Abstract
The past few decades have witnessed the remarkable progress of cancer immunotherapy. Neoantigens, also known as tumor-specific antigens, are novel antigens originating from tumor-specific alterations such as genomic mutations, dysregulated RNA splicing, and post-translational modifications. Neoantigens, recognized as non-self entities, trigger immune responses that evade central and peripheral tolerance mechanisms. With the notable strides in cancer genomics facilitated by next-generation sequencing technologies, neoantigens have emerged as a promising avenue for tumor-specific immunotherapy grounded in genomic profiling-based precision medicine. Furthermore, a growing number of preclinical and clinical investigations are harnessing the potential synergies between neoantigens and other immunotherapies such as adoptive cell therapy and immune checkpoint inhibitors. In this review, we will provide a comprehensive perspective encompassing the trajectory of neoantigens, neoantigen design strategies, and the diverse array of clinical applications inherent in immunotherapy strategies centered around neoantigens. Moreover, we delve into the inherent prospects and challenges that accompany the clinical adoption of neoantigen-based immunotherapies while also putting forth potential solutions to address these challenges.
1. Introduction
The field of cancer research has witnessed remarkable advancements over the past few decades, unveiling intricate dynamics between malignant cells and the immune system. Amid the myriad of novel avenues that have surfaced, the exploration of neoantigens has emerged as a captivating frontier within cancer immunotherapy. Neoantigens, originating from somatic mutations within the tumor genome, possess an unparalleled capacity to trigger precise immune responses, potentially reshaping the landscape of personalized cancer treatment.
At the core of virtually all immunotherapeutic strategies lies the induction and activation of tumor-specific T cells. Neoantigens, aptly named, represent the distinctive epitopes that emerge from modified gene products and novel proteins resulting from mutations within the genome’s coding regions. These neoantigens are presented for recognition by T cells after being processed [1]. Compared to various tumor-associated antigens (TAAs), neoantigens provide a distinct advantage. While TAAs exhibit elevated levels on tumor cells but are also expressed at lower levels on healthy cells, neoantigens are expressed in the tumor tissues and absent in the normal tissues. Given that TAAs remain non-mutated self-antigens, their recognition can be hampered by central T cell tolerance mechanisms, potentially accounting for the subdued T cell responses. In contrast, T cells primed to target neoantigens can circumvent the suppressive impacts of negative selection within the thymus, due to the pronounced antigenicity conferred by somatic mutations within tumors [2,3,4]. This unique attribute of neoantigens mitigates the risk of “off-target” harm to normal tissues and circumvents the constraints of central or peripheral tolerance, offering an individualized vaccine capable of stimulating the activation of tumor-specific T cells [5].
In the context of neoantigen-based immunotherapy, synthetically engineered neopeptides are administered to patients with the aim of triggering an immune response, particularly engaging CD8+ and CD4+ T cells to recognize the neoantigens and target and eliminate tumor cells [6]. The effectiveness of neoantigens hinges upon various factors, with tumor mutation burden (TMB) and the presentation and recognition of neoantigens being of paramount importance. A higher TMB is anticipated to yield a greater pool of tumor-specific antigens, enhancing the likelihood of inducing tumor antigen-specific T cells. To validate this hypothesis, a multitude of studies have been designed, stratifying immunotherapy-treated patients based on TMB levels. The outcomes consistently reveal that patients with higher TMB experience improved results, including enhanced progression-free survival and overall survival [7,8]. Within the intricate landscape of tumor neoantigens, genetic anomalies in tumor cells—ranging from somatic point mutations and insertions to deletions and chromosomal translocations—undergo transcription and translation, ultimately giving rise to mutated peptides. These peptides subsequently undergo hydrolysis and are presented by major histocompatibility complex (MHC) molecules, facilitating their recognition by T cells [9]. Factors such as peptide splicing, the antigen processing and presentation machinery, and peptide affinity can influence MHC identification. Moreover, T cell recognition can be influenced by the extent of tumor infiltrating lymphocytes, culminating in the determination of the neoantigen’s potential to elicit a robust immune response [10].
To date, a wide array of neoantigen-based vaccines have undergone evaluation in patients with various types of tumors. These vaccines encompass peptide, nucleic acid, and dendritic cell (DC) vaccine modalities. Peptide and nucleic acid vaccines primarily derive from predicted neopeptides resulting from somatic mutations, such as single nucleotide variations, frameshift insertions or deletions, and gene fusions. In contrast, DC vaccines are produced by loading dendritic cells with neoantigens. Several techniques have been employed for this purpose, including pulsing with synthetic peptides, transfection using mRNA, and pulsing with autologous whole tumor lysate [11].
In this review, we offer an encompassing overview of the evolution of neoantigens, the pipelines for predicting these novel antigens, and the diverse clinical applications of immunotherapy strategies centered around neoantigens. Additionally, we delve into the prospects and hurdles inherent in the clinical adoption of immunotherapies rooted in neoantigens while also putting forth potential solutions to address these challenges.
2. History of Neoantigen and Neoepitope
The history of neoantigen and neoepitope discovery spans several decades and reflects the evolving landscape of cancer research, immunology, and genomics. The journey to uncover these novel targets for cancer immunotherapy is characterized by pivotal milestones that have gradually transformed our understanding of tumor-specific antigens and their potential therapeutic applications.
2.1. Early Exploration (1970s–1990s)
The early years of cancer immunology focused primarily on TAAs that were shared between cancer cells and normal tissues. Efforts to develop immunotherapies centered on these antigens, often yielding modest success due to the risk of off-target effects. For instance, in Ramarathinam et al., it was reported that multiple lineages of tumors are not cross protected even though they share the common unmutated tumor antigen P1A that are recognized by cytolytic T lymphocyte (CTL) [12]. In the other study, the transgenic mice were produced to expresses the T cell receptors (TCR) from CTL that recognized P1A; however, the T cells were not able to reject tumors that expresses P1A antigen [13]. In an attempt to elucidate the suboptimal T cell therapeutic efficacy, a hypothesis was formulated, suggesting that the host may have developed mechanisms to either eliminate or functionally restrain the T cells capable of targeting self-tissues, owing to their expression of P1A.
However, during this period, researchers began to recognize the importance of antigens derived from unique somatic mutations present in cancer cells. In Monach et al., strong evidence was provided that a unique tumor antigen derived from amino acid substitution in a cellular protein led to remarkably higher T cell stimulation in comparison to the wild-type peptide [14].
