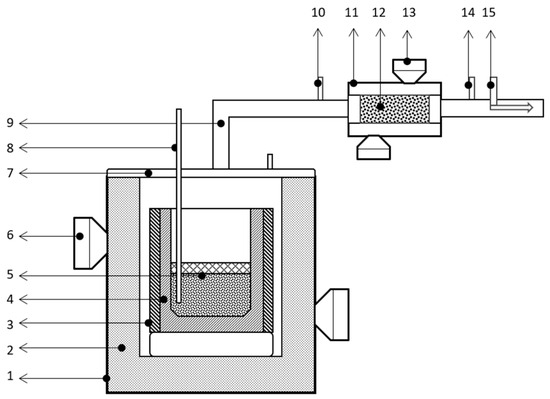
Figure 3.
MW laboratory furnace. 1. Furnace shell (steel); 2. insulating material (alumina); 3. MW susceptor material (SiC); 4. melting crucible (graphite, SiC, BN); 5. batch; 6. MW furnace magnetrons/generators; 7. furnace lid (steel); 8. thermocouple (K or S type); 9. gases/NOx exhaust pipe (flexible, steel); 10,14. gases/NOx capture nozzles; 11. thermal filter for tartar/neutralization of gases; 12. MW susceptor material (SiC balls/foam); 13. MW magnetrons thermal filter; 15. Venturi tube.
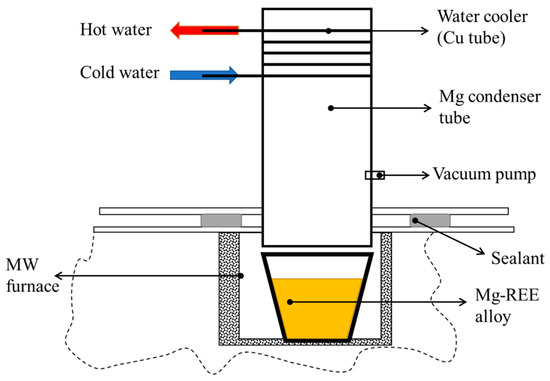
Figure 4.
Mg distiller apparatus set-up for the MW furnace.
All HDDs were manually dismantled (Figure 5), a critical step for characterizing end-of-life (EoL) devices to gather information about their structure and to separate components for chemical analysis, as in a 2020 article [57].
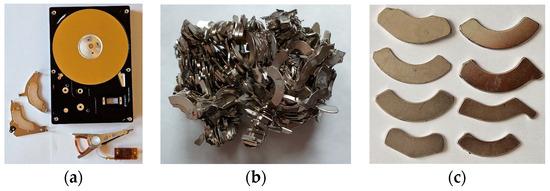
Figure 5.
(a) Dismantled HDD; (b,c) Permanent magnets from VCM, before and after demagnetization.
Our experiment’s flowsheet is presented in Figure 6 and has three main steps: the pretreatment of magnets (demagnetization, removal of protective film and crushing), the liquid metal extraction using Mg as an extractant, and the Mg distillation (Figure 6).
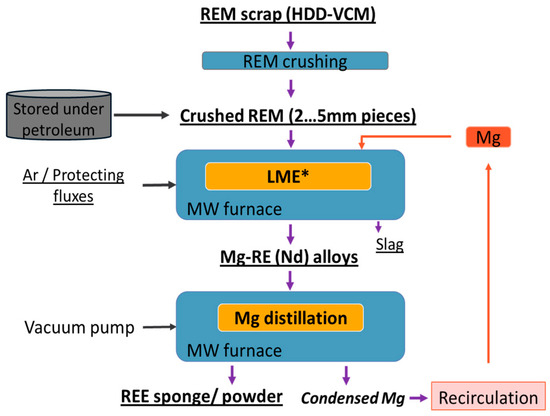
Figure 6.
Flowsheet for recovery of Nd from HDD magnets.
2.1. Pretreatment of Magnets
The sintered magnets were demagnetized in an electric furnace at a temperature of 350 °C for 15 min. Then, the protective film (Ni, Ni alloy or stainless steel) was removed from the HDD magnets by scraping. After this, they were hand-shredded into pieces of approx. 2–5 mm. These operations were performed under a film of gas lamp to prevent oxidation. A gas lamp was also used for storing magnets.
