Tumor Initiation and Progression in People Living on Antiretroviral Therapies
Abstract
1. Introduction
2. Mechanisms of Tumor Initiation in PLWHA
2.1. HIV-Related Factors
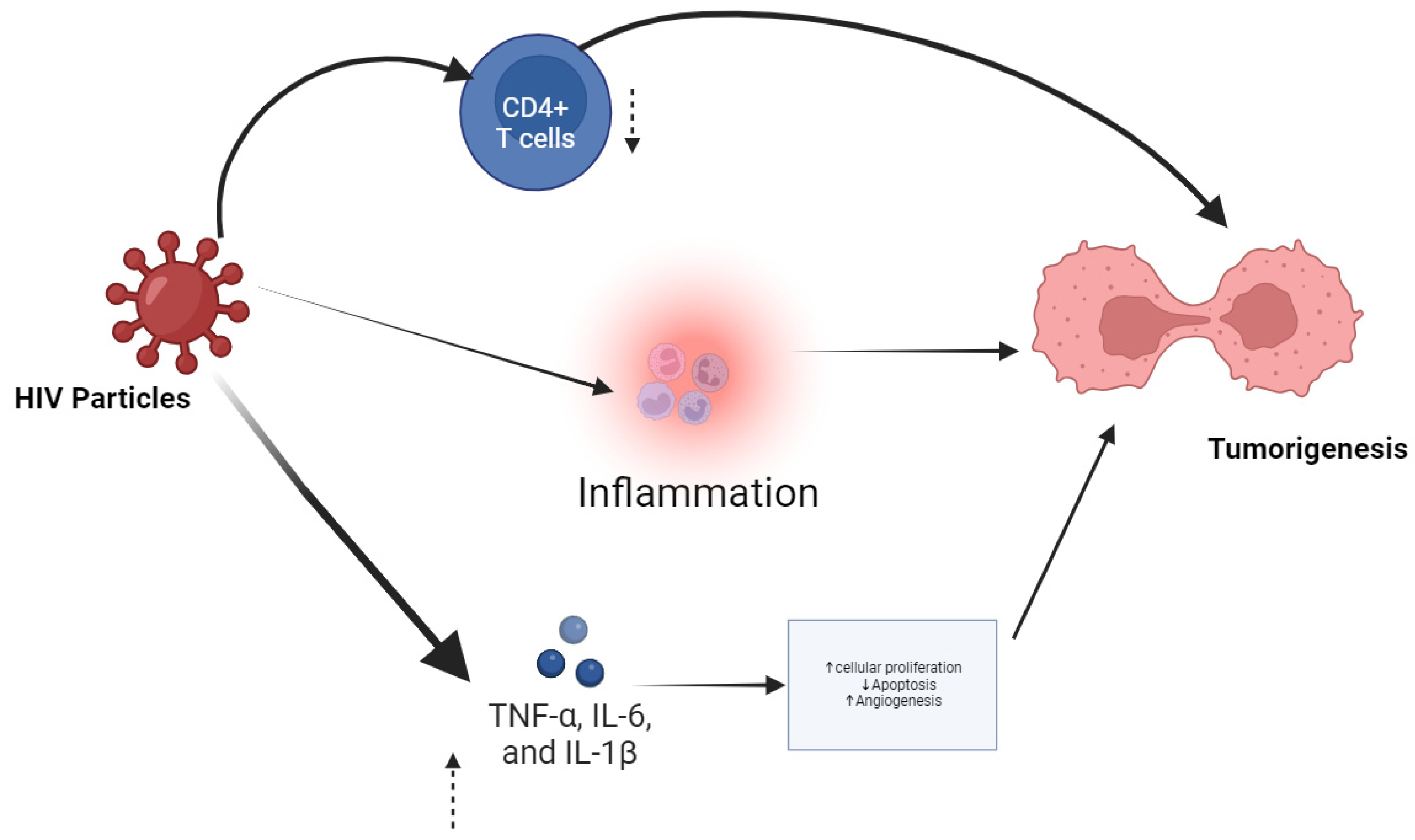
2.1.1. Immunosuppression and Chronic Inflammation
2.1.2. Direct Oncogenic Effects of HIV Proteins
2.2. ART-Related Factors
2.2.1. Impact of ART on Immune Restoration
2.2.2. Potential Mutagenic Effects of ART Drugs
2.2.3. Customizing ART Regimens to Mitigate Cancer Risk
2.3. Co-Infections and Oncogenic Viruses
2.3.1. Human Papillomavirus
2.3.2. Epstein–Barr Virus
2.3.3. Kaposi’s Sarcoma-Associated Herpesvirus
2.3.4. Hepatitis B and C Viruses
3. Types of Cancers in People Living with HIV and AIDS on Antiretroviral Therapy
3.1. AIDS-Defining Cancers
3.1.1. Kaposi’s Sarcoma
3.1.2. Non-Hodgkin Lymphoma
3.1.3. Cervical Cancer
3.2. Non-AIDS-Defining Cancers
3.2.1. Lung Cancer
3.2.2. Liver Cancer
3.2.3. Anal Cancer
3.2.4. Hodgkin Lymphoma
3.2.5. Others (e.g., Colorectal Cancer, Breast Cancer)
4. Tumor Progression and Antiretroviral Therapy
4.1. Antiretroviral Therapy’s Influence on Cancer Progression
4.1.1. Immune Reconstitution Inflammatory Syndrome (IRIS) and Cancer Progression
4.1.2. Antiretroviral Therapy Adherence and Viral Suppression
4.2. Antiretroviral Therapy-Related Toxicity and Cancer Progression
4.2.1. Long-Term Toxicities of Antiretroviral Therapy Drugs
4.2.2. Metabolic Complications
4.3. Drug Interactions and Cancer Treatment
4.3.1. Antiretroviral Therapy and Chemotherapy Interactions
4.3.2. Impact on Efficacy and Toxicity of Cancer Therapies
5. Epidemiological Studies and Data
5.1. Incidence and Prevalence of Cancers in People Living with HIV and AIDS
5.1.1. Comparative Studies Between PLWHA and the General Population
5.1.2. Trends over Time with the Advent of Antiretroviral Therapy
5.2. Geographic and Demographic Variations
5.2.1. Differences by Region and Access to ART
5.2.2. Variations by Age, Sex, and Race
6. Prevention and Management Strategies
6.1. Screening and Early Detection
