Cutting-Edge Microwave Sensors for Vital Signs Detection and Precise Human Lung Water Level Measurement
Abstract
1. Introduction
2. Vital Signs Detection
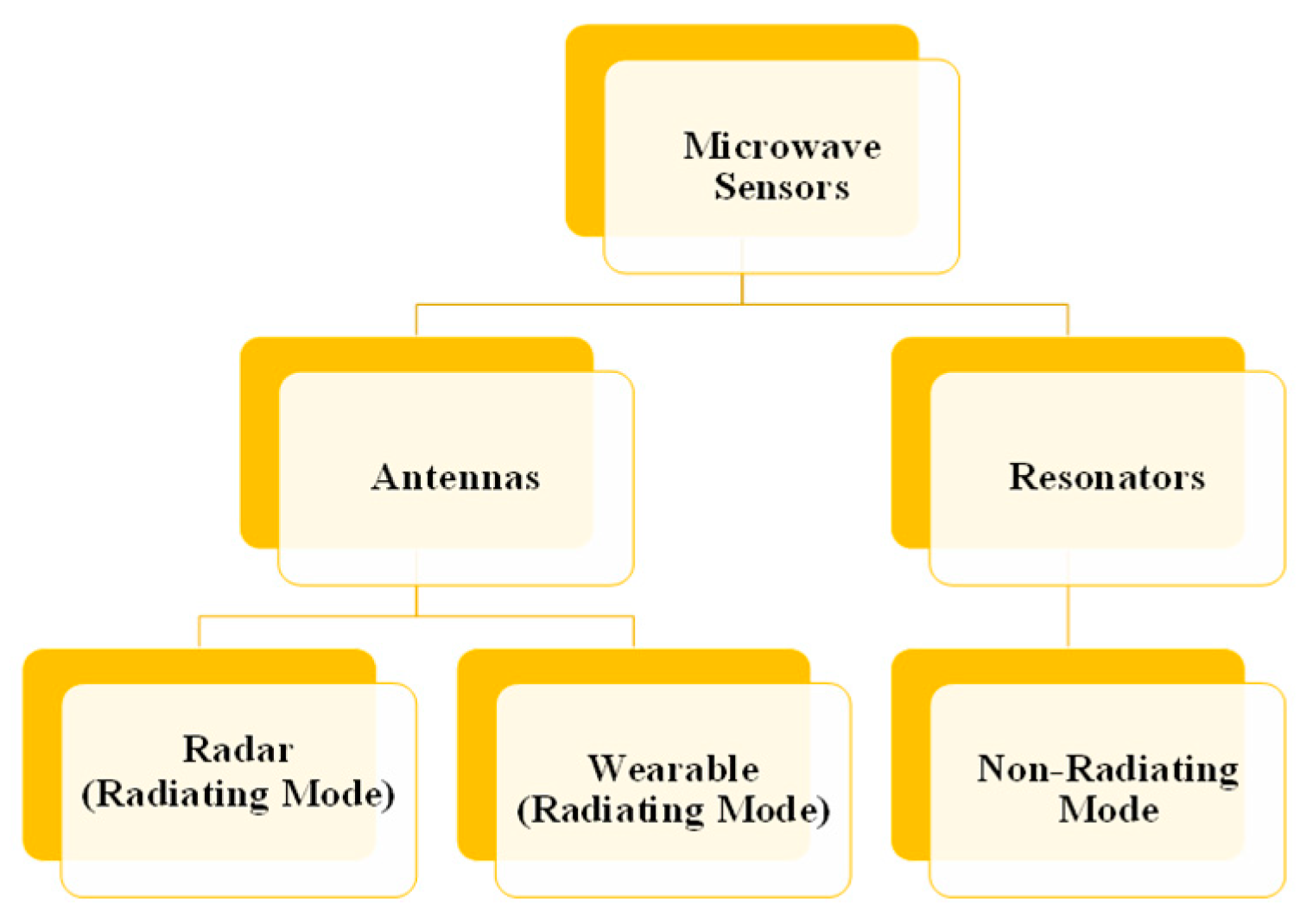
2.1. Types of Microwave Sensors
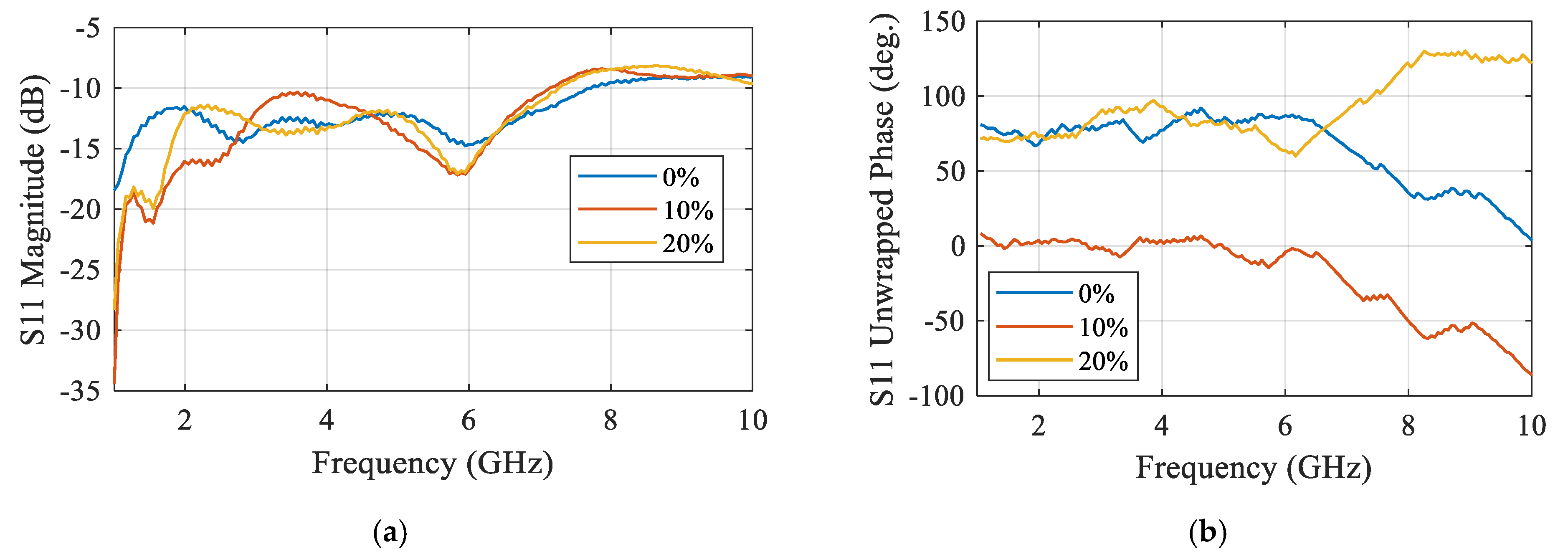
2.1.1. Techniques Based on Microwave Resonators
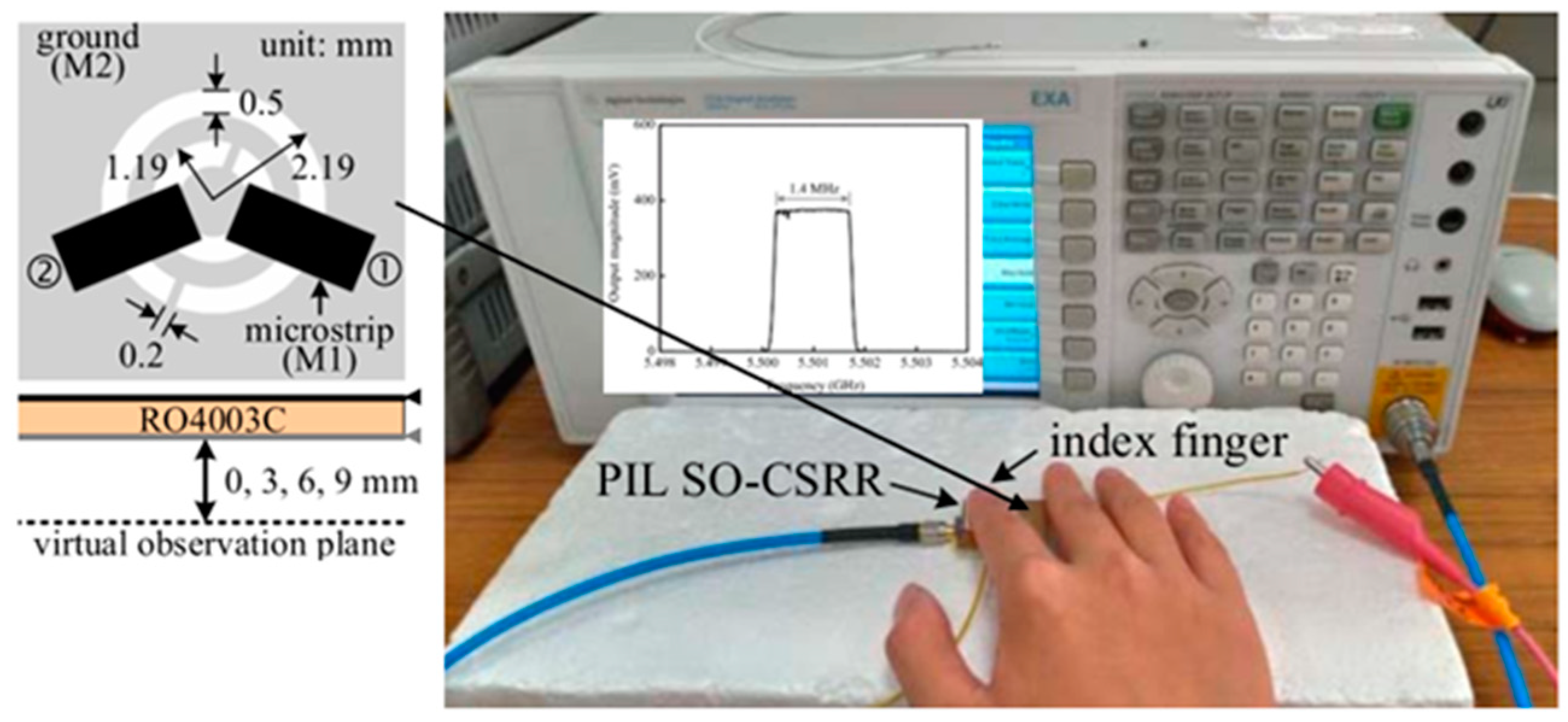
2.1.2. Antenna
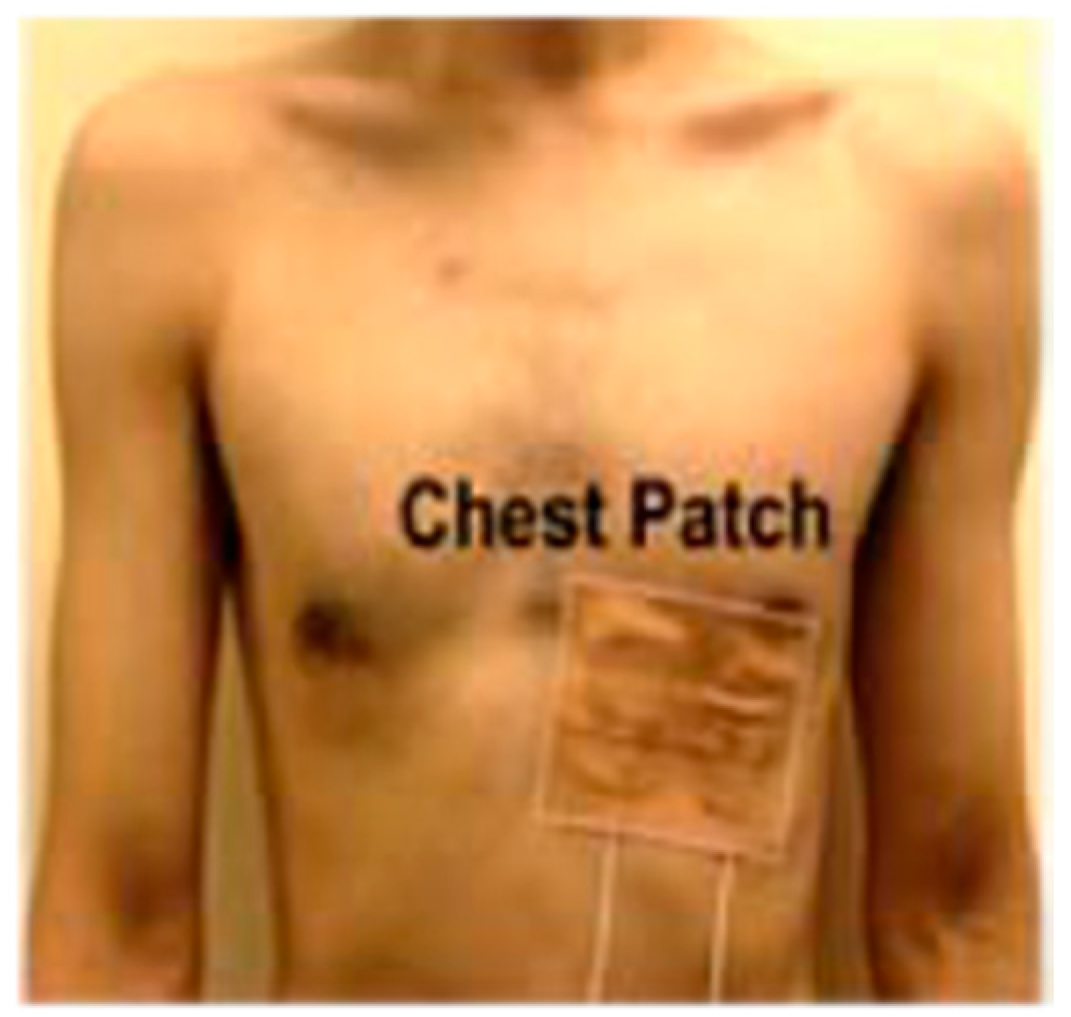
2.2. Vital Signs Detection Methods
2.2.1. Traditional Contact-Based Vital Signs Collection
- (a)
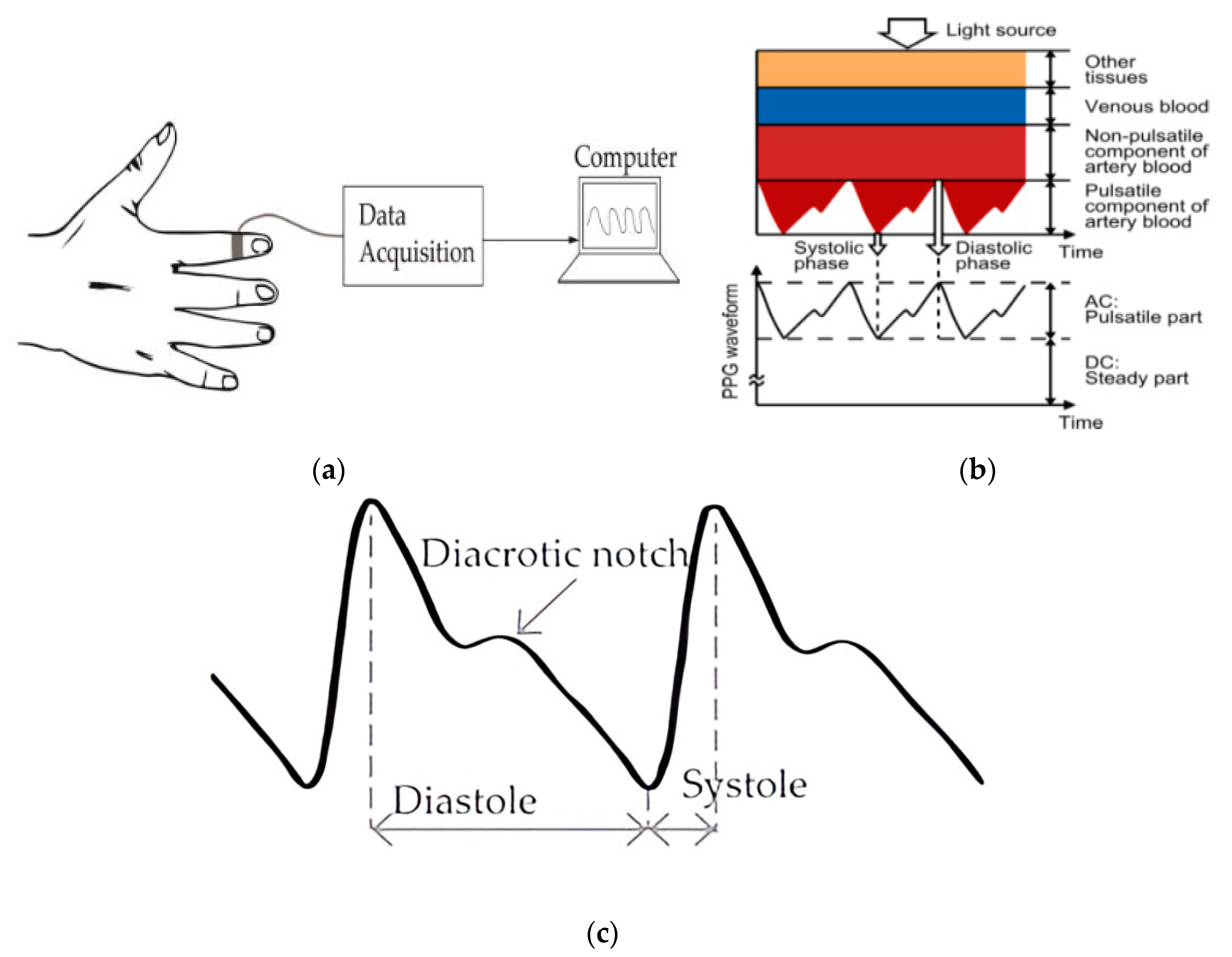
- Electrocardiography (ECG)

- (b)
- Photoplethysmography
- (c)
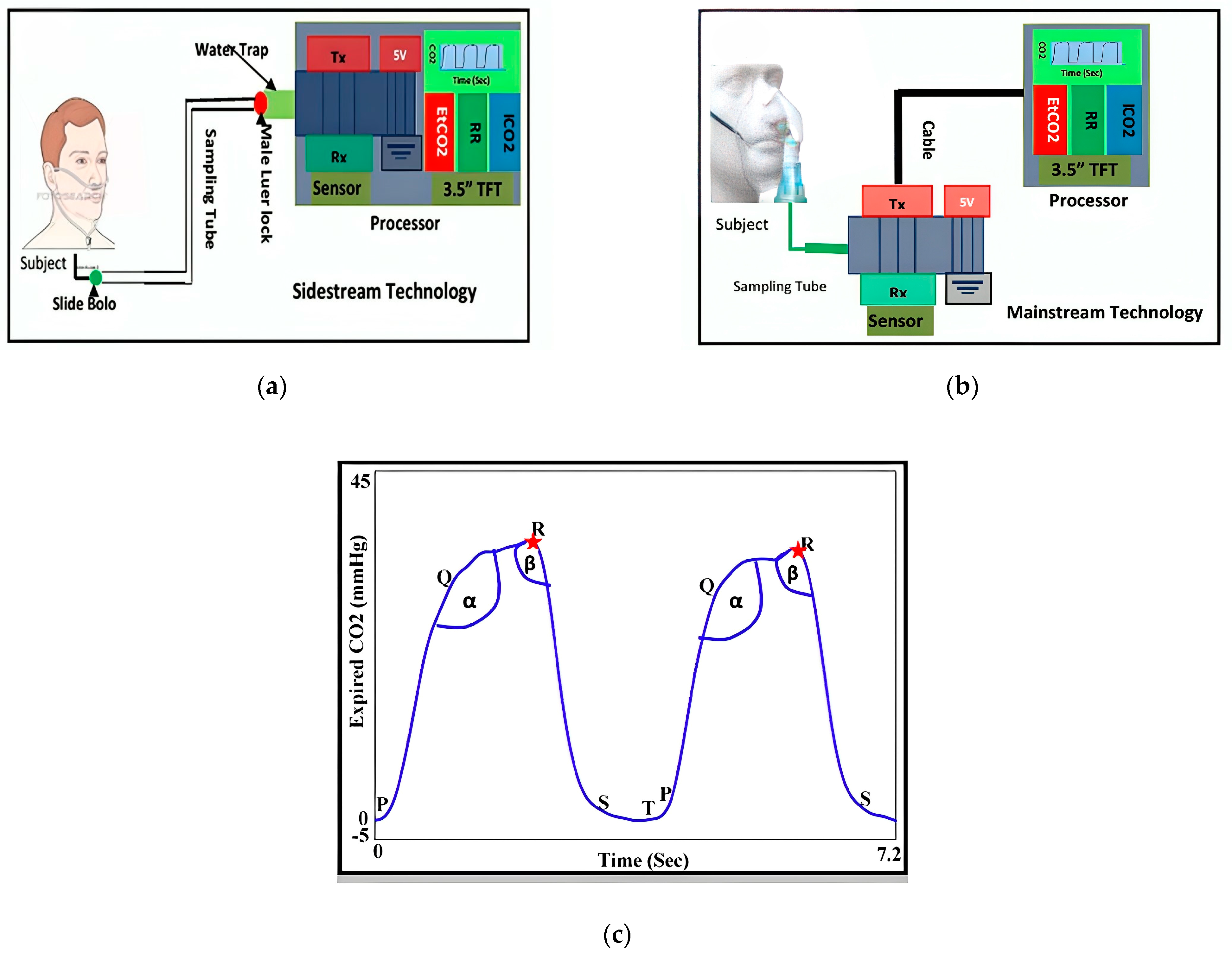
- Methods Based on Temperature, Humidity, and Air Components
- ➢
- Air Components-Based Technique
- ➢
- Air Temperature-Based Technique
- ➢
- Air Humidity-Based Technique
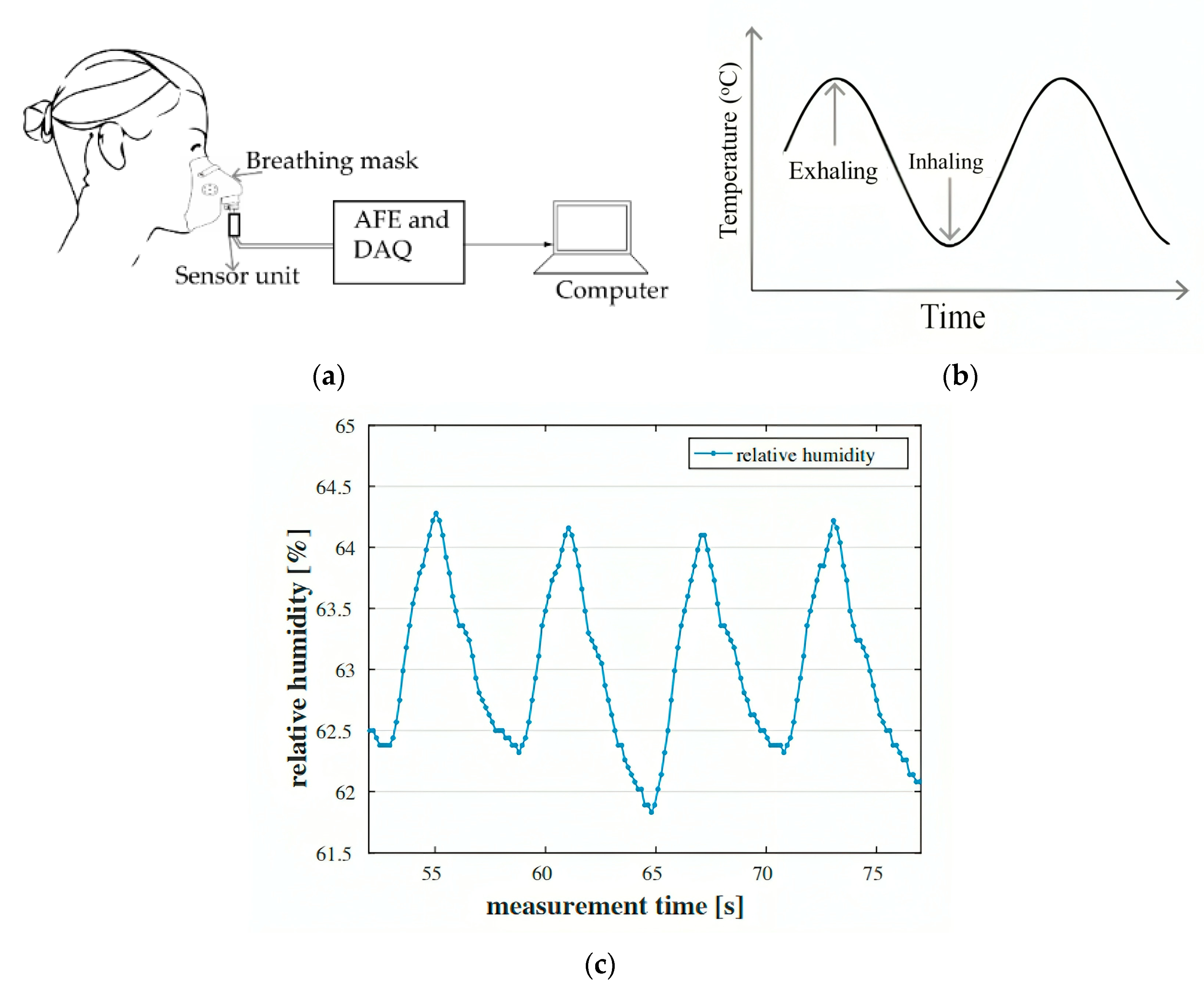
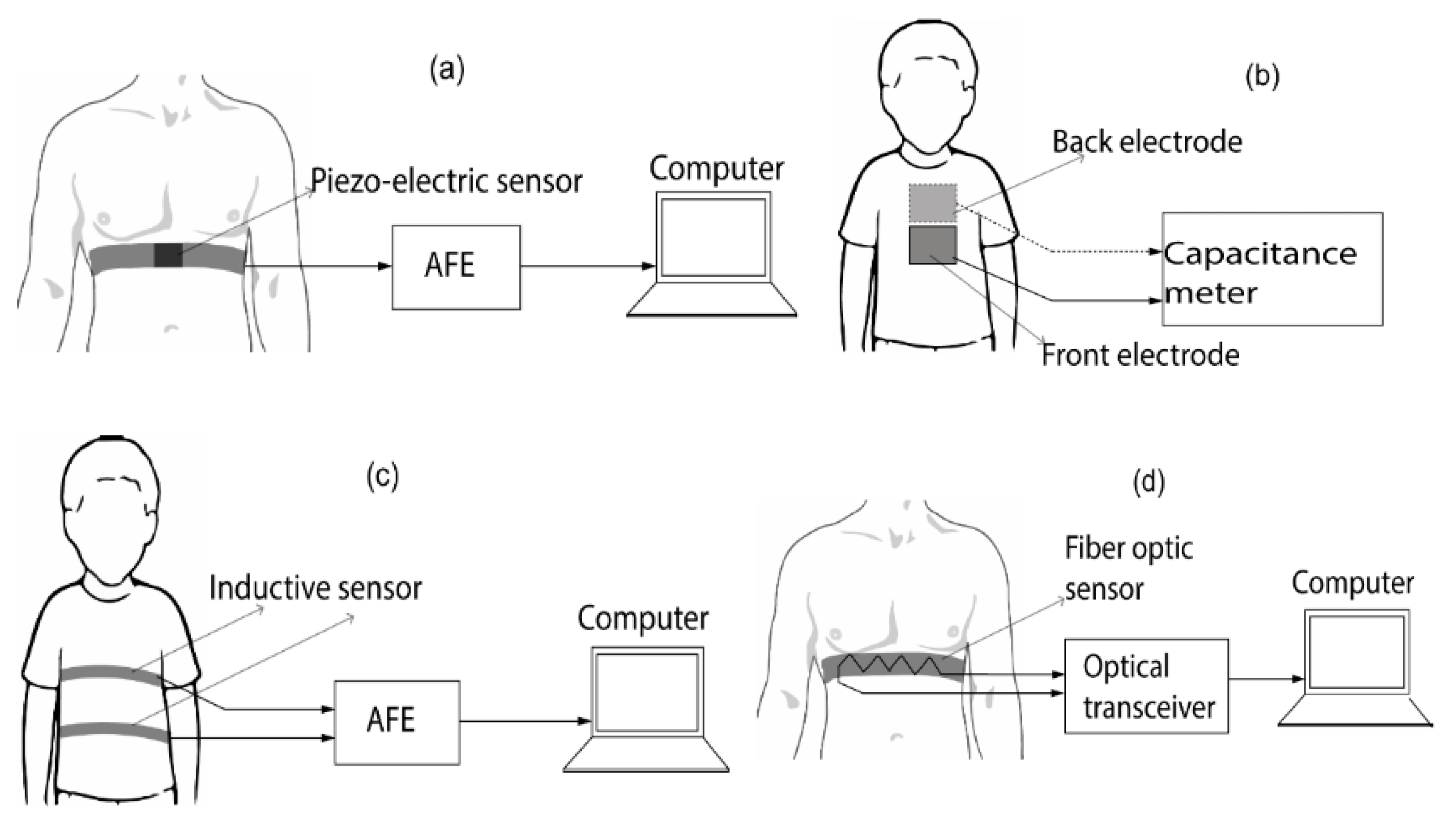
2.2.2. Chest-Wall Mechanical Displacement Sensing Methods
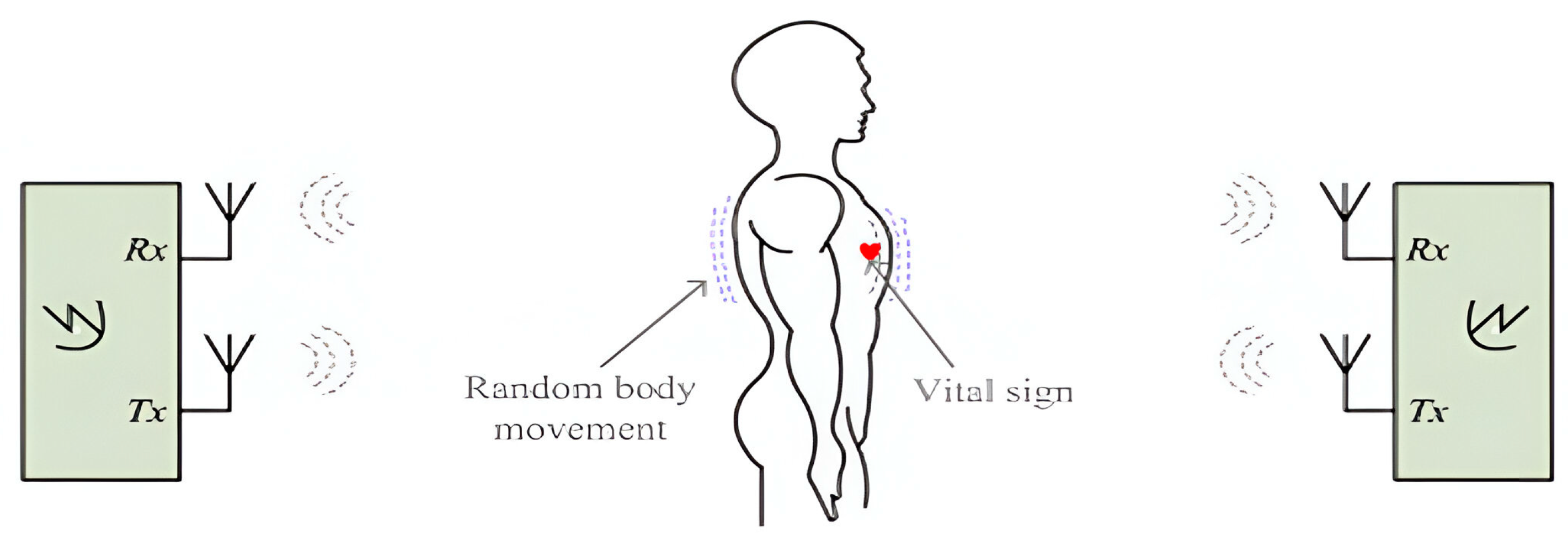
2.2.3. Contactless Vital Signs Monitoring Employing Radar Methods
- (a)
- Radar with Continuous Waves (CW)
- (b)
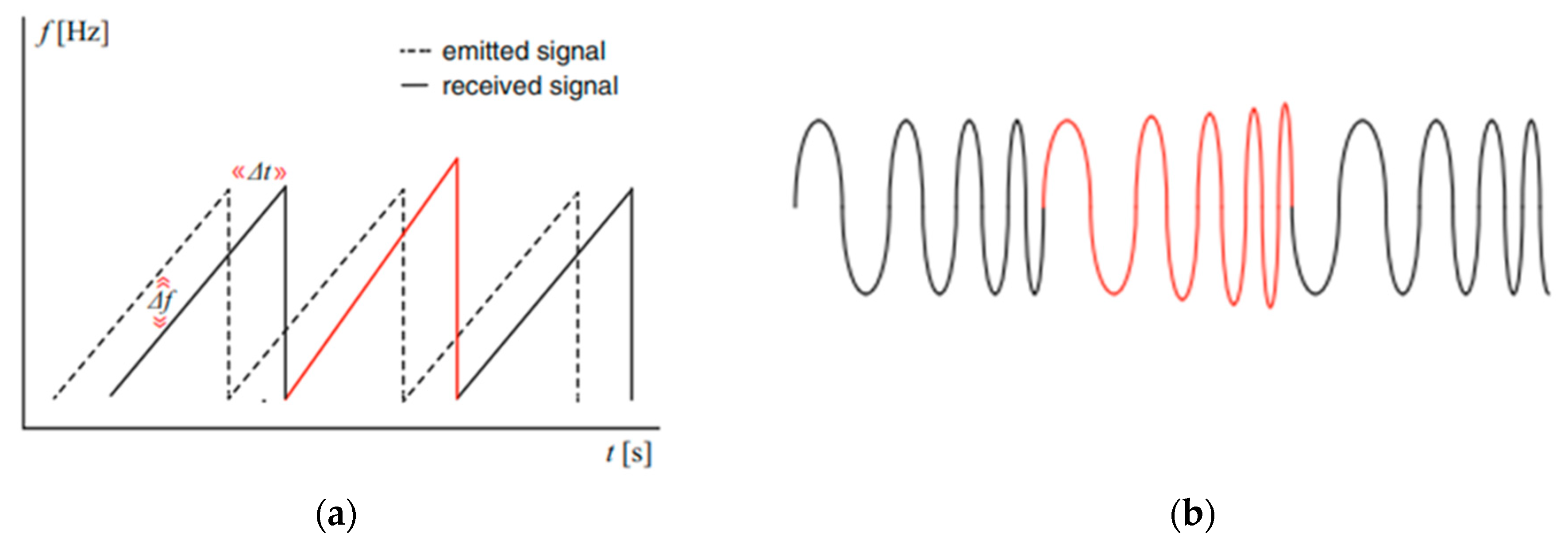
- Radar with frequency modulation continuous wave (FMCW)
- (c)
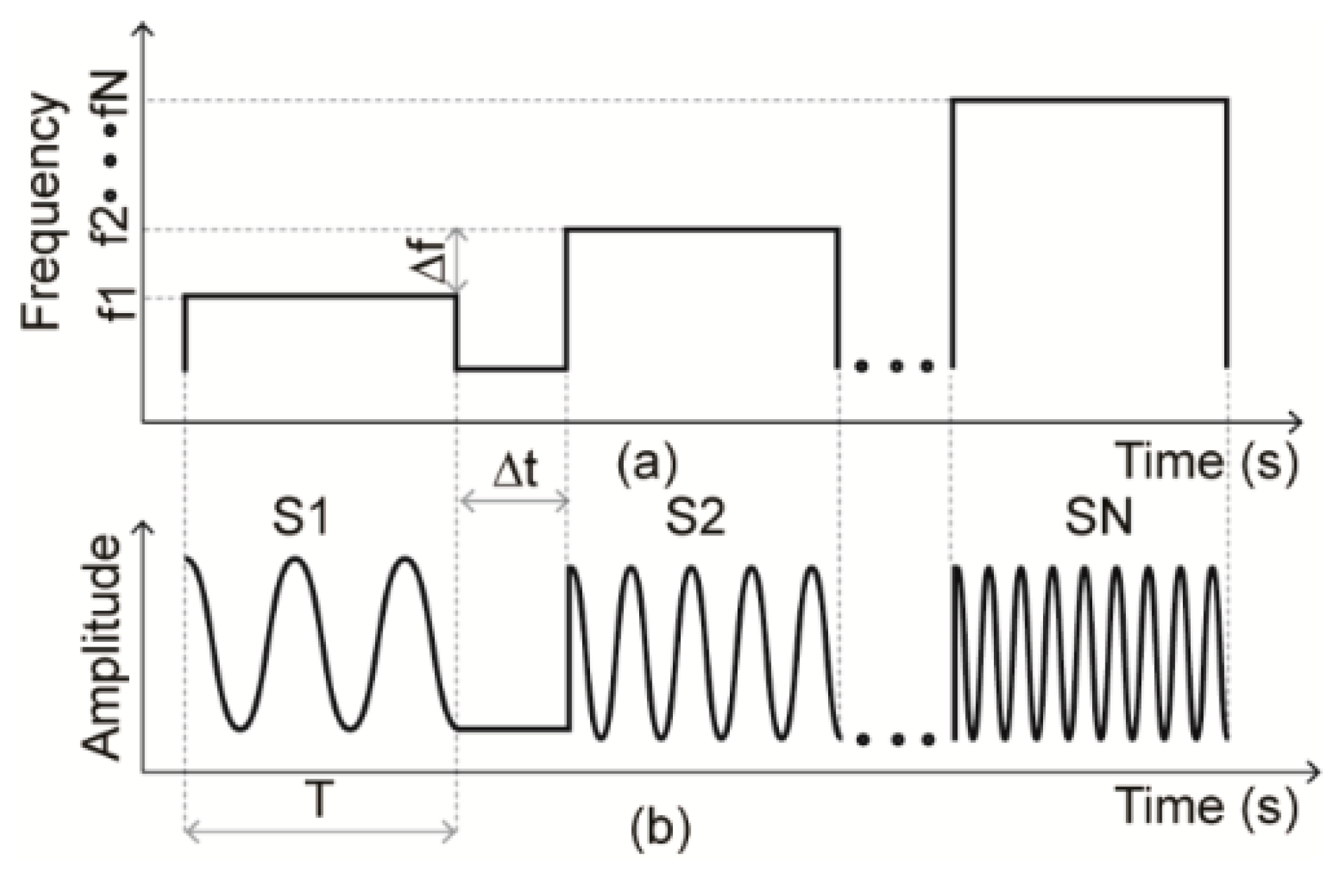
- SFCW Radar: Stepped-Frequency Continuous Wave
- (d)
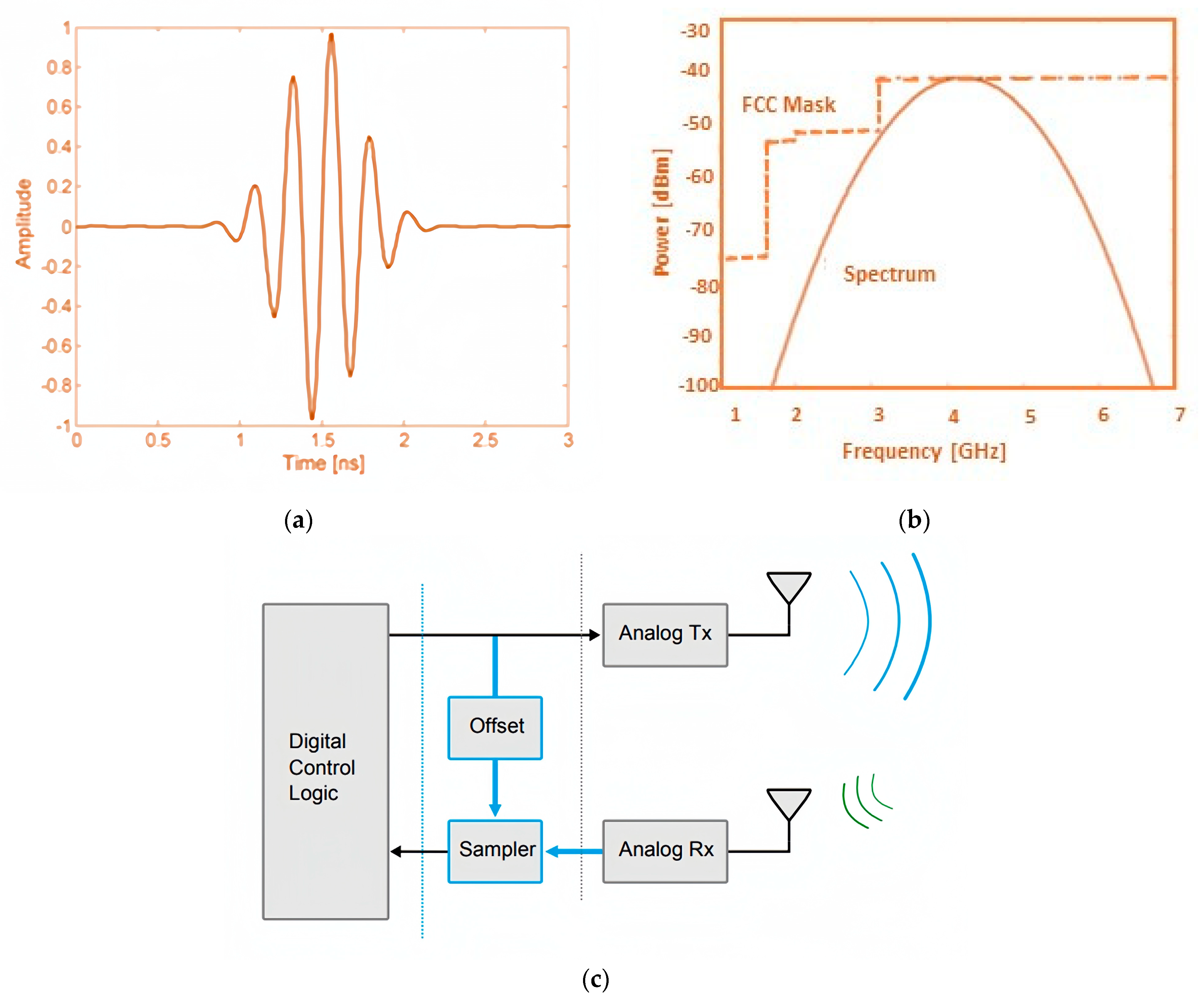
- Pulse-Based Ultra-Wideband (UWB) Radar
- (e)
- Techniques for Cancelling Random Body Movement (RBM) in Doppler Radar
2.2.4. Advancements in Signal Processing
