Abstract
Antiretroviral treatment (ART) has revolutionized the management of the human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS), enabling long-term viral load (VL) suppression in patients. Despite the proven effectiveness of ART, a significant proportion of patients with HIV receiving ART fail to achieve viral load suppression (VLS). This study aimed to identify factors associated with low VLS in the Tanganyika province. An unmatched case–control study was conducted from January 2022 to June 2023, including 22 care facilities with viral load data. Data were collected from patient records. For each reviewed record, the patient was invited for an interview upon providing informed consent. Data were analyzed using SPSS version 27. In a multivariable binary logistic regression model, variables with a p-value < 0.05 and a 95% confidence interval for the adjusted odds ratio were considered significantly associated with unsuppressed VL. A total of 462 individuals, including 156 cases and 306 controls, were included in the study. The mean age (standard deviation) of participants was 42.12 (±11.6) years. The following covariates were significantly associated with unsuppressed VL: poor HIV status disclosure to a confidant [adjusted OR = 2.10, 95% CI (1.33–3.31), p = 0.001], poor ART adherence [adjusted OR = 2.01, 95% CI (1.25–3.23), p = 0.004], ART interruption [adjusted OR = 3.43, 95% CI (2.00–5.88), p < 0.001], no participation in support groups [adjusted OR = 2.16, 95% CI (1.25–3.71), p = 0.005], baseline WHO clinical stage 3 and 4 [adjusted OR = 2.24, 95% CI (1.32–3.79), p = 0.003], opportunistic infections (OIs) [adjusted OR = 2.30, 95% CI (1.27–4.16), p = 0.006], and non-communicable chronic diseases (NCDs) [adjusted OR = 2.30, 95% CI (1.10–4.79), p = 0.026]. Given the clear association between several factors and unsuppressed VL, prevention should involve the implementation of innovative strategies targeting at-risk patient groups. Strengthening the monitoring of these factors among active patients at each appointment is recommended to achieve this goal.
1. Introduction
ART has revolutionized the management of HIV/AIDS, enabling long-term VLS in many patients [1,2,3]. Despite the proven efficacy of ART, a significant proportion of patients with HIV receiving ART fail to achieve VLS [4,5].
Globally, according to a World Health Organization (WHO) report, the unsuppressed viral load rate varies by region [6]. In 2021, 30% of patients on ART aged 15 years and over did not achieve VL suppression worldwide [7]. In the WHO regions of West and Central Africa and East and Southern Africa, the VL unsuppressed rates were 38% (2020) and 28% (2021) among patients ≥15 years old on ART, respectively [7,8]. In the Democratic Republic of Congo (DRC), approximately 11% of people living with HIV (PLHIV) on ART had unsuppressed VL among those tested for VL in 2021 [9]. Similar trends are observed in the Tanganyika province, and this situation remains a concern, as reports indicate that viral load non-suppression (VLNS) increased from 20% to 34% between 2021 and 2022 [10].
Unsuppressed VL is a major challenge for HIV/AIDS control programs due to its consequences [11], such as an increased risk of virus transmission, progression to advanced HIV disease (AHD), emergence of drug-resistant HIV strains, and increased mortality [12,13,14].
Identifying factors associated with unsuppressed VL will enable the development of interventions, improve patients’ quality of life and life expectancy, decrease new infection rates and the emergence of ARV-resistant strains, and support a reduction in HIV-related morbidity and mortality [4,12,13,15].
This study was aimed at identifying potential factors associated with unsuppressed VL among patients on ART in the Tanganyika province from January 2022 to June 2023. No similar study has been conducted in the province. Several studies [5,14,16,17,18,19,20,21,22], have identified multiple factors linked to VLNS: (a) non-disclosure of HIV status, (b) poor treatment adherence, (c) distance from care facilities, (d) low adherence, (e) ART interruption, (f) stigma, and (g) WHO clinical stage. Considering the specific context of the Tanganyika province, other factors could be associated with unsuppressed VL.
2. Materials and Methods
This was an unmatched case–control study conducted in patients with HIV receiving ART from 1 January 2022 to 30 June 2023 in the Tanganyika province, in the eastern region of the DRC. The province comprises 11 health zones and 263 health areas, of which 110 (42%) have integrated HIV activities. In 2023, the province had a population of 3,570,874 inhabitants, with an active cohort of 5624 patients on ART and an HIV prevalence rate of 2.3% [23]. All the facilities with HIV activities and with VL results were included in the study (13 general referral hospitals and nine health centers). All of these health care facilities were located in the 8/11 (73%) health zones with VL results, namely, Kalemie, Nyemba, Moba, Nyunzu, Kabalo, Kongolo, Kansimba, and Manono.
The study included the following cases: patients ≥18 years old enrolled in an ART program for at least six months, with a documented unsuppressed VL (≥1000 copies/mL) during the period from January 2022 to 30 June 2023. Controls were identified among the patients at the same health facilities with a documented suppressed VL (<1000 copies/mL) during the same period.
