The Current Role of Collagen Patch Augmentation in Rotator Cuff Pathology: A Narrative Review
Abstract
1. Introduction
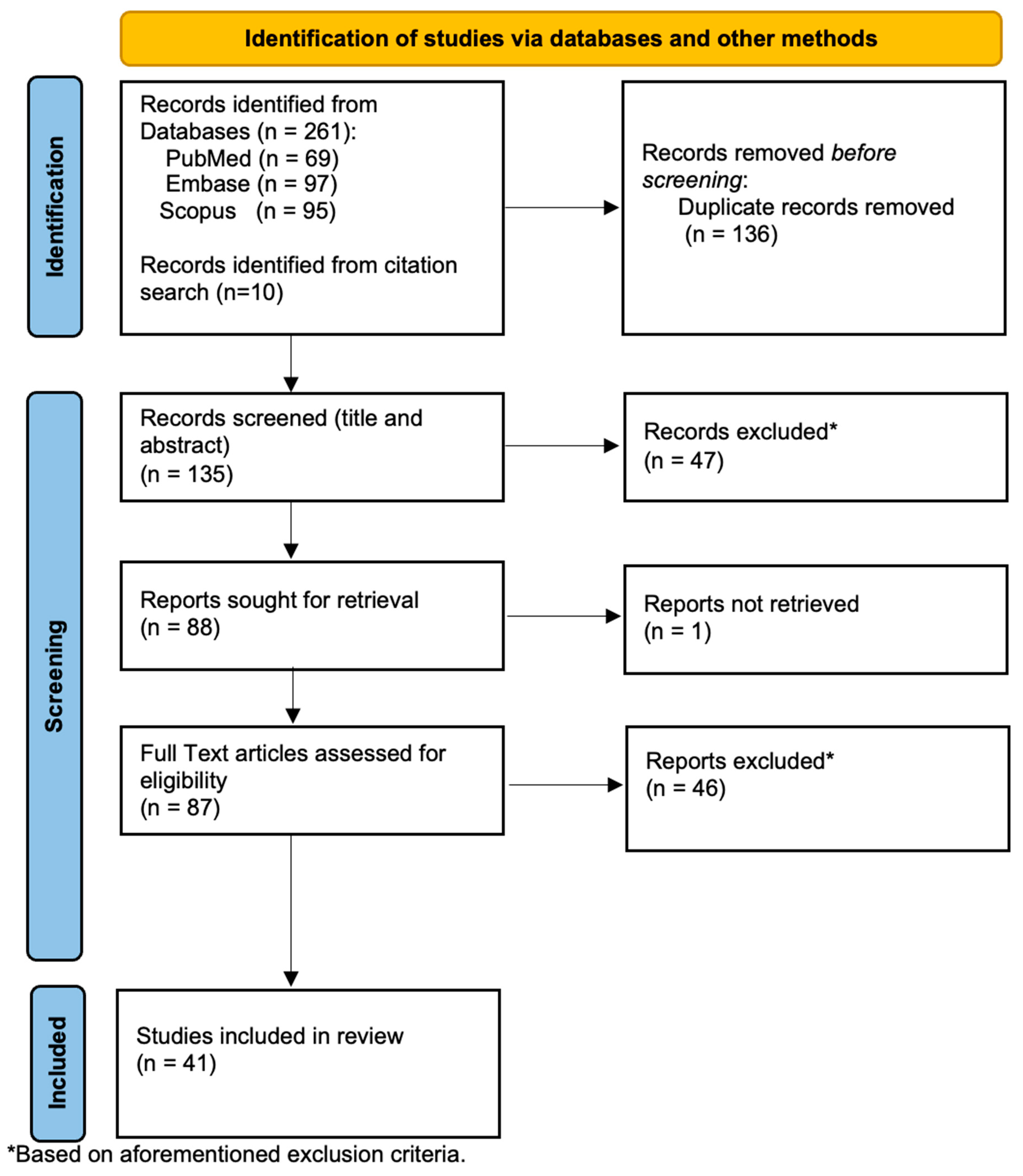
2. Methods
2.1. Eligibility Criteria
2.2. Identification and Selection of Studies
3. Discussion
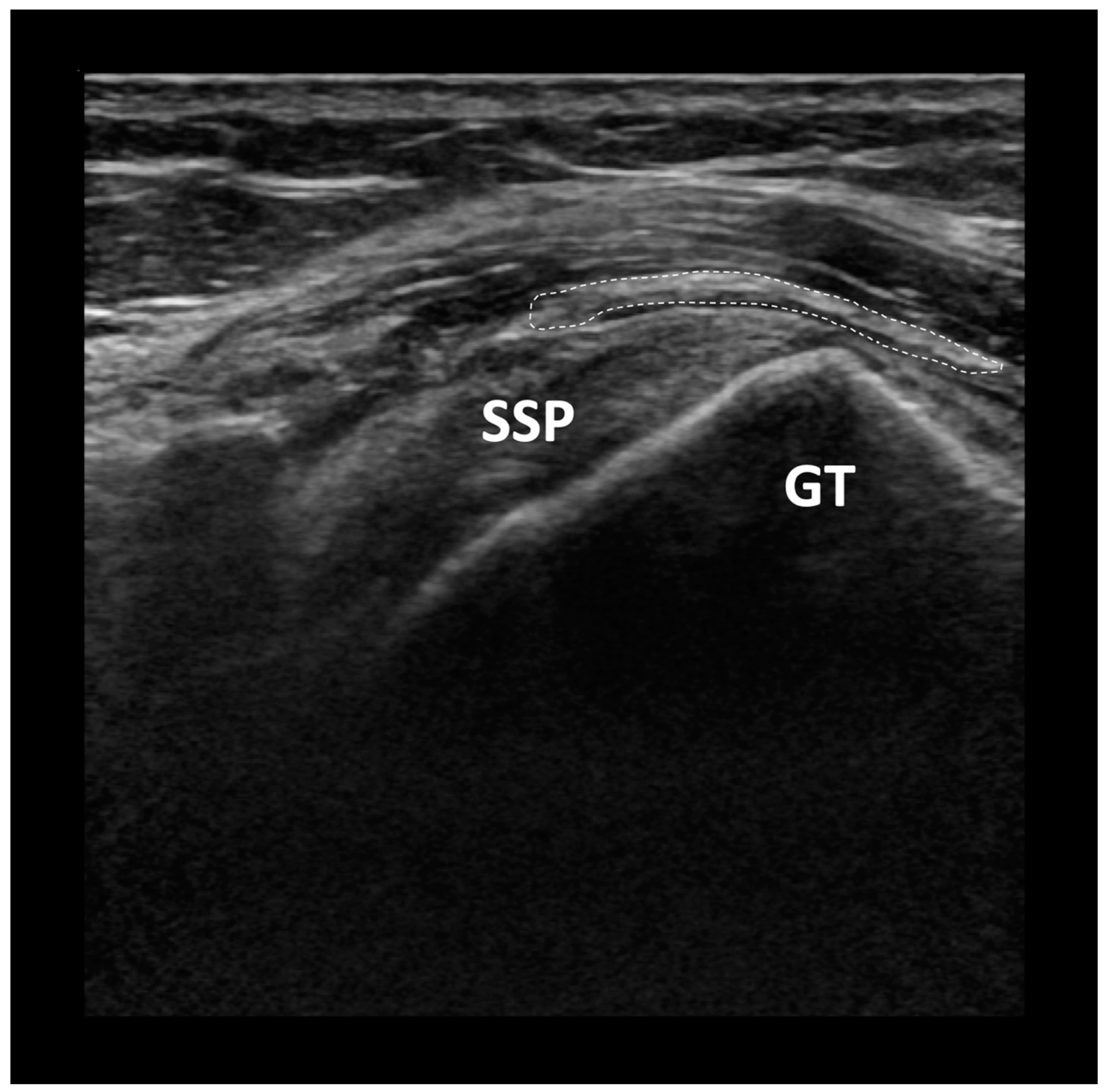
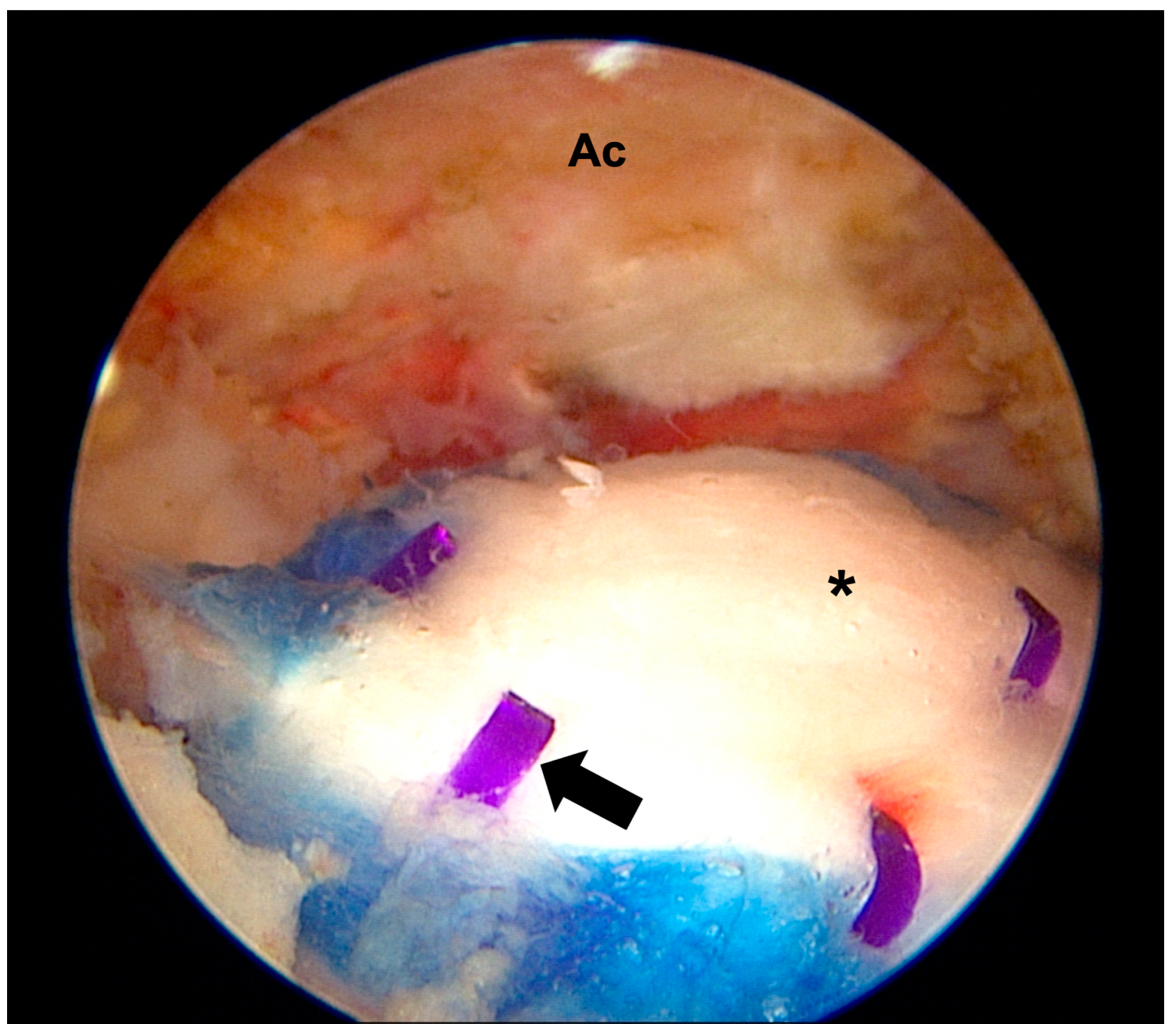
3.1. Properties
3.2. Safety and Complications
3.3. Stiffness
3.4. Re-Tear Rates
3.5. Clinical Outcomes
3.6. Economic Impact
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hinsley, H.; Ganderton, C.; Arden, N.K.; Carr, A.J. Prevalence of rotator cuff tendon tears and symptoms in a Chingford general population cohort, and the resultant impact on UK health services: A cross-sectional observational study. BMJ Open 2022, 12, e059175. [Google Scholar] [CrossRef] [PubMed]
- Reilly, P.; Macleod, I.; Macfarlane, R.; Windley, J.; Emery, R. Dead men and radiologists don’t lie: A review of cadaveric and radiological studies of rotator cuff tear prevalence. Ann. R. Coll. Surg. Engl. 2006, 88, 116–121. [Google Scholar] [CrossRef]
- Minagawa, H.; Yamamoto, N.; Abe, H.; Fukuda, M.; Seki, N.; Kikuchi, K.; Kijima, H.; Itoi, E. Prevalence of symptomatic and asymptomatic rotator cuff tears in the general population: From mass-screening in one village. J. Orthop. 2013, 10, 8–12. [Google Scholar] [CrossRef]
- Khoschnau, S.; Milosavjevic, J.; Sahlstedt, B.; Rylance, R.; Rahme, H.; Kadum, B. High prevalence of rotator cuff tears in a population who never sought for shoulder problems: A clinical, ultrasonographic and radiographic screening study. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Teunis, T.; Lubbers, B.; Reilly, B.T.; Ring, D. A systematic review and pooled analysis of the prevalence of rotator cuff disease with increasing age. J. Shoulder Elb. Surg. 2014, 23, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- MacDermid, J.C.; Ramos, J.; Drosdowech, D.; Faber, K.; Patterson, S. The impact of rotator cuff pathology on isometric and isokinetic strength, function, and quality of life. J. Shoulder Elb. Surg. 2004, 13, 593–598. [Google Scholar] [CrossRef]