6.1.1. Guidelines for Cancer Screening in People Living with HIV and AIDS
- Cervical cancer: regular Pap smears and HPV testing for HIV-positive women are essential for early detection of cervical cancer. Screening should start at the time of HIV diagnosis and be repeated annually [76].
- Anal cancer: for high-risk groups, such as men who have sex with men (MSM) and individuals with a history of anal HPV infection, anal Pap smears or high-resolution anoscopies are recommended [77].
- Liver cancer: screening for hepatocellular carcinoma (HCC) with ultrasound and alpha-fetoprotein (AFP) levels is advised for PLWHA co-infected with hepatitis B or C viruses [78].
- Other cancers: regular screenings for breast, colorectal, and lung cancers should be conducted according to general population guidelines, with adjustments based on individual risk factors and health status.
6.1.2. Role of Regular Monitoring and Check-Ups
6.2. Modifying ART Regimens
6.2.1. Selection of ART Drugs with Lower Carcinogenic Potential
6.2.2. Addressing ART-Related Toxicities
6.3. Integrative Cancer Care
6.3.1. Multidisciplinary Approach to Cancer Treatment
6.3.2. Supportive Care and Quality of Life
- Pain management and symptom control: using medications, physical therapy, and complementary therapies to alleviate symptoms.
- Nutritional support: ensuring adequate nutrition to support immune function and recovery.
- Psychosocial support: providing counseling, support groups, and mental health services to address emotional and psychological needs.
- Palliative care: offering palliative care services to improve comfort and quality of life for patients with advanced cancer [80].
6.4. Vaccination and Prophylaxis
6.4.1. HPV Vaccination
6.4.2. Prophylactic Treatments for Co-Infections
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deeks, S.G.; Overbaugh, J.; Phillips, A.; Buchbinder, S. HIV infection. Nat. Rev. Dis. Primers 2015, 1, 15035. [Google Scholar] [CrossRef] [PubMed]
- Hiv/Aids. Available online: https://www.who.int/news-room/questions-and-answers/item/hiv-aids (accessed on 11 July 2024).
