Abstract
Since 1995, it has been known that carbohydrate drinks (CHDs) can be safely consumed two to three hours (2–3 h) preoperatively. Furthermore, preoperative CHDs significantly benefit many outcomes, such as thirst, hunger, and insulin resistance. Patients, however, still fast excessively. This study aimed to determine if a CHD, consumed 2–3 h preoperatively, impacts postoperative inflammation compared to a placebo drink or fasting. This was achieved through analysing the levels of interleukin-6, C-reactive peptide, and serum albumin 10–24 h postoperatively. We conducted a systematic review of randomised control trials. We comprehensively searched the Embase, MEDLINE and Web of Science databases, identified 473 studies, and, after screening, were left with 10 randomised control trials. Our meta-analyses found a significantly lower mean interleukin-6 level of −21.26 pg/mL ((95% CI −33.37, −9.15); p = 0.0006) postoperatively in patients given a preoperative CHD compared to fasting and a significantly higher mean serum albumin level of 2.56 g/L ((95% CI 1.41, 3.71); p < 0.0001) postoperatively in patients given a preoperative CHD compared to a placebo. Our results therefore show that a CHD, consumed 2–3 h preoperatively, lowers proinflammatory cytokine levels and increases serum albumin levels. Thus, our study reinforces guideline recommendations to give patients a CHD 2–3 h preoperatively for improved outcomes.
1. Introduction
The historical consensus for over a century was that patients should fast from midnight until their operation to prevent the aspiration of gastric contents into the stomach following general anaesthesia [1,2]. However, the futility of this practice was demonstrated by Nygren et al. [2] in 1995 and Lobo et al. [3] in 2009 as they discovered the complete emptying of gastric contents up to three hours (3 h) after the consumption of a carbohydrate drink (CHD). Furthermore, Brady et al. [4] found that gastric contents and pH are no different in patients fasting from midnight and those who consume a CHD 2 h before an operation. Consequently, since 2005, surgical guidelines have consistently recommended that patients should continue to consume CHDs up to 2–3 h preoperatively [5,6,7,8,9]. Furthermore, consuming a preoperative CHD is more beneficial than fasting [10].
Preoperative CHDs reduce patients’ weakness, hunger, and thirst without increasing the risk of aspiration when compared to fasting [10]. Furthermore, intravenous infusions are not as beneficial as CHDs [10], especially for thirst [10], which is the most troublesome complication of fasting [10,11]. Preoperative CHDs may also shorten the length of hospital stay [12] and thus reduce the overall cost of care. However, despite the safety of preoperative CHDs and the wide range of benefits, there appears to be a consistent lack of conformity to this recommendation [13,14]. A recent audit by Degeeter et al. [14], for example, found that patients had an average fluid fasting time of just under 10 h and thus were subject to unnecessary discomfort [14]. Such extensive fasting also increases the catabolic state of patients [15] and appears to increase their postoperative inflammation [16], thus impacting their recovery.
Surgery is very traumatic to the body, and the acute immune cells respond via triggering a systemic inflammatory response [17]. This postsurgical inflammatory response has been suggested as the main cause of severe complications, such as organ failure and death [18,19]. Cytokines, such as interleukin-6 (IL-6), are released by macrophages in response to surgery [17] and stimulate the liver hepatocytes to produce and release acute phase proteins (APPs), such as C-reactive peptide (CRP) [17], which trigger a systematic inflammatory response [17]. IL-6 is one of the most useful markers of inflammation as it has been shown to independently predict postsurgical complications, such as sepsis and mortality [18,20,21]. Furthermore, CRP is another commonly used marker of inflammation post surgically [22,23]. Additionally, the liver’s increased production of APPs results in the reduced production and release of transporter proteins, such as albumin; hence, a reduction in serum albumin is another useful marker of inflammation [24,25]. Two systematic reviews [22,23] have previously investigated the impact of a preoperative CHD on postoperative inflammation, but they only included CRP levels as an inflammatory marker and also did not include all relevant trials. Thus, an updated systematic review is required which investigates different postoperative inflammatory markers.
The first primary outcome of this systematic review is to determine whether a CHD, consumed 2–3 h before an elective operation, reduces 10–24 h postoperative IL-6 levels when compared to patients who are made to fast ≥6 h preoperatively. The second primary outcome is the same as the first, except the control comparison to the CHD is a non-carbohydrate-containing placebo drink (PD) consumed at the same time. There are also four secondary outcomes: (i) to determine whether a CHD, consumed 2–3 h before an elective operation, reduces 10–24 h postoperative CRP levels when compared to patients who are made to fast ≥6 h preoperatively; (ii) the same as (i), except the control comparison to the CHD is a non-carbohydrate-containing PD consumed at the same time; (iii) to determine whether a CHD, consumed 2–3 h before an elective operation, increases the 10–24 h postoperative serum albumin levels when compared to patients who are made to fast ≥6 h preoperatively; (iv) the same as (iii), except the control comparison to the CHD is a non-carbohydrate-containing PD consumed at the same time.
2. Materials and Methods
2.1. Literature Search
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [26] were followed to undertake this systematic review. On 4 June 2023, two independent researchers (K.Z.H. and Z.A.) comprehensively searched the Embase, MEDLINE, and Web of Science databases, with no date restrictions. Furthermore, reference lists were screened, and Google Scholar was scoured to identify additional studies. The searches utilised Boolean operators, and the following terms were included in all database searches: (“Oral carbohydrate” OR “Carbohydrate drink” OR “CHD” OR “Preoperative carbohydrate”) AND (“C-reactive protein” OR “C-reactive peptide” OR “CRP” OR “Inflammation” OR “Inflammatory” OR “Interleukin-6” OR “Interleukin 6” OR “IL-6” OR “IL6” OR “Albumin”)) AND (“Operation” OR “Surgery” OR “Preoperative” OR “Operative”). These terms were applied to all fields, ensuring all relevant studies were captured.
