Impact of Antidiabetic Medication on Therapy Outcomes in Metastatic Urothelial Cancer Patients Receiving Enfortumab Vedotin Monotherapy
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic Characterization of the Study Population
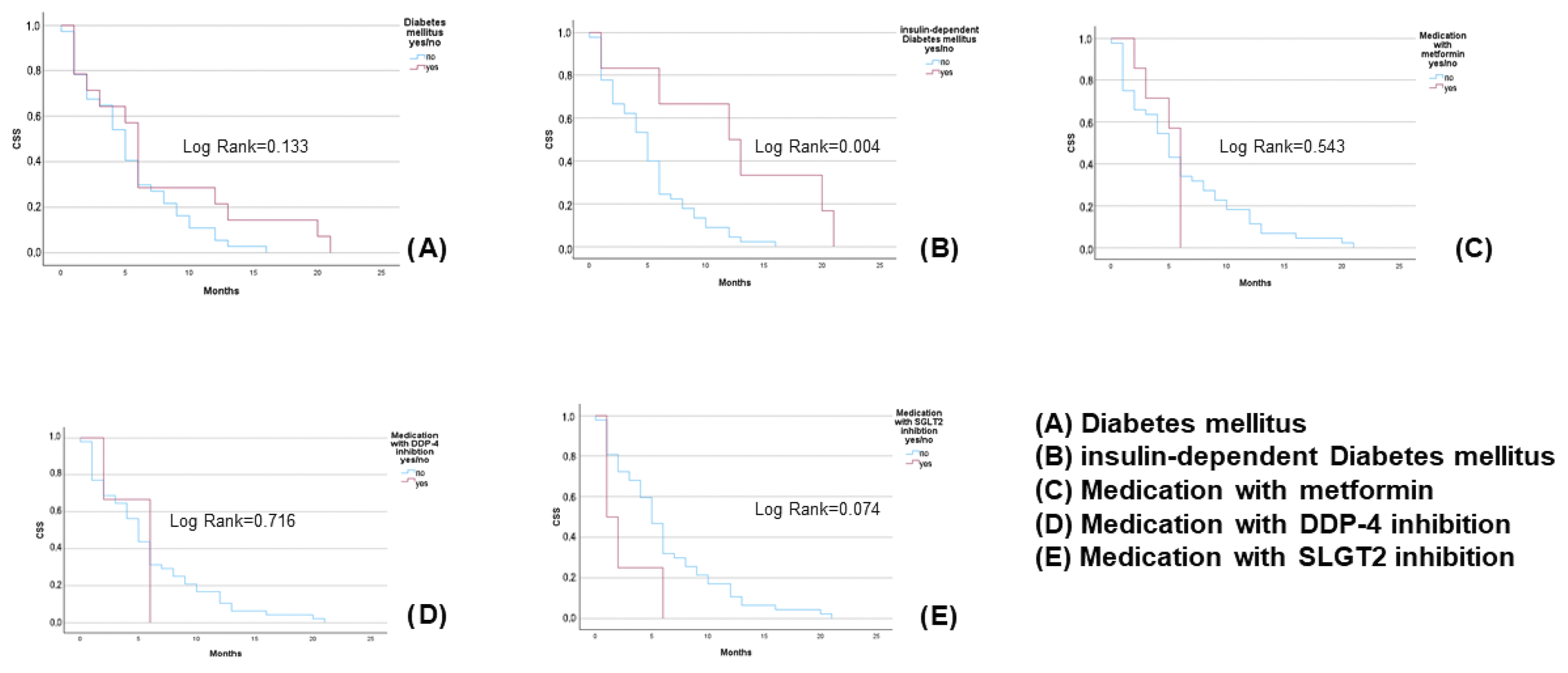
3.2. Diabetes Mellitus, Antidiabetic Drugs, and Response Rates
3.3. Subgroup Analysis of UTUC
4. Discussion
4.1. Metformin and Cancer-Specific Outcomes
- –
- Firstly, is metformin a driver of carcinogenesis in bladder cancer?
- –
- Secondly, does metformin’s interaction with bladder cancer treatment lead to worse response or outcomes?