- Parikh, N.; Martinez, D.J.; Winer, I.; Costa, L.; Dua, D.; Trueman, P. Direct and indirect economic burden associated with rotator cuff tears and repairs in the US. Curr. Med. Res. Opin. 2021, 37, 1199–1211. [Google Scholar] [CrossRef]
- Rajalekshmi, R.; Agrawal, D.K. Understanding Fibrous Tissue in the Effective Healing of Rotator Cuff Injury. J. Surg. Res. 2024, 7, 215–228. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Z.; Song, W.; Cai, Z.; Ding, Z.; Chen, D.; Xia, F.; He, Y. Inhibition of IKKβ/NF-κB signaling facilitates tendinopathy healing by rejuvenating inflamm-aging induced tendon-derived stem/progenitor cell senescence. Mol. Ther. Nucleic Acids 2022, 27, 562–576. [Google Scholar] [CrossRef]
- Chaudhury, S.; Holland, C.; Thompson, M.S.; Vollrath, F.; Carr, A.J. Tensile and shear mechanical properties of rotator cuff repair patches. J. Shoulder Elb. Surg. 2012, 21, 1168–1176. [Google Scholar] [CrossRef]
- Zumstein, M.-A.; Lädermann, A.; Raniga, S.; Schär, M.-O. The biology of rotator cuff healing. Orthop. Traumatol. Surg. Res. 2017, 103 (Suppl. S1), S1–S10. [Google Scholar] [CrossRef]
- Ruiz Ibán, M.Á.; García, N.M.; Moros, M.S.; Diaz, H.J.; Hernando, S.A.; Ruiz, D.R.; Vaquero, C.C.; Rosas, O.M.L.; Del Monte, B.G.; Ávila, L.J.L. Augmentation of a Transosseous-Equivalent Repair in Posterosuperior Nonacute Rotator Cuff Tears with a Bioinductive Collagen Implant Decreases the Retear Rate at One Year: A Randomized Controlled Trial. Arthrosc. J. Arthrosc. Relat. Surg. 2023, 40, 1760–1773. [Google Scholar] [CrossRef] [PubMed]
- Rohman, L.; Snow, M. Use of biologics in rotator cuff disorders: Current concept review. J. Clin. Orthop. Trauma 2021, 19, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.; Davies, M. Rotator Cuff Disease: Treatment Options and Considerations. Sports Med. Arthrosc. Rev. 2018, 26, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Reilly, P.; Amis, A.A.; Wallace, A.L.; Emery, R.J. Supraspinatus tears: Propagation and strain alteration. J. Shoulder Elb. Surg. 2003, 12, 134–138. [Google Scholar] [CrossRef]
