Abstract
The increasing use of e-cigarettes (ECs) has raised public health concerns due to the observed cytotoxic effects in both in vitro and in vivo studies. Infants and young children, being particularly vulnerable groups, exhibit heightened susceptibility to potential hazards arising from maternal use of ECs, as well as exposure to second-hand and third-hand aerosols emitted by ECs. Melanocytes are neural-crest-derived cells that regulate multiple biological functions. Melanocyte death, triggered by chemical exposure, is a known etiological cause of pigmentation abnormalities and neurodevelopmental disorders. Prior reports have demonstrated nicotine-induced differential cytotoxicity to neonatal human melanocytes derived from lightly pigmented (LP) and darkly pigmented (DP) donors. We recently reported that the vehicle base propylene glycol (PG) in e-liquid can alter the functions of LP melanocytes. However, to date, the effects of e-liquid flavors on LP and DP cells remain unexplored. Hence, in this preliminary study, a panel of twenty EC refill liquids comprising ten popular flavors (strawberry, grape, banana, vanilla, butterscotch, cinnamon, menthol, chocolate, cola, and tobacco), where each flavored e-liquid contained either 0 or 18 mg/mL nicotine, was examined for in vitro cytotoxicity to neonatal human melanocytes derived from LP and DP donors. Our results reveal that of the ten flavors, five (menthol, cinnamon, vanilla, tobacco, and banana) were highly cytotoxic, with their half-maximal inhibitory concentration (IC50) values within the tested concentration ranges. Moreover, the cytotoxicity of the specific flavors menthol, cinnamon, and vanilla was enhanced in the presence of nicotine, indicative of interactive effects, with nicotine and flavor contributing to greater melanocyte injury. The cytotoxicity of menthol (both with and without nicotine) and cinnamon (without nicotine) e-liquids was found to be higher in LP cells as compared to DP cells. In contrast, nicotine-containing vanilla e-liquid induced higher cytotoxicity in DP cells than LP cells. Only three flavors, cola (without nicotine), strawberry (without nicotine), and chocolate (without nicotine), were non-cytotoxic to both LP and DP cells. The findings that popular flavors in e-liquids induced moderate to high degree of melanocytotoxicity even in the absence of nicotine suggests that ECs are not harmless. This information may assist EC users identify particular flavors in refill liquids that may be detrimental to melanocytes. A first-screen identification of flavors in e-liquids that show a racial/ethnicity dependence can provide a baseline to identify cytotoxicity concentration ranges for popular flavors and help inform the regulatory guidelines for EC toxicity to young children and youth.
1. Introduction
Electronic cigarettes (ECs) are widely used electronic gadgets powered by batteries that serve as a substitute for traditional combustible cigarettes [1,2]. EC devices use heating coils to vaporize the e-liquid or e-juice contained in the cartridge, creating an aerosol that imitates cigarette smoke without the process of combustion. E-liquids typically include a blend of propylene glycol (PG), vegetable glycerin (VG), nicotine, and flavorings [3,4]. The data from 2021 shows that the rate of EC use among children and young adults aged 8–20 years in 69 countries and territories was 7.8% within the past 30 days [5]. According to a 2023 survey [6], EC products were the most widespread tobacco product commonly used by students in middle and high school, with a usage rate of 7.7% (equivalent to 2.13 million students). Over 95% of children begin nicotine use via the use of flavored products, and the presence of flavors significantly contributes to the continuation of vaping habits [7,8]. The belief that the use of flavored ECs can aid in weight control and appetite suppression is also one of the key motivators for youth, especially high school students, to initiate EC [9,10]. The cost-effectiveness of different EC products compared to tobacco cigarettes, the social media influence and advertising targeting youngsters, and the wider variety of flavors that allow user control and the ability to mix and match multiple flavors add to the appeal of ECs [11]. Children exposed to EC advertisements depicting flavored EC as more appealing than unflavored ones are more inclined to experiment with ECs [12]. Additionally, the perception that ECs are innocuous or less injurious than conventional cigarettes and other tobacco products has played a vital role in the increased use of ECs [13,14,15]. Moreover, non-smokers may experiment with ECs due to the assumption that ECs are safe to use [16,17].
