Immune Modulatory Effects of Probiotic Streptococcus thermophilus on Human Monocytes
Abstract
:1. Introduction
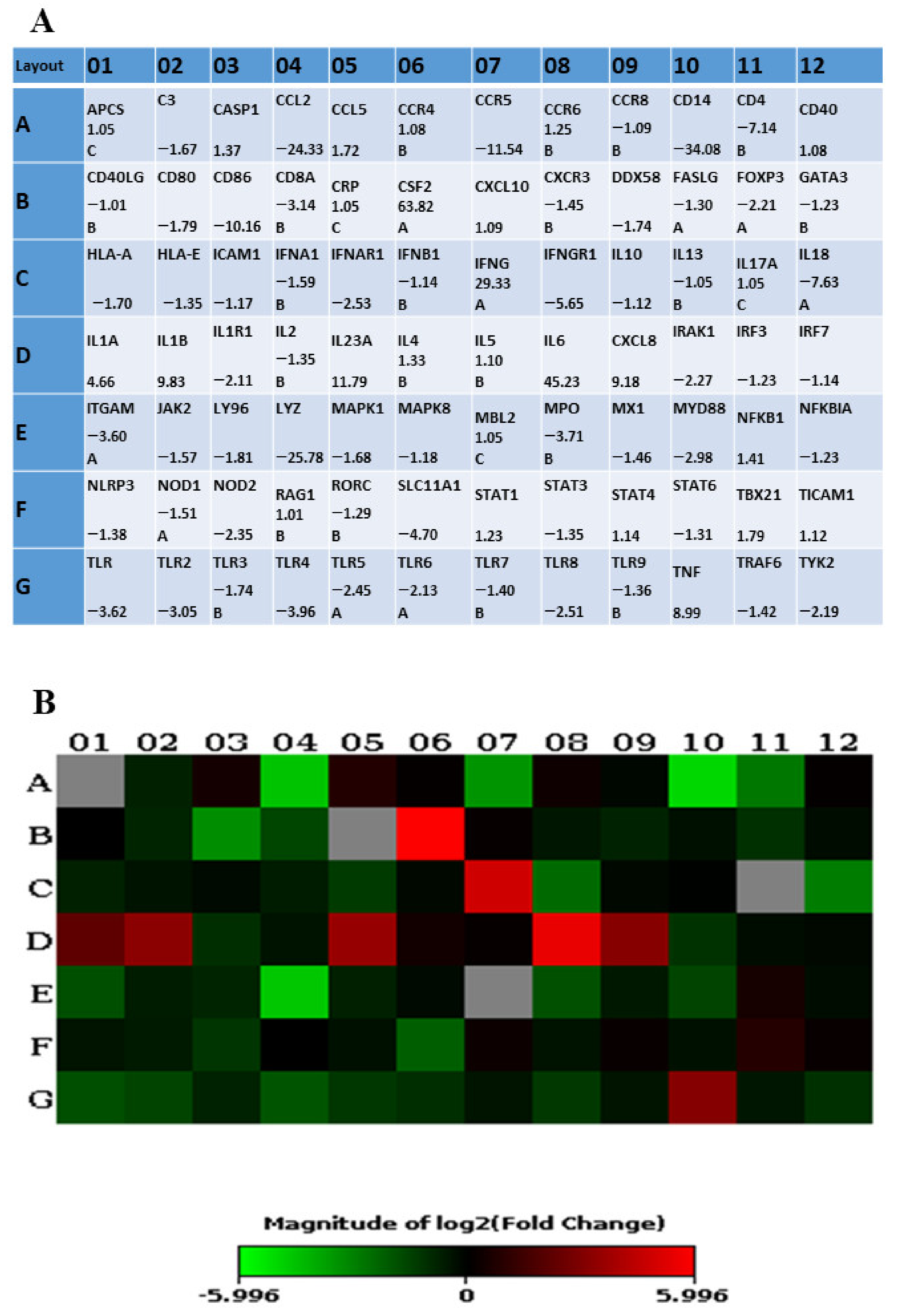
2. Results
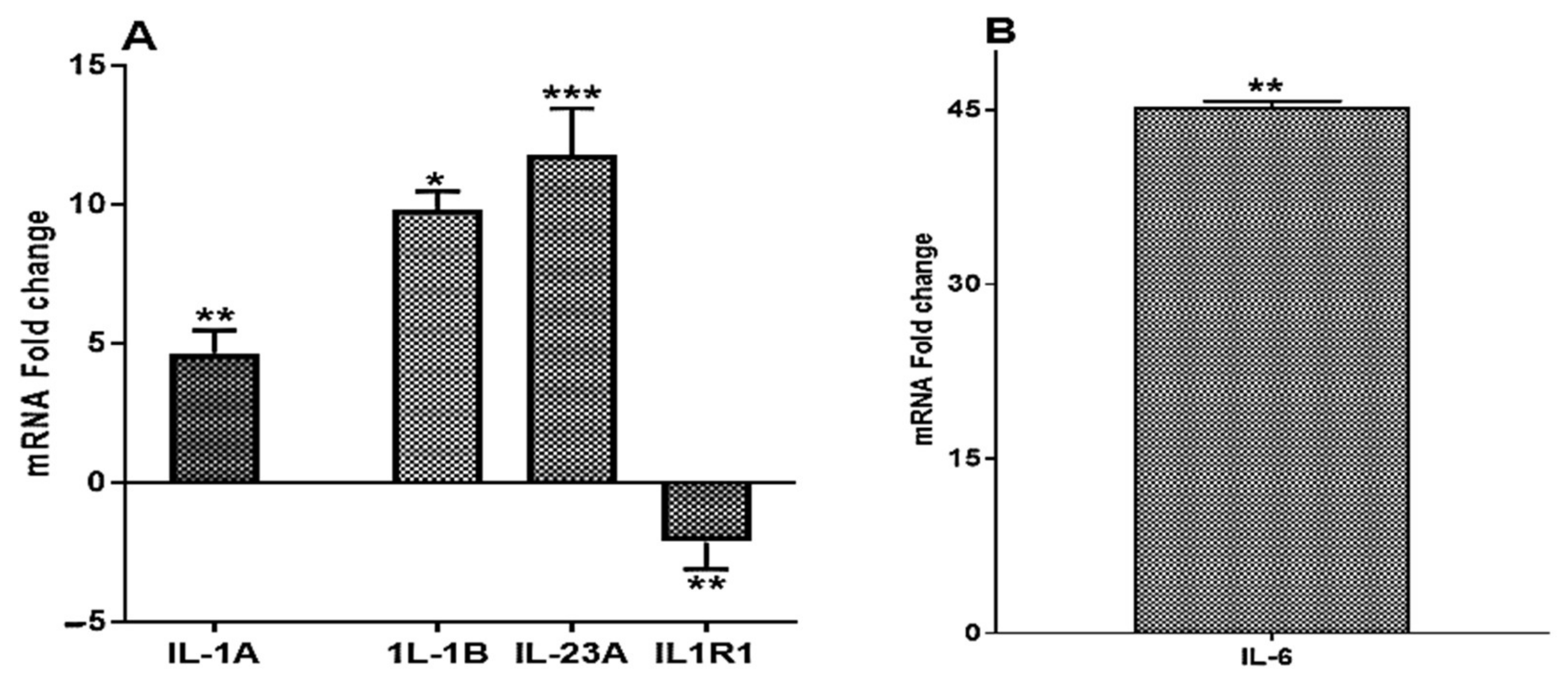
2.1. ST285 Alters Cytokine Gene Expression Levels of Monocytes
2.1.1. ST285 Causes Upregulation of IL-1α, IL-1β, IL-6, and IL-23 and Downregulation of IL-1R1
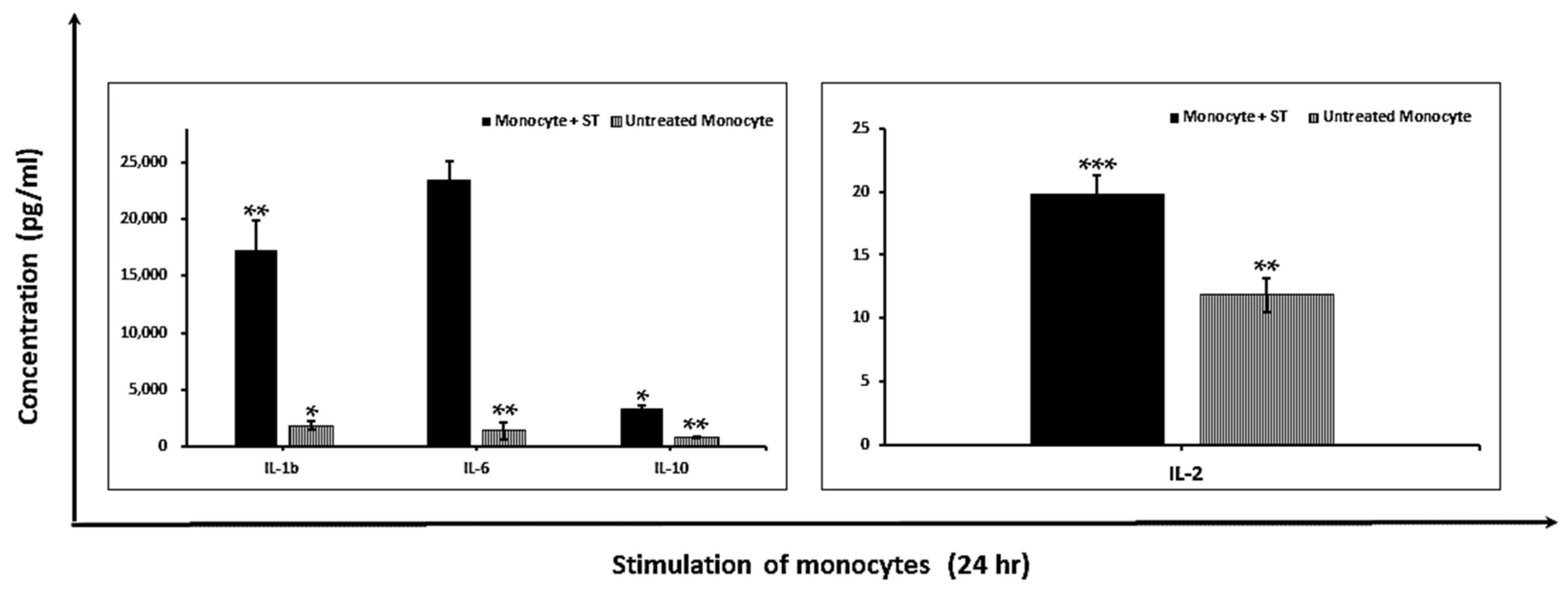
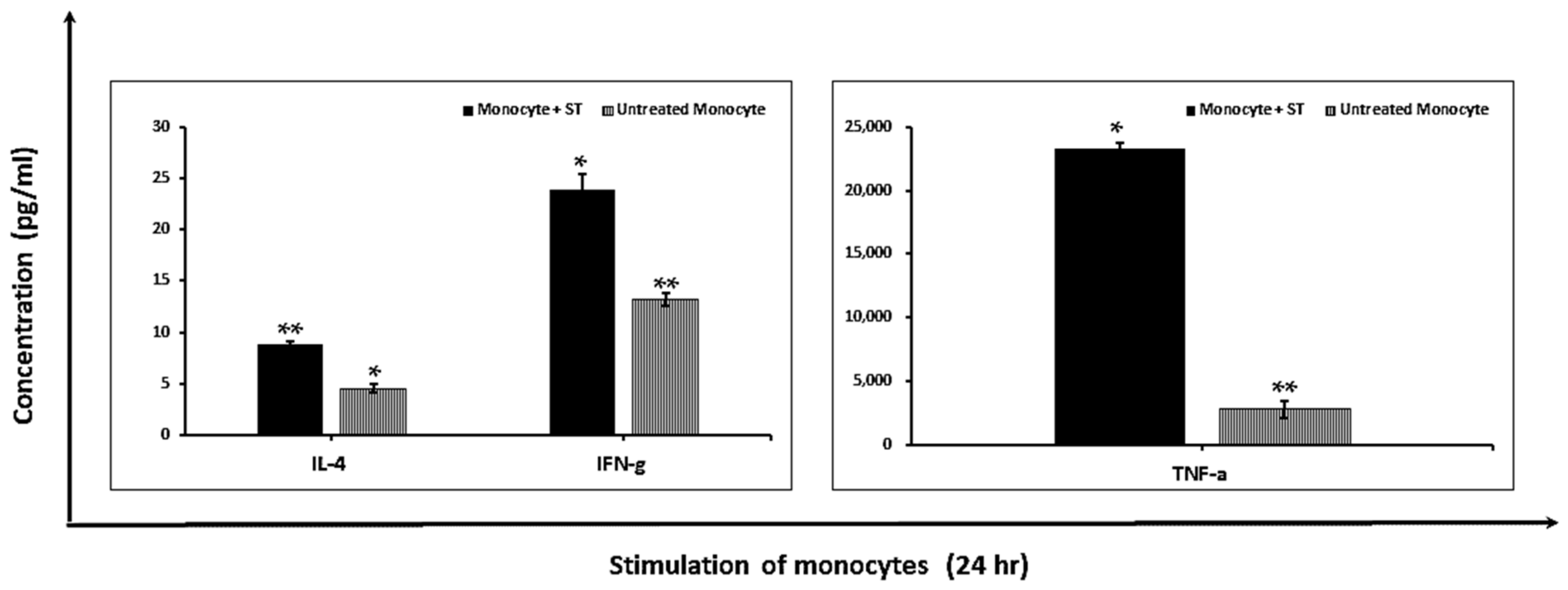
2.1.2. Modulation of Pro-Inflammatory Cytokines
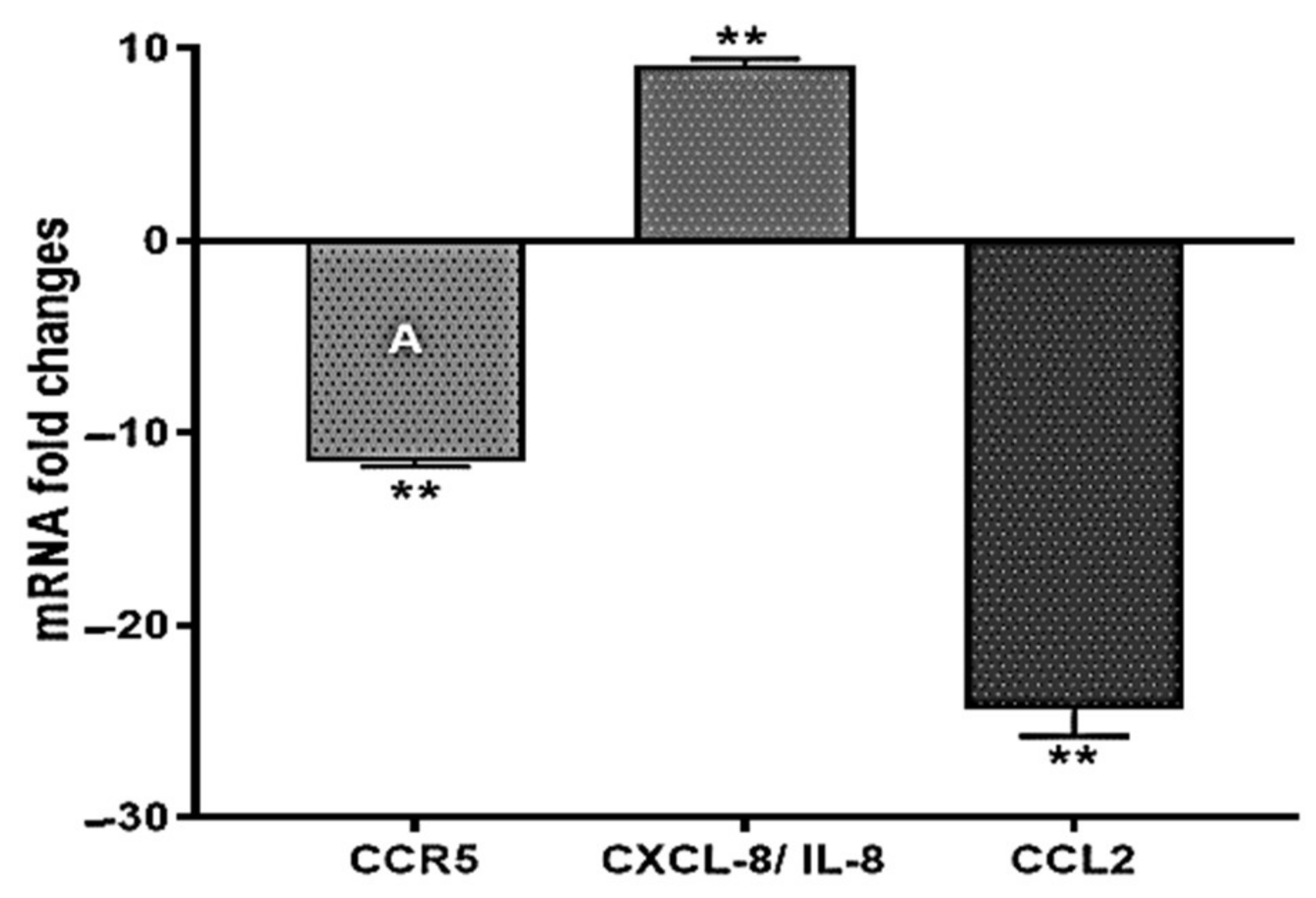
2.2. ST285 Alters Chemokine Gene Expression Levels of Monocytes
2.3. Significant Upregulation of Colony Stimulating Factor mRNA Expression Levels
2.4. ST285 Alters Toll-Like Receptor Gene Expression Levels of Monocytes
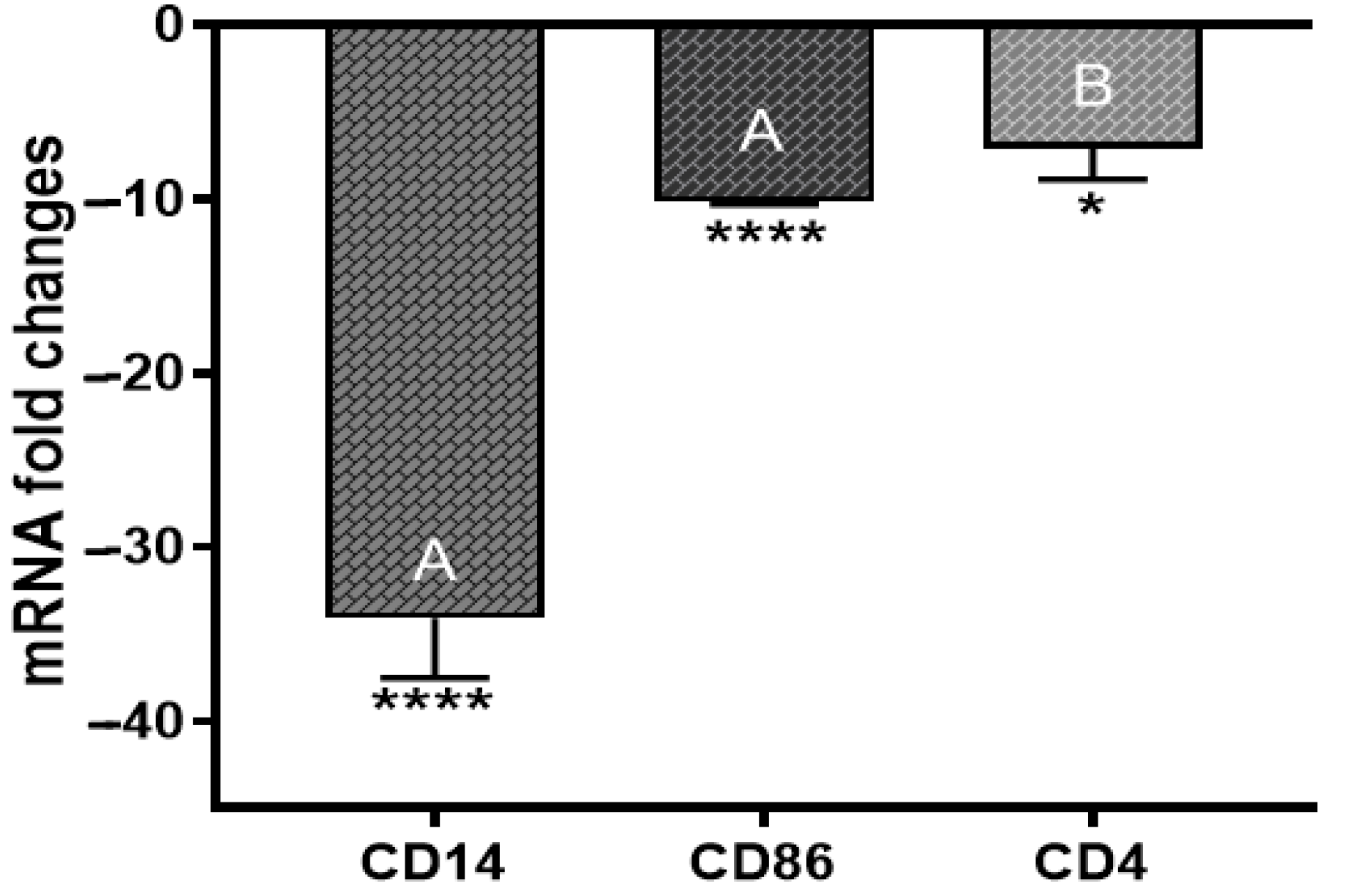
2.5. Cell Surface Markers CD14, CD86, and CD4 mRNA Expression Levels
2.6. Changes to Other Innate and Adaptive Molecules, mRNA Expression Levels
3. Discussion
3.1. ST285 Induces IL-1β and IL-6 and Downregulates IL-1R1
3.2. ST285 Changes the Expression of Cytokines Involved in Inflammation and Defense against Bacteria
3.3. ST285 Activates mRNA Expression of CXCL8 and Downregulates CCR5 and CCL2
3.4. ST285 Significantly Upregulates mRNA Expression Level of Colony Stimulating Factor
3.5. ST285 Downregulates mRNA Expression Levels of Toll-Like Receptors
3.6. ST285 Downregulates Cell Surface Markers CD14, CD86, and CD4
3.7. ST285 Differentially Downregulates mRNA Expression Level of Other Innate and Adaptive Immune Response Markers and Chemokines
4. Material and Methods
4.1. Bacterial Strains
4.1.1. Preparation of Live Bacterial Suspensions
4.1.2. Enumeration of Bacterial Cells
4.2. Isolation of Monocytes from Buffy Coats
4.3. Stimulation of Monocytes with ST285
4.4. RNA Extraction from Monocytes
4.5. Assessing Changes in the Expression of Genes Associated with Innate and Adaptive Immunity
4.6. Data Analysis
4.7. Statistical Analysis
4.8. Cytokine Production Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dargahi, N.; Johnson, J.; Donkor, O.; Vasiljevic, T.; Apostolopoulos, V. Immunomodulatory effects of Streptococcus thermophilus on U937 monocyte cell cultures. J. Funct. Foods 2018, 49, 241–249. [Google Scholar] [CrossRef]
- Dargahi, N.; Katsara, M.; Tselios, T.; Androutsou, M.-E.; De Courten, M.; Matsoukas, J.; Apostolopoulos, V. Multiple Sclerosis: Immunopathology and Treatment Update. Brain Sci. 2017, 7, 78. [Google Scholar] [CrossRef] [Green Version]
- Stagg, J.; Divisekera, U.; Duret, H.; Sparwasser, T.; Teng, M.; Darcy, P.; Smyth, M. CD73-Deficient Mice Have Increased Antitumor Immunity and Are Resistant to Experimental Metastasis. Cancer Res. 2011, 71, 2892–2900. [Google Scholar] [CrossRef] [Green Version]