2.2. Genomic Revolution (2000s–Early 2010s)
From the end of 1990s to 2000s, studies were blooming and provided more insights of neoantigens as the therapeutic targets for cancer treatment. In Lennerz et al., T cell responses against the five neoantigens generated by somatic point mutations in the patient’s melanoma was observed and predominated in comparison to that against TAAs [15]. In another study, Rosernberg et al. reported a melanoma patient who underwent complete regression after the adoptive transfer of ex vivo expanded, tumor-infiltrating lymphocytes. Screening of the autologous tumor cell cDNA library revealed the immune response of tumor-infiltrating lymphocytes (TILs) to two novel mutated genes, respectively, growth arrest-specific gene 7 and glyceraldehyde-3-phosphate dehydrogenase gene transcripts. The T cells targeting these two novel mutated genes were found in the expanded TILs and persisted in the tumor and blood in the patient [16].
While emerging studies supported the profound potential of T cells recognizing neoantigens, the advent of high-throughput DNA sequencing technologies revolutionized cancer research. With the ability to sequence entire-cancer genomes, researchers gained insights into the genomic alterations driving tumorigenesis. One example was in 2008, when Allison et al. designed an in silico-based approach and high throughput post hoc analysis to examine whether the somatic mutations in human breast and colon cancers had the potential to generate novel epitopes that might serve as targets for immune responses [17]. Around this time, genomic revolution paved the way for the identification and prediction of neoantigens, which are unique to individual tumors and arise from somatic mutations in the coding regions of the genome.
2.3. Neoantigen Prediction Algorithms (Mid 2010s)
In this period, genomic and bioinformatics continued to evolve and provided clear evidence that they could be used in identification of neoantigens for potential T cell stimulation. When genome sequencing data became available, computational methods could be used to predict neoantigens. These algorithms analyze tumor DNA sequences to identify mutations that could lead to altered protein sequences, and subsequently predict which of these alterations are likely to give rise to neoepitopes—the small, recognizable portions of neoantigens that T cells can target. The pipeline of next-generation sequencing, in silico neoepitope prediction and development of immunological assays emerged and was proved as an effective approach.
In Rosernberg et al., exome sequencing was applied for identification of the mutated proteins expressed in the melanoma of patients who received adoptively transferred autologous TILs. The mutated neoepitopes were verified using an MHC-binding algorithm and evaluated for TIL recognition [18]. In Sahin et al., next-generation sequencing was applied to identify nonsynonymous somatic point mutations from B16F10 murine melanoma cells, with the immunogenicity and specificity of the selected mutations being validated by immunizing mice, with peptides containing the mutated epitopes. In comparison to the wild-type sequences, these mutated peptides elicited stronger immunogenic responses, which clearly indicated the efficacy of neopeptide immunization in therapeutic settings [19]. With this methodology, Schreiber et al. were able to predict and validate neoantigens from highly immunogenic methylcholanthrene-induced sarcomas derived from immunodeficient Rag2(−/−) mice [20].
2.4. Validation and Clinical Application (Late 2010s–Present)
The late 2010s witnessed the translation of neoantigen discovery into clinical applications. Researchers began identifying neoantigens in patients with different cancer types and demonstrated that they could trigger immune responses. Clinical trials were designed to test personalized neoantigen-based vaccines and adoptive T cell therapies. Notably, these trials demonstrated the feasibility of inducing potent and specific anti-tumor immune responses in a subset of patients. One example was the T cell transfer therapy targeting mutant KRAS G12D conducted by Rosernberg’s group, which led to tumor regression in patients with metastatic colorectal cancers [21]. In another study conducted by Wu’s group, vaccines targeting predicted personal tumor neoantigens were administered in melanoma patients and led to no recurrences in four out of six vaccinated patients. The other two patients with recurrent diseases were treated with anti-programmed cell death-1 (PD-1) and had complete regression [22]. Moreover, in the clinical trial NCT01174121, the TILs recognizing a mutation in the erbb2 interacting protein (ERBB2IP) were used for adoptive cell transfer, and the treated patient achieved a remission in target lesions with prolonged stabilization [23].
Neoantigens have become a focal point in the development of cancer immunotherapies. As immunotherapy strategies such as immune checkpoint inhibitors and Chimeric Antigen Receptor T (CAR-T) cell therapies gained prominence, the potential synergies between these approaches and neoantigen-targeted therapies became evident [24,25,26]. Combining these strategies has the potential to enhance the effectiveness of cancer treatment by leveraging the immune system’s natural ability to recognize and destroy cancer cells.
3. Neoantigen Design and Development
Predicting and identifying neoantigens involves a multidisciplinary approach encompassing genomics, bioinformatics, and immunology.
3.1. Tumor Biopsy and Next-Generation Sequencing
Upon diagnosis of the patient, the process initiates with the selection of tumor tissue samples. These samples are meticulously curated to represent areas abundantly populated by tumor cells. Histological evaluation is of paramount importance in distinguishing cancerous tissue from healthy tissue and offers valuable insights into the tumor’s histopathological attributes, encompassing its type, grade, and stage. Once histological analysis definitively confirms the presence of tumor cells, the selection of tissue becomes pivotal. Careful consideration is given to identifying tumor-rich regions for subsequent genomic and proteomic analyses. These regions are expected to harbor the specific genetic mutations and neoantigens pertinent to the patient’s cancer, rendering them the focal point for personalized immunotherapies.
The tumor is biopsied, and both the cancerous and normal tissues are sequenced. Whole exome sequencing (WES), which is a powerful genomic technique, focuses on sequencing the coding regions, or exons, of genes in an individual’s genome. While the majority of the human genome consists of non-coding regions, exons are where most disease-associated mutations are found. By sequencing only the exonic regions, WES enables researchers to identify single nucleotide variations, small insertions, and deletions [27,28]. Unlike WES, which focuses on the genome’s DNA, transcriptome analyses, or RNA sequencing (RNA-seq) examines the transcripts that are produced from genes and serve as templates for protein synthesis. Specifically, RNA-Seq can detect alternative splicing events, where different exons are included or excluded from the final mRNA transcript and result in the production of diverse protein isoforms. Additionally, RNA-Seq captures gene fusions arising from chromosomal rearrangements, which lead to the expression of fusion transcripts that are translated into fusion proteins with unique antigenic properties. RNA-seq involves extracting RNA molecules from cells or tissues and converting them into complementary DNA (cDNA) through reverse transcription. These cDNA fragments are then sequenced to quantify the abundance of various RNA molecules [29]. The incorporation of RNA-Seq into mutanome analyses enables prioritization of highly expressed mutations over nonexpressed variants, resulting in a more refined selection of potential vaccine candidates.