The US Food and Drug Administration (FDA) enforced guidelines that forbid the sale of flavored cartridge-based ECs that are attractive to children, such as fruit and mint flavors [18]. However, this policy does not extend to disposable ECs, which are allowed to have the same flavors restricted in pod-based ECs [19]. As a result, a significant number of EC users who acquired their devices from retail sources indicated that they used disposable EC devices. In addition, disposable EC products are typically more affordable than other devices, such as pod-based systems and tanks [20]. This affordability factor contributes to their widespread availability and accessibility. EC usage in homes leads to the exposure of inhabitants, including children, to secondhand EC vapor, independent of the specific features of EC use [21]. Passive exposure to EC aerosol from an EC user in the home of a pregnant mother (EC non-user) and her 3-year-old child exhibited detectable biomarkers of EC [22]. A separate study [23] quantified the amount of nicotine present in wristbands worn by children who had been exposed to secondhand EC vapor from their caretakers. The results indicated that these children had a greater exposure than children whose caregivers did not use ECs. Furthermore, infants and toddlers exhibit heightened susceptibility owing to their penchant for crawling and inquisitive tendencies, which exposes them to surfaces, including floors, walls, and windows that have been found to harbor residual nicotine resulting from indirect exposure to EC vapor, commonly referred to as third-hand exposure [24]. Collectively, these studies highlight that children are susceptible to passive EC use by second or third-hand exposures. Ingestion of liquid nicotine present in EC liquids has previously been shown to result in poisoning in children <6 years of age [25]. The sale of bulk high-nicotine e-liquids, commonly found in 30 mL bottles, poses a potential danger of poisoning for children [26]. Additionally, the accidental spilling or handling of nicotine e-liquids can result in its easy penetration through the skin; a study [27] showed that a 10 min exposure was sufficient for nicotine to enter through the skin, and despite rinsing the skin, the nicotine was still detectable.
Melanocytes, originating from the embryonic neural crest cells, are distributed across several regions of the human body, such as the skin, hair, eyes, inner ears, oral cavity, heart, and brain [28]. The exposure of melanocytes to EC chemicals may induce cell death, which can adversely impact various functions of melanocytes, including pigmentation, photoprotection, organ development and function, immunological function, and antioxidant and anti-inflammatory properties [29]. Melanocytic disorders encompass a spectrum of conditions encompassing aberrations in pigmentation, immune responses, sensory modalities, or the development of malignancies [30]. The phenomenon of melanocyte cell death, induced by the presence of exogenous chemicals, can give rise to various pigmentation disorders, most notably vitiligo. These, in turn, also impact the function of other sensory organs and might cause neurodevelopmental disorders [31]. Hence, investigating the cytotoxic effects of EC chemicals on melanocytes is significant.
The rising use of ECs by pregnant women, driven in part by the perception that ECs carry little or no risk in comparison to traditional cigarettes, raises concerns over the potential for fetal exposure [32]. Nicotine has been shown to be linked to developmental toxicity in mouse and human models [33,34]. In addition, nicotine [35], flavors [35], and PG [36,37] have demonstrated neurotoxicity in zebrafish embryos. Therefore, it is imperative to consider the potential melanocytotoxicity. Intriguingly, only a few studies have explored the effects of EC constituents, nicotine, or PG/VG on human melanocytes, while the effect of EC flavorings remains unaddressed. For example, studies by Delijewski et al. [38,39,40] have shown that nicotine selectively induces greater cytotoxicity to melanocytes from African-American newborn donors compared to Caucasian newborn donors, as well as perturbed melanogenesis and induced oxidative stress and biochemical changes. We recently showed that different ratios of PG/VG vehicle e-liquid distinctly affect human melanocytes from a Caucasian newborn donor, with high PG-containing e-liquids being severely cytotoxic, while non-cytotoxic concentrations elevated melanin production in cells, resulting in hyperpigmentation [41].
Multiple studies have previously established the racial discrepancy in EC use, revealing that African Americans had greater rates of EC use compared to Caucasians [42,43,44,45,46,47]. A higher proportion of individuals from the Black community reported using EC for smoking cessation purposes, while those from the White community mostly used EC as a recreational device in non-smoking places [48]. Furthermore, it was shown that Blacks had a higher preference for menthol-flavored ECs compared to Whites [48], mirroring the larger demand for menthol in traditional cigarettes among Blacks [49]. Menthol has also been shown to inhibit CYP2A6 enzymatic activity, thus affecting nicotine metabolism in vivo [50]. Other flavoring chemicals, cinnamaldehyde, and vanillin, have also been shown to inhibit the activity of CYP2A6, with cinnamaldehyde exhibiting a greater inhibitory effect than vanillin [51]. However, in the same study [51], the flavoring chemical isoamyl acetate, which is typically found in banana-flavored e-liquids, did not impact the enzymatic activity of CYP2A6, suggesting that only a specific subset of flavors can inhibit the activity. Hence, the use of ECs containing the flavorings of cinnamaldehyde, vanilla, and menthol is expected to decrease nicotine clearance in vivo by lowering nicotine metabolism, as a result of which the frequency of cigarette smoking can be lowered. This is beneficial for users who were prior smokers or current smokers who initiated EC use solely for smoking cessation. However, because there is a racial disparity wherein Caucasians prefer non-mentholated ECs for recreational purposes [48], coupled with their high nicotine metabolism owing to higher CYP2A6 activity (as compared to African-Americans), their use of EC is likely to increase with potential for greater exposure to these flavors. African Americans have higher melanin content in their melanocytes than Caucasians, which causes increased nicotine binding to melanin with prolonged retention and potential for long-term cytotoxicity [52,53]. These racial/ethnic differences in quantities of melanin pigment might modulate the cytotoxic effects of flavoring chemicals differentially in cells derived from different ethnicities due to the known capacity of melanin to bind with chemicals [54].