The sample size was determined using OpenEpi version 3.01, referencing factors associated with VLNS identified in a case–control study conducted in Ethiopia in 2022 [22].
From the retained independent variables, the “presence of OI” variable resulted in the largest sample size. Based on patient data with VL results, including 571 subjects, we retained a minimum sample size of 282 (94 cases and 188 controls) calculated from the “treatment interruption” variable. To increase the study’s precision and avoid type II errors, we opted to use an exhaustive sample for the cases; thus, the expected sample size became 173 cases and 346 controls, totaling 519 subjects. A simple random sampling technique was used to select the controls (Table 1).

Table 1.
Calculation of the minimum sample size based on selected variables.
Study Variables
The dependent variable was non-suppressed VL. In this study, the non-suppression of VL was defined as a VL measurement with a value equal to or greater than 1000 copies/mL [1,6].
Independent variables were grouped into sociodemographic characteristics (age, sex, religion, marital status…), individual and behavioral characteristics (HIV status disclosure, history of stigma, no participation in support groups…), clinical and biological characteristics (adherence to ART, history of ART interruption, use of cotrimoxazole chemoprophylaxis, use of TB-preventive therapy, nutritional status, duration of treatment at first viral load, WHO clinical stage at ART initiation, history of OI, TB-HIV co-infection, history of chronic non-communicable diseases), and system-related characteristics (ARV stock-outs in the last 12 months, history of irregular biological monitoring, type of technical facilities, presence of psychosocial care services, distance between home and care facility). VL measurement in Tanganyika province is performed using dry blood spot samples collected on filter paper and sent to the reference laboratory in Kinshasa, where analyses are conducted using the Abbott m2000RT platform.
Adherence was measured using three parameters based on the Morisky Medication Adherence Scale (MMAS) [24,25], which has been adapted for use in the context of our study. The adhesion measurement parameters are as follows:
- Self-reported adherence related to missed doses;
- Pill count to determine if missed daily doses were ≥2/30 monthly doses;
- History of poor adherence reported in patient records before viral load testing.
All three parameters were scored one point for every “Yes” response. For interpretation, an overall score of 0 indicated good adherence, and an overall score between 1 and 3 indicated poor adherence.
Data collection, processing, and analysis: Two main techniques were used for data collection: interviews and document reviews based on a structured electronic questionnaire configured using the Kobocollect application v2024.1.3. Five trained nurses performed the interviews and the data collection. All the study team members were trained on the objectives of the study, study procedures and SOPs, and related ethics considerations and principles, including good clinical practices and confidentiality.
The research team obtained prior authorizations (from the dissertation director, Ethics Committee (ESP/CE/122/2023), politico-administrative authorities, Provincial Health Division, health zone, and health care facilities).
Data processing and analysis were performed using SPSS version 27.0.
Descriptive analysis: Quantitative variables are presented as mean and standard deviation. The distribution of quantitative variables such as age was verified by the Kolmogorov–Smirnov test. All categorical variables are presented as frequency tables and percentages.
Bivariate analyses: Variables with a p-value less than 0.20 in simple binary logistic regression were candidates for multivariable analysis.
Multivariable analyses: The odds ratio with 95% confidence interval was calculated, and independent variables with p-values of less than 0.05 in multivariable logistic regression analysis were considered significantly associated with unsuppressed VL.
Ethical Considerations: The entire process (from protocol design to report writing) of this study was conducted in accordance with basic ethical principles, namely, respect for persons, beneficence, and justice. This study was reviewed and approved by the Ethics Committee of the School of Public Health, University of Kinshasa (ESP/CE/122/2023). Verbal informed consent was obtained individually from participants after explaining the study’s objectives and benefits. Throughout data collection and processing, participant confidentiality and anonymity were maintained.
3. Results
3.1. Participation Flow
A total of 519 participants were expected in the study, consisting of 173 cases and 346 controls. However, 57 subjects did not participate in the study, resulting in a non-consent rate of 11.0%. Ultimately, 462 participants consented, including 156 cases and 306 controls, yielding a response rate of 89.0% (Figure 1).
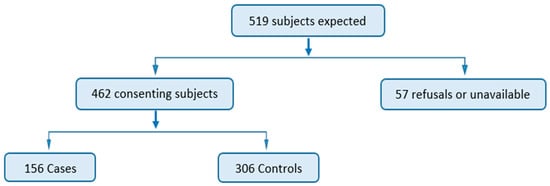
Figure 1.
Participant flow diagram.
An 11% non-response rate means 57 subjects did not participate in the study; 17 (5 cases and 12 controls) did not consent and 40 (12 cases and 28 controls) were not available. This did not affect the validity of the study because the minimum sample size required was exceeded (n = 462), whereas it was calculated at 282 (cases and controls). The ratio of cases to controls moved from the expected 1/2 ratio to 1/1.96.