3.2. Somatic Mutation Detection
Mutation calling is a computational process that follows the sequencing of DNA (WES) or cDNA (RNA-seq) to identify genetic variations or mutations within the genome. The goal is to distinguish between the naturally occurring variations and mutations that might contribute to disease. The process involves several steps, including aligning sequenced reads to a reference genome, identifying areas with discrepancies (variations), and filtering out background noise and false positives. Currently, specialized algorithms like Varscan, SomaticSniper, Strelka, and MuTect2 GATK are employed to identify somatic mutations by comparing tumor and normal sequences. They identify somatic mutations that are unique to the tumor and not present in the individual’s normal tissue. Filters are applied to remove common germline variations and retain tumor-specific alterations. By comparing the tumor genome (WES) or transcriptome (RNA-seq) with the corresponding normal tissue, researchers can pinpoint mutations that have arisen during tumorigenesis [30].
Following mutation calling, the subsequent annotation steps are crucial for neoantigen prediction. Annotation involves assigning functional and contextual information to the identified variants using tools like ANNOVAR, Variant Effect Predictor (VEP), or SnpEff. These tools help determine whether a variant falls within a protein-coding region, its effect on the amino acid sequence, and its potential impact on protein structure and function. Subsequently, the annotated variants are further analyzed to identify mutations that generate altered peptide sequences (neoepitopes) capable of binding to MHC molecules and eliciting an immune response. The integration of mutation calling and comprehensive variant annotation lay the foundation for accurately predicting potential neoantigens that can be harnessed for personalized cancer immunotherapy strategies [31,32].
3.3. Neoantigen Prediction
Epitope prediction algorithms play a pivotal role in neoantigen prediction by evaluating the likelihood that a given peptide sequence, derived from a mutated protein, will bind to MHC molecules with sufficient affinity to be presented to T cells. Currently, multiple tools such as SYFPEITHI, IEDB, and NetMHCpan are extremely useful in offering unique features in epitope-binding prediction. For instance, SYFPEITHI calculates the binding affinity of peptides to MHC class I molecules. It employs a scoring system based on experimental binding data to predict the likelihood of peptide-MHC interaction. This approach enables the identification of potential neoepitopes that have a high probability of being presented by MHC molecules and recognized by T cells. SYFPEITHI’s approach, while valuable, is often constrained by the availability of experimental binding data for a diverse range of MHC alleles [33]. While SYFPEITHI, Rankpep, and BIMAS served as pioneering prediction tools, the field has seen the emergence of more refined alternatives. Among these, NetMHC stands out as one of the most widely utilized and rigorously validated algorithms available today. NetMHC employs artificial neural networks to predict peptide binding across various MHCI variants, yielding the predicted IC50 as an output. The accuracy of neural network-based methods relies on the training set’s quality and size, thus performing better for more prevalent alleles. Notably, a refined version known as NetMHCpan expands the training dataset to encompass data from diverse species, enhancing the accuracy of predictions for less common MHC alleles [34].
Currently, the most useful epitope prediction algorithms are those focusing on peptide binding to MHC class I molecules. The MHCI antigen presentation pathway plays a central role in presenting peptides derived from endogenous cellular proteins to CD8+ T cells. Intracellular proteins undergo proteasomal processing, yielding 8–11 amino acid peptides that are subsequently transported into the endoplasmic reticulum (ER) by the transporter associated with antigen processing. There, they associate with newly synthesized class I molecules, forming stable peptide–MHCI complexes that are transported to the cell surface [35,36]. On the other hand, MHC class II antigen presentation involves the presentation of peptides derived from exogenous antigens, often proteins internalized through endocytosis or phagocytosis. In the endosomal compartments of antigen-presenting cells, these antigens are processed into peptide fragments, and a subset of these peptides binds to MHC II molecules within the groove created by the α and β chains. The resulting peptide-MHC II complex is then transported to the cell surface, where it is presented to CD4+ T helper cells. While prediction algorithms for MHCI neoantigens have flourished, MHC II neoantigens have posed challenges due to their diverse lengths (ranging from 13 to 25 amino acids) and increased binding complexity. As a result, there is a relative scarcity of binding-affinity training data and fewer algorithms available for predicting MHC II neoantigens [3].
3.4. Neoantigen Prioritization
Subsequent to neoantigen prediction using NetMHCpan, a pivotal step involves the comprehensive prioritization of neoepitopes, ensuring the selection of those with the highest potential to trigger a robust immune response. While NetMHCpan aids in identifying peptide sequences likely to bind to MHC molecules, further criteria are considered to assess their immunogenicity. Among the steps in neoepitope prioritization, the predicted binding affinity holds significance, as neoepitopes displaying strong MHC binding are more likely to be presented to immune cells. Tools like MHCflurry and MHCconsortium can also refine binding affinity predictions, aiding in the identification of top candidates. Additionally, assessing the conservation of the mutated amino acid across species using tools such as SIFT and PolyPhen enhances the understanding of its potential functional impact. Estimating epitope abundance is presently achieved through an indirect assessment involving the quantification of RNA expression levels. Mutations can be identified through tumor-to-normal DNA comparisons undergo bioinformatic scrutiny to gauge their immunogenic potential. The subsequent estimation of candidate immune stimulatory peptide levels is facilitated by RNA-Seq analysis. Prioritization based on gene expression levels, using databases like The Cancer Genome Atlas and Genotype-Tissue Expression, adds another layer of insight into neoepitope selection. Furthermore, considering the antigen processing machinery’s efficiency, tools like NetChop and NetCTLpan evaluate proteasomal cleavage and T cell processing, respectively. Neoepitopes arising from frameshift mutations and non-synonymous alterations are often prioritized due to their potential to generate immunogenic peptides. Tailoring the prioritization based on tumor heterogeneity and patient-specific HLA type refines the strategy [37].
3.5. Neoantigen Validation
Validation of immunogenic neoantigens can be performed in many ways, among which the most common methodologies included are mass spectrometry, tetramer/multimer staining, and ELISpot, ELISA, or intracellular cytokine staining. By eluting bound peptides and identifying using tumor-specific variant libraries, mass spectrometry is able to profile the neoantigens presented on the MHC molecules. Its high sensitivity enables the detection of even minute quantities of antigens, making it well-suited for the task. Moreover, it offers an unbiased approach, capable of identifying a wide range of neoantigens without prior knowledge of their sequences. Mass spectrometry can also provide quantitative data about the abundance of neoantigens, which is valuable for assessing their significance. However, it comes with certain complexities. Specialized equipment and expertise are prerequisites, making it less accessible for some laboratories. Additionally, sample preparation can be time-consuming and technically challenging. It is worth noting that mass spectrometry primarily detects neoantigens presented on MHC class I molecules, limiting its applicability to this subset of antigens [38].
