Is the Gut Microbiome a Target for Adjuvant Treatment of COVID-19?
Abstract
:1. Introduction
2. Gastrointestinal Symptoms Caused by SARS-CoV-2
3. Detection of SARS-CoV-2 Infection of the Gastrointestinal Tract via Swabs or Stool Samples
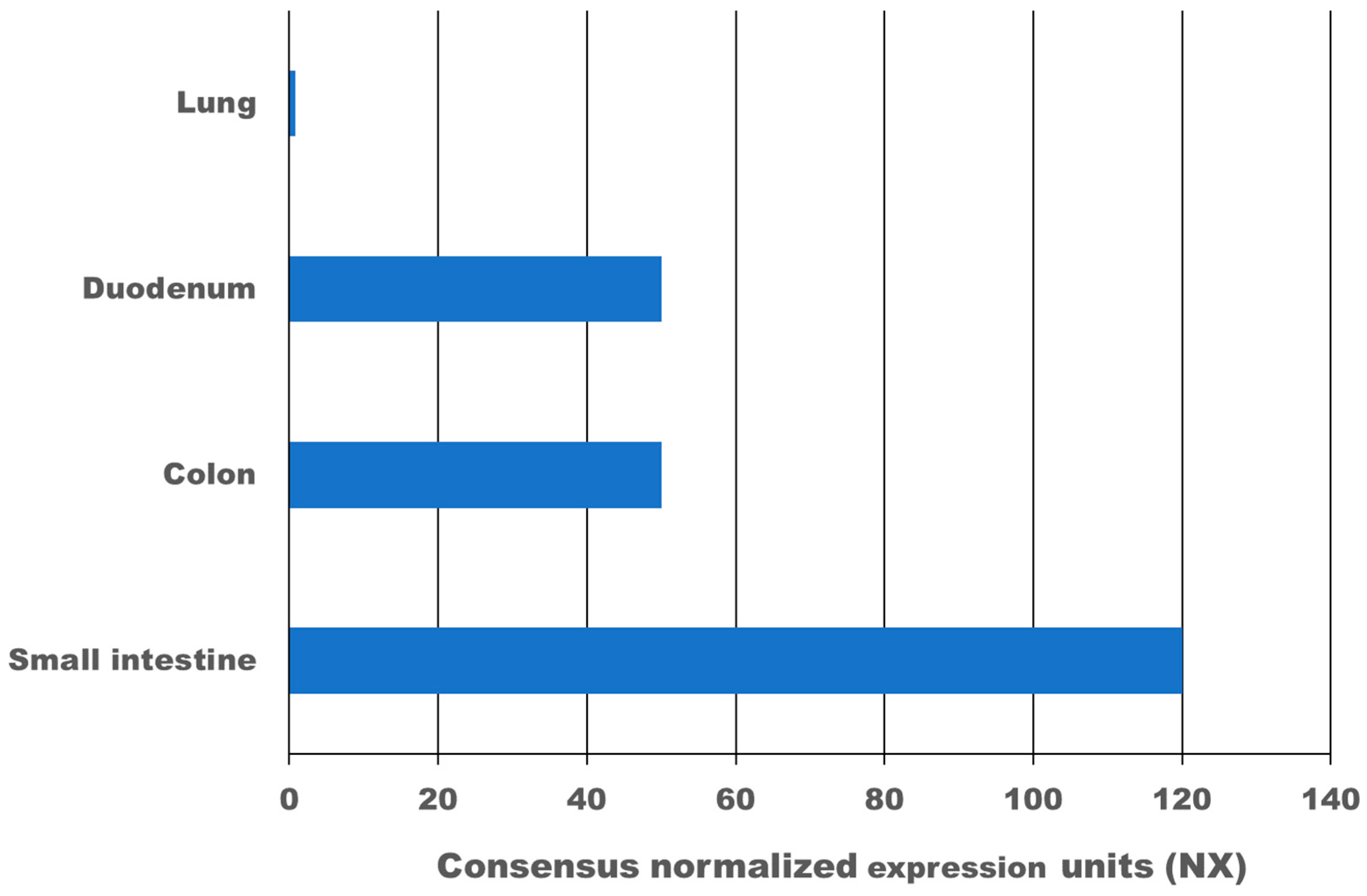
4. Expression of ACE2 and TMPRSS2 in the Gastrointestinal Tract
5. Faecal Transmission of SARS-CoV-2
6. Gut Inflammation in COVID-19 Patients
7. SARS-CoV-2 and the Gut Microbiome
8. Targeting the Gut Microbiome as Adjunctive Therapy for COVID-19
9. Is There a Link between Changes in the Gut Microbiome in COVID-19 Patients and Chronic COVID-19 Symptoms?
10. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Hilpert, K.; Mikut, R. Is there a connection between gut microbiome dysbiosis occurring in COVID-19 patients and post-COVID-19 symptoms? Front. Microbiol. 2021, 12, 2564. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. In Coronaviruses Methods and Protocols; Springer: New York, NY, USA, 2015; pp. 1–23. [Google Scholar]
- Wood, E.N. An apparently new syndrome of porcine epidemic diarrhoea. Veter. Rec. 1977, 100, 243–244. [Google Scholar] [CrossRef]
- Jung, K.; Annamalai, T.; Lu, Z.; Saif, L.J. Comparative pathogenesis of US porcine epidemic diarrhea virus (PEDV) strain PC21A in conventional 9-day-old nursing piglets vs. 26-day-old weaned pigs. Veter. Microbiol. 2015, 178, 31–40. [Google Scholar] [CrossRef]
- Saif, L.J.; Wang, Q.; Vlasova, A.N.; Jung, K.; Xiao, S. Coronaviruses. In Diseases of Swine; Wiley: Hoboken, NJ, USA, 2019; pp. 488–523. [Google Scholar]