2.2. Inclusion and Exclusion Criteria
The articles obtained from the database searches were screened according to our inclusion and exclusion criteria (Table 1). Studies were not restricted by language, date, nor sample size. Diabetic patients were excluded as they require specific preoperative regimes to manage their abnormal glucose metabolism and thus cannot be pooled with non-diabetic patients.

Table 1.
Inclusion and exclusion criteria.
2.3. Data Collection
All studies obtained from the database searches were transferred to Microsoft Excel using each database’s export function. Then, each of the two researchers (K.Z.H. and Z.A.) independently screened the titles and abstracts and selected relevant studies for full-text analysis. Each researcher then read the full articles of the selected studies and excluded studies that did not meet the inclusion and exclusion criteria. Any disagreements were resolved after the selection process through discussion between the two researchers. Where data were missing or unavailable, authors were contacted on multiple occasions to provide raw data, but we received no responses.
2.4. Data Extraction
One of the researchers (K.Z.H.) extracted the data from the included studies. The following demographics data were extracted from each study: location, year of publication, number of patients, type of surgery, patient groupings, carbohydrate content of drinks, non-carbohydrate content of drinks, timing of drinks, volume of drinks, and length of fasting. Also, the following outcomes data were collected: mean IL-6 and/or CRP and/or serum albumin levels 10–24 h postoperatively and confidence intervals (CIs) (standard deviation (SD)). Finally, any additional information to assist in the risk of bias (RoB) calculation and discussion was taken from each study.
2.5. Risk of Bias
Two researchers (K.Z.H. and Z.A.) independently judged the RoB for each study using the Cochrane Risk of Bias Tool 2 (RoB2) designed for randomised control trials (RCTs) [27]. RoB2 has five domains that focus on these potential sources of bias: the process of randomisation, any deviations from predetermined interventions, missing data, how the outcomes were measures, and how the results were reported [27]. For every study, each domain was given one of the following bias risk scores: low risk, some concerns, or high risk [27]. Each study was then given an overall RoB score, and any disagreements were resolved through discussion between the two researchers.
2.6. Statistical Analysis
Where three or more RCTs reported the same outcome using comparable approaches, data were pooled to perform a meta-analysis using RevMan 4.0 software (Cochrane, London, UK), employing a random effects model, reporting mean differences and confidence intervals. Heterogeneity in the studies was assessed using I2, Chi2, and Tau2 statistics.
3. Results
3.1. Study Selection
A total of 458 studies were obtained from the three database searches, and an additional 15 studies were identified through reference screening and searching Google Scholar. These studies’ titles and abstracts were screened, and 386 were subsequently excluded. Duplicates were then removed from the remaining 87 studies, leaving 31 at the end of the screening phase. Full texts were obtained, when possible, for the remaining 31 studies, and a further 21 were excluded due to a lack of conformity to the inclusion and exclusion criteria. These were excluded for the following reasons: the intervention and/or control were inappropriate; absent relevant inflammatory marker results or inappropriate collection times; absent mean results with CIs; the journal was discontinued, thus, the article was absent; and the inclusion of diabetes patients. The remaining 10 studies [16,19,24,28,29,30,31,32,33,34] were all included in the systematic review and meta-analysis. A PRISMA flowchart detailing the study selection process is provided in Figure 1.
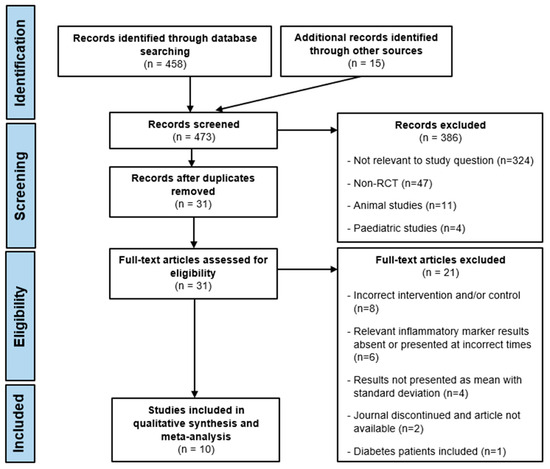
Figure 1.
PRISMA flow diagram.
3.2. Study Characteristics
The 10 studies [16,19,24,28,29,30,31,32,33,34] included are all RCTs that ran between 2010 and 2021 and were conducted in six different countries: China, Korea, Bosnia and Herzegovina, Turkey, Czech Republic, and Brazil. Also, all together, these trials included 718 patients (317 males and 401 females) and involved the following surgeries: prostatectomy, femur fracture repair, hip surgery, gynaecological surgery, colorectal surgery, lumbar discectomy, laminectomy, cholecystectomy, and inguinal hernia repair. Seven trials gave the CHD 2 h perioperatively [16,28,30,31,32,33,34], one 2–3 h preoperatively [19], and the remaining two 3 h preoperatively [24,29]. Additionally, six trials used a pure CHD [16,19,30,31,32,33], whereas the remaining four used a CHD mixed sometimes with protein, vitamins, and minerals [24,28,29,34]. Table 2 includes the detailed demographic information of each study.

