Calcium Signaling Involves Na+/H+ Exchanger and IP3 Receptor Activation in T. cruzi Epimastigotes
Abstract
1. Introduction
2. Results
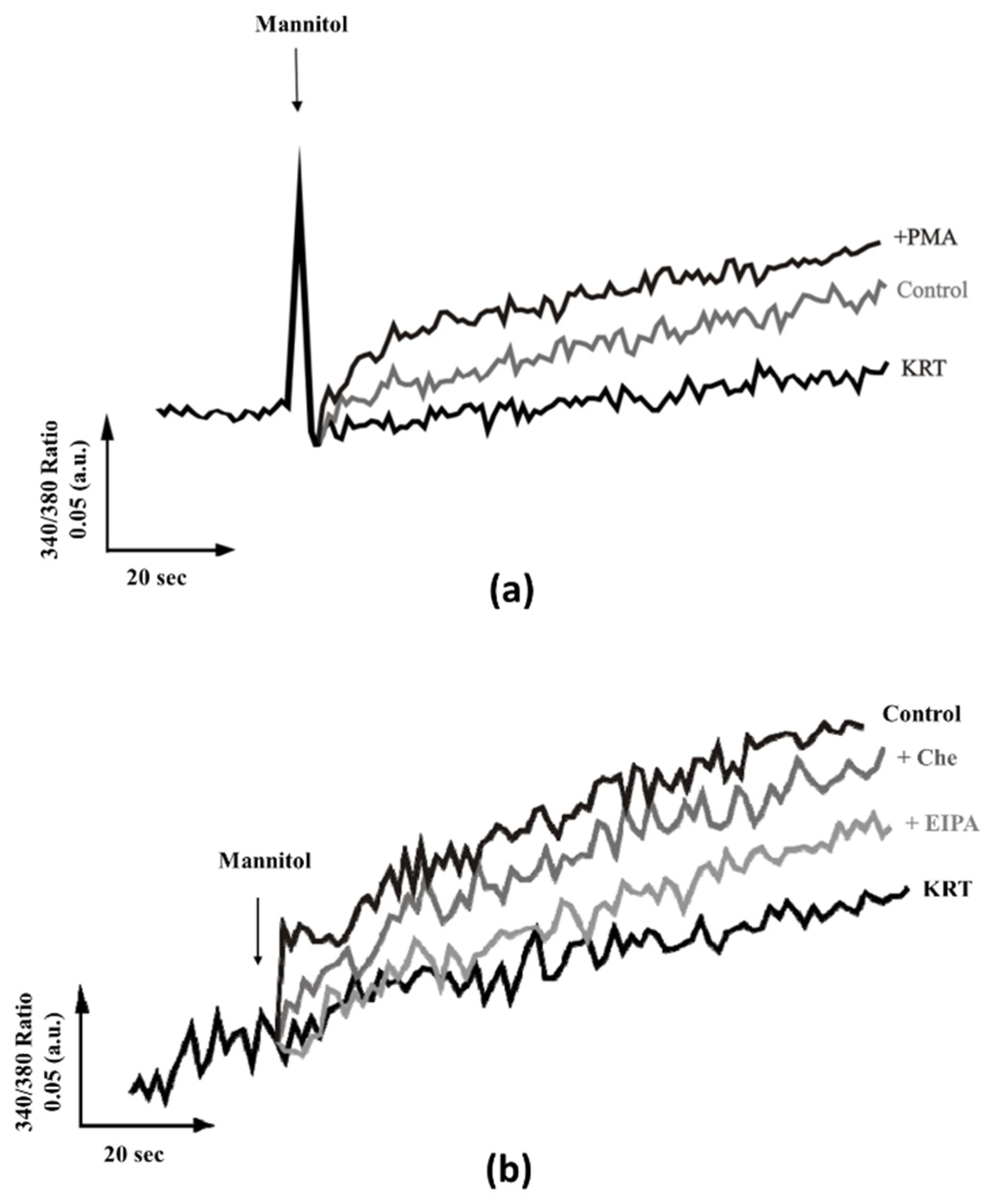
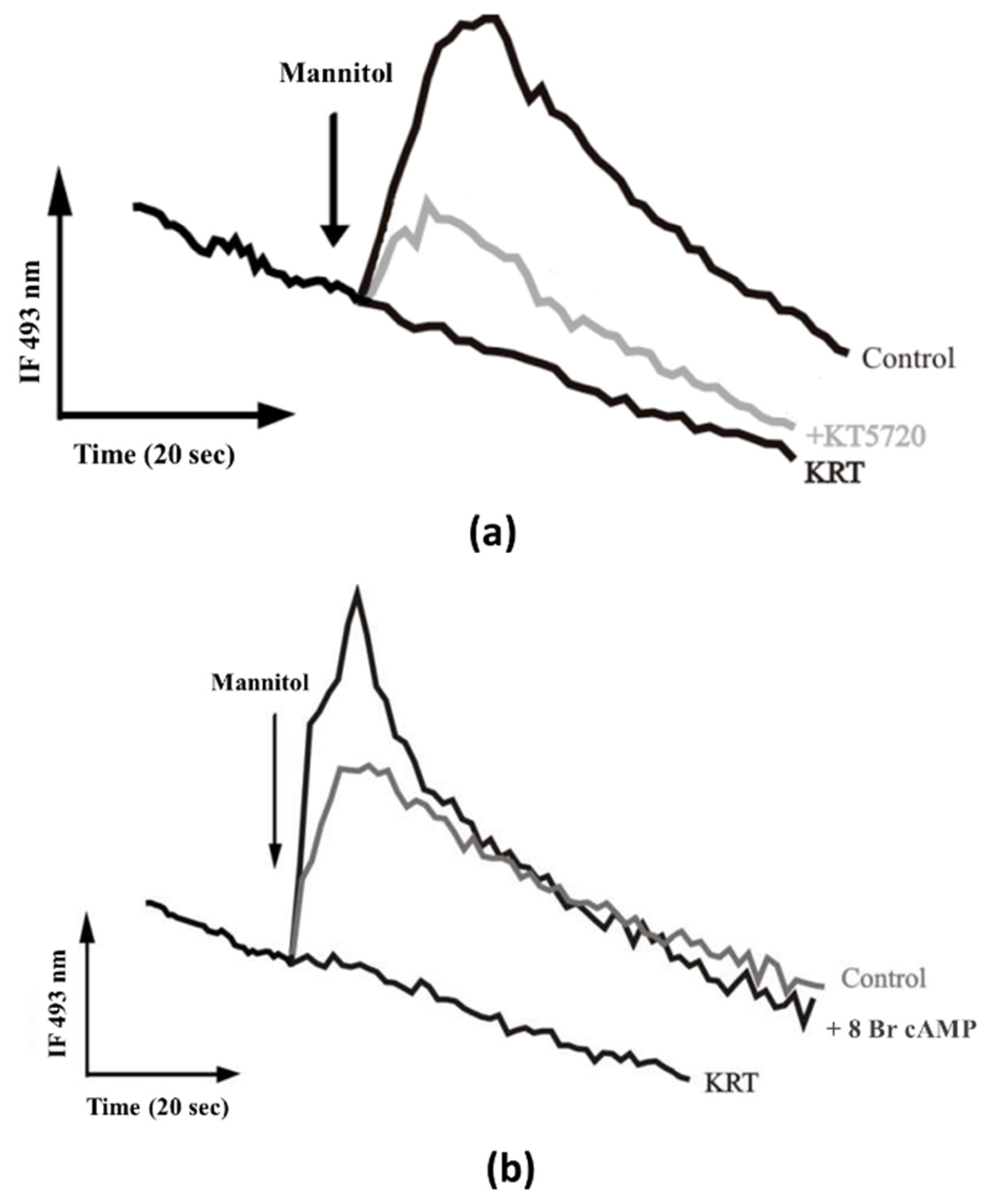
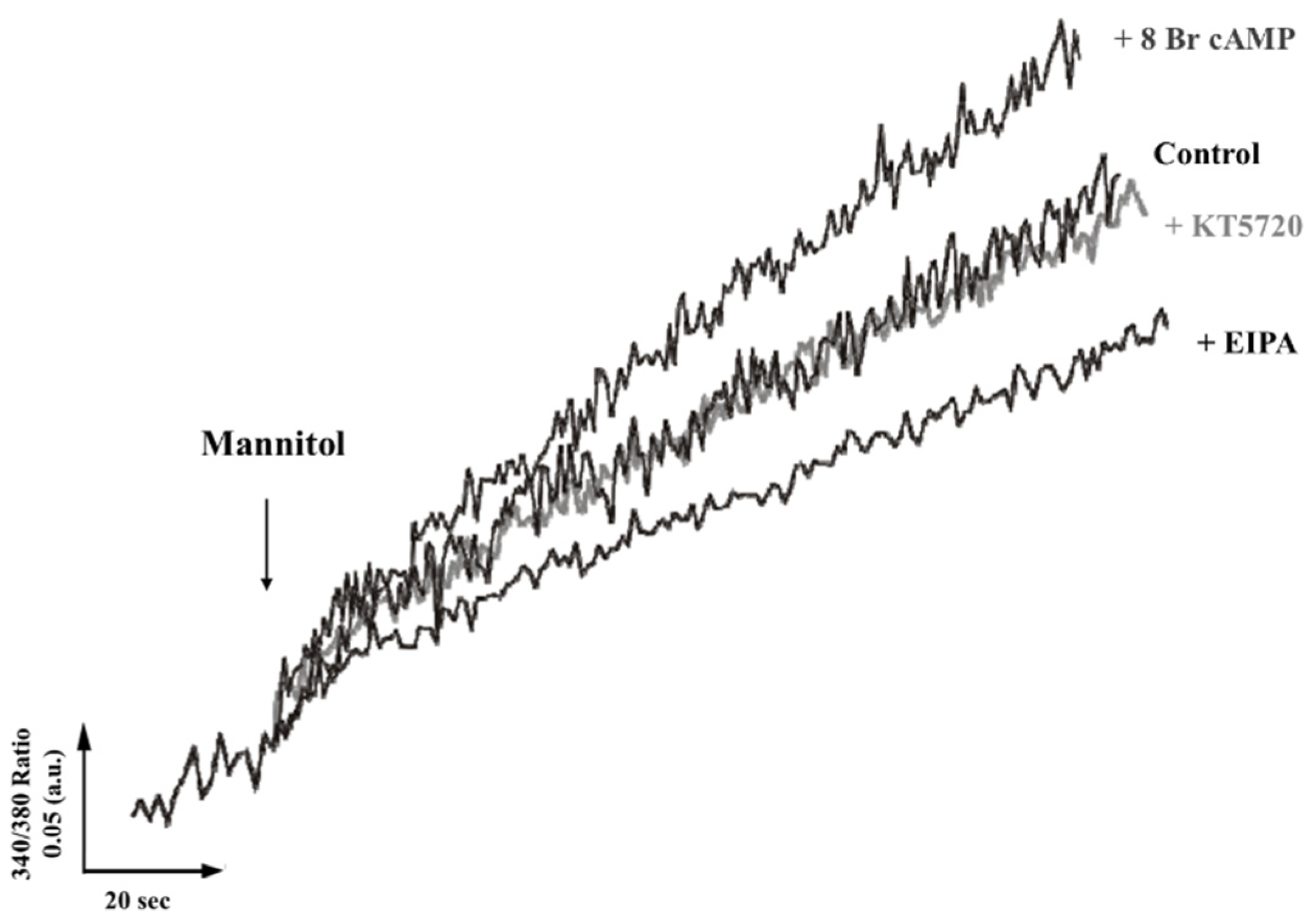
2.1. Mobilization of Intracellular Calcium through Activity of a Na+/H+ Exchanger Regulated by PKC and PKA
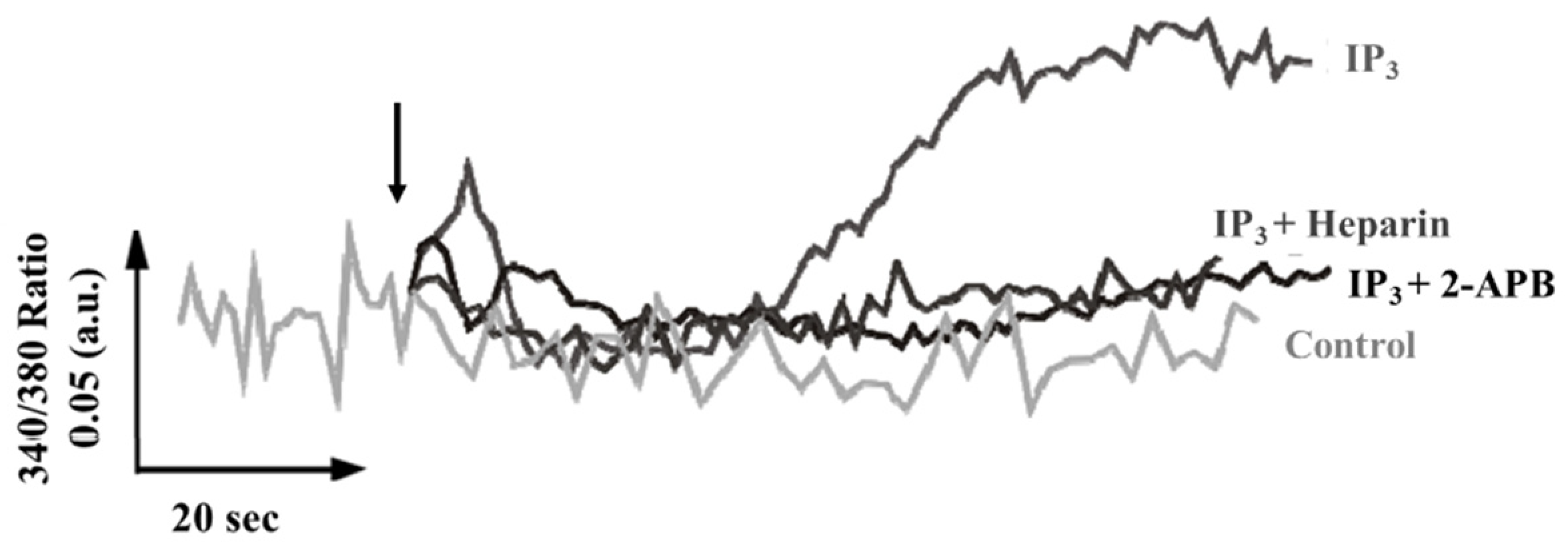
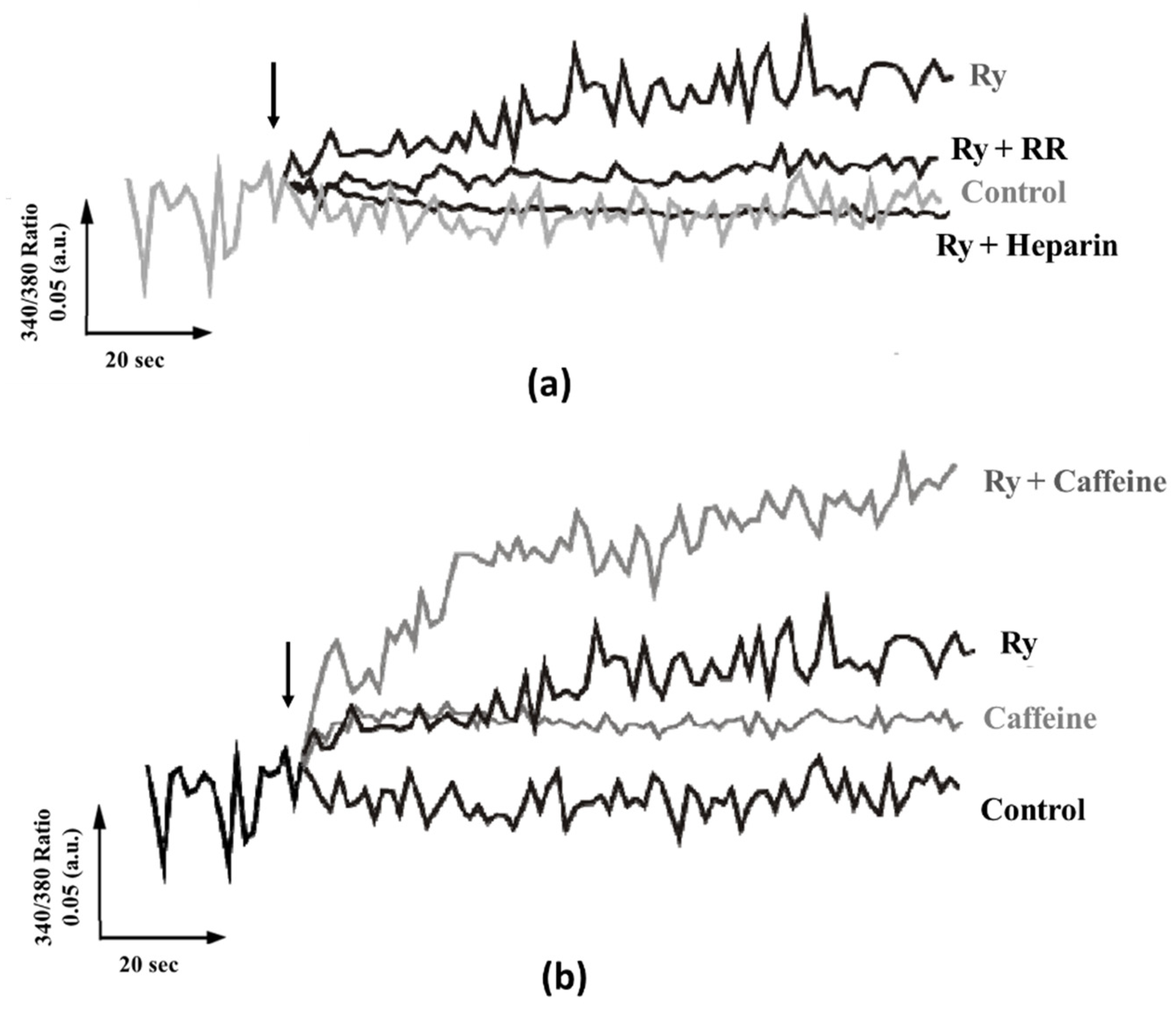
2.2. Calcium Release through Channels Belonging to the IP3/Ry Receptor Superfamily
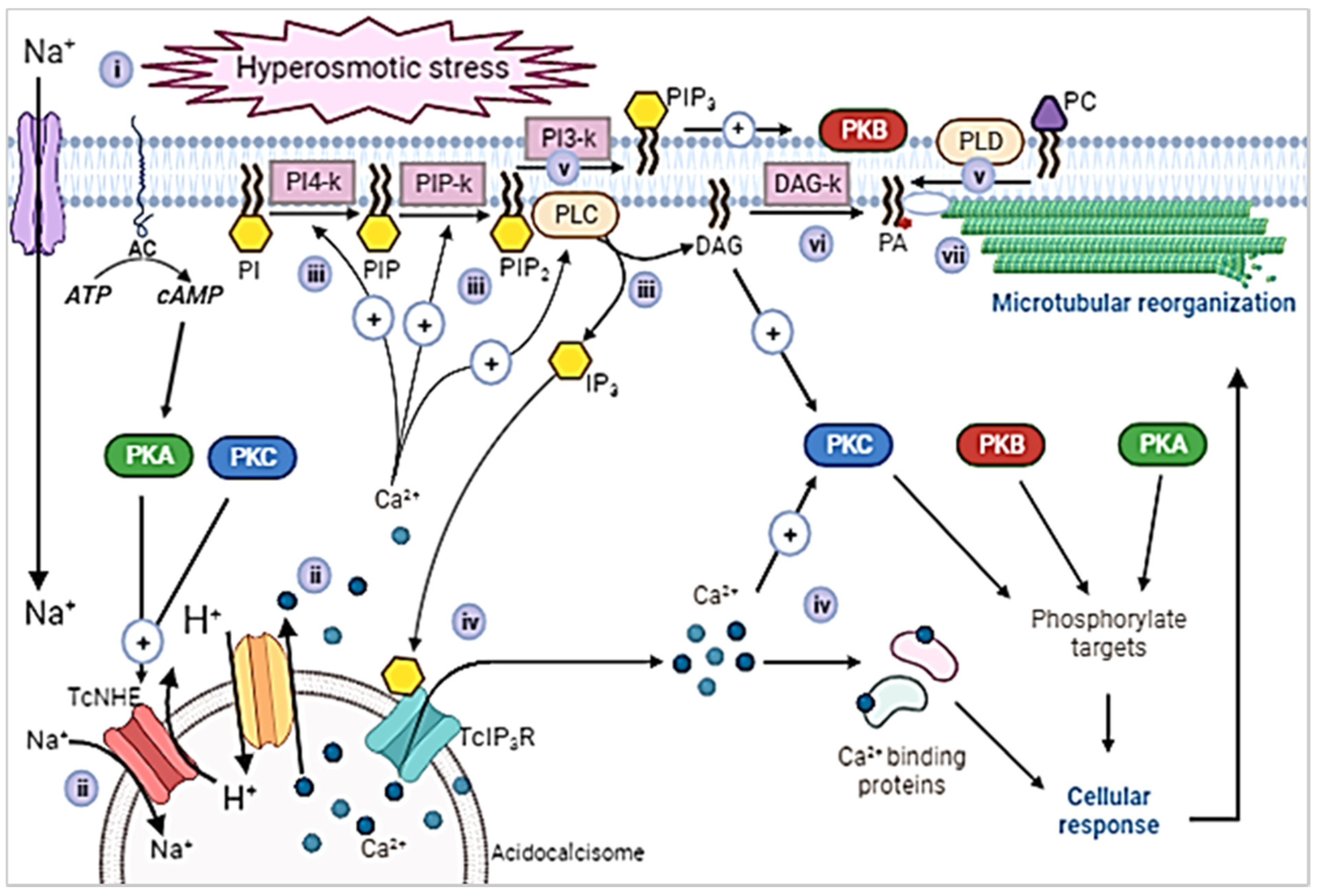