- Longo, U.G.; Carnevale, A.; Piergentili, I.; Berton, A.; Candela, V.; Schena, E.; Denaro, V. Retear rates after rotator cuff surgery: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2021, 22, 749. [Google Scholar] [CrossRef]
- Zhao, J.; Luo, M.; Pan, J.; Liang, G.; Feng, W.; Zeng, L.; Yang, W.; Liu, J. Risk factors affecting rotator cuff retear after arthroscopic repair: A meta-analysis and systematic review. J. Shoulder Elb. Surg. 2021, 30, 2660–2670. [Google Scholar] [CrossRef]
- Routledge, J.C.; Saber, A.Y.; Pennington, N.; Gupta, N. Re-Tear Rates Following Rotator Cuff Repair Surgery. Cureus 2023, 15, e34426. [Google Scholar] [CrossRef]
- Barber, F.A. PRP as an Adjunct to Rotator Cuff Tendon Repair. Sports Med. Arthrosc. Rev. 2018, 26, 42–47. [Google Scholar] [CrossRef]
- Ajrawat, P.; Dwyer, T.; Almasri, M.; Veillette, C.; Romeo, A.; Leroux, T.; Theodoropoulos, J.; Nauth, A.; Henry, P.; Chahal, J. Bone marrow stimulation decreases retear rates after primary arthroscopic rotator cuff repair: A systematic review and meta-analysis. J. Shoulder Elb. Surg. 2019, 28, 782–791. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y. Efficacy of bone marrow stimulation in arthroscopic repair of full thickness rotator cuff tears: A meta-analysis. J. Orthop. Surg. Res. 2019, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Van Kampen, C.; Arnoczky, S.; Parks, P.; Hackett, E.; Ruehlman, D.; Turner, A.; Schlegel, T. Tissue-engineered augmentation of a rotator cuff tendon using a reconstituted collagen scaffold: A histological evaluation in sheep. Muscle Ligaments Tendons J. 2019, 3, 229–235. [Google Scholar] [CrossRef]
- Turner, A.S. Experiences with sheep as an animal model for shoulder surgery: Strengths and shortcomings. J. Shoulder Elb. Surg. 2007, 16, S158–S163. [Google Scholar] [CrossRef] [PubMed]