- Okur, G.S.; Yazici, Z.; Albayrak, H.; Meral, Y. Rotavirus and Coronavirus Prevalances in Healthy Calves and Calves with Diarrhoea. Available online: https://www.researchgate.net/publication/287890228 (accessed on 29 April 2020).
- Rehman, S.U.; Shafique, L.; Ihsan, A.; Liu, Q. Evolutionary trajectory for the emergence of novel coronavirus SARS-CoV-2. Pathogens 2020, 9, 240. Available online: https://www.mdpi.com/2076-0817/9/3/240 (accessed on 28 April 2020). [CrossRef] [Green Version]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.-R.; Liu, J.; Liao, Z.-G.; Zhou, J.; Peng, H.-W.; Gong, F.; Hu, J.-F.; Zhou, Y. COVID-19 and gastroenteric manifestations. World J. Clin. Cases 2021, 9, 4990–4997. [Google Scholar] [CrossRef]
- Perisetti, A.; Goyal, H.; Gajendran, M.; Boregowda, U.; Mann, R.; Sharma, N. Prevalence, mechanisms, and implications of gastrointestinal symptoms in COVID-19. Front. Med. 2020, 7, 588711. [Google Scholar] [CrossRef]
- Fang, D.; Ma, J.; Guang, J.; Wang, M.; Song, Y.; Tian, D. Manifestations of digestive system in hospitalized patients with novel coronavirus pneumonia in Wuhan, China: A single-center, descriptive study. Chin. J. Dig. 2020, 40, E005. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Tian, Y.; Rong, L.; Nian, W.; He, Y. Review article: Gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment. Pharmacol. Ther. 2020, 51, 843–851. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Kumar, V.C.S.; Mukherjee, S.; Harne, P.S.; Subedi, A.; Ganapathy, M.K.; Patthipati, V.S.; Sapkota, B. Novelty in the gut: A systematic review and meta-analysis of the gastrointestinal manifestations of COVID-19. BMJ Open Gastroenterol. 2020, 7, 417. Available online: http://bmjopengastro.bmj.com/ (accessed on 5 October 2020).
