Abstract
Repetitive mild traumatic brain injuries (mTBIs) contribute to inflammation-induced neurodegeneration. Cycloxygenase (COX) enzymes produce inflammatory cytokines that influence the microglia response to neurotrauma. Celecoxib is a selective COX-2 inhibitor that is prescribed in some conditions of mTBI to alleviate symptoms of concussion, and has shown benefits in neurodegenerative conditions. We investigated molecular pathways of neuroinflammation in response to celecoxib treatment in a mouse model of repetetive mTBI. Fifteen mTBIs were delivered over 23 days in adult male C57BL/6J mice in one of four groups (control, celecoxib without impact, celecoxib with impact, and vehicle with impact). Cognitive function was assessed at 48 h and three months following the final mTBI. Morris Water Maze testing revealed impaired hippocampal spatial learning performance in the celecoxib treatment with the impact group compared to the vehicle with impact control in the acute phase, with celecoxib treatment providing no improvement compared with the control at chronic testing; mRNA analysis of the cerebral cortex and hippocampus revealed expression change, indicating significant improvement in microglial activation, inflammation, excitotoxicity, and neurodegeneration at chronic measurement. These data suggest that, in the acute phase following injury, celecoxib protected against neuroinflammation, but exacerbated clinical cognitive disturbance. Moreover, while there was evidence of neuroprotective alleviation of mTBI pathophysiology at chronic measurement, there remained no change in clinical features.
1. Introduction
Mild traumatic brain injuries (mTBI) may result in persistent cognitive dysfunction [1]. Repetitive subconcussive impacts have been identified in driving the development of chronic clinical symptoms, which may progress to neurodegenerative diseases, such as chronic traumatic encephalopathy (CTE) [2]. Prolonged secretion of inflammatory molecules and dysregulated synaptic influx of calcium and neurotransmitters are key processes associated with the accumulation of damage to neurons and glia that underpin the aetiology of neurodegenerative conditions [3,4]. The development of pharmacological agents to moderate these processes has been identified as a key clinical priority, but to date there are no therapies that have shown consistent effectiveness as treatment.
Non-steroidal anti-inflammatory drugs (NSAIDs) exert their anti-inflammatory effect by inhibiting cyclooxygenase (COX)-1 and COX-2 pathways induced by trauma [5]. Inhibition of COX-1 reduces prostaglandin biosynthesis and inhibits platelet aggregation, which may protect in the acute phase of injury. However, these actions may result in adverse effects, such as gastric ulceration, bleeding, and renal dysfunction [6,7]. Alternatively, inhibition of COX-2 promotes anti-inflammatory effects without these adverse effects by reducing (i) prostaglandins and thromboxanes, (ii) cytokines, and (iii) proteases. This distinction has led to the focused development of selective COX-2 inhibitors, including celecoxib. Celecoxib demonstrates expected analgesic and anti-inflammatory effects, however, there are also studies that provide evidence of neuroprotection from COX inhibition in conditions of neurotoxicity, neurodegeneration, and demyelination [8,9,10,11,12]. Notably, these protective effects are not strictly resultant from reduced inflammation, as increased neuronal survival following COX-2 inhibition was not associated specifically with decreased mRNA inflammatory signaling [13]. COX-2 activity catalyzes the production of prostaglandin E2 (PGE2), which stimulates the release of glutamate from astrocytes, leading to excitotoxic effects [14], which may be a key driver of neuroinflammation that may result in dementia [15]. The process of increased excitotoxicity through COX-2 PGE2 and tumor necrosis factor (TNF) has also been demonstrated in the pathophysiology of epilepsy [12]. The review by Rawat et al. (2019) proposed that COX-2 activation, resultant PGE2 production, and related TNF release concomitantly triggered microglial and astrocyte-induced neuroinflammation. The effects of COX-2 selective inhibitors appear to show a dual effect on neuroinflammation and neuroexcitability via separate and distinct mechanisms, with inhibition of PGE2 production quieting the calcium glutamate axis of seizures with cellular inflammation and toxicity suppressed through reduced pro-inflammatory cytokine production [12]. Thus, the complexity of neuroinflammation in these disorders is linked not just to changes in COX-2 enzyme production, but the linked inflammatory mediator pool that is generated.
Interest in COX enzymes as a therapeutic strategy targeting neuroinflammation and cognitive decline originated from epidemiological studies reporting a protective effect of NSAIDs in Alzheimer’s disease (AD) patients [16,17]. Evidence of a potential therapeutic effect has been shown in animal models of neurodegeneration, with celecoxib treatment providing a protective effect on learning and memory deficits [18]. A rat model of moderate TBI treatment with COX-2 inhibitor nimesulide inhibited COX-2 elevation following injury, leading to significantly improved cognitive performance compare to vehicle treated TBI animals [19]. In a model of AD, celecoxib administration resulted in reduced microglial and astrocyte upregulation that prevented behavioral dysfunction and normalized biochemical and neurotransmitter changes [15]. Large human trials of selective COX-2 inhibitors have been unsuccessful in showing benefit in memory decline in AD [20,21], although these studies involved individuals in the late stages of disease progression, with advanced development of neurofibrillary tangles and neuritic plaques. There are distinct shared cellular mechanisms of inflammation between CTE and AD [22], which supports the need for further investigation of the COX-derived pathways in CTE therapeutics given the extensive studies using COX blockade in models of AD. In a model of focal penetrating TBI, COX-2 inhibition by diclofenac was shown to decrease the degree of apoptosis in injured rats [23]. COX-2 expression was increased at the site of injury, however, the full effects of diclofenac were not solely via inhibition of COX-2, but also via indirect linked inflammatory modulation of microglial cells and astrocytes [23]. This signals a potential avenue to also target mTBI via COX-2 selective therapies, which may not be directly linked to COX-2 induction. While celecoxib has not been used in clinical research in the area of brain insult, due to the inflammatory cascade following neurological trauma of concussion, it is hypothesized that this compound will have protective effects.
Based on these previous investigations, this study aimed to investigate the role of celecoxib treatment in the prevention of inflammation and neurodegeneration induced by repetitive mTBI. Inflammation signaling was examined in the context of markers of neurodegeneration to determine if celecoxib has a positive effect on measures of glial cell activation, glutamate receptor regulation, and axonal neurodegeneration. In addition to molecular response, we measured the subsequent neurodegeneration and cognitive disturbance induced by head injury via Morris Water Maze (MWM) measures of spatial learning and memory at acute and chronic time points.
2. Materials and Methods
2.1. Animals and General Overview
Experimental procedures were approved by the Animal Ethics Committee of Central Queensland University (CQU AEC 0000021124) under guidelines from the National Medical Research Council of Australia. The ARRIVE guidelines were adhered to for the design and reporting of the study. A total of 64 male C57BL/6J mice (Animal Resource Centre, Canning Vale, WA, Australia) were housed in a constant 12:12 h light–dark cycle, with the temperature controlled at 22 ± 2 °C. Mice were housed four to six per cage, and had food and water access as permitted ad libitum. At the time of arrival, mice were eight weeks old and undertook a two-week habituation period to allow environment acclimatization before the initiation of the study protocol.