Complementary to mass spectrometry, tetramer or multimer staining facilitates the visualization and quantification of neoantigen-specific T cells. This technique employs fluorescently labeled MHC–peptide complexes to detect and enumerate neoantigen-specific T cell populations, offering insights into their abundance and specificity and confirming the presence of T cells capable of recognizing the neoantigens. However, there are limitations to consider. Tetramer and multimer staining require prior knowledge of the neoantigens of interest and the availability of corresponding tetramers/multimers. Custom production of these reagents can be expensive and time-consuming. Moreover, these techniques may have limited sensitivity in detecting rare T cell populations, posing challenges in studies where low-frequency neoantigen-specific T cells are of interest [39].
Functional validation techniques like ELISpot, ELISA, and intracellular cytokine staining assess the ability of neoantigens to stimulate T cell responses. With synthesized neoepitopes, ELISpot and ELISA quantify interferon-gamma secretion or cytokine production in response to neoantigens, providing quantitative data on T cell activation. Meanwhile, intracellular cytokine staining detects cytokine production within T cells, corroborating their activation status. However, they do have limitations to consider. ELISpot and ELISA may lack the specificity of tetramer staining and mass spectrometry, potentially leading to false-positive results. Intracellular cytokine staining, while capable of detecting functional responses, may not directly identify neoantigen-specific T cells. Additionally, the sensitivity of these techniques may be limited, particularly in detecting low-frequency neoantigen-specific T cell populations [40].
In conclusion, the choice of neoantigen validation technique should align with the specific goals of the study, available resources, and the nature of the neoantigens under investigation. Each technique has its strengths and limitations, and combining multiple methods can provide a comprehensive assessment of neoantigen-specific immune responses, enhancing the overall validation process.
4. Studies of Neoantigens in Immunotherapy
Immunotherapy has been proven to be a groundbreaking approach in cancer treatment via harnessing the body’s own immune system to combat tumors. Aiming at enhancing the immune system’s ability to recognize and target cancer cells, examples of current immunotherapy include checkpoint inhibitors, adoptive T cell therapy (including CAR-T cell therapy), and oncolytic virotherapy [26,41]. Neoantigens, known for their high immunogenicity, possess significant potential to elicit a robust immune response. When these neoantigens are displayed on the surfaces of cancer cells through MHC molecules, they can be recognized by both CD8+ and CD4+ T cells, essential components of the adaptive immune system. This recognition sets off a series of immune responses, including T cell activation and the mobilization of other immune cells to the tumor microenvironment, aimed at identifying and eliminating the cancer cells presenting these neoantigens.
Neoantigens have emerged as promising targets for cancer immunotherapy, holding the potential to revolutionize the landscape of personalized treatment strategies. Numerous clinical trials and studies are underway to explore the therapeutic efficacy of neoantigen-based interventions either as standalone treatments or in combination with other immunotherapies (Table 1).

Table 1.
Clinical trials of neoantigen in immunotherapy.
In clinical trials focusing on neoantigen-based immunotherapy, personalized vaccines are designed to elicit robust T cell responses against patient-specific neoepitopes. These vaccines are formulated by selecting neoantigens predicted to bind strongly to the patient’s MHC molecules and thus maximize T cell activation. One pioneering example is the trial (NCT01970358) initiated by Ott et al., wherein the predicted long peptides 3, targeting up to 20 neoantigens admixed with the Toll-like receptor 3 and melanoma differentiation-associated protein 5 agonist poly-ICLC4 (Hiltonol), were administered to patients with previously untreated high-risk melanoma (stage IIIB/C and IVM1a/b) in a phase I study. Four out of the six patients under vaccination had no recurrence after 25 months, whereas the other two with recurrent disease underwent tumor regression after the addition of anti-PD-1 therapy [22]. The study demonstrated the feasibility and safety of the approach and highlighted the potential of neoantigen vaccines to induce antitumor immune responses. Moreover, the ongoing WINTHER trial (NCT01856296) explores the integration of whole exome sequencing and transcriptome analysis to guide the selection of neoantigens for personalized cancer vaccines. These endeavors underscore the evolving precision of neoantigen-targeted strategies [43].
Combination therapies that harness the potential of neoantigens alongside other immunotherapies have also garnered considerable attention, with the goal of achieving synergistic efficacy. Neoantigen-targeted therapies can be integrated with immune checkpoint inhibitors, such as anti-PD-1 or anti-CTLA-4 antibodies, to enhance the efficacy of immune responses. For instance, the NCT02950766 trial assesses the combination of a personalized neoantigen vaccine with immune checkpoint inhibitors in patients with stage III/IV clear cell renal cell carcinoma. More specifically, the approach involved the inclusion of subcutaneously administered ipilimumab adjacent to the vaccination site, targeted at pre-specified cohorts. This strategy was intended to enhance T cell priming and activation at the local draining lymph node. Among all nine patients, the study successfully manufactured and administered a median of 15 vaccinating peptides per subject, with a range of 8 to 19 peptides. These peptides were designed to target a median of 13 unique mutations per patient, spanning a range of 7 to 17 mutations [50]. There were no instances of dose-limiting toxicities observed, and, notably, no occurrences of disease recurrences were recorded within this high-risk population. Additionally, combining neoantigen-based vaccines with adoptive T cell therapy holds promise in augmenting the patient’s immune response against tumors (Figure 1). Innovative technologies like RNA-based vaccines have further expanded the scope of neoantigen immunotherapy. RNA vaccines encoding neoantigen sequences are designed to stimulate potent immune responses against cancer cells. In one clinical trial (NCT03313778) involving 13 patients with high-risk resectable solid tumors, and a separate cohort of 20 patients with unresectable advanced-stage solid tumors, mRNA-4157, an RNA-based neoantigen vaccine encapsulated in lipid nanoparticles, underwent testing, respectively, as monotherapy and in combination with pembrolizumab in these two cohorts. Intriguingly, the latter group included 12 patients who had experienced disease progression on prior immune checkpoint inhibitor (ICI) therapy. Encouragingly, no instances of dose-limiting toxicities or severe grade 3–4 adverse events were reported. Noteworthy outcomes emerged in the cohort treated with the combination, where six clinical responses were documented among the 20 patients, culminating in an overall response rate of 30%. Of particular significance, two of the 12 patients who had previously undergone ICIs showcased clinical responses, illustrating the potential of this approach to benefit patients who had previously exhibited disease progression on ICI therapies [51]. As clinical trials and studies continue to provide insights into the potential of neoantigen-based interventions, the integration of these novel strategies with established immunotherapies offers a promising avenue to enhance antitumor immune responses and improve patient outcomes. The growing body of evidence underscores the dynamic nature of neoantigen-based immunotherapy and its potential to shape the future of precision cancer treatment. However, challenges persist, including the identification of optimal neoantigens for each patient, addressing tumor heterogeneity, and ensuring consistent manufacturing of personalized vaccines.
