Decreased Effectiveness of a Novel Opioid Withdrawal Protocol Following the Emergence of Medetomidine as a Fentanyl Adulterant
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Population
2.3. Exposure Classification
2.4. Outcomes
2.5. Measurements and Analysis
3. Results
3.1. Patient Characteristics
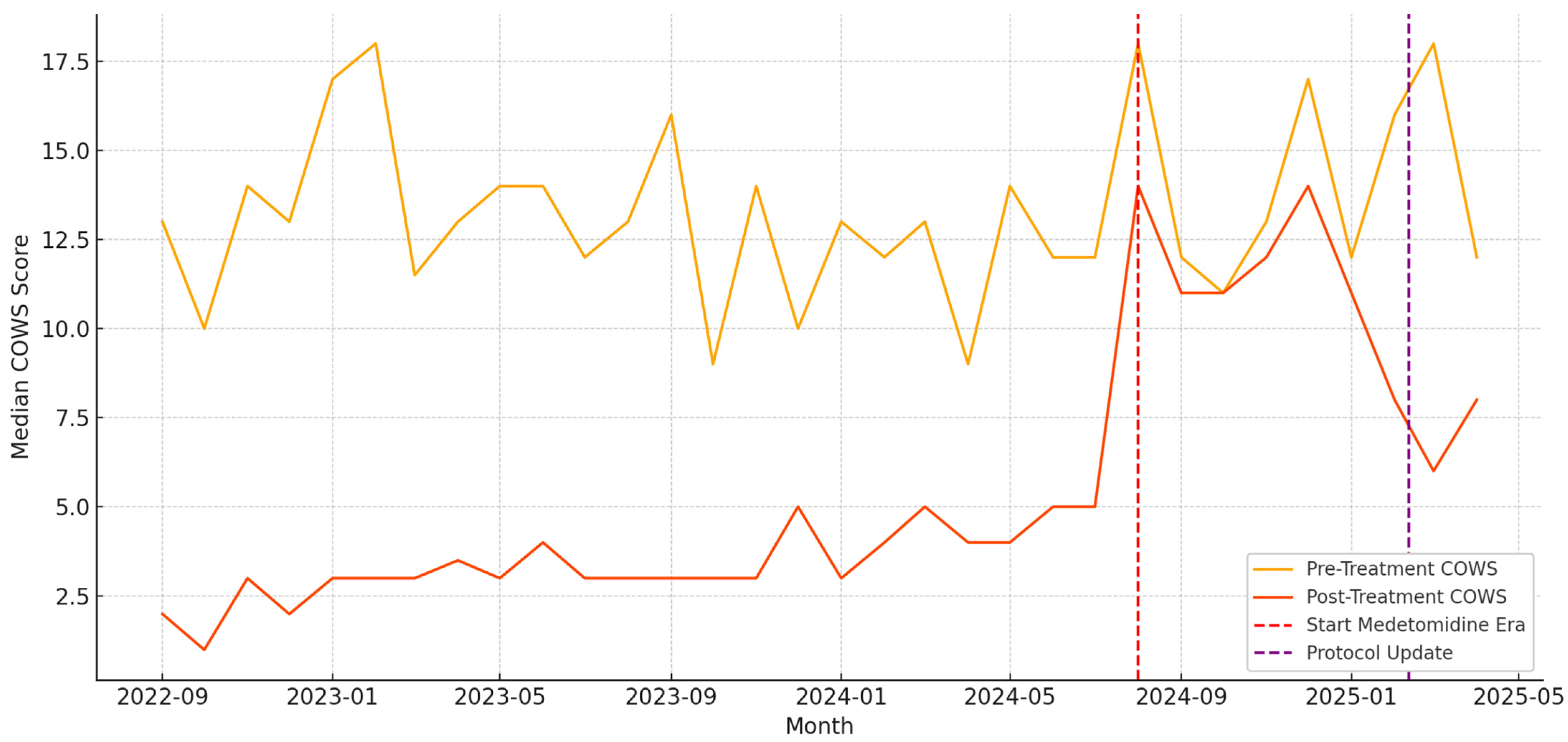
3.2. Withdrawal Treatment and COWS Outcomes
3.3. Secondary Outcomes: Subgroup, Disposition, and Adverse Events
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ED | Emergency Department |
| ME | Medetomidine Era |
| XE | Xylazine Era |
| OUD | Opioid Use Disorder |
| COWS | Clinical Opioid Withdrawal Scale |
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
Appendix A. Categorization of Chief Complaints by Assigned Presentation Category
| Chief Complaint | Assigned Category |
| ABDOMINAL INJURY | Trauma |
| ABDOMINAL PAIN | General Medical/Other |
| ABNORMAL LAB | General Medical/Other |
| ABRASION | Trauma |
| ABSCESS | Skin/Soft Tissue Infection |
| ABSCESS DRAINAGE | Skin/Soft Tissue Infection |
| ALCOHOL INTOXICATION | Psychiatric, Behavioral, and Social Needs |
| ALTERED MENTAL STATUS | General Medical/Other |
| ANGIOEDEMA | General Medical/Other |
| ANKLE PAIN | General Medical/Other |
| ANXIETY | Psychiatric, Behavioral, and Social Needs |
| ARM INJURY | Trauma |
| ARM PAIN | General Medical/Other |
| ARM SWELLING | Skin/Soft Tissue Infection |
| ASSAULT | Trauma |
| ASTHMA | General Medical/Other |
| BACK PAIN | General Medical/Other |
| BLACK OR BLOODY STOOL | General Medical/Other |
| BLOOD INFECTION | General Medical/Other |
| BURN | Trauma |
| CAST CHECK | General Medical/Other |
| CELLULITIS | Skin/Soft Tissue Infection |
| CHEST PAIN | General Medical/Other |
| CHEST TIGHTNESS | General Medical/Other |
| CHILLS | General Medical/Other |
| COLD EXPOSURE | Psychiatric, Behavioral, and Social Needs |
| CONSTIPATION | General Medical/Other |
| COUGH | General Medical/Other |
| COUGHING UP BLOOD | General Medical/Other |
| CYST | General Medical/Other |
| DEBRIDEMENT | General Medical/Other |
| DENTAL PAIN | General Medical/Other |
| DEPRESSION | Psychiatric, Behavioral, and Social Needs |
| DETOX | Opioid Withdrawal |
| DIALYSIS TREATMENT | General Medical/Other |
| DIARRHEA | General Medical/Other |
| DIFFICULTY WALKING | General Medical/Other |
| DIZZINESS | General Medical/Other |
| DRUG OVERDOSE | Psychiatric, Behavioral, and Social Needs |
| DRUG PROBLEM | Psychiatric, Behavioral, and Social Needs |
| DYSURIA | General Medical/Other |
| EARACHE | General Medical/Other |
| ELBOW PAIN | General Medical/Other |
| ENDOCARDITIS | General Medical/Other |