3. Discussion
- i.
- Hyperosmotic stress produced by urine presence activates TcNHE1 in acidocalcisomes, either by a cytosolic Na+ gradient or as part of the mechanism to regulate the cytoplasmatic pH [21]. The TcNHE1 activity could also be positively regulated by PKA, previously activated by cAMP produced by adenyl cyclase [34].
- ii.
- iii.
- The released Ca2+ acts as cofactor of lipid kinases whose activity is also stimulated by hyperosmotic stress [6,41]. Thus, the sequential activity of phosphoinositide kinases [5] leads to increment in the levels of phosphatidylinositol-4,5-bisphosphate (PIP2). Ca2+ also activates PLC, which uses PIP2 as a substrate to form DAG and IP3 [4].
- iv.
- The released IP3 binds to TcIP3R [7] present in acidocalcisomes [16], producing a second release of Ca2+ (this study, [20]). Subsequently, DAG and Ca2+ activate PKC [9] which triggers other signaling pathways [8]. Ca2+ also produces activation of other mechanisms that lead to cellular responses [28,33].
- v.
- In addition to being a substrate of PLC, the incremented levels of PIP2 could also act as a cofactor for phospholipase D (PLD) which would in turn increment the levels of phosphatidic acid (PA) (Santander et al., unpublished). Consistently with unpublished results and observations from our group, non-classic PLDs homologous were recently described in T. cruzi [43]. PIP2 can also be a substrate of TcPI3K, an enzyme that produces phosphatidylinositol-3,4,5-trisphosphate (PIP3) which could trigger the PKB/Akt signaling pathway [5].
- vi.
- DAG is also a substrate for DAGK, producing PA. DAGK activity is highly incremented in intermediate forms during epi- to trypomastigote differentiation under hyperosmotic stress conditions. In agreement, dephosphorylation of PA by the action of phosphatidate phosphatases (PAPs) is inhibited [41].
- vii.
- Incremented PA could act as part of the dynamic complex that binds microtubules to plasma membrane [41]. That binding would be required to maintain the parasite flagellum in an extended stage in intermediate forms [40], as part of the rearrangement that the subpellicular array of trypanosomes undergoes in morphological transitions [44]. In addition, PA could act as precursor of CDP-DAG, required to complete the PI cycle [45].