2.2. Groups and Dosing
There were two separate study arms for this study: An acute branch where animals were sacrificed 48 h following final impact, and a chronic branch where animals were sacrificed 90 days following final impact (Figure 1). Using the random number generator function of Excel, mice were randomized to one of four treatment groups: control with vehicle (VEH + CON, n = 8), control with celecoxib (CEL + CON, n = 8), impacts with celecoxib (CEL + IMP, n = 8), or impacts with vehicle (VEH + IMP, n = 8). CEL was dissolved in saline and delivered at a dose of 50 mg kg−1 day−1, which is equivalent to previous mouse dosages. The vehicle groups received saline only. Vehicle or CEL were administered via subcutaneous injection, and were given for 14 days prior to the first impact and throughout the impact and testing schedule until the time of sacrifice of the acute groups. The body weight of each mouse was assessed before the commencement of CEL administration, weekly during mTBI administration and at time of euthanasia. There were no significant differences in weight between impacted and non-impacted groups at all time points (data not shown). Impacts or anesthesia for all groups were delivered across a 23-day span, on a rotation of three impact/sham days followed by two rest days (Figure 2A).
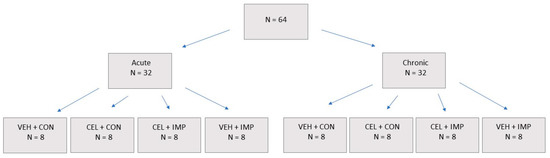
Figure 1.
Sixty-four total mice were used for the study, with N = 8 randomly allocated to each group.
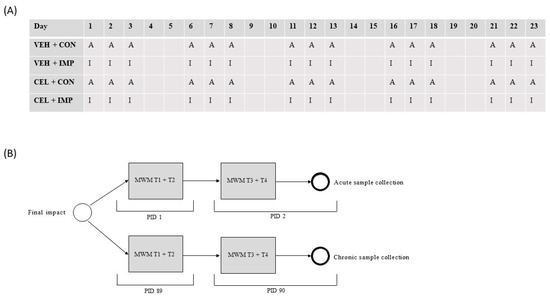
Figure 2.
(A) All mice were involved in either sham anesthesia or impact conditions in 15 out of 23 days. A = anesthetic only; I = impact. (B) Behavioral testing involved MWM trial 1 + 2 on PID 1 for acute groups and PID 89 for chronic groups, followed by MWM trial 3 + 4 and sample collection on PID 2 for acute groups and PID 90 for chronic groups.
Following final injury, a subset of animals were assessed for behavioral changes, and samples were collected (Figure 2B). No animal in any group died during impact or in the recovery phase, there was no evidence of bleeding or skull fracture at post-mortem analysis of any animal, and as such, no animals were removed from the study.
2.3. mTBI Modelling
We exposed animals to mTBI and associated acceleration forces, modelled on what is observed in human mTBI, such as in contact sports [24]; mTBI was administered via an apparatus designed and built for this purpose, as previously described [25]. An enclosed inhalation chamber (1 L) containing 0.5 mL of isoflurane (Zoetis, Rhodes, NSW, Australia) in a cotton ball (yielding a steady 4% concentration) was used to anesthetize the mice. Inhalation between 1 to 2 min resulted in light anesthesia, as determined by lack of response to tail pinch. A steel weight (12 mm diameter) of 25 g was used for impacting the skull, dropped from a height of 1 m, and guided through a PVC tube (15 mm diameter). A small rubber cap (1 × 10 mm) was attached to the bottom of the weight to restrict the contact zone. Prior to mTBI, the mouse was positioned chest down on the apparatus platform, which consisted of two magnetically adjoined acetate panels. This platform had a weight limit of 33 g, so that the platform collapsed upon impact, resulting in minimal platform resistance applied to the head of the mouse. The head was positioned under the vertical tube, through which the impact weight protruded. Specific alignment was such that the weight made contact between the bregma and lambda intersection. Upon impact, the mouse fell and rotated about a horizontal axis, and landed in a supine position on the padded landing area 10 cm below the stage, which was composed of a sponge cushion (15 cm length × 9 cm width × 7.5 cm depth). The impact weight was tethered to the guide tube by a commercially available braided nylon line (Spear and Jackson, Melbourne, VIC, Australia), restricting the fall of the weight so that it could continue downward no more than 1 cm beyond the starting position of the dorsal surface of the skull, thereby avoiding unintentional secondary contact. The mouse was immediately moved to a recovery heating pad, and recovery was monitored.
2.4. Neurological and Spatial Learning Assessments
To assess neurological restoration following mTBI, the time to recover righting reflex (RR) was monitored following isoflurane-induced anesthesia (controls) or mTBI. Mice were placed in a supine position on the recovery pad, and the time taken for the animal to adopt a prone position was recorded after each impact. RR time was calculated from the discontinuation of isoflurane inhalation to the first sign of righting.
MWM was used to provide measurement of hippocampal-dependent spatial learning and memory in mTBI and control groups [26]. The test was conducted in a circular tank with a diameter of 110 cm, with highly visual cues fixed at locations around the pool. The pool was filled with water (temperature 27 +/− 1 °C) made opaque with nontoxic, water-soluble Tempera paint (Fine Art Supplies, Auckland, New Zealand). A round platform with a diameter of 10 cm was hidden 1 cm below the surface of the water in the northern quadrant. As described by Mannix and colleagues, a total of four trials were administered across two consecutive days, with a 6 h interval provided between trials on the same day [27]. Each trial consisted of three attempts to reach the hidden platform, with a start position from each of the quadrants that did not contain the platform (south, east, and west). For each trial, a random order of start positions was selected, and this was held consistent for each animal across the trial. The trials occurred on PID 1 and 2 for the acute groups, and PID 89 and 90 for chronic animals (n = 4 per group). For each attempt, mice were given a maximum test duration of 60 s to find and remain on the hidden platform. Mice that did not locate the platform within the allocated time were guided to the platform and allowed to rest for 10 s. On PID 2 and 90, all animals also underwent a probe trial, which involved removal of the hidden platform from the pool. Mice were placed in the pool opposite the target quadrant (the quadrant where the platform had been) and had a time limit of 30 s to search for the platform. Time spent in the target quadrant was assessed, as described previously [26]. The researcher assessing MWM was blinded to the condition of the animal. Animals were assessed for motor function deficits using Kinovea 0.8.15 software (open source) to track swim speed and time spent in the goal quadrant during the probe trial.
2.5. Sample Collection
Inhalation of isoflurane between 4 to 6 min resulted in euthanasia, and death was confirmed by a lack of pedal reflex. Immediately following cessation of vital signs, the mouse was decapitated, and the brain was removed and weighed. There were no significant differences in brain weights across any group (p = 0.062). The brain was washed in ice-cold oxygenated (95% O2, 5% CO2) artificial cerebrospinal fluid (CSF) containing 118.0 mM of NaCl, 3.5 mM of KCl, 1.3 mM of MgCl2, 26.2 mM of NaHCO3, 1.0 mM of NaH2PO4, 2.5 mM of CaCl2, and 11.0 mM of glucose before being rapidly dissected on a frozen dissection platform for hippocampus and cerebral cortex sections. Sections were frozen at −80 °C for genetic analysis. To enable blinding conditions, collection tubes were coded so that group names were not accessible to the investigators undertaking sample analysis. Coding information was secured on the lead investigator’s computer, and the codes were only accessed after the samples were analyzed.