Hence, the current study aimed to identify if there was an ethnicity-dependent differential cytotoxic effect of the flavored e-liquids in melanocytes from Caucasian (LP) and African-American (DP) donors. In addition, we aimed to identify the cytotoxic effects of EC flavors on LP and DP cells in the absence and presence of nicotine to identify if nicotine amplified cytotoxicity. The novelty of the study lies in evaluating the cytotoxicity of flavored e-liquids, with and without nicotine, on melanocyte cultures derived from newborn donors with varying pigmentation levels. This is significant because neural crest cells, which give rise to melanocytes, play a role in the development of various organs and are implicated in several diseases. While only preliminary, the results of this study possess potential relevance to the emerging concerns surrounding the health impacts of flavored ECs.
2. Materials and Methods
2.1. Materials
E-liquids of ten diverse flavors (strawberry, grape, banana, vanilla, butterscotch, chocolate, cola, tobacco, cinnamon, and menthol), each without nicotine (−) and with 18 mg/mL nicotine (+), were procured from an online vendor, namely, My Freedom Smokes (MFS, Charlotte, NC, USA). All these 20 e-liquids were prepared in a vehicle base of propylene glycol/vegetable glycerin (PG/VG) as 80/20% v/v, as stated by the vendor. The label names of the e-liquids purchased were Ripe Strawberry, Grape, Banana Pudding, French Vanilla, Butterscotch, Chocolate, Cola, Captain’s Choice tobacco, Cinnamon Roll, and Fresh Menthol; the description of these e-liquids and flavor class has been listed in our previous study [55]. Pure PG and VG were procured from the same vendor and combined in an 80/20% v/v ratio to prepare the vehicle base (PG/VG 80/20% v/v) for vehicle control in the e-liquid experiments. An unflavored nicotine base (in PG/VG 75/25% v/v) was purchased from the same vendor as a nicotine control. MTS assay (CellTiter 96® AQueous One Solution) was purchased from Promega Corporation (Madison, WI, USA).
2.2. Cell Culture
Human epidermal melanocytes from a lightly pigmented neonatal donor (HEMn-LP; [56]) and darkly-pigmented neonatal donor (HEMn-DP; [57]) were purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA). Both cells were cultured using medium 254 (Cascade Biologics, Portland, OR, USA) supplemented with 1% human melanocyte growth supplement (HMGS, Cascade Biologics) and 1% penicillin–streptomycin antibiotics (Gibco™) at 37 °C in a 95% air–5% CO2 incubator in a humidified atmosphere.
2.3. MTS Cytotoxicity Assay
DP or LP cells (2 × 104 cells/well in 0.2 mL culture medium) were cultured in a 96-well plate for 24 h. After 24 h, the culture medium was replaced by a fresh medium containing different concentrations of e-liquids (0.5–2% v/v) diluted in the culture medium from the neat e-liquid (considered as 100%), and cultures were maintained for another 48 h. At the end of treatments, the culture medium was aspirated and replaced with 100 µL of culture medium containing 20 µL of MTS reagent, and the culture plates were incubated for 2 h at 37 °C. Subsequently, 100 µL were aliquoted to a new 96-well plate, and the absorbance readings were recorded at 490 nm using a Versamax™ microplate reader (Molecular Devices, San Jose, CA, USA). Cell viability was calculated from treatment group absorbance values normalized to untreated control and reported as percentages.
2.4. Statistical Analysis
For analysis of groups, one-way ANOVA followed by Dunnett’s or Tukey’s multiple comparisons test was performed using GraphPad Prism version 10.1.1 for Windows, GraphPad Software (San Diego, CA, USA). Statistical significance was considered at p < 0.05; data are reported as mean ± SD. The half-maximal inhibitory concentration (IC50) values were calculated in GraphPad Prism with nonlinear regression from the log (inhibitor) versus response, with variable slope (four parameters) analysis with top and bottom constraints of 100 and 0, respectively. The IC50 values were compared using Student’s t-test.
3. Results
The effects of unflavored vehicle bases (PG/VG) were evaluated to rule out any possible contributions of cytotoxicity from them before evaluating the flavored e-liquids. Treatment with PG/VG e-liquid over the 0.5–2% concentration range showed no cytotoxicity to DP cells (Figure 1A) and LP cells (Figure 1B).
