Cell-Based Therapies for the Treatment of Traumatic Brain Injury: Promises and Trajectories
Abstract
1. Epidemiology of Traumatic Brain Injury
2. Pathophysiology of Traumatic Brain Injury
3. Therapeutics for TBI
4. Endogenous Cell Therapy
5. Exogenous Cell Therapies
5.1. Sources of Exogenous Cells
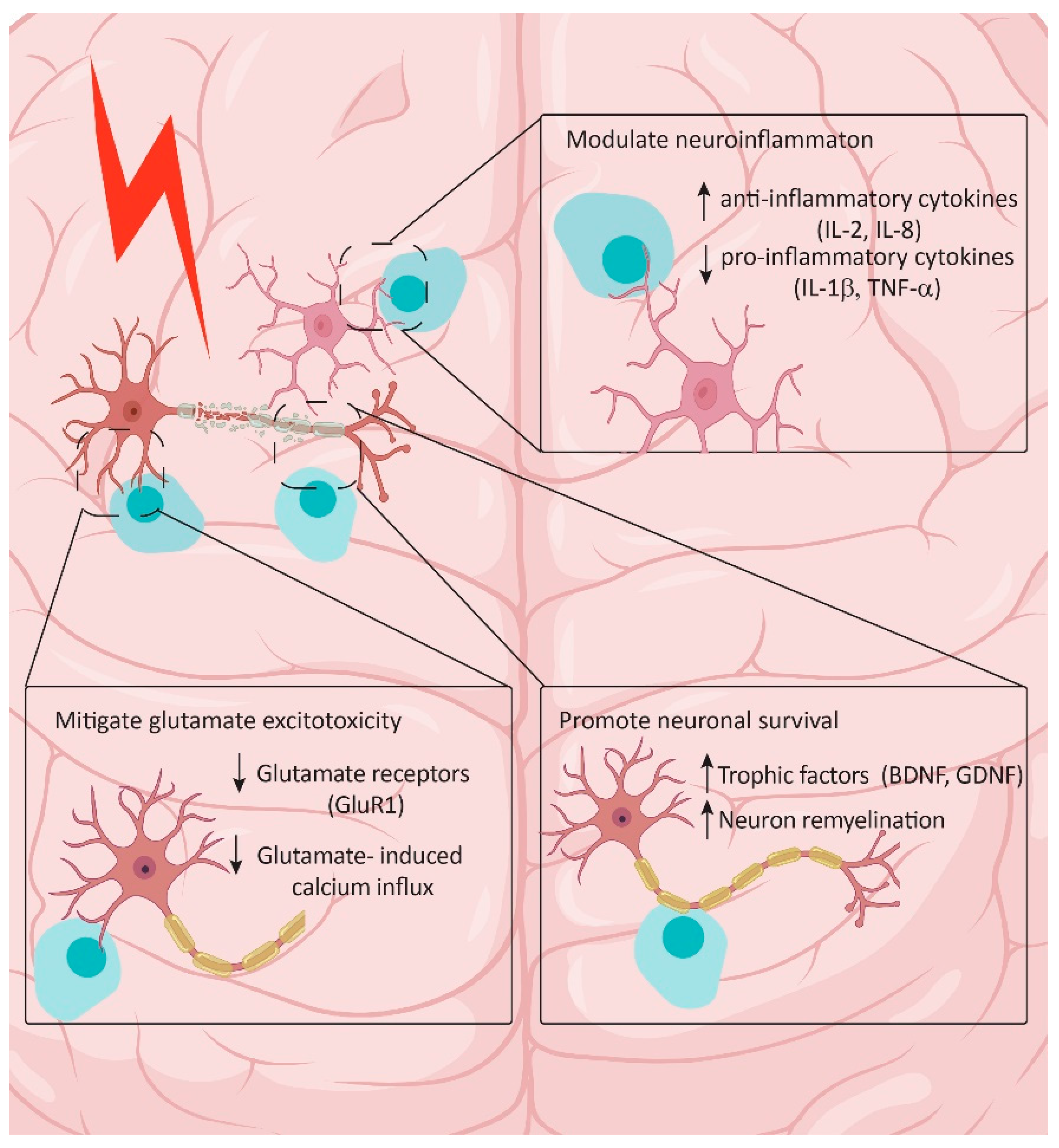
5.2. Therapeutic Potential of Exogenous Cells
6. Challenges and Strategies to Improve Clinical Translation
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation; National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention: Atlanta, GA, USA, 2015. [Google Scholar]
- Roozenbeek, B.; Maas, A.I.; Menon, D.K. Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 2013, 9, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.I.; Stocchetti, N.; Bullock, R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008, 7, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Traumatic Brain Injury in the United States: A Report to Congress. 1999. Available online: https://www.cdc.gov/traumaticbraininjury/pdf/tbi_in_the_us.pdf (accessed on 1 March 2024).
