Overview of COVID-19 Disease: Virology, Epidemiology, Prevention Diagnosis, Treatment, and Vaccines
Abstract
1. Introduction
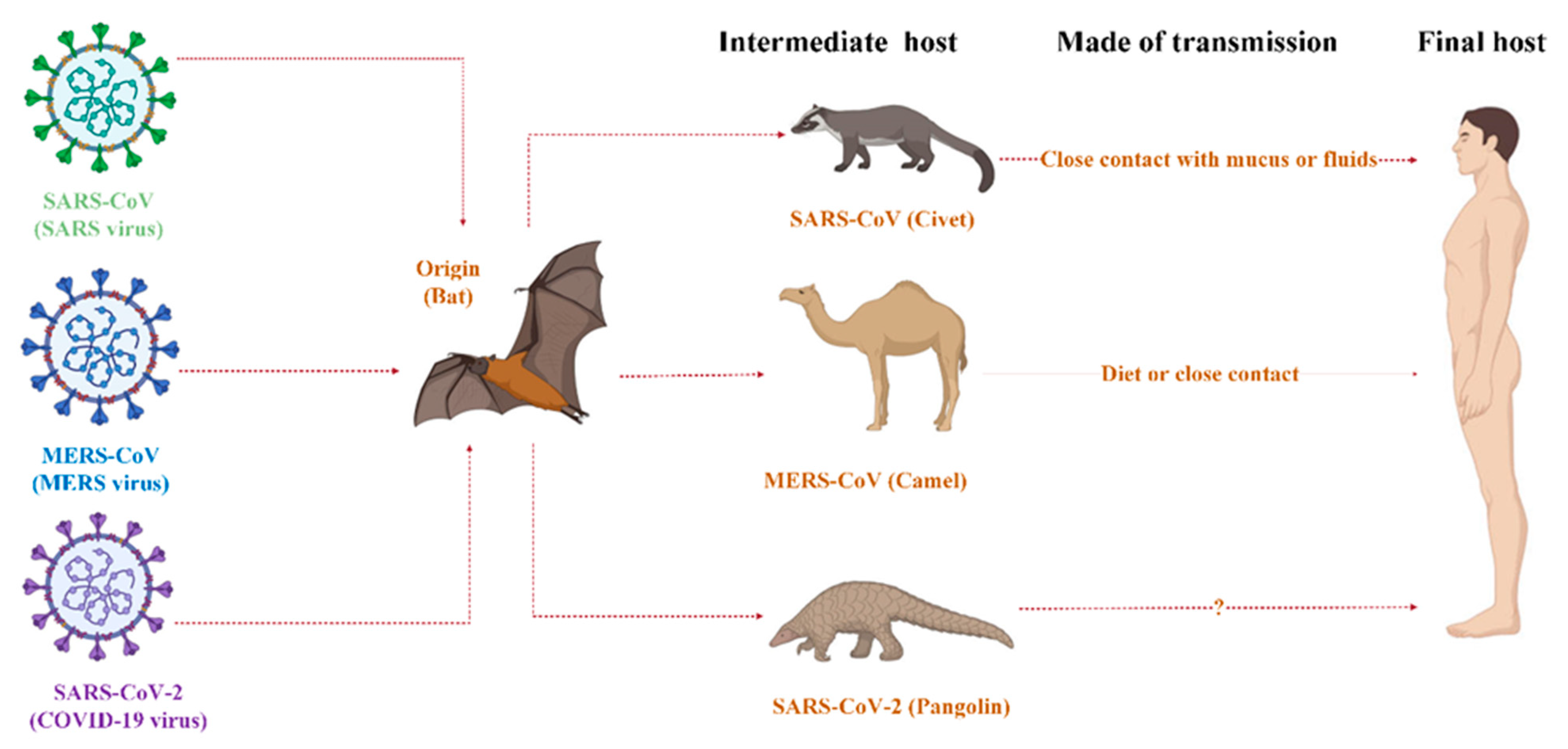
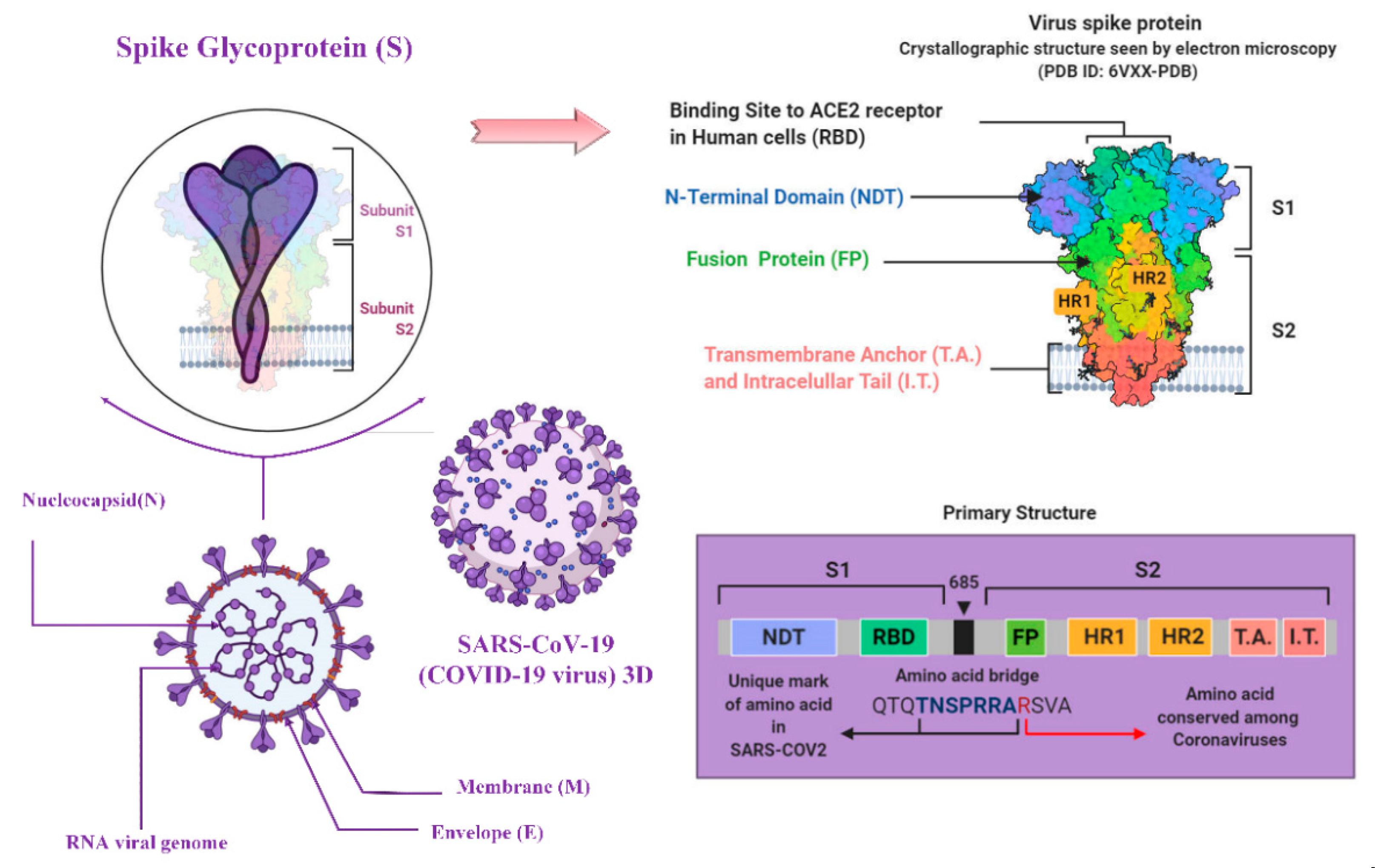
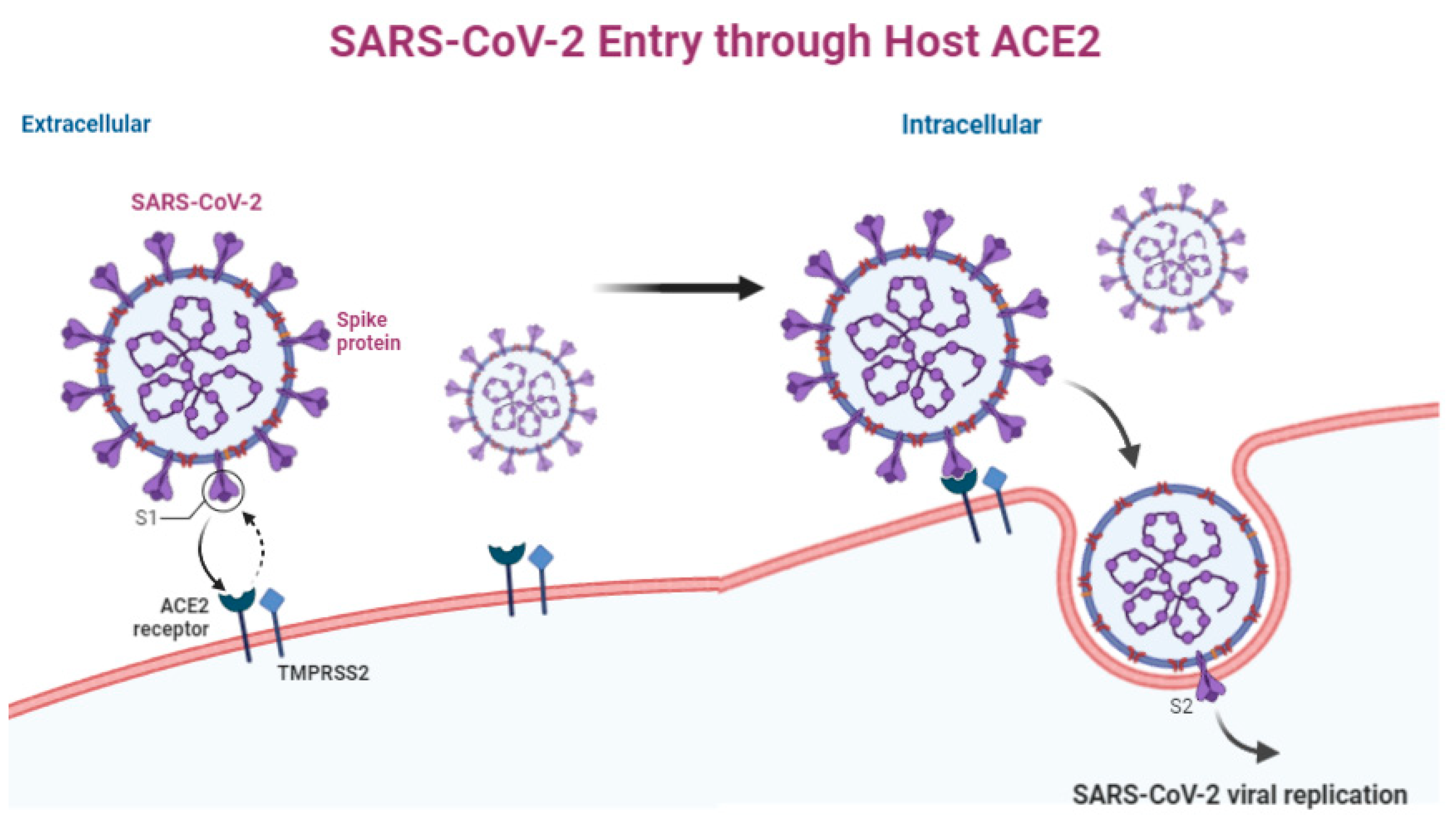
2. Virology
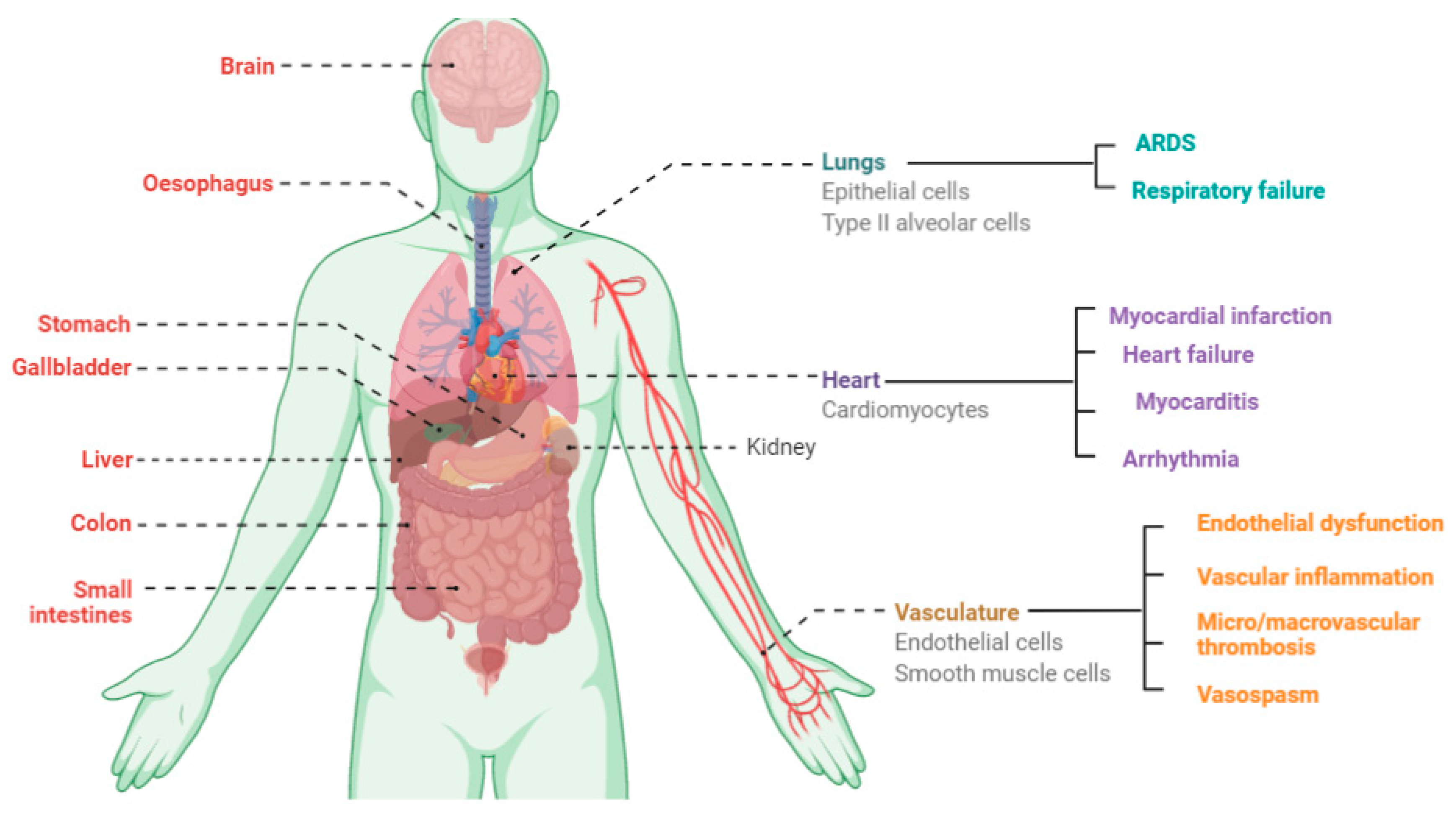
3. Symptoms
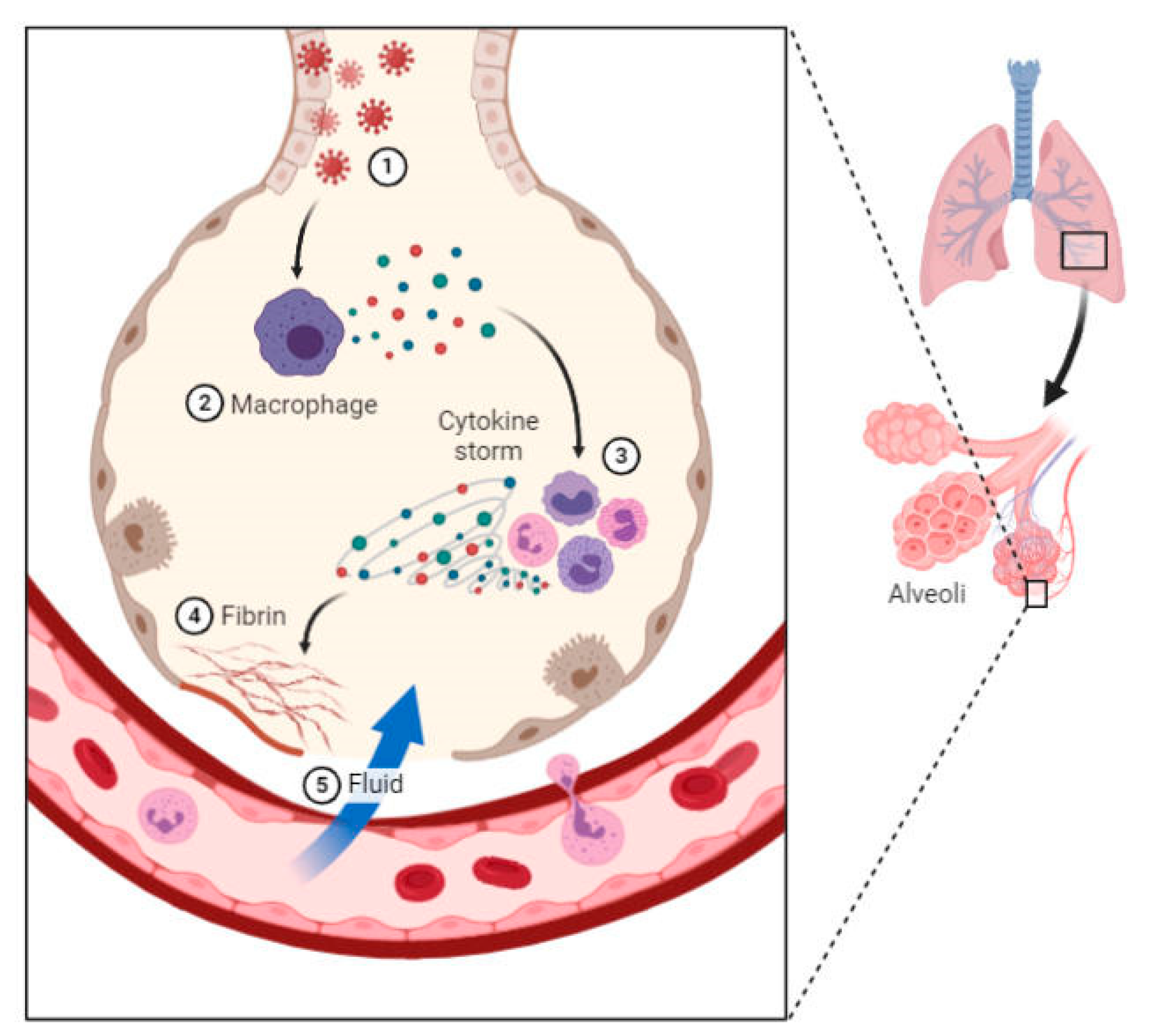
- (1)
- Infection of lung cells by COVID-19.
- (2)
- Cytokine production through virus detection by immune cells (macrophages).
- (3)
- Creating a cycle of inflammation in lung cells by further uptake of immune cells (white blood cells) through the cytokine phenomenon.
- (4)
- Fibrin formation and further damage.
- (5)
- Filling of the lung cavities due to the infiltration of fluids into the weak blood vessels, followed by respiratory damage.
4. Transmission Routes
- (1)
- Make sure the patient is in a convenient place.
- (2)
- The necessity of proper use of personal protective equipment (PPE).
- (3)
- Restrict the transportation and movement of patients as much as possible.
- (4)