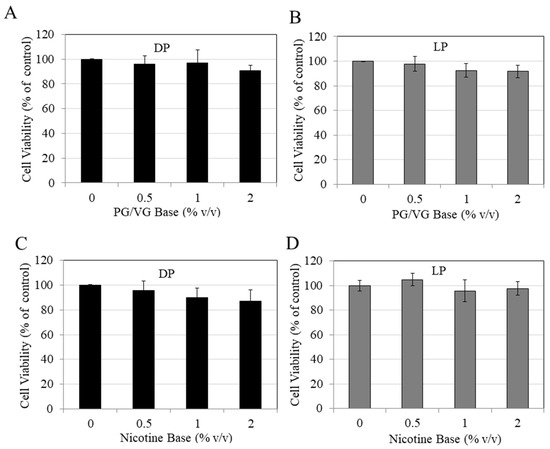
Figure 1.
Effects of PG/VG vehicle (0.5, 1, and 2%) on the viability of (A) darkly pigmented (DP) and (B) lightly pigmented (LP) melanocytes. Effects of unflavored nicotine base (0.5, 1, and 2%) on the viability of (C) DP cells and (D) LP cells. Data for (A–C) is mean ± SD of at least three independent experiments, while data for (D) is mean ± SD of one of two independent experiments in triplicate. One-way ANOVA with Dunnett’s test; no significance was found between groups.
The results of the unflavored nicotine base also showed no toxicity to DP cells (Figure 1C) or LP cells (Figure 1D). These results show that the unflavored nicotine-free e-liquid and nicotine-containing e-liquid do not affect the viability of either LP or DP cells.
Next, the results of flavored e-liquids from flavors of strawberry, vanilla, grape, butterscotch, and banana are summarized in Figure 2. Strawberry-flavored e-liquid without nicotine did not cause cytotoxicity to DP or LP cells at any concentration. However, nicotine-containing strawberry e-liquid significantly lowered viability by 33.05% at 2% in DP cells, with no change in the viability of LP cells (Figure 2A).
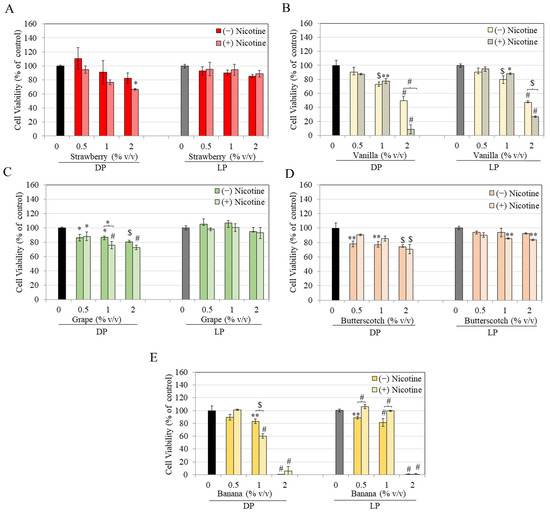
Figure 2.
Viability of DP and LP melanocytes in the presence of nicotine-free (−) and nicotine-rich (+) e-liquids of flavors: (A) strawberry, (B) vanilla, (C) grape, (D) butterscotch, and (E) banana, over 48 h, as measured by MTS assay. All data are mean ± SD (n = 3); one-way ANOVA with Tukey’s test. * p < 0.05; ** p < 0.01; $ p < 0.001 and # p < 0.0001 vs. Ctrl.
Vanilla-flavored e-liquid without or with nicotine caused significant cytotoxicity in both LP and DP cells at 1 and 2% concentrations. Cell viabilities were significantly diminished by 26.7% and 50.03% in DP cells. In comparison, they were diminished by 19.77% and 52.57% in LP cells at 1 and 2%, respectively (Figure 2B). In the case of nicotine-containing vanilla e-liquid, viabilities were significantly diminished by 22.49% and 91.12% in DP cells, while they were diminished by 11.8% and 72.84% in LP cells at 1 and 2%, respectively (Figure 2B). Interestingly, viabilities of 2% vanilla e-liquid containing nicotine were significantly lower than corresponding nicotine-free e-liquid at 2% in both LP and DP cells (Figure 2B), indicating increased cytotoxicity due to nicotine. However, as the unflavored nicotine e-liquid at 2% does not result in cytotoxicity (based on earlier data) in LP and DP cells, it can be concluded that nicotine synergistically increased the cytotoxicity of vanilla e-liquids in both LP and DP cells. Overall, DP cells showed greater sensitivity to cytotoxicity by vanilla flavor in the presence of nicotine than LP cells.
Grape-flavored e-liquid significantly lowered viability of DP cells by 13.46%, 12.97%, and 19.37% at concentrations of 0.5, 1, and 2%, while the viability of LP cells was unaffected (Figure 2C). Nicotine-containing grape-flavored e-liquid significantly lowered DP cell viability by 11.72%, 24.02%, and 27.09% at 0.5, 1, and 2%, respectively, with no changes in LP cells at any concentration (Figure 2C). Taken together, grape e-liquid with or without nicotine was cytotoxic in DP cells, while it was nontoxic in LP cells over the range tested.