- Joseph, B.; Obaid, O.; Dultz, L.; Black, G.; Campbell, M.; Berndtson, A.E.; Costantini, T.; Kerwin, A.; Skarupa, D.; Burruss, S.; et al. Validating the Brain Injury Guidelines: Results of an American Association for the Surgery of Trauma prospective multi-institutional trial. J. Trauma. Acute Care Surg. 2022, 93, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.Y.; Hwang, S.L.; Lee, I.C.; Chen, I.T.; Lee, K.T.; Lin, C.L. Trends and outcome predictors after traumatic brain injury surgery: A nationwide population-based study in Taiwan. J. Neurosurg. 2014, 121, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, D.B.; Holcomb, J.; Abraham, E.; Atkins, J.; Sopko, G.; Working Group on Trauma, R. Working Group on Trauma Research Program summary report: National Heart Lung Blood Institute (NHLBI), National Institute of General Medical Sciences (NIGMS), and National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health (NIH), and the Department of Defense (DOD). J. Trauma Acute Care Surg. 2004, 57, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.P.; Hayes, R.L.; Newcomb, J.K. Head Trauma: Basic, Preclinical, and Clinical Directions; Wiley-Liss: Hoboken, NJ, USA, 2001. [Google Scholar]
- Maas, A.I.R.; Menon, D.K.; Manley, G.T.; Abrams, M.; Akerlund, C.; Andelic, N.; Aries, M.; Bashford, T.; Bell, M.J.; Bodien, Y.G.; et al. Traumatic brain injury: Progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022, 21, 1004–1060. [Google Scholar] [CrossRef] [PubMed]
- Theadom, A.; Starkey, N.J.; Dowell, T.; Hume, P.A.; Kahan, M.; McPherson, K.; Feigin, V.; Group, B.R. Sports-related brain injury in the general population: An epidemiological study. J. Sci. Med. Sport. 2014, 17, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.D. The clinical spectrum of sport-related traumatic brain injury. Nat. Rev. Neurol. 2013, 9, 222–230. [Google Scholar] [CrossRef]
- Stern, R.A.; Daneshvar, D.H.; Baugh, C.M.; Seichepine, D.R.; Montenigro, P.H.; Riley, D.O.; Fritts, N.G.; Stamm, J.M.; Robbins, C.A.; McHale, L.; et al. Clinical presentation of chronic traumatic encephalopathy. Neurology 2013, 81, 1122–1129. [Google Scholar] [CrossRef]
- Roberts, A.H. Brain Damage in Boxers: A Study of the Prevalence of Traumatic Encephalopathy among Ex-Professional Boxers; Pitman Medical & Scientific Pub. Co.: London, UK, 1969. [Google Scholar]
- Smith, D.H.; Johnson, V.E.; Trojanowski, J.Q.; Stewart, W. Chronic traumatic encephalopathy—Confusion and controversies. Nat. Rev. Neurol. 2019, 15, 179–183. [Google Scholar] [CrossRef]
- Davis, A.E. Mechanisms of traumatic brain injury: Biomechanical, structural and cellular considerations. Crit. Care Nurs. Q. 2000, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Haddad, S.H.; Arabi, Y.M. Critical care management of severe traumatic brain injury in adults. Scand. J. Trauma. Resusc. Emerg. Med. 2012, 20, 12. [Google Scholar] [CrossRef]
- Willis, E.F.; MacDonald, K.P.A.; Nguyen, Q.H.; Garrido, A.L.; Gillespie, E.R.; Harley, S.B.R.; Bartlett, P.F.; Schroder, W.A.; Yates, A.G.; Anthony, D.C.; et al. Repopulating Microglia Promote Brain Repair in an IL-6-Dependent Manner. Cell 2020, 180, 833–846 e816. [Google Scholar] [CrossRef] [PubMed]
- Weston, N.M.; Sun, D. The Potential of Stem Cells in Treatment of Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2018, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.S.; Perfilieva, E.; Bjork-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef]