- Utilization of disposable or patient care equipment as much as possible.
- (5)
- Prioritization disinfection and scouring of rooms.
- (1)
- The necessity of keeping the patient in the air infection isolated room (AIIR).
- (2)
- limitation on the movement of health care personnel to patients’ rooms.
- (3)
- The need for proper use of personal protective equipment (PPE).
- (4)
- Confine movement and transport of patients.
- (5)
- Immunization of people suspected of COVID-19 from unguarded contact.
5. Prevention
6. Epidemiology
7. Diagnosis
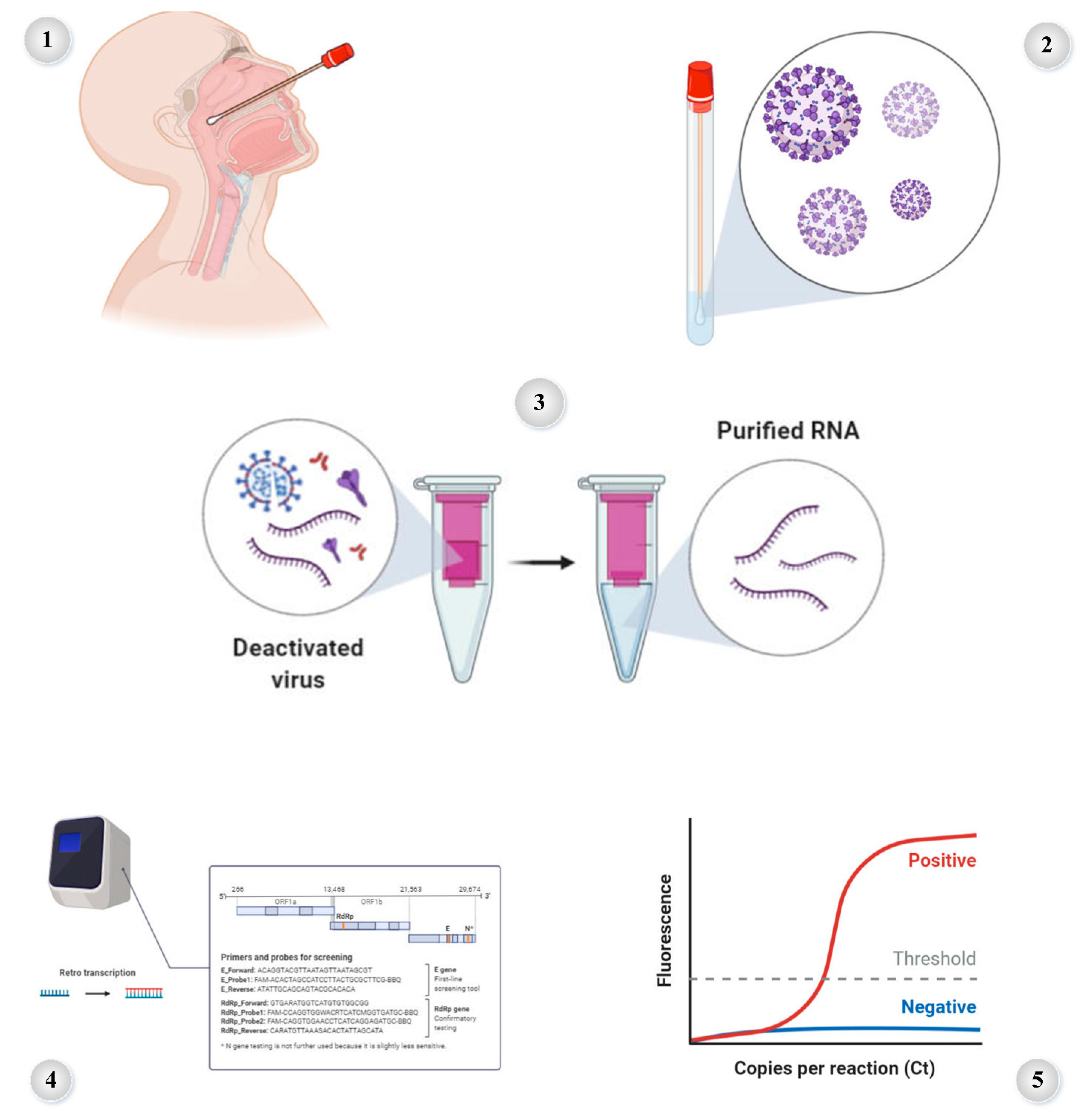
7.1. RT-PCR Method
7.2. CT-Scan
7.3. The Serological Antibody Blood Test
- (1)
- Sample loading: add a drop of blood or serum in the sample well (S).
- (2)
- Buffer loading: add dilution phosphate saline buffer to sample well.
- (3)
- Sample incubation: capillary action moves sample across lateral flow test.
- (4)
- Antibody-antigen recognition: antibodies with specificity for COVID-19 bind to gold COVID-19-antigen conjugates in the conjugate pad.