- Cheung, K.S.; Hung, I.F.; Chan, P.P.; Lung, K.C.; Tso, E.; Liu, R.; Ng, Y.Y.; Chu, M.Y.; Chung, T.W.; Tam, A.R.; et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology 2020, 159, 81–95. [Google Scholar] [CrossRef]
- Parasa, S.; Desai, M.; Chandrasekar, V.T.; Patel, H.K.; Kennedy, K.F.; Roesch, T.; Spadaccini, M.; Colombo, M.; Gabbiadini, R.; Artifon, E.L.; et al. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: A systematic review and meta-analysis. JAMA Netw. Open 2020, 3, e2011335. Available online: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2767009 (accessed on 27 August 2020). [CrossRef]
- Rokkas, T. Gastrointestinal involvement in COVID-19: A systematic review and meta-analysis. Ann. Gastroenterol. 2020, 33, 355–365. [Google Scholar] [CrossRef]
- Dorrell, R.D.; Dougherty, M.K.; Barash, E.L.; Lichtig, A.E.; Clayton, S.B.; Jensen, E.T. Gastrointestinal and hepatic manifestations of COVID-19: A systematic review and meta-analysis. JGH Open 2021, 5, 107–115. [Google Scholar] [CrossRef]
- Silva, F.A.F.D.; Brito, B.B.D.; Santos, M.L.C.; Marques, H.S.; Silva, R.T.D.; Carvalho, L.S.D.; Vieira, E.S.; Oliveira, M.V.; Melo, F.F.D. COVID-19 gastrointestinal manifestations: A systematic review. Rev. Soc. Bras. Med. Trop. 2020, 53, 1–11. [Google Scholar] [CrossRef]
- Zhang, W.; Du, R.-H.; Li, B.; Zheng, X.-S.; Yang, X.-L.; Hu, B.; Wang, Y.-Y.; Xiao, G.-F.; Yan, B.; Shi, Z.-L.; et al. Molecular and serological investigation of 2019-nCoV infected patients: Implication of multiple shedding routes. Emerg. Microbes Infect. 2020, 9, 386–389. [Google Scholar] [CrossRef] [Green Version]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020, 6, 1831–1833. [Google Scholar] [CrossRef]
- Lo, I.L.; Lio, C.F.; Cheong, H.H.; Lei, C.I.; Cheong, T.H.; Zhong, X.; Tian, Y.; Sin, N.N. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int. J. Biol. Sci. 2020, 16, 1698–1707. Available online: http://www.ijbs.com//creativecommons.org/licenses/by/4.0/ (accessed on 3 September 2020). [CrossRef] [Green Version]
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Deng, Q.; Zhang, G.; Wu, K.; Ni, L.; Yang, Y.; Liu, B.; Wang, W.; Wei, C.; et al. The presence of SARS-CoV-2 RNA in feces of COVID-19 patients. J Med. Virol. 2020, 92, 833–840. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, C.; Song, Y.; Zhu, S.; Wang, D.; Zhang, H.; Han, G.; Weng, Y.; Xu, J.; Xu, J.; et al. Excretion of SARS-CoV-2 through faecal specimens. Emerg. Microbes. Infect. 2020, 9, 2501–2508. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA J. Am. Med. Assoc. 2020, 323, 1843–1844. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Jiang, X.; Zhang, Z.; Huang, S.; Zhang, Z.; Fang, Z.; Gu, Z.; Gao, L.; Shi, H.; Mai, L.; et al. Gut immunity gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020, 69, 997–1001. Available online: http://gut.bmj.com/ (accessed on 4 May 2020). [CrossRef] [PubMed]
- Zhang, N.; Gong, Y.; Meng, F.; Bi, Y.; Yang, P. Virus Shedding Patterns in Nasopharyngeal and Fecal Specimens of COVID-19 Patients. Sci. China Life Sci. 2021, 64, 486–488. Available online: https://doi.org/10.1101/2020.03.28.20043059 (accessed on 12 May 2020). [CrossRef] [PubMed]
- Jiang, X.; Luo, M.; Zou, Z.; Wang, X.; Chen, C.; Qiu, J. Asymptomatic SARS-CoV-2 infected case with viral detection positive in stool but negative in nasopharyngeal samples lasts for 42 days. J. Med. Virol. 2020, 92, 1807–1809. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Zhu, B.; Liang, H.; Fang, C.; Gong, Y.; Guo, Q.; Sun, X.; Zhao, D.; Shen, J.; et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020, 26, 502–505. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Cui, X.; Zhao, X.; Wang, J.; Zheng, J.; Zheng, G.; Guo, W.; Cai, C.; He, S.; Xu, Y. Detectable SARS-CoV-2 viral RNA in feces of three children during recovery period of COVID-19 pneumonia. J. Med. Virol. 2020, 92, 909–914. [Google Scholar] [CrossRef]
- Byrne, A.W.; McEvoy, D.; Collins, A.B.; Hunt, K.; Casey, M.; Barber, A.; Butler, F.; Griffin, J.; Lane, E.A.; McAloon, C.; et al. Inferred duration of infectious period of SARS-CoV-2: Rapid scoping review and analysis of available evidence for asymptomatic and symptomatic COVID-19 cases. BMJ Open 2020, 10, e039856. Available online: http://bmjopen.bmj.com/ (accessed on 3 September 2020). [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. Available online: https://pubmed.ncbi.nlm.nih.gov/32142651/ (accessed on 14 June 2021). [CrossRef]
- Hikmet, F.; Méar, L.; Edvinsson, Å.; Micke, P.; Uhlén, M.; Lindskog, C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020, 16, e9610. [Google Scholar] [CrossRef]
- TMPRSS2 Gene—GeneCards. TMPS2 Protein TMPS2 Antibody. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=TMPRSS2 (accessed on 9 September 2021).