- Stagg, A.J.; Hart, A.L.; Knight, S.C.; A Kamm, M. Interactions between dendritic cells and bacteria in the regulation of intestinal immunity. Best Pr. Res. Clin. Gastroenterol. 2004, 18, 255–270. [Google Scholar] [CrossRef]
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A.D. Probiotics, Prebiotics and Immunomodulation of Gut Mucosal Defences: Homeostasis and Immunopathology. Nutrients 2013, 5, 1869–1912. [Google Scholar] [CrossRef]
- Kinross, J.; Von Roon, A.C.; Holmes, E.; Darzi, A.; Nicholson, J.K. The human gut microbiome: Implications for future health care. Curr. Gastroenterol. Rep. 2008, 10, 396–403. [Google Scholar] [CrossRef]
- Michałkiewicz, J.; Krotkiewski, M.; Gackowska, L.; Wyszomirska-Gołda, M.; Helmin-Basa, A.; Dzierżanowska, D.; Madaliński, K. Immunomodulatory Effects of Lactic Acid Bacteria on Human Peripheral Blood Mononuclear Cells. Microb. Ecol. Health Dis. 2003, 15, 185–192. [Google Scholar] [CrossRef]
- Maassen, C.B.; van Holten-Neelen, C.; Balk, F.; Bak-Glashouwer, M.-J.H.D.; Leer, R.J.; Laman, J.D.; Boersma, W.J.; Claassen, E. Strain-dependent induction of cytokine profiles in the gut by orally administered Lactobacillus strains. Vaccine 2000, 18, 2613–2623. [Google Scholar] [CrossRef]
- Fink, L.; Zeuthen, L.H.; Christensen, H.R.; Morandi, B.; Frøkiær, H.; Ferlazzo, G. Distinct gut-derived lactic acid bacteria elicit divergent dendritic cell-mediated NK cell responses. Int. Immunol. 2007, 19, 1319–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asarat, M.; Apostolopoulos, V.; Vasiljevic, T.; Donkor, O. Short-Chain Fatty Acids Regulate Cytokines and Th17/Treg Cells in Human Peripheral Blood Mononuclear Cellsin vitro. Immunol. Investig. 2016, 45, 205–222. [Google Scholar] [CrossRef] [Green Version]
- Salazar, N.; Prieto, A.; Leal, J.A.; Mayo, B.; Bada-Gancedo, J.C.; Reyes-Gavilan, C.D.L.; Ruas-Madiedo, P. Production of exopolysaccharides by Lactobacillus and Bifidobacterium strains of human origin, and metabolic activity of the producing bacteria in milk. J. Dairy Sci. 2009, 92, 4158–4168. [Google Scholar] [CrossRef] [Green Version]
- Dargahi, N.; Johnson, J.; Donkor, O.; Vasiljevic, T.; Apostolopoulos, V. Immunomodulatory effects of probiotics: Can they be used to treat allergies and autoimmune diseases? Maturitas 2019, 119, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Asarat, M.; Apostolopoulos, V.; Vasiljevic, T.; Donkor, O. Short-chain fatty acids produced by synbiotic mixtures in skim milk differentially regulate proliferation and cytokine production in peripheral blood mononuclear cells. Int. J. Food Sci. Nutr. 2015, 66, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Asarat, M.; Vasiljevic, T.; Apostolopoulos, V.; Donkor, O. Short-Chain Fatty Acids Regulate Secretion of IL-8 from Human Intestinal Epithelial Cell Lines in vitro. Immunol. Investig. 2015, 44, 678–693. [Google Scholar] [CrossRef] [PubMed]