Butterscotch-flavored e-liquid significantly lowered the viability of DP cells by 21.44%, 22.94%, and 25.88%, at concentrations of 0.5, 1 and 2%, respectively, with no changes in LP cell viability (Figure 2D). However, in the presence of nicotine, butterscotch-flavored e-liquid significantly diminished the viability of LP cells by 14.37% and 16.38% at 1 and 2%, respectively, in DP cells; viability was significantly diminished by 29.06% only at the highest concentration of 2% (Figure 2D). Overall, DP cells showed greater sensitivity to cytotoxicity by butterscotch flavor as compared to LP cells.
Banana-flavored e-liquid caused significantly lowered viability of DP cells by 16.4% and 99.64% at 1 and 2%, respectively. In comparison, the viability of LP cells was significantly diminished by 11.16%, 18.68%, and 99.75% at 0.5, 1, and 2%, respectively (Figure 2E). However, in the presence of nicotine, banana-flavored e-liquid showed greater cytotoxicity to DP cells with significant diminutions of 39.52% and 93.74% at 1 and 2%, respectively (Figure 2E). Interestingly, cytotoxicity of banana flavor was rescued by nicotine-containing liquid at 0.5 and 1% in LP cells (Figure 2E).
The results of the remaining five flavored e-liquids are summarized in Figure 3. Chocolate-flavored e-liquid without nicotine did not cause any significant changes in the viability of DP or LP cells at any concentration. However, nicotine-containing chocolate e-liquid significantly lowered LP cell viability by 25.49% and 22.18% at 1 and 2%, respectively, with no change in the viability of DP cells (Figure 3A).
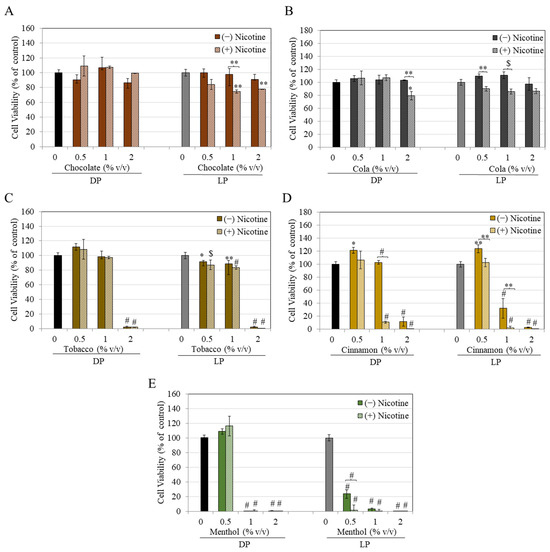
Figure 3.
Viability of DP and LP melanocytes in the presence of nicotine-free (−) and nicotine-rich (+) e-liquids of flavors: (A) chocolate, (B) cola, (C) tobacco, (D) cinnamon, and (E) menthol over 48 h, as measured by MTS assay. All data are mean ± SD (n = 3); one-way ANOVA with Tukey’s test. * p < 0.05; ** p < 0.01; $ p < 0.001 and # p < 0.0001 vs. Ctrl.
Cola-flavored e-liquid did not cause any significant alterations in viability of DP and LP cells (Figure 3B). However, in the presence of nicotine, cola e-liquid at 2% significantly decreased the viability of DP cells by 20.83%; this decrease was also significant as compared to the corresponding concentration of nicotine-free cola e-liquid (Figure 3B). Interestingly, in LP cells, the viabilities of nicotine-containing cola e-liquid at 0.5% and 1% were significantly lower relative to the corresponding concentrations of nicotine-free e-liquid, although the values were not significantly different from the untreated control (Figure 3B). These results suggest that nicotine might interact with cola e-liquid to alter viability, in contrast to nicotine-free liquid.
Tobacco-flavored e-liquid markedly diminished the viability of DP cells by 98.30%, only at the highest concentration of 2%. In comparison, the viability of LP cells was significantly diminished by 8.16%, 11.22%, and 97.99% at 0.5, 1, and 2%, respectively (Figure 3C). Nicotine-containing tobacco e-liquid showed similar results to that of nicotine-free tobacco in both cells; viability of DP cells was significantly diminished by 98.35% at 2%, while in LP cells, viability was significantly diminished by 12.79%, 16.54%, and 99.62% at 0.5, 1, and 2%, respectively (Figure 3C).