- Zhang, H.; Kang, Z.; Gong, H.; Xu, D.; Wang, J.; Li, Z.; Li, Z.; Cui, X.; Xiao, J.; Zhan, J.; et al. Digestive system is a potential route of COVID-19: An analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut 2020, 69, 1010–1018. [Google Scholar] [CrossRef]
- Horrocks, W.H. Experiments made to determine the conditions under which “specific” bacteria derived from sewage may be present in the air of ventilating pipes, drains, inspection chambers, and sewers. In Proceedings of the Royal Society of London. Series B, Containing Papers of a Biological Character; The Royal Society: London, UK, 1907; Volume 79, pp. 255–266. [Google Scholar]
- Jessen, C.U. Airborne Microorganisms: Occurrance and Control; GEC Gad: Copenhagen, Denmark, 1955. [Google Scholar]
- Wells, W.F. On air-borne infection: Study II. Droplets and droplet nuclei. Am. J. Epidemiol. 1934, 20, 611–618. [Google Scholar] [CrossRef]
- Darlow, H.M.; Bale, W.R. Infective hazards of water-closets. Lancet 1959, 273, 1196–1200. [Google Scholar] [CrossRef]
- Blair, M. Ceramic Water Closets; Bloomsbury Publishing: New York, NY, USA, 2008. [Google Scholar]
- Gerba, C.P.; Wallis, C.; Melnick, J.L. Microbiological hazards of household toilets: Droplet production and the fate of residual organisms. Appl. Microbiol. 1975, 30, 229–237. [Google Scholar] [CrossRef]
- Barker, J.; Vipond, I.; Bloomfield, S. Effects of cleaning and disinfection in reducing the spread of Norovirus contamination via. environmental surfaces. J. Hosp. Infect. 2004, 58, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.; Jones, M.V. The potential spread of infection caused by aerosol contamination of surfaces after flushing a domestic toilet. J. Appl. Microbiol. 2005, 99, 339–347. [Google Scholar] [CrossRef]
- Best, E.; Sandoe, J.; Wilcox, M. Potential for aerosolization of Clostridium difficile after flushing toilets: The role of toilet lids in reducing environmental contamination risk. J. Hosp. Infect. 2012, 80, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Newsom, S.W.B. Microbiology of hospital toilets. Lancet 1972, 300, 700–703. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.; Zhu, S.; Shu, C.; Wang, D.; Song, J.; Song, Y.; Zhen, W.; Feng, Z.; Wu, G.; et al. Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19). China CDC Wkly. 2020, 2, 123–124. [Google Scholar] [CrossRef]
- Xu, K.; Cai, H.; Shen, Y.; Ni, Q.; Chen, Y.; Hu, S.; Li, J.; Wang, H.; Yu, L.; Huang, H.; et al. Management of corona virus disease-19 (COVID-19): The Zhejiang experience. J. Zhejiang Univ. 2020, 49, 147–157. [Google Scholar]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Xiao, A.; Zhang, J.; Gu, X.; Lee, W.L.; Kauffman, K.; Hanage, W.; Matus, M.; Ghaeli, N.; Endo, N. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. medRxiv 2020, 5, e00614–e00620. [Google Scholar] [CrossRef]
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D.; et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 2020, 582, 557–560. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Lloyd-Smith, J.O.; Munster, V.J.; Bushmaker, T.; Morris, D.H.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; et al. Aerosol and surface stability of HCoV-19 (SARS-CoV-2) compared to SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. Available online: https://www.nejm.org/doi/10.1056/NEJMc2004973 (accessed on 17 April 2020). [CrossRef] [PubMed]
- Ong, S.W.X.; Tan, Y.K.; Chia, P.Y.; Lee, T.H.; Ng, O.T.; Wong, M.S.Y.; Marimuthu, K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA J. Am. Med. Assoc. 2020, 323, 1610–1612. Available online: https://jamanetwork.com/journals/jama/fullarticle/2762692 (accessed on 28 April 2020). [CrossRef] [PubMed] [Green Version]
