Clinical Efficacy and Safety of Antiviral Drugs in the Extended Use against COVID-19: What We Know So Far
Abstract
:1. Introduction
2. Methods
Search and Data Collection
3. Promising Antiviral Drugs against SARS-CoV-2: Results from Human Studies
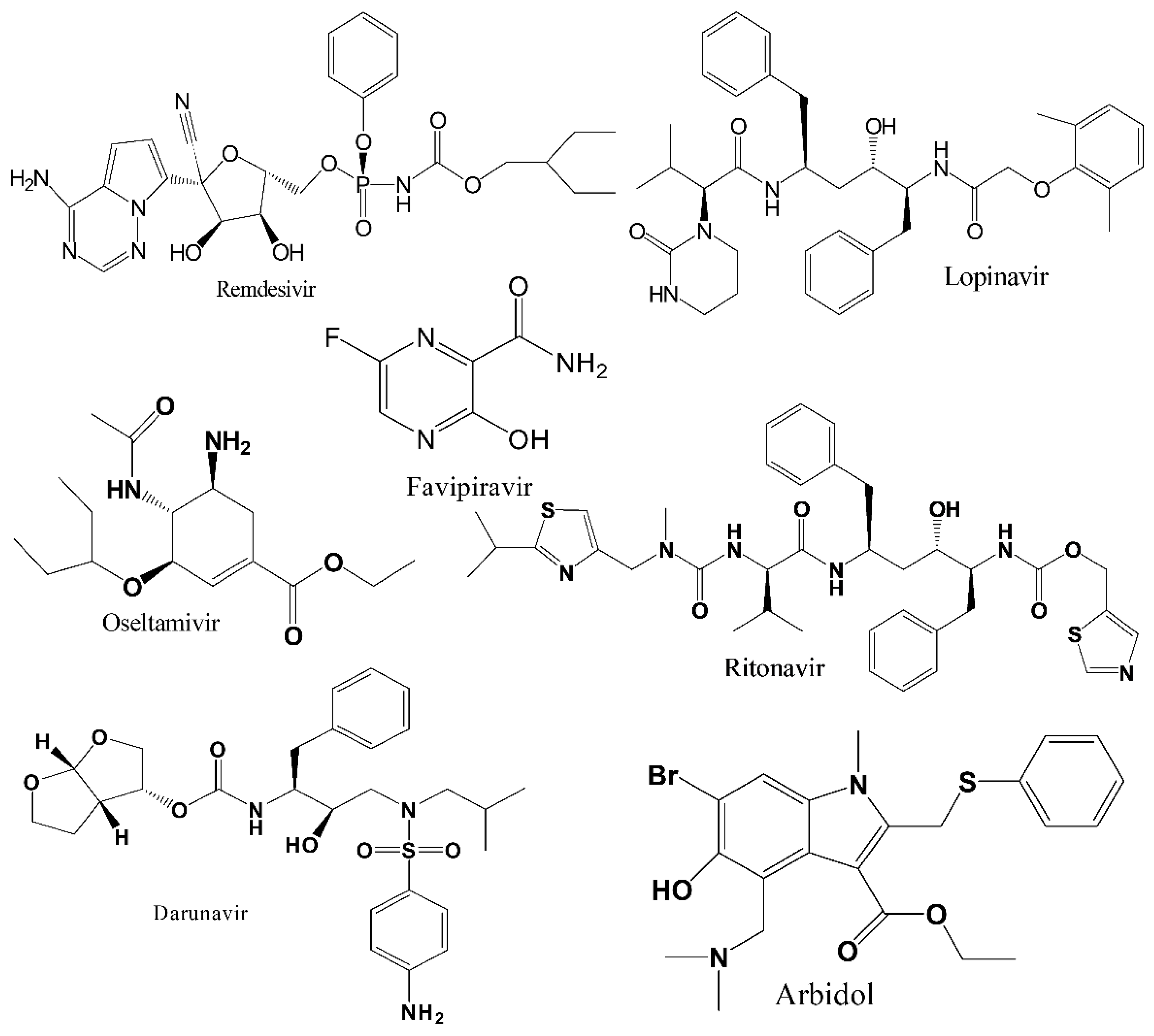
3.1. Remdesivir (RDV)
3.2. Favipiravir (FPV)
3.3. Interferons (IFNs)
3.4. Lopinavir-Ritonavir (LPV/RTV)
3.5. Arbidol (ARB)
3.6. Oseltamivir
3.7. Darunavir (DRV)
4. Discussion and Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RDV | remdesivir |
| FPV | favipiravir |
| LPV-RTV | lopinavir-ritonavir |
| INF | interferon |
| rhIFN-α | recombinant human interferon alpha |
| rSIFN | recombinant super compound interferon |
| TFF2 | trefoil factor 2 |
| ARB | arbidol |
| DRV | darunavir |
| DRV/c | darunavir/cobicisitat |
| HCQ | hydroxychloroquine |
| CQ | chloroquine |
| RCT | randomized controlled trial |
| LHQW | Lianhuaqingwen |
| LQ | Lianhua Qingwen |
| CP | convalescent plasma |
| BCN | baricitinib |
| RBV | ribavirin |
| SFV/DCV | sofosbuvir/daclatasvir |
| N | number |
| T | treatment (group) |
| C | control (group) |
| SOC | standard of care |
| CT | computed tomography |
| IV | intravenous |
| NA | not available |
| SC | subcutaneous |
| GI | gastrointestinal |
| 95% CI | 95% confidence interval |
| HR | hazard ratio |
| OR | odds ratio |
| RR | relative risk |
| ROA | route of administration |
| ADR | adverse drug reaction |
| ADRS | acute respiratory distress syndrome |
| ALT | alanine aminotransferase |
| RdRp | RNA-dependent RNA polymerase |
| IQR | interquartile range |
| PCR | polymerase chain reaction |
| ECG | electrocardiogram |
References
- Ashraf, B.N. Economic impact of government interventions during the COVID-19 pandemic: International evidence from financial markets. J. Behav. Exp. Financ. 2020, 27, 100371. [Google Scholar] [CrossRef]
- Hossain, M.J.; Ahmmed, F.; Rahman, S.; Sanam, S.; Emran, T.B.; Mitra, S. Impact of online education on fear of academic delay and psychological distress among university students following one year of COVID-19 outbreak in Bangladesh. Heliyon 2021, 7, e07388. [Google Scholar] [CrossRef]
- Gollakner, R.; Capua, I. Is COVID-19 the first pandemic that evolves into a panzootic? Vet. Ital. 2020, 56, 11–12. [Google Scholar] [CrossRef]
- Yoo, H.S.; Yoo, D. COVID-19 and veterinarians for one health, zoonotic- and reverse-zoonotic transmissions. J. Vet. Sci. 2020, 21, e51. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Singhai, M.; Garg, S.; Shah, D.; Sood, V.; Singh, S.K. The missing pieces in the jigsaw and need for cohesive research amidst coronavirus infectious disease 2019 global response. Med. J. Armed Forces India 2020, 76, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Lorusso, A. Novel human coronavirus (SARS-CoV-2): A lesson from animal coronaviruses. Vet. Microbiol. 2020, 244, 108693. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Weekly Operational Update on COVID-19—20 July 2021; World Health Organization: Geneva, Switzerland, 2021; pp. 1–13. [Google Scholar]
- Hossain, M.J.; Kuddus, M.R.; Rahman, S.M.A. Knowledge, attitudes, and behavioral responses toward COVID-19 during early phase in bangladesh: A questionnaire-based study. Asia Pac. J. Public Health 2021, 33, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Our World in Data. Statistics and Research. Coronavirus Disease 2019 (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 30 August 2021).
- Forni, G.; Mantovani, A.; COVID-19 Commission of Accademia Nazionale dei Lincei, Rome. COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19). Available online: https://stacks.cdc.gov/view/cdc/88624 (accessed on 30 August 2021).