| EXTREMITY WEAKNESS | General Medical/Other |
| EYE DRAINAGE | General Medical/Other |
| EYE INFECTION | General Medical/Other |
| EYE PAIN | General Medical/Other |
| EYE PROBLEM | General Medical/Other |
| EYE TRAUMA | Trauma |
| FACIAL SWELLING | General Medical/Other |
| FAILURE TO THRIVE | Psychiatric, Behavioral, and Social Needs |
| FALL | Trauma |
| FEVER | General Medical/Other |
| FINGER INJURY | Trauma |
| FINGER PAIN | General Medical/Other |
| FLANK PAIN | General Medical/Other |
| FLU SYMPTOMS | General Medical/Other |
| FOOT BLISTER | General Medical/Other |
| FOOT INJURY | Trauma |
| FOOT PAIN | General Medical/Other |
| FOOT SWELLING | General Medical/Other |
| FOOT WOUND CHECK | General Medical/Other |
| FOREIGN BODY | General Medical/Other |
| FOREIGN BODY IN SKIN | General Medical/Other |
| FROSTBITE | Trauma |
| GENERALIZED BODY ACHES | General Medical/Other |
| GENITAL WARTS | General Medical/Other |
| GROIN PAIN | General Medical/Other |
| GROIN SWELLING | General Medical/Other |
| GUN SHOT WOUND | Trauma |
| HAND INFECTION | Skin/Soft Tissue Infection |
| HAND INJURY | Trauma |
| HAND PAIN | General Medical/Other |
| HEAD LICE | General Medical/Other |
| HEADACHE | General Medical/Other |
| HERNIA | General Medical/Other |
| HIP PAIN | General Medical/Other |
| HOMELESS | Psychiatric, Behavioral, and Social Needs |
| HYPERGLYCEMIA | General Medical/Other |
| HYPERTENSION | General Medical/Other |
| HYPOGLYCEMIA | General Medical/Other |
| INFECTION | Skin/Soft Tissue Infection |
| INGESTION | Psychiatric, Behavioral, and Social Needs |
| INTOXICATED | Psychiatric, Behavioral, and Social Needs |
| JAW PAIN | General Medical/Other |
| JOINT SWELLING | General Medical/Other |
| KNEE INJURY | Trauma |
| KNEE PAIN | General Medical/Other |
| LEG INJURY | Trauma |
| LEG PAIN | General Medical/Other |
| LEG PROBLEM | General Medical/Other |
| LEG SWELLING | General Medical/Other |
| MEDICAL COMPLAINT | General Medical/Other |
| MEDICATION REFILL | Psychiatric, Behavioral, and Social Needs |
| MOTOR VEHICLE vs. PEDESTRIAN | Trauma |
| MOTOR VEHICLE CRASH | Trauma |
| MRSA | Skin/Soft Tissue Infection |
| MULTIPLE SCLEROSIS | General Medical/Other |
| NASAL CONGESTION | General Medical/Other |
| NAUSEA | General Medical/Other |
| NECK INJURY | Trauma |
| NECK PAIN | General Medical/Other |
| NUMBNESS | General Medical/Other |
| OPEN WOUND | Skin/Soft Tissue Infection |
| OSTEOMYELITIS | General Medical/Other |
| OTHER | General Medical/Other |
| PAIN | General Medical/Other |
| PAIN WITH BREATHING | General Medical/Other |
| PALPITATIONS | General Medical/Other |
| PNEUMONIA | General Medical/Other |