3.2. Sociodemographic Characteristics
The mean age (standard deviation) of the participants was 42.12 (±11.6) years. Of the 156 cases, 66.7% were women and 33.3% were men, resulting in a sex ratio of 1/2 (male/female). In both participant groups, females constituted approximately six out of ten cases (66.7%) and seven out of ten controls (69.0%).
The predominant age group was 34 to 49 years, accounting for 44.9% of the cases and 48.7% of the controls. Three-quarters of both cases (75.6%) and controls (74.8%) lived in urban areas. Married individuals comprised the majority, with 41.7% of the cases (65 individuals) and 48.0% of the controls (147 individuals). The majority of participants had secondary education (54.5% of the cases and 51.0% of the controls). Protestantism was the most represented religion among cases (32%), while among controls, Protestantism and Revival Churches were equally represented (Table 2).

Table 2.
Sociodemographic characteristics of respondents in the Tanganyika province, January 2022 to 30 June 2023.
3.3. Binary Logistic Regression in Bivariate Analyses
Regarding the sociodemographic characteristics, the history of internal displacement due to conflict (p = 0.013) was selected for multivariable analysis. Several behavioral characteristics were considered for multivariable analysis: non-disclosure of serological status to a confidant (p < 0.001), history of stigma (p = 0.001), non-participation in support groups (p < 0.001), history of self-medication using traditional methods (p < 0.001), and history of alcohol consumption (p < 0.001). Clinical and biological characteristics such as poor adherence to ART (p < 0.001), history of ART interruption (p < 0.001), TB-preventive treatment (TPT) (p = 0.016), underweight (p = 0.004), WHO clinical stage 3 and 4 at ART initiation (p < 0.001), history of OIs (p < 0.001), and history of chronic non-communicable diseases (p = 0.008) were selected for multivariable analysis. Regarding system-related characteristics, only a distance between the residence and the health care center greater than 5 km (p = 0.045) was used for multivariable analysis (Table 3).

Table 3.
Simple binary logistic regression analysis of factors associated with unsuppressed viral load among adult patients with HIV in the Tanganyika province from January 2022 to 30 June 2023.
3.4. Multivariable Logistic Regression Analysis
After adjusting for potential confounders using the backward elimination method in the multivariable logistic regression model, several factors remained significantly associated with unsuppressed VL. The most adjusted model included the following variables: non-disclosure of serological status to a confidant (p = 0.001), poor adherence to ART (p = 0.004), ART interruption (p < 0.001), non-participation in a support group (p = 0.005), WHO clinical stage 3 and 4 at ART initiation (p = 0.003), history of OIs (p < 0.006), and non-communicable chronic diseases (p = 0.026).
The results showed that HIV-positive patients on ART who had not disclosed their serological status to a confidant had two times the odds of having unsuppressed VL compared to those who had disclosed their status [AOR = 2.10, 95% CI (1.33–3.31)]. Patients with poor adherence to ART also had twice the odds of having unsuppressed VL compared to those with good adherence [AOR = 2.01; 95% CI (1.25–3.23)]. Patients with a history of ART interruption had 3.4 times the odds of having unsuppressed VL than those who had not interrupted their ART [AOR = 3.43; 95% CI (2.00–5.88)]. Additionally, patients who were not members of a support group were twice as likely to have unsuppressed VL compared to those who were members of support groups [AOR = 2.16; 95% CI (1.25–3.71)]. It also emerged that patients with HIV who were at WHO clinical stage 3 or 4 at ART initiation [AOR = 2.24; 95% CI (1.32–3.79)] had two times the odds of having unsuppressed VL compared to those with WHO clinical stage 1 or 2. Furthermore, patients with a history of OIs had 2.3 times higher odds of having unsuppressed VL compared to those without an history of OIs [AOR = 2.30; 95% CI (1.27–4.16)]. HIV-positive patients on ART with a history of non-communicable chronic diseases (NCDs) [AOR = 2.30; 95% CI (1.10–4.79)] had 2.3 times the odds of having unsuppressed VL compared to those without a history of NCDs (Table 4).

Table 4.
Multivariable logistic regression analysis of factors associated with viral load non-suppression among adult patients with HIV in the Tanganyika province from January 2022 to 30 June 2023.
4. Discussion
This study was initiated to identify factors associated with unsuppressed VL among HIV-positive patients receiving ART in the Tanganyika province.