- (5)
- COVID-19 antibody detection: sample enters testing well (T), and COVID-19 antibody–antigen complex binds to immobilized anti-human lgG/IgM antibodies.
- (6)
- Control antibody detection: rabbit antibody-gold conjugate binds to immobilized anti-rabbit lgG antibodies.
- (7)
- Interpreting results: Positive: one strip each in C well and T well, Negative = one strip in C well.
7.4. Artificial Intelligence (AI)
8. The Role of Nanotechnology in Diagnostics and Treatment of COVID-19
- (1)
- Design of safe personal protective equipment (PPE) to prevent infection and increase healthcare workers’ safety.
- (2)
- Production of antiviral disinfectants and surface coatings that inactivate the virus and prevent its spread.
- (3)
- Design of precise and sensitive nano-based sensors for rapid detection of infection or immunological response.
- (4)
- Production of new drugs to increase activity, reduce toxicity, and continuous release.
- (5)
- Targeting drug delivery.
- (6)
- Vaccination production (enhancement of humoral and cellular immune responses).
9. Treatment
10. Vaccines
- (1)
- Active ingredients Viral or bacterial antigens that directly stimulate the immune system but cannot cause disease.
- (2)
- Adjuvants Aluminum salts in small quantities that help to boost the immune response to the vaccine.
- (3)
- Antibiotics prevent contamination by bacteria during the vaccine manufacturing process.
- (4)
- Stabilizers Sugar/gelatin keeps the valuable vaccine until it is administered to a patient.
- (5)
- Preservatives Thimerosal prevents dangerous bacterial or fungal contamination (only used for influenza vaccines).
- (6)
- Trace components Residual inactivating ingredients such as formaldehyde, and residual cell culture materials (present in small quantities that do not pose a safety concern).
11. The Effect of COVID-19 on Pregnant Women
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khaykelson, D.; Raviv, U. Studying viruses using solution x-ray scattering. Biophys. Rev. 2020, 12, 41–48. [Google Scholar] [CrossRef]
- Gardner, P. Virus infections and respiratory disease of childhood. Arch. Dis. Child. 1968, 43, 629. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Payne, S. Family Coronaviridae. Viruses 2017, 149, 149–158. [Google Scholar]
- Pal, M.; Berhanu, G.; Desalegn, C.; Kandi, V. Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): An Update. Cureus 2020, 12, e7423. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, D.; Kusov, Y.; Nian, Y.; Ma, Q.; Wang, J.; Von Brunn, A.; Leyssen, P.; Lanko, K.; Neyts, J. α-Ketoamides as broad-spectrum inhibitors of coronavirus and enterovirus replication: Structure-based design, synthesis, and activity assessment. J. Med. Chem. 2020, 63, 4562–4578. [Google Scholar] [CrossRef]
- Shivanika, C.; Kumar, D.; Ragunathan, V.; Tiwari, P.; Sumitha, A. Molecular docking, validation, dynamics simulations, and pharmacokinetic prediction of natural compounds against the SARS-CoV-2 main-protease. J. Biomol. Struct. Dyn. 2020. [Google Scholar] [CrossRef]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef]
- Mittal, A.; Manjunath, K.; Ranjan, R.K.; Kaushik, S.; Kumar, S.; Verma, V. COVID-19 pandemic: Insights into structure, function, and hACE2 receptor recognition by SARS-CoV-2. PLoS Pathog. 2020, 16, e1008762. [Google Scholar] [CrossRef] [PubMed]
- Neerukonda, S.N.; Katneni, U. A Review on SARS-CoV-2 Virology, Pathophysiology, Animal Models, and Anti-Viral Interventions. Pathogens 2020, 9, 426. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.-Y.; Yang, J.-S.; Kim, J.W.; Kim, V.N.; Chang, H. The architecture of SARS-CoV-2 transcriptome. Cell 2020, 181, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, D.; Fielding, B.C. Coronavirus envelope protein: Current knowledge. Virol. J. 2019, 16, 1–22. [Google Scholar] [CrossRef]
- Kang, S.; Yang, M.; Hong, Z.; Zhang, L.; Huang, Z.; Chen, X.; He, S.; Zhou, Z.; Zhou, Z.; Chen, Q. Crystal structure of SARS-CoV-2 nucleocapsid protein RNA binding domain reveals potential unique drug targeting sites. Acta Pharm. Sin. B 2020, 10, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.; Hirsch, M.; Bloom, A. Coronavirus disease 2019 (COVID-19): Epidemiology, virology, and prevention. Lancet Infect. Dis. 2020, 1, 2019–2020. [Google Scholar]
- BioRender. An In-Depth Look into the Structure of the SARS-CoV2 Spike Glycoprotein. 2021. Available online: https://app.biorender.com/biorender-templates/figures/5e99f5395fd61e0028682c01/t-5f1754e62baea000aee86904-an-in-depth-look-into-the-structure-of-the-sars-cov2-spike-g (accessed on 1 August 2020).
- BioRender. SARS-CoV-2 Targeting of ACE2 Receptor and Entry in Infected Cell. 2020. Available online: https://app.biorender.com/biorender-templates/figures/5e99f5395fd61e0028682c01/t-5f9ae195d8c66d00a335fc7f-sars-cov-2-targeting-of-ace2-receptor-and-entry-in-infected- (accessed on 1 August 2020).
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef]
- Foresta, C.; Rocca, M.; Di Nisio, A. Gender susceptibility to COVID-19: A review of the putative role of sex hormones and X chromosome. J. Endocrinol. Investig. 2020, 2020, 1–6. [Google Scholar] [CrossRef]
- Vinciguerra, M.; Greco, E. Sars-CoV-2 and black population: ACE2 as shield or blade? Infect. Genet. Evol. 2020, 84, 104361. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Letko, M.; Marzi, A.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef] [PubMed]
- BioRender. Expression of ACE2 Receptor in Human Host Tissues. 2020. Available online: https://app.biorender.com/biorender-templates/figures/5e99f5395fd61e0028682c01/t-5f9ae2721197d300aa746c11-expression-of-ace2-receptor-in-human-host-tissues (accessed on 10 August 2020).
- Dhama, K.; Sharun, K.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus Disease 2019–COVID-19, 2020. Clin. Microbiol. Rev. 2020. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Gavriatopoulou, M.; Korompoki, E.; Fotiou, D.; Ntanasis-Stathopoulos, I.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Organ-specific manifestations of COVID-19 infection. Clin. Exp. Med. 2020, 20, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, F.; Prasad, K.; Kumar, V. Neurological manifestations of COVID-19: Available evidences and a new paradigm. J. Neurovirol. 2020, 26, 619–630. [Google Scholar] [CrossRef]
- Grasselli, G.; Tonetti, T.; Protti, A.; Langer, T.; Girardis, M.; Bellani, G.; Laffey, J.; Carrafiello, G.; Carsana, L.; Rizzuto, C.; et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: A multicentre prospective observational study. Lancet Respir. Med. 2020, 8, 1201–1208. [Google Scholar] [CrossRef]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 37. [Google Scholar] [CrossRef]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Visualizing What COVID-19 Does to Your Body. Available online: https://www.weforum.org/agenda/2020/04/this-graphic-shows-what-COVID-19-does-to-your-body/ (accessed on 11 April 2020).
- Hu, Z.; Ge, Q.; Jin, L.; Xiong, M. Artificial intelligence forecasting of COVID-19 in China. arXiv 2020, arXiv:200207112. [Google Scholar]
- BioRender. Cytokine Storm. 2020. Available online: https://app.biorender.com/biorender-templates/figures/5e99f5395fd61e0028682c01/t-5f177d0f77526300ae48799f-cytokine-storm (accessed on 3 July 2020).
- Sharif, A.S. Transmission Routes of COVID-19: A Review of the Evidence. J. Pediatr. Nephrol. 2020, 8. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Smith, D.W. COVID-19: A novel zoonotic disease caused by a coronavirus from China: What we know and what we don’t. Microbiol. Aust. 2020. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Ji, M.; Pei, F.; Zhao, Q.; Zhou, Y.; Hong, Y.; Han, S.; Wang, J.; Wang, Q.; et al. Transmission Routes Analysis of SARS-CoV-2: A Systematic Review and Case Report. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Falahi, S.; Kenarkoohi, A. Transmission routes for SARS-CoV-2 infection: Review of evidence. New Microbes New Infect. 2020, 38, 100778. [Google Scholar] [CrossRef]
- Transmission of SARS-CoV-2: Implications for Infection Prevention Precautions. Available online: https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions (accessed on 9 July 2020).
- Liu, J.; Liao, X.; Qian, S.; Yuan, J.; Wang, F.; Liu, Y.; Wang, Z.; Wang, F.-S.; Liu, L.; Zhang, Z. Community transmission of severe acute respiratory syndrome coronavirus 2, Shenzhen, China, 2020. Emerg. Infect. Dis. 2020, 26, 1320. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.W.X.; Tan, Y.K.; Chia, P.Y.; Lee, T.H.; Ng, O.T.; Wong, M.S.Y.; Marimuthu, K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA 2020, 323, 1610–1612. [Google Scholar] [PubMed]
- Ji, W.; Li, X.; Chen, S.; Ren, L. Transmission of SARS-CoV-2 via fomite, especially cold chain, should not be ignored. Proc. Natl. Acad. Sci. USA 2021, 118, e2026093118. [Google Scholar] [CrossRef]
- Transmission-Based Precautions. Available online: https://www.cdc.gov/infectioncontrol/basics/transmission-based-precautions.html (accessed on 1 July 2020).
- WHO. Infection Prevention and Control of Epidemic-and Pandemic-Prone Acute Respiratory Infections in Health Care; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- WHO. Advice on the Use of Masks in the Context of COVID-19: Interim Guidance, 5 June 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Asadi, S.; Bouvier, N.; Wexler, A.S.; Ristenpart, W.D. The Coronavirus Pandemic and Aerosols: Does COVID-19 Transmit via Expiratory Particles? Taylor & Francis: Abingdon, UK, 2020. [Google Scholar]
- Asadi, S.; Wexler, A.S.; Cappa, C.D.; Barreda, S.; Bouvier, N.M.; Ristenpart, W.D. Aerosol emission and superemission during human speech increase with voice loudness. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Science Brief: SARS-CoV-2 and Potential Airborne Transmission. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/scientific-brief-sars-cov-2.html (accessed on 5 October 2020).
- Xu, Y.; Li, X.; Zhu, B.; Liang, H.; Fang, C.; Gong, Y.; Guo, Q.; Sun, X.; Zhao, D.; Shen, J. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020, 26, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Hindson, J. COVID-19: Faecal–oral transmission? Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 259. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Tao, W.; Flavell, R.A.; Zhu, S. Potential intestinal infection and faecal-oral transmission of SARS-CoV-2. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 269–283. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Xue, Y. Fecal specimen diagnosis 2019 novel coronavirus–infected pneumonia. J. Med. Virol. 2020, 92, 680–682. [Google Scholar] [PubMed]
- Xu, X.; Chen, P.; Wang, J.; Feng, J.; Zhou, H.; Li, X.; Zhong, W.; Hao, P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020, 63, 457–460. [Google Scholar] [CrossRef]
- Güner, H.R.; Hasanoğlu, I.; Aktaş, F. COVID-19: Prevention and control measures in community. Turk. J. Med. Sci. 2020, 50, 571–577. [Google Scholar] [CrossRef] [PubMed]
- How to Protect Yourself & Others. Available online: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html (accessed on 8 March 2021).
- Adhikari, S.P.; Meng, S.; Wu, Y.-J.; Mao, Y.-P.; Ye, R.-X.; Wang, Q.-Z.; Sun, C.; Sylvia, S.; Rozelle, S.; Raat, H. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: A scoping review. Infect. Dis. Poverty 2020, 9, 1–12. [Google Scholar]
- BioRender. COVID-19 Safety Information. 2020. Available online: https://app.biorender.com/biorender-templates/figures/5e99f5395fd61e0028682c01/t-5e7cac8aa4038d00b05a8adb-COVID-19-safety-information (accessed on 18 March 2020).
- About COVID-19 Epidemiology. Available online: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/about-epidemiology/index.html (accessed on 1 July 2020).