4.2. Insulin-Dependent Diabetes and Hyperglycaemia
4.3. Considerations for the UTUC Subgroup
4.4. Study Limitations
4.5. Mechanistic Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ripamonti, E.; Azoulay, L.; Abramovicz, M.; Platt, R.W.; Siussa, S. A systematic review of observational studies of the association between pioglitazone use and bladder cancer. Diabet. Med. 2019, 36, 22–35. [Google Scholar] [CrossRef]
- Mehtälä, J.; Khanfir, H.; Bennett, D.; Ye, Y.; Korhonen, P.; Hoti, F. Pioglitazone use and risk of bladder cancer: A systematic literature review and meta-analysis of observational studies. Diabetol. Int. 2019, 10, 24–36. [Google Scholar] [CrossRef]
- Turner, R.M.; Kwok, C.S.; Chen-Turner, C.; Maduakor, C.A.; Singh, S.; Loke, Y.K. Thiazolidinediones and associated risk of bladder cancer: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2014, 78, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. A review on thiazolidinediones and bladder cancer in human studies. J. Environ. Sci. Health Part. 2014, 32, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Schneidewind, L.; Sommerhalder, B.; Willi, D.; Rönnau, C.; Uhlig, A.; Kiss, B. Association of glitazones and bladder cancer: A rapid review. Urologie 2025, 64, 669–677. [Google Scholar] [CrossRef]
- Yoshimura, R.; Matsuyama, M.; Segawa, Y.; Hase, T.; Mitsuhashi, M.; Tsuchida, T.; Wada, S.; Kawahito, Y.; Sano, H.; Nakatani, T. Expression of peroxisome proliferator-activated receptors (PPARs) in human urinary bladder carcinoma and growth inhibition by its agonists. Int. J. Cancer 2003, 104, 597–602. [Google Scholar] [CrossRef]
- Plumber, S.A.; Tate, T.; Al-Ahmadi, H.; Chen, X.; Choi, W.; Basar, M.; Lu, C.; Viny, A.; Batourina, E.; Li, J.; et al. Rosiglitazone and trametinib exhibit potent anti-tumor activity in a mouse model of muscle invasive bladder cancer. Nat. Commun. 2024, 15, 6538. [Google Scholar] [CrossRef]
- Chang, K.; Delavan, H.M.; Zhu, J.; Kasap, C.; Yip, E.; Lodha, R.; Sheng-You, L.; Porten, S.; Friedlander, T.; Koshkin, V.; et al. Modulating the PPARγ pathway to augment NECTIN4-targeting chimeric antigen receptor (CAR) T cell therapy. In Proceedings of the AACR Special Conference on Bladder Cancer: Transforming the Field, Charlotte, NC, USA, 17–20 May 2024; Volume 30. [Google Scholar]
- Yu, E.Y.; Petrylak, D.P.; O´Donnel, P.H.; Lee, J.L.; van der Heijden, M.S.; Loriot, Y.; Stein, M.N.; Necchi, A.; Kojima, T.; Harrison, M.R.; et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2021, 22, 872–882. [Google Scholar] [CrossRef]
- Yajima, S.; Hirose, K.; Masuda, H. Enfortumab Vedotin with or Without Pembrolizumab in Metastatic Urothelial Carcinoma: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2025, 8, e250250. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Powles, T.; Sonpavde, G.P.; Loriot, Y.; Duran, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Mamtani, R.; et al. EV-301 long-term outcomes: 24-month findings from the phase III trial of enfortumab vedotin versus chemotherapy in patients with previously treated advanced urothelial carcinoma. Ann. Oncol. 2023, 34, 1047–1054. [Google Scholar] [CrossRef]