- Hossain, M.J. Is Bangladesh moving toward herd immunity? Current COVID-19 perspective. Bangladesh J. Infect. Dis. 2020, 7 (Suppl. S2), S63–S66. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Hossain, M.J.; Kuddus, M.R.; Rashid, M.A.; Sultan, M.Z. Understanding and dealing the SARS-CoV-2 infection: An updated concise review. Bangladesh Pharm. J. 2021, 24, 61–75. [Google Scholar] [CrossRef]
- Godwin, M.; Ruhland, L.; Casson, I.; MacDonald, S.; Delva, D.; Birtwhistle, R.; Lam, M.; Seguin, R. Pragmatic controlled clinical trials in primary care: The struggle between external and internal validity. BMC Med. Res. Methodol. 2003, 3, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viveiros Rosa, S.G.; Santos, W.C. Clinical trials on drug repositioning for COVID-19 treatment. Rev. Panam. Salud Publica Pan Am. J. Public Health 2020, 44, e40. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Oprea, T.I.; Bauman, J.E.; Bologa, C.G.; Buranda, T.; Chigaev, A.; Edwards, B.S.; Jarvik, J.W.; Gresham, H.D.; Haynes, M.K.; Hjelle, B.; et al. Drug repurposing from an academic perspective. Drug Discov. Today Ther. Strateg. 2011, 8, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Senanayake, S.L. Drug repurposing strategies for COVID-19. Future Drug Discov. 2020, 2, FDD40. [Google Scholar] [CrossRef]
- Young, B.E.; Ong, S.W.X.; Kalimuddin, S.; Low, J.G.; Tan, S.Y.; Loh, J.; Ng, O.-T.; Marimuthu, K.; Ang, L.W.; Mak, T.M.; et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA 2020, 323, 1488–1494. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Parvathaneni, V.; Gupta, V. Utilizing drug repurposing against COVID-19—Efficacy, limitations, and challenges. Life Sci. 2020, 259, 118275. [Google Scholar] [CrossRef]
- Marra, F.; Smolders, E.J.; El-Sherif, O.; Boyle, A.; Davidson, K.; Sommerville, A.J.; Marzolini, C.; Siccardi, M.; Burger, D.; Gibbons, S.; et al. Recommendations for Dosing of Repurposed COVID-19 Medications in Patients with Renal and Hepatic Impairment. Drugs R&D 2021, 21, 9–27. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, F.; Tang, J.; Nussinov, R.; Cheng, F. Artificial intelligence in COVID-19 drug repurposing. Lancet Digit. Health 2020, 2, e667–e676. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Leist, S.R.; Schäfer, A.; Won, J.; Brown, A.J.; Montgomery, S.A.; Hogg, A.; Babusis, D.; Clarke, M.O.; et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.-X.; et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef]
- Antinori, S.; Cossu, M.V.; Ridolfo, A.L.; Rech, R.; Bonazzetti, C.; Pagani, G.; Gubertini, G.; Coen, M.; Magni, C.; Castelli, A.; et al. Compassionate remdesivir treatment of severe Covid-19 pneumonia in intensive care unit (ICU) and Non-ICU patients: Clinical outcome and differences in post-treatment hospitalisation status. Pharmacol. Res. 2020, 158, 104899. [Google Scholar] [CrossRef]
- Pasquini, Z.; Montalti, R.; Temperoni, C.; Canovari, B.; Mancini, M.; Tempesta, M.; Pimpini, D.; Zallocco, N.; Barchiesi, F. Effectiveness of remdesivir in patients with COVID-19 under mechanical ventilation in an Italian ICU. J. Antimicrob. Chemother. 2020, 75, 3359–3365. [Google Scholar] [CrossRef] [PubMed]
- Aiswarya, D.; Arumugam, V.; Dineshkumar, T.; Gopalakrishnan, N.; Lamech, T.M.; Nithya, G.; Sastry, B.V.R.H.; Vathsalyan, P.; Dhanapriya, J.; Sakthirajan, R. Use of Remdesivir in Patients With COVID-19 on Hemodialysis: A Study of Safety and Tolerance. Kidney Int. Rep. 2021, 6, 586–593. [Google Scholar] [CrossRef]
- Olender, S.A.; Perez, K.K.; Go, A.S.; Balani, B.; Price-Haywood, E.G.; Shah, N.S.; Wang, S.; Walunas, T.L.; Swaminathan, S.; Slim, J.; et al. Remdesivir for Severe Coronavirus Disease 2019 (COVID-19) Versus a Cohort Receiving Standard of Care. Clin. Infect. Dis. 2020, ciaa1041. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.D.; Lye, D.C.B.; Hui, D.S.; Marks, K.M.; Bruno, R.; Montejano, R.; Spinner, C.D.; Galli, M.; Ahn, M.-Y.; Nahass, R.G.; et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N. Engl. J. Med. 2020, 383, 1827–1837. [Google Scholar] [CrossRef]
- Garibaldi, B.T.; Wang, K.; Robinson, M.L.; Zeger, S.L.; Roche, K.B.; Wang, M.-C.; Alexander, G.C.; Gupta, A.; Bollinger, R.; Xu, Y. Effectiveness of remdesivir with and without dexamethasone in hospitalized patients with COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Hayakawa, K.; Matsunaga, N.; Terada, M.; Suzuki, S.; Ohtsu, H.; Asai, Y.; Kitajima, K.; Saito, S.; Uemura, Y.; et al. Efficacy of remdesivir in Japanese patients hospitalised with COVID-19: A large observational study using the COVID-19 Registry Japan. medRxiv 2021. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; López, J.R.A.; Cattelan, A.M.; Viladomiu, A.S.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A.; et al. Effect of Remdesivir vs. Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 1048–1057. [Google Scholar] [CrossRef]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N. Engl. J. Med. 2020, 384, 795–807. [Google Scholar] [CrossRef]
- Falcão, F.; Viegas, E.; Carmo, I.; Soares, J.; Falcao, M.; Solano, M.; Cavaco, P.; Mendes, D.; Rijo, J.; Povoa, P.; et al. A prospective, observational study to evaluate adverse drug reactions in patients with COVID-19 treated with remdesivir or hydroxychloroquine: A preliminary report. Eur. J. Hosp. Pharm. 2021, 28, 248–253. [Google Scholar] [CrossRef]
- Goldberg, E.; Zvi, H.B.; Sheena, L.; Sofer, S.; Krause, I.; Sklan, E.H.; Shlomai, A. A real-life setting evaluation of the effect of remdesivir on viral load in COVID-19 patients admitted to a large tertiary centre in Israel. Clin. Microbiol. Infect. 2021, 27, 917.e1–917.e4. [Google Scholar] [CrossRef]
- Padilla, R.; Arquiette, J.; Mai, Y.; Singh, G.; Galang, K.; Liang, E. Clinical Outcomes of COVID-19 Patients Treated with Convalescent Plasma or Remdesivir Alone and in Combination at a Community Hospital in California’s Central Valley. J. Pharm. Pharm. Sci. 2021, 24, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Yang, M.; Liu, D.; Chen, J.; Shu, D.; Xia, J.; Liao, X.; Gu, Y.; Cai, Q.; Yang, Y.; et al. Experimental Treatment with FPVipiravir for COVID-19: An Open-Label Control Study. Engineering 2020, 6, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Y.; Huang, J.; Yin, P.; Cheng, Z.; Wu, J.; Chen, S.; Zhang, Y.; Chen, B.; Lu, M.; et al. Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Lou, Y.; Liu, L.; Yao, H.; Hu, X.; Su, J.; Xu, K.; Luo, R.; Yang, X.; He, L.; Lu, X.; et al. Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and FPVipiravir in COVID-19 Patients: An Exploratory Randomized, Controlled Trial. Eur. J. Pharm. Sci. 2021, 157, 105631. [Google Scholar] [CrossRef]
- The Indian Express. COVID-19: Glenmark’s FPVipiravir Shows Encouraging Results in Phase 3 Clinical Trial. Available online: https://www.newindianexpress.com/nation/2020/jul/23/covid-19-glenmarks-FPVipiravir-shows-encouraging-results-in-phase-3-clinical-trial-2173500.html (accessed on 14 July 2021).
- The Daily Star. Covid-19 Patients: Favipiravir Effective in Dhaka Trial. Available online: https://www.thedailystar.net/backpage/news/covid-19-patients-FPVipiravir-effective-dhaka-trial-1927321 (accessed on 14 July 2021).
- Ucan, A.; Cerci, P.; Efe, S.; Akgun, H.; Ozmen, A.; Yagmuroglu, A.; Bilgin, M.; Avci, D. Benefits of treatment with Favipiravir in hospitalized patients for COVID-19: A retrospective observational case–control study. Virol. J. 2021, 18, 102. [Google Scholar] [CrossRef] [PubMed]
- Alamer, A.; Alrashed, A.A.; Alfaifi, M.; Alosaimi, B.; AlHassar, F.; Almutairi, M.; Howaidi, J.; Almutairi, W.; Mohzari, Y.; Sulaiman, T.; et al. Effectiveness and safety of Favipiravir compared to supportive care in moderately to critically ill COVID-19 patients: A retrospective study with propensity score matching sensitivity analysis. Curr. Med. Res. Opin. 2021, 37, 1085–1097. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, C.; Zhu, Q.; Chen, X.; Chen, G.; Sun, W.; Xiao, Z.; Du, W.; Yao, J.; Li, G.; et al. Favipiravir in the treatment of patients with SARS-CoV-2 RNA recurrent positive after discharge: A multicenter, open-label, randomized trial. Int. Immunopharmacol. 2021, 97, 107702. [Google Scholar] [CrossRef]