Table 2.
Demographics table of included studies.
3.3. Risk of Bias
RoB was calculated for each study using the RoB2 tool [27], and the individual studies and overall results are presented in Figure 2. Overall, six studies had a high RoB [24,29,30,31,32,33], and the remaining four had some concerns (Figure 2) [16,19,28,34]. The high risks were either due to some deviation in patient management after randomisation without incorporating an intention to treat analysis [27], resulting in missing data (five trials), [24,29,31,32,33] and/or due to problems with the randomisation process (two trials) [24,30]. Additionally, as the studies did not specify whether the measured and reported inflammatory markers were in line with a predetermined protocol, they all had some concerns of bias in the selection of the reported results.
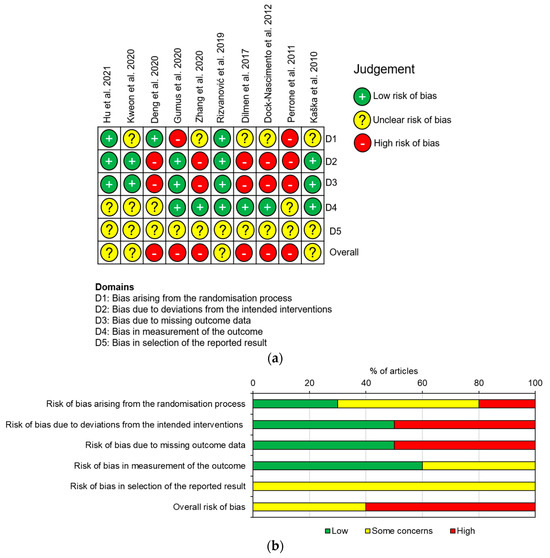
Figure 2.
RoB of the included RCTs according to the RoB2: (a) demonstrates the RoB of each individual study; (b) demonstrates the overall RoB for all the studies [16,19,24,28,29,30,31,32,33,34].
3.4. Primary Outcomes
Regarding the first primary outcome, six RCTs [16,19,28,31,32,33] measured IL-6 levels 10–24 h postoperatively in fasting patients and those who consumed a preoperative CHD. All of the RCTs gave the CHD 2 h preoperatively, except Hu et al. [19], which gave the CHD 2–3 h preoperatively. Furthermore, they all measured IL-6 levels around 24 h postoperatively, except Dock-Nascimento et al. [33], which measured the IL-6 level 10 h postoperatively. Additionally, patients fasted 6–8 h preoperatively across the six RCTs. All six RCTs reported a lower postoperative mean IL-6 level in the CHD group, and Hu et al. [19], Zhang et al. [31], and Rizvanović et al. [16] reported significantly lower mean values. A meta-analysis of the pooled data demonstrated a significantly lower mean IL-6 level of −21.26 pg/mL ((95% CI −33.37, −9.15); p = 0.0006) 10–24 h postoperatively in patients who consumed a CHD 2–3 h preoperatively compared to patients who were made to fast 6–8 h preoperatively (Figure 3).
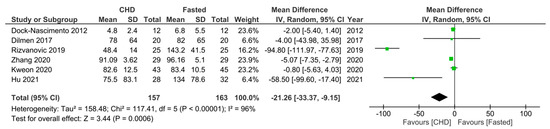
Figure 3.
Forest plot to show 10–24 h postoperative IL-6 levels (pg/mL) in fasting patients and those who consumed a CHD 2–3 h preoperatively [16,19,28,31,32,33].
Regarding the second primary outcome, two RCTs [19,33] measured IL-6 levels 10–24 h postoperatively in patients who consumed a preoperative CHD or PD. The mean ± SD for each can be found in Table 3. Both studies gave the drinks 2 h preoperatively and Hu et al. [19] measured the IL-6 24 h postoperatively whereas Dock-Nascimento et al. [33] 10 h postoperatively. Both reported a lower mean IL-6 level in the CHD group, but only Hu et al. [19] reported a significantly lower mean. Given only two RCTs were identified, a meta-analysis was not conducted as it would have a low power [35].

Table 3.
Postoperative IL-6 levels (pg/mL) for primary outcome 2 (CHD vs. placebo patients).
3.5. Secondary Outcomes
Regarding the first secondary outcome, five RCTs [16,30,31,33,34] measured CRP levels 10–24 h postoperatively in fasting patients and those who consumed a preoperative CHD. All the trials gave the CHD 2 h preoperatively and all measured the CRP level around 24 h postoperatively, except that of Dock-Nascimento et al. [33], which measured the CRP level 10 h postoperatively. Additionally, patients fasted 8–12 h preoperatively across the five RCTs. All the RCTs, except that of Gumus et al. [30], reported a lower mean CRP level in the CHD group, with significantly lower mean levels in Zhang et al. [31], Rizvanović et al. [16], and Dock-Nascimento et al. [33]. However, Gumus et al. [30] reported a significantly higher postoperative mean CRP level in the CHD group. A meta-analysis of the pooled data demonstrated a non-significantly lower mean CRP level of −5.02 mg/L ((95% CI −11.52, 1.49); p = 0.13) 10–24 h postoperatively in patients who consumed a CHD 2 h preoperatively compared to patients who were made to fast 8–12 h preoperatively (Figure 4).