- Powles, T.; Valderrama, B.P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J.; et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. New Engl. J. Med. 2024, 390, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.; Flemming, K.; McInnes, E.; Oliver, S.; Craig, J. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med. Res. Methodol. 2012, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Villaruz, L.C.; Socinski, M.A. The clinical viewpoint: Definitions, limitations of RECIST, practical considerations of measurement. Clin. Cancer Res. 2013, 19, 2629–2636. [Google Scholar] [CrossRef]
- Makkar, N.; Ostrom, Q.T.; Kruchko, C.; Barnholtz-Sloan, J.S. A comparison of relative survival and cause-specific survival methods to measure net survival in cancer populations. Cancer Med. 2018, 7, 4773–4780. [Google Scholar] [CrossRef]
- Hu, J.; Chen, J.; Cui, Y.; Zhu, Y.; Ren, W.; Zhou, X.; Long-Fei, L.; He-Qun, C.; Xiong-Bing, Z. Association of metformin intake with bladder cancer risk and oncologic outcomes in type 2 diabetes mellitus patients—A systematic review and meta-analysis. Medicine 2018, 97, 30. [Google Scholar] [CrossRef]
- McFarland, M.S.; Cripps, R. Diabetes Mellitus and Increased Risk of Cancer: Focus on Metformin and the Insulin Analogs. Pharmacotherapy 2010, 30, 1159–1178. [Google Scholar] [CrossRef]
- Sondergaard, C.S.; Esquivel, P.N.; Dalamaga, M.; Magkos, F. Use of Antihyperglycemic Drugs and Risk of Cancer in Patients with Diabetes. Curr. Oncol. Rep. 2023, 25, 29–40. [Google Scholar] [CrossRef]
- Shen, Z.; Xue, D.; Wang, K.; Zhang, F.; Shi, J.; Jia, B.; Yang, D.; Zhang, Q.; Zhang, S.; Jiang, H.; et al. Metformin exerts an antitumor effect by inhibiting bladder cancer cell migration and growth and promoting apoptosis through the PI3K/AKT/mTOR pathway. BMC Urology 2022, 22, 79. [Google Scholar] [CrossRef]
- Najafi, F.; Rajati, F.; Sarokhani, D.; Babandpour, M.; Moradinazar, M. The Relationship between Metformin Consumption and Cancer Risk: An Updated Umbrella Review of Systematic Reviews and Meta-Analyses. Int. J. Prev. Med. 2023, 14, 90. [Google Scholar] [PubMed]
- Van Hattum, J.W.; de Ruiter, B.M.; Oddens, J.R.; de Reijke, T.M.; Wilmink, J.W.; Molenaar, R.J. The Effect of Metformin on Bladder Cancer Incidence and Outcomes: A Systematic Review and Meta-Analysis. Bladder Cancer 2022, 8, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Elleisy, M.; Zettl, H.; Dräger, D.L.; Hakenberg, O.W. The Impact of Diabetes and Antidiabetics on the Obesity Paradox in Renal Cell Cancer. Urol. Int. 2024, 109, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Elleisy, M.; Zettl, H.; Dräger, D.L.; Hakenberg, O.W. The Impact of Diabetes and Antidiabetics on Uro-Oncological Disease Outcomes: A Single-Center Experience. Urol. Int. 2025, 1–17. [Google Scholar] [CrossRef]
- Zschaebitz, S.; Casuscelli, J.; Büttner, T.; Darr, C.; Volk, A.-L.; Hennig, M.; Holzwarth, N.; Zengerling, F.; Dib, M.; Schlack, K.; et al. Enfortumab vedotin plus pembrolizumab in metastatic urothelial carcinoma: First results on outcomes and safety in a German multicenter real-world patient cohort (GUARDIANS). J. Clin. Oncol. 2025, 43, 715. [Google Scholar] [CrossRef]
- Pandolfo, S.D.; Cilio, S.; Aveta, A.; Wu, Z.; Cerrato, C.; Napolitano, L.; Lasorsa, F.; Lucarelli, G.; Verze, P.; Siracusano, S.; et al. Upper Tract Urothelial Cancer: Guideline of Guidelines. Cancers 2024, 16, 1115. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Wang, L.; Paul, A.; Raman, J.D.; Necchi, A.; Psutka, S.P.; Buonerba, C.; Zargar, H.; Black, P.C.; et al. Neoadjuvant systemic therapy in patients undergoing nephroureterectomy for urothelial cancer: A multidisciplinary systematic review and critical analysis. Minerva Urol. Nephrol. 2022, 74, 518–527. [Google Scholar] [CrossRef]