- Singhal, T. A review of coronavirus disease-2019 (COVID-19). Indian J. Pediatrics 2020, 87, 281–286. [Google Scholar]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef]
- Meo, S.; Alhowikan, A.; Al-Khlaiwi, T.; Meo, I.; Halepoto, D.; Iqbal, M.; Usmani, A.; Hajjar, W.; Ahmed, N. Novel coronavirus 2019-nCoV: Prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur. Rev. Med. Pharm. Sci. 2020, 24, 2012–2019. [Google Scholar]
- Breastfeeding Advice during the COVID-19 Outbreak. Available online: http://www.emro.who.int/nutrition/news/breastfeeding-advice-during-the-covid-19-outbreak.html (accessed on 1 June 2020).
- Breastfeeding and COVID-19. Available online: https://www.who.int/news-room/commentaries/detail/breastfeeding-and-COVID-19 (accessed on 23 June 2020).
- World Health Organization. Clinical Management of COVID-19: Interim Guidance 27 May 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Disease Burden of Influenza. Available online: https://www.cdc.gov/flu/about/burden/index.html (accessed on 10 March 2020).
- Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Available online: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-COVID-19-final-report.pdf (accessed on 1 February 2020).
- Consensus Document on the Epidemiology of Severe Acute Respiratory Syndrome (SARS). Available online: https://www.who.int/csr/sars/en/WHOconsensus.pdf (accessed on 1 October 2003).
- Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Available online: https://www.who.int/emergencies/mers-cov/en/ (accessed on 19 January 2018).
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.; Lau, E.H.; Wong, J.Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, H.; Tabrizi, A.H.H.; Foroohi, F. Determination of COVID-19 prevalence with regards to age range of patients referring to the hospitals located in western Tehran, Iran. Gene Rep. 2020, 21, 100910. [Google Scholar] [CrossRef]
- He, F.; Deng, Y.; Li, W. Coronavirus disease 2019: What we know? J. Med. Virol. 2020, 92, 719–725. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Bonanad, C.; García-Blas, S.; Tarazona-Santabalbina, F.; Sanchis, J.; Bertomeu-González, V.; Fácila, L.; Ariza, A.; Núñez, J.; Cordero, A. The effect of age on mortality in patients with COVID-19: A metanalysis with 611,583 subjects. J. Am. Med. Dir. Assoc. 2020, 21, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Backer, J.A.; Klinkenberg, D.; Wallinga, J. Incubation period of 2019 novel coronavirus. 2019,-nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Eurosurveillance 2020, 25, 2000062. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.A. Age as a Risk Factor of COVID-19 Mortality in the Philippines. SSRN 2020. [Google Scholar] [CrossRef]
- Chinnadurai, R.; Ogedengbe, O.; Agarwal, P.; Money-Coomes, S.; Abdurrahman, A.Z.; Mohammed, S.; Kalra, P.A.; Rothwell, N.; Pradhan, S. Older age and frailty are the chief predictors of mortality in COVID-19 patients admitted to an acute medical unit in a secondary care setting-a cohort study. BMC Geriatr. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Wang, S.X.; Wang, Y.; Lu, Y.B.; Li, J.Y.; Song, Y.J.; Nyamgerelt, M.; Wang, X.X. Diagnosis and treatment of novel coronavirus pneumonia based on the theory of traditional Chinese medicine. J. Integr. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bhopal, S.S.; Bhopal, R. Sex differential in COVID-19 mortality varies markedly by age. Lancet 2020, 396, 532–533. [Google Scholar] [CrossRef]
- Jin, J.-M.; Bai, P.; He, W.; Wu, F.; Liu, X.-F.; Han, D.-M.; Liu, S.; Yang, J.-K. Gender differences in patients with COVID-19: Focus on severity and mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Omori, R.; Matsuyama, R.; Nakata, Y. The age distribution of mortality from novel coronavirus disease (COVID-19) suggests no large difference of susceptibility by age. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Brandén, M.; Aradhya, S.; Kolk, M.; Härkönen, J.; Drefahl, S.; Malmberg, B.; Rostila, M.; Cederström, A.; Andersson, G.; Mussino, E. Residential context and COVID-19 mortality among adults aged 70 years and older in Stockholm: A population-based, observational study using individual-level data. Lancet Healthy Longev. 2020, 1, e80–e88. [Google Scholar] [CrossRef]
- Hoffmann, C.; Wolf, E. Older age groups and country-specific case fatality rates of COVID-19 in Europe, USA and Canada. Infection 2020, 49, 111–116. [Google Scholar] [CrossRef]
- Borges, G.M.; Nepomuceno, M.R. A contribuição da demografia para os estudos de mortalidade em tempos de pandemia. Rev. Bras. Estud. Popul. 2020, 37. [Google Scholar] [CrossRef]
- Qin, J.; You, C.; Lin, Q.; Hu, T.; Yu, S.; Zhou, X.-H. Estimation of incubation period distribution of COVID-19 using disease onset forward time: A novel cross-sectional and forward follow-up study. Sci. Adv. 2020, 6, eabc1202. [Google Scholar] [CrossRef]
- Imai, N.; Dorigatti, I.; Cori, A.; Donnelly, C.; Riley, S.; Ferguson, N. Report 2: Estimating the Potential Total Number of Novel Coronavirus Cases in Wuhan City, China; Imperial College School of Public Health: London, UK, 2020. [Google Scholar]
- Linton, N.M.; Kobayashi, T.; Yang, Y.; Hayashi, K.; Akhmetzhanov, A.R.; Jung, S.-M.; Yuan, B.; Kinoshita, R.; Nishiura, H. Incubation Period and Other Epidemiological Characteristics of 2019 Novel Coronavirus Infections with Right Truncation: A Statistical Analysis of Publicly Available Case Data. J. Clin. Med. 2020, 9, 538. [Google Scholar] [CrossRef]
- Böhmer, M.M.; Buchholz, U.; Corman, V.M.; Hoch, M.; Katz, K.; Marosevic, D.V.; Böhm, S.; Woudenberg, T.; Ackermann, N.; Konrad, R. Investigation of a COVID-19 outbreak in Germany resulting from a single travel-associated primary case: A case series. Lancet Infect. Dis. 2020, 20, 920–928. [Google Scholar] [CrossRef]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology 2020, 296, 200642. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus. 2019,-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-R.; Cao, Q.-D.; Hong, Z.-S.; Tan, Y.-Y.; Chen, S.-D.; Jin, H.-J.; Tan, K.-S.; Wang, D.-Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Natl. Sci. Rev. 2020, 7, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, B.; Heymann, D.L. SARS to novel coronavirus–old lessons and new lessons. Epidemiol. Infect. 2020, 148, e22. [Google Scholar] [CrossRef] [PubMed]
- BioRender. COVID-19 Diagnostic Test through RT-PCR. 2020. Available online: https://app.biorender.com/biorender-templates/figures/5e99f5395fd61e0028682c01/t-5e8f21be13f46000ab9f6806-COVID-19-diagnostic-test-through-rt-pcr (accessed on 9 April 2020).
- Harmon, S.A.; Sanford, T.H.; Xu, S.; Turkbey, E.B.; Roth, H.; Xu, Z.; Yang, D.; Myronenko, A.; Anderson, V.; Amalou, A. Artificial intelligence for the detection of COVID-19 pneumonia on chest CT using multinational datasets. Nat. Commun. 2020, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Agarwal, P.P. Chest imaging appearance of COVID-19 infection. Radiology 2020, 2, e200028. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, H.; Xie, J.; Lin, M.; Ying, L.; Pang, P.; Ji, W. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology 2020, 296, 200432. [Google Scholar] [CrossRef]
- Polsinelli, M.; Cinque, L.; Placidi, G. A Light CNN for detecting COVID-19 from CT scans of the chest. arXiv 2020, arXiv:200412837. [Google Scholar] [CrossRef]
- Parekh, M.; Donuru, A.; Balasubramanya, R.; Kapur, S. Review of the chest CT differential diagnosis of ground-glass opacities in the COVID era. Radiology 2020, 297, 202504. [Google Scholar] [CrossRef]
- Zheng, C. Time course of lung changes at chest CT during recovery from Coronavirus Disease 2019 (COVID-19). Radiology 2020, 295, 715–721. [Google Scholar]
- Meng, H.; Xiong, R.; He, R.; Lin, W.; Hao, B.; Zhang, L.; Lu, Z.; Shen, X.; Fan, T.; Jiang, W. CT imaging and clinical course of asymptomatic cases with COVID-19 pneumonia at admission in Wuhan, China. J. Infect. 2020, 81, e33–e39. [Google Scholar] [CrossRef]
- Silva, P.; Luz, E.; Silva, G.; Moreira, G.; Silva, R.; Lucio, D.; Menotti, D. COVID-19 detection in CT images with deep learning: A voting-based scheme and cross-datasets analysis. Inform. Med. Unlocked 2020, 20, 100427. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, D.; Yang, P.; Poon, L.L.M.; Wang, Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020, 20, 411–412. [Google Scholar] [CrossRef]
- Miller, S.; Chiu, C.Y.; Rodino, K.G.; Miller, M.B. Point-Counterpoint: Should We Be Performing Metagenomic Next-Generation Sequencing for Infectious Disease Diagnosis in the Clinical Laboratory? J. Clin. Microbiol. 2019, 58. [Google Scholar] [CrossRef]
- Peeling, R.W.; Wedderburn, C.J.; Garcia, P.J.; Boeras, D.; Fongwen, N.; Nkengasong, J.; Sall, A.; Tanuri, A.; Heymann, D.L. Serology testing in the COVID-19 pandemic response. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Ghaffari, A.; Meurant, R.; Ardakani, A. COVID-19 serological tests: How well do they actually perform? Diagnostics 2020, 10, 453. [Google Scholar] [CrossRef]
- The Worldwide Test for COVID-19. Available online: https://www.globalbiotechinsights.com/articles/20247/the-worldwide-test-for-COVID-19 (accessed on 26 March 2020).
- BioRender. COVID-19 Serologic Diagnostic Test through Antibody Detection. 2020. Available online: https://app.biorender.com/biorender-templates/figures/5e99f5395fd61e0028682c01/t-5e8f2287bbf15200b1d9143a-COVID-19-serologic-diagnostic-test-through-antibody-detectio (accessed on 2 January 2020).