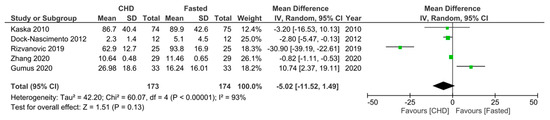
Figure 4.
Forest plot to show 10–24 h postoperative CRP levels (mg/L) in fasting patients and those who consumed a CHD 2 h preoperatively [16,30,31,33,34].
Regarding the second secondary outcome, three RCTs [24,29,33] measured CRP levels 10–24 h postoperatively in patients who consumed a preoperative CHD or PD. Perrone et al. [24] and Deng et al. [29] gave the drinks 3 h preoperatively and measured the CRP levels 24 h postoperatively, whereas Dock-Nascimento et al. [33] gave the drinks 2 h preoperatively and measured the CRP level 10 h postoperatively. All three RCTs reported a lower mean CRP level postoperatively in the CHD group, with only Deng et al. [29] reporting a significantly lower mean level. A meta-analysis of the pooled data demonstrated a non-significantly lower mean CRP level of −7.32 mg/L ((95% CI −18.53, 3.89); p = 0.20) 10–24 h postoperatively in patients who consumed a CHD 2–3 h preoperatively compared to patients who consumed a PD (Figure 5).
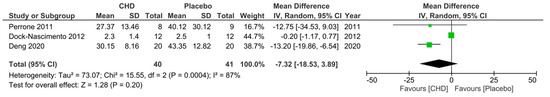
Figure 5.
Forest plot to show 10–24 h postoperative CRP levels (mg/L) in patients who consumed a CHD and PD 2–3 h preoperatively [24,29,33].
Regarding the third secondary outcome, three RCTs [16,30,33] measured serum albumin levels 10–24 h postoperatively in fasting patients and those who consumed a preoperative CHD. All the RCTs gave the CHD 2 h preoperatively and measured the serum albumin levels around 24 h postoperatively, except that of Dock-Nascimento et al. [33], which measured the serum albumin level 10 h postoperatively. Additionally, patients fasted 8–12 h preoperatively across the three RCTs. For the CHD group, Gumus et al. [30] reported a non-significantly lower mean serum albumin level, Dock-Nascimento et al. [33] reported the exact same one, and Rizvanović et al. [16] reported a significantly higher postoperative mean serum albumin level. A meta-analysis of the pooled data demonstrated no significant difference (0.06, 95% CI [−2.74, 2.87], p = 0.97) in the 10–24 h postoperative serum albumin level between patients who consumed a CHD 2 h preoperatively compared to patients who were made to fast 8–12 h preoperatively (Figure 6).
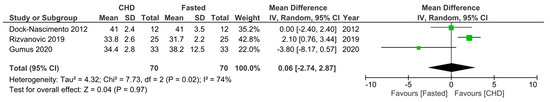
Figure 6.
Forest plot to show 10–24 h postoperative serum albumin levels (g/L) in fasting patients and those who consumed a CHD 2 h preoperatively [16,30,33].
Regarding the fourth secondary outcome, three RCTs [24,29,33] measured serum albumin levels 10–24 h postoperatively in patients who consumed a preoperative CHD or PD. Perrone et al. [24] and Deng et al. [29] gave the drinks 3 h preoperatively and measured serum albumin levels 24 h postoperatively, whereas Dock-Nascimento et al. [33] gave the drinks 2 h preoperatively and measured the serum albumin level 10 h postoperatively. They all demonstrated a higher mean serum albumin level postoperatively in the CHD group, with Deng et al. [29] reporting a significantly higher mean level. A meta-analysis of the pooled data demonstrated a significantly higher mean serum albumin level of 2.56 g/L ((95% CI 1.41, 3.71); p < 0.0001) 10–24 h postoperatively in patients who consumed a CHD 2–3 h preoperatively compared to patients who consumed a PD (Figure 7).
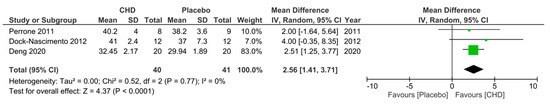
Figure 7.
Forest plot to show 10–24 h postoperative serum albumin levels (g/L) in patients who consumed a CHD and PD 2–3 h preoperatively [24,29,33].
4. Discussion
This systematic review aimed to determine whether a CHD, consumed 2–3 h preoperatively, would reduce postoperative inflammation compared to a PD or fasting. Through conducting a meta-analysis, we found a significantly lower postoperative IL-6 level in patients given a preoperative CHD compared to fasting patients. Furthermore, we also found, through another meta-analysis, a significantly higher postoperative serum albumin level in patients given a preoperative CHD compared to a PD. This is, thus, the first systematic review suggesting significantly lower postoperative inflammation following a preoperative CHD compared to a PD and compared to fasting. As postoperative inflammation [18,19] and specifically IL-6 levels [18,20,21] are independent risk factors for postoperative complications and mortality, these results reinforce the many guidelines that recommend a CHD 2–3 h preoperatively [5,6,7,8,9]. Our other secondary outcomes, however, demonstrated non-significantly lower CRP levels following a preoperative CHD compared to a PD or fasting and no significant difference in the postoperative serum albumin level following a preoperative CHD compared to fasting. This reduces the strength of the hypothesis that a preoperative CHD reduces postoperative inflammation; however, when considering the other benefits of a preoperative CHD [10,11,12] and our significant IL-6 and albumin results, clinicians should ensure they give patients a CHD 2–3 h preoperatively and not prolong their fasting period.