2.2. MW LME
Molten Mg was used as the rare earth metal extraction agent for LME. Magnesium has many advantages as an extractant: melting point of 922 K (649 °C); great solubility of Nd in molten Mg compared to Fe and B (solubility for Nd is 68 at% and for Fe is 0.035 at%); high vapor pressure of over 1073 K (0.045 atm, 1073 K/800 °C); ability to be recovered and reused by condensing as a pure solid; and effortless elimination and transportation of Mg through the gas phase [50,51,54,55,56].
The chemical analysis of Mg used in selective REE extraction is presented in Table 3.

Table 3.
Magnesium chemical analysis.
The chemical composition of the protective fluxes used to protect the metal bath during the REE smelting–extraction experiments is presented in Table 4. Different flux compositions were used to avoid high viscosity and/or high vaporization pressures. The most suitable flux compositions were selected depending on the temperature for the best protection of the metal bath. It was observed that F3 flux (CaF2, AlF3, B2O3, MgF2) begins to evaporate at 900 °C; this means it is suitable only for experiments with lower temperature. A good protection of the metal bath was found to be a mixture of salts (LiCl, LiF) in combination with a stream of dry and purified inert gas (Ar—0.5 to 1 L/min.). As an additional protection, inert gas (Ar) was used [58].

Table 4.
The composition of the protective fluxes used in the experimental works [wt.%].
2.3. Distillation of Mg-REE Ingots
The separation of REE from obtained Mg-REE alloys was performed by the vacuum Mg distillation process. This method can be used to recover REEs in metallic form and reduce energy usage [59].
The significant difference in vapor pressure between magnesium and neodymium (Mg—0.73 atm, Nd—10−5 atm, 1300 K) is crucial for their separation using vacuum distillation. Magnesium’s high vapor pressure means it transitions into the gas phase at relatively low pressures and elevated temperatures, while neodymium, with its low vapor pressure, remains in the solid or liquid phase under the same conditions. In this case, vacuum distillation has many benefits: selective evaporation (Mg vaporizes while Nd remains behind, allowing for their physical separation), energy efficiency (the selective removal of Mg reduces the need for more energy-intensive chemical or reduction processes), purity (the method ensures minimal contamination since Mg is effectively isolated as a vapor, leaving Nd in its metallic state).
The parameters for vacuum distillation were chosen based on previous experiments. The experiments lasted 12 h each; the crucible charge was 1 kg of Mg-REE alloy, the temperature was set to 900 °C, and the pressure to 450 mmHg.
3. Results and Discussion
The chemical composition of the samples is presented in Table 1. Their chemical composition is not uniform and varies within a fair range: Fe (50–65.1%), Nd (22.5–26.5%), Pr (3.3–4.8%), Dy (0.71–1.32%), Ni (1.26–1.93%), Co (0.1–2.09%) and B (0.89–1.03%).
Microwave heating enhances REE extraction by targeting materials for faster, energy-efficient processing. Liquid metal extraction, using magnesium’s low melting point and strong REE affinity, reduces energy use, speeds processing, and efficiently recycles complex NdFeB magnets. The effects of Mg/REE ratio and melt temperature on the extraction behavior of the Nd from Nd-Fe-B magnet scraps were investigated through several experiments. The parameters and the resulting REE composition are shown in Table 5 and Figure 7.

Table 5.
Experimental conditions and results for microwave LME of REE.

Figure 7.
Mg-REE alloy ingot samples.
The other rare metals are present in low concentrations in our samples as can be observed in Table 6: Pr, 3.3–4.8%, Dy, 0.71–1.32%, Ce, 0.06–0.12%. The rest of the REEs occur in concentrations less than 0.1%; all of these have been formed in the slag.

Table 6.
Chemical composition of Mg-Nd representative samples obtained through microwave LME.

Table 7.
Chemical composition of recovered Nd from Mg distillation.
A wide fluctuation in the Nd content, ranging from 6.9 to 43.2 wt.%, was observed in the experimental samples (Table 7 and Figure 8). The extracted content of Nd increased with the Mg/REE ratio and melting temperature. The higher the temperature, the more the purity of the recovered neodymium increases towards 99 wt.%. The values for Nd content almost doubled with just 50 °C degrees increase in process temperature. Both temperature intervals determined remarkably high differences in the extraction efficiency, so it can be affirmed that process temperature is the main factor in deciding the recovery capacity of REEs by the LME method.