Cinnamon-flavored e-liquid significantly lowered the viability of DP cells by 88.41% at 2%. In comparison, the viability of LP cells was significantly diminished by 67.83% and 97.14% at 1 and 2%, respectively (Figure 3D). Interestingly, cinnamon at 0.5% significantly increased viability by 21.38% and 23.73% in DP and LP cells, respectively. In the presence of nicotine, cinnamon-flavored e-liquid significantly lowered viability by 89.32% and 99.60% in DP cells at 1 and 2%, respectively, while in LP cells, viability was similarly lowered; diminutions of 97.78% and 99.71% were obtained at 1 and 2%, respectively (Figure 3D). Overall, LP cells showed greater susceptibility to damage by cinnamon e-liquid as compared to DP cells.
Menthol-flavored e-liquid significantly decreased the viability of DP cells by 99.92% and 99.61% at concentrations of 1 and 2%, respectively. In comparison, the viability of LP cells was significantly diminished by 76.25%, 96.47%, and 99.66% at 0.5, 1, and 2%, respectively (Figure 3E). Nicotine-containing menthol e-liquid significantly lowered DP cell viability by 99.55% and 99.97% at 1 and 2%, respectively, while it significantly diminished viability of LP cells by 98.4%, 99.83%, and 99.86% at 0.5, 1, and 2%, respectively (Figure 3E). Overall, LP cells showed greater sensitivity to cytotoxicity by menthol e-liquid both without and with nicotine.
Figure 4 summarizes the description of cytotoxic (moderate and high) and non-cytotoxic e-liquids in both LP and DP cells. Five e-liquids [(butterscotch (−), cola (+), strawberry (+), grape (−), and grape (+)] and one e-liquid [chocolate (+)] induced moderate cytotoxicity in DP and LP cells, respectively (Figure 4A). Butterscotch (+) e-liquid induced moderate cytotoxicity to both LP and DP cells. Ten e-liquids [vanilla (−), vanilla (+), banana (−), banana (+), tobacco (−), tobacco (+), cinnamon (−), cinnamon (+), menthol (−), and menthol (+)] induced high cytotoxicity to both LP and DP cells (Figure 4A). One e-liquid, chocolate (+), was non-cytotoxic to DP cells, while five e-liquids [(butterscotch (−), strawberry (+), cola (+), grape (−), and grape (+)] were non-cytotoxic to LP cells (Figure 4B). Three e-liquids [chocolate (−), cola (−), and strawberry (−)] were non-cytotoxic to both LP and DP cells (Figure 4B).
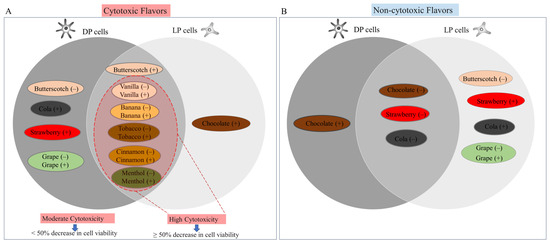
Figure 4.
Schematic Venn diagram showing the flavored e-liquids that were (A) cytotoxic and (B) non-cytotoxic over the concentration range (0.5–2%) in DP and LP melanocytes; flavors with (+) sign denote nicotine-containing while with (−) sign denote nicotine-free flavors; flavors shown within the red dotted shape indicate highly cytotoxic flavors that lowered cell viability by ≥50%.
The IC50 values for cytotoxicity of flavored nicotine-rich (+) and nicotine-free (−) e-liquids were determined and are presented in Table 1. The other five flavors (grape, strawberry, chocolate, cola, and butterscotch) did not reach IC50 in the tested concentration range; hence, they were not determined. The results showed that the mean IC50 values of cinnamon (−) and menthol (−) e-liquids were 0.98% and 0.34%, respectively, in LP cells. These values were significantly lower than the corresponding IC50 values in DP cells (cinnamon (−)IC50: 1.77%; menthol (−) IC50: 0.89%), suggesting that LP cells are more sensitive to these two flavors than DP cells (Table 1). The results further showed that in DP cells, vanilla (+) (IC50: 1.26%) and cinnamon (+) (IC50: 0.86%) e-liquids were significantly more cytotoxic than their corresponding nicotine-free e-liquids (vanilla (−) IC50: 1.97%; cinnamon (−) IC50: 1.77%). Furthermore, in LP cells, vanilla (+) e-liquid (IC50: 1.58%) and menthol (+) (IC50: 0.04%) e-liquids were significantly more cytotoxic than the ones without nicotine (vanilla (−) IC50: 1.94%; menthol (−) IC50: 0.34%). Curiously, we saw a contrasting pattern in the presence of cinnamon and menthol flavors in both cell types. Specifically, it was observed that the presence or absence of nicotine did not significantly alter the cytotoxic effects of menthol on DP cells. Similarly, the cytotoxicity of cinnamon on LP cells remained comparable, regardless of the presence or absence of nicotine.

Table 1.
IC50 values (as % v/v) of cytotoxicity induced by different e-liquids in DP and LP cells.