Six systematic reviews and meta-analyses [22,23,36,37,38,39] have previously been conducted measuring different non-inflammatory outcomes in patients who fast or are given a CHD or PD preoperatively. Tong et al. [23] reported no significant differences in the levels of postoperative thirst, hunger, and anxiety between the three cohorts, whereas Cheng et al. [39] reported significantly better outcomes with a larger number of patients given a preoperative CHD compared to fasting patients and significantly better hunger levels in patients given a preoperative CHD compared to a PD. Additionally, Tong et al. [23] demonstrated a significant reduction in postoperative infection in patients given a preoperative CHD compared to fasting patients, whilst Cheng et al. [39] found no significant differences. Furthermore, Ricci et al. [22] found no significant differences in postoperative morbidity amongst any of the three cohorts. Finally, none of the systematic reviews [22,23,36,37,38,39] found significant differences in intraoperative aspiration between fasting patients and those given a preoperative drink, highlighting the safety of preoperative drinks. It appears any clear drink, given 2–3 h preoperatively, is safe and may improve patient satisfaction [39] and the rates of postoperative infection [23], and thus unnecessarily fasting for longer than 2–3 h is inappropriate for patients.
The four systematic reviews [23,36,38,39] that reported insulin resistance found it to be significantly lower in patients given a preoperative CHD compared to a PD. Insulin resistance itself is an independent risk factor for postoperative complications [40] and results in protein depletion [41], reduced wound healing efficiency [33], a weakened immune system [33], and muscle fatigue, thus impeding recovery [37]. The significant reductions in insulin resistance postoperatively after a preoperative CHD [23,36,38,39] support the guideline recommendations of a CHD given 2–3 h preoperatively over a PD [5,6,7,8,9].
Two systematic reviews, those of Ricci et al. [22] and Tong et al. [23], were both published in 2022 and both only included postoperative CRP levels as a marker of inflammation. However, there a number of reasons an updated CRP search and analysis was justified in our study. Firstly, Ricci et al. [22] only compared outcomes after abdominal surgeries, whereas this systematic review includes all elective surgeries. Additionally, Ricci et al. [22] did not conduct separate meta-analyses for placebo-controlled and fasting-controlled RCTs. Furthermore, Ricci et al. [22] and Tong et al. [23] included an RCT by Braga et al. [42], despite patients in both the intervention and control groups being given a carbohydrate-containing drink. Ricci et al. [22] concluded that a CHD infused with antioxidants is the most optimal preoperative CHD but only included one RCT with an antioxidant-infused CHD, thus limiting the quality of this result. Additionally, Tong et al. [23] included an RCT by Pexe-Mechado et al. [43], which measured the CRP levels on the second postoperative day, despite claiming all CRP level measurements must be within the first 24 h. Tong et al. [23], like our systematic review, separated the fasting-controlled and placebo-controlled RCTs for separate analysis and also found no significant differences in the postoperative CRP levels between these cohorts. Our CRP analysis, however, included RCTs by Gumus et al. [30] and Deng et al. [29] that were not included in the previous systematic reviews’ [22,23] CRP meta-analyses, thus providing a more comprehensive and updated result.
Our study found significant reductions in postoperative inflammation. For example, we found significantly lower IL-6 levels postoperatively in patients given a preoperative CHD compared to fasting patients, and significantly higher serum albumin levels in patients given a preoperative CHD compared to a PD. As mentioned previously, less inflammation postoperatively improves recovery and reduces complications [18,19]. Hence, we urge clinicians to follow the many guidelines that recommend giving patients a CHD 2–3 h preoperatively [5,6,7,8,9] and stop the harmful practice of excessively and unnecessarily making patients fast [13,14]. As for lowering IL-6 levels through preoperative CHD loading, this topic needs more studies to assess clinical outcomes to demonstrate a reduction in morbidity.
Despite studies consistently demonstrating complete gastric emptying up to 3 h after the consumption of a CHD [2,3] and guidelines since 2005 recommending a CHD 2–3 h preoperatively [5,6,7,8,9], clinicians persistently prolong fluid fasting before elective procedures [13,14]. For example, an audit in 2014 [13] and another in 2023 [14] found average fluid-fasting times of over 9.5 h before an operation. This was partly due to misinformed staff believing prolonged fasting is more beneficial and advising patients to fast from the midnight before their operation [13,14]. Furthermore, patients themselves, despite being given the correct advice, prolonged their fast due to a lack of appreciation of the dangers of fasting excessively or an unwillingness to alter their normal eating habits [13]. Interestingly, de Aguilar-Nascimento et al. [44] found that different patient demographics, such as age, sex, or type of hospital they were admitted into, were not significant predictors of excessive fasting, highlighting that all surgical patients are at risk. Hence, surgical staff must be adequately educated to dispel incorrectly held beliefs in order to ensure patients are properly informed [13,14]. Furthermore, patients should always be given written advice as this has been shown to improve retention [45] and significantly reduce unnecessary fasting [13]. However, de Putte et al. [46] found that 6.2% of patients presented to their operation in a non-fasted state due to, in 72% of cases, late fluid consumption. Thus, patient education needs to be clear and accurate, preventing the overconsumption of fluids and intraoperative pulmonary aspiration. However, this is a rare event, and the included studies were not powered adequately to be able to assess this adequately.