Seven variables remained significantly associated with unsuppressed VL. The non-disclosure of serological status to a confidant was statistically significantly associated with unsuppressed VL. This result aligns with other previous studies conducted in the African context such as Ethiopia and in Tanzania [5,18], where patients who did not disclose their HIV status had 5 and 3.3 times the odds of having unsuppressed VL compared to those who had disclosed their status. This could be due to the fear of being rejected by society or becoming victims of stigma and discrimination if they share their HIV status [26], with a negative impact on adherence. It could also be exacerbated by the lack of psychosocial support and inability to receive help or support from close ones or family, as well as the poor implementation of disclosure policies by providers, especially for adolescents and young children [18,20]. Conversely, two other cross-sectional studies from 2019 conducted in South Africa among HIV-positive women and another in the DRC among pregnant and breastfeeding HIV-positive women found that not disclosing seropositivity to male partners was not significantly associated with detectable viremia [19,27]. The difference in these results might be due to the fact that this study followed a case–control design, while the studies with contrary findings were cross-sectional with different inclusion criteria.
Patients with poor adherence to ART had also twice the odds of having unsuppressed VL compared to those with good adherence. A previous study with a similar design conducted in Ethiopia found that clients with poor medication adherence were 2.44 and 1.11 times more likely to have unsuppressed VL compared to those with better adherence [22]. Similar results were found in another study, where a low level of ART adherence was associated with a 2.5 times higher likelihood of having unsuppressed VL than that for good adherence [21].
In our study, patients with a history of ART interruption had 3.4 times higher odds of having unsuppressed VL than those who did not have an interruption in their treatment. Similar results were observed in previous studies. In a study conducted in Guatemala, treatment interruptions of ≥7 days were associated with an increased odds of unsuppressed VL [15]. Additionally, a case–control study conducted in Ethiopia showed that patients with a history of treatment interruption were 2.4 times more likely to have unsuppressed VL [22]. During ART interruption, plasma viral rebound occurs relatively quickly (2 to 4 weeks) in most patients [28].
HIV-positive patients on ART not participating in a support group in this study had twice the odds of having unsuppressed VL compared to those who did belong to support groups. According to a previous study conducted in Uganda, it was found that unsuppressed VL was significantly associated with not belonging to a support group [16]. It might also be linked to the lack of psychosocial support, inability to receive help from peers, and poor dissemination of the existence of support groups by providers, especially for the benefit of adolescents and young children [18,20]. Previous studies have shown that belonging to a support group with other peers allows patients to feel hope through shared experiences and testimonies [29].
HIV-positive patients on ART who were at WHO clinical stage 3 or 4 at the beginning of ART had 2.2 times the odds of having unsuppressed VL compared to those at WHO clinical stage 1 or 2. Similarly, a study conducted in Ethiopia found that patients at WHO clinical stages 3 and 4 at the time of ART initiation were almost twice as likely to fail first-line treatment because the advanced WHO clinical stages are often associated with high VL [11]. In contrast, another study conducted in Senegal, which aimed to identify the association between WHO clinical stage and virological failure, defined as a VL > 1000 copies after adherence counseling, found that virological failure was not associated with WHO clinical stages at inclusion (p = 0.29) [30].
Our study showed that the HIV-positive patients on ART with a history of OIs had 2.4 times higher odds of unsuppressed VL compared to those without such history. Similarly, a study conducted in South Africa found that being diagnosed with tuberculosis during ART (p < 0.0001) was associated with unsuppressed VL [31]. Likewise, a multi-country study (Uganda, Malawi, Zimbabwe, and South Africa) found that recent hospital admission for OIs was associated with 2.5 times and almost 2 times higher odds of detectable viremia, respectively [19]. Additionally, Temesgen Getaneh et al., (2022), [32] conducted a systematic review and meta-analysis in Ethiopia on 15 primary studies reporting the impact of OIs (tuberculosis) on unsuppressed virology in adults living with HIV. They found that the risk of unsuppressed VL was significantly higher in adults living with HIV-TB co-infection compared to that in adults living with HIV alone [32]. Certain OIs such as tuberculosis, cytomegalovirus infections, and other gastrointestinal infections can trigger a systemic inflammatory response and chronic immune activation in patients living with HIV. This inflammation can disrupt the immune response and promote viral replication, thus compromising VL [33,34,35,36]. Additionally, some medications used to treat OIs can interact with ART, affecting drug plasma concentration and efficacy. These drug interactions can compromise the effectiveness of ART and lead to unsuppressed VL. For example, rifampicin, used in tuberculosis treatment, can induce the metabolism of some antiretrovirals, thereby reducing their effectiveness [37,38,39].