| POOR APPETITE | General Medical/Other |
| POST-OP PROBLEM | General Medical/Other |
| PREGNANCY PROBLEM | General Medical/Other |
| PSYCHIATRIC EVALUATION | Psychiatric, Behavioral, and Social Needs |
| RAPID HEART RATE | General Medical/Other |
| RASH | General Medical/Other |
| RECURRENT SKIN INFECTIONS | Skin/Soft Tissue Infection |
| RESPIRATORY DISTRESS | General Medical/Other |
| RIB INJURY | General Medical/Other |
| RING REMOVAL | General Medical/Other |
| RULE OUT STROKE | General Medical/Other |
| SEIZURES | General Medical/Other |
| SEPSIS OUTREACH | General Medical/Other |
| SEXUAL ASSAULT | Trauma |
| SHORTNESS OF BREATH | General Medical/Other |
| SHOULDER PAIN | General Medical/Other |
| SINUSITIS | General Medical/Other |
| SKIN PROBLEM | Skin/Soft Tissue Infection |
| SOCIAL DETERMINANTS SCREENING | Psychiatric, Behavioral, and Social Needs |
| SORE THROAT | General Medical/Other |
| SPASMS | General Medical/Other |
| STAB WOUND | Trauma |
| STROKE ALERT | General Medical/Other |
| SUICIDAL | Psychiatric, Behavioral, and Social Needs |
| SUICIDE ATTEMPT | Psychiatric, Behavioral, and Social Needs |
| SWELLING | General Medical/Other |
| SWELLING HEAD/NECK | General Medical/Other |
| SYNCOPE | General Medical/Other |
| TRAUMA | Trauma |
| URI | General Medical/Other |
| URINARY PROBLEM | General Medical/Other |
| VAGINAL BLEEDING | General Medical/Other |
| VAGINAL BLEEDING—PREGNANT | General Medical/Other |
| VASCULAR ACCESS PROBLEM | General Medical/Other |
| VENOUS THROMBOSIS | General Medical/Other |
| VOMITING | General Medical/Other |
| VOMITING BLOOD | General Medical/Other |
| WEAKNESS—GENERALIZED | General Medical/Other |
| WELLNESS VISIT | Psychiatric, Behavioral, and Social Needs |
| WITHDRAWAL | Opioid Withdrawal |
| WOUND CARE | Skin/Soft Tissue Infection |
| WOUND CHECK | Skin/Soft Tissue Infection |
| WOUND DEHISCENCE | Skin/Soft Tissue Infection |
| WOUND INFECTION | Skin/Soft Tissue Infection |
| WRIST PAIN | General Medical/Other |
References
- Quijano, T.; Crowell, J.; Eggert, K.; Clark, K.; Alexander, M.; Grau, L.; Heimer, R. Xylazine in the drug supply: Emerging threats and lessons learned in areas with high levels of adulteration. Int. J. Drug Policy 2023, 120, 104154. [Google Scholar] [CrossRef] [PubMed]
- DeBord, J.S.; Shinefeld, J.; Russell, R.; Denn, M.; Quinter, A.; Logan, B.K.; Teixeira da Silva, D.; Krotulski, A.J. Drug Checking—Quarterly Report; Center for Forensic Science Research and Education: Philadelphia, PA, USA, 2023; Available online: https://www.cfsre.org/images/content/reports/drug_checking/2023_Q1_and_Q2_Drug_Checking_Quarterly_Report_CFSRE_NPS_Discovery.pdf (accessed on 9 April 2025).