- Wang, C.; Liu, F.; Liu, Y.Y.; Zhao, C.H.; You, Y.; Wang, L.; Zhang, J.; Wei, B.; Ma, T.; Zhang, Q.; et al. Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell Res. 2011, 21, 1534–1550. [Google Scholar] [CrossRef]
- Ernst, A.; Alkass, K.; Bernard, S.; Salehpour, M.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; Frisen, J. Neurogenesis in the striatum of the adult human brain. Cell 2014, 156, 1072–1083. [Google Scholar] [CrossRef]
- Nunes, M.C.; Roy, N.S.; Keyoung, H.M.; Goodman, R.R.; McKhann, G., 2nd; Jiang, L.; Kang, J.; Nedergaard, M.; Goldman, S.A. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat. Med. 2003, 9, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Lojewski, X.; Hermann, A.; Wegner, F.; Arauzo-Bravo, M.J.; Hallmeyer-Elgner, S.; Kirsch, M.; Schwarz, J.; Scholer, H.R.; Storch, A. Human adult white matter progenitor cells are multipotent neuroprogenitors similar to adult hippocampal progenitors. Stem Cells Transl. Med. 2014, 3, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Behnan, J.; Stangeland, B.; Langella, T.; Finocchiaro, G.; Tringali, G.; Meling, T.R.; Murrell, W. Identification and characterization of a new source of adult human neural progenitors. Cell Death Dis. 2017, 8, e2991. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Enikolopov, G.; Chen, J. Moderate traumatic brain injury promotes proliferation of quiescent neural progenitors in the adult hippocampus. Exp. Neurol. 2009, 219, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, S.; Goings, G.E.; Soderstrom, K.E.; Szele, F.G.; Kozlowski, D.A. Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain Res. 2005, 1053, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; McGinn, M.J.; Zhou, Z.; Harvey, H.B.; Bullock, M.R.; Colello, R.J. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp. Neurol. 2007, 204, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.A.; Tajiri, N.; Shinozuka, K.; Ishikawa, H.; Sanberg, P.R.; Sanchez-Ramos, J.; Song, S.; Kaneko, Y.; Borlongan, C.V. Combination therapy of human umbilical cord blood cells and granulocyte colony stimulating factor reduces histopathological and motor impairments in an experimental model of chronic traumatic brain injury. PLoS ONE 2014, 9, e90953. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.; Cheng, A.; Hoyles, A.; Jervis, E.; Morshead, C.M. Cyclosporin A has direct effects on adult neural precursor cells. J. Neurosci. 2010, 30, 2888–2896. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, P.; Mahmud, N.; Sinai, L.; Azimi, A.; Fatt, M.; Wondisford, F.E.; Miller, F.D.; Morshead, C.M. Activating Endogenous Neural Precursor Cells Using Metformin Leads to Neural Repair and Functional Recovery in a Model of Childhood Brain Injury. Stem Cell Rep. 2015, 5, 166–173. [Google Scholar] [CrossRef]
- Heinrich, C.; Bergami, M.; Gascon, S.; Lepier, A.; Vigano, F.; Dimou, L.; Sutor, B.; Berninger, B.; Gotz, M. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Rep. 2014, 3, 1000–1014. [Google Scholar] [CrossRef]
- Liu, X.; Wu, C.; Zhang, Y.; Chen, S.; Ding, J.; Chen, Z.; Wu, K.; Wu, X.; Zhou, T.; Zeng, M.; et al. Hyaluronan-based hydrogel integrating exosomes for traumatic brain injury repair by promoting angiogenesis and neurogenesis. Carbohydr. Polym. 2023, 306, 120578. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Mothe, A.; Khazaei, M.; Badhiwala, J.H.; Gilbert, E.A.; van der Kooy, D.; Morshead, C.M.; Tator, C.; Fehlings, M.G. The leading edge: Emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury. Stem Cells Transl. Med. 2020, 9, 1509–1530. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Kong, X.; Acosta, S.; Sava, V.; Borlongan, C.; Sanchez-Ramos, J. Granulocyte colony-stimulating factor promotes behavioral recovery in a mouse model of traumatic brain injury. J. Neurosci. Res. 2016, 94, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Miao, Z.; Ye, Y.; Zhao, P.; Fan, L.; Bao, Z.; Tu, Y.; Li, C.; Chao, H.; Xu, X.; et al. Curcumin alleviates neuroinflammation, enhances hippocampal neurogenesis, and improves spatial memory after traumatic brain injury. Brain Res. Bull. 2020, 162, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.R.; Smith, C.; Harris, B.T.; Costine, B.A.; Duhaime, A.C. Maturation-dependent response of neurogenesis after traumatic brain injury in children. J. Neurosurg. Pediatr. 2013, 12, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdallah, N.M.; Slomianka, L.; Vyssotski, A.L.; Lipp, H.P. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol. Aging 2010, 31, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Daniels, S.B.; Lennington, J.B.; Notti, R.Q.; Conover, J.C. The aging neurogenic subventricular zone. Aging Cell 2006, 5, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Nguyen, T.; Ihrie, R.A.; Mirzadeh, Z.; Tsai, H.H.; Wong, M.; Gupta, N.; Berger, M.S.; Huang, E.; Garcia-Verdugo, J.M.; et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 2011, 478, 382–386. [Google Scholar] [CrossRef]
- Paredes, M.F.; James, D.; Gil-Perotin, S.; Kim, H.; Cotter, J.A.; Ng, C.; Sandoval, K.; Rowitch, D.H.; Xu, D.; McQuillen, P.S.; et al. Extensive migration of young neurons into the infant human frontal lobe. Science 2016, 354, aaf7073. [Google Scholar] [CrossRef]
- Conover, J.C.; Todd, K.L. Development and aging of a brain neural stem cell niche. Exp. Gerontol. Aug. 2017, 94, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Ritzel, R.M.; Doran, S.J.; Glaser, E.P.; Meadows, V.E.; Faden, A.I.; Stoica, B.A.; Loane, D.J. Old age increases microglial senescence, exacerbates secondary neuroinflammation, and worsens neurological outcomes after acute traumatic brain injury in mice. Neurobiol. Aging 2019, 77, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; FitzGerald, K.T.; Giordano, J. On the Viability and Potential Value of Stem Cells for Repair and Treatment of Central Neurotrauma: Overview and Speculations. Front. Neurol. 2018, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Sanal, M.G. A highly efficient method for generation of therapeutic quality human pluripotent stem cells by using naive induced pluripotent stem cells nucleus for nuclear transfer. SAGE Open Med. 2014, 2, 2050312114550375. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Doi, A.; Wen, B.; Ng, K.; Zhao, R.; Cahan, P.; Kim, J.; Aryee, M.J.; Ji, H.; Ehrlich, L.I.; et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010, 467, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lehle, J.D.; McCarrey, J.R. Source cell-type epigenetic memory persists in induced pluripotent cells but is lost in subsequently derived germline cells. Front. Cell Dev. Biol. 2024, 12, 1306530. [Google Scholar] [CrossRef] [PubMed]
- Kawabori, M.; Weintraub, A.H.; Imai, H.; Zinkevych, I.; McAllister, P.; Steinberg, G.K.; Frishberg, B.M.; Yasuhara, T.; Chen, J.W.; Cramer, S.C.; et al. Cell Therapy for Chronic TBI: Interim Analysis of the Randomized Controlled STEMTRA Trial. Neurology 2021, 96, e1202–e1214. [Google Scholar] [CrossRef]
- Cox, C.S., Jr.; Baumgartner, J.E.; Harting, M.T.; Worth, L.L.; Walker, P.A.; Shah, S.K.; Ewing-Cobbs, L.; Hasan, K.M.; Day, M.C.; Lee, D.; et al. Autologous bone marrow mononuclear cell therapy for severe traumatic brain injury in children. Neurosurgery 2011, 68, 588–600. [Google Scholar] [CrossRef]