- Okoye, A.A.; Picker, L.J. CD4(+) T-cell depletion in HIV infection: Mechanisms of immunological failure. Immunol. Rev. 2013, 254, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Alum, E.U.; Uti, D.E.; Ugwu, O.P.-C.; Alum, B.N. Toward a cure—Advancing HIV/AIDs treatment modalities beyond antiretroviral therapy: A Review. Medicine 2024, 103, e38768. [Google Scholar] [CrossRef]
- SeyedAlinaghi, S.; Afsahi, A.M.; Moradi, A.; Parmoon, Z.; Habibi, P.; Mirzapour, P.; Dashti, M.; Ghasemzadeh, A.; Karimi, E.; Sanaati, F.; et al. Current ART, determinants for virologic failure and implications for HIV drug resistance: An umbrella review. AIDS Res. Ther. 2023, 20, 74. [Google Scholar] [CrossRef]
- Kilcrease, C.; Yusuf, H.; Park, J.; Powell, A.; James, L.; Oates, J.; Lmsw, B.D.; Weld, E.D.; Dooley, K.E.; Arrington-Sanders, R.; et al. Realizing the promise of long-acting antiretroviral treatment strategies for individuals with HIV and adherence challenges: An illustrative case series. AIDS Res. Ther. 2022, 19, 56. [Google Scholar] [CrossRef]
- Lee, S.O.; Lee, J.E.; Lee, S.; Lee, S.H.; Kang, J.S.; Son, H.; Lee, H.; Kim, J. Nationwide population-based incidence of cancer among patients with HIV/AIDS in South Korea. Sci. Rep. 2022, 12, 9974. [Google Scholar] [CrossRef]
- Trickey, A.; Zhang, L.; Sabin, C.A.; Sterne, J.A.C. Life expectancy of people with HIV on long-term antiretroviral therapy in Europe and North America: A cohort study. Lancet Healthy Longev. 2022, 3, S2. [Google Scholar] [CrossRef]
- Krump, N.A.; You, J. Molecular mechanisms of viral oncogenesis in humans. Nat. Rev. Microbiol. 2018, 16, 684–698. [Google Scholar] [CrossRef]
- Mohanty, K.; Cheung, H.W.; Stafford, K.A.; Riedel, D.J. Care Outcomes in People Living with HIV and Cancer. Curr. Treat. Options Infect. Dis. 2021, 13, 83–99. [Google Scholar] [CrossRef]
- Grafodatskaya, D.; Cytrynbaum, C.; Weksberg, R. The health risks of ART. EMBO Rep. 2013, 14, 129–135. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, X.; Yi, Y.; Wang, X.; Zhu, L.; Shen, Y.; Lin, D.; Wu, C. Tumor initiation and early tumorigenesis: Molecular mechanisms and interventional targets. Signal Transduct. Target. Ther. 2024, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Cao, W.; Li, T. HIV-Related Immune Activation and Inflammation: Current Understanding and Strategies. J. Immunol. Res. 2021, 2021, 7316456. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Isaguliants, M.; Bayurova, E.; Avdoshina, D.; Kondrashova, A.; Chiodi, F.; Palefsky, J.M. Oncogenic Effects of HIV-1 Proteins, Mechanisms Behind. Cancers 2021, 13, 305. [Google Scholar] [CrossRef]
- Clark, E.; Nava, B.; Caputi, M. Tat is a multifunctional viral protein that modulates cellular gene expression and functions. Oncotarget 2017, 8, 27569–27581. [Google Scholar] [CrossRef]
- Rasola, A.; Gramaglia, D.; Boccaccio, C.; Comoglio, P.M. Apoptosis enhancement by the HIV-1 Nef protein. J. Immunol. 2001, 166, 81–88. [Google Scholar] [CrossRef]
- Staudt, R.P.; Alvarado, J.J.; Emert-Sedlak, L.A.; Shi, H.; Shu, S.T.; Wales, T.E.; Engen, J.R.; Smithgall, T.E. Structure, function, and inhibitor targeting of HIV-1 Nef-effector kinase complexes. J. Biol. Chem. 2020, 295, 15158–15171. [Google Scholar] [CrossRef]
- De Clercq, J.; Rutsaert, S.; De Scheerder, M.-A.; Verhofstede, C.; Callens, S.; Vandekerckhove, L. Benefits of antiretroviral therapy initiation during acute HIV infection. Acta Clin. Belg. 2022, 77, 168–176. [Google Scholar] [CrossRef]
- Hileman, C.O.; Funderburg, N.T. Inflammation, Immune Activation, and Antiretroviral Therapy in HIV. Curr. HIV/AIDS Rep. 2017, 14, 93–100. [Google Scholar] [CrossRef]