- Varshneya, N.B.; Thakrar, A.P.; Hobelmann, J.G.; Dunn, K.E.; Huhn, A.S. Evidence of buprenorphine-precipitated withdrawal in persons who use fentanyl. J. Addict. Med. 2022, 16, e265–e268. [Google Scholar] [CrossRef] [PubMed]
- London, K.; Li, Y.; Kahoud, J.L.; Cho, D.; Mulholland, J.; Roque, S.; Stugart, L.; Gillingham, J.; Borne, E.; Slovis, B. Tranq dope: Characterization of an ED cohort treated with a novel opioid withdrawal protocol in the era of fentanyl/xylazine. Am. J. Emerg. Med. 2024, 85, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Teixeira da Silva, D.; LaBoy, C.; Franklin, F. Hospitals and Behavioral Health Providers Are Reporting Severe and Worsening Presentations of Withdrawal Among People Who Use Drugs (PWUD) in Philadelphia; Philadelphia Department of Public Health, Health Alert: Philadelphia, PA, USA, 2024. Available online: https://hip.phila.gov/document/4874/PDPH-HAN-00444A-12-10-2024.pdf/ (accessed on 9 April 2025).
- Virtanen, R.; Savola, J.M.; Saano, V.; Nyman, L. Characterization of the selectivity, specificity and potency of medetomidine as an α2-adrenoceptor agonist. Eur. J. Pharmacol. 1988, 150, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Yaygıngül, R.; Belge, A. The comparison of clinical and cardiopulmonary effects of xylazine, medetomidine and detomidine in dogs. Ankara Univ. Vet. Fak. Derg. 2018, 65, 313–322. [Google Scholar] [CrossRef]
- Sugarman, O.K.; Shah, H.; Whaley, S.; McCourt, A.; Saloner, B.; Bandara, S. A content analysis of legal policy responses to xylazine in the illicit drug supply in the United States. Int. J. Drug Policy 2024, 129, 104472. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; London, K.; Murphy, L.; Casey, E.; Durney, P.; Arora, M.; McKeever, R.; Tasillo, A.; Goodstein, D.; Hart, B.; et al. Notes from the field: Suspected medetomidine withdrawal syndrome among fentanyl-exposed patients—Philadelphia, Pennsylvania, September 2024–January 2025. MMWR Morb. Mortal. Wkly. Rep. 2025, 74, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Kukoyi, A.T.; Coker, S.A.; Lewis, L.D.; Nierenberg, D.W. Two cases of acute dexmedetomidine withdrawal syndrome following prolonged infusion in the intensive care unit: Report of cases and review of the literature. Hum. Exp. Toxicol. 2013, 32, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.R.; Ling, W. The clinical opiate withdrawal scale (COWS). J. Psychoact. Drugs 2003, 35, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.; Agwuncha, C.; Wilson, C.; Schrecker, J.; Holt, A.; Heltsley, R. Withdrawal signs and symptoms among patients positive for fentanyl with and without xylazine. J. Addict. Med. 2025, 19, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Kariisa, M.; O’Donnell, J.; Kumar, S.; Mattson, C.L.; Goldberger, B.A. Illicitly Manufactured Fentanyl–Involved Overdose Deaths with Detected Xylazine—United States, January 2019–June 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.S.; Buchanan, J.; Aldy, K.; Shulman, J.; Krotulski, A.J.; Walton, S.E.; Logan, B.K.; Wax, P.M.; Campleman, S.; Brent, J.A.; et al. Notes from the Field: Detection of Medetomidine Among Patients Evaluated in Emergency Departments for Suspected Opioid Overdoses—Missouri, Colorado, and Pennsylvania, September 2020–December 2023. CDC MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Philadelphia Department of Public Health. Health Alert: Medetomidine Detected in Philadelphia’s Illicit Drug Supply. 13 May 2024. Available online: https://hip.phila.gov/document/4421/PDPH-HAN-0441A-05-13-24.pdf (accessed on 6 October 2024).