- Udwadia, Z.F.; Singh, P.; Barkate, H.; Patil, S.; Rangwala, S.; Pendse, A.; Kadam, J.; Wu, W.; Caracta, C.F.; Tandon, M. Efficacy and safety of Favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int. J. Infect. Dis. 2021, 103, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Wang, T.; Chen, L.; Chen, X.; Li, L.; Qin, X.; Li, H.; Luo, J. The Effect of Recombinant Human Interferon Alpha Nasal Drops to Prevent COVID-19 Pneumonia for Medical Staff in an Epidemic Area. Curr. Top. Med. Chem. 2021, 21, 920–927. [Google Scholar] [CrossRef]
- Pandit, A.; Bhalani, N.; Bhushan, B.L.S.; Koradia, P.; Gargiya, S.; Bhomia, V.; Kansagra, K. Efficacy and safety of pegylated interferon alfa-2b in moderate COVID-19: A phase II, randomized, controlled, open-label study. Int. J. Infect. Dis. 2021, 105, 516–521. [Google Scholar] [CrossRef]
- Yu, J.; Lu, X.; Tong, L.; Shi, X.; Ma, J.; Lv, F.; Wu, J.; Pan, Q.; Yang, J.; Cao, H.; et al. Interferon-α-2b aerosol inhalation is associated with improved clinical outcomes in patients with coronavirus disease-2019. Br. J. Clin. Pharmacol. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Feld, J.J.; Kandel, C.; Biondi, M.J.; Kozak, R.A.; Zahoor, M.A.; Lemieux, C.; Borgia, S.M.; Boggild, A.K.; Powis, J.; McCready, J.; et al. Peginterferon lambda for the treatment of outpatients with COVID-19: A phase 2, placebo-controlled randomised trial. Lancet Respir. Med. 2021, 9, 498–510. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Li, Q.; Wen, L.; Zhang, R. Clinical Features of 69 Cases With Coronavirus Disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 769–777. [Google Scholar] [CrossRef] [Green Version]
- Lian, N.; Xie, H.; Lin, S.; Huang, J.; Zhao, J.; Lin, Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: A retrospective study. Clin. Microbiol. Infect. 2020, 26, 917–921. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, Z.; Xu, T.; Chen, C.; Yang, G.; Zha, T.; Lu, J.; Xue, Y. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J. Infect. 2020, 81, e21–e23. [Google Scholar] [CrossRef]
- Chen, W.; Yao, M.; Fang, Z.; Lv, X.; Deng, M.; Wu, Z. A study on clinical effect of Arbidol combined with adjuvant therapy on COVID-19. J. Med. Virol. 2020, 92, 2702–2708. [Google Scholar] [CrossRef]
- Liu, Q.; Fang, X.; Tian, L.; Vankadari, N.; Chen, X.; Wang, K.; Li, D.; Dai, X.; Xu, F.; Shen, L.; et al. Arbidol treatment with reduced mortality of adult patients with COVID-19 in Wuhan, China: A retrospective cohort study. medRxiv 2021. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Ke, C.; Yue, D.; Li, W.; Hu, Z.; Liu, W.; Hu, S.; Wang, S.; Liu, J. Effectiveness of Arbidol for COVID-19 Prevention in Health Professionals. Front. Public Health 2020, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; He, X.; Liu, W.; Kan, J.; He, L.; Zhao, J.; Chen, C.; Zhang, J.; Chen, S. Antiviral Abidol is Associated with the Reduction of In-Hospital Mortality in COVID-19 Patients. Cardiol. Discov. 2021, 1, 37–43. [Google Scholar]
- Tan, J.; Yuan, Y.; Xu, C.; Song, C.; Liu, D.; Ma, D.; Gao, Q. A retrospective comparison of drugs against COVID-19. Virus Res. 2021, 294, 198262. [Google Scholar] [CrossRef]
- Moreno, G.; Rodríguez, A.; Sole-Violán, J.; Martín-Loeches, I.; Díaz, E.; Bodí, M.; Reyes, L.F.; Gómez, J.; Guardiola, J.; Trefler, S.; et al. Early oseltamivir treatment improves survival in critically ill patients with influenza pneumonia. ERJ Open Res. 2021, 7, 00888–2020. [Google Scholar] [CrossRef]
- Deng, L.; Xiong, Y.; Chen, T.; Zhang, Y.; Luo, M.; Gao, S.; Mo, P.; Hospital, Z.; Song, S.; Hospital Zhiyong, Z.; et al. Role of Darunavir/cobicisitat in the Treatment of COVID-19: Initial Virological and Clinical Findings. Res. Sq. 2021, 2021, 1–23. [Google Scholar] [CrossRef]
- Kim, E.J.; Choi, S.H.; Park, J.S.; Kwon, Y.S.; Lee, J.; Kim, Y.; Lee, S.Y.; Choi, E.Y. Use of Darunavir-Cobicistat as a Treatment Option for Critically Ill Patients with SARS-CoV-2 Infection. Yonsei Med. J. 2020, 61, 826–830. [Google Scholar] [CrossRef]
- Chen, J.; Xia, L.; Liu, L.; Xu, Q.; Ling, Y.; Huang, D.; Huang, W.; Song, S.; Xu, S.; Shen, Y.; et al. Antiviral Activity and Safety of Darunavir/Cobicistat for the Treatment of COVID-19. Open Forum Infect. Dis. 2020, 7, ofaa241. [Google Scholar] [CrossRef]
- Guner, R.; Hasanoglu, I.; Kayaaslan, B.; Aypak, A.; Akinci, E.; Bodur, H.; Eser, F.; Kaya Kalem, A.; Kucuksahin, O.; Ates, I.; et al. Comparing ICU admission rates of mild/moderate COVID-19 patients treated with hydroxychloroquine, FPVipiravir, and hydroxychloroquine plus FPVipiravir. J. Infect. Public Health 2021, 14, 365–370. [Google Scholar] [CrossRef]
- Dabbous, H.M.; Abd-Elsalam, S.; El-Sayed, M.H.; Sherief, A.F.; Ebeid, F.F.S.; El Ghafar, M.S.A.; Soliman, S.; Elbahnasawy, M.; Badawi, R.; Tageldin, M.A. Efficacy of Favipiravir in COVID-19 treatment: A multi-center randomized study. Arch. Virol. 2021, 166, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Kocayiğit, H.; Süner, K.Ö.; Tomak, Y.; Demir, G.; Yaylacı, S.; Dheir, H.; Güçlü, E.; Erdem, A.F. Observational study of the effects of FPVipiravir vs. Lopinavir/Ritonavir on clinical outcomes in critically Ill patients with COVID-19. J. Clin. Pharm. Ther. 2021, 46, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Dabbous, H.M.; El-Sayed, M.H.; El Assal, G.; Elghazaly, H.; Ebeid, F.F.S.; Sherief, A.F.; Elgaafary, M.; Fawzy, E.; Hassany, S.M.; Riad, A.R.; et al. Safety and efficacy of Favipiravir versus hydroxychloroquine in management of COVID-19: A randomised controlled trial. Sci. Rep. 2021, 11, 7282. [Google Scholar] [CrossRef]
- Khamis, F.; Al Naabi, H.; Al Lawati, A.; Ambusaidi, Z.; Al Sharji, M.; Al Barwani, U.; Pandak, N.; Al Balushi, Z.; Al Bahrani, M.; Al Salmi, I.; et al. Randomized controlled open label trial on the use of FPVipiravir combined with inhaled interferon beta-1b in hospitalized patients with moderate to severe COVID-19 pneumonia. Int. J. Infect. Dis. 2021, 102, 538–543. [Google Scholar] [CrossRef]
- Kim, J.-W.; Kim, E.J.; Kwon, H.H.; Jung, C.Y.; Kim, K.C.; Choe, J.-Y.; Hong, H.-L. Lopinavir-ritonavir versus hydroxychloroquine for viral clearance and clinical improvement in patients with mild to moderate coronavirus disease 2019. Korean J. Intern. Med. 2020, 36, S253–S263. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, S.O.; Heo, J.; Kim, D.W.; Park, M.R.; Son, H.; Kim, D.; Kim, K.-H.; Lee, S.; Lee, S.H. Comparative outcomes of lopinavir/ritonavir and hydroxychloroquine for the treatment of coronavirus disease 2019 with mild to moderate severity. Res. Sq. 2020, 2020, 1–20. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, Z.; Guo, Y.; Shi, J.; Pei, G.; Yao, Y.; Liao, W.; Zeng, R. Lopinavir/ritonavir is associated with pneumonia resolution in COVID-19 patients with influenza coinfection: A retrospective matched-pair cohort study. J. Med. Virol. 2021, 93, 472–480. [Google Scholar] [CrossRef]
- Lecronier, M.; Beurton, A.; Burrel, S.; Haudebourg, L.; Deleris, R.; Le Marec, J.; Virolle, S.; Nemlaghi, S.; Bureau, C.; Mora, P.; et al. Comparison of hydroxychloroquine, lopinavir/ritonavir, and standard of care in critically ill patients with SARS-CoV-2 pneumonia: An opportunistic retrospective analysis. Crit. Care 2020, 24, 1–9. [Google Scholar] [CrossRef]
- Lan, X.; Shao, C.; Zeng, X.; Wu, Z.; Xu, Y. Lopinavir-ritonavir alone or combined with arbidol in the treatment of 73 hospitalized patients with COVID-19: A pilot retrospective study. Int. J. Clin. Pharmacol. Ther. 2021, 59, 378–385. [Google Scholar] [CrossRef]
- Gao, G.; Wang, A.; Wang, S.; Qian, F.; Chen, M.; Yu, F.; Zhang, J.; Wang, X.; Ma, X.; Zhao, T.; et al. Brief Report: Retrospective Evaluation on the Efficacy of Lopinavir/Ritonavir and Chloroquine to Treat Nonsevere COVID-19 Patients. J. Acquir. Immune Defic. Syndr. 2020, 85, 239–243. [Google Scholar] [CrossRef]
- Karolyi, M.; Pawelka, E.; Mader, T.; Omid, S.; Kelani, H.; Ely, S.; Jilma, B.; Baumgartner, S.; Laferl, H.; Ott, C.; et al. Hydroxychloroquine versus lopinavir/ritonavir in severe COVID-19 patients: Results from a real-life patient cohort. Wien. Klin. Wochenschr. 2020, 133, 284–291. [Google Scholar] [CrossRef]