- Bohr, A.; Memarzadeh, K. The rise of artificial intelligence in healthcare applications. Artif. Intell. Healthc. 2020. [Google Scholar] [CrossRef]
- Lalmuanawma, S.; Hussain, J.; Chhakchhuak, L. Applications of machine learning and artificial intelligence for COVID-19 (SARS-CoV-2) pandemic: A review. Chaos Solitons Fractals 2020, 139, 110059. [Google Scholar] [CrossRef] [PubMed]
- DeGrave, A.J.; Janizek, J.D.; Lee, S.-I. AI for radiographic COVID-19 detection selects shortcuts over signal. medRxiv 2020. [Google Scholar] [CrossRef]
- Yang, Z.; Zeng, Z.; Wang, K.; Wong, S.-S.; Liang, W.; Zanin, M.; Liu, P.; Cao, X.; Gao, Z.; Mai, Z.; et al. Modified SEIR and AI prediction of the epidemics trend of COVID-19 in China under public health interventions. J. Thorac. Dis. 2020, 12, 165–174. [Google Scholar] [CrossRef]
- Vaishya, R.; Javaid, M.; Khan, I.H.; Haleem, A. Artificial Intelligence (AI) applications for COVID-19 pandemic. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Javaid, M.; Vaishya, R. Effects of COVID 19 pandemic in daily life. Curr. Med. Res. Pract. 2020, 10, 78–79. [Google Scholar] [CrossRef]
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Li, V.Y.C.; Chen, H.; Mubareka, S.; Gubbay, J.B.; Chan, W.C.W. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 2020, 14, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Tang, Q.-L.; Shang, Y.-X.; Liang, S.-B.; Yang, M.; Robinson, N.; Liu, J.-P. Can Chinese Medicine Be Used for Prevention of Corona Virus Disease 2019 (COVID-19)? A Review of Historical Classics, Research Evidence and Current Prevention Programs. Chin. J. Integr. Med. 2020, 26, 243–250. [Google Scholar] [CrossRef]
- Haleem, A.; Vaishya, R.; Javaid, M.; Khan, I.H. Artificial Intelligence (AI) applications in orthopaedics: An innovative technology to embrace. J. Clin. Orthop. Trauma 2020, 11, S80–S81. [Google Scholar] [CrossRef]
- Stebbing, J.; Phelan, A.; Griffin, I.; Tucker, C.; Oechsle, O.; Smith, D.; Richardson, P. COVID-19: Combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020, 20, 400–402. [Google Scholar] [CrossRef]
- Sweeney, Y. Tracking the debate on COVID-19 surveillance tools. Nat. Mach. Intell. 2020, 2, 301–304. [Google Scholar] [CrossRef]
- Pham, Q.V.; Nguyen, D.C.; Huynh-The, T.; Hwang, W.J.; Pathirana, P.N. Artificial Intelligence (AI) and Big Data for Coronavirus (COVID-19) Pandemic: A Survey on the State-of-the-Arts. IEEE Access 2020. [Google Scholar] [CrossRef]
- Nguyen, T.T. Artificial intelligence in the battle against coronavirus (COVID-19): A survey and future research directions. arXiv 2020, arXiv:2008.07343. [Google Scholar]
- Wang, R.; Pan, W.; Jin, L.; Li, Y.; Geng, Y.; Gao, C.; Chen, G.; Wang, H.; Ma, D.; Liao, S. Artificial intelligence in reproductive medicine. Reproduction 2019, 158, R139–R154. [Google Scholar] [CrossRef] [PubMed]
- Kulikowski, C.A. Beginnings of artificial intelligence in medicine (AIM): Computational artifice assisting scientific inquiry and clinical art–with reflections on present aim challenges. Yearb. Med. Inform. 2019, 28, 249. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef]
- Bobdey, S.; Ray, S. Going viral–COVID-19 impact assessment: A perspective beyond clinical practice. J. Mar. Med. Soc. 2020, 22, 9. [Google Scholar] [CrossRef]
- Gozes, O.; Frid-Adar, M.; Greenspan, H.; Browning, P.D.; Zhang, H.; Ji, W.; Bernheim, A.; Siegel, E. Rapid AI development cycle for the coronavirus (COVID-19) pandemic: Initial results for automated detection & patient monitoring using deep learning CT image analysis. arXiv 2020, arXiv:200305037. [Google Scholar]
- Pirouz, B.; Shaffiee Haghshenas, S.; Shaffiee Haghshenas, S.; Piro, P. Investigating a serious challenge in the sustainable development process: Analysis of confirmed cases of COVID-19 (new type of coronavirus) through a binary classification using artificial intelligence and regression analysis. Sustainability 2020, 12, 2427. [Google Scholar] [CrossRef]
- Wan, K.H.; Huang, S.S.; Young, A.L.; Lam, D.S.C. Precautionary measures needed for ophthalmologists during pandemic of the coronavirus disease 2019 (COVID-19). Acta Ophthalmol. 2020, 98, 221–222. [Google Scholar] [CrossRef] [PubMed]
- Smeulders, A.; Van Ginneken, A. An analysis of pathology knowledge and decision making for the development of artificial intelligence-based consulting systems. Anal. Quant. Cytol. Histol. 1989, 11, 154–165. [Google Scholar]
- Choi, Y.H.; Han, H.-K. Nanomedicines: Current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharm. Investig. 2018, 48, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.V.; Pereira, A.E.; de Oliveira, J.L.; Carvalho, L.B.; Guilger-Casagrande, M.; de Lima, R.; Fraceto, L.F. How can nanotechnology help to combat COVID-19? Opportunities and urgent need. J. Nanobiotechnol. 2020, 18, 1–23. [Google Scholar] [CrossRef]
- Querido, M.M.; Aguiar, L.; Neves, P.; Pereira, C.C.; Teixeira, J.P. Self-disinfecting surfaces and infection control. Colloids Surf. B Biointerfaces 2019, 178, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Dyshlyuk, L.; Babich, O.; Ivanova, S.; Vasilchenco, N.; Prosekov, A.; Sukhikh, S. Suspensions of metal nanoparticles as a basis for protection of internal surfaces of building structures from biodegradation. Case Stud. Constr. Mater. 2020, 12, e00319. [Google Scholar] [CrossRef]
- Chan, W.C. Nano Research for COVID-19. ACS Nano 2020, 14, 3719–3720. [Google Scholar] [CrossRef] [PubMed]
- CDC. Coronavirus Disease 2019 (COVID19)—Transmission. Centers for Disease Control and Prevention. 2020. Available online: https://www.cdc.gov/coronavirus/2019ncov/preventgettingsick/howcovidspreads.html (accessed on 20 August 2020).
- Yetisen, A.K.; Qu, H.; Manbachi, A.; Butt, H.; Dokmeci, M.R.; Hinestroza, J.P.; Skorobogatiy, M.; Khademhosseini, A.; Yun, S.H. Nanotechnology in textiles. ACS Nano 2016, 10, 3042–3068. [Google Scholar] [CrossRef] [PubMed]
- Spagnol, C.; Fragal, E.H.; Pereira, A.G.; Nakamura, C.V.; Muniz, E.C.; Follmann, H.D.; Silva, R.; Rubira, A.F. Cellulose nanowhiskers decorated with silver nanoparticles as an additive to antibacterial polymers membranes fabricated by electrospinning. J. Colloid Interface Sci. 2018, 531, 705–715. [Google Scholar] [CrossRef]
- Nikaeen, G.; Abbaszadeh, S.; Yousefinejad, S. Application of nanomaterials in treatment, anti-infection and detection of coronaviruses. Nanomedicine 2020, 15, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Santiago, I. Trends and innovations in biosensors for COVID-19 mass testing. ChemBioChem 2020, 21, 1–11. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, X.; Han, L.; Chen, T.; Wang, L.; Li, H.; Li, S.; He, L.; Fu, X.; Chen, S. Reverse transcription loop-mediated isothermal amplification combined with nanoparticles-based biosensor for diagnosis of COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Sun, X.; Wang, T.; Cai, D.; Hu, Z.; Liao, H.; Zhi, L.; Wei, H.; Zhang, Z.; Qiu, Y.; Wang, J. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020, 53, 38–42. [Google Scholar] [CrossRef]
- Lung, P.; Yang, J.; Li, Q. Nanoparticle formulated vaccines: Opportunities and challenges. Nanoscale 2020, 12, 5746–5763. [Google Scholar] [CrossRef]
- Shin, M.D.; Shukla, S.; Chung, Y.H.; Beiss, V.; Chan, S.K.; Ortega-Rivera, O.A.; Wirth, D.M.; Chen, A.; Sack, M.; Pokorski, J.K.; et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020, 15, 646–655. [Google Scholar] [CrossRef]
- Espeseth, A.S.; Cejas, P.J.; Citron, M.P.; Wang, D.; DiStefano, D.J.; Callahan, C.; O’Donnell, G.; Galli, J.D.; Swoyer, R.; Touch, S. Modified mRNA/lipid nanoparticle-based vaccines expressing respiratory syncytial virus F protein variants are immunogenic and protective in rodent models of RSV infection. NPJ Vaccines 2020, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Medhi, R.; Srinoi, P.; Ngo, N.; Tran, H.-V.; Lee, T.R. Nanoparticle-Based Strategies to Combat COVID-19. ACS Appl. Nano Mater. 2020, 3, 8557–8580. [Google Scholar] [CrossRef]
- Moitra, P.; Alafeef, M.; Dighe, K.; Frieman, M.B.; Pan, D. Selective Naked-Eye Detection of SARS-CoV-2 Mediated by N Gene Targeted Antisense Oligonucleotide Capped Plasmonic Nanoparticles. ACS Nano 2020, 14, 7617–7627. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kang, B.-H.; Kang, M.; Chung, D.R.; Yi, G.-S.; Lee, L.P.; Jeong, K.-H. Nanoplasmonic On-Chip PCR for Rapid Precision Molecular Diagnostics. ACS Appl. Mater. Interfaces 2020, 12, 12533–12540. [Google Scholar] [CrossRef]
- Pinals, R.L.; Ledesma, F.; Yang, D.; Navarro, N.; Jeong, S.; Pak, J.E.; Kuo, L.; Chuang, Y.C.; Cheng, Y.W.; Sun, H.Y.; et al. Rapid SARS-CoV-2 Detection by Carbon Nanotube-Based Near-Infrared Nanosensors. medRxiv 2020. [Google Scholar] [CrossRef]
- Vadlamani, B.S.; Uppal, T.; Verma, S.C.; Misra, M. Functionalized TiO2 Nanotube-Based Electrochemical Biosensor for Rapid Detection of SARS-CoV-2. Sensors 2020, 20, 5871. [Google Scholar] [CrossRef]
- Balagna, C.; Perero, S.; Percivalle, E.; Nepita, E.V.; Ferraris, M. Virucidal effect against coronavirus SARS-CoV-2 of a silver nanocluster/silica composite sputtered coating. Open Ceram. 2020. [Google Scholar] [CrossRef]
- Gong, P.; He, X.; Wang, K.; Tan, W.; Xie, W.; Wu, P.; Li, H. Combination of functionalized nanoparticles and polymerase chain reaction-based method for SARS-CoV gene detection. J. Nanosci. Nanotechnol. 2008, 8, 293–300. [Google Scholar] [CrossRef]
- Gorshkov, K.; Susumu, K.; Chen, J.; Xu, M.; Pradhan, M.; Zhu, W.; Hu, X.; Breger, J.C.; Wolak, M.; Oh, E. Quantum Dot-Conjugated SARS-CoV-2 Spike Pseudo-Virions Enable Tracking of Angiotensin Converting Enzyme 2 Binding and Endocytosis. ACS Nano 2020, 14, 12234–12247. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Niu, S.; Li, H.; Tian, Y.; Yu, S.; Yu, F.; Wu, Y.; Liu, L.-E. Highly Sensitive Fluorescence-Linked Immunosorbent Assay for the Determination of Human IgG in Serum Using Quantum Dot Nanobeads and Magnetic Fe3O4 Nanospheres. ACS Omega 2020, 5, 23229–23236. [Google Scholar] [CrossRef] [PubMed]
- Tortella, G.R.; Rubilar, O.; Diez, M.C.; Padrão, J.; Zille, A.; Pieretti, J.C.; Seabra, A.B. Multifunctional nano-magnetic particles assisted viral RNA-extraction protocol for potential detection of COVID-19. Mater. Res. Innov. 2020. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, H.; Song, W.; Ru, X.; Zhou, W.; Yu, X. A simple magnetic nanoparticles-based viral RNA extraction method for efficient detection of SARS-CoV-2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Cordaro, A.; Neri, G.; Sciortino, M.T.; Scala, A.; Piperno, A. Graphene-Based Strategies in Liquid Biopsy and in Viral Diseases Diagnosis. Nanomaterials 2020, 10, 1014. [Google Scholar] [CrossRef] [PubMed]
- Torrente-Rodríguez, R.M.; Lukas, H.; Tu, J.; Min, J.; Yang, Y.; Xu, C.; Rossiter, H.B.; Gao, W. SARS-CoV-2 RapidPlex: A Graphene-Based Multiplexed Telemedicine Platform for Rapid and Low-Cost COVID-19 Diagnosis and Monitoring. Matter 2020, 3, 1981–1998. [Google Scholar] [CrossRef]
- Zumla, A.; Hui, D.S.; Azhar, E.I.; Memish, Z.A.; Maeurer, M. Reducing mortality from 2019-nCoV: Host-directed therapies should be an option. Lancet 2020, 395, e35–e36. [Google Scholar] [CrossRef]
- Bhavana, V.; Thakor, P.; Singh, S.B.; Mehra, N.K. COVID-19: Pathophysiology, treatment options, nanotechnology approaches, and research agenda to combating the SARS-CoV2 pandemic. Life Sci. 2020, 261, 118336. [Google Scholar] [CrossRef]
- Tufan, A.; Güler, A.A.; Matucci-Cerinic, M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk. J. Med. Sci. 2020, 50, 620–632. [Google Scholar] [CrossRef]
- Therapeutic Management of Adults with COVID-19. Available online: https://www.covid19treatmentguidelines.nih.gov/therapeutic-management/ (accessed on 11 February 2021).