- Arnoczky, S.P.; Bishai, S.K.; Schofield, B.; Sigman, S.; Bushnell, B.D.; Hommen, J.P.; Van Kampen, C. Histologic Evaluation of Biopsy Specimens Obtained After Rotator Cuff Repair Augmented with a Highly Porous Collagen Implant. Arthrosc. J. Arthrosc. Relat. Surg. 2017, 33, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Chacon, J.A.; Cuenca-Espierrez, J.; Roda-Rojo, V.; Martin-Martinez, A.; Calderon-Meza, J.M.; Alvarez-Alegret, R.; Martin-Hernandez, C. Bioinductive collagen implants facilitate tendon regeneration in rotator cuff tears. J. Exp. Orthop. 2022, 9, 53. [Google Scholar] [CrossRef]
- Bokor, D.J.; Sonnabend, D.; Deady, L.; Cass, B.; Young, A.; Van Kampen, C.; Arnoczky, S. Evidence of healing of partial-thickness rotator cuff tears following arthroscopic augmentation with a collagen implant: A 2-year MRI follow-up. Muscles Ligaments Tendons J. 2016, 6, 16–25. [Google Scholar] [CrossRef]
- Schlegel, T.F.; Abrams, J.S.; Bushnell, B.D.; Brock, J.L.; Ho, C.P. Radiologic and clinical evaluation of a bioabsorbable collagen implant to treat partial-thickness tears: A prospective multicenter study. J. Shoulder Elb. Surg. 2018, 27, 242–251. [Google Scholar] [CrossRef]
- Sano, H.; Wakabayashi, I.; Itoi, E. Stress distribution in the supraspinatus tendon with partial-thickness tears: An analysis using two-dimensional finite element model. J. Shoulder Elb. Surg. 2006, 15, 100–105. [Google Scholar] [CrossRef]
- Bey, M.J.; Ramsey, M.L.; Soslowsky, L.J. Intratendinous strain fields of the supraspinatus tendon: Effect of a surgically created articular-surface rotator cuff tear. J. Shoulder Elb. Surg. 2002, 11, 562–569. [Google Scholar] [CrossRef]
- Chen, Q. Two-dimensional finite element proof-of-concept modeling on rotator cuff tear scaffold efficacy. In Technical Report from the Material and Structural Testing Core; Mayo Clinic: Rochester, MN, USA, 2011. [Google Scholar]
- Thon, S.G.; O’malley, L.; O’brien, M.J.; Savoie, F.H. Evaluation of Healing Rates and Safety with a Bioinductive Collagen Patch for Large and Massive Rotator Cuff Tears: 2-Year Safety and Clinical Outcomes. Am. J. Sports Med. 2019, 47, 1901–1908. [Google Scholar] [CrossRef]