Figure 8.
Raw Nd sponge.
The Mg/REE ratio signifies the content of REEs added to the melt. It is observed that a lower ratio at lower process temperature has negligible effect on the efficiency. Once the temperature is raised over 800 °C, the recovery level varies with several percentage differences on the initial Mg/REE ratio. At 800 °C, when the Mg/REE ratio decreases from 0.5 to 0.25, the Nd percentage in the ingot increases with almost 9 wt.%. The same significant effect is met at 900 °C, when the difference is of about 6 wt.% (Figure 9a). The improved content of Nd at higher temperatures could be justified by the increased element diffusion in the melt. However, the highest Pr content was extracted at a lower Mg/REE ratio and lower temperature. The solubility limit of Pr in Mg is significantly lower so the increase in temperature does not have the same effect as for Nd.
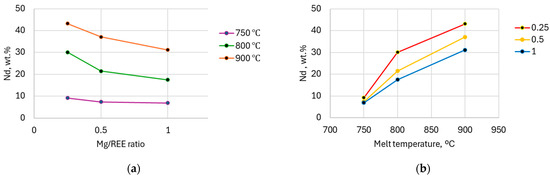
Figure 9.
The variation in Nd concentration in recovered Nd-based sponge with varied parameters: (a) Mg/REE ratio; (b) melting temperature.
From the point of view of temperature variation, it can be observed that with increasing temperature, the percentage of Nd in the Nd-based sponge also increases (Figure 9b).
It was found that ≈97% of the Nd in the scrap was extracted from 2 to 5 mm crushed scrap at 800 °C for 8 h using a LiF-LiCl-MgF2 protecting flux in a furnace Ar atmosphere.
Vacuum distillation efficiently separates REEs from magnesium alloys by capitalizing on differences in vapor pressures, facilitating high-purity recovery while reducing energy consumption. The distillation process removed most of the impurities from the alloy while obtaining high Nd concentrations. Alloy ingot samples with high Nd content were selected for the distillation process: samples no. 4 to no. 9 from Table 6. The results of the chemical analysis of recovered neodymium sponge samples (Figure 8) are outlined in Table 7. The distillation efficiency is found in the conventional limits. A high-grade product is obtained which shows a great interest for the direct extraction of REE from recycled permanent magnets.
It should be mentioned that there were temperature and pressure variations, considering that the installation is a prototype mounted on the microwave furnace. Therefore, the temperature and pressure could not be fully controlled. However, the aim of the project is to test the viability of the process of recycling HDD magnets in a microwave field.
We also conducted an LCA study on this process flow, and we compared the results with others. Table 8 is a snippet of the LCA study poster we presented at an international conference [60]. In the compared article, Karal et al. [61] conducted and then analyzed a process to recover Nd from NdFeB magnets through pretreatment, chemical leaching and Nd metal precipitation; their conclusion was that economically and environmentally speaking, recovery of Nd “gained more positive points” than raw magnet production. Our conclusion is that our Nd recovery route has even more “positive points”. Also, for better use of resources and lower negative impacts, renewable resources and capturing and neutralizing the heat treatment gases are to be considered.

Table 8.
Comparison of impact categories between Karal et al.’s study (raw and recovered Nd) [61] and ours [60].
This research demonstrates that liquid metal extraction process, conducted in an MW furnace and followed by vacuum distillation, could be a viable and inexpensive method for recovery of the expensive rare earth element Nd from NdFeB permanent magnets scraps.
The novelty in this process flow is combining the liquid metal extraction with microwave technology. Works are in progress to demonstrate that the resulting material is suitable for reuse in new NdFeB magnets.