- Purwandari, U.; Vasiljevic, T. Rheological properties of fermented milk produced by a single exopolysaccharide producing Streptococcus thermophilus strain in the presence of added calcium and sucrose. Int. J. Dairy Technol. 2009, 62, 411–421. [Google Scholar] [CrossRef]
- Dicaro, S.; Tao, H.; Grillo, A.; Elia, C.; Gasbarrini, G.; Sepulveda, A.; Gasbarrini, A. Effects of Lactobacillus GG on genes expression pattern in small bowel mucosa. Dig. Liver Dis. 2005, 37, 320–329. [Google Scholar] [CrossRef]
- Hols, P.; Hancy, F.; Fontaine, L.; Grossiord, B.; Prozzi, D.; Leblond-Bourget, N.; Decaris, B.; Bolotin, A.; Delorme, C.; Duskoehrlich, S. New insights in the molecular biology and physiology of Streptococcus thermophilus revealed by comparative genomics. FEMS Microbiol. Rev. 2005, 29, 435–463. [Google Scholar]
- Uriot, O.; Denis, S.; Junjua, M.; Roussel, Y.; Dary-Mourot, A.; Blanquet-Diot, S. Streptococcus thermophilus: From yogurt starter to a new promising probiotic candidate? J. Funct. Foods 2017, 37, 74–89. [Google Scholar] [CrossRef]
- Mennigen, R.; Nolte, K.; Rijcken, E.; Utech, M.; Loeffler, B.; Senninger, N.; Bruewer, M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am. J. Physiol. Liver Physiol. 2009, 296, G1140–G1149. [Google Scholar]
- Dai, C.; Zheng, C.Q.; Meng, F.J.; Zhou, Z.; Sang, L.X.; Jiang, M. VSL#3 probiotics exerts the anti-inflammatory activity via PI3k/Akt and NF-κB pathway in rat model of DSS-induced colitis. Mol. Cell. Biochem. 2013, 374, 1–11. [Google Scholar]
- Kim, H.G.; Kim, N.R.; Gim, M.G.; Lee, J.M.; Lee, S.Y.; Ko, M.Y.; Kim, J.Y.; Han, S.H.; Chung, D.K. Lipoteichoic acid isolated from Lactobacillus plantarum inhibits lipopolysaccharide-induced TNF-α production in THP-1 cells and endotoxin shock in mice. J. Immunol. 2008, 180, 2553–2561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vliagoftis, H.; Kouranos, V.; Betsi, G.I.; Falagas, M.E. Probiotics for the treatment of allergic rhinitis and asthma: Systematic review of randomized controlled trials. Ann. Allergy Asthma Immunol. 2008, 101, 570–579. [Google Scholar] [CrossRef]
- Latvala, S.; Pietilä, T.E.; Veckman, V.; Kekkonen, R.A.; Tynkkynen, S.; Korpela, R.; Julkunen, I. Potentially probiotic bacteria induce efficient maturation but differential cytokine production in human monocyte-derived dendritic cells. World J. Gastroenterol. 2008, 14, 5570–5583. [Google Scholar] [CrossRef]