3. Lung Water Level Measurement
3.1. Traditional Techniques
3.2. Ultrasound Imaging
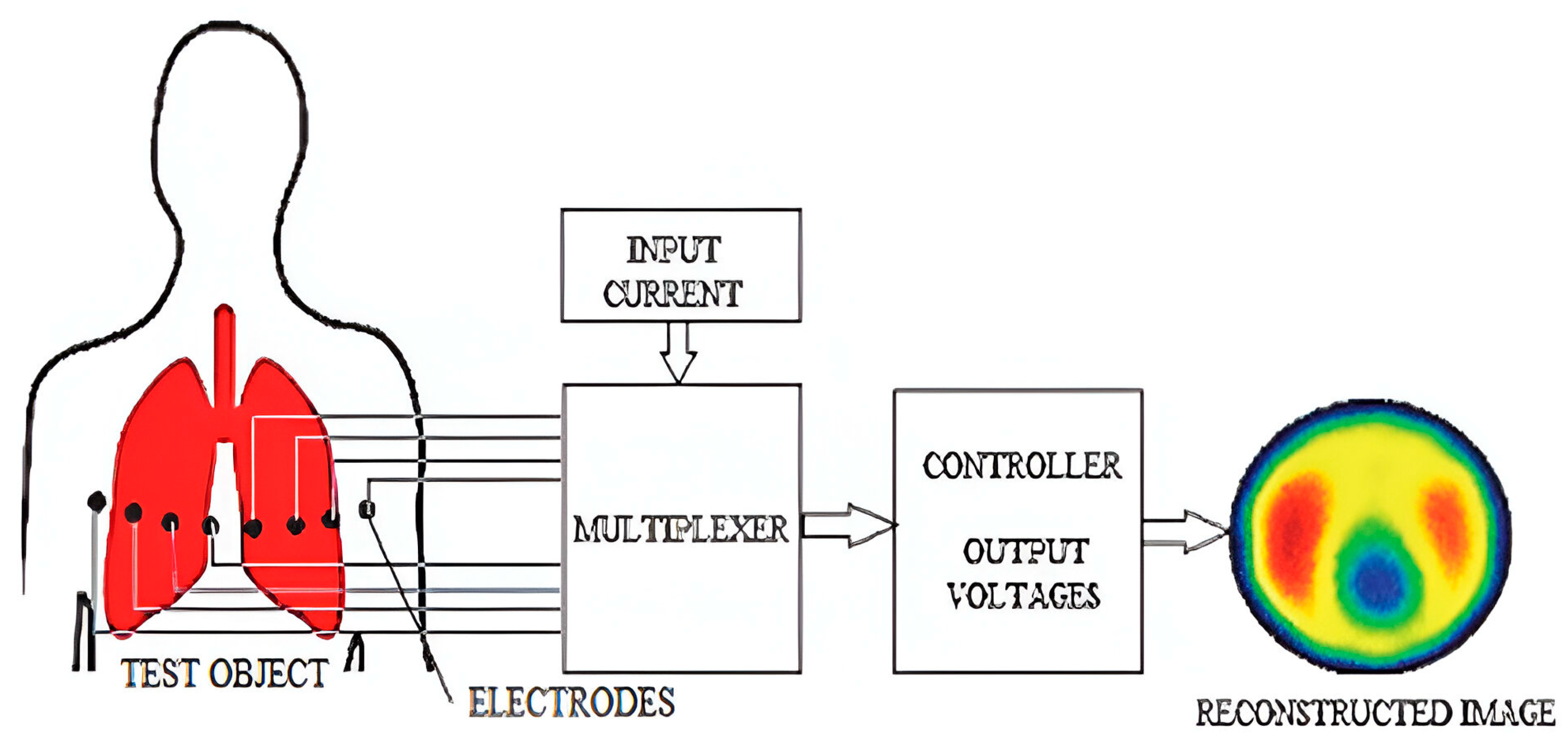
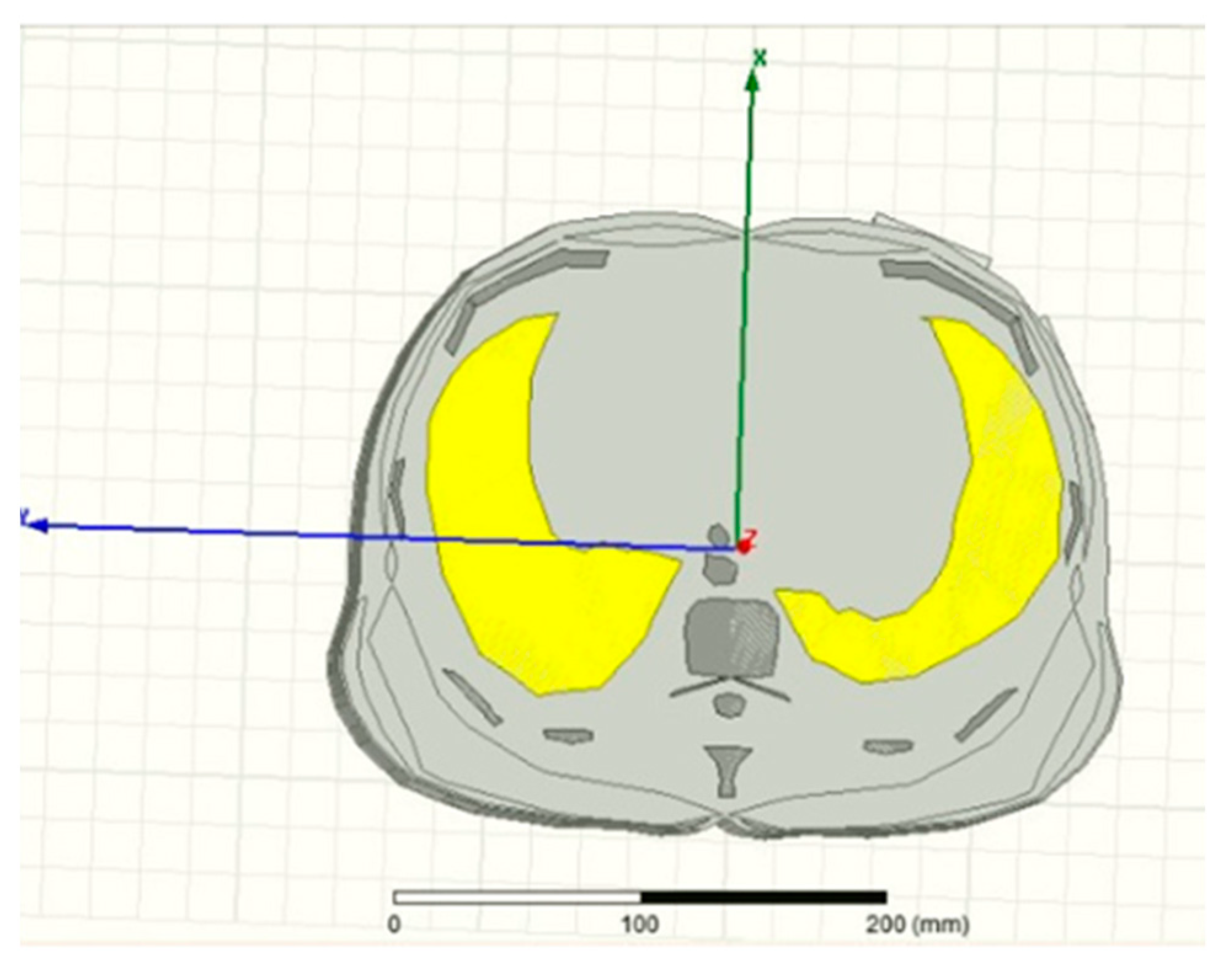
3.3. Bioimpedance and Electrical Impedance Tomography (EIT)
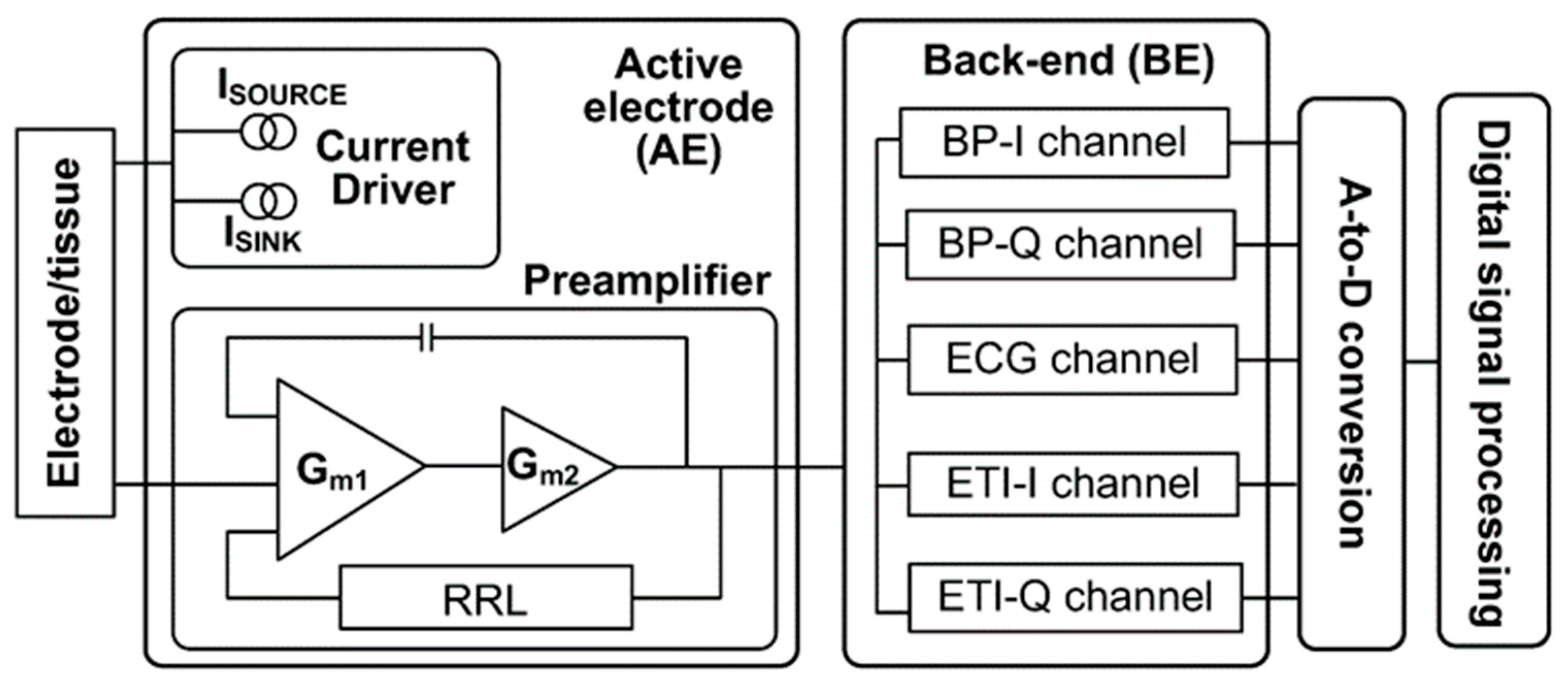
3.4. Microwave Sensors-Based Technique
4. Vital Signs Radar-Based Techniques versus Traditional Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhiah el Diehn, I.; Obermaisser, R.; Abuteir, M.; Darabkh, K.A. A Reliable System-of-Systems Healthcare Monitoring Framework. IEEE Access 2023, 11, 145679–145691. [Google Scholar]
- Zhang, E.; Trujillo, R.; Templeton, J.M.; Poellabauer, C. A Study on Mobile Crowd Sensing Systems for Healthcare Scenarios. IEEE Access 2023, 11, 40325–140347. [Google Scholar] [CrossRef]
- Marzouk, H.M.; Abd El-Hameed, A.S.; Allam, A.; Abdel-Rahman, A.B.A. Design of Non-Invasive Glucose Measurement Sensor. In Proceedings of the 2022 10th International Japan-Africa Conference on Electronics, Communications, and Computations (JAC-ECC), Alexandria, Egypt, 19–20 December 2022; pp. 212–215. [Google Scholar]
- Xu, Z.; Rodriguez-Villegas, E. Power Transfer Mattress Based System for Perpetually Operating Physiological Monitoring Wearables. IEEE Trans. Biomed. Circuits Syst. 2023, 18, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Yavari, E. Distortion Reduction and Signal Estimation in Doppler Radar Physiological Monitoring Systems. Ph.D. Thesis, University of Hawaii, Manoa, HI, USA, 2015. [Google Scholar]