- Yang, X.; Su, B.; Zhang, X.; Liu, Y.; Wu, H.; Zhang, T. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: Challenges of immunological non-responders. J. Leukoc. Biol. 2020, 107, 597–612. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, S.; Advani, D.; Khosla, A.; Kumar, P.; Ambasta, R.K. Unboxing the molecular modalities of mutagens in cancer. Environ. Sci. Pollut. Res. Int. 2022, 29, 62111–62159. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W.; Day, B.J.; Copeland, W.C. Mitochondrial toxicity of NRTI antiviral drugs: An integrated cellular perspective. Nat. Rev. Drug Discov. 2003, 2, 812–822. [Google Scholar] [CrossRef]
- Desai, M.; Iyer, G.; Dikshit, R.K. Antiretroviral drugs: Critical issues and recent advances. Indian. J. Pharmacol. 2012, 44, 288–298. [Google Scholar] [CrossRef]
- Urbina, A.E.; McGowan, J.P.; Fine, S.M.; Vail, R.; Merrick, S.T.; Radix, A.; Hoffmann, C.J.; Gonzalez, C.J. Selecting an Initial ART Regimen; Johns Hopkins University: Baltimore, MD, USA, 2022. [Google Scholar]
- Lustberg, M.B.; Kuderer, N.M.; Desai, A.; Bergerot, C.; Lyman, G.H. Mitigating long-term and delayed adverse events associated with cancer treatment: Implications for survivorship. Nat. Rev. Clin. Oncol. 2023, 20, 527–542. [Google Scholar] [CrossRef]
- Zapatka, M.; Borozan, I.; Brewer, D.S.; Iskar, M.; Grundhoff, A.; Alawi, M.; Desai, N.; Sültmann, H.; Moch, H.; Cooper, C.S.; et al. The landscape of viral associations in human cancers. Nat. Genet. 2020, 52, 320–330. [Google Scholar] [CrossRef]
- Verma, M.; Erwin, S.; Abedi, V.; Hontecillas, R.; Hoops, S.; Leber, A.; Bassaganya-Riera, J.; Ciupe, S.M. Modeling the Mechanisms by Which HIV-Associated Immunosuppression Influences HPV Persistence at the Oral Mucosa. PLoS ONE 2017, 12, e0168133. [Google Scholar] [CrossRef]
- Young, L.S.; Murray, P.G. Epstein-Barr virus and oncogenesis: From latent genes to tumours. Oncogene 2003, 22, 5108–5121. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Y.; Wang, C.; Gan, R. Signaling pathways of EBV-induced oncogenesis. Cancer Cell Int. 2021, 21, 93. [Google Scholar] [CrossRef]
- Jary, A.; Veyri, M.; Gothland, A.; Leducq, V.; Calvez, V.; Marcelin, A.-G. Kaposi’s Sarcoma-Associated Herpesvirus, the Etiological Agent of All Epidemiological Forms of Kaposi’s Sarcoma. Cancers 2021, 13, 6208. [Google Scholar] [CrossRef]
- Motlhale, M.; Sitas, F.; Bradshaw, D.; Chen, W.C.; Singini, M.G.; de Villiers, C.B.; Lewis, C.M.; Muchengeti, M.; Waterboer, T.; Mathew, C.G.; et al. Epidemiology of Kaposi’s sarcoma in sub-Saharan Africa. Cancer Epidemiol. 2022, 78, 102167. [Google Scholar] [CrossRef]
- Shen, C.; Jiang, X.; Li, M.; Luo, Y. Hepatitis Virus and Hepatocellular Carcinoma: Recent Advances. Cancers 2023, 15, 533. [Google Scholar] [CrossRef] [PubMed]
- Spengler, U.; Fischer, H.-P.; Caselmann, W.H. Chapter 34—Liver Disease Associated with Viral Infections. In Zakim and Boyer’s Hepatology, 6th ed.; Boyer, T.D., Manns, M.P., Sanyal, A.J., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2012; pp. 629–643. [Google Scholar] [CrossRef]
- Suk-Ouichai, C.; Coghill, A.E.; Schabath, M.B.; Sanchez, J.A.; Chahoud, J.; Necchi, A.; Giuliano, A.R.; Spiess, P.E. A clinical overview of people living with HIV and genitourinary cancer care. Nat. Rev. Urol. 2024, 21, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Dalla Pria, A.; Pinato, D.J.; Bracchi, M.; Bower, M. Recent advances in HIV-associated Kaposi sarcoma. F1000Res 2019, 8, 970. [Google Scholar] [CrossRef] [PubMed]