- Barad, S.J. Severe subacromial-subdeltoid inflammation with rice bodies associated with implantation of a bio-inductive collagen scaffold after rotator cuff repair. J. Shoulder Elb. Surg. 2019, 28, e190–e192. [Google Scholar] [CrossRef] [PubMed]
- Root, K.T.; Wright, J.O.; Mandato, N.; Stewart, B.D.; Moser, M.W. Subacromial-Subdeltoid Bursitis with Rice Bodies After Rotator Cuff Repair with a Collagen Scaffold Implant: A Case Report. JBJS Case Connect. 2023, 13, e22.00565. [Google Scholar] [CrossRef]
- Ciccone, W.J.; Motz, C.; Bentley, C.; Tasto, J.P. Bioabsorbable implants in orthopaedics: New developments and clinical applications. J. Am. Acad. Orthop. Surg. 2001, 9, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.N.; Amin, N.H. Letter to the Editor regarding Barad: “Severe subacromial-subdeltoid inflammation with rice bodies associated with implantation of a bio-inductive collagen scaffold after rotator cuff repair”. J. Shoulder Elb. Surg. 2020, 29, e93–e94. [Google Scholar] [CrossRef]
- Bushnell, B.D.; Bishai, S.K.; Krupp, R.J.; McMillan, S.; Schofield, B.A.; Trenhaile, S.W.; McIntyre, L.F. Treatment of Partial-Thickness Rotator Cuff Tears with a Resorbable Bioinductive Bovine Collagen Implant: 1-Year Results from a Prospective Multicenter Registry. Orthop. J. Sports Med. 2021, 9, 23259671211027850. [Google Scholar] [CrossRef] [PubMed]
- Ting, R.S.; Loh, Y.C.; Rosenthal, R.; Zhong, K.; Al-Housni, H.S.; Shenouda, M.; Hackett, L.; Lam, P.H.; Murrell, G.A. Revision Rotator Cuff Repair with versus without an Arthroscopically Inserted Onlay Bioinductive Implant in Workers’ Compensation Patients. Orthop. J. Sports Med. 2023, 11, 23259671231175883. [Google Scholar] [CrossRef] [PubMed]
- Yeazell, S.; Lutz, A.; Bohon, H.; Shanley, E.; Thigpen, C.A.; Kissenberth, M.J.; Pill, S.G. Increased stiffness and reoperation rate in partial rotator cuff repairs treated with a bovine patch: A propensity-matched trial. J. Shoulder Elb. Surg. 2022, 31, S131–S135. [Google Scholar] [CrossRef]