- Marzouk, H.M.; Hameed, A.S.A.E.; Allam, A.; Pokharel, R.K.; Rahman, A.B.A. A new rectangular dielectric resonator sensor for glucose measurement: Design, modeling, and experimental validation. Int. J. Circuit Theory Appl. 2023, 52, 3040–3051. [Google Scholar] [CrossRef]
- Marzouk, H.M.; Abd El-Hameed, A.S.; Allam, A.; Pokharel, R.K.; Abdel-Rahman, A.B. Circular DGS Resonator for Non-Invasive Sensing of Diabetes. In Proceedings of the 2023 17th European Conference on Antennas and Propagation (EuCAP), Florence, Italy, 26–31 March 2023; pp. 1–5. [Google Scholar]
- Perron, R.R.; Iskander, M.F.; Seto, T.B.; Huang, G.C.; Bibb, D.A. Electromagnetics in medical applications: The cardiopulmonary stethoscope journey. In The World of Applied Electromagnetics: In Appreciation of Magdy Fahmy Iskander; Springer: Berlin/Heidelberg, Germany, 2018; pp. 443–479. [Google Scholar]
- Gagarin, R.; Celik, N.; Huang, G.C.; Iskander, M.F. Microwave Stethoscope, a new noninvasive multiple vital signs sensor: Human clinical trials. In Proceedings of the 2012 IEEE International Symposium on Antennas and Propagation, Chicago, IL, USA, 8–14 July 2012; pp. 1–2. [Google Scholar]
- Staub, N.C. Measurement of lung water content. J. Microw. Power 1983, 18, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Celik, N.; Gagarin, R.; Huang, G.C.; Iskander, M.F.; Berg, B.W. Microwave stethoscope: Development and benchmarking of a vital signs sensor using computer-controlled phantoms and human studies. IEEE Trans. Biomed. Eng. 2013, 61, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Korostynska, O.; Mason, A.; Al-Shamma’a, A. Microwave sensors for the noninvasive monitoring of industrial and medical applications. Sens. Rev. 2014, 34, 182–191. [Google Scholar] [CrossRef]
- Tseng, C.H.; Wu, C.Z. A novel microwave phased-and perturbation-injection-locked sensor with self-oscillating complementary split-ring resonator for finger and wrist pulse detection. IEEE Trans. Microw. Theory Tech. 2020, 68, 1933–1942. [Google Scholar] [CrossRef]
- Leong, C.; Xiao, Z.; Yun, Z.; Iskander, M.F.I. Non-Invasive assessment of lung water content using chest patch RF sensors: A computer study using NIH patients CT scan database and AI classification algorithms. IEEE Access 2023, 11, 13058–13066. [Google Scholar] [CrossRef]
- Atlef, J.L. Principles of Clinical Electrocardiography. Anesthesiology 1980, 52, 195. [Google Scholar] [CrossRef]
- Hsu, C.C.; Lin, B.S.; He, K.Y.; Lin, B.S. Design of a Wearable 12-Lead Noncontact Electrocardiogram Monitoring System. Sensors 2019, 19, 1509. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Schumann, A.; Müller, J.; Bär, K.J.; Rose, G. ECG derived respiration: Comparison of time-domain approaches and application to altered breathing patterns of patients with schizophrenia. Physiol. Meas. 2017, 38, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Hallfors, N.; Jaoude, M.A.; Liao, K.; Ismail, M.; Isakovic, A. Graphene oxide—Nylon ECG sensors for wearable IoT healthcare. In Proceedings of the 2017 Sensors Networks Smart and Emerging Technologies (SENSET), Beirut, Lebanon, 12–14 September 2017. [Google Scholar]