- New York State Office of Addiction Services and Supports. Another Potent Sedative, Medetomidine, Now Appearing in Illicit Drug Supply. 31 May 2024. Available online: https://oasas.ny.gov/advisory-may-31-2024 (accessed on 6 October 2024).
- Krotulski, A.J.; Shinefeld, J.; Moraff, C.; Wood, T.; Walton, S.E.; DeBord, J.S.; Denn, M.T.; Quinter, A.D.; Logan, B.K. Medetomidine Rapidly Proliferating Across USA—Implicated in Recreational Opioid Drug Supply & Causing Overdose Outbreaks. CFSRE NPS Discovery Public Alert. 20 May 2024. Available online: https://www.cfsre.org/nps-discovery/public-alerts/medetomidine-rapidly-proliferating-across-usa-implicated-in-recreational-opioid-drug-supply-causing-overdose-outbreaks (accessed on 6 October 2024).
- Sood, N.; Dhillon, G. Illicit Medetomidine Use with Fentanyl. The Hospitalist. 1 January 2025. Available online: https://www.the-hospitalist.org/hospitalist/article/38385/addiction-medicine/illicit-medetomidine-use-with-fentanyl/ (accessed on 30 March 2025).
- Sood, N. Rise of illicit medetomidine use: A worrisome trend. Am. J. Addict. 2025, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Scheinin, H.; Virtanen, R.; MacDonald, E.; Lammintausta, R.; Scheinin, M. Medetomidine—A Novel Alpha2-Adrenoceptor Agonist: A Review of Its Pharmacodynamic Effects. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1989, 13, 635–651. [Google Scholar] [CrossRef] [PubMed]
- Papich, M.G. Medetomidine Hydrochloride. In Saunders Handbook of Veterinary Drugs, 4th ed.; Papich, M.G., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2016; pp. 481–483. [Google Scholar]
- New York City Department of Health and Mental Hygiene. What Is Xylazine? (Frequently Asked Questions). May 2023. Available online: https://www.nyc.gov/assets/doh/downloads/pdf/basas/xylazine-faq.pdf (accessed on 30 March 2025).