- Donkor, O.N.; Ravikumar, M.; Proudfoot, O.; Day, S.L.; Apostolopoulos, V.; Paukovics, G.; Vasiljevic, T.; Nutt, S.L.; Gill, H. Cytokine profile and induction of T helper type 17 and regulatory T cells by human peripheral mononuclear cells after microbial exposure. Clin. Exp. Immunol. 2012, 167, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, S.J.; Kumar, P. Cross-Talk Between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis. Front. Immunol. 2019, 10, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dargahi, N.; Johnson, J.; Apostolopoulos, V. Streptococcus thermophilus alters the expression of genes associated with innate and adaptive immunity in human peripheral blood mononuclear cells. PLoS ONE 2020, 15, e0228531. [Google Scholar] [CrossRef] [Green Version]
- Dargahi, N.; Matsoukas, J.; Apostolopoulos, V. Streptococcus thermophilus ST285 Alters Pro-Inflammatory to Anti-Inflammatory Cytokine Secretion against Multiple Sclerosis Peptide in Mice. Brain Sci. 2020, 10, 126. [Google Scholar] [CrossRef] [Green Version]
- Gaston, J.D.; Bischel, L.L.; Fitzgerald, L.A.; Cusick, K.D.; Ringeisen, B.R.; Pirlo, R.K. Gene Expression Changes in Long-Term in Vitro Human Blood-Brain Barrier Models and Their Dependence on a Transwell Scaffold Materia. J. Healthc. Eng. 2017, 2017, 5740975. [Google Scholar] [CrossRef]
- Abubaker, J.; Tiss, A.; Abu-Farha, M.; Al-Ghimlas, F.; Al-Khairi, I.; Baturcam, E.; Cherian, P.; Elkum, N.; Hammad, M.; John, J.; et al. DNAJB3/HSP-40 Cochaperone Is Downregulated in Obese Humans and Is Restored by Physical Exercise. PLoS ONE 2013, 8, e69217. [Google Scholar] [CrossRef] [Green Version]
- Goad, J.; Ko, Y.-A.; Kumar, M.; Syed, S.; Tanwar, P.S. Differential Wnt signaling activity limits epithelial gland development to the anti-mesometrial side of the mouse uterus. Dev. Biol. 2017, 423, 138–151. [Google Scholar] [CrossRef]
- Ben-Sasson, S.Z.; Hu-Li, J.; Quiel, J.; Cauchetaux, S.; Ratner, M.; Shapira, I.; Dinarello, C.A.; Paul, W.E. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc. Natl. Acad. Sci. USA 2009, 106, 7119–7124. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1beta secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Choy, E.; Rose-John, S. Interleukin-6 as a Multifunctional Regulator: Inflammation, Immune Response, and Fibrosis. J. Scleroderma Relat. Disord. 2017, 2, S1–S5. [Google Scholar] [CrossRef] [Green Version]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [Green Version]
- Garbers, C.; Hermanns, H.M.; Schaper, F.; Müller-Newen, G.; Grötzinger, J.; Rose-John, S.; Scheller, J. Plasticity and cross-talk of Interleukin 6-type cytokines. Cytokine Growth Factor Rev. 2012, 23, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H.; Takeuchi, O.; Teraguchi, S.; Matsushita, K.; Uehata, T.; Kuniyoshi, K.; Satoh, T.; Saitoh, T.; Matsushita, M.; Standley, D.M.; et al. The IκB kinase complex regulates the stability of cytokine-encoding mRNA induced by TLR–IL-1R by controlling degradation of regnase-1. Nat. Immunol. 2011, 12, 1167–1175. [Google Scholar] [CrossRef]