2.6. Quantitative Real-Time Reverse Transcriptase PCR
The mRNA was extracted from tissue homogenates of the hippocampus and cerebral cortex of mTBI and control groups (n = 4 per group at 48 h or 90 days after injury) using the phenol-chloroform method [28]. Sample concentration and purity were evaluated using a spectrophotometer (NanoDrop 2000c, Thermo Fisher Scientific, Wilmington, DE, USA) (mean ± SD 260/230 spectral ratio: 1.92 ± 0.22; mean ± SD 260/280 spectral ratio: 2.00 ± 0.05). Complementary DNA was synthesized using a Superscript III First-Strand Synthesis System for reverse transcriptase-PCR according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA, USA) and run in a thermal cycler (T100 Thermal Cycler, Bio-Rad, Gladesville, NSW, Australia). Samples and negative controls were prepared in duplicate using Taqman universal PCR master mix and run using a thermal cycler (Rotor-Gene Q, Qiagen, Venlo, The Netherlands). The following Taqman gene expression assays were used (Applied Biosystems catalog numbers): mouse MAPT (Mm00521990_m1), GFAP (Mm01253030_m1), AIF1 (Mm00479862_g1), GRIA1 (Mm00433753_m1), TARDBP (Mm01257504_g1), TNF (Mm00443258_m1), NEFL (Mm01315666_m1), and gene products were normalized to endogenous mouse GAPDH (Mm99999915_g1). Relative expression for Taqman-analyzed transcripts was calculated using the delta-delta Ct method [29].
2.7. Statistical Analyses
Group numbers for behavioral and laboratory tests were calculated via an a priori power analysis using α of 0.05, power of 0.8, and means and SD from previous laboratory pilot data. Statistical analyses were performed using IBM SPSS Statistics for Windows Version 25.0 (IBM Corp, Armonk, NY, USA). Data were evaluated for normality (Shapiro–Wilk test or Kolomogov–Smirnov test) prior to statistical testing. As all data were parametric, one-way ANOVA with Tukey post-hoc tests (alpha < 0.05) was used to assess for statistically significant differences. All data are presented as means with standard deviations.
3. Results
Mice receiving mTBIs in the VEH + IMP and the CEL + IMP groups showed no signs of convulsions or physical stress following impacts, indicating that our model sufficiently mimicked the mild impact forces typically seen in sub-concussive injury. Due to the asymptomatic nature of the injuries, no mice were withdrawn from the study on ethical grounds. Mice tolerated the administration of CEL with no side effects.
3.1. Righting Reflex
The time required to regain consciousness was assessed immediately following impact or anesthetization on all 15 impact days, and results can be seen for each group (Figure 3). The VEH + IMP group demonstrated a delay in the return to consciousness compared with VEH + CON following impacts one through four (p < 0.05), with no differences seen between these groups following impacts five through 15. Significant differences were seen in the time required to regain consciousness between the VEH + IMP and CEL + IMP groups following impacts four and 13 (p < 0.05), with no significant difference following other impacts. There were significant differences between CEL + CON and CEL + IMP following impacts one through three, eight, 13, and 14 (p < 0.05). There were no differences following any impact between the VEH + CON and the CEL + CON groups.
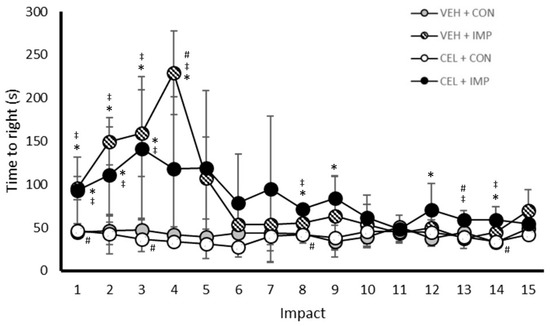
Figure 3.
Time to regain righting reflex (seconds) following impact or sham control anesthesia. Values are reported as mean (± SD); * p < 0.05 difference compared with VEH + CON; ‡ p < 0.05 difference with CEL + CON; # p < 0.05 difference compared with CEL + IMP. N = 5 per group.
3.2. Spatial Learning and Memory
Acute testing of hippocampal-dependent spatial learning and memory indicated that there was progressive improvement in the ability to find the hidden platform with each successive trial for the control and impact groups, but not the celecoxib impact treatment group (Figure 4A). There were no differences between groups for trial 1. The group mean time difference to find the hidden platform at trial 2, 3, and 4 was significant for the VEH + CON group compared with the VEH + IMP and CEL + IMP groups (p < 0.05).
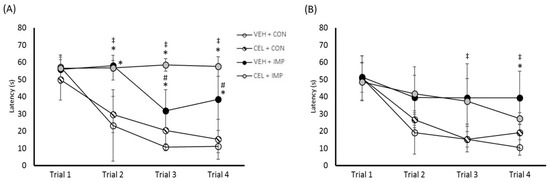
Figure 4.
(A) Time to find the hidden platform (seconds) in the MWM at acute testing. (B) Time to find the hidden platform in the MWM at chronic testing. Values are reported as mean (± SD); * p < 0.05 difference compared with VEH + CON; ‡ p < 0.05 difference with CEL + CON; # p < 0.05 difference compared with CEL + IMP. N = 4–5 per group.
Interestingly, there were group differences between the impact groups, VEH + IMP and CEL + IMP, at trials 3 and 4 (p < 0.05), however, this represented increased detriment as a result of the celecoxib treatment, rather than an improvement.
In the three months of testing, no group differences in platform finding ability were seen at trial 1 and 2 (Figure 4B). VEH + IMP was different than VEH + CON at trial 4 (p < 0.05). The differences seen between VEH + IMP and CEL + IMP in the acute testing had diminished at the chronic testing time point, and while celecoxib treatment was not shown to be detrimental to performance at this time, there were no beneficial effects. In probe trials where the hidden platform was removed, there were no group differences in time spent in the goal quadrant for any group at either acute or chronic testing (data not shown).
3.3. Quantitative Reverse Transcription Polymerase Chain Reaction Analysis
The results derived from the molecular analysis have been condensed into tables that contain all F and p-values for the ANOVAs with corresponding post hoc testing where required for the molecular analysis, involving four treatment groups, seven genes, two tissue types, and two time points (Supplementary Table S1a,b). The expression of genes was different in the cortex and hippocampus, and displayed unique expression at acute time points compared with chronic animals. Celecoxib treatment following impact was effective in many genes in providing a neuroprotective effect relating to reduced excitotoxicity, inflammation, glial activation, and neuronal damage. Key genetic changes that were seen included measures of glial activation response to injury, glial acidic fibrillary protein (GFAP), and allograft inflammatory factor 1 (AIF1), which were increased in the VEH + IMP group in the cortex at acute measurement (p < 0.05) and in the hippocampus at chronic measurement (p < 0.05). In both genes the CEL + IMP treatment group had reduced expression that was not different than control levels (p > 0.05). Inflammation response as measured by TNF expression was also increased in the acute cortex of the VEH + IMP group (p < 0.05), with no difference compared to the control for the CEL + IMP group (p > 0.05), while VEH + IMP and CEL + IMP were significantly different (p < 0.05). Microtubule associated protein tau (MAPT) expression was significantly different between VEH + IMP and CEL + IMP for both tissues at both time points (p < 0.05). Detailed results pertaining to treatment effects in cortex and hippocampal mRNA expression change for acute and chronic mice can be seen in Figure 5A–D.