In the present study, HIV-positive patients on ART with a history of non-communicable chronic diseases (NCDs) had 2.4 times the odds of unsuppressed VL compared to those without such history. Similarly, in a study conducted in Morocco, unsuppressed VL was significantly associated with the presence of diabetes [40]. In systematic reviews of earlier studies conducted before 2017 by J. Nansseu et al., (2018) [41,42], there were significant relationships between ART use and certain NCDs such as diabetes or pre-diabetes. However, a meta-analysis on several heterogeneous studies published between 2008 and 2016, which presented a moderate risk of bias, did not reveal any significant association between NCDs such as diabetes and HIV or antiretroviral therapy [42,43]. Medications used to treat NCDs can interact with antiretrovirals, affecting their efficacy. Drug interactions such as the inhibition of specific enzymes can compromise VL suppression in patients living with HIV [44]. Antihypertensive drugs (calcium channel blockers, beta-blockers, angiotensin II receptor antagonists…) and antidiabetic drugs (thiazolidinediones, sulfonylureas, biguanides…) in the presence of protease inhibitors and/or nucleos(t)idic transcriptase inhibitors may have adverse effects on the tolerability of ART [45,46,47], leading to reduced ARV efficacy, which can result in unsuppressed VL [48]. NCDs are often associated with chronic systemic inflammation, which can increase immune activation and promote viral replication in patients living with HIV, thus compromising VL suppression [49]. Moreover, NCDs can lead to immune dysfunction in patients living with HIV, which can compromise the body’s ability to control viral replication despite ART [50]. Beyond these points, unsuppressed VL in HIV-positive patients on ART with a history of NCDs could be linked to the risk factors for these diseases. Factors such as harmful alcohol use, recreational drug use, age, weight, and high blood sugar levels can negatively influence ART adherence and observance, thereby reducing ART efficacy and success.
Limitations and Strengths of the Study
There are limitations to this study. To minimize information bias (memory) due to a lack of recall of certain exposures and the underestimation or overestimation of factors, we combined the file review with a structured face-to-face interview. We also limited the number of years prior to the study to one and a half.
In selecting cases and controls without matching, we did not account for certain confounding factors that could influence our results. To minimize the effect of confounding, we conducted multivariable logistic regression, which allows each variable to be interpreted independently, keeping other variables constant. To reduce selection bias, we increased the sample size by taking an exhaustive sample of cases while randomly selecting the controls.
In addition to interviews, the study was based on a review of patient records or secondary data, which could affect the reliability of the data. To minimize this information bias, we coupled the record review with structured individual interviews.
The choice of variables allowed us to examine multiple risk factors simultaneously. While the influence of confounding factors on the association between certain potential risk factors and VLNS was possible, it was minimized by multivariable analysis. Using exhaustive sampling, the prevalent and incident cases are likely to be included in the analysis. Thus, taking prevalent cases has the drawback that factors associated with survival after developing unsuppressed VL may appear to be protective. Due to our case–control design, we could not determine the causality of the identified factors.
Considering the limited availability of data, we decided to calculate the sample size based on the “ART interruption” factor, which indicated 282 participants. By selecting this estimator and maximizing the utilization of available data, we included 462 participants, exceeding the minimum requirement of 282. This choice might mitigate the potential limitation concerning the statistical power of the study.
5. Conclusions
Unsuppressed VL remains a major challenge in the Tanganyika province despite the free availability of antiretroviral treatment. This study identified groups of patients likely to have an unsuppressed VL. Given the clear association between several factors and unsuppressed VL, including the non-disclosure of HIV status to a confidant, poor adherence, a history of ART interruption, non-membership in a support group, clinical stages 3 and 4 at the start of ART, a history of OIs, and the presence of associated NCDs, a response should be considered.
Prevention should rely on the implementation of innovative strategies that consider patient groups likely to be affected by unsuppressed VL. To achieve this, it is desirable to enhance the monitoring of these factors among active patients at each appointment.
Author Contributions
Conceptualization, M.L. and J.D.; methodology, M.L., R.I. and J.D.; software, M.L.; validation, J.D., M.L. and R.I.; formal analysis, M.L., R.I. and J.D.; investigation, M.L.; resources, A.L.; data curation, M.L.; writing—original draft preparation, M.L., J.D., R.I., A.L. and D.N.; writing—review and editing, M.L., J.D. and R.I.; visualization, M.L.; supervision, J.D.; project administration, M.L.; funding acquisition, A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Kinshasa School of Public Health through a grant from the EDCTP2 capacity development for disease outbreak and epidemic response in sub-Saharan Africa (GRANT ID: CSA2020E-3123).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the School of Public Health of the University of Kinshasa, Democratic Republic of Congo (ESP/CE/122/2023, 5 September 2023), for studies involving human subjects.
Informed Consent Statement
All participants provided their informed consent prior to participation by completing an informed consent form outlining the aims and benefits of the study.
Data Availability Statement
Study data are currently unavailable due to restrictions imposed by the National Program for the Fight against AIDS (PNLS). Some study data may be made available upon reasonable request after permission to share has been obtained from the relevant PNLS authorities.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- WHO. Information Strategique VIH Pour Impact; WHO: Geneva, Switzerland, 2021; Volume 27, ISBN 978-92-4-003446-4. [Google Scholar]
- Aubry, P.P.; Gaüzère, D.B.-A. Infection Par Le VIH et Tropiques. Aide Soignante 2022, 32, 20–23. [Google Scholar] [CrossRef]
- WHO. Essentiels Sur Le VIH/SIDA. Available online: https://www.who.int/fr/news-room (accessed on 7 July 2024).