- Sun, K.-Y.; Xu, D.-H.; Xie, C.; Plummer, S.; Tang, J.; Yang, X.F.; Ji, X.H. Lactobacillus paracasei modulates LPS-induced inflammatory cytokine release by monocyte-macrophages via the up-regulation of negative regulators of NF-kappaB signaling in a TLR2-dependent manner. Cytokine 2017, 92, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Balzaretti, S.; Taverniti, V.; Guglielmetti, S.; Fiore, W.; Minuzzo, M.; Ngo, H.N.; Ngere, J.B.; Sadiq, S.; Humphreys, P.; Laws, A.P. A Novel Rhamnose-Rich Hetero-exopolysaccharide Isolated from Lactobacillus paracasei DG Activates THP-1 Human Monocytic Cells. Appl. Environ. Microbiol. 2017, 83, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, H.; Larsson, P.; Wold, A.E.; Rudin, A. Pattern of Cytokine Responses to Gram-Positive and Gram-Negative Commensal Bacteria Is Profoundly Changed when Monocytes Differentiate into Dendritic Cells. Infect. Immun. 2004, 72, 2671–2678. [Google Scholar] [CrossRef] [Green Version]
- Schoenborn, J.R.; Wilson, C.B. Regulation of Interferon-γ During Innate and Adaptive Immune Responses. Adv. Immunol. 2007, 95, 41–101. [Google Scholar]
- Shida, K.; Suzuki, T.; Kiyoshima-Shibata, J.; Shimada, S.-I.; Nanno, M. Essential Roles of Monocytes in Stimulating Human Peripheral Blood Mononuclear Cells with Lactobacillus casei To Produce Cytokines and Augment Natural Killer Cell Activity. Clin. Vaccine Immunol. 2006, 13, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Wiese, M.; Eljaszewicz, A.; Andryszczyk, M.; Gronek, S.; Gackowska, L.; Kubiszewska, I.; Kaszewski, W.; Helmin-Basa, A.; Januszewska, M.; Motyl, I.; et al. Immunomodulatory effects of Lactobacillus plantarum and helicobacter pylori CagA+on the expression of selected superficial molecules on monocyte and lymphocyte and the synthesis of cytokines in whole blood culture. J. Physiol. Pharmacol. 2012, 63, 217–224. [Google Scholar]
- Kim, C.H.; Kim, H.G.; Kim, J.Y.; Kim, N.R.; Jung, B.J.; Jeong, J.H.; Chung, D.K. Probiotic genomic DNA reduces the production of pro-inflammatory cytokine tumor necrosis factor-alpha. FEMS Microbiol. Lett. 2012, 328, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Goritzka, M.; Durant, L.; Pereira, C.; Salek-Ardakani, S.; Openshaw, P.; Johansson, C. Alpha/Beta Interferon Receptor Signaling Amplifies Early Proinflammatory Cytokine Production in the Lung during Respiratory Syncytial Virus Infection. J. Virol. 2014, 88, 6128–6136. [Google Scholar] [CrossRef] [Green Version]