4. Materials and Methods
Author Contributions
Funding
Conflicts of Interest
References
- Berridge, M.J. Inositol trisphosphate and calcium signalling mechanisms. Biochim. Biophys. Acta 2009, 1793, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Scarpelli, P.H.; Pecenin, M.F.; Garcia, C.R.S. Intracellular Ca2+ Signaling in Protozoan Parasites: An Overview with a Focus on Mitochondria. Int. J. Mol. Sci. 2021, 22, 469. [Google Scholar] [CrossRef]
- Docampo, R.; Huang, G. Calcium signaling in trypanosomatid parasites. Cell Calcium. 2015, 57, 194–202. [Google Scholar] [CrossRef]
- Nozaki, T.; Toh-e, A.; Fujii, M.; Yagisawa, H.; Nakazawa, M.; Takeuchi, T. Cloning and characterization of a gene encoding phosphatidyl inositol-specific phospholipase C from Trypanosoma cruzi. Mol. Biochem. Parasitol. 1999, 102, 283–295. [Google Scholar] [CrossRef]
- Gimenez, A.M.; Gesumaria, M.C.; Schoijet, A.C.; Alonso, G.D.; Flawia, M.M.; Racagni, G.E.; Machado, E.E. Phosphatidylinositol kinase activities in Trypanosoma cruzi epimastigotes. Mol. Biochem. Parasitol. 2015, 203, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Santander, V.; Bollo, M.; Machado-Domenech, E. Lipid kinases and Ca2+ signaling in Trypanosoma cruzi stimulated by a synthetic peptide. Biochem. Biophys. Res. Commun. 2002, 293, 314–320. [Google Scholar] [CrossRef]
- Hashimoto, M.; Enomoto, M.; Morales, J.; Kurebayashi, N.; Sakurai, T.; Hashimoto, T.; Nara, T.; Mikoshiba, K. Inositol 1,4,5-trisphosphate receptor regulates replication, differentiation, infectivity and virulence of the parasitic protist Trypanosoma cruzi. Mol. Microbiol. 2013, 87, 1133–1150. [Google Scholar] [CrossRef] [PubMed]
- Belaunzaran, M.L.; Lammel, E.M.; Gimenez, G.; Wainszelbaum, M.J.; de Isola, E.L. Involvement of protein kinase C isoenzymes in Trypanosoma cruzi metacyclogenesis induced by oleic acid. Parasitol. Res. 2009, 105, 47–55. [Google Scholar] [CrossRef]
- Gomez, M.L.; Ochatt, C.M.; Kazanietz, M.G.; Torres, H.N.; Tellez-Inon, M.T. Biochemical and immunological studies of protein kinase C from Trypanosoma cruzi. Int. J. Parasitol. 1999, 29, 981–989. [Google Scholar] [CrossRef]
- Gomez, M.L.; Erijman, L.; Arauzo, S.; Torres, H.N.; Tellez-Inon, M.T. Protein kinase C in Trypanosoma cruzi epimastigote forms: Partial purification and characterization. Mol. Biochem. Parasitol. 1989, 36, 101–108. [Google Scholar] [CrossRef]
- D’Angelo, M.A.; Montagna, A.E.; Sanguineti, S.; Torres, H.N.; Flawia, M.M. A novel calcium-stimulated adenylyl cyclase from Trypanosoma cruzi, which interacts with the structural flagellar protein paraflagellar rod. J. Biol. Chem. 2002, 277, 35025–35034. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Inon, M.T.; Ulloa, R.M.; Torruella, M.; Torres, H.N. Calmodulin and Ca2+-dependent cyclic AMP phosphodiesterase activity in Trypanosoma cruzi. Mol. Biochem. Parasitol. 1985, 17, 143–153. [Google Scholar] [CrossRef]
- Moreno, S.N.; Docampo, R. The role of acidocalcisomes in parasitic protists. J. Eukaryot. Microbiol. 2009, 56, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Ulrich, P.N.; Storey, M.; Johnson, D.; Tischer, J.; Tovar, J.A.; Moreno, S.N.; Orlando, R.; Docampo, R. Proteomic analysis of the acidocalcisome, an organelle conserved from bacteria to human cells. PLoS Pathog. 2014, 10, e1004555. [Google Scholar] [CrossRef] [PubMed]
- Benaim, B.; Garcia, C.R. Targeting calcium homeostasis as the therapy of Chagas’ disease and leishmaniasis—A review. Trop Biomed. 2011, 28, 471–481. [Google Scholar]
- Lander, N.; Chiurillo, M.A.; Storey, M.; Vercesi, A.E.; Docampo, R. CRISPR/Cas9-mediated endogenous C-terminal Tagging of Trypanosoma cruzi Genes Reveals the Acidocalcisome Localization of the Inositol 1,4,5-Trisphosphate Receptor. J. Biol. Chem. 2016, 291, 25505–25515. [Google Scholar] [CrossRef] [PubMed]