- PNMLS & PNLS. Baromètre Analytique de La Lutte Contre Le VIH/SIDA En République Démocratique Du Congo. Available online: https://www.pnmls.cd/ (accessed on 7 July 2024).
- Mundamshimu, J.S.; Malale, K.; Benson, K.R.; Gunda, D.W.; Bwemelo, L.; Mwashiuya, M.; Omar, S.S.; Mlowe, N.; Kiyumbi, M.; Ngocho, J.S.; et al. Failure to Attain HIV Viral Suppression After Intensified Adherence Counselling—What Can We Learn About Its Factors? Infect. Drug Resist. 2023, 16, 1885–1894. [Google Scholar] [CrossRef]
- WHO. Lignes Directices Unifiées Sur Les Services de Dépistage Du VIH; WHO: Geneva, Switzerland, 2019; pp. 1–284. ISBN 978-92-4-001633-0. [Google Scholar]
- ONUSIDA. Rapport Mondial Actualisé Sur Le Sida 2022. Available online: https://www.unaids.org/fr (accessed on 7 July 2024).
- ONUSIDA. La Réponse Au VIH En Afrique Occidentale et Centrale. Available online: https://www.unaids.org/fr (accessed on 7 July 2024).
- PNLS. Revue Annuelle 2021 Sur Le VIH/SIDA Du 30 Mars Au 03 Avril 2022. 2022; unpublished.
- BPC-PNLS. Rapport Annuel 2022 PNLS Tanganyika. 2023; unpublished.
- Tesema, S.T.; Reshad, M.; Abebe, E. Determinants of First-Line Antiretroviral Treatment Failure among Adult Patients on Treatment in Mettu Karl Specialized Hospital, South West Ethiopia; a Case Control Study. PLoS ONE 2021, 16, 1–13. [Google Scholar] [CrossRef]
- WHO. Des Tests de Diagnostic Moléculaire de l’Infection à VIH Destinés à Améliorer l’accès à La Mesure de La Charge Virale et Au Diagnostic Du VIH Chez Le Nourrisson; WHO: Geneva, Switzerland, 2019; pp. 1–48. ISBN 978-92-4-000411-5. [Google Scholar]
- ONUSIDA. Suivi Mondial de La Lutte Contre Le SIDA 2023. Available online: https://www.unaids.org/fr (accessed on 7 July 2024).
- Ortíz, D.W.; Roberts-Sano, O.; Marroquin, H.E.; Larson, L.; Franco, K.B.; Spec, A.; Melendez, J.R.; Pinzón, R.; Samayoa, A.J.; Mejia-Chew, C.; et al. Factors Associated with Viremia in People Living with HIV on Antiretroviral Therapy in Guatemala. AIDS Res. Ther. 2021, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Afrane, A.K.A.; Goka, B.Q.; Renner, L.; Yawson, A.E.; Owiafe, S.N.; Agyeman, S.; Sagoe, K.W.C. HIV Virological Non-Suppression and Its Associated Factors in Children on Antiretroviral Therapy at a Major Treatment Centre in Southern Ghana: A Cross-Sectional Study. BMC Infect. Dis. 2021, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.P.; Talbert, M.; Herbert, E.; Mugisha, M.K.; Herbert, A.E. Factors Associated with HIV Viral Suppression among Adolescents in Kabale District, South Western Uganda. PLoS ONE 2022, 17, 1–20. [Google Scholar] [CrossRef]
- Maena, J.; Thomas, A.B.; Mukiza, N.; Kuteesa, C.N.; Kakumba, R.M.; Kataike, H.; Kizito, S.; Babirye, J.A. Rita Nakalega Determinants of Viral Load Non-Suppression among Adolescents in Mbale District, Eastern Rural Uganda. BMC Rech. Thérapie Sur Le Sida 2021, 18, 1–9. [Google Scholar] [CrossRef]
- Meshesha, H.M.; Nigussie, Z.M.; Asrat, A.; Mulatu, K. Determinants of Virological Failure among Adults on First-Line Highly Active Antiretroviral Therapy at Public Health Facilities in Kombolcha Town, Northeast, Ethiopia: A Case–Control Study. BMJ Open 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Atuhaire, P.; Hanley, S.; Yende-Zuma, N.; Aizire, J.; Stranix-Chibanda, L.; Makanani, B.; Milala, B.; Haseena, C.; Taha, T.; Fowler, M.G. Factors Associated with Unsuppressed Viremia in Women Living with HIV on Lifelong ART in the Multi-Country US-PEPFAR PROMOTE Study: A Cross-Sectional Analysis. PLoS ONE 2019, 14, 1–14. [Google Scholar] [CrossRef]