- Baggiolini, M.; Clark-Lewis, I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992, 307, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Masotti, A.I.; Buckley, N.; Champagne, C.P.; Tompkins, T.; Green-Johnson, J. Effects of soy and dairy ferments on monocyte viability, cytokine production and cell surface molecule expression: Impact in a low-shear modeled microgravity system. In Proceedings of the 61st International Astronautical Congress 2010, IAC 2010, Prague, Czech Republic, 27 September–1 October 2010. [Google Scholar]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interf. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Lee, I.; Wang, L.; Wells, A.D.; Ye, Q.; Han, R.; Dorf, M.E.; Kuziel, W.A.; Rollins, B.J.; Chen, L.; Hancock, W.W. Blocking the Monocyte Chemoattractant Protein-1/CCR2 Chemokine Pathway Induces Permanent Survival of Islet Allografts through a Programmed Death-1 Ligand-1-Dependent Mechanism. J. Immunol. 2003, 171, 6929–6935. [Google Scholar] [CrossRef] [Green Version]
- Malashchenko, V.V.; Meniailo, M.E.; Shmarov, V.; Gazatova, N.D.; Melashchenko, O.B.; Goncharov, A.G.; Seledtsova, G.V.; Seledtsov, V.I. Direct anti-inflammatory effects of granulocyte colony-stimulating factor (G-CSF) on activation and functional properties of human T cell subpopulations in vitro. Cell. Immunol. 2018, 325, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Sugitharini, V.; Shahana, P.; Prema, A.; Berla Thangam, E. TLR2 and TLR4 co-activation utilizes distinct signaling pathways for the production of Th1/Th2/Th17 cytokines in neonatal immune cells. Cytokine 2016, 85, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Pröll, M.; Hölker, M.; Tholen, E.; Tesfaye, D.; Looft, C.; Schellander, K.; Cinar, M.U. Alveolar macrophage phagocytic activity is enhanced with LPS priming, and combined stimulation of LPS and lipoteichoic acid synergistically induce pro-inflammatory cytokines in pigs. Innate Immun. 2013, 19, 631–643. [Google Scholar] [CrossRef]
- Sugitharini, V.; Pavani, K.; Prema, A.; Thangam, E.B. TLR-mediated inflammatory response to neonatal pathogens and co-infection in neonatal immune cells. Cytokine 2014, 69, 211–217. [Google Scholar] [CrossRef]
- Galli, R.; Starace, D.; Busà, R.; Angelini, D.F.; Paone, A.; De Cesaris, P.; Filippini, A.; Sette, C.; Battistini, L.; Ziparo, E.; et al. TLR Stimulation of Prostate Tumor Cells Induces Chemokine-Mediated Recruitment of Specific Immune Cell Types. J. Immunol. 2010, 184, 6658–6669. [Google Scholar] [CrossRef] [PubMed]
- Bron, P.; Tomita, S.; Mercenier, A.; Kleerebezem, M. Cell surface-associated compounds of probiotic lactobacilli sustain the strain-specificity dogma. Curr. Opin. Microbiol. 2013, 16, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Van Baarlen, P.; Wells, J.M.; Kleerebezem, M. Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol. 2013, 34, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-C.; Tomita, S.; Kleerebezem, M.; Bron, P.A. The quest for probiotic effector molecules—Unraveling strain specificity at the molecular level. Pharmacol. Res. 2013, 69, 61–74. [Google Scholar] [CrossRef]