- Hsieh, J.C.; Hsu, M.W. A cloud computing based 12-lead ECG telemedicine service. BMC Med. Inform. Decis. 2012, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- 12-Lead ECG Systems in Clinical Trials. Available online: https://www.clinicaltrialsarena.com/features/12-lead-ecg-systems-in-clinical-trials/ (accessed on 20 December 2019).
- Majumder, S.; Chen, L.; Marinov, O.; Chen, C.H.; Mondal, T.; Deen, M.J. Noncontact Wearable Wireless ECG Systems for Long-Term Monitoring. IEEE Rev. Biomed. Eng. 2018, 11, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Bayasi, N.; Tekeste, T.; Saleh, H.; Mohammad, B.; Khandoker, A.; Ismail, M. Low-Power ECG-Based Processor for Predicting Ventricular Arrhythmia. IEEE Trans. Very Large Scale Integr. VLSI Syst. 2016, 24, 1962–1974. [Google Scholar] [CrossRef]
- Yasin, M.; Tekeste, T.; Saleh, H.; Mohammad, B.; Sinanoglu, O.; Ismail, M. Ultra-Low Power, Secure IoT Platform for Predicting Cardiovascular Diseases. IEEE Trans. Circuits Syst. I Reg. Papers 2017, 64, 2624–2637. [Google Scholar] [CrossRef]
- Sinha, R. An Approach for Classifying ECG Arrhythmia Based on Features Extracted from EMD and Wavelet Packet Domains. Master Dissertation. 2012. Available online: https://www.researchgate.net/publication/287200946_An_Approach_for_Classifying_ECG_Arrhythmia_Based_on_Features_Extracted_from_EMD_and_Wavelet_Packet_Domains (accessed on 3 March 2020).