5. Limitations
There are several limitations within our study that warrant further discussion. For example, although we used a pre-determined protocol and accurately followed it, the protocol was not pre-registered in an appropriate database, and, hence, any lack of deviation from the original protocol cannot be proven. Furthermore, our predetermined 10–24 h postoperative inclusion criteria for inflammatory marker levels resulted in all the meta-analyses including RCTs that measured inflammatory markers up to 14 h apart. The wide time period was adopted to ensure all RCTs that measured the inflammatory markers in the first postoperative day were captured; however, it introduced a degree of heterogeneity. There were also some limitations with our results.
Six of the RCTs had a high risk of bias, thus potentially reducing the validity of the results [47]. Additionally, three of our meta-analyses only included three RCTs, including our significant serum albumin result, thus limiting the power of these results [35]. The small number of included trials is due to the recency of RCTs investigating postoperative inflammation in these patients, with the first trial being conducted in 2010 [34]. We are confident, however, that our systematic review included all up-to-date relevant RCTs.
6. Conclusions
This systematic review investigated whether a CHD, given 2–3 h preoperatively, reduces postoperative IL-6 and CRP levels and prevents a reduction in serum albumin levels compared to a PD or fasting. We found that IL-6 levels were significantly reduced whilst CRP levels were lower in CHD groups but did not reach significance. Post-operative serum albumin levels were significantly higher in patients who had a CHD preoperative than those who fasted. This is the first systematic review to demonstrate a significant reduction in some of the postoperative inflammatory markers following a preoperative CHD, and, hence, these results reinforce the guideline recommendations [5,6,7,8,9] of giving patients a CHD 2–3 h before an elective operation.
Author Contributions
Conceptualization, K.Z.H.; methodology, K.Z.H. and Z.A.; validation, K.Z.H. and Z.A.; formal analysis, K.Z.H. and Z.A.; investigation, K.Z.H. and Z.A.; data curation, K.Z.H. and Z.A.; writing—original draft preparation, K.Z.H.; writing—review and editing, K.Z.H. and Z.A.; supervision, Z.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to it being a systematic review of previously published trials. This was confirmed from the NHS Health Research Authority (UK) decision tool.
Informed Consent Statement
Patient consent was waived due to this study being a systematic review of previously published trials; thus, no patient or member of the public was involved in any stage.
Data Availability Statement
All data generated are included in the article.
Acknowledgments
The authors would like to express their gratitude to ‘The Arthur Thomson Charitable Trust’ for kindly awarding an intercalated bursary to K.Z.H. to help fund the master’s degree programme. However, this bursary did not at all influence the creation of this article, its study design, nor the decision to publish.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maltby, J.R. Fasting from midnight—The history behind the dogma. Best Pract. Res. Clin. Anaesthesiol. 2006, 20, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Nygren, J.; Thorell, A.; Jacobsson, H.; Larsson, S.; Schnell, P.-O.; Hylén, L.; Ljungqvist, O. Preoperative gastric emptying. Effects of anxiety and oral carbohydrate administration. Ann. Surg. 1995, 222, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Lobo, D.N.; Hendry, P.O.; Rodrigues, G.; Marciani, L.; Totman, J.J.; Wright, J.W.; Preston, T.; Gowland, P.; Spiller, R.C.; Fearon, K.C.H. Gastric emptying of three liquid oral preoperative metabolic preconditioning regimens measured by magnetic resonance imaging in healthy adult volunteers: A randomised double-blind, crossover study. Clin. Nutr. ESPEN 2009, 28, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.C.; Kinn, S.; Stuart, P.; Ness, V. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst. Rev. 2003, 4, CD004423. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Harsanyi, L.; Laviano, A.; Ljungqvist, O.; Soeters, P.; DGEM; Jauch, K.W.; Kemen, M.; Hiesmayr, J.M.; et al. ESPEN Guidelines on Enteral Nutrition: Surgery including Organ Transplantation. Clin. Nutr. ESPEN 2006, 25, 224–244. [Google Scholar] [CrossRef]
- Feldheiser, A.; Aziz, O.; Baldini, G.; Cox, B.P.B.W.; Fearon, K.C.H.; Feldman, L.S.; Gan, T.J.; Kennedy, R.H.; Ljungqvist, O.; Lobo, D.N.; et al. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: Consensus statement for anaesthesia practice. Acta Anaesthesiol. Scand. 2016, 60, 289–334. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Scott, M.J.; Schwenk, W.; Demartines, N.; Roulin, D.; Francis, N.; McNaught, C.E.; MacFie, J.; Liberman, A.S.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colonic Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations. World J. Surg. 2013, 37, 259–284. [Google Scholar] [CrossRef]