- Hajebi, A.; Motevalian, S.A.; Rahimi-Movaghar, A.; Sharifi, V.; Amin-Esmaeili, M.; Radgoodarzi, R.; Hefazi, M. Major anxiety disorders in Iran: Prevalence, sociodemographic correlates and service utilization. BMC Psychiatry 2018, 18, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glatzová, D.; Cebecauer, M. Dual Role of CD4 in Peripheral T Lymphocytes. Front. Immunol. 2019, 10, 618. [Google Scholar] [CrossRef]
- Huang, J.; Gao, X.; Li, S.; Cao, Z. Recruitment of IRAK to the interleukin 1 receptor complex requires interleukin 1 receptor accessory protein. Proc. Natl. Acad. Sci. USA 1997, 94, 12829–12832. [Google Scholar] [CrossRef] [Green Version]
- Sohn, S.J.; Barrett, K.; Van Abbema, A.; Chang, C.; Kohli, P.B.; Kanda, H.; Smith, J.; Lai, Y.; Zhou, A.; Zhang, B.; et al. A Restricted Role for TYK2 Catalytic Activity in Human Cytokine Responses Revealed by Novel TYK2-Selective Inhibitors. J. Immunol. 2013, 191, 2205–2216. [Google Scholar] [CrossRef]
- Ishizaki, M.; Akimoto, T.; Muromoto, R.; Yokoyama, M.; Ohshiro, Y.; Sekine, Y.; Maeda, H.; Shimoda, K.; Oritani, K.; Matsuda, T. Involvement of tyrosine kinase-2 in both the IL-12/Th1 and IL-23/Th17 axes in vivo. J. Immunol. 2011, 187, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Thayer, T.; Wilson, S.B.; Mathews, C.E. Use of Nonobese Diabetic Mice to Understand Human Type 1 Diabetes. Endocrinol. Metab. Clin. N. Am. 2010, 39, 541–561. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.D.; Marrero, I.G.; Gros, P.; Zaghouani, H.; Wicker, L.S.; Sercarz, E.E. Slcllal enhances the autoimmune diabetogenic T-cell response by altering processing and presentation of pancreatic islet antigens. Diabetes 2009, 58, 156–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, L.C.; Day, A.S.; Pearson, J.; Barclay, M.L.; Gearry, R.B.; Roberts, R.L.; Bentley, R.W. SLC11A1 polymorphisms in inflammatory bowel disease and Mycobacterium avium subspecies paratuberculosis status. World J. Gastroenterol. 2010, 16, 5727–5731. [Google Scholar] [CrossRef] [PubMed]
- Archer, N.S.; Nassif, N.; Obrien, B. Genetic variants of SLC11A1 are associated with both autoimmune and infectious diseases: Systematic review and meta-analysis. Genes Immun. 2015, 16, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husson-Kao, C.; Mengaud, J.; Cesselin, B.; van Sinderen, D.; Benbadis, L.; Chapot-Chartier, M.-P. The Streptococcus thermophilus Autolytic Phenotype Results from a Leaky Prophage. Appl. Environ. Microbiol. 2000, 66, 558–565. [Google Scholar] [CrossRef] [Green Version]
- Faisal, M.; Dargahi, N.; Vasiljevic, T.; Donkor, O.N. Immunomodulatory properties of selectively processed prawn protein fractions assessed using human peripheral blood mononuclear cells. Int. J. Food Sci. Technol. 2020, 55, 795–804. [Google Scholar] [CrossRef] [Green Version]