- Suárez-Lledó, A.; Padullés, A.; Lozano, T.; Cobo-Sacristán, S.; Colls, M.; Jódar, R. Management of Tizanidine Withdrawal Syndrome: A Case Report. Clin. Med. Insights Case Rep. 2018, 11, 1179547618758022. [Google Scholar] [CrossRef] [PubMed]
- Knapp, T.; DiLeonardo, O.; Maul, T.; Hochwald, A.; Li, Z.; Hossain, J.; Lowry, A.; Parker, J.; Baker, K.; Wearden, P.; et al. Dexmedetomidine Withdrawal Syndrome in Children in the PICU: Systematic Review and Meta-Analysis. Pediatr. Crit. Care Med. 2024, 25, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; McLaughlin, K.C.; Romero, N.; Crowley, K.E. Evaluation of Dexmedetomidine Withdrawal and Management After Prolonged Infusion. Clin. Ther. 2024, 46, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
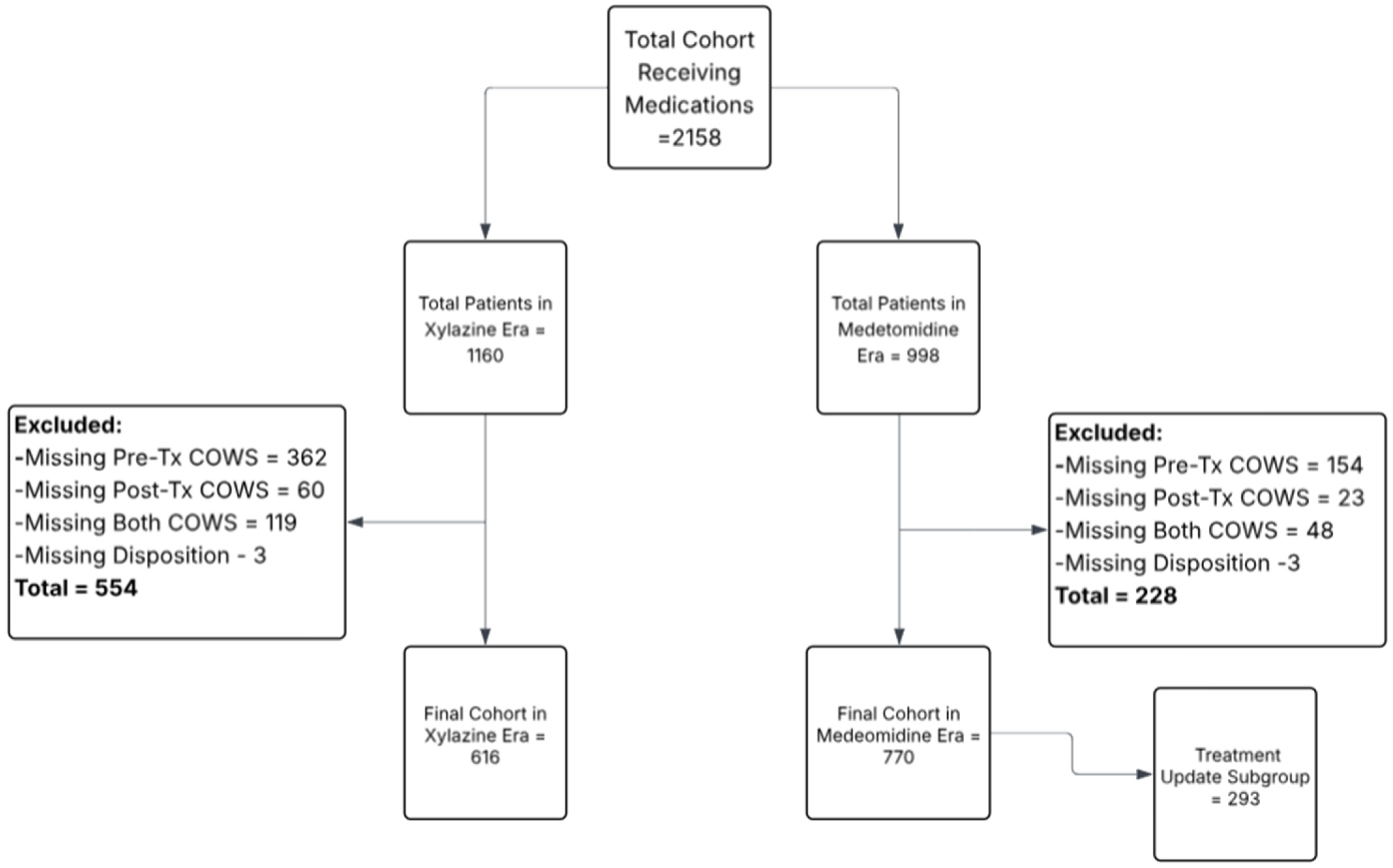

| Characteristic | Xylazine Era (Total, N = 1160) | Medetomidine Era (Total, N = 998) | Xylazine Era (Final, N = 616) | Medetomidine Era (Final, N = 770) |
|---|---|---|---|---|
| Age, mean ± SD | 39.8 ± 9.2 | 40.9 ± 9.6 | 39.6 ± 9.2 | 41.1 ± 10.0 |
| Female, n (%) | 408 (35.2%) | 351 (35.2%) | 213 (34.6%) | 275 (35.7%) |
| White, n (%) | 885 (76.3%) | 779 (78.1%) | 467 (75.8%) | 599 (77.8%) |
| Black, n (%) | 122 (10.5%) | 117 (11.7%) | 66 (10.7%) | 87 (11.3%) |
| Latinx, n (%) | 115 (9.9%) | 69 (6.9%) | 65 (10.6%) | 55 (7.1%) |
| 2 + Races, n (%) | 13 (1.1%) | 4 (0.4%) | 6 (1.0%) | 4 (0.5%) |
| Asian, n (%) | 3 (0.3%) | 4 (0.4%) | 1 (0.2%) | 3 (0.4%) |