- Lammel, E.M.; Barbieri, M.A.; Wilkowsky, S.E.; Bertini, F.; Isola, E.L. Trypanosoma cruzi: Involvement of intracellular calcium in multiplication and differentiation. Exp. Parasitol. 1996, 83, 240–249. [Google Scholar] [CrossRef]
- Bollo, M.; Venera, G.; de Jimenez Bonino, M.B.; Machado-Domenech, E. Binding of nicotinic ligands to and nicotine-induced calcium signaling in Trypanosoma cruzi. Biochem. Biophys. Res. Commun. 2001, 281, 300–304. [Google Scholar] [CrossRef]
- Bollo, M.; Bonansea, S.; Machado, E.E. Involvement of Na+/H+ exchanger in the calcium signaling in epimastigotes of Trypanosoma cruzi. FEBS Lett. 2006, 580, 2686–2690. [Google Scholar] [CrossRef]
- Marchesini, N.; Bollo, M.; Hernandez, G.; Garrido, M.N.; Machado-Domenech, E.E. Cellular signalling in Trypanosoma cruzi: Biphasic behaviour of inositol phosphate cycle components evoked by carbachol. Mol. Biochem. Parasitol. 2002, 120, 83–91. [Google Scholar] [CrossRef]
- Bonansea, S.; Usorach, M.; Gesumaria, M.C.; Santander, V.; Gimenez, A.M.; Bollo, M.; Machado, E.E. Stress response to high osmolarity in Trypanosoma cruzi epimastigotes. Arch. Biochem. Biophys. 2012, 527, 6–15. [Google Scholar] [CrossRef]
- Ulloa, R.M.; Mesri, E.; Esteva, M.; Torres, H.N.; Tellez-Inon, M.T. Cyclic AMP-dependent protein kinase activity in Trypanosoma cruzi. Biochem. J. 1988, 255, 319–326. [Google Scholar]
- Huang, H.; Werner, C.; Weiss, L.M.; Wittner, M.; Orr, G.A. Molecular cloning and expression of the catalytic subunit of protein kinase A from Trypanosoma cruzi. Int. J. Parasitol. 2002, 32, 1107–1115. [Google Scholar] [CrossRef]
- Huang, H.; Weiss, L.M.; Nagajyothi, F.; Tanowitz, H.B.; Wittner, M.; Orr, G.A.; Bao, Y. Molecular cloning and characterization of the protein kinase A regulatory subunit of Trypanosoma cruzi. Mol. Biochem. Parasitol. 2006, 149, 242–245. [Google Scholar] [CrossRef]
- Bao, Y.; Weiss, L.M.; Braunstein, V.L.; Huang, H. Role of protein kinase A in Trypanosoma cruzi. Infect. Immun. 2008, 76, 4757–4763. [Google Scholar] [CrossRef] [PubMed]
- Tagoe, D.N.; Kalejaiye, T.D.; de Koning, H.P. The ever unfolding story of cAMP signaling in trypanosomatids: Vive la difference! Front. Pharmacol. 2015, 6, 185. [Google Scholar] [CrossRef]
- Chiurillo, M.A.; Lander, N.; Vercesi, A.E.; Docampo, R. IP3 receptor-mediated Ca2+ release from acidocalcisomes regulates mitochondrial bioenergetics and prevents autophagy in Trypanosoma cruzi. Cell Calcium. 2020, 92, 102284. [Google Scholar] [CrossRef] [PubMed]
- Docampo, R.; Huang, G. The IP3 receptor and Ca2+ signaling in trypanosomes. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118947. [Google Scholar] [CrossRef]
- Mackrill, J.J. Ryanodine receptor calcium release channels: An evolutionary perspective. Adv. Exp. Med. Biol. 2012, 740, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Nass, R.; Rao, R. Novel localization of a Na+/H+ exchanger in a late endosomal compartment of yeast. Implications for vacuole biogenesis. J. Biol. Chem. 1998, 273, 21054–21060. [Google Scholar] [CrossRef]
- Wang, H.; Silva, N.L.; Lucchesi, P.A.; Haworth, R.; Wang, K.; Michalak, M.; Pelech, S.; Fliegel, L. Phosphorylation and regulation of the Na+/H+ exchanger through mitogen-activated protein kinase. Biochemistry 1997, 36, 9151–9158. [Google Scholar] [CrossRef] [PubMed]
- Wiederkehr, M.R.; Zhao, H.; Moe, O.W. Acute regulation of Na/H exchanger NHE3 activity by protein kinase C: Role of NHE3 phosphorylation. Am. J. Physiol. 1999, 276, C1205–C1217. [Google Scholar] [CrossRef] [PubMed]
- Schoijet, A.C.; Sternlieb, T.; Alonso, G.D. Signal Transduction Pathways as Therapeutic Target for Chagas Disease. Curr. Med. Chem. 2019, 26, 6572–6589. [Google Scholar] [CrossRef]
- Shemarova, I.V. cAMP-dependent signal pathways in unicellular eukaryotes. Crit. Rev. Microbiol. 2009, 35, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Jager, A.V.; De Gaudenzi, J.G.; Mild, J.G.; Mc Cormack, B.; Pantano, S.; Altschuler, D.L.; Edreira, M.M. Identification of novel cyclic nucleotide binding proteins in Trypanosoma cruzi. Mol. Biochem. Parasitol. 2014, 198, 104–112. [Google Scholar] [CrossRef]