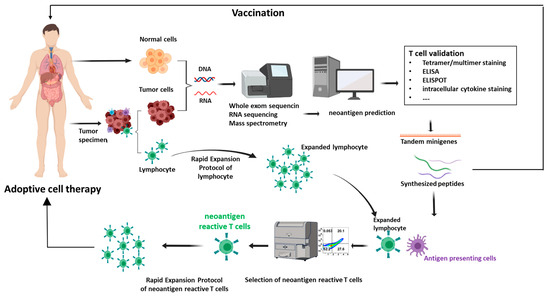
Figure 1.
Neoantigen identification and validation and application in adoptive cell therapy (ACT). Patient samples, encompassing both tumor and normal cells, are gathered and subjected to whole exome DNA sequencing or RNA sequencing. Subsequently, a variants calling procedure is executed to identify the alterations within protein-encoding regions, followed with neoantigens computational predictions and prioritization. The validation process involves a range of methods such as tetramer assays, Enzyme-linked immunosorbent spot (ELISPOT) assays, and T-cell activation assays. The identified neoantigens can be harnessed for the development of immunotherapies, often taking the form of cancer vaccines or ACT. In the realm of cancer vaccines, neoantigens can be administered in diverse formats, including DNA, mRNA, or peptide-based constructs. In the context of ACT, T cells are extracted from either tumor tissues or peripheral blood. Through coculture with antigen-presenting cells (APCs) primed with neoantigens, neoantigen-specific T cells are identified. These cells then undergo an expansion process facilitated by rapid expansion protocols. Upon achieving a substantial quantity, the expanded T cells are reintroduced into the patient’s system, instigating a concerted effort to suppress the tumor.
For instance, the number of neoantigens included in a cancer vaccine can vary depending on several factors, including the patient’s individual tumor profile, the vaccine design, and the specific immunotherapy approach. There exists no universally fixed or average number of neoantigens deemed optimal for all patients or cancer types. Researchers aim to identify and target neoantigens that are unique to an individual’s tumor. This means that the number of neoantigens included in a vaccine can vary from one patient to another, from a few hundred to thousands, depending on the number of relevant neoantigens identified in the patient’s tumor. This variability stems from the fact that some patients exhibit a greater abundance of immunogenic neoantigens, whereas others may present fewer candidates. Therefore, it becomes crucial to discern and prioritize the most efficacious neoepitopes for clinical impact, given that administering the identified number of neo-peptides directly to patients is not feasible.
The quantity of neoantigens incorporated into these vaccines can also differ from one study to another. To provide insights into this variability, certain successful studies serve as examples. For instance, in the study by Keskin et al., each patient received up to 20 peptides, grouped into four pools of 3–5 peptides. This approach aimed to segregate peptides binding to the same MHC allele into different pools, thereby mitigating potential antigen competition at the draining lymph nodes [52]. In the other study, NEO-PV-01, a personalized vaccine consisted of up to 20 synthesized peptides, each ranging from 14 to 35 amino acids in length, was administered to patients [46]. Alternatively, some studies have employed vaccines comprising 5–10 synthetically manufactured peptides [53]. It is worth noting that the optimal number of neoantigens for a vaccine may evolve as our comprehension of neoantigens and cancer immunotherapy advances, and this number will likely continue to be tailored to each patient’s tumor characteristics.
Given the rarity of shared cancer mutations between tumors and the unique nature of patients’ immune systems, neoantigen vaccines must be patient specific and custom manufactured. Typically, the production timeline for neoantigen vaccines in studies conducted so far falls between 12 to 24 weeks [52,53]. Urgently addressing methods to expedite this manufacturing process is crucial to deliver effective treatments to patients, maximizing the vaccine’s potential to stimulate an anti-tumor immune response.
5. Conclusions and Discussion
In recent decades, our comprehension of cancer’s intricate complexities and available treatment avenues has grown substantially. The journey of neoantigens, from their initial discovery to their practical applications in clinical settings, represents a transformative shift in cancer treatment paradigms. This field continues to expand and refine, marked by the optimization and standardization of processes, ranging from neoepitope identification and prediction to the translation into clinical studies. As research and clinical trials progress, the realization that capitalizing on the immune system’s innate ability to target cancer-specific neoantigens holds the potential to inaugurate a novel era of precise and potent cancer therapies.
While neoantigen-based strategies exhibit remarkable potential, several challenges persist on the path toward achieving optimal tumor eradication. First, despite notable advancements in streamlining the neoantigen identification and prediction pipeline, the production of personalized neoantigens for patient treatment remains time-consuming and costly. This expense primarily stems from the sequencing process and the preparation of materials meeting good manufacturing practice standards. Although recent reductions in the cost of DNA and RNA sequencing have been achieved by pooling numerous samples into a single sequencing run, this cost-effective approach is not applicable to the development of personalized cancer vaccines, which are typically tailor-made for small patient cohorts. Given that neoantigens are often considered for patients with advanced cancer, the question arises whether patients can feasibly await neoantigen production. Administering neoantigens at an earlier cancer stage could provide a viable solution to mitigate this concern. Furthermore, due to the extended manufacturing timeline, the use of neoantigen vaccines typically occurs well after the initiation of ICIs. Nevertheless, commencing simultaneous treatment with ICIs and the vaccine earlier in the treatment trajectory may offer a higher likelihood of treatment synergy.
Second, cancers are known to have a high degree of heterogeneity and a capacity of metastasis, which causes difficulties in cancer therapy. The intricate defense mechanisms that cancer cells employ to evade immune recognition pose a significant hurdle. In response, combining neoantigen therapies with other approaches emerges as a promising strategy to surmount tumor immune evasion. Studies underscore the effectiveness of employing adjuvant therapies to enhance immune cell infiltration and reshape the tumor microenvironment. For example, cytokines like interferon-gamma and granulocyte-macrophage colony-stimulating factor have been shown to synergize with neoantigens, augmenting their efficacy [54,55]. Among the adjuvant drugs, immune checkpoint blockade drugs targeting PD-1/PD-L1 or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) are most widely used. While the combination therapy provides enhanced effectiveness in the treatment for early stage tumors, less optimal results are observed with more advanced tumors. Immune evasion and resistance to therapies have been postulated and often stem from the dynamic shifts in tumor antigen expression, the loss of HLA, or the downregulation of HLA expression. This prompts contemplation on the necessity of establishing the minimum count of neoepitopes required for administration and methods of restoration of the HLA expression in cancer cells, potentially offering a strategic approach to curtailing the emergence of antigen loss variants [56].