- Marzaioli, V.; Hurtado-Nedelec, M.; Pintard, C.; Tlili, A.; Marie, J.-C.; Monteiro, R.C.; Gougerot-Pocidalo, M.-A.; Dang, P.M.-C.; El-Benna, J. NOX5 and p22phox are 2 novel regulators of human monocytic differentiation into dendritic cells. Blood 2017, 130, 1734–1745. [Google Scholar] [CrossRef]
- Kaur, R.; Casey, J.; Pichichero, M. Differences in Innate Immune Response Gene Regulation in the Middle Ear of Children who are Otitis Prone and in those not Otitis Prone. Am. J. Rhinol. Allergy 2016, 30, e218–e223. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhang, T.; Xiu, H.-H.; Liu, J.-X.; Ma, Y.; Xu, K.-Q.; Huang, W.-Q. Protective effect of aspirin-triggered resolvin D1 on hepatic ischemia/reperfusion injury in rats: The role of miR-146b. Int. Immunopharmacol. 2017, 51, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhong, L.; Zhong, S.; Xian, R.; Yuan, B. miR-203 protects microglia mediated brain injury by regulating inflammatory responses via feedback to MyD88 in ischemia. Mol. Immunol. 2015, 65, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.M.; Preisser, T.M.; Pereira, V.B.; Zurita-Turk, M.; De Castro, C.P.; Da Cunha, V.P.; De Oliveira, R.P.; Gomes-Santos, A.C.; De Faria, A.M.C.; Machado, D.C.C.; et al. Lactococcus lactis carrying the pValac eukaryotic expression vector coding for IL-4 reduces chemically-induced intestinal inflammation by increasing the levels of IL-10-producing regulatory cells. Microb. Cell Factories 2016, 15, 1–18. [Google Scholar] [CrossRef]
- Yannakakis, M.-P.; Simal, C.; Tzoupis, H.; Rodi, M.; Dargahi, N.; Prakash, M.; Mouzaki, A.; Platts, J.A.; Apostolopoulos, V.; Tselios, T. Design and Synthesis of Non-Peptide Mimetics Mapping the Immunodominant Myelin Basic Protein (MBP83–96) Epitope to Function as T-Cell Receptor Antagonists. Int. J. Mol. Sci. 2017, 18, 1215. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dargahi, N.; Johnson, J.C.; Apostolopoulos, V. Immune Modulatory Effects of Probiotic Streptococcus thermophilus on Human Monocytes. Biologics 2021, 1, 396-415. https://doi.org/10.3390/biologics1030023
Dargahi N, Johnson JC, Apostolopoulos V. Immune Modulatory Effects of Probiotic Streptococcus thermophilus on Human Monocytes. Biologics. 2021; 1(3):396-415. https://doi.org/10.3390/biologics1030023
Chicago/Turabian StyleDargahi, Narges, Joshua C. Johnson, and Vasso Apostolopoulos. 2021. "Immune Modulatory Effects of Probiotic Streptococcus thermophilus on Human Monocytes" Biologics 1, no. 3: 396-415. https://doi.org/10.3390/biologics1030023
APA StyleDargahi, N., Johnson, J. C., & Apostolopoulos, V. (2021). Immune Modulatory Effects of Probiotic Streptococcus thermophilus on Human Monocytes. Biologics, 1(3), 396-415. https://doi.org/10.3390/biologics1030023