- Ozawa, T. Modulation of ryanodine receptor Ca2+ channels (Review). Mol. Med. Rep. 2010, 3, 199–204. [Google Scholar] [CrossRef]
- Lovett, J.L.; Marchesini, N.; Moreno, S.N.; Sibley, L.D. Toxoplasma gondii microneme secretion involves intracellular Ca2+ release from inositol 1,4,5-triphosphate (IP(3))/ryanodine-sensitive stores. J. Biol. Chem. 2002, 277, 25870–25876. [Google Scholar] [CrossRef]
- Prole, D.L.; Taylor, C.W. Identification of intracellular and plasma membrane calcium channel homologues in pathogenic parasites. PLoS ONE 2011, 6, e26218. [Google Scholar] [CrossRef]
- Benaim, G.; Paniz-Mondolfi, A.E.; Sordillo, E.M.; Martinez-Sotillo, N. Disruption of Intracellular Calcium Homeostasis as a Therapeutic Target against Trypanosoma cruzi. Front. Cell Infect. Microbiol. 2020, 10, 46. [Google Scholar] [CrossRef]
- Gimenez, A.M.; Machado, E.E. Trypanosoma cruzi: Morphological and microtubular changes related to epimastigote differentiation under hyperosmotic stress. SOJ Microbiol. Infect. Dis. 2016, 4, 1–6. [Google Scholar] [CrossRef]
- Gimenez, A.M.; Santander, V.S.; Villasuso, A.L.; Pasquare, S.J.; Giusto, N.M.; Machado, E.E. Regulation of phosphatidic acid levels in Trypanosoma cruzi. Lipids 2011, 46, 969–979. [Google Scholar] [CrossRef]
- Vercesi, A.E.; Docampo, R. Sodium-proton exchange stimulates Ca2+ release from acidocalcisomes of Trypanosoma brucei. Biochem. J. 1996, 315 Pt 1, 265–270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Plonski, N.M.; Bissoni, B.; Arachchilage, M.H.; Romstedt, K.; Kooijman, E.E.; Piontkivska, H. Shedding light on lipid metabolism in Kinetoplastida: A phylogenetic analysis of phospholipase D protein homologs. Gene 2018, 656, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.N.; de Graffenried, C.L. More than Microtubules: The Structure and Function of the Subpellicular Array in Trypanosomatids. Trends Parasitol. 2019, 35, 760–777. [Google Scholar] [CrossRef]
- Booth, L.A.; Smith, T.K. Lipid metabolism in Trypanosoma cruzi: A review. Mol. Biochem. Parasitol. 2020, 240, 111324. [Google Scholar] [CrossRef] [PubMed]
- Machado de Domenech, E.E.; Garcia, M.; Garrido, M.N.; Racagni, G. Phospholipids of Trypanosoma cruzi: Increase of polyphosphoinositides and phosphatidic acid after cholinergic stimulation. FEMS Microbiol. Lett. 1992, 74, 267–270. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Saleem, H.; Tovey, S.C.; Molinski, T.F.; Taylor, C.W. Interactions of antagonists with subtypes of inositol 1,4,5-trisphosphate (IP3) receptor. Br. J. Pharmacol. 2014, 171, 3298–3312. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usorach, M.; Gimenez, A.M.; Peppino Margutti, M.; Racagni, G.E.; Machado, E.E. Calcium Signaling Involves Na+/H+ Exchanger and IP3 Receptor Activation in T. cruzi Epimastigotes. Biologics 2021, 1, 384-395. https://doi.org/10.3390/biologics1030022
Usorach M, Gimenez AM, Peppino Margutti M, Racagni GE, Machado EE. Calcium Signaling Involves Na+/H+ Exchanger and IP3 Receptor Activation in T. cruzi Epimastigotes. Biologics. 2021; 1(3):384-395. https://doi.org/10.3390/biologics1030022
Chicago/Turabian StyleUsorach, Melina, Alba Marina Gimenez, Micaela Peppino Margutti, Graciela E. Racagni, and Estela E. Machado. 2021. "Calcium Signaling Involves Na+/H+ Exchanger and IP3 Receptor Activation in T. cruzi Epimastigotes" Biologics 1, no. 3: 384-395. https://doi.org/10.3390/biologics1030022
APA StyleUsorach, M., Gimenez, A. M., Peppino Margutti, M., Racagni, G. E., & Machado, E. E. (2021). Calcium Signaling Involves Na+/H+ Exchanger and IP3 Receptor Activation in T. cruzi Epimastigotes. Biologics, 1(3), 384-395. https://doi.org/10.3390/biologics1030022