- Shi, N.; Guo, L.; Liu, B.; Bian, Y.; Chen, R.; Chen, S.; Chen, Y.; Chen, Y.; Cong, X.; Dong, G.; et al. Efficacy and safety of Chinese herbal medicine versus Lopinavir-Ritonavir in adult patients with coronavirus disease 2019: A non-randomized controlled trial. Phytomedicine 2021, 81, 153367. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Li, Y.; Xie, Z.; Lin, W.; Cai, W.; Wen, C.; Guan, Y.; Mo, X.; Wang, J.; Wang, Y.; Peng, P.; et al. Efficacy and Safety of Lopinavir/Ritonavir or Arbidol in Adult Patients with Mild/Moderate COVID-19: An Exploratory Randomized Controlled Trial. Med 2020, 1, 105–113.e4. [Google Scholar] [CrossRef]
- Hung, I.F.-N.; Lung, K.-C.; Tso, E.Y.-K.; Liu, R.; Chung, T.W.-H.; Chu, M.-Y.; Ng, Y.-Y.; Lo, J.; Chan, J.; Tam, A.R.; et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial. Lancet 2020, 395, 1695–1704. [Google Scholar] [CrossRef]
- Huang, Y.-Q.; Tang, S.-Q.; Xu, X.-L.; Zeng, Y.-M.; He, X.-Q.; Li, Y.; Harypursat, V.; Lu, Y.-Q.; Wan, Y.; Zhang, L.; et al. No Statistically Apparent Difference in Antiviral Effectiveness Observed Among Ribavirin Plus Interferon-Alpha, Lopinavir/Ritonavir Plus Interferon-Alpha, and Ribavirin Plus Lopinavir/Ritonavir Plus Interferon-Alpha in Patients With Mild to Moderate Coronavirus Disease 2019: Results of a Randomized, Open-Labeled Prospective Study. Front. Pharmacol. 2020, 11, 1071. [Google Scholar] [CrossRef]
- Horby, P.W.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Emberson, J.; Palfreeman, A.; Raw, J.; Elmahi, E.; Prudon, B.; et al. Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2020, 396, 1345–1352. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Asiri, A.Y.; Assiri, A.M.; Balkhy, H.H.; Al Bshabshe, A.; Al Jeraisy, M.; Mandourah, Y.; Azzam, M.H.A.; Bin Eshaq, A.M.; Al Johani, S.; et al. Interferon Beta-1b and Lopinavir–Ritonavir for Middle East Respiratory Syndrome. N. Engl. J. Med. 2020, 383, 1645–1656. [Google Scholar] [CrossRef]
- Sevilla-Castillo, F.; Roque-Reyes, O.J.; Romero-Lechuga, F.; Gómez-Núñez, M.F.; Castillo-López, M.; Medina-Santos, D.; Román, P.O.; Flores-Hernández, J.R.; Méndez-Coca, J.D.; Montaño-Olmos, D.; et al. Both Chloroquine and Lopinavir/Ritonavir Are Ineffective for COVID-19 Treatment and Combined Worsen the Pathology: A Single-Center Experience with Severely Ill Patients. Biomed. Res. Int. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Malhani, A.A.; Enani, M.A.; Sharif-Askari, F.S.; Alghareeb, M.R.; Bin-Brikan, R.T.; AlShahrani, S.A.; Halwani, R.; Tleyjeh, I.M. Combination of (interferon beta-1b, lopinavir/ritonavir and ribavirin) versus FPVipiravir in hospitalized patients with non-critical COVID-19: A cohort study. PLoS ONE 2021, 16, e0252984. [Google Scholar] [CrossRef]
- Lepage, M.-A.; Rozza, N.; Kremer, R.; Grunbaum, A. Safety and effectiveness concerns of lopinavir/ritonavir in COVID-19 affected patients: A retrospective series. Clin. Toxicol. 2021, 59, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Jaenigen, B.; Wagner, D.; Rieg, S.; Hornuss, D.; Biever, P.M.; Kern, W.V.; Walz, G. Therapy with lopinavir/ritonavir and hydroxychloroquine is associated with acute kidney injury in COVID-19 patients. PLoS ONE 2021, 16, e0249760. [Google Scholar] [CrossRef]
- Fu, W.; Liu, Y.; Liu, L.; Hu, H.; Cheng, X.; Liu, P.; Song, Z.; Zha, L.; Bai, S.; Xu, T.; et al. An open-label, randomized trial of the combination of IFN-κ plus TFF2 with standard care in the treatment of patients with moderate COVID-19. EClinicalMedicine 2020, 27, 100547. [Google Scholar] [CrossRef]
- Li, C.; Luo, F.; Liu, C.; Xiong, N.; Xu, Z.; Zhang, W.; Yang, M.; Wang, Y.; Liu, D.; Yu, C.; et al. Effect of a genetically engineered interferon-alpha versus traditional interferon-alpha in the treatment of moderate-to-severe COVID-19: A randomised clinical trial. Ann. Med. 2021, 53, 391–401. [Google Scholar] [CrossRef]
- Li, H.; Xiong, N.; Li, C.; Gong, Y.; Liu, L.; Yang, H.; Tan, X.; Jiang, N.; Zong, Q.; Wang, J.; et al. Efficacy of ribavirin and interferon-α therapy for hospitalized patients with COVID-19: A multicenter, retrospective cohort study. Int. J. Infect. Dis. 2021, 104, 641–648. [Google Scholar] [CrossRef]
- Darazam, I.A.; Shokouhi, S.; Pourhoseingholi, M.A.; Irvani, S.S.N.; Mokhtari, M.; Shabani, M.; Amirdosara, M.; Torabinavid, P.; Golmohammadi, M.; Hashemi, S.; et al. Role of interferon therapy in severe COVID-19: The COVIFERON randomized controlled trial. Sci. Rep. 2021, 11, 8059. [Google Scholar] [CrossRef]
- Fang, J.; Li, H.; Du, W.; Yu, P.; Guan, Y.-Y.; Ma, S.-Y.; Liu, D.; Chen, W.; Shi, G.-C.; Bian, X.-L. Efficacy of Early Combination Therapy With Lianhuaqingwen and Arbidol in Moderate and Severe COVID-19 Patients: A Retrospective Cohort Study. Front. Pharmacol. 2020, 11, 560209. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.Y.; Xie, Z.W.; Li, Y.P.; Deng, X.L.; Chen, X.T.; Cao, Y.; Ou, X.; Lin, W.Y.; Li, F.; Cai, W.P.; et al. Real-world efficacy and safety of lopinavir/ritonavir and arbidol in treating with COVID-19: An observational cohort study. Zhonghua Nei Ke Za Zhi 2020, 59, E012. [Google Scholar] [CrossRef]
- Deng, L.; Li, C.; Zeng, Q.; Liu, X.; Li, X.; Zhang, H.; Hong, Z.; Xia, J. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J. Infect. 2020, 81, e1–e5. [Google Scholar] [CrossRef]
- Chen, J.; Lin, S.; Niu, C.; Xiao, Q. Clinical evaluation of Shufeng Jiedu Capsules combined with umifenovir (Arbidol) in the treatment of common-type COVID-19: A retrospective study. Expert Rev. Respir. Med. 2020, 15, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.K.; Hao, S.L.; Ma, J.H.; Wei, G.Y.; Song, K.Y.; Tang, C.; Gao, Y.F.; Liang, S.Q.; Du, W.J. Observation on clinical effect of Shufeng Jiedu Capsule combined with Arbidol Hydrochloride Capsule in treatment of COVID-19. Chin. Tradit. Herb. Drugs 2020, 51, 1167–1170. [Google Scholar] [CrossRef]
- Yu, P.; Li, Y.Z.; Wan, S.B.; Wang, Y. Effects of Lianhua Qingwen Granules Plus Arbidol on Treatment of Mild Corona Virus Disease-19. Chin. Pharm. J. 2020, 55, 1042–1045. [Google Scholar] [CrossRef]
- Xi, W.-N.; Jin, D.; Sun, K.; Yu, R.-Y.; Yao, X.-B.; Zou, B.-S.; Song, Z.-Y.; Yang, A.-Y.; Luo, R.-X.; Liu, Y.; et al. Treatment with Arbidol and Moxifloxacin in Ordinary and Severe Adult Patients Infected with COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Huang, H.; Guan, L.; Yang, Y.; Le Grange, J.M.; Tang, G.; Xu, Y.; Yuan, J.; Lin, C.; Xue, M.; Zhang, X.; et al. Chloroquine, arbidol (umifenovir) or lopinavir/ritonavir as the antiviral monotherapy for COVID-19 patients: A retrospective cohort study. Res. Sq. 2020, 2020, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Huang, J.; Fan, Z.; Huang, W.; Qi, M.; Lin, X.; Song, W.; Yi, L. Arbidol/IFN-α2b therapy for patients with corona virus disease 2019: A retrospective multicenter cohort study. Microbes Infect. 2020, 22, 200–205. [Google Scholar] [CrossRef]
- Nojomi, M.; Yassin, Z.; Keyvani, H.; Makiani, M.J.; Roham, M.; Laali, A.; Dehghan, N.; Navaei, M.; Ranjbar, M. Effect of Arbidol (Umifenovir) on COVID-19: A randomized controlled trial. BMC Infect. Dis. 2020, 20, 954. [Google Scholar] [CrossRef] [PubMed]
- Ghaderkhani, S.; Khaneshan, A.S.; Salami, A.; Alavijeh, P.E.; Kouchak, H.E.; Khalili, H.; Naghi, S.A.A.; Ahmadinejad, Z.; Rasolinejad, M.; Hajiabdolbaghi, M.; et al. Efficacy and Safety of Arbidol in Treatment of Patients with COVID-19 Infection: A Randomized Clinical Trial. Res. Sq. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Li, M.; Yu, T.; Zhu, J.; Wang, Y.; Yang, Y.; Zhao, K.; Yi, Y.; He, J.; Li, C.; He, J. Comparison of the antiviral effect of Arbidol and Chloroquine in treating COVID-19. Ann. Palliat. Med. 2021, 10, 3307–3312. [Google Scholar] [CrossRef]
- Chiba, S. Effect of early oseltamivir on outpatients without hypoxia with suspected COVID-19. Wien. Klin. Wochenschr. 2020, 133, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Meriglier, E.; Rivoisy, C.; Hessamfar, M.; Bernard, N.; Aureau, I.; Lapoirie, J.; Contis, A.; Sacher, F.; Sacristan, B.; Lahouati, M.; et al. Safety of hydroxychloroquine and darunavir or lopinavir in COVID-19 infection. J. Antimicrob. Chemother. 2021, 76, 482–486. [Google Scholar] [CrossRef]