Author Contributions
Conceptualization, R.-R.P.; methodology: S.A.F. and M.B.; validation: R.-R.P. and M.B.; formal analysis, R.-R.P. and M.B.; investigation, S.A.F., M.B., I.C.B. and L.L.; resources, R.-R.P.; writing—original draft preparation, S.A.F., M.B. and I.C.B.; writing—review and editing, S.A.F., R.-R.P. and M.B.; supervision, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in the frame of the COFUND-ERAMIN-3-MW4REMAM-2 Contract 309/2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank Olivier Jay, project director from FADDTORY sprl Belgium, Sorin Mircea Axinte from Daily Sources and Resources SRL and Dumitru Mitrica from National Institute of Non-Ferrous and Rare Metals.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Binnemans, K.; McGuiness, P.P.; Jones, P.T. Rare-earth recycling needs market intervention. Nat. Rev. Mater. 2021, 6, 459–461. [Google Scholar] [CrossRef]
- Habibzadeh, A.; Kucuker, M.A.; Gökelma, M. Review on the Parameters of Recycling NdFeB Magnets via a Hydrogenation Process. ACS Omega 2023, 8, 17431–17445. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.K. Acquaintance. In Chemical Metallurgy: Principles and Practice; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; pp. 4–7. ISBN 3-527-30376-6. [Google Scholar]
- Hower, J.C.; Granite, E.J.; Mayfield, D.B.; Lewis, A.S.; Finkelman, R.B. Notes on Contributions to the Science of Rare Earth Element Enrichment in Coal and Coal Combustion Byproducts. Minerals 2016, 6, 32. [Google Scholar] [CrossRef]
- El-Taher, A. Rare earth elements content in geological samples from eastern desert, Egypt, determined by instrumental neutron activation analysis. Appl. Radiat. Isot. 2010, 68, 1859–1863. [Google Scholar] [CrossRef]
- Dutta, T.; Kim, K.-H.; Uchimiya, M.; Kwon, E.E.; Jeon, B.-H.; Deep, A.; Yun, S.-T. Global demand for rare earth resources and strategies for green mining. Environ. Res 2016, 150, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-L.; Fan, H.-R.; Liu, X.; Meng, J.; Butcher, A.R.; Yann, L.; Yang, K.-F.; Li, X.-C. Global rare earth elements projects: New developments and supply chains. Ore Geol. Rev. 2023, 157, 105428. [Google Scholar] [CrossRef]
- Distribution of Rare Earth Element Consumption Worldwide in 2022, by End Use. Available online: https://www.statista.com/statistics/604190/distribution-of-rare-earth-element-consumption-worldwide-by-end-use/ (accessed on 30 November 2024).
- International Energy Agency (IEA), Global Critical Minerals Outlook 2024. Available online: https://www.iea.org/reports/global-critical-minerals-outlook-2024 (accessed on 6 December 2024).
- Britannica. Available online: https://www.britannica.com/science/magnet (accessed on 22 August 2024).
- Lucas, J.; Lucas, P.; Le Mercier, T.; Rollat, A.; Davenport, W. Rare Earths: Science, Technology, Production and Use, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780444627445. [Google Scholar] [CrossRef]
- King, A.H.; Eggert, R.G. Chapter 10—Critical materials for permanent magnets. In Modern Permanent Magnets; Electronic and Optical Materials, Woodhead Publishing: Cambridge, MA, USA, 2022; pp. 343–370. [Google Scholar] [CrossRef]
- Herbst, J.F.; Croat, J.J.; Pinkerton, F.E.; Yelon, W.B. Relationships between crystal structure and magnetic properties in Nd2Fe14B. Phys. Rev. B 1984, 29, 4176–4178. [Google Scholar] [CrossRef]
- Sagawa, M.; Fujimura, S.; Togawa, N.; Yamamoto, H.; Matsuura, Y. New material for permanent magnets on a base of Nd and Fe. J. Appl. Phys. 1984, 55, 2083–2087. [Google Scholar] [CrossRef]