- Mansourabadi, A.H.; Sadeghalvad, M.; Mohammadi-Motlagh, H.-R.; Rezaei, N. The immune system as a target for therapy of SARS-CoV-2: A systematic review of the current immunotherapies for COVID-19. Life Sci. 2020, 258, 118185. [Google Scholar] [CrossRef]
- Chorin, E.; Dai, M.; Shulman, E.; Wadhwani, L.; Bar-Cohen, R.; Barbhaiya, C.; Aizer, A.; Holmes, D.; Bernstein, S.; Spinelli, M.; et al. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nat. Med. 2020, 26, 808–809. [Google Scholar] [CrossRef]
- University of Liverpool. COVID-19 Drug interactions. 2020. Available online: https://www.covid19-druginteractions.org/ (accessed on 24 September 2020).
- Pasin, L.; Navalesi, P.; Zangrillo, A.; Kuzovlev, A.; Likhvantsev, V.; Hajjar, L.A.; Fresilli, S.; Lacerda, M.V.G.; Landoni, G. Corticosteroids for Patients with Coronavirus Disease 2019 (COVID-19) with Different Disease Severity: A Meta-Analysis of Randomized Clinical Trials. J. Cardiothorac. Vasc. Anesth. 2021, 35, 578–584. [Google Scholar] [CrossRef] [PubMed]
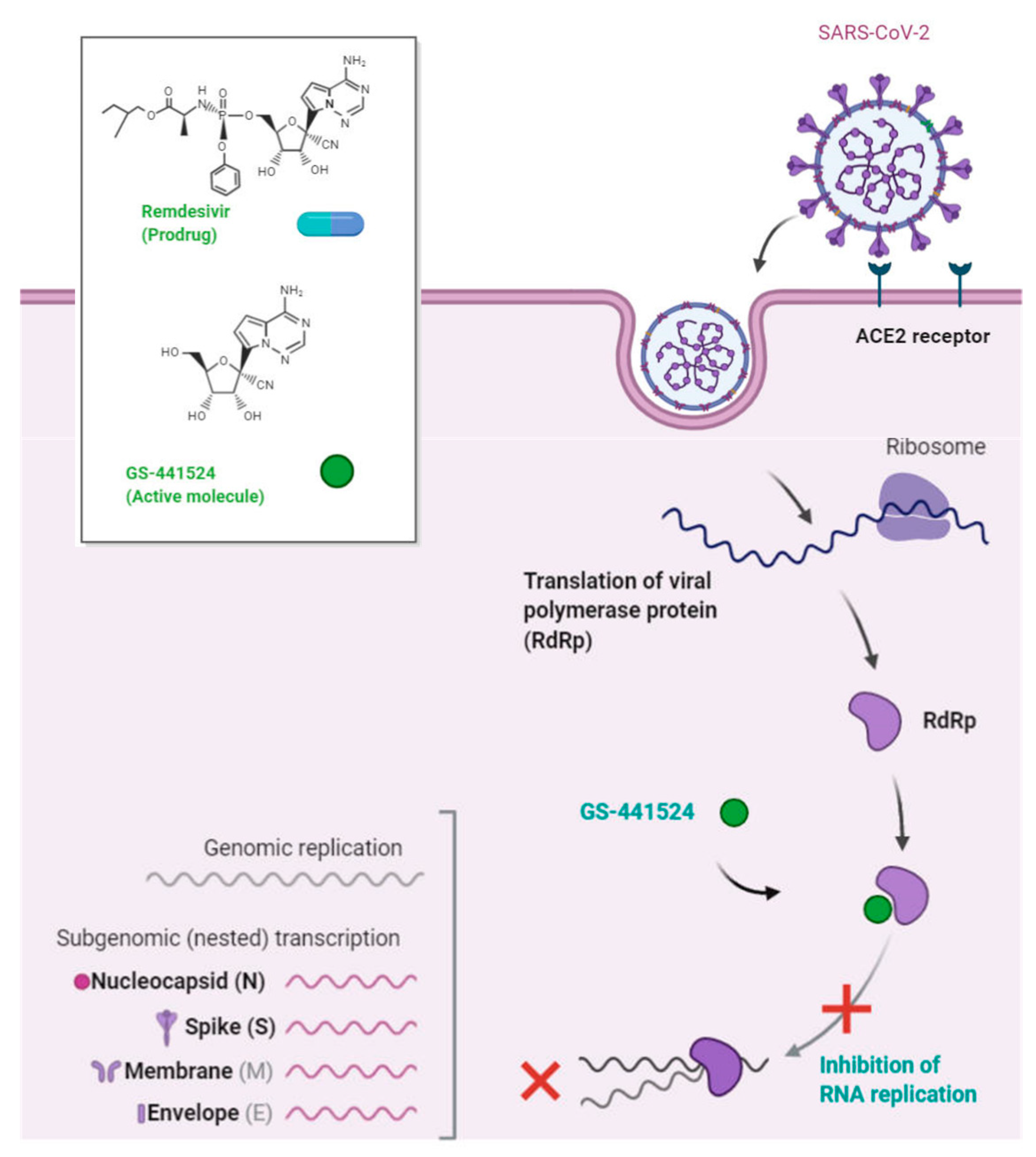
- BioRender. Remdesivir: Potential Repurposed Drug Candidate for COVID-19 (Portrait). 2020. Available online: https://app.biorender.com/biorender-templates/figures/5e99f5395fd61e0028682c01/t-5ea056e2183b5d00b114231f-remdesivir-potential-repurposed-drug-candidate-for-COVID-19- (accessed on 1 April 2020).
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S. Remdesivir for the treatment of COVID-19. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus. 2019,-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Owa, A.B.; Owa, O.T. Lopinavir/ritonavir use in COVID-19 infection: Is it completely non-beneficial? J. Microbiol. Immunol. Infect. 2020, 53, 674–675. [Google Scholar] [CrossRef]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef]
- Burwick, R.M.; Yawetz, S.; Stephenson, K.E.; Collier, A.Y.; Sen, P.; Blackburn, B.G.; Kojic, E.M.; Hirshberg, A.; Suarez, J.F.; Sobieszczyk, M.E.; et al. Compassionate Use of Remdesivir in Pregnant Women with Severe COVID-19. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Dabbous, H.M.; Abd-Elsalam, S.; El-Sayed, M.H.; Sherief, A.F.; Ebeid, F.F.S.; El Ghafar, M.S.A.; Soliman, S.; Elbahnasawy, M.; Badawi, R.; Tageldin, M.A. Efficacy of favipiravir in COVID-19 treatment: A multi-center randomized study. Arch. Virol. 2021, 166, 949–954. [Google Scholar] [CrossRef]
- Agrawal, U.; Raju, R.; Udwadia, Z.F. Favipiravir: A new and emerging antiviral option in COVID-19. Med. J. Armed Forces India 2020, 76, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Immune-Based Therapy. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/ (accessed on 11 February 2021).
- Wang, X.; Guo, X.; Xin, Q.; Pan, Y.; Hu, Y.; Li, J.; Chu, Y.; Feng, Y.; Wang, Q. Neutralizing antibodies responses to SARS-CoV-2 in COVID-19 inpatients and convalescent patients. Clin. Infect. Dis. 2020, 71, 2688–2694. [Google Scholar] [CrossRef]
- Vincent, M.J.; Bergeron, E.; Benjannet, S.; Erickson, B.R.; Rollin, P.E.; Ksiazek, T.G.; Seidah, N.G.; Nichol, S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005. [Google Scholar] [CrossRef]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chloroquine or Hydroxychloroquine with or without Azithromycin. Available online: https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/chloroquine-or-hydroxychloroquine-with-or-without-azithromycin/ (accessed on 9 October 2020).
- Nguyen, L.S.; Dolladille, C.; Drici, M.D.; Fenioux, C.; Alexandre, J.; Mira, J.P.; Moslehi, J.J.; Roden, D.M.; Funck-Brentano, C.; Salem, J.E. Cardiovascular Toxicities Associated with Hydroxychloroquine and Azithromycin: An Analysis of the World Health Organization Pharmacovigilance Database. Circulation 2020, 142, 303–305. [Google Scholar] [CrossRef]
- Shakoory, B.; Carcillo, J.A.; Chatham, W.W.; Amdur, R.L.; Zhao, H.; Dinarello, C.A.; Cron, R.Q.; Opal, S.M. Interleukin-1 Receptor Blockade Is Associated with Reduced Mortality in Sepsis Patients with Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit. Care Med. 2016, 44, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Mercuro, N.J.; Yen, C.F.; Shim, D.J.; Maher, T.R.; McCoy, C.M.; Zimetbaum, P.J.; Gold, H.S. Risk of QT Interval Prolongation Associated with Use of Hydroxychloroquine with or without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.S.; Dufort, E.M.; Udo, T.; Wilberschied, L.A.; Kumar, J.; Tesoriero, J.; Weinberg, P.; Kirkwood, J.; Muse, A.; DeHovitz, J.; et al. Association of Treatment with Hydroxychloroquine or Azithromycin with In-Hospital Mortality in Patients with COVID-19 in New York State. JAMA 2020, 323, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- Vitamin C, COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/adjunctive-therapy/vitamin-c/ (accessed on 3 November 2020).
- Tahir Ul Qamar, M.; Alqahtani, S.M.; Alamri, M.A.; Chen, L.-L. Structural basis of SARS-CoV-2 3CL(pro) and anti-COVID-19 drug discovery from medicinal plants. J. Pharm. Anal. 2020, 10, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Meini, S.; Pagotto, A.; Longo, B.; Vendramin, I.; Pecori, D.; Tascini, C. Role of Lopinavir/Ritonavir in the Treatment of COVID-19: A Review of Current Evidence, Guideline Recommendations, and Perspectives. J. Clin. Med. 2020, 9, 2050. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, J.; Ma, C.; Yue, Y.; Zou, Z.; Yu, C.; Yin, F. Link between community-acquired pneumonia and vitamin D levels in older patients. Zeitschrift fur Gerontologie und Geriatrie 2016, 51, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.N.Y.; Atkinson, S.C.; Wang, C.; Lee, A.; Bogoyevitch, M.A.; Borg, N.A.; Jans, D.A. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antivir. Res. 2020, 177, 104760. [Google Scholar] [CrossRef] [PubMed]
- Baxter, D. Active and passive immunity, vaccine types, excipients and licensing. Occup. Med. 2007, 57, 552–556. [Google Scholar] [CrossRef]
- Marovich, M.; Mascola, J.R.; Cohen, M.S. Monoclonal Antibodies for Prevention and Treatment of COVID-19. JAMA 2020, 324, 131–132. [Google Scholar] [CrossRef]
- Jaworski, J.P. Neutralizing monoclonal antibodies for COVID-19 treatment and prevention. Biomed. J. 2020. [Google Scholar] [CrossRef]
- Faust, S.; Horby, P.; Lim, W.S.; Emberson, J.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.D.; Brightling, C.E.; Ustianowski, A. Effect of dexamethasone in hospitalized patients with COVID-19–preliminary report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Zhou, Q.; Wei, X.-S.; Xiang, X.; Wang, X.; Wang, Z.-H.; Chen, V.; Shannon, C.P.; Tebbutt, S.J.; Kollmann, T.R.; Fish, E.N. Interferon-a2b treatment for COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Cao, Y.; Wei, J.; Zou, L.; Jiang, T.; Wang, G.; Chen, L.; Huang, L.; Meng, F.; Huang, L.; Wang, N.; et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J. Allergy Clin. Immunol. 2020, 146, 137–146.e133. [Google Scholar] [CrossRef]
- Samaee, H.; Mohsenzadegan, M.; Ala, S.; Maroufi, S.S.; Moradimajd, P. Tocilizumab for treatment patients with COVID-19: Recommended medication for novel disease. Int. Immunopharmacol. 2020, 89, 107018. [Google Scholar] [CrossRef]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus Remdesivir for Hospitalized Adults with COVID-19. N. Engl. J. Med. 2020, 384, 795–807. [Google Scholar] [CrossRef]
- The COVID-19 Treatment Guidelines Panel’s Statement on the Emergency Use Authorization of the Bamlanivimab Plus Etesevimab Combination for the Treatment of COVID-19. Available online: https://www.covid19treatmentguidelines.nih.gov/statement-on-bamlanivimab-plus-etesevimab-eua/ (accessed on 21 April 2021).
- Matthay, M.A.; Thompson, B.T. Dexamethasone in hospitalised patients with COVID-19: Addressing uncertainties. Lancet Respir. Med. 2020, 8, 1170–1172. [Google Scholar] [CrossRef]
- Corticosteroids. Available online: https://www.covid19treatmentguidelines.nih.gov/immunomodulators/corticosteroids/ (accessed on 3 November 2020).