- Cox, C.S., Jr.; Hetz, R.A.; Liao, G.P.; Aertker, B.M.; Ewing-Cobbs, L.; Juranek, J.; Savitz, S.I.; Jackson, M.L.; Romanowska-Pawliczek, A.M.; Triolo, F.; et al. Treatment of Severe Adult Traumatic Brain Injury Using Bone Marrow Mononuclear Cells. Stem Cells 2017, 35, 1065–1079. [Google Scholar] [CrossRef]
- Cox, C.S., Jr.; Notrica, D.M.; Juranek, J.; Miller, J.H.; Triolo, F.; Kosmach, S.; Savitz, S.I.; Adelson, P.D.; Pedroza, C.; Olson, S.D.; et al. Autologous bone marrow mononuclear cells to treat severe traumatic brain injury in children. Brain 2024, 147, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Hellewell, S.C.; Mondello, S.; Conquest, A.; Shaw, G.; Madorsky, I.; Deng, J.V.; Little, L.; Kobeissy, F.; Bye, N.; Bellomo, R.; et al. Erythropoietin Does Not Alter Serum Profiles of Neuronal and Axonal Biomarkers After Traumatic Brain Injury: Findings From the Australian EPO-TBI Clinical Trial. Crit. Care Med. 2018, 46, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Kumar Mishra, S.; Khushu, S.; Gangenahalli, G. Neuroprotective response and efficacy of intravenous administration of mesenchymal stem cells in traumatic brain injury mice. Eur. J. Neurosci. 2021, 54, 4392–4407. [Google Scholar] [CrossRef]
- Kim, J.T.; Kim, T.Y.; Youn, D.H.; Han, S.W.; Park, C.H.; Lee, Y.; Jung, H.; Rhim, J.K.; Park, J.J.; Ahn, J.H.; et al. Human embryonic stem cell-derived cerebral organoids for treatment of mild traumatic brain injury in a mouse model. Biochem. Biophys. Res. Commun. 2022, 635, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.L.; Hu, Y.; Zhang, P.; Zhang, Z.; Li, L.H.; Gao, G.D.; Zhou, X.F.; Wang, T.H. Neural Stem Cell Transplantation Promotes Functional Recovery from Traumatic Brain Injury via Brain Derived Neurotrophic Factor-Mediated Neuroplasticity. Mol. Neurobiol. 2018, 55, 2696–2711. [Google Scholar] [CrossRef] [PubMed]
- Yousefifard, M.; Rahimi-Movaghar, V.; Nasirinezhad, F.; Baikpour, M.; Safari, S.; Saadat, S.; Moghadas Jafari, A.; Asady, H.; Razavi Tousi, S.M.; Hosseini, M. Neural stem/progenitor cell transplantation for spinal cord injury treatment; A systematic review and meta-analysis. Neuroscience 2016, 322, 377–397. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Deeb, G.; Rahal, R.; Atwi, K.; Mondello, S.; Marei, H.E.; Gali, A.; Sleiman, E. Mesenchymal Stem Cells in the Treatment of Traumatic Brain Injury. Front. Neurol. 2017, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Papazian, I.; Kyrargyri, V.; Evangelidou, M.; Voulgari-Kokota, A.; Probert, L. Mesenchymal Stem Cell Protection of Neurons against Glutamate Excitotoxicity Involves Reduction of NMDA-Triggered Calcium Responses and Surface GluR1, and Is Partly Mediated by TNF. Int. J. Mol. Sci. 2018, 19, 651. [Google Scholar] [CrossRef]
- Duan, T.Q.; Gao, Z.L.; Luo, A.X.; Chen, D.; Tong, J.B.; Huang, J.F. Adipose mesenchymal stem cell-derived extracellular vesicles reduce glutamate-induced excitotoxicity in the retina. Neural Regen. Res. 2023, 18, 2315–2320. [Google Scholar] [CrossRef]
- Amirbekyan, M.; Adhikarla, V.; Cheng, J.P.; Moschonas, E.H.; Bondi, C.O.; Rockne, R.C.; Kline, A.E.; Gutova, M. Neuroprotective potential of intranasally delivered L-myc immortalized human neural stem cells in female rats after a controlled cortical impact injury. Sci. Rep. 2023, 13, 17874. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.A.; Abbas, A.Y.; Imam, M.U.; Saidu, Y.; Bilbis, L.S. Efficacy of stem cell secretome in the treatment of traumatic brain injury: A systematic review and meta-analysis of preclinical studies. Mol. Neurobiol. 2022, 59, 2894–2909. [Google Scholar] [CrossRef] [PubMed]