- Yu, I.T.; Li, Y.; Wong, T.W.; Tam, W.; Chan, A.; Lee, J.H.; Leung, D.Y.; Ho, T. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N. Engl. J. Med. 2004, 350, 1731–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.; Wei, J.; Yuan, J.; Guo, J.; Zhang, Y.; Hang, J.; Qu, Y.; Qian, H.; Zhuang, Y.; Chen, X.; et al. Probable evidence of fecal aerosol transmission of SARS-CoV-2 in a high-rise building. Ann. Intern. Med. 2020, 173, 974–980. [Google Scholar] [CrossRef]
- Effenberger, M.; Grabherr, F.; Mayr, L.; Schwaerzler, J.; Nairz, M.; Seifert, M.; Hilbe, R.; Seiwald, S.; Scholl-Buergi, S.; Fritsche, G.; et al. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut 2020, 69, 1543–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuken, P.A.; Wüst, M.; Löffler, B.; Bauer, M.; Stallmach, A. Letter: SARS-CoV-2-induced gastrointestinal inflammation. Aliment. Pharmacol. Ther. 2020, 52, 1748–1749. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7753696/pdf/APT-52-1750.pdf (accessed on 14 June 2021).
- Oliva, A.; Miele, M.C.; Di Timoteo, F.; De Angelis, M.; Mauro, V.; Aronica, R.; Al Ismail, D.; Ceccarelli, G.; Pinacchio, C.; D’Ettorre, G.; et al. Persistent systemic microbial translocation and intestinal damage during coronavirus disease-19. Front. Immunol. 2021, 12, 2810. [Google Scholar] [CrossRef]
- Prasad, R.; Patton, M.J.; Floyd, J.L.; Vieira, C.P.; Fortmann, S.; DuPont, M.; Harbour, A.; See, J.R.C.; Wright, J.; Lamendella, R.; et al. Plasma microbiome in COVID-19 subjects: An indicator of gut barrier defects and dysbiosis. bioRxiv 2021. Available online: https://www.biorxiv.org/content/10.1101/2021.04.06.438634v1 (accessed on 9 September 2021).
- Tsitsiklis, A.; Shoshana, Z.B.; Byrne, A.; Devoe, C.; Levan, S.; Rackaityte, E.; Sunshine, S.; Mick, E.; Ghale, R.; Jauregui, A.; et al. Impaired immune signaling and changes in the lung microbiome precede secondary 1 bacterial pneumonia in COVID-19. Res. Sq. 2021, 1, rs-3. [Google Scholar]
- Gaibani, P.; Viciani, E.; Bartoletti, M.; Lewis, R.E.; Tonetti, T.; Lombardo, D.; Castagnetti, A.; Bovo, F.; Horna, C.S.; Ranieri, M.; et al. The lower respiratory tract microbiome of critically ill patients with COVID-19. Sci. Rep. 2021, 11, 10103. [Google Scholar] [CrossRef] [PubMed]
- Haiminen, N.; Utro, F.; Seabolt, E.; Parida, L. Functional profiling of COVID-19 respiratory tract microbiomes. Sci. Rep. 2021, 11, 6433. [Google Scholar] [CrossRef] [PubMed]
- Keely, S.; Talley, N.J.; Hansbro, P.M. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 2012, 5, 7–18. Available online: www.nature.com/mi (accessed on 3 September 2020). [CrossRef] [Green Version]
- Dumas, A.; Bernard-Raichon, L.; Poquet, Y.; Lugo, G.; Neyrolles, O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell. Microbiol. 2018, 20, e12966. [Google Scholar] [CrossRef] [Green Version]
- Dhar, D.; Mohanty, A. Gut microbiota and Covid-19 possible link and implications. Virus Res. 2020, 285, 198018. [Google Scholar] [CrossRef]
- Baud, D.; Dimopoulou Agri, V.; Gibson, G.R.; Reid, G.; Giannoni, E. Using probiotics to flatten the curve of coronavirus disease COVID-2019 pandemic. Front. Public Health 2020, 8, 186. [Google Scholar] [CrossRef]
- Ebrahimi, K.H. SARS-CoV-2 spike glycoprotein-binding proteins expressed by upper respiratory tract bacteria may prevent severe viral infection. FEBS Lett. 2020, 594, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Dysbiosis—Wikipedia. Available online: https://en.wikipedia.org/wiki/Dysbiosis (accessed on 9 September 2021).