- Mhlanga, T.T.; Jacobs, B.K.; Decroo, T.; Govere, E.; Bara, H.; Chonzi, P.; Sithole, N.; Apollo, T.; Damme, W.V.; Simbarashe, R.; et al. Virological Outcomes and Risk Factors for Non-Suppression for Routine and Repeat Viral Load Testing after Enhanced Adherence Counselling during Viral Load Testing Scale-up in Zimbabwe: Analytic Cross-Sectional Study Using Laboratory Data from 2014 to 2018. Chem. Ind. For. Prod. 2022, 19, 1–13. [Google Scholar] [CrossRef]
- Desta, A.A.; Woldearegay, T.W.; Futwi, N.; Gebrehiwot, G.T.; Gebru, G.G.; Berhe, A.A.; Godefay, H. HIV Virological Non-Suppression and Factors Associated with Non-Suppression among Adolescents and Adults on Antiretroviral Therapy in Northern Ethiopia: A Retrospective Study. BMC Infect. Dis. 2020, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jaleta, F.; Bekele, B.; Kedir, S.; Hassan, D.; Getahun, A.; Tadesse, L.; Garoma, G.; Itefa, K.; Gerenfe, T.; Botoré, A.; et al. Predictors of Unsuppressed Viral Load among Adults on Follow up of Antiretroviral Therapy at Selected Public and Private Health Facilities of Adama Town: Unmached Case-Control Study. BMC Publique Health 2022, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- PNLS. Direction Nationale Plan Strategique Sectoriel Santé de Lutte Contre Le VIH/SIDA 2018–2021. 2018; unpublished.
- Morisky, D.E.; Ang, A.; Krousel-Wood, M.; Ward, H.J. Predictive Validity of a Medication Adherence Measure in an Outpatient Setting. J. Clin. Hypertens. 2008, 10, 348–354. [Google Scholar] [CrossRef]
- Morisky, D.E.; Green, L.W.; Levine, D.M. Concurrent and Predictive Validity of a Self-Reported Measure of Medication Adherence. Med. Care 1986, 24, 67–74. [Google Scholar] [CrossRef] [PubMed]
- WHO. Lignes Directrices Unifiées Sur Les Informations Stratégiques; WHO: Geneva, Switzerland, 2015; pp. 1–310. ISBN 978-92-4-250875-8. [Google Scholar]
- Yotebieng, M.; Mpody, C.; Ravelomanana, N.L.; Tabala, M.; Malongo, F.; Kawende, B.; Ntangu, P.; Behets, F.; Okitolonda, E. HIV Viral Suppression among Pregnant and Breastfeeding Women in Routine Care in the Kinshasa Province: A Baseline Evaluation of Participants in CQI-PMTCT Study. J. Int. AIDS Soc. 2019, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mdluli, T.; Li, Y.; Pinyakorn, S.; Francisco, L.V.; Vasan, S.; Rolland, M. Acute HIV-1 Infection Viremia Associate with Rebound upon Treatment Interruption. Med 2022, 3, 622–635.e3. [Google Scholar] [CrossRef] [PubMed]
- Arnold, E.A.; Weeks, J.; Benjamin, M.; Stewart, W.R.; Pollack, L.M.; Kegeles, S.M.; Operario, D. Identifying Social and Economic Barriers to Regular Care and Treatment for Black Men Who Have Sex with Men and Women (BMSMW) and Who Are Living with HIV: A Qualitative Study from the Bruthas Cohort. BMC Health Serv. Res. 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Cisse, V.; Niang, I.; Diallo, K.; Senghor, G.; Diop, S.; Manga, N. Facteurs Associés à l’échec Virologique Chez Les Patients Infectés Par Le VIH Suivis Dans Le District Sanitaire de Oussouye, Région de Ziguinchor Au Sénégal. Médecine Mal. Infect. 2019, 49, S146. [Google Scholar] [CrossRef]
- Soogun, A.O.; Kharsany, A.B.; Zewotir, T.; North, D.; Ogunsakin, R.E. Identifying Potential Factors Associated with High HIV Viral Load in KwaZulu-Natal, South Africa Using Multiple Correspondence Analysis and Random Forest Analysis. BMC Med. Res. Methodol. 2022, 22, 1–16. [Google Scholar] [CrossRef]
- Getaneh, T.; Negesse, A.; Dessie, G.; Desta, M. The Impact of Tuberculosis Co-Infection on Virological Failure among Adults Living with HIV in Ethiopia: A Systematic Review and Meta-Analysis. J. Clin. Tuberc. Other Mycobact. Dis. 2022, 27, 100310. [Google Scholar] [CrossRef]
- Brenchley, J.M.; Price, D.A.; Schacker, T.W.; Asher, T.E.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D.; et al. Microbial Translocation Is a Cause of Systemic Immune Activation in Chronic HIV Infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Lackner, A.A.; Mohan, M.; Veazey, R.S. The Gastrointestinal Tract and AIDS Pathogenesis. Natl. Inst. Health 2009, 136, 1966–1978. [Google Scholar] [CrossRef] [PubMed]