- Nazareth, L.; Shelper, T.B.; Chacko, A.; Basu, S.; Delbaz, A.; Lee, J.Y.P.; Chen, M.; St John, J.A.; Ekberg, J.A.K. Key differences between olfactory ensheathing cells and Schwann cells regarding phagocytosis of necrotic cells: Implications for transplantation therapies. Sci. Rep. 2020, 10, 18936. [Google Scholar] [CrossRef] [PubMed]
- Roet, K.C.; Verhaagen, J. Understanding the neural repair-promoting properties of olfactory ensheathing cells. Exp. Neurol. 2014, 261, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Xia, Q.J.; Ba, Y.C.; Wang, T.Y.; Li, N.N.; Zou, Y.; Shang, F.F.; Zhou, X.F.; Wang, T.H.; Fu, X.M.; et al. Transplantation of olfactory ensheathing cells promotes the recovery of neurological functions in rats with traumatic brain injury associated with downregulation of Bad. Cytotherapy 2014, 16, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yin, Z.; Yue, S.; An, Y.; Wang, X.; Zhou, S.; Meng, L.; Jin, B. Effect of valproic acid combined with transplantation of olfactory ensheathing cells modified by neurotrophic 3 gene on nerve protection and repair after traumatic brain injury. Neuropeptides 2024, 103, 102389. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Wang, X.; Wang, X.; Wang, L.; Wang, X.; Wu, S.; Wan, Z. Autologous bone marrow mesenchymal stem cell therapy in the subacute stage of traumatic brain injury by lumbar puncture. Exp. Clin. Transplant. 2013, 11, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, Y.; Chen, L.; Liang, W. Safety of neural stem cell transplantation in patients with severe traumatic brain injury. Exp. Ther. Med. 2017, 13, 3613–3618. [Google Scholar] [CrossRef] [PubMed]
- Kondziolka, D.; Wechsler, L.; Goldstein, S.; Meltzer, C.; Thulborn, K.R.; Gebel, J.; Jannetta, P.; DeCesare, S.; Elder, E.M.; McGrogan, M.; et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology 2000, 55, 565–569. [Google Scholar] [CrossRef]
- Duma, C.; Kopyov, O.; Kopyov, A.; Berman, M.; Lander, E.; Elam, M.; Arata, M.; Weiland, D.; Cannell, R.; Caraway, C.; et al. Human intracerebroventricular (ICV) injection of autologous, non-engineered, adipose-derived stromal vascular fraction (ADSVF) for neurodegenerative disorders: Results of a 3-year phase 1 study of 113 injections in 31 patients. Mol. Biol. Rep. 2019, 46, 5257–5272. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, H.; Dai, G.; Wang, X.; Hua, R.; Liu, X.; Wang, P.; Chen, G.; Yue, W.; An, Y. Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res. 2013, 1532, 76–84. [Google Scholar] [CrossRef] [PubMed]
- McCrea, M.A.; Cramer, S.C.; Okonkwo, D.O.; Mattke, S.; Paadre, S.; Bates, D.; Nejadnik, B.; Giacino, J.T. Determining minimally clinically important differences for outcome measures in patients with chronic motor deficits secondary to traumatic brain injury. Expert. Rev. Neurother. 2021, 21, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Ariza de Schellenberger, A.; Kratz, H.; Farr, T.D.; Lowa, N.; Hauptmann, R.; Wagner, S.; Taupitz, M.; Schnorr, J.; Schellenberger, E.A. Labeling of mesenchymal stem cells for MRI with single-cell sensitivity. Int. J. Nanomed. 2016, 11, 1517–1535. [Google Scholar] [CrossRef] [PubMed]