- Guerra, K.C.; Crane, J.S. Cutaneous Vascular Malignancies, Angiosarcoma and Kaposi Sarcoma; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Liu, P.; Tang, Z.; Lan, G.; Zhu, Q.; Chen, H.; You, Y.; Yang, X.; Liang, S.; Chen, Y.; Xing, H.; et al. Early antiretroviral therapy on reducing HIV transmission in China: Strengths, weaknesses and next focus of the program. Sci. Rep. 2018, 8, 3431. [Google Scholar] [CrossRef]
- Shingleton, J.; Wang, J.; Baloh, C.; Dave, T.; Davis, N.; Happ, L.; Jadi, O.; Kositsky, R.; Li, X.; Love, C.; et al. Non-Hodgkin Lymphomas: Malignancies Arising from Mature B Cells. Cold Spring Harb. Perspect. Med. 2021, 11, a034843. [Google Scholar] [CrossRef]
- Indari, O.; Ghosh, S.; Bal, A.S.; James, A.; Garg, M.; Mishra, A.; Karmodiya, K.; Jha, H.C. Awakening the sleeping giant: Epstein-Barr virus reactivation by biological agents. Pathog. Dis. 2024, 82, ftae002. [Google Scholar] [CrossRef]
- Höft, M.A.; Burgers, W.A.; Riou, C. The immune response to SARS-CoV-2 in people with HIV. Cell. Mol. Immunol. 2024, 21, 184–196. [Google Scholar] [CrossRef]
- Okunade, K.S. Human papillomavirus and cervical cancer. J. Obstet. Gynaecol. 2020, 40, 602–608. [Google Scholar] [CrossRef]
- Wilson, E.M.P.; Sereti, I. Immune restoration after antiretroviral therapy: The pitfalls of hasty or incomplete repairs. Immunol. Rev. 2013, 254, 343–354. [Google Scholar] [CrossRef]
- Sigel, K.; Makinson, A.; Thaler, J. Lung cancer in persons with HIV. Curr. Opin. HIV AIDS 2017, 12, 31–38. [Google Scholar] [CrossRef]
- Manosuthi, W.; Charoenpong, L.; Santiwarangkana, C. A retrospective study of survival and risk factors for mortality among people living with HIV who received antiretroviral treatment in a resource-limited setting. AIDS Res. Ther. 2021, 18, 71. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Kanwal, F.; Llovet, J.M. Global trends in hepatocellular carcinoma epidemiology: Implications for screening, prevention and therapy. Nat. Rev. Clin. Oncol. 2023, 20, 864–884. [Google Scholar] [CrossRef]
- Neshat, S.Y.; Quiroz, V.M.; Wang, Y.; Tamayo, S.; Doloff, J.C. Liver Disease: Induction, Progression, Immunological Mechanisms, and Therapeutic Interventions. Int. J. Mol. Sci. 2021, 22, 6777. [Google Scholar] [CrossRef]
- Wieland, U.; Kreuter, A. Anal cancer risk: HPV-based cervical screening programmes. Lancet Infect. Dis. 2019, 19, 799–800. [Google Scholar] [CrossRef]
- Barroso, L.F.; Stier, E.A.; Hillman, R.; Palefsky, J. Anal Cancer Screening and Prevention: Summary of Evidence Reviewed for the 2021 Centers for Disease Control and Prevention Sexually Transmitted Infection Guidelines. Clin. Infect. Dis. 2022, 74, S179–S192. [Google Scholar] [CrossRef]
- Navarro, J.-T.; Moltó, J.; Tapia, G.; Ribera, J.-M. Hodgkin Lymphoma in People Living with HIV. Cancers 2021, 13, 4366. [Google Scholar] [CrossRef]
- Montaño, M.A.; Chagomerana, M.B.; Borok, M.; Painschab, M.; Uldrick, T.S.; Bender Ignacio, R.A. Impact of Antiretroviral Therapy on Cancer Treatment Outcomes among People Living with HIV in Low- and Middle-Income Countries: A Systematic Review. Curr. HIV/AIDS Rep. 2021, 18, 105–116. [Google Scholar] [CrossRef]
- Marino, A.; Pavone, G.; Martorana, F.; Fisicaro, V.; Motta, L.; Spampinato, S.; Celesia, B.M.; Cacopardo, B.; Vigneri, P.; Nunnari, G. Navigating the Nexus: HIV and Breast Cancer—A Critical Review. Int. J. Mol. Sci. 2024, 25, 3222. [Google Scholar] [CrossRef]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef]