The cytotoxicity of five nicotine-free e-liquids in DP cells may be ranked based on their IC50 values: menthol (−) > banana (−) > tobacco (−) > cinnamon (−) > vanilla (−). The order of cytotoxicity of these e-liquids in LP cells is as follows: menthol (−) > cinnamon (−) > banana (−) > tobacco (−) > vanilla (−). Next, the order of cytotoxicity for nicotine-containing e-liquids in DP cells is menthol (+) = cinnamon (+) > banana (+) > vanilla (+) > tobacco (+), while the order for these e-liquids in LP cells is menthol (+) > cinnamon (+) > tobacco (+) > banana (+) > vanilla (+). Overall, menthol emerged as the most cytotoxic flavor among the ten flavors that were examined.
4. Discussion
Our study results show that out of the 20 e-liquids analyzed, 16 e-liquids exhibited cytotoxic properties toward DP cells. Among these, 10 e-liquids demonstrated a high degree of cytotoxicity, while the remaining 6 e-liquids displayed a moderate level of cytotoxicity. Similarly, when examining the response of LP cells to the e-liquids, it was found that 12 e-liquids exhibited cytotoxic effects. Among these, 10 e-liquids were highly cytotoxic, whereas the remaining 2 e-liquids displayed a moderate level of cytotoxicity. Given that LP cells exhibited more pronounced cytotoxic effects when exposed to menthol (with or without nicotine) and cinnamon (without nicotine) e-liquids compared to DP cells, it is likely that the higher melanin levels in DP cells provide protection against the cytotoxicity induced by these two flavors. Nevertheless, when nicotine is present, the increased melanin levels in DP cells do not protect against cytotoxicity caused by cinnamon, implying that nicotine potentiates the cytotoxic effects of cinnamon e-liquid in the presence of higher melanin levels. In contrast, nicotine potentiates the cytotoxic effects of menthol e-liquid in the presence of low melanin.
In this study, we used a concentration range between 0.5 and 2% for e-liquids; these concentrations are within the range used in other studies that have used 0.25–0.5% [58] or even lower concentrations of 0.01, 0.1, and 0.3% [59]. Concentrations higher than 2% were not examined for cytotoxicity as the physiological concentrations of EC are lower than that, although in our previous study [41], we reported that the 80/20 PG/VG e-liquid exhibited an IC50 value of 3.76% in HEMn-LP cells after a 72 h exposure. In the current study, as we did not examine concentrations > 2%, it is unknown if nicotine-containing PG/VG e-liquid will show cytotoxicity at concentrations > 2%. Interestingly, other studies have reported on the effects of nicotine in HEMn-DP cells [39] and HEMn-LP cells [40]; the authors showed that nicotine, when added to cultures for 24 h, was 2.95-fold more cytotoxic to HEMn-DP cells (IC50: 2.52 mM) than HEMn-LP cells (IC50: 7.43 mM). The authors attributed the higher cytotoxicity of nicotine towards HEMn-DP cells to the presence of higher melanin in them, which causes nicotine binding with melanin and retention in cells, eliciting greater cytotoxicity over time. Accordingly, the cytotoxicity of vehicle PG/VG, flavors, flavor-vehicle adducts, nicotine, or nicotine adducts can vary depending on the pigmentation content of the cells. It should be noted that the HEMn-DP cells used in the current study also contain significantly higher melanin than HEMn-LP cells [60]. In our experiments, nicotine was not evaluated as a pure standard. Instead, it was present in a mixture form with the PG/VG vehicle. Moreover, our experiments were conducted over a duration of 48 h and with the maximum tested concentration of 2%, which is equivalent to 2.2 mM nicotine, which is below the 24 h IC50 values of nicotine in both LP and DP cells as observed in previous studies.
Our results of the protective effect of nicotine in decreasing cytotoxicity of banana e-liquid in LP cells are reminiscent of the results of previous studies [61,62]. For example, in a study where the authors used e-liquid aerosol condensates, they showed that flavored e-liquid (different flavor) condensates containing 18 mg/mL nicotine rescued cytotoxicity of nicotine-free flavored e-liquid condensates in airway epithelial cells [61]. Another study [62] reported that the in vivo administration of nicotine-free e-liquid of a different flavor (tobacco) to rats decreased the viability of their hippocampal neurons significantly, but administration of the same EC liquid containing 18 mg/mL nicotine rescued the cytotoxicity by significant amounts. Interestingly, rat cortical cells that were harvested in the same study showed no change in cell viability after exposure to these two e-liquids, indicating a cell-type-specific cytotoxic effect. Melanocytes share similarities with cells of neural lineage as they are embryonically derived from neural crest [63,64]. Another study showed that mouse cranial neural crest cells that were exposed to aerosols generated from six differently flavored nicotine-containing e-liquids responded with impaired vascular and cartilage differentiation in the case of two e-liquids that shared similar flavor additives of strawberry and cream [65].