- Stingeni, L.; Cerulli, E.; Spalletti, A.; Mazzoli, A.; Rigano, L.; Bianchi, L.; Hansel, K. The role of acrylic acid impurity as a sensitizing component in electrocardiogram electrodes. Contact Dermat. 2015, 73, 44–48. [Google Scholar] [CrossRef]
- Özkaya, E.; Bozkurt, P.K. Allergic contact dermatitis caused by self-adhesive electrocardiography electrodes: A rare case with concomitant roles of nickel and acrylates. Contact Dermat. 2014, 70, 121–123. [Google Scholar] [CrossRef]
- Deswysen, A.C.; Zimerson, E.; Goossens, A.; Bruze, M.; Baeck, M. Allergic contact dermatitis caused by self-adhesive electrocardiography electrodes in an infant. Contact Dermat. 2013, 69, 379–381. [Google Scholar] [CrossRef]
- Nemati, E.; Deen, M.; Mondal, T. A wireless wearable ECG sensor for long-term applications. IEEE Commun. Mag. 2012, 50, 36–43. [Google Scholar] [CrossRef]
- Arcelus, A.; Sardar, M.; Mihailidis, A. Design of a capacitive ECG sensor for unobtrusive heart rate measurements. In Proceedings of the 2013 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Minneapolis, MN, USA, 13 July 2013. [Google Scholar]
- Khayatzadeh, M.; Zhang, X.; Tan, J.; Liew, W.S.; Lian, Y. A 0.7-V 17.4-_W 3-lead wireless ECG SoC. IEEE Trans. Biomed. Circuits Syst. 2013, 7, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Jevon, P. Procedure for recording a standard 12-lead electrocardiogram. Br. J. Nurs. 2010, 19, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Charlton, P.H.; Birrenkott, D.A.; Bonnici, T.; Pimentel, M.A.; Johnson, A.E.; Alastruey, J.; Tarassenko, L.; Watkinson, P.J.; Beale, R.; Clifton, D.A. Breathing Rate Estimation from the Electrocardiogram and Photoplethysmogram: A Review. IEEE Rev. Biomed. Eng. 2018, 11, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Bailón, R.; Sörnmo, L.; Laguna, P. Advanced Methods and Tools for ECG Data Analysis. Artech House Lond. 2006, 1, 215–243. [Google Scholar]
- Berntson, G.G.; Cacioppo, J.T.; Quigley, K.S. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology 1993, 30, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1. [Google Scholar] [CrossRef] [PubMed]
- Massaroni, C.; Nicolò, A.; Presti, D.L.; Sacchetti, M.; Silvestri, S.; Schena, E. Contact-Based Methods for Measuring Respiratory Rate. Sensors 2019, 19, 908. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Maeda, Y.; Sekine, M.; Yoshida, M. Wearable Photoplethysmographic Sensors—Past and Present. Electronics 2014, 3, 282–302. [Google Scholar] [CrossRef]
- Shao, D.; Liu, C.; Tsow, F.; Yang, Y.; Du, Z.; Iriya, R.; Yu, H.; Tao, N. Noncontact Monitoring of Blood Oxygen Saturation Using Camera and Dual-Wavelength Imaging System. IEEE Trans. Biomed. Eng. 2016, 63, 1091–1098. [Google Scholar] [CrossRef]
- Humphreys, K.; Ward, T.; Markham, C. Noncontact simultaneous dual wavelength photoplethysmography: A further step toward noncontact pulse oximetry. Rev. Sci. Instrum. 2007, 78, 044304. [Google Scholar] [CrossRef]
- Gastel, M.V.; Verkruysse, W.; Haan, G.D. Data-Driven Calibration Estimation for Robust Remote Pulse-Oximetry. Appl. Sci. 2019, 9, 3857. [Google Scholar] [CrossRef]
- Meredith, D.J.; Clifton, D.; Charlton, P.; Brooks, J.; Pugh, C.W.; Tarassenko, L. Photoplethysmographic derivation of respiratory rate: A review of relevant physiology. J. Med. Eng. Technol. 2011, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Reisner, A.; Shaltis, P.A.; Mccombie, D.; Asada, H.H. Utility of the Photoplethysmogram in Circulatory Monitoring. Anesthesiology 2008, 108, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, D.; Esparza, A.; Ghamari, M.; Soltanpur, C.; Nazeran, H. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int. J. Biosens. Bioelectron. 2018, 4, 195–202. [Google Scholar] [PubMed]
- Fletcher, R.R.; Chamberlain, D.; Paggi, N.; Deng, X. Implementation of smart phone video plethysmography and dependence on lighting parameters. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 3747–3750. [Google Scholar]
- Guzman, J.H.; Couderc, J.P.; Tsouri, G.R. Accurate Hemodynamic Sensing using Video Plethysmography with High Quality Cameras. In Proceedings of the 2019 13th International Symposium on Medical Information and Communication Technology (ISMICT), Oslo, Norway, 8–10 May 2019; pp. 1–6. [Google Scholar]
- Kwon, S.; Kim, J.; Lee, D.; Park, K. ROI analysis for remote photoplethysmography on facial video. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 4938–4941. [Google Scholar]
- Singh, O.P.; Howe, T.A.; Malarvili, M. Real-time human respiration carbon dioxide measurement device for cardiorespiratory assessment. J. Breath Res. 2018, 12, 026003. [Google Scholar] [CrossRef] [PubMed]
- Höppe, P. Temperatures of expired air under varying climatic conditions. Int. J. Biometeorol. 1981, 25, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Venkateshan, S. Measurements of Temperature. In Mechanical Measurements, 2nd ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 103–182. [Google Scholar]
- Van Herwaarden, A.W.; Sarro, P.M. Thermal sensors based on the Seebeck effect. Sens. Actuators 1986, 10, 321–346. [Google Scholar] [CrossRef]
- Huang, Y.P.; Young, M.S.; Tai, C.C. Noninvasive respiratory monitoring system based on the piezoceramic transducer’s pyroelectric effect. Rev. Sci. Instrum. 2008, 79, 035103. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.P.; Huang, K.N. Monitoring of breathing rate by a piezofilm sensor using pyroelectric e_ect. In Proceedings of the 2013 1st International Conference on Orange Technologies (ICOT), Tainan, Taiwan, 12–16 March 2013; pp. 99–102. [Google Scholar]
- Liang, Y.; Mazzolini, A.P.; Stoddart, P.R. Fibre Bragg grating sensor for respiratory monitoring. In Proceedings of the ACOFT/AOS 2006—Australian Conference on Optical Fibre Technology/Australian Optical Society, Melbourne, VIC, Australia, 10–13 July 2006; pp. 75–77. [Google Scholar]
- Massaroni, C.; Presti, D.L.; Saccomandi, P.; Caponero, M.A.; Damato, R.; Schena, E. Fiber Bragg Grating Probe for Relative Humidity and Respiratory Frequency Estimation: Assessment During Mechanical Ventilation. IEEE Sens. J. 2018, 18, 2125–2130. [Google Scholar] [CrossRef]
- Kano, S.; Dobashi, Y.; Fujii, M. Silica Nanoparticle-Based Portable Respiration Sensor for Analysis of Respiration Rate, Pattern, and Phase During Exercise. IEEE Sens. Lett. 2018, 2, 1–4. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, Y.E.; Li, P.; Zhang, Y. Facile Fabrication of MoS2-Modified SnO2 Hybrid Nanocomposite for Ultrasensitive Humidity Sensing. ACS Appl. Mater. Interfaces 2016, 8, 14142–14149. [Google Scholar] [CrossRef] [PubMed]
- Iacoponi, S.; Massaroni, C.; Presti, D.L.; Saccomandi, P.; Caponero, M.; Damato, R.; Schena, E. Polymer-coated fiber optic probe for the monitoring of breathing pattern and respiratory rate. In Proceedings of the 2018, 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 1616–1619. [Google Scholar]
- Tang, Y.; Li, Z.; Ma, J.; Wang, L.; Yang, J.; Du, B.; Yu, Q.; Zu, X. Highly sensitive surface acoustic wave (SAW) humidity sensors based on sol–gel SiO2 films: Investigations on the sensing property and mechanism. Sens. Actuators B Chem. 2015, 215, 283–291. [Google Scholar] [CrossRef]
- Scholz, R.; Bracio, B.R.; Brutscheck, M.; Trommler, P. Non-invasive respiratory rate detection in spontaneous respiration by humidity measurement. In Proceedings of the 2017 28th Irish Signals and Systems Conference (ISSC), Killarney, Ireland, 20–21 June 2017; pp. 1–6. [Google Scholar]
- Moll, J.M.; Wright, V. An objective clinical study of chest expansion. Ann. Rheum. Dis. 1972, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, A.; Critello, C.; Pullano, S. Theory, technology and applications of piezoresistive sensors: A review. Sens. Actuators A-Phys. 2018, 281, 156–175. [Google Scholar] [CrossRef]
- Rossi, D.D.; Carpi, F.; Lorussi, F.; Scilingo, E.P.; Tognetti, A. Electroactive fabrics and wearable man–machine interfaces. In Wearable Electronics and Photonics, 1st ed.; Tao, X., Ed.; Woodhead Publishing: Cambridge, UK, 2005; pp. 59–80. [Google Scholar]
- Egami, Y.; Suzuki, K.; Tanaka, T.; Yasuhara, T.; Higuchi, E.; Inoue, H. Preparation and characterization of conductive fabrics coated uniformly with polypyrrole nanoparticles. Synth. Met. 2011, 161, 219–224. [Google Scholar] [CrossRef]
- Ghasemzadeh, H.; Ostadabbas, S.; Guenterberg, E.; Pantelopoulos, A. Wireless Medical-Embedded Systems: A Review of Signal-Processing Techniques for Classification. IEEE Sens. J. 2013, 13, 423–437. [Google Scholar] [CrossRef]
- Kundu, S.K.; Kumagai, S.; Sasaki, M. A Wearable Capacitive Sensor for Monitoring Human Respiratory Rate. Jpn. J. Appl. Phys. 2013, 52, 04CL05. [Google Scholar] [CrossRef]