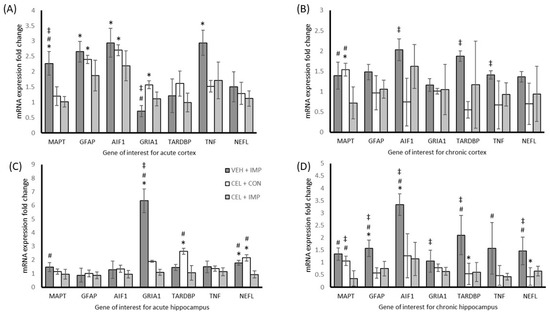
Figure 5.
(A) The mRNA expression fold change relative to VEH + CON of genes in the cortex at acute testing. (B) The mRNA expression fold change relative to VEH + CON of genes in the cortex at chronic testing. (C) The mRNA expression fold change relative to VEH + CON of genes in the hippocampus at acute testing. (D) The mRNA expression fold change relative to VEH + CON of genes in the hippocampus at chronic testing. Normalized to Gapdh (± SD); * p < 0.05 difference compared with VEH + CON; ‡ p < 0.05 difference with CEL + CON; # p < 0.05 difference compared with CEL + IMP. N = 4 per group.
4. Discussion
This study investigates the preventative effects of celecoxib on the COX-2 induced inflammatory response and neurodegeneration following mTBI. COX-2 inhibition has shown protection in conditions of neurotoxicity, neurodegeneration, focal penetrating TBI, epilepsy, and demyelination [8,9,10,11,12,23]. We also assessed the molecular response of astrocytes and neuronal/axonal damage to celecoxib treatment. We delivered mild impacts repeated over a sustained timeframe, and we were able to induce the head acceleration forces that contact sports athletes may be subjected to by using a model that has shown pathology and cognitive decline indicative of CTE [25].
Analysis of behavior and cognition provides insight into the interplay between repeated subclinical mTBI and COX-2 suppression. A key comparison in RR data is in the differences between the VEH + CON group and the two impact groups (the VEH + IMP and CEL + IMP groups). The vehicle impact group displayed significant latency to regain RR following impacts one through three, however, the impact group treated with CEL had additional deficits following impacts eight, nine, 12, and 14. In this way, treating with CEL resulted in a prolonged poorer outcome relative to the vehicle treatment. In the same way, the CEL-treated impact group had prolonged delays compared to the CEL control group.
In the MWM, the VEH + IMP mice displayed spatial learning and memory deficits in both the acute and chronic phases of testing compared with mice that had anesthesia, but not impact. This provides a baseline to compare our model with clinical studies, where post-concussion syndrome in humans affects cognition in initial recovery, and repetitive mTBI causes chronic disturbances, as observed in retired professional football players [30] and athletes [31]. Upon this acute and chronic cognitive impairment, we provided CEL treatment with the intention to target COX-mediated inflammation and improve function performance. Previous studies have found that CEL was effective in attenuating COX-2 expression and maintaining cognitive performance in a TBI model using adult rats [32], and, in conditions of intracerebral hemorrhage in rats, CEL reduced sensorimotor deficit [33], with better functional recovery shown the earlier the drug was administered. While the changes in COX-2 expression are increased in models of brain injury [34], therapeutics from the NSAIDs class often do not alter COX-2 expression, and the therapeutic outcome appears to have both COX-2 specific and COX-2 non-specific anti-inflammatory actions [23]. However, in our study, in acute MWM testing, the CEL + IMP group showed additional cognitive detriment compared with the impact group receiving only the vehicle, with no improvement in the ability to learn the test or remember the location displayed across the four trials. In chronic testing, the CEL + IMP group showed some minor improvement across the progression of the trials, however, this was not significantly different from the vehicle treated impact group, indicating no effect of CEL treatment. When looking broadly at the performance of other COX-2 inhibitors, there have been several other preclinical investigations that have reported the inability to improve measures of cognition following TBI [35], as was found in this study. An explanation of this may be that, under conditions of physiological homeostasis, COX-2 is expressed in the hippocampus and cerebral cortex by glutamatergic neurons, and may be required in regulating postsynaptic signaling and plasticity processes involved in memory and learning [36]. For this reason, cognitive impairment in processes involving hippocampus structure have been seen following selective inhibition of COX-2 enzymes independent of trauma [37,38].
The mTBI sets in motion inflammation responses that are intended to provide protection, but can become pathological if chronically upregulated [31]. Microglia initiate these responses, which include the production of cytokines, conversion of arachidonic acid to prostaglandins, and release of excitotoxins, which are intended to provide repair following insult and diminish to baseline levels thereafter [39]. Circumstances of repetitive or severe trauma contribute to prolonged microglial activation and the development of chronic inflammation that results in neurodegeneration involving neurons and glia [40]. The anti-inflammatory effects of celecoxib through COX-2 inhibition has been shown to decrease microglial activation in the hippocampus in a rat model of dementia [15]. Our study sought to investigate this microglial response in the context of CTE modelling by analyzing AIF1 gene expression. In the absence of celecoxib treatment, our VEH + IMP group showed a significant increase in expression in the cortex at both acute and chronic time points and in the hippocampus at three months. When celecoxib was administered with impact, AIF1 expression in both the cortex and hippocampus for all groups was reduced and significantly different than VEH + IMP. However, in the acute cortex, celecoxib was also shown to have the effect of increasing AIF1 levels relative to VEH + CON. The demonstrated reduction in AIF1 levels in CEL + IMP groups provides evidence of a microglial mediating effect, and this is thought to be particularly important in the chronic phase of injury. Microglia display two distinct phenotypes, and while the acute response is likely to provide beneficial delivery of pro-regenerative and neuroprotective substrates, it is likely that chronically activated microglia alter to primarily destructive functions [41].
Another mechanism for inflammation following mTBI is via astroglial cells, which crosstalk with microglia to meet the ongoing demands of the CNS [40]. Like microglia, increased astrocyte activation is initiated to provide neuroregenerative support after mTBI [42], but severe or repetitive injury causes a chronic response that contributes to neuroinflammation cascades and pathological glial scarring at the injury site, leading to detrimental functional outcomes [43]. GFAP is an accepted measure of astrocyte activity in human head trauma, and in this study, GFAP mRNA elevation in acute cortex and chronic hippocampus tissues was observed, in line with data from previous animal mTBI studies [44]. In the CEL + IMP groups, the effects of impact were mediated by celecoxib, with levels not significantly different from the non-impacted control group. This effect is in line with previous data showing a reduction in astrocyte activation following administration of celecoxib in a pre-clinical model of dementia [15].
An important validation of COX-2 related inflammation in our study is the increase of TNF in both cortex tissues and in the chronic hippocampus following mTBI. The production of cytokines is one of three key pathways induced by COX-2 following head injury, and TNF is a key pro-inflammatory cytokine that provides a downstream measure of COX-2 induction. TNF is also involved in the glial cell response described above, both as an initiator and a product of increased mobilization of microglia and astrocytes following CNS injury [45]. We found TNF mRNA expression in the impact only group to follow the same pattern as astrocyte and microglial upregulation. Likewise, in the CEL + IMP group, we saw the same reduction in TNF mRNA expression as in AIF1 and GFAP, reflecting further evidence of the effectiveness of the role of celecoxib in extinguishing inflammation in these pathways. While TNF, AIF1, and GFAP responded to injury and treatment in concert, the extent of causation is subject to further exploration. This potentially links inflammatory mediators, such as PGE2, TNF, and IL-6, as the most likely downstream mediators, which has been demonstrated in other models of brain injury and inflammation, such as epilepsy [12].