- Davoudi-Monfared, E.; Rahmani, H.; Khalili, H.; Hajiabdolbaghi, M.; Salehi, M.; Abbasian, L.; Kazemzadeh, H.; Yekaninejad, M.S. A Randomized Clinical Trial of the Efficacy and Safety of Interferon β-1a in Treatment of Severe COVID-19. Antimicrob. Agents Chemother. 2020, 64, e01061-20. [Google Scholar] [CrossRef]
- Qu, J.; Li, G.-H.; Wang, J.-J.; He, G.-F.; Huang, J.-J.; Chen, Y.; Qu, Q.; Chen, X.-Y.; Lu, Q. Comparative effectiveness of Lopinavir/Ritonavir-based regimens in COVID-19. Clin. Exp. Pharmacol. Physiol. 2021, 48, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Parkar, J.; Ansari, A.; Vora, A.; Talwar, D.; Tiwaskar, M.; Patil, S.; Barkate, H. Role of FPVipiravir in the treatment of COVID-19. Int. J. Infect. Dis. 2021, 102, 501–508. [Google Scholar] [CrossRef]
- Duyan, M.; Ozturan, I.U. Acute Psychosis in COVID-19: Is It Due to FPVipiravir Treatment or Acute Viral Illness? SN Compr. Clin. Med. 2021, 3, 1627–1629. [Google Scholar] [CrossRef]
- Agrawal, U.; Raju, R.; Udwadia, Z.F. FPVipiravir: A new and emerging antiviral option in COVID-19. Med. J. Armed Forces India 2020, 76, 370–376. [Google Scholar] [CrossRef]
- Kaur, R.J.; Charan, J.; Dutta, S.; Sharma, P.; Bhardwaj, P.; Sharma, P.; Lugova, H.; Krishnapillai, A.; Islam, S.; Haque, M.; et al. Favipiravir Use in COVID-19: Analysis of Suspected Adverse Drug Events Reported in the WHO Database. Infect. Drug Resist. 2020, 13, 4427–4438. [Google Scholar] [CrossRef]
- De Andrea, M.; Ravera, R.; Gioia, D.; Gariglio, M.; Landolfo, S. The interferon system: An overview. Eur. J. Paediatr. Neurol. 2002, 6, A41–A46. [Google Scholar] [CrossRef] [Green Version]
- Jakimovski, D.; Kolb, C.; Ramanathan, M.; Zivadinov, R.; Weinstock-Guttman, B. Interferon β for Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a032003. [Google Scholar] [CrossRef]
- Hasselbalch, H.C.; Skov, V.; Kjær, L.; Ellervik, C.; Poulsen, A.; Poulsen, T.D.; Nielsen, C.H. COVID-19 as a mediator of interferon deficiency and hyperinflammation: Rationale for the use of JAK1/2 inhibitors in combination with interferon. Cytokine Growth Factor Rev. 2021, 60, 28–45. [Google Scholar] [CrossRef]
- Yuan, J.; Zou, R.; Zeng, L.; Kou, S.; Lan, J.; Li, X.; Liang, Y.; Ding, X.; Tan, G.; Tang, S.; et al. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm. Res. 2020, 69, 599–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Chen, V.; Shannon, C.P.; Wei, X.-S.; Xiang, X.; Wang, X.; Wang, Z.-H.; Tebbutt, S.J.; Kollmann, T.R.; Fish, E.N. Interferon-α2b Treatment for COVID-19. Front. Immunol. 2020, 11, 1061. [Google Scholar] [CrossRef]
- Wang, N.; Zhan, Y.; Zhu, L.; Hou, Z.; Liu, F.; Song, P.; Qiu, F.; Wang, X.; Zou, X.; Wan, D.; et al. Retrospective Multicenter Cohort Study Shows Early Interferon Therapy Is Associated with FPVorable Clinical Responses in COVID-19 Patients. Cell Host Microbe 2020, 28, 455–464.e2. [Google Scholar] [CrossRef]
- Kaplan, S.S.; Hicks, C.B. Lopinavir/ritonavir in the treatment of human immunodeficiency virus infection. Expert Opin. Pharmacother. 2005, 6, 1573–1585. [Google Scholar] [CrossRef]
- Podzamczer, D.; King, M.S.; Klein, C.E.; Flexner, C.; Katlama, C.; Havlir, D.V.; Letendre, S.L.; Eron, J.J.; Brun, S.C.; Bernstein, B. High-Dose Lopinavir/Ritonavir in Highly Treatment-Experienced HIV-1 Patients: Efficacy, Safety, and Predictors of Response. HIV Clin. Trials 2015, 8, 193–204. [Google Scholar] [CrossRef]
- Meini, S.; Pagotto, A.; Longo, B.; Vendramin, I.; Pecori, D.; Tascini, C. Role of Lopinavir/Ritonavir in the Treatment of Covid-19: A Review of Current Evidence, Guideline Recommendations, and Perspectives. J. Clin. Med. 2020, 9, 2050. [Google Scholar] [CrossRef]
- Choi, M.J.; Kang, M.; Shin, S.Y.; Noh, J.Y.; Cheong, H.J.; Kim, W.J.; Jung, J.; Song, J.Y. Comparison of antiviral effect for mild-to-moderate COVID-19 cases between lopinavir/ritonavir versus hydroxychloroquine: A nationwide propensity score-matched cohort study. Int. J. Infect. Dis. 2021, 102, 275–281. [Google Scholar] [CrossRef]
- Yadollahzadeh, M.; Eskandari, M.; Roham, M.; Zamani, F.; Laali, A.; Yassin, Z.; Zeiaei, M.T.; Rahimian, N.; Moetamed, N.; Aliakbar, A.; et al. Evaluation of Sovodak (Sofosbuvir/Daclatasvir) Treatment Outcome in COVID-19 Patient’s Compared with Kaletra (Lopinavir/ritonavir): A Randomized Clinical Trial. Res. Sq. 2021, 2021, 1–18. [Google Scholar] [CrossRef]
- Hossain, M.J.; Rahman, S.M.A. Repurposing therapeutic agents against SARS-CoV-2 infection: Most promising and neoteric progress. Expert Rev. Anti. Infect. Ther. 2020, 19, 1009–1027. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, R.; Zhang, H.; Liu, J.; Xu, M.; Hu, H.; Li, Y.; Zhao, L.; Li, W.; Sun, X.; et al. The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discov. 2020, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, W.; Peng, B.; Peng, W.; Zhang, Y.; Wang, Y.; Wan, Y.; Chang, J.; Mao, L.; Miao, X.; et al. Potential of Arbidol for Post-exposure Prophylaxis of COVID-19 Transmission: A Preliminary Report of a Retrospective Cohort Study. Curr. Med. Sci. 2020, 40, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Chen, Y.; Yuan, J.; Yi, P.; Ding, C.; Wu, W.; Li, Y.; Ni, Q.; Zhou, R.; Li, X.; et al. Clinical Efficacy of Arbidol in Patients with 2019 Novel Coronavirus-Infected Pneumonia: A Retrospective Cohort Study. SSRN Electron. J. 2020, 2020, 1–28. [Google Scholar] [CrossRef]
- Yadegarinia, D.; Tehrani, S.; Abolghasemi, S.; Zarghi, A.; Sali, S.; Zolfaghari, F. Evaluation of the Efficacy of Arbidol in Comparison with the Standard Treatment Regimen of Hospitalized Patients with Covid-19: A Randomized Clinical Trial. Arch. Clin. Infect. Dis. 2020, 15, 1–6. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Zhu, B.; Zeng, J.; Hong, W.; He, X.; Chen, J.; Zheng, H.; Qiu, S.; Deng, Y.; et al. Associations of clinical characteristics and antiviral drugs with viral RNA clearance in patients with COVID-19 in Guangzhou, China: A retrospective cohort study. medRxiv 2020. [Google Scholar] [CrossRef]
- McClellan, K.; Perry, C. Oseltamivir: A review of its use in influenza. Drugs 2001, 61, 263–283. [Google Scholar] [CrossRef] [PubMed]
- Whitley, R.J.; Hayden, F.G.; Reisinger, K.S.; Young, N.; Dutkowski, R.; Ipe, D.; Mills, R.G.; Ward, P. Oral oseltamivir treatment of influenza in children. Pediatr. Infect. Dis. J. 2001, 20, 127–133. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Sanders, J.M.; Monogue, M.L.; Jodlowski, T.Z.; Cutrell, J.B. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 323, 1824–1836. [Google Scholar] [CrossRef]
- Wu, R.; Wang, L.; Kuo, H.-C.D.; Shannar, A.; Peter, R.; Chou, P.J.; Li, S.; Hudlikar, R.; Liu, X.; Liu, Z.; et al. An Update on Current Therapeutic Drugs Treating COVID-19. Curr. Pharmacol. Rep. 2020, 6, 56–70. [Google Scholar] [CrossRef]
- Mancilla-Galindo, J.; García-Méndez, J.Ó.; Marquéz-Sánchez, J.; Reyes-Casarrubias, R.E.; Aguirre-Aguilar, E.; Rocha-González, H.I.; Kammar-García, A. All-cause mortality among patients treated with repurposed antivirals and antibiotics for COVID-19 in Mexico City: A real-world observational study. EXCLI J. 2021, 20, 199–222. [Google Scholar] [CrossRef]
- Deeks, E.D. Darunavir/Cobicistat/Emtricitabine/Tenofovir Alafenamide: A Review in HIV-1 Infection. Drugs 2018, 78, 1013–1024. [Google Scholar] [CrossRef]
- Chowdhury, K.H.; Chowdhury, R.; Mahmud, S.; Tareq, A.M.; Hanif, N.B.; Banu, N.; Reza, A.S.M.; Emran, T.B.; Simal-Gandara, J. Drug repurposing approach against novel coronavirus disease (COVID-19) through virtual screening targeting SARS-CoV-2 main protease. Biology 2021, 10, 2. [Google Scholar] [CrossRef]
- Halder, U.C. Predicted antiviral drugs Darunavir, Indinavir and Rimantadine can potentially bind to neutralize COVID-19 conserved proteins. Res. Sq. 2020, 2020, 1–21. [Google Scholar] [CrossRef]
- OECD. Policy Responses to Coronavirus (COVID-19). Coronavirus (COVID-19) Vaccines for Developing Countries: An Equal Shot at Recovery. Available online: https://www.oecd.org/coronavirus/policy-responses/coronavirus-covid-19-vaccines-for-developing-countries-an-equal-shot-at-recovery-6b0771e6/ (accessed on 27 July 2021).