- Retory, Y.; Niedzialkowski, P.; Picciotto, C.D.; Bonay, M.; Petitjean, M. New Respiratory Inductive Plethysmography (RIP) Method for Evaluating Ventilatory Adaptation during Mild Physical Activities. PLoS ONE 2016, 11, e0151983. [Google Scholar] [CrossRef]
- Collop, N.A.; Tracy, S.L.; Kapur, V.; Mehra, R.; Kuhlmann, D.; Fleishman, S.A.; Ojile, J.M. Obstructive Sleep Apnea Devices for Out-Of-Center (OOC) Testing: Technology Evaluation. J. Clin. Sleep Med. 2011, 7, 531–548. [Google Scholar] [CrossRef]
- Kaplan, V.; Zhang, J.; Russi, E.; Bloch, K. Detection of inspiratory flow limitation during sleep by computer assisted respiratory inductive plethysmography. Eur. Respir. J. 2000, 15, 570. [Google Scholar] [CrossRef]
- Massaroni, C.; Saccomandi, P.; Schena, E. Medical Smart Textiles Based on Fiber Optic Technology: An Overview. J. Funct. Biomater. 2015, 6, 204–221. [Google Scholar] [CrossRef] [PubMed]
- Quandt, B.M.; Scherer, L.J.; Boesel, L.F.; Wolf, M.; Bona, G.-L.; Rossi, R.M. Body-Monitoring and Health Supervision by Means of Optical Fiber-Based Sensing Systems in Medical Textiles. Adv. Healthc. Mater. 2014, 4, 330–355. [Google Scholar] [CrossRef] [PubMed]
- Chethana, K.; Prasad, A.S.G.; Omkar, S.N.; Asokan, S. Fiber bragg grating sensor based device for simultaneous measurement of respiratory and cardiac activities. J. Biophoton. 2016, 10, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, N.; Massaroni, C.; Presti, D.L.; Saccomandi, P.; Tomaso, G.D.; Zollo, L.; Perego, P.; Andreoni, G.; Schena, E. Wearable textile based on silver plated knitted sensor for respiratory rate monitoring. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018. [Google Scholar]
- Naranjo-Hernández, D.; Talaminos-Barroso, A.; Reina-Tosina, J.; Roa, L.; Barbarov-Rostan, G.; Cejudo-Ramos, P.; Márquez-Martín, E.; Ortega-Ruiz, F. Smart Vest for Respiratory Rate Monitoring of COPD Patients Based on Non-Contact Capacitive Sensing. Sensors 2018, 18, 2144. [Google Scholar] [CrossRef] [PubMed]
- Clarenbach, C.F.; Senn, O.; Brack, T.; Kohler, M.; Bloch, K.E. Monitoring of Ventilation During Exercise by a Portable Respiratory Inductive Plethysmograph. Chest 2005, 128, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Ciocchetti, M.; Massaroni, C.; Saccomandi, P.; Caponero, M.; Polimadei, A.; Formica, D.; Schena, E. Smart Textile Based on Fiber Bragg Grating Sensors for Respiratory Monitoring: Design and Preliminary Trials. Biosensors 2015, 5, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K. Respiration Rate Measurement Based on Impedance Pneumography. In Application Report SBAA181; Texas Instruments: Dallas, TX, USA, 2011. [Google Scholar]
- Wang, F.T.; Chan, H.L.; Wang, C.L.; Jian, H.M.; Lin, S.H. Instantaneous Respiratory Estimation from Thoracic Impedance by Empirical Mode Decomposition. Sensors 2015, 15, 16372–16387. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, A.S.; Lenahan, J.L.; Izadnegahdar, R.; Ansermino, J.M. A Systematic Review of Tools to Measure Respiratory Rate in Order to Identify Childhood Pneumonia. Am. J. Respir. Crit. Care Med. 2018, 197, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Chen, K.; Dai, Y.; Zhang, S. Utility of transthoracic impedance and novel algorithm for sleep apnea screening in pacemaker patient. Sleep Breath. 2018, 23, 741–746. [Google Scholar] [CrossRef]
- Reinvuo, T.; Hannula, M.; Sorvoja, H.; Alasaarela, E.; Myllyla, R. Measurement of respiratory rate with high-resolution accelerometer and emfit pressure sensor. In Proceedings of the 2006 IEEE Sensors Applications Symposium, Houston, TX, USA, 7–9 February 2006; pp. 192–195. [Google Scholar]
- Phan, D.H.; Bonnet, S.; Guillemaud, R.; Castelli, E.; Thi, N.Y.P. Estimation of respiratory waveform and heart rate using an accelerometer. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 4916–4919. [Google Scholar]
- Bates, A.; Ling, M.J.; Mann, J.; Arvind, D. Respiratory Rate and Flow Waveform Estimation from Tri-axial Accelerometer Data. In Proceedings of the 2010 International Conference on Body Sensor Networks, Singapore, 7–9 June 2010; pp. 144–150. [Google Scholar]
- Liu, G.Z.; Guo, Y.W.; Zhu, Q.S.; Huang, B.Y.; Wang, L. Estimation of Respiration Rate from Three-Dimensional Acceleration Data Based on Body Sensor Network. Telemed. E-Health 2011, 17, 705–711. [Google Scholar] [CrossRef]
- Passaro, V.M.N.; Cuccovillo, A.; Vaiani, L.; Carlo, M.D.; Campanella, C.E. Gyroscope Technology and Applications: A Review in the Industrial Perspective. Sensors 2017, 17, 2284. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Jung, S. Gyro sensor drift compensation by Kalman filter to control a mobile inverted pendulum robot system. In Proceedings of the 2009 IEEE International Conference on Industrial Technology, Gippsland, VIC, Australia, 10–13 February 2009; pp. 1–6. [Google Scholar]
- Yoon, J.W.; Noh, Y.S.; Kwon, Y.S.; Kim, W.K.; Yoon, H.R. Improvement of Dynamic Respiration Monitoring Through Sensor Fusion of Accelerometer and Gyro-sensor. J. Electr. Eng. Technol. 2014, 9, 334–343. [Google Scholar] [CrossRef]
- Milici, S.; Lazaro, A.; Villarino, R.; Girbau, D.; Magnarosa, M. Wireless Wearable Magnetometer-Based Sensor for Sleep Quality Monitoring. IEEE Sens. J. 2018, 18, 2145–2152. [Google Scholar] [CrossRef]
- Yu, H.W.; Kim, H.K.; Kim, T.; Bae, K.M.; Seo, S.M.; Kim, J.M.; Kang, T.J.; Kim, Y.H. Self-Powered Humidity Sensor Based on Graphene Oxide Composite Film Intercalated by Poly(Sodium 4-Styrenesulfonate). ACS Appl. Mater. Interfaces 2014, 6, 8320–8326. [Google Scholar] [CrossRef] [PubMed]
- Cesareo, A.; Previtali, Y.; Biffi, E.; Aliverti, A. Assessment of Breathing Parameters Using an Inertial Measurement Unit (IMU)-Based System. Sensors 2018, 19, 88. [Google Scholar] [CrossRef] [PubMed]
- Vidjak, K.; Farina, L.; Ruvio, G.; O’Halloran, M.; Cavagnaro, M. Dielectric properties of healthy ex vivo ovine lung tissue at microwave frequencies. IEEE Trans. Dielectr. Electr. Insul. 2023, 30, 1162–1169. [Google Scholar] [CrossRef]
- Dicke, R.H. The measurement of thermal radiation at microwave frequencies. Rev. Sci. Instrum. 1946, 17, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Thurn, K.; Ebelt, R.; Vossiek, M. Noise in Homodyne FMCW radar systems and its e_ects on ranging precision. In Proceedings of the 2013 IEEE MTT-S International Microwave Symposium Digest (MTT), Seattle, WA, USA, 2–7 June 2013; pp. 1–3. [Google Scholar]
- Wang, G.; Munoz-Ferreras, J.M.; Gu, C.; Li, C.; Gomez-Garcia, R. Application of Linear-Frequency-Modulated Continuous-Wave (LFMCW) Radars for Tracking of Vital Signs. IEEE Trans. Microw. Theory Tech. 2014, 62, 1387–1399. [Google Scholar] [CrossRef]
- Wisland, D.T.; Granhaug, K.; Pleym, J.R.; Andersen, N.; Stoa, S.; Hjortland, H.A. Remote monitoring of vital signs using a CMOS UWB radar transceiver. In Proceedings of the 2016 14th IEEE International New Circuits and Systems Conference (NEWCAS), Vancouver, BC, USA, 26–29 June 2016; pp. 1–4. [Google Scholar]
- Li, C.; Lin, J. Complex signal demodulation and random body movement cancellation techniques for non-contact vital sign detection. In Proceedings of the 2008 IEEE MTT-S International Microwave Symposium Digest, Atlanta, GA, USA, 15–20 June 2008; pp. 567–570. [Google Scholar]
- Tang, M.-C.; Wang, F.-K.; Horng, T.-S. Single Self-Injection-Locked Radar with Two Antennas for Monitoring Vital Signs With Large Body Movement Cancellation. IEEE Trans. Microw. Theory Tech. 2017, 65, 5324–5333. [Google Scholar] [CrossRef]
- Mostafanezhad, I.; Yavari, E.; Boric-Lubecke, O.; Lubecke, V.M.; Mandic, D.P. Cancellation of Unwanted Doppler Radar Sensor Motion Using Empirical Mode Decomposition. IEEE Sens. J. 2013, 13, 1897–1904. [Google Scholar] [CrossRef]
- Kazemi, S.; Ghorbani, A.; Amindavar, H.; Li, C. Cyclostationary approach to Doppler radar heart and respiration rates monitoring with body motion cancelation using Radar Doppler System. Biomed. Signal Process. 2014, 13, 79–88. [Google Scholar] [CrossRef]
- Wei, J.; Huang, L.; Tong, P.; Tan, B.; Bai, J.; Wu, Z. Realtime multi-target vital sign detection with 79ghz fmcw radar. In Proceedings of the 2020 IEEE MTT-S International Wireless Symposium (IWS), Shanghai, China, 20–23 September 2020; pp. 1–3. [Google Scholar]
- Dwinanda, A.R.; Pramudita, A.A.; Nugroho, B.S.; Dhiyani, A.A. In Effects of Drone Height Fluctuations on Detection of Respiratory Vital Signs Using FMCW Radar. In Proceedings of the 2023 IEEE International Symposium on Antennas and Propagation (ISAP), Kuala Lumpur, Malaysia, 30 October–2 November 2023; pp. 1–2. [Google Scholar]
- Numan, P.E.; Park, H.; Lee, J.; Kim, S. Machine learning-based joint vital signs and occupancy detection with IR-UWB sensor. IEEE Sens. J. 2023, 23, 7475–7482. [Google Scholar] [CrossRef]