GRIA1 mRNA expression was assessed in this study, as previous murine studies have shown spatial working memory to be dependent on the activation of glutamate-specific AMPA receptors expressing the GluA1 subunit [46]; mTBI initiates glutamate excitotoxicity that is driven by impaired neuronal calcium regulation, and leads to postsynaptic dendrite damage [47], with the hippocampus showing particular vulnerability to damage [48]. To counter the damage caused by the build-up of glutamate in the synaptic cleft, upregulation of GluA1 receptors is initiated to normalize levels. In humans, spatial working memory involves information processing in the prefrontal cortex, and is assisted by the hippocampus, whereas, in mice, it is primarily a function of the hippocampus [49]. COX-2 increases in the brain in situations of increased excitotoxicity in vivo [50], and COX-2 inhibition has been shown to have a beneficial effect in models of excitotoxicity [51]. Our results show significant hippocampal excitotoxicity in VEH + IMP mice at 48 h, with increased GRIA1 expression evidence of the attempts to clear excessive glutamate. Notably, CEL + IMP mice had significantly reduced GRIA1 levels compared to the group receiving impact but not treatment, such that CEL + IMP levels were not different at 48 h from non-impacted control groups. The lack of improvement in MWM measures of spatial working memory in the CEL treated group following normalization of GRIA1 levels point to other signaling influences at work in spatial working memory disruption.
Transactive response DNA binding protein 43 (TDP-43) provides maintenance of neuronal cytoskeleton assembly, and abnormal signaling can occur from axonal damage and ionic calcium dysregulation that results from mTBI. This disruption affects the structure and organization of microtubules and neurofilaments [52], and these processes are involved in conditions of chronic neurodegeneration [53]. Pathological TDP-43 is rarely seen in the acute phases of injury or disease, and shows prevalence in chronic disease progression [54]. Indeed, in progressed cases of CTE, TDP-43 protein aggregation is a key diagnostic feature at autopsy [55]. When assessing the TARDBP gene that codes for TDP-43, we saw no significant changes in the acute phase in either the cortex or hippocampus, as may be expected from human data. However, in both tissues, TARDBP was significantly elevated at three months in the impact group receiving only the vehicle. We saw a variability in effectiveness of the CEL treatment, whereby the CEL + IMP levels were significantly lower compared to VEH + IMP in the hippocampus, but failed to reach significance in the cortex. There was a large variability of individual response to CEL treatment in the cortex, which impacted the significance of response.
Incorrect TDP-43 signaling impairs protein and organelle transport within the neuron, and leads to clumping of axonal debris that contributes to neurofibrillary tangles (NFTs) involving microtubule-associated protein tau [56,57]. Tau (coded by the MAPT gene) is an important regulator of axon construction [58], and conditions of trauma that cause axonal damage lead to compromised tau integrity, such that the presence of pathological NFTs comprised of hyper-phosphorylated tau is a key diagnostic characteristic of CTE [55]. For our study, in the cortex at 48 h, there was upregulation of MAPT mRNA levels in the VEH + IMP group that was significantly different from non-impacted control groups and the CEL + IMP group. The VEH + IMP expression change was less pronounced in the cortex at three months and in the hippocampus at both time points, however, there remained significant differences compared to the celecoxib-treated impact group in each of these conditions. This response provides some evidence of a neuroprotective mechanism that may maintain tau function and organization within the axon. Celecoxib treatment has shown protective effects in other neurodegenerative conditions where abnormal tau is implicated [15], however, the tau-mediating involvement of COX-2 inhibition in these processes has not previously been shown. We further investigated axonal neurodegeneration via neurofilament light (NF-L), which is the cytoskeleton structural protein that is prominent in white matter tracts [59]. NF-L has been shown as a sensitive marker of neurofilament breakdown that is initiated by mTBI and found in serum, CSF, and brain tissue [60]. NF-L in our study was measured by NEFL mRNA expression, and was found to be significantly elevated in the untreated impact group in the acute and chronic hippocampus, but was not different in the cortex at either time point. This NF-L involvement is notable, as serum levels are recognized as a valuable measure of concussive and subconcussive injury in both the initial phase following injury and in follow-up during prolonged recovery [61]. When comparing the CEL + IMP group, levels were not different from non-impacted controls, and showed a significant reduction from VEH + IMP at both time points. This provides further evidence of the protective role of celecoxib in mitigating impaired axonal integrity via cytoskeleton structural maintenance.
This study shows an anti-inflammatory and neuroprotective response of COX-2 inhibitors in mRNA expression, but impairment of cognitive function. Previous preclinical TBI studies have shown that functional improvement and measures of histological neuronal damage are not always correlated [62,63]. The initial inflammatory response following injury is intended to provide protection, and in altering the COX-2 release following injury, there may be unintended deleterious consequences. For example, brain prostaglandin levels rise quickly in response to injury, and the reflective increase in thromboxane may reduce the risk of brain hemorrhage [50]. In this way, it is hypothesized that inhibiting the production of PGE2 with NSAID therapy exacerbates the neuroinflammatory response, and that COX-2 inhibition may elicit more detriment than benefit in some cases. Selective COX-2 blockage has been previously shown to increase the expression of several inflammatory genes in the microglia of mice [64]. It is possible that in removing some of the adaptive processes with the introduction of celecoxib, there was an acute exacerbation of secondary damage processes.
Previous studies have investigated the role of CEL in TBI [32] and intracerebral hemorrhage [33]. However, this is the first study we are aware of that has aimed to treat repetitive mTBI with prophylactic CEL. When administering the drug after injury, the efficacy of achieving initiation within the tight post-injury window is difficult to achieve, and if this opportunity is missed, the effectiveness of treatment is vastly reduced [35]. As the safety profile of CEL is well-established, we aimed to administer the drug in the context of contact sports, which rarely provides injuries of a severe nature requiring hospitalization, but contribute their own risk of seemingly innocuous, frequent impacts that have now been implicated as a risk factor for developing neurodegenerative disease [65]. We hypothesized that prophylactic treatment would increase effectiveness in this model, but from our results and the proposed protective mechanisms of compounds such as PGE2 in the acute phase, this administration appears inappropriate. Nonetheless, there may be a role for delayed COX-2 inhibition to derive benefit from the chronic neuroprotective effects that we demonstrated, and this would be a valuable future investigation.
A limitation of this study is that we did not directly measure the temporal profile of COX-2 expression and the attenuation that CEL affects. While evidence of COX-2 elevation in the cortex and hippocampus has been reported in moderate TBI models [66], and examination of COX-2 response has been undertaken in a mild TBI model [67], knowledge of COX-2 expression in this repetitive mTBI model would be valuable for future investigations. Additionally, it would have been valuable to assess other cytokines and COX-2 products, such as PGE2. A further limitation is that molecular markers were not also assessed for change in protein expression. This study served as a point-of-concept investigation, and a comprehensive evaluation of changes in protein levels was beyond the scope of assessment and should be a target for future studies. Another limitation is that only male animals were used in this study and that investigation of sex differences was not undertaken, as has been recommended for pre-clinical studies [68]. Studies show that the COX-2 induced and derived brain injury effect tends to be male-specific [23]. The role of age was also not considered in this study, and the neurodegenerative mechanisms described here would be useful to consider in juvenile and aged mice in future studies.