- Bari, M.S.; Hossain, M.J.; Akhter, S.; Emran, T.B. Delta variant and black fungal invasion: A bidirectional assault might worsen the massive second/third stream of COVID-19 outbreak in South-Asia. Ethics Med. Public Health 2021, 19, 100722. [Google Scholar] [CrossRef]
- Williamson, B.N.; Feldmann, F.; Schwarz, B.; Meade-White, K.; Porter, D.P.; Schulz, J.; Van Doremalen, N.; Leighton, I.; Yinda, C.K.; Pérez-Pérez, L.; et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature 2020, 585, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Nagata, T.; Lefor, A.K.; Hasegawa, M.; Ishii, M. Favipiravir: A new medication for the Ebola virus disease pandemic. Dis. Med. Public Health Prep. 2015, 9, 79–81. [Google Scholar] [CrossRef]
- Sharun, K.; Tiwari, R.; Dhama, K.; Emran, T.B.; Rabban, A.A.; Al Mutair, A. Emerging SARS-CoV-2 variants: Impact on vaccine efficacy and neutralizing antibodies. Hum. Vaccines Immunother. 2021, 17, 1–4. [Google Scholar] [CrossRef]
- Hossain, M.J. Impact of COVID-19 pandemic among health care providers in Bangladesh: A systematic review. Bangladesh J. Infect. Dis. 2020, 7 (Suppl. S2), S8–S15. [Google Scholar] [CrossRef]
- Hossain, M.J.; Islam, M.S.; Shahriar, S.; Sanam, S.; Emran, T.B.; Khatun, C.S.; Islam, M.R.; Mitra, S.; Dhama, K. Comedication of rabeprazole sodium causes potential drug-drug interaction with diabetic drug linagliptin: In-vitro and in-silico approaches. J. Exp. Biol. Agric. Sci. 2021, 9, 528–542. [Google Scholar] [CrossRef]
- Lai, C.C.; Wang, C.Y.; Hsueh, P.R. Coinfections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J. Microbiol. Immunol. Infect. 2020, 53, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Liu, Y.; Zhong, Q.; Zhang, K.; Xu, Y.; Wang, Z. Lopinavir/ritonavir and interferon combination therapy may help shorten the duration of viral shedding in patients with COVID-19: A retrospective study in two designated hospitals in Anhui, China. J. Med. Virol. 2020, 92, 2666–2674. [Google Scholar] [CrossRef] [PubMed]
| Drug | Reference | Study Type | Country | n | ROA | Results | ADR | Interpretation |
|---|---|---|---|---|---|---|---|---|
| RDV | Beigel et al. [28] | RCT (NCT04280705) | USA, Denmark, UK, Greece, Germany, Korea, Mexico, Spain, Japan, and Singapore | 1059 patients (T = 541 and C = 521) | IV | RDV showed shortened recovery time (from 15 to 11 days) and reduced mortality rate by 4.8% | Serious ADR: T vs. C = 24.6% vs. 31.6% | Warrants RCT |
| RDV | Grein et al. [29] | Cohort | USA, Europe, Canada, and Japan | 61 | IV | 68% patients (n = 36 out of 53) showed clinical improvement | Multiple organ dysfunction syndrome, septic shock, acute kidney injury, and hypotension | Warrants RCT |
| RDV | Antinori et al. [30] | Open label (Observational) | Italy | 35 patients (ICU = 18 and Non-ICU = 17) | IV | Observed more beneficial effect for non-ICU patients after 10 days of therapy | Hyper-transaminasemia and acute kidney injury | Warrants RCT |
| RDV | Pasquini et al. [31] | Retrospective | Italy | 51 patients (T = 25, C = 26) | IV | Significantly reduced fatality rate (56% vs. 92%; p < 0.001) and improved survival rate (OR = 3.506, 95% CI = 1.768 to 6.954; p < 0.001) | NA | Significant survaival might be associated with RDV use |
| RDV | Aiswarya et al. [32] | Cohort | India | 48 | IV | Early treatment of RDV (within 48 hrs) reduced recovery and discharge time with safety and good tolerability. | Acute kidney injury and hypotension | Warrants RCT |
| RDV | Olender et al. [33] | Open level (NCT04292899) | USA, Italy, Spain, Germany, Hong Kong, Singapore, S. Korea, and Taiwan | 1130 patients (T = 312 and C = 818) | IV | RDV exerted 15.4% more recovery (adjusted odds ratio (aOR = 2.03; 95% CI = 1.34–3.08, p = 0.001) and reduced mortality of 4.9% (aOR = 0.38; 95% CI = 0.22–0.68, p = 0.001) in severe COVID-19 patients. | NA | Warrants RCT |
| RDV | Goldman et al. [34] | RCT (NCT04292899) | USA, Italy, Spain, Germany, Hong Kong, Singapore, South Korea and Taiwan | 397 patients (10-day course for 200 and 5-day course for 197) | IV | No significant difference between 5- or 10-day course | Nausea (9%), worsening respiratory failure (8%), elevated alanine aminotransferase level (7%), and constipation (7%). | Warrants RCT |
| RDV | Garibaldi et al. [35] | Retrospective | USA | 2309 patients (RDV = 158, RDV + CTS = 184 and C = 1957) | NA | Clinical improvement time is shorter in RDV group compared to control group. | Increased levels of liver enzyme or bilirubin (n = 4), kidney failure of unclear cause (n = 2), nausea (n = 1), epistaxis and tachycardia (n = 1), neck and mouth itching (n = 1) | Warrants further study |
| RDV | Tsuzuki et al. [36] | Observational study | Japan | 269 patients (T = 74 and C = 195) | NA | No statistical significance was observed in fatality rate or length of hospital stay | Elevation of liver enzyme and rash (n = 2) | Warrants further study |
| RDV | Wang et al. [37] | RCT (NCT04257656) | China | 237 patients (T = 158 and C = 79) | IV | No significant time difference for clinical improvement between the two groups (HR = 1.23, 95% CI = 0.87–1.75) | Adverse effects were reported for both groups; T = 102 (66%) and C = 50 (64%) | Numerical time reduction to clinical benefit was reported that warrants larger study |
| RDV | Spinner et al. [38] | RCT (NCT04292730) | USA, Europe, and Asia | 596 patients (T: 10-day course for 197, 5-day course for 199, and C: 200) | IV | No statistically significant change between T and C (p = 0.18 by Wilcoxon rank sum test) | Nausea (10% vs. 3%), hypokalemia (6% vs. 2%), and headache (5% vs. 3%) | RCT is warranted |
| RDV + BCN | Kalil et al. [39] | RCT (NCT04401579) | USA, UK, Singapore, South Korea, Mexico, Japan, Spain, and Denmark | 1033 patients (T = 515 and C = 518) | IV, oral | At 28-day fatality rate: T = 5.1% and C = 7.8% (HR for death = 0.65; 95% CI, 0.39 to 1.09). | Some common ADRs like hyperglycemia, anemia, decreased lymphocyte count, and acute kidney injury were present in both groups | The combination therapy was superior to only RDV. |
| RDV | Falcao et al. [40] | Observational cohort study | Lisbon, Portugal | 149 patients (HCQ = 101 and RDV = 48) | Oral | ADRs were more significant the in case of HCQ (47.5%) than RDV (12.5%). | RDV group developed ADRs like hepatobiliary disorder (8.3%), acute renal failure, nervous system disorder, and others (2.1%). | Warrants larger sample size and follow-up study |
| RDV | Goldberg et al. [41] | Real-life observational | Israel | 142 patients (T = 29 and C = 113) | IV | Reduced hospitalization in case of both non-intubated and intubated patients (3.1 vs. 1.4 days, respectively; p > 0.05) | NA | Warrants further study as sample size was small |
| RDV/CP/RDV + CP | Padilla et al. [42] | Retrospective observational study | California, USA | 106 patients (RDV = 11, CP = 53, and RDV + CP = 42) | IV | Survival rate was higher with RDV alone than with combination therapy or CP alone. | NA | Warrants further study |
| FPV | Cai et al. [43] | Open level | China | 80 patients (T = 35 and C = 45) | Oral | FPV showed significant improvement rate over the control (91.43% vs. 62.22%, respectively, p = 0.004). | T vs. C = 4 (11.43%) vs. 25 (55.56%), respectively; p < 0.001 | Warrants RCT |
| FPV | Chen et al. [44] | RCT (ChiCTR2000030254). | China | 240 patients (T = 120, C = 120) | NA | T showed shorter latency recovery from fever (difference: 1.70 days, p < 0.0001) and cough (difference: 1.75 days, p < 0.0001) | Increased serum uric acid 16 (13.79%) in FPVipiravir group, p = 0.0014 | Adverse effects are mild and manageable. |
| FPV | Lou et al. [45] | RCT (ChiCTR 2000029544). | China | 30 patients (FPV = 10 Baloxavir marboxil = 10 and control = 10) | Oral | The viral loading was 77%, 70%, and 100% and median time was 14, 14, and 15, respectively after 14-day treatment. | Respiratory failure (14), triglyceride (20), liver function abnormality (18), rash (7), and diarrhea (4) | No proven benefit was observed either addition of baloxavir marboxil or FPV under the trial dosage. |
| FPV | The New Indian Express [46] | RCT | India | 150 (FPV = 75 and control = 75) | Oral | Primarily FPV showed 28.6% numerical faster viral clearance | NA | Warrants RCT |
| FPV | The Daily Star [47] | RCT | Bangladesh | 50 | Oral | FPV showed 44% more viral clearance than placebo | No significant side effect was reported. | Warrants RCT |
| FPV | Ucan et al. [48] | Retrospective cohort | Turkey | 144 (FPV + HCQ (early) = 48, FPV + HQ (late) = 48 and HQ = 48) | Oral | Early starting of FPV had an impact on PCR negativity and the progression of the disease. | Diarrhea, nausea, and vomiting | Needs RCT |
| FPV | Alamer et al. [49] | Retrospective | Saudi Arabia | 457 (FPV = 234 and C = 223) | Oral | Improvement of discharge rate and less progression to ventilation; no effect on mortality in severe cases | Acute kidney injury, increased ALT, AST, bilirubin, cardiovascular effects, constipation, seizure, hypercalcemia, hyperphosphatemia, and hypermagnesemia | Warrants RCT |
| FPV | Zhao et al. [50] | RCT | China | 55 (FPV = 36 and C = 19) | Oral | Improvement in virus shedding and CRP decreasing | Elevated AST and ALT, hyperuricemia, hypernatremia, diarrhea, and nausea. | Warrants RCT |
| FPV | Udwadia et al. [51] | RCT | India | 150 patients (T = 75 and C = 75) | Oral | Median required time of reducing viral load between T and C was 5 days vs. 7 days and clinically median cure time was 3 days vs. 5 days, respectively | ADR: T vs. C = 36% vs. 8%, respectively | T showed significant improvement in time to clinical recovery |
| rhIFN-α | Meng et al. [52] | RTC (NCT04320238) | China | 2944 patients (low risk = 2415 and high risk = 529) | Nasal drop | Observed negative clinical symptoms for pneumonia in both high- and low-risk groups after 28 days | Flu-like symptoms (burning pain and itching), allergic reactions (rash, nausea, chest distress, palpitation, and flushing) | Warrants RCT |