- Zuo, T.; Zhang, F.; Lui, G.C.; Yeoh, Y.K.; Li, A.Y.; Zhan, H.; Wan, Y.; Chung, A.C.; Cheung, C.P.; Chen, N.; et al. Alterations in gut microbiota of patients with covid-19 during time of hospitalization. Gastroenterology 2020, 159, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Chen, Y.; Wu, Z.; Chen, Y.; Gao, H.; Lv, L.; Guo, F.; Zhang, X.; Luo, R.; Huang, C.; et al. Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin. Infect. Dis. 2020, 71, 2669–2678. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Zhan, H.; Zhang, F.; Liu, Q.; Tso, E.Y.; Lui, G.C.; Chen, N.; Li, A.; Lu, W.; Chan, F.K.; et al. Alterations in fecal fungal microbiome of patients with covid-19 during time of hospitalization until discharge. Gastroenterology 2020, 159, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Wang, H.; Cui, G.; Lu, H.; Wang, L.; Luo, H.; Chen, X.; Ren, H.; Sun, R.; Liu, W.; et al. Alterations in the human oral and gut microbiomes and lipidomics in COVID-19. Gut 2021, 70, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Gou, W.; Fu, Y.; Yue, L.; Chen, G.-D.; Cai, X.; Shuai, M.; Xu, F.; Yi, X.; Chen, H.; Zhu, Y.; et al. Gut microbiota, inflammation and molecular signatures of host response to infection. J. Genet. Genom. 2021, in press. [Google Scholar] [CrossRef]
- Vighi, G.; Marcucci, F.; Sensi, L.; DI Cara, G.; Frati, F. Allergy and the gastrointestinal system. Clin. Exp. Immunol. 2008, 153, 3–6. [Google Scholar] [CrossRef]
- Hummel, S.; Veltman, K.; Cichon, C.; Sonnenborn, U.; Schmidt, M.A. Differential targeting of the E-cadherin/β-catenin complex by gram-positive probiotic lactobacilli improves epithelial barrier function. Appl. Environ. Microbiol. 2012, 78, 1140–1147. [Google Scholar] [CrossRef] [Green Version]
- Zelaya, H.; Alvarez, S.; Kitazawa, H.; Villena, J. Respiratory antiviral immunity and immunobiotics: Beneficial effects on inflammation-coagulation interaction during influenza virus infection. Front. Immunol. 2016, 7, 633. [Google Scholar] [CrossRef] [Green Version]
- D’Ettorre, G.; Ceccarelli, G.; Marazzato, M.; Campagna, G.; Pinacchio, C.; Alessandri, F.; Ruberto, F.; Rossi, G.; Celani, L.; Scagnolari, C.; et al. Challenges in the management of SARS-CoV2 infection: The role of oral bacteriotherapy as complementary therapeutic strategy to avoid the progression of COVID-19. Front Med. 2020, 7, 389. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Borrazzo, C.; Pinacchio, C.; Santinelli, L.; Innocenti, G.P.; Cavallari, E.N.; Celani, L.; Marazzato, M.; Alessandri, F.; Ruberto, F.; et al. Oral bacteriotherapy in patients with COVID-19: A retrospective cohort study. Front Nutr. 2021, 7, 341. [Google Scholar] [CrossRef] [PubMed]
- Oxygen-Ozone as Adjuvant Treatment in Early Control of COVID-19 Progression and Modulation of the Gut Microbial Flora—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04366089 (accessed on 13 August 2021).
- Efficacy of L. Plantarum and P. Acidilactici in Adults with SARS-CoV-2 and COVID-19—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04517422 (accessed on 13 August 2021).
- Efficacy of Intranasal Probiotic Treatment to Reduce Severity of Symptoms in COVID19 Infection—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04458519 (accessed on 13 August 2021).
- Efficacy of Probiotics in Reducing Duration and Symptoms of COVID-19—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04621071 (accessed on 13 August 2020).
- Evaluation of the Probiotic Lactobacillus Coryniformis K8 on COVID-19 Prevention in Healthcare Workers—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04366180 (accessed on 12 August 2021).