- Zhu, H.-M.; Li, Y.; Li, B.-Y.; Yang, S.; Peng, D.; Yang, X.; Sun, X.-L.; Zhang, M. Effect of methylprednisolone in severe and critical COVID-19: Analysis of 102 cases. World J. Clin. Cases 2020, 8, 5952. [Google Scholar] [CrossRef] [PubMed]
- Antithrombotic Therapy, COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/adjunctive-therapy/antithrombotic-therapy/ (accessed on 11 February 2021).
- Vitamin D, COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/adjunctive-therapy/vitamin-d/ (accessed on 17 July 2020).
- Science, M.; Maguire, J.L.; Russell, M.L.; Smieja, M.; Walter, S.D.; Loeb, M. Low serum 25-hydroxyvitamin D level and risk of upper respiratory tract infection in children and adolescents. Clin. Infect. Dis. 2013, 57, 392–397. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Zinc, COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/adjunctive-therapy/zinc/ (accessed on 11 February 2021).
- National Institutes of Health. Office of Dietary Supplements. Zinc Fact Sheet for Health Professionals. 2020. Available online: https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/ (accessed on 26 June 2020).
- Pfizer and Biontech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis from Phase 3 Study. Pfizer Press Release. 9 November 2020. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-vaccine-candidate-against (accessed on 9 November 2020).
- Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints. Pfizer Press Release. 18 November 2020. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-COVID-19-vaccine (accessed on 18 November 2020).
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2020, 397, 99–111. [Google Scholar] [CrossRef]
- AZD1222 Vaccine Met Primary Efficacy Endpoint in Preventing COVID-19. AstraZeneca Press Release. 23 November 2020. Available online: https://www.astrazeneca.com/media-centre/press-releases/2020/azd1222hlr.html (accessed on 23 November 2020).
- BioRender. Clinical Phase Vaccine Candidates for COVID-19. 2020. Available online: https://app.biorender.com/biorender-templates/figures/5e99f5395fd61e0028682c01/t-5ea83be2d3420200adea0b6a-clinical-phase-vaccine-candidates-for-COVID-19 (accessed on 15 April 2020).
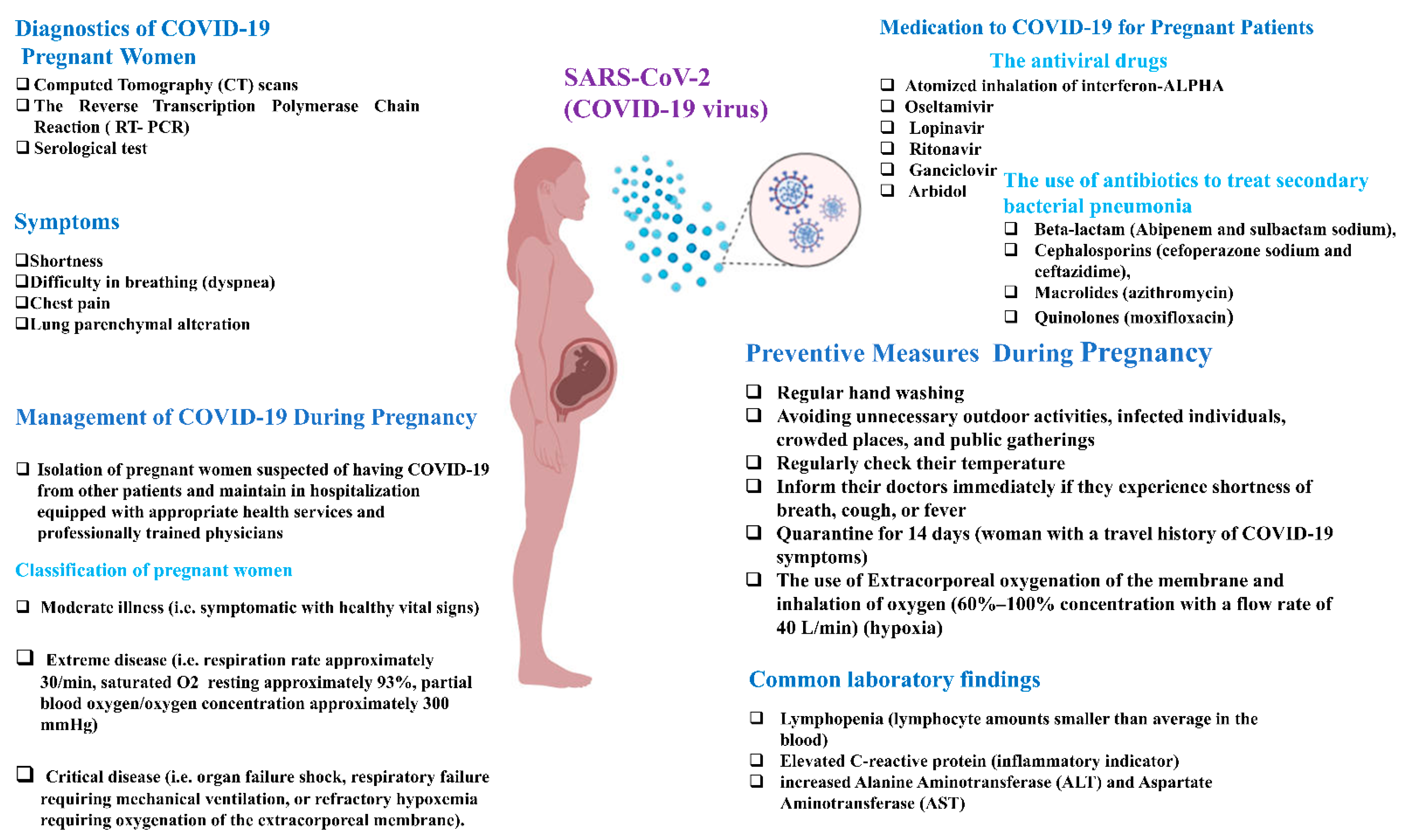
- Wastnedge, E.A.N.; Reynolds, R.M.; van Boeckel, S.R.; Stock, S.J.; Denison, F.C.; Maybin, J.A.; Critchley, H.O.D. Pregnancy and COVID-19. Physiol. Rev. 2021, 101, 303–318. [Google Scholar] [CrossRef]
- Shek, C.C.; Ng, P.C.; Fung, G.P.G.; Cheng, F.W.T.; Chan, P.K.S.; Peiris, M.J.S.; Lee, K.H.; Wong, S.F.; Cheung, H.M.; Li, A.M.; et al. Infants Born to Mothers with Severe Acute Respiratory Syndrome. Pediatrics 2003, 112, e254. [Google Scholar] [CrossRef] [PubMed]
- Dashraath, P.; Wong, J.L.J.; Lim, M.X.K.; Lim, L.M.; Li, S.; Biswas, A.; Choolani, M.; Mattar, C.; Su, L.L. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020, 222, 521–531. [Google Scholar] [CrossRef]
- Vaccination Considerations for People who are Pregnant or Breastfeeding. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html (accessed on 28 April 2021).
- Schwartz, D.A.; Graham, A.L. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: Lessons from SARS, MERS, and other human coronavirus infections. Viruses 2020, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef] [PubMed]
- Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7). Chin. Med. J. 2020, 133, 1087–1095. [CrossRef] [PubMed]
- Liu, W.; Wang, Q.; Zhang, Q.; Chen, L.; Chen, J.; Zhang, B.; Lu, Y.; Wang, S.; Xia, L.; Huang, L.; et al. Coronavirus Disease 2019 (COVID-19) During Pregnancy: A Case Series. Preprints 2020, 2020, 020373. [Google Scholar]
- Yan, J.; Guo, J.; Fan, C.; Juan, J.; Yu, X.; Li, J.; Feng, L.; Li, C.; Chen, H.; Qiao, Y.; et al. Coronavirus disease 2019 in pregnant women: A report based on 116 cases. Am. J. Obs. Gynecol. 2020, 223, 111.e111–111.e114. [Google Scholar] [CrossRef]
- Rahaman, S.T. A Review on the Effect of COVID-19 in Pregnant Women. Pharm. Biomed. Res. 2020, 6, 17–26. [Google Scholar] [CrossRef]
- Li, N.; Han, L.; Peng, M.; Lv, Y.; Ouyang, Y.; Liu, K.; Yue, L.; Li, Q.; Sun, G.; Chen, L.; et al. Maternal and Neonatal Outcomes of Pregnant Women with Coronavirus Disease 2019 (COVID-19) Pneumonia: A Case-Control Study. Clin. Infect. Dis. 2020, 71, 2035–2041. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q.; et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020, 395, 809–815. [Google Scholar] [CrossRef]
- Sills, E.S.; Wood, S.H. An Experimental Model for Peri-conceptual COVID-19 Pregnancy Loss and Proposed Interventions to Optimize Outcomes. Int. J. Mol. Cell Med. 2020, 9, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Peng, L.; Siddique, R.; Nabi, G.; Xue, M.; Liu, J.; Han, G. Impact of COVID-19 infection on pregnancy outcomes and the risk of maternal-to-neonatal intrapartum transmission of COVID-19 during natural birth. Infect. Control. Hosp. Epidemiol. 2020, 41, 748–750. [Google Scholar] [CrossRef]


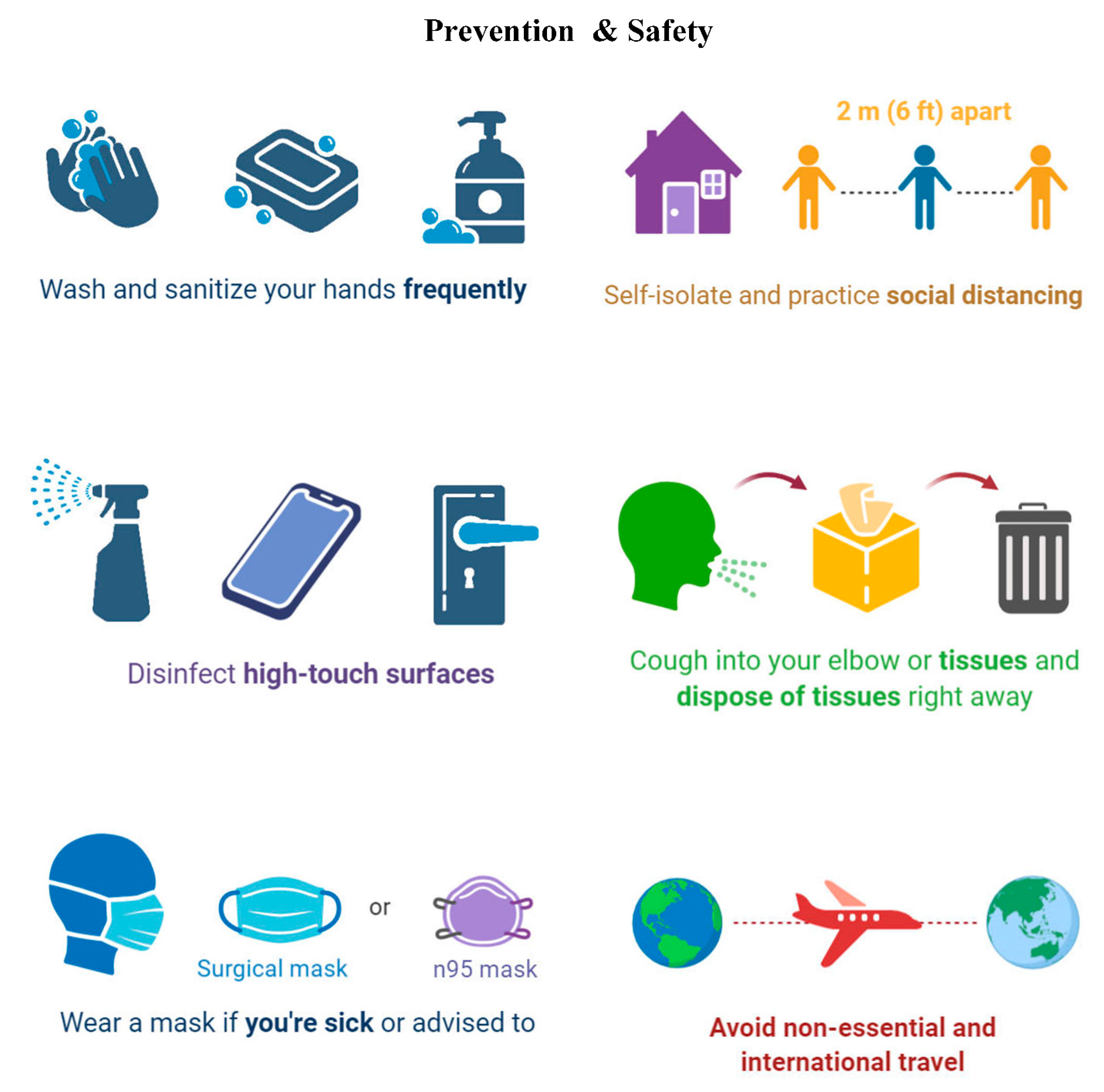




| Everyone Should | Description |
|---|---|
| Wash your hands |
|
| Stay away from close contact |
|
| The necessity of using masks to cover the mouth and nose in the face of others |
|
| Cover coughs and sneezes |
|
| Clean and disinfect |
|
| Monitor Your Health Daily |
|
| Protect Your Health This Flu Season |
|
| Disease | Disease-Causing Pathogen | RO Basic Reproductive Number | CFR Case Fatality Rate | Incubation Time | Hospitalization Rate | Community Attack Rate | Annual Infected Global |
|---|---|---|---|---|---|---|---|
| SARS |  SARS-CoV | 3 | 9.6–11% | 2–7 days | Most cases | 10–60% | 8098 (in 2003) |
| MERS |  MERS-CoV | 0.3–0.8 | 34.4% | 6 days | Most cases | 4–13% | 420 |
| Flu |  Influenza virus | 1.3 | 0.05–0.1% | 1–4 days | 2% | 10–20% | ~1 billion |
| COVID-19 |  SARS-CoV-2 | 2.0–2.5 | ~3.4% | 4–14 days | ~19% | 30–40% | N/A ongoing |
| Indicators | Description |
|---|---|
|
|
| |
| |
|
| Properties | Description |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Properties | Advantages | Disadvantages |