In conclusion, there is a pressing need for neuroprotective strategies in mTBI management, and this study investigated the role of suppressing the COX-2 pathway and potential non-COX-2 specific anti-inflammatory effects congruent with a common class effect. This study provided evidence of the effectiveness of celecoxib in reducing the neurodegenerative response of mobilized microglia, astrocytes, and components of axonal breakdown, including tau and NF-L. However, like previous studies that have investigated non-specific NSAIDS, the selective COX-2 inhibitor did not show improvement in functional measures. This indicates that the anti-inflammatory action aimed at reducing the impairment imparted by mTBI is not effective in preventing damage across complex secondary injury cascades, and thus impairment of function. This study shows that targeting COX-2, even in the most advantageous scenario of pre-treating with the drug and providing head trauma at arguably sub-concussive levels, is unlikely to be an effective treatment in mitigating damage, but not a direct behavioral improvement. In moving forward, the heterogeneity of brain injuries provides challenges for the continuing work that is required in developing therapies.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/traumacare1010003/s1, Table S1: (A) Statistical results obtained from the one-way ANOVAs for gene expression changes for the two brain regions at the acute sacrifice time (48-hour post-mTBI) (significant differences p < 0.05 are in bold); (B) Statistical results obtained from the one-way ANOVAs for gene expression changes for the two brain regions at the chronic sacrifice time (3 months post-mTBI) (significant differences p < 0.05 are in bold).
Author Contributions
M.H.: Conceptualization, methodology, investigation, formal analysis, writing–original draft preparation; R.V.: Supervision, writing–reviewing and editing; A.S.: Supervision, writing–reviewing and editing; A.F.: Resources, reviewing and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Australian Government Research Training Scheme. The funding source was not involved in any of the following: study design; collection, analysis, and interpretation of data; writing of the report; or decision to submit the article for publication.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Animal Ethics Committee of Central Queensland University (CQU AEC 0000021124 on 3 August 2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the reported results can be found at DOI: 10.17632/tj8mf3htkc.1.
Acknowledgments
The authors would like to express their gratitude to Kristal Parmenter and Peta-Anne Clark for their time and assistance with the laboratory experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rivara, F.P.; Graham, R. Sports-Related Concussions in Youth: Report from the institute of medicine and national research council. JAMA 2014, 311, 239–240. [Google Scholar] [CrossRef]
- Montenigro, P.H.; Baugh, C.M.; Daneshvar, D.H.; Mez, J.; Budson, A.E.; Au, R.; Katz, D.I.; Cantu, R.C.; Stern, R.A. Clinical subtypes of chronic traumatic encephalopathy: Literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimer Res. Ther. 2014, 6, 68. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, J.E.; Begbie, F.D.; Trojanowski, J.Q.; Smith, D.H.; Stewart, W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 2013, 136, 28–42. [Google Scholar] [CrossRef]
- Smith, C.; Gentleman, S.M.; Leclercq, P.D.; Murray, L.S.; Griffin, W.S.T.; Graham, D.I.; Nicoll, J.A.R. The neuroinflammatory response in humans after traumatic brain injury. Neuropathol. Appl. Neurobiol. 2013, 39, 654–666. [Google Scholar] [CrossRef]
- Chakraborti, A.K.; Garg, S.K.; Kumar, R.; Motiwala, H.F.; Jadhavar, P.S. Progress in COX-2 inhibitors: A journey so far. Curr. Med. Chem. 2010, 17, 1563–1593. [Google Scholar] [CrossRef] [PubMed]
- Allison, M.C.; Howatson, A.G.; Torrance, C.J.; Lee, F.D.; Russell, R.I. Gastrointestinal Damage Associated with the Use of Nonsteroidal Antiinflammatory Drugs. N. Engl. J. Med. 1992, 327, 749–754. [Google Scholar] [CrossRef]
- Clive, D.M.; Stoff, J.S. Renal Syndromes Associated with Nonsteroidal Antiinflammatory Drugs. N. Engl. J. Med. 1984, 310, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Blasko, I.; Apochal, A.; Boeck, G.; Hartmann, T.; Grubeck-Loebenstein, B.; Ransmayr, G. Ibuprofen Decreases Cytokine-Induced Amyloid Beta Production in Neuronal Cells. Neurobiol. Dis. 2001, 8, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Pompl, P.N.; Ho, L.; Bianchi, M.; McManus, T.; Qin, W.; Pasinetti, G.M. A therapeutic role for cyclooxygenase-2 inhibitors in a transgenic mouse model of amyotrophic lateral sclerosis. FASEB J. 2003, 17, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Salzberg-Brenhouse, H.C.; Chen, E.-Y.; Emerich, D.F.; Baldwin, S.; Hogeland, K.; Ranelli, S.; LaFreniere, D.; Perdomo, B.; Novak, L.; Kladis, T.; et al. Inhibitors of Cyclooxygenase-2, but Not Cyclooxygenase-1 Provide Structural and Functional Protection against Quinolinic Acid-Induced Neurodegeneration. J. Pharmacol. Exp. Ther. 2003, 306, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Katsuki, H.; Nishiwaki, M.; Kume, T.; Kaneko, S.; Akaike, A. Lipopolysaccharide-induced dopaminergic cell death in rat midbrain slice cultures: Role of inducible nitric oxide synthase and protection by indomethacin. J. Neurochem. 2003, 86, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Rawat, C.; Kukal, S.; Dahiya, U.R.; Kukreti, R. Cyclooxygenase-2 (COX-2) inhibitors: Future therapeutic strategies for epilepsy management. J. Neuroinflamm. 2019, 16, 197. [Google Scholar] [CrossRef]
- Teismann, P.; Tieu, K.; Choi, D.; Wu, D.; Naini, A.; Hunot, S.; Vila, M.; Jackson-Lewis, V.; Przedborski, S. Cyclooxygenase-2 is instrumental in parkinson’s disease neurodegeneration. Proc. Natl. Acad. Sci. USA 2003, 100, 5473–5478. [Google Scholar] [CrossRef] [PubMed]