- Tang, H.; Bohannon, L.; Lew, M.; Jensen, D.; Jung, S.-H.; Zhao, A.; Sung, A.D.; Wischmeyer, P.E. Randomised, double-blind, placebo-controlled trial of probiotics to eliminate COVID-19 transmission in exposed household contacts (PROTECT-EHC): A Clinical trial protocol. BMJ Open 2021, 11, e047069. [Google Scholar] [CrossRef] [PubMed]
- Effect of Lactobacillus on the Microbiome of Household Contacts Exposed to COVID-19—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04399252 (accessed on 12 August 2021).
- Synbiotic Therapy of Gastrointestinal Symptoms During Covid-19 Infection—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04420676 (accessed on 13 August 2021).
- Biliński, J.; Winter, K.; Jasiński, M.; Szczęś, A.; Bilinska, N.; Mullish, B.H.; Małecka-Panas, E.; Basak, G.W. Rapid resolution of COVID-19 after faecal microbiota transplantation. Gut 2021, 1–2. [Google Scholar] [CrossRef]
- The Impact of Fecal Microbiota Transplantation as an Immunomodulation on the Risk Reduction of COVID-19 Disease Progression with Escalating Cytokine Storm and Inflammatory Parameter—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04824222 (accessed on 16 August 2021).
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Sacco, S.; Togha, M. Gut-brain Axis and migraine headache: A comprehensive review. J. Headache Pain 2020, 21, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galland, L. The gut microbiome and the brain. J. Med. Food. 2014, 17, 1261–1272. Available online: https://pubmed.ncbi.nlm.nih.gov/25402818/ (accessed on 19 June 2021). [CrossRef]
- Matenchuk, B.A.; Mandhane, P.J.; Kozyrskyj, A.L. Sleep, circadian rhythm, and gut microbiota. Sleep Med. Rev. 2020, 53, 101340. Available online: https://pubmed.ncbi.nlm.nih.gov/32668369/ (accessed on 19 June 2021). [CrossRef] [PubMed]
- Molina-Torres, G.; Rodriguez-Arrastia, M.; Roman, P.; Labraca, M.N.S.; Cardona, D. Stress and the gut microbiota-brain axis. Behav. Pharmacol. 2019, 30, 187–200. [Google Scholar] [CrossRef]
- Ogawa, Y.; Miyoshi, C.; Obana, N.; Yajima, K.; Hotta-Hirashima, N.; Ikkyu, A.; Kanno, S.; Soga, T.; Fukuda, S.; Yanagisawa, M. Gut microbiota depletion by chronic antibiotic treatment alters the sleep/wake architecture and sleep EEG power spectra in mice. Sci. Rep. 2020, 10, 19554. [Google Scholar] [CrossRef] [PubMed]
- Peirce, J.M.; Alviña, K. The role of inflammation and the gut microbiome in depression and anxiety. J. Neurosci. Res. 2019, 97, 1223–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poroyko, V.A.; Carreras, A.; Khalyfa, A.; Khalyfa, A.A.; Leone, V.; Peris, E.; Almendros, I.; Gileles-Hillel, A.; Qiao, Z.; Hubert, N.; et al. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci. Rep. 2016, 6, 35405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.P.; Easson, C.; Lyle, S.M.; Kapoor, R.; Donnelly, C.P.; Davidson, E.J.; Parikh, E.; Lopez, J.V.; Tartar, J.L. Gut microbiome diversity is associated with sleep physiology in humans. PLoS ONE 2019, 14, e0222394. [Google Scholar] [CrossRef] [PubMed]
- Boehme, M.; Guzzetta, K.E.; Bastiaanssen, T.F.S.; van de Wouw, M.; Moloney, G.M.; Gual-Grau, A.; Spichak, S.; Olavarría-Ramírez, L.; Fitzgerald, P.; Morillas, E.; et al. Microbiota from young mice counteracts selective age-associated behavioral deficits. Nat. Aging 2021, 1, 666–676. [Google Scholar] [CrossRef]
- Vestad, B.; Ueland, T.; Lerum, T.V.; Dahl, T.B.; Holm, K.; Barratt-Due, A.; Kasine, T.; Dyrhol-Riise, A.M.; Stiksrud, B.; Tonby, K.; et al. Gut microbiota alterations in patients with persistent respiratory dysfunction three months after severe COVID-19. medRxiv 2021. Available online: https://www.medrxiv.org/content/10.1101/2021.07.13.21260412v1 (accessed on 9 September 2021).