| IFN-alpha 2b | Pandit et al. [53] | RCT | India | 40 (T = 20 and C = 20) | SC | Better clinical improvement (IFN = 95% and C = 68.42%) and viral shedding (IFN = 95% and C = 68%). | Respiratory distress, hypoxia, nausea, vomiting, mouth dryness, and headache | Further confirmatory studies needed |
| IFN-alpha 2b | Yu et al. [54] | Retrospective | China | 1401 (T = 852 and C = 549) | Inhalation | Lower viral shedding time, improved clinical outcome | NA | Warrants RCT |
| IFN-lambda | Feld et al. [55] | RCT | Canada | 60 (IFN = 30 and C = 30) | SC | Shortening of viral shedding duration, prevention of clinical deterioration, and acceleration of viral decline. | Confusion, rectal bleeding, pneumonia, and pulmonary embolism | Warrants larger studies |
| ARB | Wang et al. [56] | Cohort | China | 69 | NA | Improved hospital discharge rate and reduced mortality rate by 7.5% | NA | Study with larger samples needed |
| ARB | Lian et al. [57] | Retrospective | China | 81 patients (ARB = 45 and control = 36) | NA | ARB showed better CT scores than control (IQR 7e14) vs. 8 (IQR 5e10), p < 0.05 | 5/45 (11%) ARB group and 3/36 (8%) control group showed digestive symptoms such as diarrhea and nausea (p = 0.49) | Warrants RCT |
| ARB | Zhu et al. [58] | Retrospective | China | 50 patients (ARB = 16 and LPV/RTV = 34) | NA | After day 14, viral load was found between (ARB vs. LPV/RTV = 0% vs. 44.1%) | NA | ARB superior to LPV/RTV |
| ARB | Chen et al. [59] | Observational | China | 62 patients (T = 42 and C = 20) | Oral | Test group reduced hospital stay duration more than control group | Nausea (test: n = 7, control: n = 3), diarrhea (test: n = 2, control: n = 1), and dizziness (test: n = 2, control: n = 1) | Warrants further study |
| ARB, oseltamivir | Liu et al. [60] | Retrospective cohort | China | 504 | NA | Both drugs reduced fatality rate | Nausea | ARB and oseltamivir reduced fatality rate. |
| ARB | Yang et al. [61] | RCT | China | 164 patients (ARB = 82 and non-ARB = 82) | Oral | In the ARB group The uninfected rate was significantly higher than in the non-ARB group. | NA | Needs a multicenter cohort study |
| ARB | Zeng et al. [62] | Retrospective cohort | China | 1019 (ARB = 788 and C = 231) | NA | ARB-treatment results in less in-hospital death for patients with severe and critical COVID-19. | NA | Warrants RCT |
| Oseltamivir | Tan et al. [63] | Cohort | China | 333 patients (oseltamivir = 14, ARB = 277, corticosteroid = 15, HCQ = 8, LPV/RTV = 14) | NA | The oseltamivir group showed a significantly shorter hospital stay duration than the ARB, corticosteroids, and lopinavir/ritonavir groups. | NA | Oseltamivir prudently considered as a combination therapy |
| Oseltamivir | Moreno et al. [64] | Observational | Spain | 2124 (T (early) = 529 and C (late) = 1595) | NA | Early treatment was associated with reduced mortality of critically ill patients infected with influenza pneumonia. | NA | Further studies needed |
| DRV/c | Deng et al. [65] | Retrospective | China | 66 patients (DRV/c = 32 and control = 34) | Oral | DRV/c significantly shortened nucleic acid conversion duration from the onset of symptoms to admission. | Respiratory failure, upset stomach | Further studies needed |
| DRV/c | Kim et al. [66] | Retrospective | South Korea | 110 patients (DRV-c group = 14 and control = 96) | NA | Overall, the DRV/c group showed a lower fatality rate than the control group (odds ratio (OR) 0.20, 95% CI = 0.04–0.89, p = 0.035). | NA | DRV-c showed significant survival benefit in critically ill Patients. |
| DRV/c | Chen et al. [67] | RCT (NCT04252274) | China | 30 patients (DRV/c = 15 and control = 15) | Oral | The difference in negative PCR conversion rate in between two groups at day 7 was DRV/c vs. control = 9/15 (60.0%) vs. 7/15 (46.7%), respectively, p = 0.72. | In the DRV/c group, diarrhea (20%), anemia, elevated transaminase levels (13.3%), and renal dysfunction (13.3%) were observed. | 5 days of DRV/c did not increase the rate of negative conversion vs. standard of care alone. |
| FPV + HQ | Guner et al. [68] | Retrospective | Turkey | 824 (HQ = 604, FPV = 100 and FPV + HQ = 120) | Oral | No statistically significant difference between the three groups | NA | Warrants RCT |
| FPV + CQ | Dabbous et al. [69] | RCT | Egypt | 96 (FPV = 48 and CQ = 46) | Oral | Improvement in hospital stay and need for mechanical ventilation in FPV groups | NA | Warrants a larger and diverse sample size |
| FPV vs. LPV/RTV | Kocayiğit et al. [70] | Observational | Turkey | 107 (FPV = 65 and RTV/LPV = 42) | Oral | Significantly shorter hospital stay in the case of FPV | Coinfection, ARDS, acute kidney disease, and MODS | Warrants larger sample |
| FPV vs. HCQ | Dabbous et al. [71] | RCT | Egypt | 100 (FPV = 50 and HCQ = 50) | Oral | No significant difference in hospital stay | Elevated D-dimer and ferritin, cardiovascular complications | Warrants larger sample |
| FPV + IFN beta-1b | Khamis et al. [72] | RCT (NCT04385095). | Oman | 89 patients (FPV + IFN beta-1b = 44 and HCQ = 45) | Oral | No significant difference in hospital stay between FPV and HCQ (7 vs. 7 days; p = 0.948), ICU transfer rate (18.2% vs. 17.8%; p = 0.960), or discharge rate (65.9% vs. 68.9%; p = 0.764), and fatality rate was 11.4% vs. 13.3%, respectively; p = 0.778 | liver injury | No notable difference was found in clinical results between FPV + inhaled IFN beta-1b and HCQ. |
| LPV/RTV | Kim et al. [73] | Cohort | Korea | 65 patients (T = 31, C = 34) | NA | No significant difference in time to clinical development between T and C groups (18 days vs. 21 days) | One serious ADR was detected for the T group and two serious ADRs were detected for the C group. | Warrants RCT |
| LPV/RTV, HCQ | Lee et al. [74] | Cohort | South Korea | 72 patients (LPV/RTV = 45 and HCQ = 27) | Oral | The LPV/RTV group showed lower failure rate (41% vs. 2%; p = 0.001) and disease progression than the HCQ group (18% vs. 44%, respectively; p = 0.03). | Diarrhea, abnormal stools, abdominal pain, nausea, vomiting, and asthenia | LPV/RTV showed more efficacy than HCQ in improving clinical symptoms |
| LPV/RTV | Yu et al. [75] | Cohort | China | 128 | NA | The significant median period of viral load reduction time between with influenza and without influenza was 17.0 vs. 12.0 days, respectively; p < 0.001. Besides, the T group showed faster pneumonia resolution than the C group (37% vs.1%; p = 0.001). | NA | Additional robust scientific studies with proper controls are needed. |
| LPV/RTV + SOC | Lecronier et al. [76] | Retrospective | Paris, France | 80 patients (LPV/RTV + SOC = 20, SOC = 22, and HCQ + SCQ = 38 patients) | Oral | No significant difference at ventilator-free days at 28 days and mortality rate (at 14- and 28-days) in these 3 groups | NA | Warrants RCT |
| LPV/RTV + ARB | Lan et al. [77] | Retrospective | China | 73 patients (T: LPV/RTV + ARB = 39 and C: LPV/RTV = 34) | Oral | The T group showed a 4.8% higher recovery rate and a 1.5-day shorter hospital stay than the C group. | NA | No benefit was observed between the two groups. |
| LPV/RTV + CQ | Gao et al. [78] | Retrospective | China | 129 patients (LPV/RTV = 51, SOC = 59, and chloroquine = 19) | Oral | Neither LPV/RTV nor CQ improved prognosis or shortened the clinical course. | NA | Warrants RCT |
| LPV/RTV | Karolyi et al. [79] | Cohort | Austria | 156 patients (T = 47 and C = 20) | IV | No significant difference in mortality rate (8.5% vs. 15%; p = 0.418), ICU admission rate (12.8% vs. 20%; p = 0.47), or hospital stay (11 days vs. 9 days, respectively; p = 0.34) was observed. | Diarrhea (n = 7) and liver enzyme elevation (n = 7) | Warrants RCT |
| LPV/RTV | Shi et al. [80] | Non-RCT (ChiCTR2000029400/ChiMCTR2000002940). | China | 60 patients (LPV/RTV = 20, Huashi Baidu Formula + LPV/RTV = 20, Huashi Baidu Formula = 20) | Oral | No significant difference was observed for clinical remission rate between the 3 groups (95% (19/20), 100% (20/20), and 100% (20/20)). | NA | Warrants larger RCT |
| LPV/RTV | Cao et al. [81] | RCT (ChiCTR2000029308) | China | 199 patients (T = 99 and C = 100) | Oral | No significant difference between LPV/RTV and SOC was observed in clinical improvement or mortality rate. | GI ADRs were more common in the treatment group. | No significant difference was observed between the two groups. |
| LPV/RTV, ARB | Li et al. [82] | RCT (NCT04252885) | China | 44 patients (LPV/RTV = 21, ARB = 16, and SOC = 7) | Oral | No significant difference between groups in antipyresis, cough alleviation, or improvement of chest computed tomography (CT) | 12 and 5 patients were suffering from ADRs in the LPV/RTV and ARB groups, respectively. | No significant benefit was observed among these three groups. |
| LPV/RTV + RBV + IFN beta-1b | Hung et al. [83] | RCT (NCT04276688) | China | 127 (combination group 86 and control (LPV/RTV) = 41) | Oral, SC | The combination group showed a significantly shorter recovery time than the control (7 days vs. 12 days, respectively; HR = 4.37, 95% CI = 1.86–10.24; p = 0.0010). | Diarrhea (52 (41%)), fever (48 (38%)), nausea (43 (34%)), and raised alanine transaminase level (18 (14%)) | Early triple combination therapy showed lower recovery time with safety than only LPV/RTV. |
| LPV/RTV + IFN-a | Huang et al. [84] | RCT (ChiCTR2000029387) | China | 101 patients (LPV/RTV + IFN-α = 36, RBV + IFN-α = 33, and LPV/RTV + RBV + IFN-α + = 32 | Oral | No statistically significant differences in viral clearance among these 3 groups (LPV/RTV + IFN-a vs. RBV + IFN-a vs. RBV + LPV + IFN-a = 12 days vs. 13 days vs. 15 days, respectively; p = 0.23) | Adverse gastrointestinal events were higher for the LPV/RTV + RBV + IFN-a group than the LPV/RTV + IFN-a and RBV + IFN-a groups | No significant difference was observed among the three groups. |