- Tappuni, A.R. Immune reconstitution inflammatory syndrome. Adv. Dent. Res. 2011, 23, 90–96. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br. J. Pharmacol. 2006, 148, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Byrd, K.K.; Hou, J.G.; Hazen, R.; Kirkham, H.; Suzuki, S.; Clay, P.G.; Bush, T.; Camp, N.M.; Weidle, P.J.; Delpino, A. Antiretroviral Adherence Level Necessary for HIV Viral Suppression Using Real-World Data. J. Acquir. Immune Defic. Syndr. 2019, 82, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Pellowski, J.A.; Price, D.M.; Harrison, A.D.; Tuthill, E.L.; Myer, L.; Operario, D.; Lurie, M.N. A Systematic Review and Meta-analysis of Antiretroviral Therapy (ART) Adherence Interventions for Women Living with HIV. AIDS Behav. 2019, 23, 1998–2013. [Google Scholar] [CrossRef]
- Holec, A.D.; Mandal, S.; Prathipati, P.K.; Destache, C.J. Nucleotide Reverse Transcriptase Inhibitors: A Thorough Review, Present Status and Future Perspective as HIV Therapeutics. Curr. HIV Res. 2017, 15, 411–421. [Google Scholar] [CrossRef]
- Koethe, J.R.; Lagathu, C.; Lake, J.E.; Domingo, P.; Calmy, A.; Falutz, J.; Brown, T.T.; Capeau, J. HIV and antiretroviral therapy-related fat alterations. Nat. Rev. Dis. Primers 2020, 6, 48. [Google Scholar] [CrossRef]
- Arcidiacono, B.; Iiritano, S.; Nocera, A.; Possidente, K.; Nevolo, M.T.; Ventura, V.; Foti, D.; Chiefari, E.; Brunetti, A. Insulin resistance and cancer risk: An overview of the pathogenetic mechanisms. Exp. Diabetes Res. 2012, 2012, 789174. [Google Scholar] [CrossRef]
- Pappan, N.; Awosika, A.O.; Rehman, A. Dyslipidemia; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Gong, Y.; Haque, S.; Chowdhury, P.; Cory, T.J.; Kodidela, S.; Yallapu, M.M.; Norwood, J.M.; Kumar, S. Pharmacokinetics and pharmacodynamics of cytochrome P450 inhibitors for HIV treatment. Expert Opin. Drug Metab. Toxicol. 2019, 15, 417–427. [Google Scholar] [CrossRef]
- Scripture, C.D.; Figg, W.D. Drug interactions in cancer therapy. Nat. Rev. Cancer 2006, 6, 546–558. [Google Scholar] [CrossRef]
- Jiang, X.-H.; Chen, X.-J.; Xie, Q.-Q.; Feng, Y.-S.; Chen, S.; Peng, J.-S. Effects of art therapy in cancer care: A systematic review and meta-analysis. Eur. J. Cancer Care 2020, 29, e13277. [Google Scholar] [CrossRef]
- Chawla, A.; Wang, C.; Patton, C.; Murray, M.; Punekar, Y.; de Ruiter, A.; Steinhart, C. A Review of Long-Term Toxicity of Antiretroviral Treatment Regimens and Implications for an Aging Population. Infect. Dis. Ther. 2018, 7, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Kartoun, U.; Stavropoulos, H.; Zambrano, J.A.; Tang, P.C. Personalized treatment options for chronic diseases using precision cohort analytics. Sci. Rep. 2021, 11, 1139. [Google Scholar] [CrossRef] [PubMed]
- Mathoma, A.; Sartorius, B.; Mahomed, S. The Trends and Risk Factors of AIDS-Defining Cancers and Non-AIDS-Defining Cancers in Adults Living with and without HIV: A Narrative Review. J. Cancer Epidemiol. 2024, 2024, 7588928. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.G.; Smith, D.; Salters, K.A.; Zhang, W.; Kanters, S.; Milan, D.; Montaner, J.S.; Coldman, A.; Hogg, R.S.; Wiseman, S.M. Overview of cancer incidence and mortality among people living with HIV/AIDS in British Columbia, Canada: Implications for HAART use and NADM development. BMC Cancer 2017, 17, 270. [Google Scholar] [CrossRef]
- DuBois, T.D.; Henry, K.A.; Siegel, S.D.; Lynch, S.M. Geographic Disparities in Cancer Incidence in the US Population Aged 20 to 49 Years, 2016–2020. Prev. Chronic Dis. 2024, 21, E32. [Google Scholar] [CrossRef]
- CDC. Cancer Data and Statistics. Cancer. 2024. Available online: https://www.cdc.gov/cancer/data/index.html (accessed on 5 September 2024).