- Bushnell, B.D.; Angelo, R.L.; Bishai, S.K.; Bravman, J.T.; Connor, P.M.; Getelman, M.H.; Harris, H.W.; McIntyre, L.F.; McMillan, S.; Trenhaile, S.W. Letter to the Editor regarding Yeazell et al: "Increased stiffness and reoperation rate in partial rotator cuff repairs treated with a bovine patch: A propensity-matched trial". J. Shoulder Elb. Surg. 2022, 31, E569–E571. [Google Scholar] [CrossRef]
- Castagna, A.; Borroni, M.; Garofalo, R.; Rose, G.D.; Cesari, E.; Padua, R.; Conti, M.; Gumina, S. Deep partial rotator cuff tear: Transtend on repair or tear completion and repair? A randomized clinical trial. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 460–463. [Google Scholar] [CrossRef]
- Franceschi, F.; Papalia, R.; Del Buono, A.; Vasta, S.; Costa, V.; Maffulli, N.; Denaro, V. Articular-sided rotator cuff tears: Which is the best repair? A three-year prospective randomised controlled trial. Int. Orthop. 2013, 37, 1487–1493. [Google Scholar] [CrossRef]
- Jeong, H.J.; Nam, K.P.; Yeo, J.H.; Rhee, S.-M.; Oh, J.H. Retear After Arthroscopic Rotator Cuff Repair Results in Functional Outcome Deterioration Over Time. Arthrosc. J. Arthrosc. Relat. Surg. 2022, 38, 2399–2412. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, H.; Maeda, K.; Matsuki, K.; Moriishi, J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J. Bone Jt. Surg. 2007, 89, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.W.; Kim, J.Y.; Lee, H.W.; Yoon, J.H.; Noh, K.-C. Clinical and Anatomical Outcomes of Arthroscopic Repair of Large Rotator Cuff Tears with Allograft Patch Augmentation: A Prospective, Single-Blinded, Randomized Controlled Trial with a Long-term Follow-up. Clin. Orthop. Surg. 2022, 14, 263–271. [Google Scholar] [CrossRef]
- De Barros, A.A.F. Bioinductive Scafold Augmentation in Complete and Massive Rotator Cuff Tears—Prospective Randomized Trial. J. Shoulder Elb. Surg. 2023, 32, e251. [Google Scholar] [CrossRef]