| LPV/RTV | RECOVERY Collaborative Group [85] | RCT (NCT04381936) | UK | 5040 patients (LPV/RTV = 1616 and standard of care = 3424) | Oral | No significant difference at 28-day fatality rate (LPV/RTV vs. SOC = 23% vs. 22%, respectively; p = 0.60) | NA | The treatment is not effective for COVID-19. |
| LPV/RTV + recombinant IFN beta-1b | Arabi et al. [86] | RCT (NCT02845843) | Saudi Arabia | 95 patients (LPV/RTV + recombinant IFN beta-1b = 43 and placebo = 52) | Oral, SC | Intervention therapy against MERS-CoV led to lower fatality rate (RR = 0.19; 95% CI = 0.05 to 0.75) than placebo | Serious ADRs: Intervention (4 (9%)) and placebo (10 (19%)) | Further study with larger sample size |
| CQ+ LPV/RTV | Sevilla-Castillo et al. [87] | Retrospective | Mexico | 61 (LPV/RTV = 27, CQ = 11, combination = 17 and C = 6) | Oral | Both the drugs were ineffective in COVID treatment; combination therapy enhanced mortality. | Increase in lactate dehydrogenase and ferritin | Warrants further investigation |
| IFN beta 1b + LPV/RTV + RBV vs. FPV | Malhani et al. [88] | Cohort | Saudi Arabia | 222 (IFN triple therapy = 68 and FPV = 154) | Oral, SC | IFN-based triple therapy decreased the mortality rate in non-critical patients. | Nausea and diarrhea | Warrants RCT |
| LPV/RTV | Lepage et al. [89] | Retrospective | Canada | 12 | Oral | Trough concentration is higher when an HIV dose regimen is used; dose tampering might be needed to avoid risk in COVID-19 patients. | GI symptoms, electrolyte imbalance, liver enzyme disturbance, and TG elevation | Warrants RCT |
| LPV/RTV+ HCQ | Schneider et al. [90] | Retrospective | Germany | 79 (Non-ICU- LPV/RTV+ HCQ = 14 and C = 14; ICU- LPV/RTV = 30 and 4C = 21) | Not mentioned | Triple therapy may cause elevated acute kidney injury in non-severe COVID patients. | Slight hematuria and proteinuria | Warrants RCT |
| IFN-k + TFF2 + SOC | Fu et al. [91] | RCT (ChiCTR2000030262) | China | 80 patients (experimental group = 40 and control group = 40) | inhalation | The experimental group exhibited significantly shorter days in viral RNA (-ve) conversion than the control group (3.8 vs. 7.40 days, respectively). | No discomfort or complication was reported. | The experimental combination with SOC is safe and superior to SOC alone. |
| rSIFN vs. traditional IFN-alpha | Li et al. [92] | RCT | China | 94 (rSIFN = 46 and IFN-alpha = 48) | Nasal (Nebulization) | Shortened the clinical improvement time (rSIFN vs. INF-alpha = 11.5 days vs. 14 days, respectively), clinical improvement rate (rSIFN = 93.5%, IFN-alpha = 77.1%), radiological improvement time (rSIFN = 8 days, IFN-alpha = 10 days), and viral shedding time (rSIFN = 7 days, IFN-alpha = 10 days) | Decreased appetite | rSIFN was associated with shorter time of clinical improvement. However, a broader-range trial is needed. |
| IFN- alpha + RBV | Li et al. [93] | Retrospective cohort | China | 2037 (RBV = 840, IFN-alpha = 214, RBV + IFN-alpha = 227, and C = 756) | NA | No significant difference in mortality rate between the two groups | Decreased hemoglobin and increased uric acid | Should avoid this combination in the treatment of COVID-19 |
| IFN-beta 1a vs. IFN-beta 1b | Darazam et al. [94] | RCT | Iran | 60 (IFN-beta 1a = 20, IFN-beta 1b = 20, and C = 20) | SC | Improved time to clinical improvement in IFN-beta 1a group | Liver injury, ARDS | Warrants larger studies |
| ARB + LHQW | Fang et al. [95] | Cohort | China | 162 | Oral | Significantly shortened nucleic acid negativity, improved chest CT, and reduced hospital stay duration | NA | Warrants RCT |
| ARB + LPV/RTV | Wen et al. [96] | Cohort | China | 178 | NA | Significant difference in proportion of deterioration changing from mild/moderate to severe/critical type at day 7 was found | ADRs were higher than the SOC group. | Warrants larger studies |
| ARB + LPV/RTV | Deng et al. [97] | Cohort | China | 33 patients (ARB + LPV/RTV = 16 and LPV/RTV = 17) | Oral | Observed significant difference in SARS-CoV-2 negativity between ARB + LPV/RTV and LPV/RTV groups at day 7 (75% vs. 35%; p < 0.05) and day 14 (94% vs. 52.9%, respectively) | Digestive upset (43.7%), such as mild diarrhea and nausea | Warrants RCT |
| ARB + Shufeng Jiedu capsules | Chen et al. [98] | Cohort | China | 200 (experimental = 100 and control = 100) | Oral | The experimental group showed significantly more efficacy in pneumonia resolution thank the control group (p < 0.05). | Nausea, allergic reaction, abdominal pain, and diarrhea were common for both groups. | Warrants RCT |
| ARB + Shufeng Jiedu capsules | Qu et al. [99] | Observational | China | 70 patients (ARB + Shufeng Jiedu capsules = 40 and ARB group = 30) | Oral | The combination group (ARB + Shufeng Jiedu capsules) reduced negative conversion time significantly more than the ARB group (p < 0.05). | NA | The combination therapy was better than the ARB alone. |
| ARB + LQ granules | Yu et al. [100] | Observational | China | 295 patients (observation group = 147 and control group = 14) | Oral | Effective rate was significantly higher in observation group than control group (80.95% vs. 64.86%, respectively) | NA | Warrants further intensive study |
| ARB + moxifloxacin | Xi et al. [101] | Cohort | China | 94 | NA | Treatment with ARB + moxifloxacin reduced viral load and inflammation. | NA | Warrants further clinical verification |
| ARB vs. CQ | Huang et al. [102] | Cohort (ChiCTR2000030931) | China | 27 patients (ARB = 11, CQ = 10, and LPV/RTV = 6) | NA | The median viral shedding interval for LPV/RTV and CQ were 13 and 5 days, respectively. Hospitalization duration was in ARB (11.7 ± 3.7 days; p < 0.001) and CQ (9.3 ± 1.8 days, respectively; p < 0.001) | 20% of patients from the CQ group (nausea, vomiting, dysphoria, or blurred vision), 9.1% of the ARB group, and 50% of the LPV/RTV group had diarrhea. | ARB and CQ reduced hospital stay and hospitalization expenses. |
| ARB + IFN-a2b | Xu et al. [103] | Non-RCT | China | 141 | Oral | No significant difference was reported in viral load clearance and hospital stay duration between ARB + IFN-a2b and IFNa-2b | NA | Warrants RCT |
| ARB vs. KALETRA | Nojomi et al. [104] | RCT (IRCT20180725040596N2) | Iran | 100 patients (ARB = 50 and KALETRA = 50) | Oral | ARB significantly reduced hospital stay duration compared to the KALETRA arm (7.2 vs. 9.6 days, respectively; p = 0.02). | Nausea and vomiting | Warrants more studies with larger sample size |
| ARB + HCQ | Ghaderkhani et al. [105] | RCT | Iran | 56 patients, 3 patients left from ARB group; ARB (n = 28) and control (n = 25) arms | NA | After 7 days, the ARB + HCQ group showed significantly faster recovery in dry cough (p = 0.001), weakness (p = 0.004), gastrointestinal symptoms (p = 0.043), and shortness of breath (p = 0.001) than the control group. | Dermatitis, GI symptoms (nausea and diarrhea), jaundice, and neurological symptoms were not observed in any of the patients after 14 days. | The combination group showed a better effect in the recovery process. |
| ARB vs. CQ | Li et al. [106] | Retrospective | China | 62 (ARB = 42 and CQ = 20) | NA | Length of hospital stay was significantly lower for the ARB than the HCQ group. | Vomiting, hepatic function impairment, and ALT elevation | Warrants RCT |
| Oseltamivir + antibacterial agents (levofloxacin + garenoxacin) | Chiba et al. [107] | Cohort | Japan | 16 patients | Oral | Early treatment with oseltamivir shortened fever duration time compared to the late treatment (31 ± 21 h vs. 94 ± 38 h, respectively; p < 0.001). | Mild side effects were present. | Early use of oseltamivir might reduce fever duration. |
| DRV/RTV + HCQ | Meriglier et al. [108] | Cohort | France | 46 patients (HCQ + DRV/RTV = 25 and HCQ + LPV/RTV = 21) | Oral | Combination therapy led to severe illness. | ECG abnormalities (n = 4), repolarization disorder (n = 3), conduction disorder (n = 1), diarrhea grade I/II (n = 8), and hepatic enzyme increased (n = 1) | Concomitant use was not safe. |
| INF beta-1a vs. medications of national protocols | Davoudi-Monfared et al. [109] | RCT (IRCT20100228003449N28) | Iran | 81 (T = 42 and C = 39) | SC | Significantly improved the discharge rate and decreased the 28-day mortality rate. | For the IFN beta-1a group: IFN-related injection reactions (19.04), neuropsychiatric problems (9.52%) | Needs more extensive study |
| Novaferon + LPV/RTV | Qu et al. [110] | Case-control study | China | 170 (male = 81 and female = 89) | NA | Novaferon + LPV/RTV exerted a lower duration of hospital stay and negative nucleic acid conversion compared to LPV/RTV or combination with IFN or ARB. | NA | Novaferon + LPV/RTV may have better efficacy than these control groups. Warrants RCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.J.; Jannat, T.; Brishty, S.R.; Roy, U.; Mitra, S.; Rafi, M.O.; Islam, M.R.; Nesa, M.L.; Islam, M.A.; Emran, T.B. Clinical Efficacy and Safety of Antiviral Drugs in the Extended Use against COVID-19: What We Know So Far. Biologics 2021, 1, 252-284. https://doi.org/10.3390/biologics1020016
Hossain MJ, Jannat T, Brishty SR, Roy U, Mitra S, Rafi MO, Islam MR, Nesa ML, Islam MA, Emran TB. Clinical Efficacy and Safety of Antiviral Drugs in the Extended Use against COVID-19: What We Know So Far. Biologics. 2021; 1(2):252-284. https://doi.org/10.3390/biologics1020016
Chicago/Turabian StyleHossain, Md. Jamal, Tabassum Jannat, Shejuti Rahman Brishty, Urmi Roy, Saikat Mitra, Md. Oliullah Rafi, Md. Rabiul Islam, Mst. Luthfun Nesa, Md. Ariful Islam, and Talha Bin Emran. 2021. "Clinical Efficacy and Safety of Antiviral Drugs in the Extended Use against COVID-19: What We Know So Far" Biologics 1, no. 2: 252-284. https://doi.org/10.3390/biologics1020016
APA StyleHossain, M. J., Jannat, T., Brishty, S. R., Roy, U., Mitra, S., Rafi, M. O., Islam, M. R., Nesa, M. L., Islam, M. A., & Emran, T. B. (2021). Clinical Efficacy and Safety of Antiviral Drugs in the Extended Use against COVID-19: What We Know So Far. Biologics, 1(2), 252-284. https://doi.org/10.3390/biologics1020016