- Xiao, F.; Hu, W.; Zhang, Z.; Li, B.; Zhu, H. Recovery of neodymium and iron from NdFeB waste by combined electrochemical–hydrometallurgical process. Sep. Purif. Technol. 2025, 353, 128301. [Google Scholar] [CrossRef]
- European Commission: Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs. Grohol, M.; Veeh, C. In Study on the Critical Raw Materials for the EU 2023—Final Report; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar] [CrossRef]
- Gaustad, G.; Williams, E.; Leader, A. Rare earth metals from secondary sources: Review of potential supply from waste and byproducts. Resour. Conserv. Recycl. 2021, 167, 105213. [Google Scholar] [CrossRef]
- Shahzabeen, A.; Ghosh, A.; Pandey, B.; Shekhar, S. Circular Economy and Sustainable Production and Consumption. In Green Circular Economy; Singh, P., Yadav, A., Chowdhury, I., Singh, R.P., Eds.; Springer: Cham, Switzerland, 2023; Volume 6, pp. 43–65. [Google Scholar] [CrossRef]
- Bassi, F.; Dias, J.G. The use of circular economy practices in SMEs across the EU. RCR Adv. 2019, 146, 523–533. [Google Scholar] [CrossRef]
- Kaya, E.E.; Kay, O.; Stopic, S.; Gürmen, S.; Friedrich, B. NdFeB Magnets Recycling Process: An Alternative Method to Produce Mixed Rare Earth Oxide from Scrap NdFeB Magnets. Metals 2021, 11, 716. [Google Scholar] [CrossRef]
- Danczak, A.; Chojnacka, I.; Matuska, S.; Marcola, K.; Leśniewicz, A.; Wełna, M.; Żak, A.; Adamski, Z.; Rycerz, L. The recycling-oriented material characterization of hard disk drives with special emphasis on NdFeB magnets. Phys Chem Miner 2018, 54, 363–376. [Google Scholar] [CrossRef]
- Jowitt, S.M.; Werner, T.T.; Weng, Z.; Mudd, G.M. Recycling of the rare earth elements. Curr. Opin. Green Sustain. Chem. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- World Economic Forum. A New Circular Vision for Electronics Time for a Global Reboot. Available online: https://www.weforum.org/publications/a-new-circular-vision-for-electronics-time-for-a-global-reboot/ (accessed on 6 December 2024).
- Blet, V.; Andreiadis, E.; Serp, J.; Miguirditchian, M. Innovative Coupled Hydrometallurgical and Pyrochemical Processes for Rare Earth Recycling. Extraction 2018, 2647–2658. [Google Scholar] [CrossRef]
- He, M.; Jin, X.; Zhang, X.; Duan, X.; Zhang, P.; Teng, L.; Liu, Q.; Liu, W. Combined pyro-hydrometallurgical technology for recovering valuable metal elements from spent lithium-ion batteries: A review of recent developments. Green Chem. 2023, 25, 6561–6580. [Google Scholar] [CrossRef]
- Holzer, A.; Zimmermann, J.; Wiszniewski, L.; Necke, T.; Gatschlhofer, C.; Öfner, W.; Raupenstrauch, H. A Combined Hydro-Mechanical and Pyrometallurgical Recycling Approach to Recover Valuable Metals from Lithium-Ion Batteries Avoiding Lithium Slagging. Batteries 2023, 9, 15. [Google Scholar] [CrossRef]
- Hassan, B. Environmental Impact of Modern Permanent Magnets. In Modern Permanent Magnets—Fundamentals and Applications; Dipti Ranjan Sahu; IntechOpen: Rijeka, Croatia, 2023; pp. 10–20. [Google Scholar] [CrossRef]
- Burkhardt, C.; van Nielenb, S.; Awaisc, M.; Bartolozzid, F.; Blomgrene, J.; Ortizf, P.; Xicotencatlb, M.B.; Degric, M.; Nayebossadric, S.; Walton, A. An overview of Hydrogen assisted (Direct) recycling of Rare earth permanent magnets. J. Magn. Magn. 2023, 588, 171475. [Google Scholar] [CrossRef]
- Lixandru, A. Recycling of Nd-Fe-B Permanent Magnets by Hydrogen Processes. Ph.D Thesis, Technische Universität Darmstadt, Darmstadt, Germany, 2018. [Google Scholar]