- Erkers, T.; Kaipe, H.; Nava, S.; Mollden, P.; Gustafsson, B.; Axelsson, R.; Ringden, O. Treatment of severe chronic graft-versus-host disease with decidual stromal cells and tracing with (111)indium radiolabeling. Stem Cells Dev. 2015, 24, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.; Sousa-Victor, P.; Jasper, H. Rejuvenating Strategies for Stem Cell-Based Therapies in Aging. Cell Stem Cell 2017, 20, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Zarriello, S.; Coats, A.; Nelson, C.; Kingsbury, C.; Gorsky, A.; Rajani, M.; Neal, E.G.; Borlongan, C.V. Stem cell therapy for neurological disorders: A focus on aging. Neurobiol. Dis. 2019, 126, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Gransee, H.M.; Zhan, W.Z.; Sieck, G.C.; Mantilla, C.B. Localized delivery of brain-derived neurotrophic factor-expressing mesenchymal stem cells enhances functional recovery following cervical spinal cord injury. J. Neurotrauma 2015, 32, 185–193. [Google Scholar] [CrossRef]
- Liu, W.G.; Wang, Z.Y.; Huang, Z.S. Bone marrow-derived mesenchymal stem cells expressing the bFGF transgene promote axon regeneration and functional recovery after spinal cord injury in rats. Neurol. Res. 2011, 33, 686–693. [Google Scholar] [CrossRef]

| # | NCT# | Phase | Status | Endogenous vs. Exogenous | Age | Tx Intervention | Primary Outcome Measure | Related References/Notes: |
|---|---|---|---|---|---|---|---|---|
| 1 | NCT02525432 | 2b | Active, not recruiting | Exogenous | 18–55 years | Intravenous autologous bone marrow mononuclear cells | Structural properties of gray/white matter on MRI | |
| 2 | NCT02028104 | 1 | Withdrawn | Exogenous | 6 Months to 65 Years | Intrathecal autologous bone marrow mononuclear cells | Change in clinical symptoms of traumatic brain injury | |
| 3 | NCT02416492 | 2 | Completed | Exogenous | 18–75 Years | Intracranial allogeneic modified bone marrow–derived mesenchymal stromal/stem cells (SB623) | Change in Fugl-Meyer Motor Scale (FMMS) | Interim analysis demonstrated safety and tolerability with significant improvement from baseline motor status at 6 months compared to controls [53] |
| 4 | NCT00254722 | 1 | Completed | Exogenous | 5 Years to 14 Years | Intravenous autologous bone marrow precursor cell | Safety | The treatment of severe TBI in children with autologous bone marrow-derived cells is safe and feasible [54] |
| 5 | NCT05951777 | 2 | Enrolling by invitation | Exogenous | 18–55 Years | Intravenous autologous adipose-derived mesenchymal stem cells | Safety | |
| 6 | NCT01575470 | 1–2 | Completed | Exogenous | 18–55 Years | Intravenous autologous bone marrow mononuclear cells | Safety | The treatment of severe, adult traumatic brain injury using an intravenously delivered autologous bone marrow mononuclear cell infusion is safe and logistically feasible [55]. |
| 7 | NCT05018832 | 1 | Not yet recruiting | Exogenous | Age not specified, Child, Adult, Older Adult | Intravenous allogeneic adult umbilical cord derived mesenchymal stem cells | Safety | |
| 8 | NCT01851083 | 2 | Completed | Exogenous | 5–17 Years | Intravenous autologous bone marrow mononuclear cells | Brain white matter and gray matter structural preservation on DTMRI | An autologous bone marrow transplant was safe and showed the potential for a decreased stay in the ICU and white matter structural preservation [56]. |
| 9 | NCT02959294 | 1 | Withdrawn | Exogenous | 16–70 Years | Parenteral autologous adipose-derived stem/stromal cells | Safety, functional outcome | |
| 10 | NCT04063215 | 1–2 | Active, not recruiting | Exogenous | 18–55 Years | Autologous adipose-derived mesenchymal stem cells | Safety | |