Thirdly, an ongoing challenge revolves around the development of efficient strategies for delivering neoantigen vaccines. While peptide-pulsed dendritic cells have demonstrated effectiveness, their use is still limited due to their preparation and expansion. Among the emerging alternatives, effective antigen-presenting cells (APCs), such as immortalized B cells, hold promise as an unlimited resource when compared to dendritic cells [26]. Meanwhile, biomaterial-based delivery systems have emerged as instrumental tools to enhance the efficacy of peptide- or mRNA-based neoantigen vaccines. A notable example is the work by Gao et al., who introduced a nanovaccine consisting of an antigen combined with a synthetic polymeric nanoparticle, PC7A NP [57]. This innovative approach triggers a robust cytotoxic T-cell response while minimizing systemic cytokine expression [58]. Impressively, these nanoparticles facilitate efficient cytosolic delivery of tumor antigens to antigen-presenting cells within draining lymph nodes. This not only enhances surface presentation but also concurrently activates type I interferon-stimulated genes, further amplifying the immune response. Similarly, synthetic high-density lipoprotein nanodiscs, known for their clinical safety and scalability, have been coupled with antigen peptides and adjuvants. This strategic coupling significantly improves the co-delivery of antigens and adjuvants to lymphoid organs and sustains antigen presentation on dendritic cells [59]. Notably, biomaterials are emerging as potent modulators for intracellular delivery and antigen processing within APCs. They are anticipated to offer precise control over balanced MHC class I and II loading of antigens, thereby eliciting optimal and potent antitumor immune responses. As the journey of neoantigens continues, these challenges beckon for innovative solutions, fueled by a collaborative effort among researchers, clinicians, and industry stakeholders. The overarching objective remains: to unlock the full potential of neoantigens, transforming them from promising concepts into effective therapies that revolutionize cancer care.
Author Contributions
T.Z. contributed to the literature review and writing. T.Z. and Z.W. contributed to the table design. T.Z. contributed to the conception and design. T.Z., E.K. and Z.W. contributed to the proofreading and edition. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
All other authors declared no conflict of interest.
Abbreviations
APCs, Antigen-presenting cells; CAR-T, Chimeric Antigen Receptor T cell; CTL, Cytolytic T lymphocyte; CTLA-4, Cytotoxic T-lymphocyte-associated protein 4; ERBB2IP, Erbb2 interacting protein; HLA, Human leukocyte antigen; ICIs, Immune checkpoint inhibitors; MHC, Major histocompatibility complex; PD-1, Programmed cell death-1; PD-L1, Programmed cell death ligand 1; RNA-seq, RNA sequencing; TAA, Tumor-associated antigen; TCR, T cell receptor; TMB, Tumor mutation burden; WES, Whole exome sequencing.
References
- Fritsch, E.F.; Burkhardt, U.E.; Hacohen, N.; Wu, C.J. Personal Neoantigen Cancer Vaccines: A Road Not Fully Paved. Cancer Immunol. Res. 2020, 8, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Shi, T.; Zhang, H.; Hu, J.; Song, Y.; Wei, J.; Ren, S.; Zhou, C. Tumor neoantigens: From basic research to clinical applications. J. Hematol. Oncol. 2019, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Richters, M.M.; Xia, H.; Campbell, K.M.; Gillanders, W.E.; Griffith, O.L.; Griffith, M. Best practices for bioinformatic characterization of neoantigens for clinical utility. Genome Med. 2019, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C.; Selitsky, S.R.; Chai, S.; Armistead, P.M.; Vincent, B.G.; Serody, J.S. Alternative tumour-specific antigens. Nat. Rev. Cancer 2019, 19, 465–478. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Scheper, W.; Kvistborg, P. Cancer Neoantigens. Annu. Rev. Immunol. 2019, 37, 173–200. [Google Scholar] [CrossRef]
- Biswas, N.; Chakrabarti, S.; Padul, V.; Jones, L.D.; Ashili, S. Designing neoantigen cancer vaccines, trials, and outcomes. Front. Immunol. 2023, 14, 1105420. [Google Scholar] [CrossRef]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.E.; Badin, F.; et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef]
- Lauss, M.; Donia, M.; Harbst, K.; Andersen, R.; Mitra, S.; Rosengren, F.; Salim, M.; Vallon-Christersson, J.; Torngren, T.; Kvist, A.; et al. Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma. Nat. Commun. 2017, 8, 1738. [Google Scholar] [CrossRef]
- Heemskerk, B.; Kvistborg, P.; Schumacher, T.N. The cancer antigenome. EMBO J. 2013, 32, 194–203. [Google Scholar] [CrossRef]
- Ho, S.Y.; Chang, C.M.; Liao, H.N.; Chou, W.H.; Guo, C.L.; Yen, Y.; Nakamura, Y.; Chang, W.C. Current Trends in Neoantigen-Based Cancer Vaccines. Pharmaceuticals 2023, 16, 392. [Google Scholar] [CrossRef]
- Carreno, B.M.; Magrini, V.; Becker-Hapak, M.; Kaabinejadian, S.; Hundal, J.; Petti, A.A.; Ly, A.; Lie, W.R.; Hildebrand, W.H.; Mardis, E.R.; et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 2015, 348, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Ramarathinam, L.; Sarma, S.; Maric, M.; Zhao, M.; Yang, G.; Chen, L.; Liu, Y. Multiple lineages of tumors express a common tumor antigen, P1A, but they are not cross-protected. J. Immunol. 1995, 155, 5323–5329. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.; Guo, Y.; Guilloux, Y.; Lee, C.; Bai, X.F.; Liu, Y. Cytotoxic T lymphocytes to an unmutated tumor rejection antigen P1A: Normal development but restrained effector function in vivo. J. Exp. Med. 1999, 189, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Monach, P.A.; Meredith, S.C.; Siegel, C.T.; Schreiber, H. A unique tumor antigen produced by a single amino acid substitution. Immunity 1995, 2, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Lennerz, V.; Fatho, M.; Gentilini, C.; Frye, R.A.; Lifke, A.; Ferel, D.; Wolfel, C.; Huber, C.; Wolfel, T. The response of autologous T cells to a human melanoma is dominated by mutated neoantigens. Proc. Natl. Acad. Sci. USA 2005, 102, 16013–16018. [Google Scholar] [CrossRef]