- Yang, Y.; Walton, A.; Sheridan, R.; Güth, K.; Gauß, R.; Gutfleisch, O.; Buchert, M.; Steenari, B.-M. REE Recovery from End-of-Life NdFeB Permanent Magnet Scrap: A Critical Review. J. Environ. Sustain. 2017, 3, 122–149. [Google Scholar] [CrossRef]
- Kaya, M. An overview of NdFeB magnets recycling technologies. Curr. Opin. Green Sustain. Chem. 2024, 46, 100884. [Google Scholar] [CrossRef]
- Burkhardt, C.; Ortiz, F.; Daoud, K.; Björnfot, T.; Ahrentorp, F.; Blomgren, J.; Walton, A. Automated High-Speed Approaches for the Extraction of Permanent Magnets from Hard-Disk Drive Components for the Circular Economy. Magnetism 2024, 4, 295–304. [Google Scholar] [CrossRef]
- Ormerod, J.; Karati, A.; Baghel, A.P.S.; Prodius, D.; Nlebedim, I.C. Sourcing, Refining and Recycling of Rare-Earth Magnets. Sustainability 2023, 15, 14901. [Google Scholar] [CrossRef]
- Simon, T.R.; Conga, L.; Zhaia, Y.; Zhua, Y.; Zhaoa, F. A semi-automatic system for efficient recovery of rare earth permanent magnets from hard disk drives. In Proceedings of the CIRP 69 of ScienceDirect 25th CIRP Life Cycle Engineering (LCE) Conference, Copenhagen, Denmark, 30 April–2 May 2018. [Google Scholar]
- Li, J.; Barwood, M.; Rahimifar, S. Robotic disassembly for increased recovery of strategically important materials from electrical vehicles. Robot. Comput. Integr. 2018, 50, 203–212. [Google Scholar] [CrossRef]
- SUSMAGPRO, Sustainable Recovery, Reprocessing and Reuse of Rare Earth Magnets in a European Circular Economy Project Update: Automated Separation of NdFeB Magnets from Waste Electrical and Electronic Equipment Scrap. Available online: https://www.susmagpro.eu/news/project-update-automated-separation-ndfeb-magnets-waste-electrical-and-electronic-equipment (accessed on 6 December 2024).
- Yang, Y. VALOMAG: Upscale of Permanent Magnet Dismantling and Recycling. Available online: https://www.tudelft.nl/me/over/afdelingen/materials-science-and-engineering/research/metals-production-refining-and-recycling/valomag-upscale-of-permanent-magnet-dismantling-and-recycling (accessed on 6 December 2024).
- OAK RIDGE National Laboratory, WORNL Licenses Rare Earth Magnet Recycling Process to Momentum Technologies. Available online: https://www.ornl.gov/news/ornl-licenses-rare-earth-magnet-recycling-process-momentum-technologies (accessed on 6 December 2024).
- Hansjosten, M.; Fleischer, J. Towards autonomous adaptive disassembly of permanent-magnet synchronous motors with industrial robots. Manuf. Lett. 2023, 35, 1336–1346. [Google Scholar] [CrossRef]
- Schuler, D.; Buchert, M.; Liu, R.; Dittrich, S.; Merz, C. Study on Rare Earths and Their Recycling: Final Report for the Greens/EFA Group in the European Parliament. Available online: http://www.oeko.de/publikationen/dok/1192.php (accessed on 22 August 2024).
- Cherkezova-Zheleva, Z.; Burada, M.; Sobetkii, A.E.; Paneva, D.; Fironda, S.A.; Piticescu, R.R. Green and Sustainable Rare Earth Element Recycling and Reuse from End-of-Life Permanent Magnets. Metals 2024, 14, 658. [Google Scholar] [CrossRef]
- Mishra, R.R.; Sharma, A.K. Microwave–material interaction phenomena: Heating mechanisms, challenges and opportunities in material processing. Compos. Part A 2016, 81, 78–97. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, D.; Jain, V.; Sharma, A.K. Microwave Processing of Materials and Applications in Manufacturing Industries: A Review. J. Manuf. Mater. 2015, 30, 1–29. [Google Scholar] [CrossRef]
- Jones, D.A.; Lelyveld, T.P.; Mavrofidis, S.D.; Kingman, S.W.; Miles, N.J. Microwave heating applications in environmental engineering—A review. RCR Adv. 2002, 34, 75–90. [Google Scholar] [CrossRef]