- Burdick, G.; Fathima, B.; Gaudiani, M.; Wolternik, T.; Haan, J.; Kasto, J.; Kolowich, P.; Muh, S.; Castle, J. Poster 174: Arthroscopic rotator cuff repair with bioinductive patch achieves equivalent patient- reported outcomes at 1 year. Orthop. J. Sports Med. 2023, 11 (7_Suppl. S3), 2325967123S00160. [Google Scholar] [CrossRef]
- Iannotti, J.P.; Deutsch, A.; Green, A.; Rudicel, S.; Christensen, J.; Marraffino, S.; Rodeo, S. Time to failure after rotator cuff repair: A prospective imaging study. J. Bone Jt. Surg. 2013, 95, 965–971. [Google Scholar] [CrossRef]
- McIntyre, L.F.; Bishai, S.K.; Brown, P.B.; Bushnell, B.D.; Trenhaile, S.W. Patient-Reported Outcomes After Use of a Bioabsorbable Collagen Implant to Treat Partial and Full-Thickness Rotator Cuff Tears. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 2262–2271. [Google Scholar] [CrossRef]
- Bushnell, B.D.; Connor, P.M.; Harris, H.W.; Ho, C.P.; Trenhaile, S.W.; Abrams, J.S. Two-year outcomes with a bioinductive collagen implant used in augmentation of arthroscopic repair of full-thickness rotator cuff tears: Final results of a prospective multicenter study. J. Shoulder Elb. Surg. 2022, 31, 2532–2541. [Google Scholar] [CrossRef]
- Zhang, T.; Ajayi, A.; Hajjar, M.; Fleckenstein, C.M.; Nolan, J.; Hasan, S.S. Arthroscopic Repair of Retracted Large and Massive Rotator Cuff Tears with and without Augmentation with a Bio-Inductive Collagen Implant Reveals Substantial and Comparable Clinical Improvement. Arthroscopy 2023, 40, 1434–1442. [Google Scholar] [CrossRef]
- Warren, J.R.; Domingo-Johnson, E.; Sorensen, A.; Cheng, A.-L.; Latz, K.; Cil, A. Bioinductive Patch as an Augmentation for Rotator Cuff Repair, a Systematic Review and Meta-Analysis. J. Shoulder Elb. Surg. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Jackson, G.R.; Bedi, A.; Denard, P.J. Graft Augmentation of Repairable Rotator Cuff Tears: An Algorithmic Approach Based on Healing Rates. Arthrosc. J. Arthrosc. Relat. Surg. 2022, 38, 2342–2347. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Kim, S.H.; Lee, Y.H.; Kim, T.I.; Oh, J.H. The Rotator Cuff Healing Index: A New Scoring System to Predict Rotator Cuff Healing After Surgical Repair. Am. J. Sports Med. 2019, 47, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Rognoni, C.; Nherera, L.M.; Garofalo, R.; Guerra, E.; Longo, U.G.; Taverna, E.; Tarricone, R. Economic Evaluation of a Bioinductive Implant for the Repair of Rotator Cuff Tears Compared with Standard Surgery in Italy. Adv. Ther. 2023, 40, 5271–5284. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, L.F.; Nherera, L.M.; Schlegel, T.F. Resorbable Bioinductive Collagen Implant Is Cost Effective in the Treatment of Rotator Cuff Tears. Arthrosc. Sports Med. Rehabil. 2023, 5, e367–e374. [Google Scholar] [CrossRef]
| Trade Name | Company | Material |
|---|---|---|
| TissueMend | Stryker (Kalamazoo, MI, USA) | Bovine Foetal Dermis |
| Regeneten | Smith & Nephew (Hertfordshire, UK) | Bovine Achilles |
| Atelocollagen | Koken (Tokyo, Japan) | Type I Bovine Collagen |
| BioMend | ZimVie (Westminster, CO, USA) | Type I Bovine Collagen |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wee, W.H.D.; Tan, Z.W.N.M.; Quek, C.X.; Yik, J.H.; Ho, S.W.L. The Current Role of Collagen Patch Augmentation in Rotator Cuff Pathology: A Narrative Review. BioMed 2024, 4, 256-267. https://doi.org/10.3390/biomed4030020
Wee WHD, Tan ZWNM, Quek CX, Yik JH, Ho SWL. The Current Role of Collagen Patch Augmentation in Rotator Cuff Pathology: A Narrative Review. BioMed. 2024; 4(3):256-267. https://doi.org/10.3390/biomed4030020
Chicago/Turabian StyleWee, Wen Hon Darren, Zhi Wei Nicholas Matthias Tan, Clara X. Quek, Jing Hui Yik, and Sean Wei Loong Ho. 2024. "The Current Role of Collagen Patch Augmentation in Rotator Cuff Pathology: A Narrative Review" BioMed 4, no. 3: 256-267. https://doi.org/10.3390/biomed4030020
APA StyleWee, W. H. D., Tan, Z. W. N. M., Quek, C. X., Yik, J. H., & Ho, S. W. L. (2024). The Current Role of Collagen Patch Augmentation in Rotator Cuff Pathology: A Narrative Review. BioMed, 4(3), 256-267. https://doi.org/10.3390/biomed4030020