- Shahid, M.; Kim, M.; Yeon, A.; Jin, P.; Kim, W.-K.; You, S.; Kim, J. Pioglitazone Alters the Proteomes of Normal Bladder Epithelial Cells but Shows No Tumorigenic Effects. Int. Neurourol. J. 2020, 24, 29–40. [Google Scholar] [CrossRef]
- Dabrowski, M. Diabetes, Antidiabetic Medications and Cancer Risk in Type 2 Diabetes: Focus on SGLT-2 Inhibitors. Int. J. Mol. Sci. 2021, 22, 1680. [Google Scholar] [CrossRef]
| Parameter | Bladder Cancer | UTUC | p Value |
|---|---|---|---|
| Mean age | 68.6 (SD 9.2) | 66.1 (SD 9.7) | 0.428 |
| Male gender | 84 (77.1%) | 9 (56.3%) | 0.075 |
| Primary metastatic disease | 53 (48.6%) | 6 (37.5%) | 0.405 |
| Mean ECOG | 0.7 (SD 0.8) | 0.6 (SD 0.6) | 0.814 |
| Mean therapy line | 2.95 (SD 0.7) | 3.0 (SD 0.9) | 0.525 |
| T Stage | cT1 13 (11.9%) pT2a 12 (11.0%) pT2b 13 (11.9%) pT3a 12 (11.0%) pT3b 29 (26.6%) pT4a 15 (13.9%) pT4b 12 (11.0%) ypT0 1 (0.9%) pTis 2 (1.8%) | cT1 4 (25.0%) pT2 2 (12.5%) pT3 8 (50.0) pT4 2 (12.5%) | 0.003 |
| Diabetes Parameters | 3 Months ORR, No vs. Yes for Diabetic Parameter (p Value) | 6 Months ORR No vs. Yes for Diabetic Parameter (p Value) | Cancer-Specific Mortality No vs. Yes for Diabetic Parameter (p Value) |
|---|---|---|---|
| Diabetes mellitus | 59.4% vs. 70.8% | 70.9% vs. 73.3% | 36.6% vs. 58.3% |
| (0.301) | (0.868) | (0.052) | |
| Insulin-dependent diabetes mellitus | 60.0% vs. 80.0% | 71.4% vs. 71.4% | 39.1% vs. 60.0% |
| (0.212) | (0.943) | (0.198) | |
| Metformin | 62.1% vs. 55.6% | 73.8% vs. 40.0% | 37.9% vs. 77.8% |
| (0.699) | (0.227) | (0.019) | |
| DDP-4 inhibitor | 62.5% vs. 40.0% | 71.6% vs. 66.6% | 40.0% vs. 60.0% |
| (0.311) | (0.952) | (0.373) | |
| SGLT2 inhibitor | 62.7% vs. 42.9% | 70.1% vs. 100.0 | 39.8% vs. 57.1% |
| (0.294) | (0.534) | (0.365) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneidewind, L.; Kiss, B.; Zengerling, F.; Uhlig, A.; Klümper, N.; Büttner, T.; Heinzelbecker, J.; Elegeert, T.; Aksoy, C.; Rönnau, C.; et al. Impact of Antidiabetic Medication on Therapy Outcomes in Metastatic Urothelial Cancer Patients Receiving Enfortumab Vedotin Monotherapy. Biologics 2025, 5, 20. https://doi.org/10.3390/biologics5030020
Schneidewind L, Kiss B, Zengerling F, Uhlig A, Klümper N, Büttner T, Heinzelbecker J, Elegeert T, Aksoy C, Rönnau C, et al. Impact of Antidiabetic Medication on Therapy Outcomes in Metastatic Urothelial Cancer Patients Receiving Enfortumab Vedotin Monotherapy. Biologics. 2025; 5(3):20. https://doi.org/10.3390/biologics5030020
Chicago/Turabian StyleSchneidewind, Laila, Bernhard Kiss, Friedemann Zengerling, Annemarie Uhlig, Niklas Klümper, Thomas Büttner, Julia Heinzelbecker, Thomas Elegeert, Cem Aksoy, Cindy Rönnau, and et al. 2025. "Impact of Antidiabetic Medication on Therapy Outcomes in Metastatic Urothelial Cancer Patients Receiving Enfortumab Vedotin Monotherapy" Biologics 5, no. 3: 20. https://doi.org/10.3390/biologics5030020
APA StyleSchneidewind, L., Kiss, B., Zengerling, F., Uhlig, A., Klümper, N., Büttner, T., Heinzelbecker, J., Elegeert, T., Aksoy, C., Rönnau, C., Schiller, T., Hahn, O., Hakenberg, O., Gakis, G., Hoffmann, M., Saar, M., & Kranz, J. (2025). Impact of Antidiabetic Medication on Therapy Outcomes in Metastatic Urothelial Cancer Patients Receiving Enfortumab Vedotin Monotherapy. Biologics, 5(3), 20. https://doi.org/10.3390/biologics5030020