- Hull, R.; Mbele, M.; Makhafola, T.; Hicks, C.; Wang, S.; Reis, R.M.; Mehrotra, R.; Mkhize-Kwitshana, Z.; Kibiki, G.; Bates, D.O.; et al. Cervical cancer in low and middle-income countries. Oncol. Lett. 2020, 20, 2058–2074. [Google Scholar] [CrossRef]
- Haque, A.T.; de González, A.B.; Chen, Y.; A Haozous, E.; Inoue-Choi, M.; Lawrence, W.R.; McGee-Avila, J.K.; Nápoles, A.M.; Pérez-Stable, E.J.; Taparra, K.; et al. Cancer mortality rates by racial and ethnic groups in the United States, 2018–2020. J. Natl. Cancer Inst. 2023, 115, 822–830. [Google Scholar] [CrossRef]
- Liu, G.; Sharma, M.; Tan, N.; Barnabas, R.V. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS 2018, 32, 795–808. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Fisher, P.G.; Gibbs, P. Early detection of cancer: Past, present, and future. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, 57–65. [Google Scholar] [CrossRef]
- Timoney, M.T.; Atrio, J.M.; McGowan, J.P.; Fine, S.M.; Vail, R.; Merrick, S.T.; Radix, A.; Hoffmann, C.J.; Gonzalez, C.J. Screening for Cervical Dysplasia and Cancer in Adults with HIV; Johns Hopkins University: Baltimore, MD, USA, 2022. [Google Scholar]
- Darragh, T.M.; Winkler, B. Anal cancer and cervical cancer screening: Key differences. Cancer Cytopathol. 2011, 119, 5–19. [Google Scholar] [CrossRef]
- Frenette, C.T.; Isaacson, A.J.; Bargellini, I.; Saab, S.; Singal, A.G. A Practical Guideline for Hepatocellular Carcinoma Screening in Patients at Risk. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Dillner, J. Early detection and prevention. Mol. Oncol. 2019, 13, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Krzyszczyk, P.; Acevedo, A.; Davidoff, E.J.; Timmins, L.M.; Marrero-Berrios, I.; Patel, M.; White, C.; Lowe, C.; Sherba, J.J.; Hartmanshenn, C.; et al. The growing role of precision and personalized medicine for cancer treatment. Technology 2018, 6, 79–100. [Google Scholar] [CrossRef]
- Jin, Q.; Ma, R.C.W. Metabolomics in Diabetes and Diabetic Complications: Insights from Epidemiological Studies. Cells 2021, 10, 2832. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Du, J. Human Papillomavirus Vaccines: An Updated Review. Vaccines 2020, 8, 391. [Google Scholar] [CrossRef]
- Sadiq, U.; Shrestha, U.; Guzman, N. Prevention of Opportunistic Infections in HIV/AIDS; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Chronic Comorbidities & Coinfections in People Living with HIV. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/treatment/chronic-comorbidities-and-coinfections (accessed on 19 July 2024).