- Oghbaei, M.; Mirzaee, O. Microwave versus conventional sintering: A review of fundamentals, advantages, and applications. J. Alloys Compd. 2010, 494, 175–189. [Google Scholar] [CrossRef]
- Dupont, D.; Binnemans, K. Recycling of Rare Earths from NdFeB Magnets Using a Combined Leaching/Extraction System Based on the Acidity and Thermomorphism of the Ionic Liquid [Hbet][Tf2N]. Green Chem. 2015, 17, 2150–2163. [Google Scholar] [CrossRef]
- Böhm, D.; Czerski, K.; Gottlieb, S.; Huke, A.; Ruprecht, G. Recovery of Rare Earth Elements from NdFeB Magnets by Chlorination and Distillation. Processes 2023, 11, 577. [Google Scholar] [CrossRef]
- Xu, Y.; Chumbley, L.S.; Laabs, L.S. Liquid metal extraction of Nd from NdFeB magnet scrap. J. Mater. Res. 2000, 15, 2296–2304. [Google Scholar] [CrossRef]
- Akahori, T.; Miyamoto, Y.; Saeki, T.; Okamoto, M.; Okabe, T.H. Optimum conditions for extracting rare earth metals from waste magnets by using molten magnesium. J. Alloys Compd. 2017, 703, 337–343. [Google Scholar] [CrossRef]
- Takeda, O.; Okabe, T.H.; Umetsu, Y. Recovery of neodymium from a mixture of magnet scrap and other scrap. J. Alloys Compd. 2006, 408–412, 387–390. [Google Scholar] [CrossRef]
- Rasheed, M.Z.; Nam, S.-W.; Cho, J.-Y.; Park, K.-T.; Kim, B.-S.; Kim, T.-S. Review of the Liquid Metal Extraction Process for the Recovery of Nd and Dy from Permanent Magnets. Metall Mater Trans B 2021, 52, 1213–1227. [Google Scholar] [CrossRef]
- Chae, H.J.; Kim, Y.D.; Kim, B.S.; Kim, J.G.; Kim, T.-S. Experimental investigation of diffusion behavior between molten Mg and Nd–Fe–B magnets. J. Alloys Compd. 2014, 586, 143–149. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Nam, S.-W.; Park, K.-T.; Kim, T.-S. Extraction Behavior of Rare Earth Element (Nd, Dy) from Rare Earth Magnet by Using Molten Magnesium. Sci. Adv. Mater. 2017, 9, 2166–2172. [Google Scholar] [CrossRef]
- Takeda, O.; Okabe, T.H.; Umetsu, Y. Phase equilibria of the system Fe–Mg–Nd at 1076 K. J. Alloys Compd. 2005, 392, 206–213. [Google Scholar] [CrossRef]
- Nam, S.-W.; Park, S.-M.; Rasheed, M.Z.; Song, M.-S.; Kim, D.-H.; Kim, T.-S. Influence of Dysprosium Compounds on the Extraction Behavior of Dy from Nd-Dy-Fe-B Magnet Using Liquid Magnesium. Metals 2021, 11, 1345. [Google Scholar] [CrossRef]
- Ueberschaar, M.; Geiping, J.; Zamzow, M.; Flamme, S.; Rotter, V.S. Assessment of element-specific recycling efficiency in WEEE pre-processing. Resour. Conserv. Recycl. 2017, 124, 25–41. [Google Scholar] [CrossRef]
- Okabe, T.H.; Takeda, O.; Fukuda, K.; Umetsu, Y. Direct Extraction and Recovery of Neodymium Metal from Magnet Scrap. Mater. Trans. 2003, 44, 798–801. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.-K.; Jeong, J.; Shin, J.-H.; Kang, Y.; Liu, R.; Kim, T.-S.; Song, M. Separation and recovery Nd and Dy from Mg-REEs alloy by vacuum distillation. J. Alloys Compd. 2023, 967, 171775. [Google Scholar] [CrossRef]
- Fironda, S.A.; Badea, C.I.; Burada, M.; Licu, L.; Piticescu, R.R.; Cherkezova-Zheleva, Z.; Axinte, S.; Jay, O. An LCA study on recycling neodymium from spent HDD permanent magnets. In Proceedings of the 7th 7TH International Conference on Emerging Technologies In Materials Engineering, Bucharest, Romania, 30–31 October 2024. [Google Scholar]
- Karal, E.; Kucuker, M.A.; Demirel, B.; Copty, N.K.; Kuchta, K. Hydrometallurgical recovery of neodymium from spent hard disk magnets: A life cycle perspective. J. Clean. Prod. 2021, 288, 125087. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