|---|---|---|
|
|
|
|
| |
|
| |
|
| |
|
| |
|
|
| Properties | Type of NPs | Description |
|---|---|---|
| Nanoparticle for diagnostic |
| |
|
| |
| ||
| ||
| ||
|
|
| Properties | Type | Description |
|---|---|---|
| Drug treatment |
| |
|
| |
|
| |
|
| |
|
| |
| Immune-Based Therapy [165] |
|
|
| ||
| Anti-SARS-CoV-2 Antibody Products |
|
|
|
| |
| Corticosteroids |
|
|
|
| |
| Adjunctive Therapy |
|
|
|
| |
|
| |
|
|
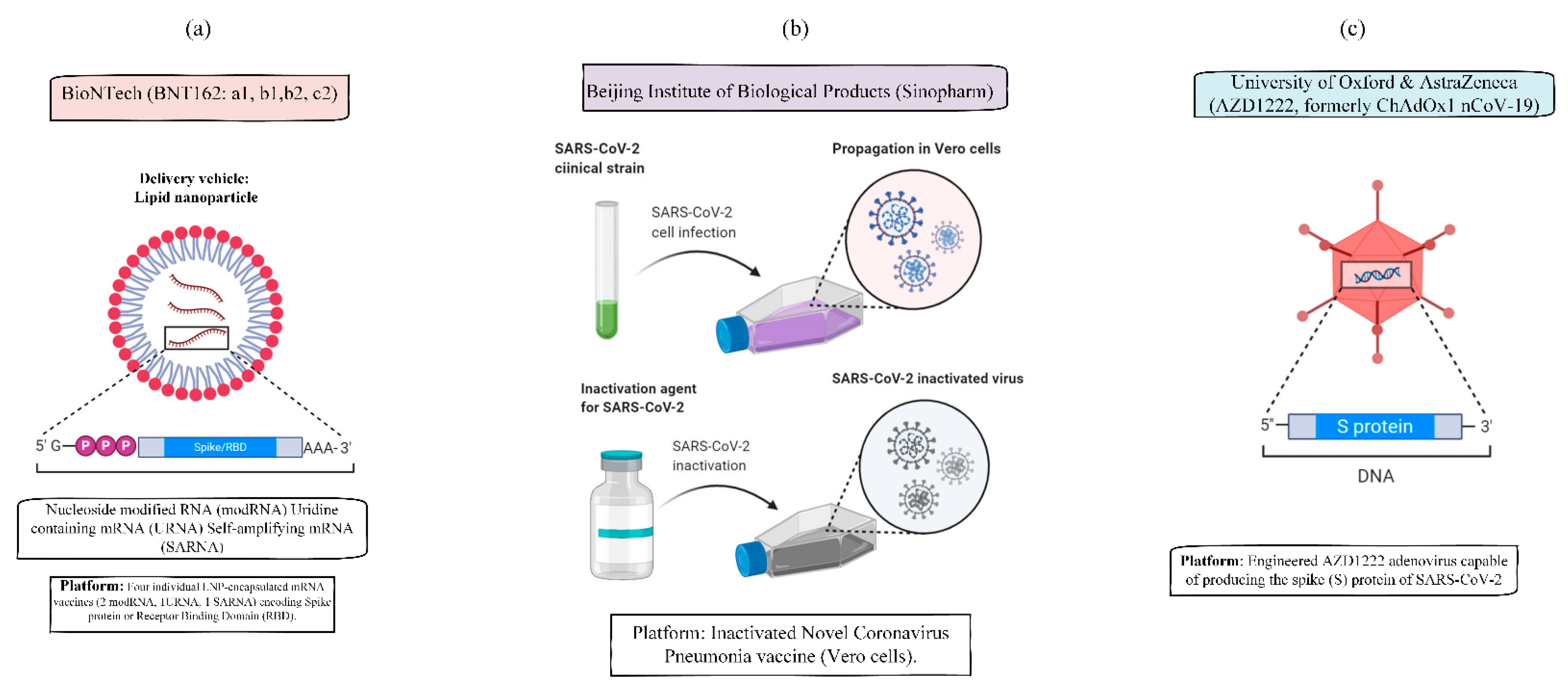
| Name | Vaccine Type | Primary Developers | Country of Origin | Authorization/Approval | Storage | Description |
|---|---|---|---|---|---|---|
| BNT162b2 | mRNA-based vaccine | Pfizer, BioNTech; Fosun Pharma | Multinational | UK, Bahrain, Canada, Mexico, US | −70 °C | BNT162b2 is a modified nucleoside mRNA-based vaccine developed by BioNTech and Pfizer. Fosun Pharma has licensed BNT162b2 in China. The vaccine is given as an intramuscular injection in two doses 21 days apart. BNT162b2 generates an immune response against SARS-CoV-2, the virus that causes COVID-19, by encoding a mutated form of the virus’s full spike protein. |
| AstraZeneca/Oxford AZD1222 | Adenovirus | The University of Oxford; AstraZeneca; IQVIA; Serum Institute of India | The UK | 2–8 °C | A safe and efficacious vaccine with more than 70% impact against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Mechanism: Replication-deficient viral vector vaccine (adenovirus from chimpanzees) | |
| Sinovac CoronaVac | Inactivated vaccine (formalin with alum adjuvant) | Sinovac | China | China | 2–8 °C | CoronaVac (formerly PiCoVacc) is a formalin-inactivated and alum-adjuvanted vaccine developed by the China-based biotechnology company Sinovac Biotech. The vaccine is administered in two doses 14 days apart. |
| Sputnik V | Non-replicating viral vector | Gamaleya Research Institute, Acellena Contract Drug Research and Development | Russia | Russia | −18 °C | The Gamaleya Research Institute in Russia and Health Ministry of the Russian Federation evaluates their non-replicating viral vector vaccine, Sputnik V (formerly Gam-COVID-Vac), in a Phase 3 trial in Russia and internationally. |
| Moderna mRNA-1273 | mRNA-based vaccine | Moderna | USA | the U.S. Food and Drug Administration’s (FDA) has authorized the emergency use of mRNA-1273, Moderna’s vaccine | 2–8 °C | Moderna developed mRNA-1273 based on prior studies of related coronaviruses such as those that cause severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS). Moderna predicts it will be able to distribute 20 million doses to the United States in December 2020, and 100 m doses globally |
| Sinopharm BBIBP-CorV | Inactivated vaccine | Beijing Institute of Biological Products; China National Pharmaceutical Group (Sinopharm) | China | China, United Arab Emirates | 2–8 °C | Sinopharm is developing a second inactivated COVID-19 vaccine candidate, BBIBP-CorV, with the Beijing Institute of Biological Products. |
| Bharat Biotech BBV152 | whole-virion β-propiolactone-inactivated | Bharat Biotech, the Indian Council of Medical Research (ICMR) and National Institute of Virology (NIV) | India | Bharat Biotech’s ‘COVAXIN™’ by DCGI-CDSCO, MoH&FW | 2–8 °C | COVAXIN is an inactivated vaccine obtained from the SARS-CoV-2 strain isolated at the NIV, Pune, an Indian virology research institute. The vaccine is used along with immune stimulants, commonly known as vaccine adjuvants (Alhydroxiquim-II), to improve immune response and longer-lasting immunity. The vaccine candidate is produced through the formulation of the inactivated virus with Kansas-based ViroVax’s Alhydroxiquim-II adjuvant. COVAXIN mainly contains 6 µg of whole-virion inactivated SARS-CoV-2 antigen (Strain: NIV-2020-770), and the other inactive components such as 250 µg aluminium hydroxide gel, 15 µg TLR 7/8 agonist (imidazoquinolinone), 2.5 mg TM 2-phenoxyethanol, and phosphate buffer saline up to 0.5 mL. |
| Johnson and Johnson vaccine (JNJ-78436735) | Viral vector | Janssen Pharmaceuticals Companies of Johnson & Johnson | USA | Johnson & Johnson COVID-19 Vaccine Authorized by U.S. FDA For Emergency Use | 2–8 °C | The J&J/Janssen vaccine was 66.3% effective in clinical trials (efficacy) at preventing laboratory-confirmed COVID-19 illness in people who had no evidence of prior infection 2 weeks after receiving the vaccine. People had the most protection 2 weeks after getting vaccinated. The vaccine had high efficacy at preventing hospitalization and death in people who did get sick. No one who got COVID-19 at least 4 weeks after receiving the J&J/Janssen vaccine had to be hospitalized. |
| Title | Objective | Description |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salahshoori, I.; Mobaraki-Asl, N.; Seyfaee, A.; Mirzaei Nasirabad, N.; Dehghan, Z.; Faraji, M.; Ganjkhani, M.; Babapoor, A.; Shadmehr, S.Z.; Hamrang, A. Overview of COVID-19 Disease: Virology, Epidemiology, Prevention Diagnosis, Treatment, and Vaccines. Biologics 2021, 1, 2-40. https://doi.org/10.3390/biologics1010002
Salahshoori I, Mobaraki-Asl N, Seyfaee A, Mirzaei Nasirabad N, Dehghan Z, Faraji M, Ganjkhani M, Babapoor A, Shadmehr SZ, Hamrang A. Overview of COVID-19 Disease: Virology, Epidemiology, Prevention Diagnosis, Treatment, and Vaccines. Biologics. 2021; 1(1):2-40. https://doi.org/10.3390/biologics1010002
Chicago/Turabian StyleSalahshoori, Iman, Noushin Mobaraki-Asl, Ahmad Seyfaee, Nasrin Mirzaei Nasirabad, Zahra Dehghan, Mehrdad Faraji, Mina Ganjkhani, Aziz Babapoor, Seyede Zahra Shadmehr, and Ali Hamrang. 2021. "Overview of COVID-19 Disease: Virology, Epidemiology, Prevention Diagnosis, Treatment, and Vaccines" Biologics 1, no. 1: 2-40. https://doi.org/10.3390/biologics1010002
APA StyleSalahshoori, I., Mobaraki-Asl, N., Seyfaee, A., Mirzaei Nasirabad, N., Dehghan, Z., Faraji, M., Ganjkhani, M., Babapoor, A., Shadmehr, S. Z., & Hamrang, A. (2021). Overview of COVID-19 Disease: Virology, Epidemiology, Prevention Diagnosis, Treatment, and Vaccines. Biologics, 1(1), 2-40. https://doi.org/10.3390/biologics1010002