| Number of COVID-19 Patients | Gastro-Intestinal Symptoms | Diarrhoea | Nausea/Vomiting | Abdominal Pain | Number of Studies Used in Meta-Analysis | Reference |
|---|---|---|---|---|---|---|
| 2477 | 13% | 7.8% | 5.5% | 2.7% | 17 | [15] |
| 4243 | 17.6% | 12.5% | 10.2% | 9.2% | 60 | [16] |
| 4805 | Not reported | 7.4% | 4.6% | Not reported | 29 | [17] |
| 5601 | 9.8% | 10.4% | 7.7% | 6.9% | 37 | [18] |
| 17,776 | 20% | 13% | 8% | 4% | 108 | [19] |
| 18,246 | Not reported | 11.5% | 6.3% | 2.3% | 43 | [20] |
| Number of COVID-19 Patients | Positive in Faeces/Anal Swabs | Duration of Positivity (Days) | Comments | Reference |
|---|---|---|---|---|
| 15 | 26.7% | 53.3% positive in oral swabs | [21] | |
| 16 | 25% (Day 0) 37.5% (Day 5) | [21] | ||
| 10 | 100% | 3–19 | [23] | |
| 73 | 53% | 1–12 | 23% stool positive and negative in respiratory samples | [22] |
| 74 | 55% | Mean of 27.9 (SD 10.7) | [24] | |
| 42 | 67% | Mean 7 (6–10) | [25] | |
| 258 | 36% | Virus isolated and sequenced from stool, infectious in monkey kidney VERO cells, and confirmation by electron microscopy (EM) | [26] | |
| 205 (153 tested for faecal samples) | 29% | [27] | ||
| 59 | 15.3% | [16] | ||
| 4243 (Meta-analysis) | 48.1% | 70% of stool positive and negative in respiratory samples | [16] | |
| 23 | 48% | [28] | ||
| 23 | 83.3% | Mean 22 | [29] |
| Number of COVID-19 Patients | Healthy Control | Age (Median) | Microbiome Investigated | Enrichment | Loss | Reference | |
|---|---|---|---|---|---|---|---|
| COVID-19 | Control | ||||||
| 15 | 15 (6 with community-acquired pneumonia) | 55 | 48 (50 for Pneumonia) | Gut (faecal sample) | opportunistic pathogens that can cause bacteraemia, including Clostridium hathewayi, Actinomyces viscosus, and Bacteroides nordii | Commensals decreased, for example, Eubacterium, Faecalibacterium prausnitzii, Roseburia, and Lachnospiraceae taxa 1* | [71] |
| 30 | 30 (24 with H1N1) | 55 | 53.5 (48.5 for H1N1) | Gut (faecal sample) | Streptococcus, Rothia, Veillonella, Erysipelatoclostridium, and Actinomyces | mean community richness and microbial diversity were significantly lower in COVID-19 and H1N1 patients 2* | [72] |
| 30 | 30 | 46 | 34 | Gut (faecal sample) | Diversity 2.5-fold higher, for example, Candida albicans, Candida auris, and Aspergillus flavus | [73] | |
| 24 | 48 | 49 | 48 | Oral cavitiy and gut (saliva and facecal sample) | Lipopolysaccharide producing bacteria increased | Microbial diversity decreased, butyric acid-producing bacteria decreased | [74] |
| 14 | 16 | 63.3 | 40.5 | Plasma (from blood) | 65% of COVID-19 patients showed atypical plasma microbiome 3* | [60] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilpert, K. Is the Gut Microbiome a Target for Adjuvant Treatment of COVID-19? Biologics 2021, 1, 285-299. https://doi.org/10.3390/biologics1030017
Hilpert K. Is the Gut Microbiome a Target for Adjuvant Treatment of COVID-19? Biologics. 2021; 1(3):285-299. https://doi.org/10.3390/biologics1030017
Chicago/Turabian StyleHilpert, Kai. 2021. "Is the Gut Microbiome a Target for Adjuvant Treatment of COVID-19?" Biologics 1, no. 3: 285-299. https://doi.org/10.3390/biologics1030017
APA StyleHilpert, K. (2021). Is the Gut Microbiome a Target for Adjuvant Treatment of COVID-19? Biologics, 1(3), 285-299. https://doi.org/10.3390/biologics1030017