- Chen, F.K.; Wang, Y.K.; Lin, H.P.; Chen, C.Y.; Yeh, S.M.; Wang, C.Y. In Feasibility study for apnea screening in patients’ homes using radar and machine learning method. In Proceedings of the 2022 IEEE 22nd International Conference on Bioinformatics and Bioengineering (BIBE), Taichung, Taiwan, 7–9 November 2022; pp. 282–287. [Google Scholar]
- Rihan, M.; Huang, L. Optimum co-design of spectrum sharing between MIMO radar and MIMO communication systems: An interference alignment approach. IEEE Trans. Veh. Technol. 2018, 67, 11667–11680. [Google Scholar] [CrossRef]
- Obadi, A.B.; Soh, P.J.; Aldayel, O.; Al-Doori, M.H.; Mercuri, M.; Schreurs, D. A survey on vital signs detection using radar techniques and processing with FPGA implementation. IEEE Circuits Syst. Mag. 2021, 21, 41–74. [Google Scholar] [CrossRef]
- Mayo Clinic. Chest X-rays. 2022. Available online: https://www.mayoclinic.org/tests-procedures/chest-x-rays/about/pac-20393494. (accessed on 20 June 2024).
- Volpicelli, G.; Mussa, A.; Garofalo, G.; Cardinale, L.; Casoli, G.; Perotto, F.; Fava, C.; Frascisco, M. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am. J. Emerg. Med. 2006, 24, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Seibel, A.; Zechner, P.M.; Berghold, A.; Holter, M.; Braß, P.; Michels, G.; Leister, N.; Gemes, G.; Donauer, R.; Giebler, R.M. B-Lines for the assessment of extravascular lung water: Just focused or semi-quantitative? Acta Anaesthesiol. Scand. 2020, 64, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Jiang, L.; Xi, X.; Jiang, Q.; Zhu, B.; Wang, M.; Xing, J.; Zhang, D. Prognostic value of extravascular lung water assessed with lung ultrasound score by chest sonography in patients with acute respiratory distress syndrome. BMC Pulm. Med. 2015, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Chiumello, D.; Mongodi, S.; Algieri, I.; Vergani, G.L.; Orlando, A.; Via, G.; Crimella, F.; Cressoni, M.; Mojoli, F. Assessment of lung aeration and recruitment by CT scan and ultrasound in acute respiratory distress syndrome patients*. Crit. Care Med. 2018, 46, 1761–1768. [Google Scholar] [CrossRef]
- Lichtenstein, D.A. Lung ultrasound in the critically ill. Ann. Intensive Care 2014, 4, 1. [Google Scholar] [CrossRef]
- Volpicelli, G.; Skurzak, S.; Boero, E.; Carpinteri, G.; Tengattini, M.; Stefanone, V.; Luberto, L.; Anile, A.; Cerutti, E.; Radeschi, G. Lung ultrasound predicts well extravascular lung water but is of limited usefulness in the prediction of wedge pressure. Anesthesiology 2014, 121, 320–327. [Google Scholar] [CrossRef]
- Grimnes, S.; Martinsen, Ø.G. Bioimpedance and Bioelectricity Basics; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Brown, B. Review of EIT systems available for medical use. In Clinical and Physiological Applications of Electrical Impedance Tomography; Holder, D., Ed.; UCL Press: London, UK, 1993; pp. 41–45. [Google Scholar]
- Chitturi, V.; Farrukh, N. Spatial resolution in electrical impedance tomography: A topical review. J. Electr. Bioimpedance 2017, 8, 66–78. [Google Scholar] [CrossRef]
- Gagarin, R.; Celik, N.; Youn, H.S.; Iskander, M.F. Microwave stethoscope: A new method for measuring human vital signs. In Proceedings of the 2011 IEEE International Symposium on Antennas and Propagation (APSURSI), Spokane, WA, USA, 3–8 July 2011. [Google Scholar]
- Gabriel, C. Compilation of the Dielectric Properties of Body Tissues at RF and Microwave Frequencies. King’s College London, Department of Physics: London, UK, 1996. [Google Scholar]
- Abd El-Hameed, A.S.; Elsheakh, D.M.; Elashry, G.M.; Abdallah, E.A. A Comparative Study of Narrow/Ultra-Wideband Microwave Sensors for the Continuous Monitoring of Vital Signs and Lung Water Level. Sensors 2024, 24, 1658. [Google Scholar] [CrossRef] [PubMed]



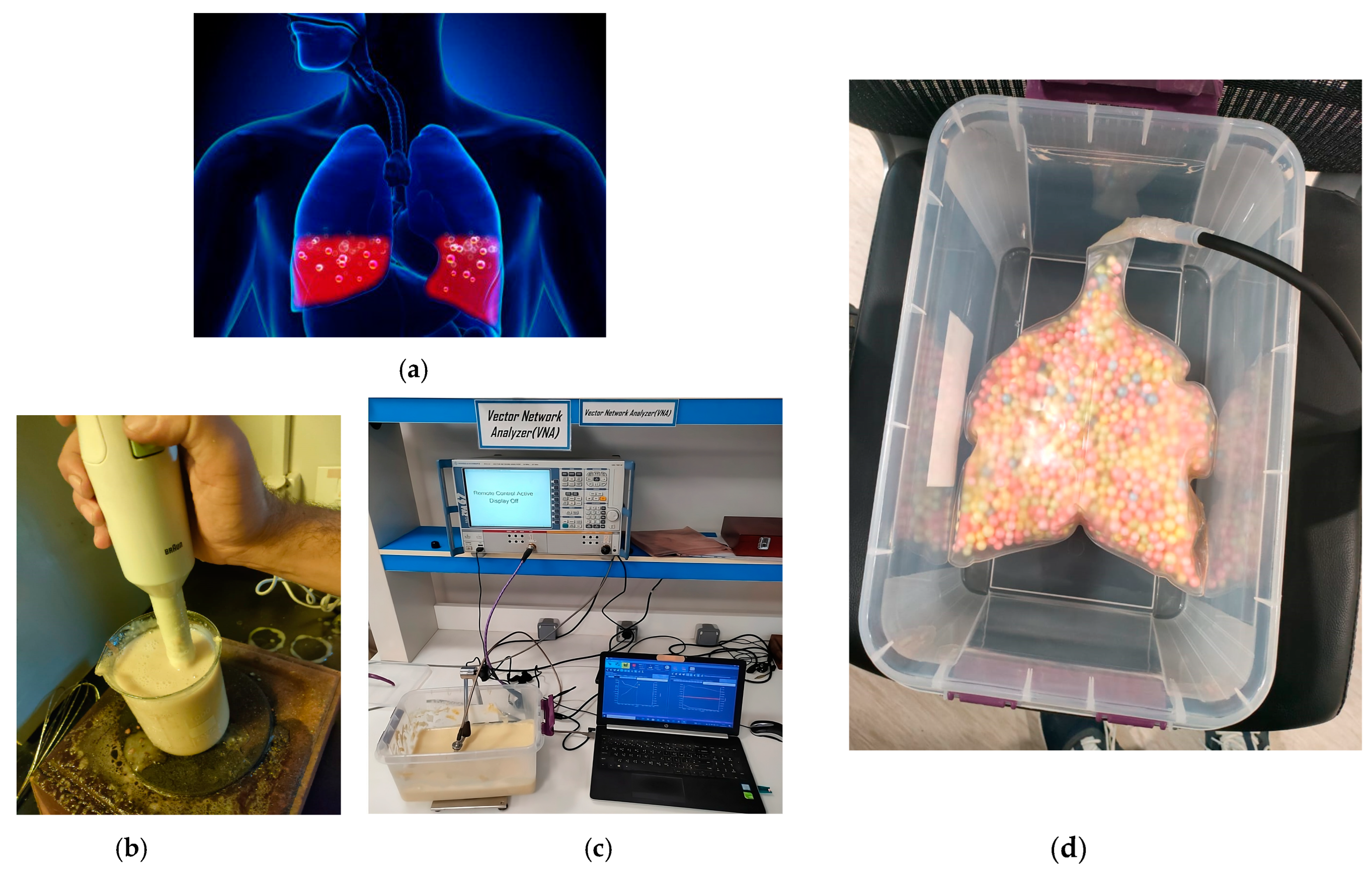
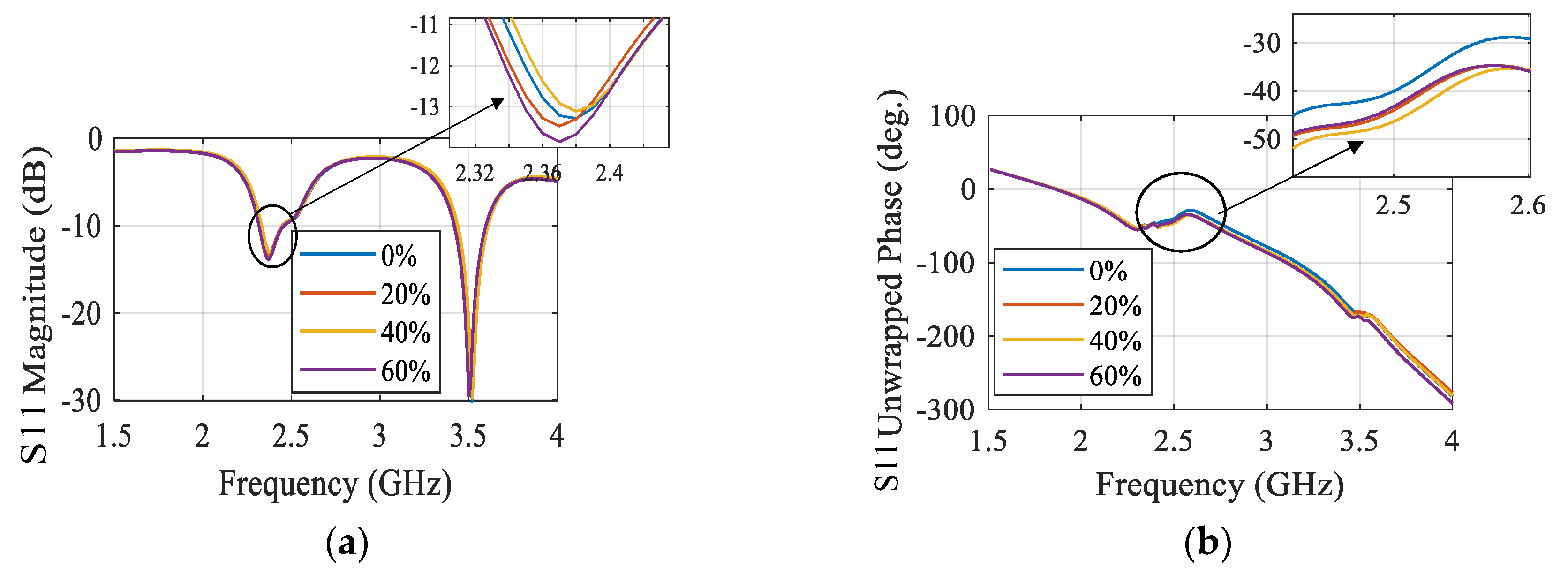
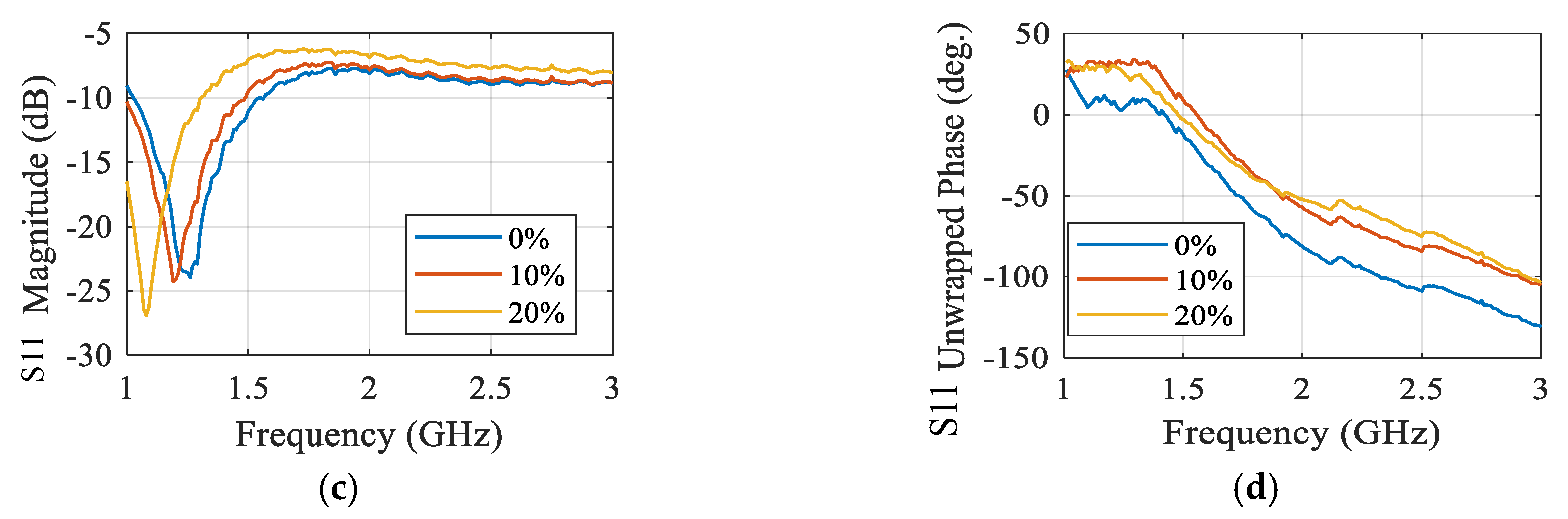
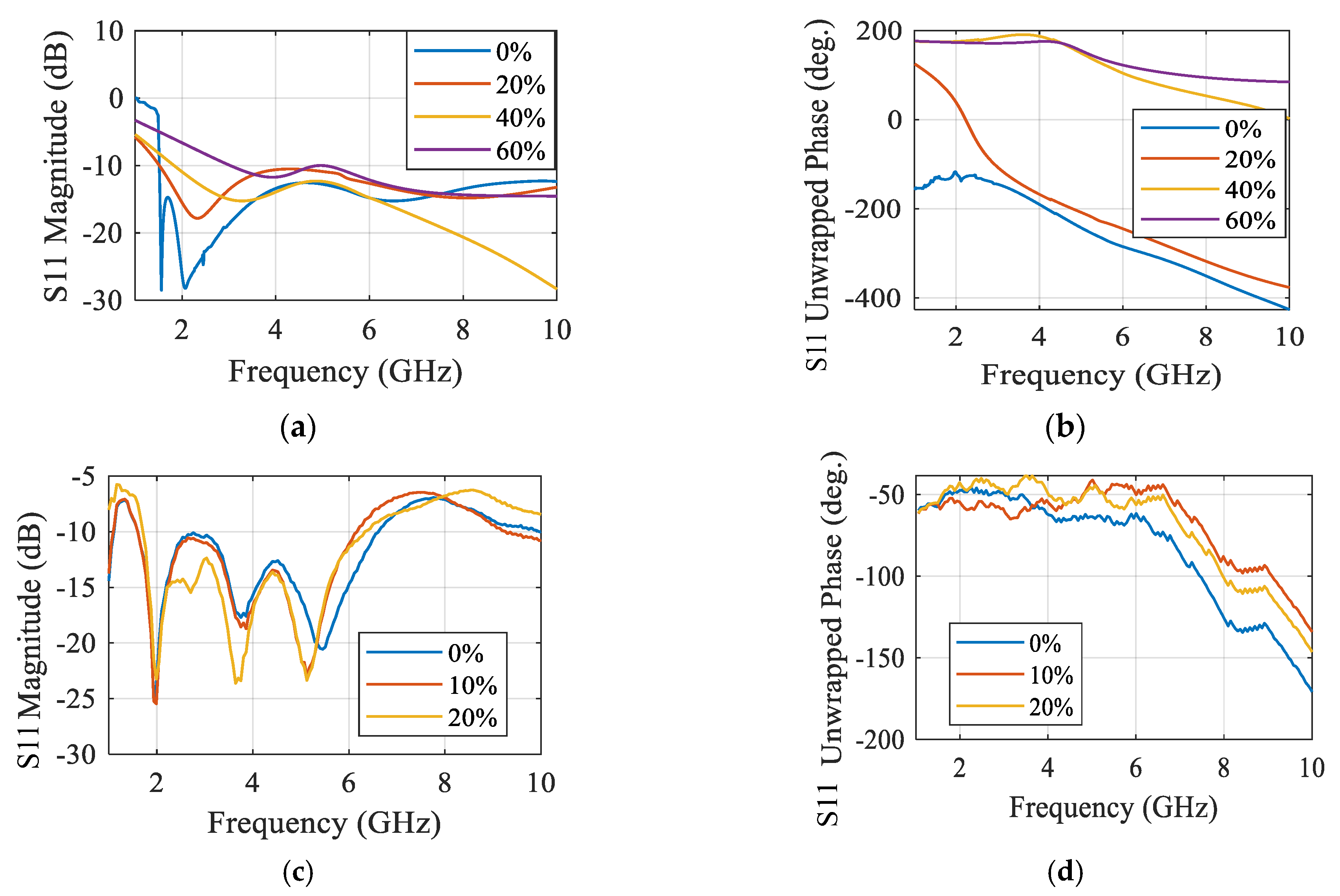
| Comparison | Radar-Based Sensors | Traditional Methods |
|---|---|---|
| Cost | -Initial Cost: Generally higher due to the advanced technology and integration required. -Long-Term Cost: Potentially lower because they often require less maintenance and fewer consumables (e.g., no need for adhesive electrodes). -Cost Efficiency: Improved over time as technology becomes more widespread and production scales up. | -Initial Cost: Typically, lower. Devices like blood pressure cuffs, thermometers, and pulse oximeters are relatively inexpensive. -Long-Term Cost: Can add up due to the need for regular replacement of parts (e.g., batteries, electrodes, cuffs) and possible maintenance. |
| Ease of Use | -Non-Intrusive: Can be used without direct contact with the body, making them very easy to use. -Setup: Generally easy to set up, often requiring minimal user intervention once installed. -Integration: Can be integrated into furniture (e.g., beds, chairs) or used in wearable formats, further simplifying use. | -Contact-Based: Often require direct contact with the skin, which can be cumbersome and uncomfortable over long periods. -Setup: Somewhat more involved, especially for devices like Holter monitors or traditional ECGs, which require proper placement of electrodes. -Usability: While generally user-friendly, repeated setup and use can be more time-consuming and intrusive compared to radar sensors. |
| Patient Compliance Effectiveness and Accuracy | -Comfort: High, as they do not require direct skin contact and can be unobtrusively integrated into daily life. -Wearability: Non-contact models are especially beneficial for patients who find wearables uncomfortable. -Long-Term Monitoring: Excellent for long-term, continuous monitoring that does not need patient involvement and encourages high compliance. | -Comfort: Variable. Devices like blood pressure cuffs or Holter monitors can be uncomfortable over time. -Wearability: Continuous wearables (e.g., Holter monitors) can be intrusive and uncomfortable, potentially reducing compliance. -Long-Term Monitoring: requires frequent patient engagement, which may reduce compliance (e.g., reattaching sensors, replacing batteries). |
| Long-Term Monitoring Scenarios | -Accuracy: can be quite accurate when monitoring factors like breathing and heart rate, but they could have trouble being as exact as clinical-grade equipment when it comes to readings. -Interference: sensitive to ambient influences and motion artefacts, which may compromise accuracy. -Continuous Monitoring: Well-suited for continuous monitoring in home settings, providing constant data without patient involvement. -Data Integration: Can be integrated with health monitoring systems for real-time data collection and analysis. -Patient Lifestyle: Minimal disruption to daily activities, which is crucial for long-term compliance. | -Accuracy: often high, particularly at institutions with strict regulations (e.g., hospitals). Gold standards include tried-and-true techniques like ECGs, sphygmomanometers, and pulse oximeters. -Interference: less influenced by external circumstances, but still vulnerable to aberrations from patient motion or incorrect sensor positioning. -Continuous Monitoring: Devices like Holter monitors provide continuous monitoring but are limited to short periods (usually 24–48 h) due to discomfort and battery life. -Data Integration: Often requires manual data retrieval and analysis, which can be cumbersome for long-term monitoring. -Patient Lifestyle: Can be disruptive, requiring frequent adjustments and maintenance. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El-Hameed, A.S.; Elsheakh, D.M.; Elashry, G.M.; Abdallah, E.A. Cutting-Edge Microwave Sensors for Vital Signs Detection and Precise Human Lung Water Level Measurement. Magnetism 2024, 4, 209-239. https://doi.org/10.3390/magnetism4030015
Abd El-Hameed AS, Elsheakh DM, Elashry GM, Abdallah EA. Cutting-Edge Microwave Sensors for Vital Signs Detection and Precise Human Lung Water Level Measurement. Magnetism. 2024; 4(3):209-239. https://doi.org/10.3390/magnetism4030015
Chicago/Turabian StyleAbd El-Hameed, Anwer S., Dalia M. Elsheakh, Gomaa M. Elashry, and Esmat A. Abdallah. 2024. "Cutting-Edge Microwave Sensors for Vital Signs Detection and Precise Human Lung Water Level Measurement" Magnetism 4, no. 3: 209-239. https://doi.org/10.3390/magnetism4030015
APA StyleAbd El-Hameed, A. S., Elsheakh, D. M., Elashry, G. M., & Abdallah, E. A. (2024). Cutting-Edge Microwave Sensors for Vital Signs Detection and Precise Human Lung Water Level Measurement. Magnetism, 4(3), 209-239. https://doi.org/10.3390/magnetism4030015