- Drachman, D.B.; Frank, K.; Dykes-Hoberg, M.; Teismann, P.; Almer, G.; Przedborski, S.; Rothstein, J.D. Cyclooxygenase 2 inhibition protects motor neurons and prolongs survival in a transgenic mouse model of ALS. Ann. Neurol. 2002, 52, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Mhillaj, E.; Morgese, M.G.; Tucci, P.; Furiano, A.; Luongo, L.; Bove, M.; Maione, S.; Cuomo, V.; Schiavone, S.; Trabace, L. Celecoxib Prevents Cognitive Impairment and Neuroinflammation in Soluble Amyloid β-treated Rats. Neuroscience 2018, 372, 58–73. [Google Scholar] [CrossRef]
- Rogers, J.; Kirby, L.C.; Hempelman, S.R.; Berry, D.L.; McGeer, P.L.; Kaszniak, A.W.; Zalinski, J.; Cofield, M.; Mansukhani, L.; Willson, P.; et al. Clinical trial of indomethacin in Alzheimer’s disease. Neurology 1993, 43, 1609. [Google Scholar] [CrossRef] [PubMed]
- Scharf, S.; Mander, A.; Ugoni, A.; Vajda, F.; Christophidis, N. A double-blind, placebo-controlled trial of diclofenac/misoprostol in alzheimer’s disease. Neurology 1999, 53, 197. [Google Scholar] [CrossRef]
- Borre, Y.; Lemstra, S.; Westphal, K.G.; Morgan, M.E.; Olivier, B.; Oosting, R.S. Celecoxib delays cognitive decline in an animal model of neurodegeneration. Behav. Brain Res. 2012, 234, 285–291. [Google Scholar] [CrossRef]
- Cernak, I.; O’Connor, C.; Vink, R. Activation of cyclo-oxygenase-2 contributes to motor and cognitive dysfunction following diffuse traumatic brain injury in rats. Clin. Exp. Pharmacol. Physiol. 2001, 28, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Schafer, K.A.; Grundman, M.; Pfeiffer, E.; Sano, M.; Davis, K.L.; Farlow, M.R.; Jin, S.; Thomas, R.G.; Thal, L.J.; et al. Effects of Rofecoxib or Naproxen vs Placebo on Alzheimer Disease Progression: A randomized controlled trial. JAMA 2003, 289, 2819–2826. [Google Scholar] [CrossRef]
- Reines, S.A.; Block, G.A.; Morris, J.C.; Liu, G.; Nessly, M.L.; Lines, C.R.; Norman, B.A.; Baranak, C.C. Rofecoxib: No effect on Alzheimer’s disease in a 1-year, randomized, blinded, controlled study. Neurology 2004, 62, 66–71. [Google Scholar] [CrossRef]
- Turner, R.C.; Lucke-Wold, B.P.; Logsdon, A.F.; Robson, M.J.; Dashnaw, M.L.; Huang, J.H.; Smith, K.E.; Huber, J.D.; Rosen, C.L.; Petraglia, A.L. The Quest to Model Chronic Traumatic Encephalopathy: A Multiple Model and Injury Paradigm Experience. Front. Neurol. 2015, 6, 222. [Google Scholar] [CrossRef]
- Dehlaghi Jadid, K.; Davidsson, J.; Lidin, E.; Hånell, A.; Angéria, M.; Mathiesen, T.; Risling, M.; Günther, M. Cox-2 inhibition by diclofenac is associated with decreased apoptosis and lesion area after experimental focal penetrating traumatic brain in-jury in rats. Front. Neurol. 2019, 10, 811. [Google Scholar] [CrossRef]
- Mannix, R.; Meehan, W.P.; Mandeville, J.; Grant, P.E.; Gray, T.; Berglass, J.; Zhang, J.; Bryant, J.; Rezaie, S.; Chung, J.Y.; et al. Clinical correlates in an experimental model of repetitive mild brain injury. Ann. Neurol. 2013, 74, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D.I.; Angoa-Pérez, M.; Kuhn, D.M. Prolonged Repetitive Head Trauma Induces a Singular Chronic Traumatic Encephalopathy-Like Pathology in White Matter Despite Transient Behavioral Abnormalities. Am. J. Pathol. 2016, 186, 2869–2886. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Mannix, R.; Berglass, J.; Berkner, J.; Moleus, P.; Qiu, J.; Andrews, N.; Gunner, G.; Berglass, L.; Jantzie, L.L.; Robinson, S.; et al. Chronic gliosis and behavioral deficits in mice following repetitive mild traumatic brain injury. J. Neurosurg. 2014, 121, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D.W. Purification of Nucleic Acids by Extraction with Phenol:Chloroform. Cold Spring Harb. Protoc. 2006, 2006. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hart, J.; Kraut, M.A.; Womack, K.B.; Strain, J.; Didehbani, N.; Bartz, E.; Conover, H.; Mansinghani, S.; Lu, H.; Cullum, C.M. Neuroimaging of Cognitive Dysfunction and Depression in Aging Retired National Football League Players: A cross-sectional study. JAMA Neurol. 2013, 70, 326–335. [Google Scholar] [CrossRef]
- De Beaumont, L.; Théoret, H.; Mongeon, D.; Messier, J.; Leclerc, S.; Tremblay, S.; Ellemberg, D.; Lassonde, M. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain 2009, 132, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.K.; Mach, S.A.; Moore, A.N. Regional Expression and Role of Cyclooxygenase-2 Following Experimental Traumatic Brain Injury. J. Neurotrauma 2000, 17, 69–81. [Google Scholar] [CrossRef]
- Chu, K.; Jeong, S.-W.; Jung, K.-H.; Han, S.-Y.; Lee, S.-T.; Kim, M.; Roh, J.-K. Celecoxib Induces Functional Recovery after Intracerebral Hemorrhage with Reduction of Brain Edema and Perihematomal Cell Death. J. Cereb. Blood Flow Metab. 2004, 24, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Girgis, H.; Palmier, B.; Croci, N.; Soustrat, M.; Plotkine, M.; Marchand-Leroux, C. Effects of selective and non-selective cyclooxygenase inhibition against neurological deficit and brain oedema following closed head injury in mice. Brain Res. 2013, 1491, 78–87. [Google Scholar] [CrossRef]
- Bergold, P.J. Treatment of traumatic brain injury with anti-inflammatory drugs. Exp. Neurol. 2016, 275 Pt 3, 367–380. [Google Scholar] [CrossRef]
- Kaufmann, W.E.; Worley, P.F.; Pegg, J.; Bremer, M.; Isakson, P. Cox-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc. Natl. Acad. Sci. USA 1996, 93, 2317–2321. [Google Scholar] [CrossRef]
- Rall, J.M.; Mach, S.A.; Dash, P.K. Intrahippocampal infusion of a cyclooxygenase-2 inhibitor attenuates memory acquisition in rats. Brain Res. 2003, 968, 273–276. [Google Scholar] [CrossRef]
- Teather, L.A.; Packard, M.G.; Bazan, N.G. Post-Training Cyclooxygenase-2 (COX-2) Inhibition Impairs Memory Consolidation. Learn. Mem. 2002, 9, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Corps, K.N.; Roth, T.L.; McGavern, D.B. Inflammation and Neuroprotection in Traumatic Brain Injury. JAMA Neurol. 2015, 72, 355–362. [Google Scholar] [CrossRef]
- Loane, D.J.; Kumar, A. Microglia in the TBI brain: The good, the bad, and the dysregulated. Exp. Neurol. 2016, 275 Pt 3, 316–327. [Google Scholar] [CrossRef]
- Clark, D.P.Q.; Perreau, V.M.; Shultz, S.R.; Brady, R.D.; Lei, E.; Dixit, S.; Taylor, J.M.; Beart, P.M.; Boon, W.C. Inflammation in Traumatic Brain Injury: Roles for Toxic A1 Astrocytes and Microglial-Astrocytic Crosstalk. Neurochem. Res. 2019, 44, 1410–1424. [Google Scholar] [CrossRef] [PubMed]