| 11 | NCT04744051 | 1 | Active, not recruiting | Exogenous | 18–65 Years | Intravenous adipose-derived stem cells | Health Status using a 36-item Short Form Health Survey (SF-36) | |
| 12 | NCT02795052 | 1 | Recruiting | Exogenous | 18 Years and older | Intravenous or intranasall autologous bone marrow-derived stem cells | Change in neurologic function, 1, 3, 6, and 12 months post treatment | |
| 13 | NCT05293873 | 1 | Unknown status | Exogenous | 20–50 Years | Transplant of autologous bone marrow-derived mononuclear cells | Adverse events—Functional independence—Extended Glasgow Outcome Scale up to 12 months post treatment | |
| 14 | NCT02742857 | 1 | Completed | Exogenous | 15–65 Years | Intrathecal mesenchymal stem cells | Reversal of brain death via clinical exam or electroencephalography | Study conducted in India, not reviewed by the Indian Council for Medical Research. Listed on the National Institute of Health’s website but not subject to American regulator governance. |
| 15 | NCT02148367 | 2 | Withdrawn | Endogenous | 18–70 Years | Erythropoietin | Number of circulating endothelial progenitor cells | Not directly aimed at enhancing neurogenesis but cited as a possible effect of Erythropoietin. Withdrawn as the primary outcome was not met [57]. |
| 16 | NCT02226848 | 2 | Withdrawn | Endogenous | 18–70 Years | Erythropoietin | Number of circulating endothelial progenitor cells | The same as above. |
| 17 | NCT02083445 | NA | Completed | Endogenous | 20–60 Years | Exercise, muscle electrostimulation, intermittent hypobaric hypoxia | Change in physical and psychological tests | The secondary outcome aims at measuring circulating progenitor cells. |
| 18 | NCT00810615 | 1-2 | Completed | Endogenous | 19–60 Years | Hyperbaric oxygen therapy | Neuropsychological tests | The secondary outcome included the measurement of CD34+ circulating stem cells. |
| 19 | NCT01239706 | 2 | Unknown status | Endogenous | 18–65 Years | Ntx-265 (Human Chorionic Gonadotropin (hCG) and erythropoietin | Safety | hCG and erythropoietin were shown to potentiate neurogenesis in preclinical models. |
| 20 | NCT03900182 | NA | Terminated | Endogenous | 18–65 Years | Hyperbaric oxygen therapy | Neuropsychological tests | The investigators mention the potential for increasing circulating progenitor cells |
| 21 | NCT01762475 | 2 | Completed | Endogenous | 18–55 Years | Sildenafil | Cerebrovascular reactivity: Blood oxygen level dependent response to hypercapnia | Measures circulating endothelial progenitor cells as the secondary outcome. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habashy, K.J.; Omais, S.; Haupt, B.; Sonabend, A.M.; Ahuja, C.S. Cell-Based Therapies for the Treatment of Traumatic Brain Injury: Promises and Trajectories. Biologics 2024, 4, 161-176. https://doi.org/10.3390/biologics4020011
Habashy KJ, Omais S, Haupt B, Sonabend AM, Ahuja CS. Cell-Based Therapies for the Treatment of Traumatic Brain Injury: Promises and Trajectories. Biologics. 2024; 4(2):161-176. https://doi.org/10.3390/biologics4020011
Chicago/Turabian StyleHabashy, Karl J., Saad Omais, Benedikt Haupt, Adam M. Sonabend, and Christopher S. Ahuja. 2024. "Cell-Based Therapies for the Treatment of Traumatic Brain Injury: Promises and Trajectories" Biologics 4, no. 2: 161-176. https://doi.org/10.3390/biologics4020011
APA StyleHabashy, K. J., Omais, S., Haupt, B., Sonabend, A. M., & Ahuja, C. S. (2024). Cell-Based Therapies for the Treatment of Traumatic Brain Injury: Promises and Trajectories. Biologics, 4(2), 161-176. https://doi.org/10.3390/biologics4020011