- Fearon, K.C.H.; Ljungqvist, O.; Von Meyenfeldt, M.; Revhaug, A.; Dejong, C.H.C.; Lassen, K.; Nygren, J.; Hausel, J.; Soop, M.; Andersen, J.; et al. Enhanced recovery after surgery: A consensus review of clinical care for patients undergoing colonic resection. Clin. Nutr. ESPEN 2005, 24, 466–477. [Google Scholar] [CrossRef]
- Nelson, G.; Fotopoulou, C.; Taylor, J.; Glaser, G.; Bakkum-Gamez, J.; Meyer, L.A.; Stone, R.; Mena, G.; Elias, K.M.; Altman, A.D.; et al. Enhanced recovery after surgery (ERAS®) society guidelines for gynecologic oncology: Addressing implementation challenges—2023 update. Gynecol. Oncol. 2023, 173, 58–67. [Google Scholar] [CrossRef]
- Bilku, D.K.; Dennison, A.R.; Hall, T.C.; Metcalfe, M.S.; Garcea, G. Role of preoperative carbohydrate loading: A systematic review. Ann. R. Coll. Surg. Engl. 2014, 96, 15–22. [Google Scholar] [CrossRef]
- Hausel, J.; Nygren, J.; Lagerkranser, M.; Hellström, P.M.; Hammarqvist, F.; Almström, C.; Lindh, A.; Thorell, A.; Ljungqvist, O. A Carbohydrate-Rich Drink Reduces Preoperative Discomfort in Elective Surgery Patients. Anesth. Analg. 2001, 93, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Noblett, S.E.; Watson, D.S.; Huong, H.; Davison, B.; Hainsworth, P.J.; Horgan, A.F. Pre-operative oral carbohydrate loading in colorectal surgery: A randomized controlled trial. Colorectal Dis. 2006, 8, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Falconer, R.; Skouras, C.; Carter, T.; Greenway, L.; Paisley, A.M. Preoperative fasting: Current practice and areas for improvement. Updates Surg. 2014, 66, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Degeeter, T.; Demey, B.; Caelenberg, E.V.; Baerdemaeker, L.D.; Coppens, M. Prospective audit on fasting status of elective ambulatory surgery patients, correlated to gastric ultrasound. Acta Chir. Belg. 2023, 123, 43–48. [Google Scholar] [CrossRef]
- Nygren, J.; Soop, M.; Thorell, A.; Efendic, S.; Nair, K.S.; Ljungqvist, O. Preoperative oral carbohydrate administration reduces postoperative insulin resistance. Clin. Nutr. ESPEN 1998, 17, 65–71. [Google Scholar] [CrossRef]
- Rizvanović, N.; Adam, V.N.; Čaušević, S.; Dervišević, S.; Delibegović, S. A randomised controlled study of preoperative oral carbohydrate loading versus fasting in patients undergoing colorectal surgery. Int. J. Color. Dis. 2019, 34, 1551–1561. [Google Scholar] [CrossRef]
- Desborough, J.P. The stress response to trauma and surgery. Br. J. Anaesth. 2000, 85, 109–117. [Google Scholar] [CrossRef]
- de Aguilar-Nascimento, J.E.; Marra, J.G.; Slhessarenko, N.; Fontes, C.J.F. Efficacy of National Nosocomial Infection Surveillance score, acute-phase proteins, and interleukin-6 for predicting postoperative infections following major gastrointestinal surgery. Sao Paulo Med. J. 2007, 125, 34–41. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, J.; Wang, F. Effects of Preoperative Carbohydrate Intake on Inflammatory Markers and Clinical Outcomes in Elderly Patients Undergoing Radical Prostatectomy: A Single-Centre, Double-Blind Randomised Controlled Trial. Front. Surg. 2021, 8, 744091. [Google Scholar] [CrossRef]
- Oka, Y.; Murata, A.; Nishijima, J.; Yasuda, T.; Hiraoka, N.; Ohmachi, Y.; Kitagawa, K.; Yasuda, T.; Toda, H.; Tanaka, N.; et al. Circulating interleukin 6 as a useful marker for predicting postoperative complications. Cytokine X 1992, 4, 298–304. [Google Scholar] [CrossRef]
- Mokart, D.; Capo, C.; Blache, J.L.; Delpero, J.R.; Houvenaeghel, G.; Martin, C.; Mege, J.L. Early postoperative compensatory anti-inflammatory response syndrome is associated with septic complications after major surgical trauma in patients with cancer. Br. J. Surg. 2002, 89, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Ingaldi, C.; Alberici, L.; Serbassi, F.; Pagano, N.; De Raffele, E.; Minni, F.; Pironi, L.; Sasdelli, A.S.; Casadei, R. Preoperative carbohydrate loading before elective abdominal surgery: A systematic review and network meta-analysis of phase II/III randomized controlled trials. Clin. Nutr. ESPEN 2022, 41, 313–320. [Google Scholar] [CrossRef]
- Tong, E.; Chen, Y.; Ren, Y.; Zhou, Y.; Di, C.; Zhou, Y.; Shao, S.; Qui, S.; Hong, Y.; Yang, L.; et al. Effects of preoperative carbohydrate loading on recovery after elective surgery: A systematic review and Bayesian network meta-analysis of randomized controlled trials. Front. Nutr. 2022, 9, 951676. [Google Scholar] [CrossRef]
- Perrone, F.; da-Silva-Filho, A.C.; Adôrno, I.F.; Anabuki, N.T.; Leal, F.S.; Colombo, T.; da Silva, B.D.; Dock-Nascimento, D.B.; Damião, A.; de Aguilar-Nascimento, J.E. Effects of preoperative feeding with a whey protein plus carbohydrate drink on the acute phase response and insulin resistance. A randomized trial. Nutr. J. 2011, 10, 66. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-Phase Proteins and Other Systemic Responses to Inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Kweon, S.-H.; Park, J.S.; Lee, Y.C. Oral Carbohydrate Administration in Patients Undergoing Cephalomedullary Nailing for Proximal Femur Fractures: An Analysis of Clinical Outcomes and Patient Satisfaction. Geriatr. Orthop. Surg. Rehabil. 2020, 11, 2151459320958609. [Google Scholar] [CrossRef]