| Other/Unknown, n (%) | 19 (1.6%) | 24 (2.4%) | 9 (1.5%) | 21 (2.7%) |
| Positive Urine Fentanyl Screen, n (%) | 893 (97.0%) | 807 (98.7%) | 515 (96.6%) | 649 (98.6%) |
| Missing Urine Fentanyl Screen | 239 | 180 | 83 | 112 |
| Xylazine Era N (%) | Medetomidine Era N (%) | p-Value | |
|---|---|---|---|
| General Medical/Other | 483 (41.6) | 419 (42.0) | p = 0.905 |
| Opioid Withdrawal | 159 (13.7) | 312 (31.3) | p < 0.001 |
| Psychiatric, Behavioral and Social Needs | 60 (5.2) | 58 (5.8) | p = 0.578 |
| Skin/Soft Tissue Infection | 386 (33.3) | 150 (15.0) | p < 0.001 |
| Trauma | 72 (6.2) | 59 (5.9) | p = 0.845 |
| Outcome Metric | Xylazine Era (n = 616) | Medetomidine Era (n = 770) | p-Value |
|---|---|---|---|
| Mean ED Encounters per Month | 26.8 | 85.6 | p < 0.001 |
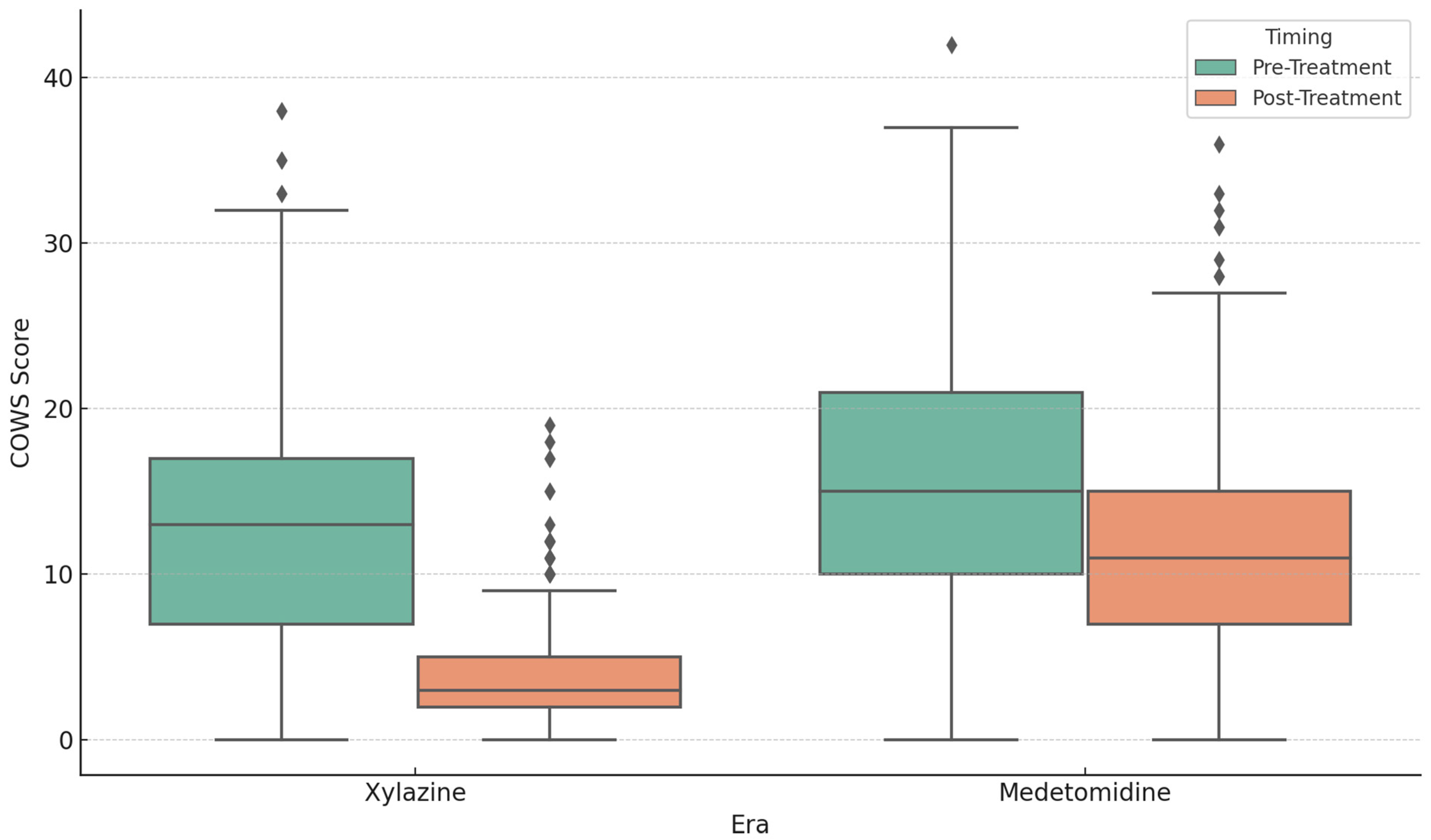
| Median Pre-Treatment COWS | 13 | 15 | p < 0.001 |
| Median Post-Treatment COWS | 4 | 11 | p < 0.001 |
| Median COWS Reduction (∆) | 9 | 5 | p < 0.001 |
| Mean COWS Reduction ± SD | 8.80 ± 7.41 | 4.39 ± 7.61 | p < 0.001 |
| % Achieving COWS ≤ 4 | 65.60% | 16.40% | p < 0.001 |
| ED Disposition | Xylazine Era (N = 616) | Medetomidine Era Before Protocol Change (BPC) (N = 477) | Medetomidine Era After Protocol Change (APC) (N = 293) | p-Value (XE vs. BPC, BPC vs. APC) |
|---|---|---|---|---|
| Admit | 410 (66.6%) | 348 (72.7%) | 131 (75.3%) | p = 0.036, p = 0.566 |