| Category | AIDS-Defining Cancers (ADCs) | Non-AIDS-Defining Cancers (NADCs) |
|---|---|---|
| Common Types | Kaposi’s sarcoma (KS), non-Hodgkin lymphoma (NHL), cervical cancer | Lung cancer, liver cancer, anal cancer, Hodgkin lymphoma |
| Etiology | Primarily linked to severe immunosuppression and oncogenic virus co-infections (e.g., Kaposi’s Sarcoma-Associated Herpesvirus (KSHV), Epstein-Barr Virus (EBV), Human Papillomavirus (HPV)) | Linked to chronic inflammation, co-infections (e.g., Human Papillomavirus (HPV), Hepatitis B Virus (HBV), Hepatitis C Virus (HCV)), lifestyle factors (e.g., smoking), and ART-related metabolic changes |
| Impact of ART | Significant reduction in incidence due to improved immune function and reduced Human Immunodeficiency Virus (HIV) viral load | Increased incidence due to longer life expectancy, persistent chronic inflammation, and ART-related factors |
| Screening and Prevention | Regular Pap smears and HPV testing for cervical cancer; monitoring for KS and NHL | Regular screenings for lung, liver, and anal cancers; HPV vaccination; liver ultrasound and Alpha-Fetoprotein (AFP) levels for Hepatocellular Carcinoma (HCC) |
| Treatment | Antiretroviral Therapy (ART) optimization, chemotherapy, radiation, surgery for cervical cancer | Multimodal approach including surgery, chemotherapy, radiation, targeted therapies, and continuous ART |
| Prognosis | Improves with ART but varies based on cancer type and stage at diagnosis | Generally poorer prognosis compared to ADCs due to later stage at diagnosis and complex interplay of factors |
| Geographic Variations | Higher prevalence in regions with limited ART access | More common in high-income countries with widespread ART access |
| Demographic Factors | Younger people living with HIV/AIDS (PLWHA) more affected; higher incidence in women for cervical cancer | Older PLWHA more affected; higher incidence in men for lung and anal cancers |
| Category | Factors | Description |
|---|---|---|
| HIV-Related Factors | Immunosuppression | Human Immunodeficiency Virus (HIV) depletes CD4+ T cells, weakening immune surveillance. |
| Chronic inflammation | Persistent immune activation promotes a tumor-friendly environment. | |
| Oncogenic HIV proteins | Proteins like Tat and Nef promote cell proliferation and inhibit apoptosis. | |
| ART-Related Factors | Incomplete immune restoration | ART does not fully normalize immune function, allowing cancer risk to persist. |
| Genotoxicity | Some Antiretroviral Therapy (ART) drugs may cause DNA damage, leading to mutations. | |
| Metabolic changes | ART-induced insulin resistance and dyslipidemia are linked to cancer risk. | |
| Co-Infections | Human Papillomavirus (HPV) | Increases risk of cervical, anal, and oropharyngeal cancers. |
| Epstein-Barr Virus (EBV) | Associated with non-Hodgkin lymphoma and Hodgkin lymphoma. | |
| Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) | Causes Kaposi’s sarcoma, particularly in immunocompromised individuals. | |
| Cancer Types | AIDS-defining cancers (ADCs) | Kaposi’s sarcoma, non-Hodgkin lymphoma, cervical cancer. |
| Non-AIDS-defining cancers (NADCs) | Lung cancer, liver cancer, anal cancer, Hodgkin lymphoma. | |
| Prevention and Management | Screening | Regular Pap smears, HPV testing, liver ultrasound, and AFP levels. |
| ART regimen modification | Selecting drugs with lower carcinogenic potential and managing toxicities. | |
| Integrative care | Multidisciplinary approach involving oncologists, HIV specialists, and primary care physicians. | |
| Vaccination | HPV vaccination to reduce the risk of HPV-related cancers. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olufemi, S.E.; Adediran, D.A.; Sobodu, T.; Adejumo, I.O.; Ajani, O.F.; Oladipo, E.K. Tumor Initiation and Progression in People Living on Antiretroviral Therapies. Biologics 2024, 4, 390-406. https://doi.org/10.3390/biologics4040024
Olufemi SE, Adediran DA, Sobodu T, Adejumo IO, Ajani OF, Oladipo EK. Tumor Initiation and Progression in People Living on Antiretroviral Therapies. Biologics. 2024; 4(4):390-406. https://doi.org/10.3390/biologics4040024
Chicago/Turabian StyleOlufemi, Seun E., Daniel A. Adediran, Temitope Sobodu, Isaac O. Adejumo, Olumide F. Ajani, and Elijah K. Oladipo. 2024. "Tumor Initiation and Progression in People Living on Antiretroviral Therapies" Biologics 4, no. 4: 390-406. https://doi.org/10.3390/biologics4040024
APA StyleOlufemi, S. E., Adediran, D. A., Sobodu, T., Adejumo, I. O., Ajani, O. F., & Oladipo, E. K. (2024). Tumor Initiation and Progression in People Living on Antiretroviral Therapies. Biologics, 4(4), 390-406. https://doi.org/10.3390/biologics4040024