- Sereti, I.; Altfeld, M. Immune Activation and HIV: An Enduring Relationship. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Appay, V.; Sauce, D. Immune Activation and Inflammation in HIV-1 Infection: Causes and Consequences. J. Pathol. 2008, 214, 231–241. [Google Scholar] [CrossRef]
- Acosta, E.P.; Kendall, M.A.; Gerber, J.G.; Alston-Smith, B.; Koletar, S.L.; Zolopa, A.R.; Agarwala, S.; Child, M.; Bertz, R.; Hosey, L.; et al. Effect of Concomitantly Administered Rifampin on the Pharmacokinetics and Safety of Atazanavir Administered Twice Daily. Antimicrob. Agents Chemother. 2007, 51, 3104–3110. [Google Scholar] [CrossRef]
- Ramautarsing, R.; Ananworanich, J. Generic and Low Dose Antiretroviral Therapy in Adults and Children: Implication for Scaling up Treatment in Resource Limited Settings. AIDS Res. Ther. 2010, 7, 1–8. [Google Scholar] [CrossRef][Green Version]
- Jens, D.; Lundgren; Gatell, J.M.; Furrer, H.; Rockstroh, J. EACS Guidelines Version 7.1. Eur. AIDS Clin. Soc. 2014, 7, 1–87. [Google Scholar]
- Hicham, T.; Ilyas, E.; Tarik, H.; Noureddine, B.; Omar, B.; Rachid, F.; Naoufal, H.; Mohammed, B. Risk Factors Associated with Unsuppressed Viral Load in HIV-1 Infected Patients at the First Antiretroviral Therapy in Morocco. Int. J. Mycobacteriolog 2019, 8, 113–117. [Google Scholar] [CrossRef]
- Nansseu, J.R.; Bigna, J.J.; Kaze, A.D.; Noubiap, J.J. Incidence and Risk Factors for Prediabetes and Diabetes Mellitus among HIV-Infected Adults on Antiretroviral Therapy. Epidemiology 2018, 29, 431–441. [Google Scholar] [CrossRef]
- Peer, N.; Nguyen, K.A.; Hill, J.; Sumner, A.E.; Cikomola, J.C.; Nachega, J.B.; Kengne, A.P. Prevalence and Influences of Diabetes and Prediabetes among Adults Living with HIV in Africa: A Systematic Review and Meta-Analysis. J. Int. AIDS Soc. 2023, 26, 1–27. [Google Scholar] [CrossRef]
- Prioreschi, A.; Munthali, R.J.; Soepnel, L.; Goldstein, J.A.; Micklesfield, L.K.; Aronoff, D.M.; Norris, S.A. Incidence and Prevalence of Type 2 Diabetes Mellitus with HIV Infection in Africa: A Systematic Review and Meta-Analysis. BMJ Open 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Izzédine, H.; Launay-Vacher, V.; Aymard, G.; Legrand, M.; Déray, G. Pharmacokinetics of Abacavir in HIV-1-Infected Patients with Impaired Renal Function. Karger 2001, 89, 62–67. [Google Scholar] [CrossRef] [PubMed]
- DiPiro, J.T.; Talbert, R.L.; Yee, G.C.; Matzke, G.R.; Wells, B.G.; Posey, L.M. Pharmacotherapy: A Pathophysiologic Approach, 9th ed.; Librería Médica Berri: Bilbao, Spain, 2014; Volume 2848, ISBN 9780071800532. [Google Scholar]
- Brunton, L.L.; Hilal-Dandan, R.; Knollmann, B.C. Chapter 7: Pharmacogenetics. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 13th ed.; McGraw-Hill Education: New York, NY, USA, 2018; ISBN 978-1-25-958473-2. [Google Scholar]
- Katzung, B.G.; Trevor, A.J. Basic & Clinical Pharmacology, 13th ed.; McGraw-Hill: New York, NY, USA, 2015; pp. 1–19. ISBN 978-0-07-182641-9. [Google Scholar]
- Kapitsinou, P.P.; Ansari, N. Acute Renal Failure in an AIDS Patient on Tenofovir: A Case Report. J. Med. Case Rep. 2008, 2, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Kuller, L.H.; Tracy, R.; Belloso, W.; De Wit, S.; Drummond, F.; Lane, H.C.; Ledergerber, B.; Lundgren, J.; Neuhaus, J.; Nixon, D.; et al. Inflammatory and Coagulation Biomarkers and Mortality in Patients with HIV Infection. PLoS Med. 2008, 5, 1496–1508. [Google Scholar] [CrossRef]
- Klatt, N.R.; Funderburg, N.T.; Brenchley, J.M. Microbial Translocation, Immune Activation, and HIV Disease. Trends Microbiol. 2013, 21, 6–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).