| ICU Admission | 35 (8.5%) | 88 (18.4%) | 22 (16.8%) | p < 0.001, p = 0.107 |
| Discharge | 182 (29.5%) | 99 (20.7%) | 33 (19.0%) | p = 0.001, p = 0.712 |
| AMA | 22 (3.6%) | 31 (6.5%) | 9 (5.2%) | p = 0.038, p = 0.669 |
| Transfer to Another Facility for Eval | 2 (0.3%) | 1 (0.2%) | 1 (0.6%) | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
London, K.S.; Durney, P.; Warrick-Stone, T.; Alexander, K.; Kahoud, J.L. Decreased Effectiveness of a Novel Opioid Withdrawal Protocol Following the Emergence of Medetomidine as a Fentanyl Adulterant. BioMed 2025, 5, 13. https://doi.org/10.3390/biomed5020013
London KS, Durney P, Warrick-Stone T, Alexander K, Kahoud JL. Decreased Effectiveness of a Novel Opioid Withdrawal Protocol Following the Emergence of Medetomidine as a Fentanyl Adulterant. BioMed. 2025; 5(2):13. https://doi.org/10.3390/biomed5020013
Chicago/Turabian StyleLondon, Kory S., Philip Durney, TaReva Warrick-Stone, Karen Alexander, and Jennifer L. Kahoud. 2025. "Decreased Effectiveness of a Novel Opioid Withdrawal Protocol Following the Emergence of Medetomidine as a Fentanyl Adulterant" BioMed 5, no. 2: 13. https://doi.org/10.3390/biomed5020013
APA StyleLondon, K. S., Durney, P., Warrick-Stone, T., Alexander, K., & Kahoud, J. L. (2025). Decreased Effectiveness of a Novel Opioid Withdrawal Protocol Following the Emergence of Medetomidine as a Fentanyl Adulterant. BioMed, 5(2), 13. https://doi.org/10.3390/biomed5020013