- Deng, Y.; Fang, Y.; Li, H.; Chen, J.; An, J.; Qiao, S.; Wang, C. A preoperative whey protein and glucose drink before hip fracture surgery in the aged improves symptomatic and metabolic recovery. Asia Pac. J. Clin. Nutr. 2020, 29, 234–238. [Google Scholar] [CrossRef]
- Gumus, K.; Aydın, G. The Effect of Preoperative Nutrition on Postoperative CRP and Albumin Levels in Patients Undergoing Laparoscopic Surgery: A Surgical Nursing Perspective. J. Perianesth. Nurs. 2020, 35, 592–596. [Google Scholar] [CrossRef]
- Zhang, Y.; Min, J. Preoperative Carbohydrate Loading in Gynecological Patients Undergoing Combined Spinal and Epidural Anesthesia. J. Investig. Surg. 2020, 33, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Dilmen, O.K.; Yentur, E.; Tunali, Y.; Balci, H.; Bahar, M. Does preoperative oral carbohydrate treatment reduce the postoperative surgical stress response in lumbar disc surgery? Clin. Neurol. Neurosurg. 2017, 153, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Dock-Nascimento, D.B.; de Aguilar-Nascimento, J.E.; Faria, M.S.M.; Caporossi, C.; Slhessarenko, N.; Waitzberg, D.L. Evaluation of the Effects of a Preoperative 2-Hour Fast with Maltodextrine and Glutamine on Insulin Resistance, Acute-Phase Response, Nitrogen Balance, and Serum Glutathione After Laparoscopic Cholecystectomy. JPEN J. Parenter. Enteral. Nutr. 2012, 36, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kaška, M.; Grosmanová, T.; Havel, E.; Hyšpler, R.; Petrová, Z.; Brtko, M.; Bareš, P.; Bareš, D.; Schusterová, B.; Pyszková, L.; et al. The impact and safety of preoperative oral or intravenous carbohydrate administration versus fasting in colorectal surgery—A randomized controlled trial. Wien. Klin. Wochenschr. 2010, 122, 23–30. [Google Scholar] [CrossRef]
- Valentine, J.C.; Pigott, T.D.; Rothstein, H.R. How Many Studies Do You Need? A Primer on Statistical Power for Meta-Analysis. J. Educ. Behav. Stat. 2010, 35, 215–247. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z.; Ying, X.; Tian, J.; Sun, T.; Yi, K.; Zhang, P.; Jing, Z.; Yang, K. Preoperative carbohydrate loading for elective surgery: A systematic review and meta-analysis. Surg. Today 2012, 42, 613–624. [Google Scholar] [CrossRef]
- Smith, M.D.; McCall, J.; Plank, L.; Herbison, G.P.; Soop, M.; Nygren, J. Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database Syst. Rev. 2014, 8, CD009161. [Google Scholar] [CrossRef]
- Amer, M.A.; Smith, M.D.; Herbison, G.P.; Plank, L.D.; McCall, J.L. Network meta-analysis of the effect of preoperative carbohydrate loading on recovery after elective surgery. Br. J. Surg. 2017, 104, 187–197. [Google Scholar] [CrossRef]
- Cheng, P.-L.; Loh, E.-W.; Chen, J.-T.; Tam, K.-W. Effects of preoperative oral carbohydrate on postoperative discomfort in patients undergoing elective surgery: A meta-analysis of randomized controlled trials. Langenbecks Arch. Surg. 2021, 406, 993–1005. [Google Scholar] [CrossRef]
- Sato, H.; Carvalho, G.; Sato, T.; Lattermann, R.; Matsukawa, T.; Schricker, T. The Association of Preoperative Glycemic Control, Intraoperative Insulin Sensitivity, and Outcomes after Cardiac Surgery. J. Clin. Endocrinol. Metab. 2010, 95, 4338–4344. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Z.; Hu, J.; Du, J.; Mitch, W.E. Insulin Resistance Accelerates Muscle Protein Degradation: Activation of the Ubiquitin-Proteasome Pathway by Defects in Muscle Cell Signaling. Endocrinology 2006, 147, 4160–4168. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.; Bissolati, M.; Rocchetti, S.; Beneduce, A.; Pecorelli, N.; Carlo, V.D. Oral preoperative antioxidants in pancreatic surgery: A double-blind, randomized, clinical trial. Nutrition 2012, 28, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Pexe-Machado, P.A.; de Oliveira, B.D.; Dock-Nascimento, D.B.; de Aguilar-Nascimento, J.E. Shrinking preoperative fast time with maltodextrin and protein hydrolysate in gastrointestinal resections due to cancer. Nutrition 2013, 29, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- de Aguilar-Nascimento, J.E.; Dias, A.L.A.; Dock-Nascimento, D.B.; Correia, M.I.T.D.; Campos, A.C.L.; Portari-Filho, P.E.; Oliveira, S.S. Actual preoperative fasting time in Brazilian hospitals: The BIGFAST multicenter study. Ther. Clin. Risk Manag. 2014, 10, 107–112. [Google Scholar] [CrossRef]
- Johnson, A.; Sandford, J.; Tyndall, J. Written and verbal information versus verbal information only for patients being discharged from acute hospital settings to home. Cochrane Database Syst. Rev. 2003, 4, CD003716. [Google Scholar] [CrossRef]
- de Putte, P.V.; Vernieuwe, L.; Jerjir, A.; Verschueren, L.; Tacken, M.; Perlas, A. When fasted is not empty: A retrospective cohort study of gastric content in fasted surgical patients. Br. J. Anaesth. 2017, 118, 363–371. [Google Scholar] [CrossRef]
- Delgado-Rodríguez, M.; Llorca, J. Bias. J. Epidemiol. Community Health 2004, 58, 635–641. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).