- Zhou, J.; Dudley, M.E.; Rosenberg, S.A.; Robbins, P.F. Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J. Immunother. 2005, 28, 53–62. [Google Scholar] [CrossRef]
- Segal, N.H.; Parsons, D.W.; Peggs, K.S.; Velculescu, V.; Kinzler, K.W.; Vogelstein, B.; Allison, J.P. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008, 68, 889–892. [Google Scholar] [CrossRef]
- Robbins, P.F.; Lu, Y.C.; El-Gamil, M.; Li, Y.F.; Gross, C.; Gartner, J.; Lin, J.C.; Teer, J.K.; Cliften, P.; Tycksen, E.; et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat. Med. 2013, 19, 747–752. [Google Scholar] [CrossRef]
- Castle, J.C.; Kreiter, S.; Diekmann, J.; Lower, M.; van de Roemer, N.; de Graaf, J.; Selmi, A.; Diken, M.; Boegel, S.; Paret, C.; et al. Exploiting the mutanome for tumor vaccination. Cancer Res. 2012, 72, 1081–1091. [Google Scholar] [CrossRef]
- Matsushita, H.; Vesely, M.D.; Koboldt, D.C.; Rickert, C.G.; Uppaluri, R.; Magrini, V.J.; Arthur, C.D.; White, J.M.; Chen, Y.S.; Shea, L.K.; et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 2012, 482, 400–404. [Google Scholar] [CrossRef]
- Tran, E.; Robbins, P.F.; Lu, Y.C.; Prickett, T.D.; Gartner, J.J.; Jia, L.; Pasetto, A.; Zheng, Z.; Ray, S.; Groh, E.M.; et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N. Engl. J. Med. 2016, 375, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Tran, E.; Turcotte, S.; Gros, A.; Robbins, P.F.; Lu, Y.C.; Dudley, M.E.; Wunderlich, J.R.; Somerville, R.P.; Hogan, K.; Hinrichs, C.S.; et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014, 344, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Suryawanshi, Y.R.; Szymczyna, B.R.; Essani, K. Neutralization of matrix metalloproteinase-9 potentially enhances oncolytic efficacy of tanapox virus for melanoma therapy. Med. Oncol. 2017, 34, 129. [Google Scholar] [CrossRef]
- Zhang, T.; Jou, T.H.; Hsin, J.; Wang, Z.; Huang, K.; Ye, J.; Yin, H.; Xing, Y. Talimogene Laherparepvec (T-VEC): A Review of the Recent Advances in Cancer Therapy. J. Clin. Med. 2023, 12, 1098. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, T.; Anderson, A.; Lee, V.; Szymura, S.; Dong, Z.; Kuang, B.; Oh, E.; Liu, J.; Neelapu, S.S.; et al. Immortalized B Cells Transfected with mRNA of Antigen Fused to MITD (IBMAM): An Effective Tool for Antigen-Specific T-Cell Expansion and TCR Validation. Biomedicines 2023, 11, 796. [Google Scholar] [CrossRef]
- Liu, X.S.; Mardis, E.R. Applications of Immunogenomics to Cancer. Cell 2017, 168, 600–612. [Google Scholar] [CrossRef]
- Thind, A.S.; Monga, I.; Thakur, P.K.; Kumari, P.; Dindhoria, K.; Krzak, M.; Ranson, M.; Ashford, B. Demystifying emerging bulk RNA-Seq applications: The application and utility of bioinformatic methodology. Brief. Bioinform. 2021, 22, bbab259. [Google Scholar] [CrossRef]
- De Mattos-Arruda, L.; Vazquez, M.; Finotello, F.; Lepore, R.; Porta, E.; Hundal, J.; Amengual-Rigo, P.; Ng, C.K.Y.; Valencia, A.; Carrillo, J.; et al. Neoantigen prediction and computational perspectives towards clinical benefit: Recommendations from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 978–990. [Google Scholar] [CrossRef]
- Cabanski, C.R.; Magrini, V.; Griffith, M.; Griffith, O.L.; McGrath, S.; Zhang, J.; Walker, J.; Ly, A.; Demeter, R.; Fulton, R.S.; et al. cDNA hybrid capture improves transcriptome analysis on low-input and archived samples. J. Mol. Diagn. 2014, 16, 440–451. [Google Scholar] [CrossRef][Green Version]
- Kim, S.; Scheffler, K.; Halpern, A.L.; Bekritsky, M.A.; Noh, E.; Kallberg, M.; Chen, X.; Kim, Y.; Beyter, D.; Krusche, P.; et al. Strelka2: Fast and accurate calling of germline and somatic variants. Nat. Methods 2018, 15, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xi, L.; Hughes, D.S.; Zhang, J.; Zhang, J.; Futreal, P.A.; Wheeler, D.A.; Wang, W. MuSE: Accounting for tumor heterogeneity using a sample-specific error model improves sensitivity and specificity in mutation calling from sequencing data. Genome Biol. 2016, 17, 178. [Google Scholar] [CrossRef] [PubMed]
- Rammensee, H.; Bachmann, J.; Emmerich, N.P.; Bachor, O.A.; Stevanovic, S. SYFPEITHI: Database for MHC ligands and peptide motifs. Immunogenetics 1999, 50, 213–219. [Google Scholar] [CrossRef]
- Lundegaard, C.; Lamberth, K.; Harndahl, M.; Buus, S.; Lund, O.; Nielsen, M. NetMHC-3.0: Accurate web accessible predictions of human, mouse and monkey MHC class I affinities for peptides of length 8–11. Nucleic Acids Res. 2008, 36, W509–W512. [Google Scholar] [CrossRef] [PubMed]
- Van Kaer, L.; Ashton-Rickardt, P.G.; Ploegh, H.L.; Tonegawa, S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4-8+ T cells. Cell 1992, 71, 1205–1214. [Google Scholar] [CrossRef]
- Robinson, J.; Halliwell, J.A.; Hayhurst, J.D.; Flicek, P.; Parham, P.; Marsh, S.G. The IPD and IMGT/HLA database: Allele variant databases. Nucleic Acids Res. 2015, 43, D423–D431. [Google Scholar] [CrossRef]