- Loane, D.J.; Kumar, A.; Stoica, B.A.; Cabatbat, R.; Faden, A.I. Progressive Neurodegeneration After Experimental Brain Trauma: Association with chronic microglial activation. J. Neuropathol. Exp. Neurol. 2014, 73, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. A polarizing question: Do M1 and M2 microglia exist? Nat. Neurosci. 2016, 19, 987. [Google Scholar] [CrossRef]
- Hiskens, M.I.; Angoa-Pérez, M.; Schneiders, A.G.; Vella, R.K.; Fenning, A.S. Modeling sports-related mild traumatic brain injury in animals—A systematic review. J. Neurosci. Res. 2019, 97, 1194–1222. [Google Scholar] [CrossRef]
- Woodcock, T.; Morganti-Kossmann, M.C. The Role of Markers of Inflammation in Traumatic Brain Injury. Front. Neurol. 2013, 4, 18. [Google Scholar] [CrossRef]
- Freudenberg, F.; Marx, V.; Seeburg, P.H.; Sprengel, R.; Celikel, T. Circuit mechanisms of GluA1-dependent spatial working memory. Hippocampus 2013, 23, 1359–1366. [Google Scholar] [CrossRef]
- Lewerenz, J.; Maher, P. Chronic glutamate toxicity in neurodegenerative diseases—What is the evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Onyszchuk, G.; Berman, N.E.J. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp. Neurol. 2008, 213, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Constantinidis, C.; Wang, X.-J. A Neural Circuit Basis for Spatial Working Memory. Neuroscientist 2004, 10, 553–565. [Google Scholar] [CrossRef]
- Gopez, J.J.; Yue, H.; Vasudevan, R.; Malik, A.S.; Fogelsanger, L.N.; Lewis, S.; Panikashvili, D.; Shohami, E.; Jansen, S.A.; Narayan, R.K.; et al. Cyclooxygenase-2-specific Inhibitor Improves Functional Outcomes, Provides Neuroprotection, and Reduces Inflammation in a Rat Model of Traumatic Brain Injury. Neurosurgery 2005, 56, 590–604. [Google Scholar] [CrossRef]
- Scali, C.; Giovannini, M.G.; Prosperi, C.; Bellucci, A.; Pepeu, G.; Casamenti, F. The selective cyclooxygenase-2 inhibitor rofecoxib suppresses brain inflammation and protects cholinergic neurons from excitotoxic degeneration in vivo. Neuroscience 2003, 117, 909–919. [Google Scholar] [CrossRef]
- Sephton, C.F.; Cenik, B.; Cenik, B.K.; Herz, J.; Yu, G. TDP-43 in central nervous system development and function: Clues to TDP-43-associated neurodegeneration. Biol. Chem. 2012, 393, 589–594. [Google Scholar] [CrossRef]
- King, A.; Sweeney, F.; Bodi, I.; Troakes, C.; Maekawa, S.; Al-Sarraj, S. Abnormal tdp-43 expression is identified in the neo-cortex in cases of dementia pugilistica, but is mainly confined to the limbic system when identified in high and moderate stages of Alzheimer’s disease. Neuropathology 2010, 30, 408–419. [Google Scholar] [CrossRef]
- McKee, A.C.; Gavett, B.E.; Stern, R.A.; Nowinski, C.J.; Cantu, R.C.; Kowall, N.W.; Perl, D.P.; Hedley-Whyte, E.T.; Price, B.; Sullivan, C.; et al. TDP-43 Proteinopathy and Motor Neuron Disease in Chronic Traumatic Encephalopathy. J. Neuropathol. Exp. Neurol. 2010, 69, 918–929. [Google Scholar] [CrossRef]
- McKee, A.C.; The TBI/CTE Group; Cairns, N.J.; Dickson, D.W.; Folkerth, R.D.; Keene, C.D.; Litvan, I.; Perl, D.P.; Stein, T.D.; Vonsattel, J.G.; et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016, 131, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Tripodis, Y.; Alvarez, V.E.; Huber, B.; Kiernan, P.T.; Daneshvar, D.H.; Mez, J.; Montenigro, P.H.; Solomon, T.M.; Alosco, M.L.; et al. Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol. Commun. 2016, 4, 112. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gonzalez, I.; Soto, C. Misfolded protein aggregates: Mechanisms, structures and potential for disease transmission. Semin. Cell Dev. Biol. 2011, 22, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Zaidi, T.; Wen, G.; Grundke-Iqbal, I.; Merz, P.; Shaikh, S.; Wisniewski, H.; Alafuzoff, I.; Winblad, B. Defective brain microtubule assembly in Alzheimer’s disease. Lancet 1986, 2, 421–426. [Google Scholar] [CrossRef]
- Al Nimer, F.; Thelin, E.; Nyström, H.; Dring, A.M.; Svenningsson, A.; Piehl, F.; Nelson, D.W.; Bellander, B.-M. Comparative Assessment of the Prognostic Value of Biomarkers in Traumatic Brain Injury Reveals an Independent Role for Serum Levels of Neurofilament Light. PLoS ONE 2015, 10, e0132177. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.E.; Khalil, M. Neurofilaments as biomarkers in multiple sclerosis. Mult. Scler. J. 2012, 18, 552–556. [Google Scholar] [CrossRef]
- Hiskens, M.I.; Schneiders, A.G.; Angoa-Pérez, M.; Vella, R.K.; Fenning, A.S. Blood biomarkers for assessment of mild traumatic brain injury and chronic traumatic encephalopathy. Biomarkers 2020, 25, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, L.S.; Sun, D.A.; Sombati, S.; Baranova, A.; Wilson, M.S.; Attkisson, E.; Hamm, R.J.; DeLorenzo, R.J. Alterations in neuronal calcium levels are associated with cognitive deficits after traumatic brain injury. Neurosci. Lett. 2008, 441, 115–119. [Google Scholar] [CrossRef]
- Scheff, S.W.; Baldwin, S.A.; Brown, R.W.; Kraemer, P.J. Morris Water Maze Deficits in Rats following Traumatic Brain Injury: Lateral Controlled Cortical Impact. J. Neurotrauma 1997, 14, 615–627. [Google Scholar] [CrossRef]
- Blais, V.; Turrin, N.P.; Rivest, S. Cyclooxygenase 2 (COX-2) inhibition increases the inflammatory response in the brain during systemic immune stimuli. J. Neurochem. 2005, 95, 1563–1574. [Google Scholar] [CrossRef]
- Stern, R.A.; Riley, D.O.; Daneshvar, D.H.; Nowinski, C.J.; Cantu, R.C.; McKee, A.C. Long-term Consequences of Repetitive Brain Trauma: Chronic Traumatic Encephalopathy. PMR 2011, 3, S460–S467. [Google Scholar] [CrossRef]
- Strauss, K.I.; Barbe, M.F.; Marshall, R.M.; Raghupathi, R.; Mehta, S.; Narayan, R.K. Prolonged Cyclooxygenase-2 Induction in Neurons and Glia Following Traumatic Brain Injury in the Rat. J. Neurotrauma 2000, 17, 695–711. [Google Scholar] [CrossRef]
- Kelso, M.L.; Scheff, S.W.; Pauly, J.R.; Loftin, C.D. Effects of genetic deficiency of cyclooxygenase-1 or cyclooxygenase-2 on functional and histological outcomes following traumatic brain injury in mice. BMC Neurosci. 2009, 10, 108. [Google Scholar] [CrossRef]
- Clayton, J.A.; Collins, F.S. Policy: NIH to balance sex in cell and animal studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).