- Rammensee, H.G.; Singh-Jasuja, H. HLA ligandome tumor antigen discovery for personalized vaccine approach. Expert Rev. Vaccines 2013, 12, 1211–1217. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, Y.; Zhang, Q.; Liu, W. Application of mass spectrometry-based MHC immunopeptidome profiling in neoantigen identification for tumor immunotherapy. Biomed. Pharmacother. 2019, 120, 109542. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Wang, X.; Kim, S.W.; Herndon, J.M.; Becker-Hapak, M.K.; Carreno, B.M.; Myers, N.B.; Sturmoski, M.A.; McLellan, M.D.; et al. Optimized polyepitope neoantigen DNA vaccines elicit neoantigen-specific immune responses in preclinical models and in clinical translation. Genome Med. 2021, 13, 56. [Google Scholar] [CrossRef]
- Yossef, R.; Tran, E.; Deniger, D.C.; Gros, A.; Pasetto, A.; Parkhurst, M.R.; Gartner, J.J.; Prickett, T.D.; Cafri, G.; Robbins, P.F.; et al. Enhanced detection of neoantigen-reactive T cells targeting unique and shared oncogenes for personalized cancer immunotherapy. JCI Insight 2018, 3, e122467. [Google Scholar] [CrossRef]
- Zhang, T.; Essani, K. Tanapoxvirus lacking the 15L gene inhibits melanoma cell growth in vitro by inducing interferon-lambda1 release. Virus Genes 2017, 53, 477–482. [Google Scholar] [CrossRef]
- Hu, Z.; Leet, D.E.; Allesoe, R.L.; Oliveira, G.; Li, S.; Luoma, A.M.; Liu, J.; Forman, J.; Huang, T.; Iorgulescu, J.B.; et al. Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma. Nat. Med. 2021, 27, 515–525. [Google Scholar] [CrossRef]
- Rodon, J.; Soria, J.C.; Berger, R.; Miller, W.H.; Rubin, E.; Kugel, A.; Tsimberidou, A.; Saintigny, P.; Ackerstein, A.; Brana, I.; et al. Genomic and transcriptomic profiling expands precision cancer medicine: The WINTHER trial. Nat. Med. 2019, 25, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Blass, E.; Ott, P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol 2021, 18, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Bauman, J.; Burris, H.; Clarke, J.; Patel, M.; Cho, D.; Gutierrez, M.; Julian, R.; Scott, A.; Cohen, P.; Frederick, J.; et al. 798 Safety, tolerability, and immunogenicity of mRNA-4157 in combination with pembrolizumab in subjects with unresectable solid tumors (KEYNOTE-603): An update. J. ImmunoTherapy Cancer 2020, 8, A477. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu-Lieskovan, S.; Chmielowski, B.; Govindan, R.; Naing, A.; Bhardwaj, N.; Margolin, K.; Awad, M.M.; Hellmann, M.D.; Lin, J.J.; et al. A Phase Ib Trial of Personalized Neoantigen Therapy Plus Anti-PD-1 in Patients with Advanced Melanoma, Non-small Cell Lung Cancer, or Bladder Cancer. Cell 2020, 183, 347–362.e324. [Google Scholar] [CrossRef]
- Coxon, A.T.; Johanns, T.M.; Dunn, G.P. An Innovative Immunotherapy Vaccine with Combination Checkpoint Blockade as a First Line Treatment for Glioblastoma in the Context of Current Treatments. Mo. Med. 2020, 117, 45–49. [Google Scholar]
- Lu, Y.C.; Zheng, Z.; Lowery, F.J.; Gartner, J.J.; Prickett, T.D.; Robbins, P.F.; Rosenberg, S.A. Direct identification of neoantigen-specific TCRs from tumor specimens by high-throughput single-cell sequencing. J. Immunother. Cancer 2021, 9, e002595. [Google Scholar] [CrossRef]
- Gillison, M.L.; Awad, M.M.; Twardowski, P.; Sukari, A.; Johnson, M.L.; Stein, M.N.; Hernandez, R.; Price, J.; Mancini, K.J.; Shainheit, M.; et al. Long term results from a phase 1 trial of GEN-009, a personalized neoantigen vaccine, combined with PD-1 inhibition in advanced solid tumors. J. Clin. Oncol. 2021, 39, 2613. [Google Scholar] [CrossRef]
- Braun, D.A.; Keskin, D.B.; Shukla, S.A.; McGregor, B.A.; Schindler, N.R.; Blass, E.; Klaeger, S.; Pomerance, L.; Sarkizova, S.; Li, S. Abstract PR015: Tumor-specific immunity generated by a personalized neoantigen vaccination incorporating locally delivered ipilimumab in renal cell carcinoma. Cancer Res. 2023, 83, PR015. [Google Scholar] [CrossRef]
- Burris, H.A.; Patel, M.R.; Cho, D.C.; Clarke, J.M.; Gutierrez, M.; Zaks, T.Z.; Frederick, J.; Hopson, K.; Mody, K.; Binanti-Berube, A.; et al. A phase I multicenter study to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in patients with resected solid tumors and in combination with pembrolizumab in patients with unresectable solid tumors. J. Clin. Oncol. 2019, 37, 2523. [Google Scholar] [CrossRef]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef]
- Mork, S.K.; Kadivar, M.; Bol, K.F.; Draghi, A.; Westergaard, M.C.W.; Skadborg, S.K.; Overgaard, N.; Sorensen, A.B.; Rasmussen, I.S.; Andreasen, L.V.; et al. Personalized therapy with peptide-based neoantigen vaccine (EVX-01) including a novel adjuvant, CAF(R)09b, in patients with metastatic melanoma. Oncoimmunology 2022, 11, 2023255. [Google Scholar] [CrossRef]
- van de Laar, L.; Coffer, P.J.; Woltman, A.M. Regulation of dendritic cell development by GM-CSF: Molecular control and implications for immune homeostasis and therapy. Blood 2012, 119, 3383–3393. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, F.; Lapenta, C.; Donati, S.; Abalsamo, L.; Barnaba, V.; Belardelli, F.; Santini, S.M.; Ferrantini, M. IFN-alpha enhances cross-presentation in human dendritic cells by modulating antigen survival, endocytic routing, and processing. Blood 2012, 119, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Luo, M.; Wang, Z.; Feng, Q.; Wilhelm, J.; Wang, X.; Li, W.; Wang, J.; Cholka, A.; Fu, Y.X.; et al. Prolonged activation of innate immune pathways by a polyvalent STING agonist. Nat. Biomed. Eng. 2021, 5, 455–466. [Google Scholar] [CrossRef]
- Luo, M.; Wang, H.; Wang, Z.; Cai, H.; Lu, Z.; Li, Y.; Du, M.; Huang, G.; Wang, C.; Chen, X.; et al. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 2017, 12, 648–654. [Google Scholar] [CrossRef]
- Kuai, R.; Ochyl, L.J.; Bahjat, K.S.; Schwendeman, A.; Moon, J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017, 16, 489–496. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).