Our results further showed that chocolate flavor was non-cytotoxic to both LP and DP cells, although LP cells were more sensitive to the moderate cytotoxicity induced by nicotine-containing chocolate e-liquid. Previous studies have reported that in human airway epithelial cells, chocolate-flavored e-liquid impaired phagocytosis and enhanced apoptosis [66], while another study [67] that examined a chocolate flavoring chemical, namely, 2,5-dimethylpyrazine, showed alterations in cellular physiology. Although chocolate-flavored ECs may appeal to children and female teenagers attempting to use them for weight reduction [68], they seem to have less attraction compared to menthol or fruit-flavored ECs. This is because the smoking frequency of cigarettes did not change for users who used chocolate-flavored ECs, whereas those who used menthol-flavored ECs experienced a decrease in the number of cigarettes smoked [69].
The findings from our study on the enhanced cytotoxicity of e-liquids containing nicotine and cinnamon, as opposed to e-liquids containing only cinnamon without nicotine, align with previous reports that have also reported similar results [55,70]. In a previous study [71], it was shown that eight varieties of cinnamon-flavored e-liquids displayed cytotoxicity toward human embryonic stem cells. The authors reported that the cytotoxicity was primarily due to the chemical cinnamaldehyde in these e-liquids. The identification of cinnamaldehyde as the key inducer of cytotoxicity has been corroborated in another study, wherein cinnamaldehyde exhibited comparable levels of cytotoxicity to those observed in e-liquids flavored with cinnamon [72]. Cinnamaldehyde exhibits remarkable chemical reactivity and electrophilic properties owing to the existence of an α,β-unsaturated carbonyl Michael acceptor moiety. A previous study showed that the anti-proliferative effect of cinnamaldehyde in melanoma cells depends on its reactivity as a Michael acceptor, as non-Michael reactive analogs did not exhibit an anti-proliferative effect [73]. A 72 h treatment with cinnamaldehyde showed IC50 values of 31 µM in A375 human melanoma cells [74]. In primary human fibroblasts and keratinocytes, cinnamaldehyde exhibited significant cytotoxicity with IC50 values of 21.4 and 17.9 µM, respectively [73]. Although the quantification of cinnamaldehyde concentration in the e-liquids utilized in this study was not performed, previous reports have indicated that the concentrations of cinnamaldehyde in cinnamon-flavored e-liquids typically exceeded 1% [75,76]. Specifically, concentrations of 11.8% (118 mg/mL or 0.89 M) and 34.3% (343 mg/mL or 2.6 M) were observed in two e-liquids [77]. Based on the supposition that our e-liquids could potentially possess concentrations of cinnamaldehyde at the upper limits of 11.8% or 34.3%, it is possible to approximate that the concentration of cinnamaldehyde in a 1% e-liquid solution employed in this study (which was a 1:100 dilution of the undiluted e-liquid) would be 8.9 mM or 26 mM, correspondingly. Evidently, these values are much higher than the usual IC50 values for cytotoxicity seen in multiple cells and explain the results of severe cytotoxicity as observed in our study. Moreover, cinnamaldehyde is a skin sensitizer and a melanocytotoxic chemical that impairs melanin synthesis and causes depigmentation [78]. Cinnamaldehyde has also been shown to induce intraoral allergy in oral products [79,80], and its inclusion in toothpaste also raises concerns due to side effects of the formation of white depigmented patches in the perioral area [81]. The application of cinnamaldehyde directly on the skin had a notable toxic effect and triggered the production of pro-inflammatory cytokines at doses of 80 mM [82]. Recent studies in the zebrafish embryo model showed that cinnamaldehyde caused oxidative-stress-dependent developmental neurotoxicity [83] and damaged bone, cartilage, and vascular development [70]. Based on these reports, it can be concluded that cinnamon e-liquids have long-term cytotoxic effects, and their use is not recommended.
Our results of non-cytotoxicity of grape-flavored e-liquid are similar to a prior study that examined grape-flavored e-liquid and its chemical flavoring compounds (ethyl acetate and ethyl butyrate) in a zebrafish model and found no toxicity of grape flavor [84]. In the same study [84], the authors also reported cytotoxicity of another flavor (Bubble gum), which shared an identical chemical composition with the grape e-liquid except that it also contained cinnamaldehyde, suggesting that cinnamaldehyde was the driver of cytotoxicity. Our results of highest cytotoxicity of menthol-flavored e-liquid in neonatal melanocytes are consistent with an earlier study that compared the effects of unvaped flavored e-liquid responses on fetal, neonatal, and adult lung cell and reported that neonatal cells displayed the highest susceptibility to damage by menthol e-liquid [85]. A recent study documented that exposure to menthol-based EC condensates induced toxicity and impaired critical physiological functions of the lung [86]. The use of menthol-flavored ECs was markedly increased following the FDA ban on fruit-flavored refillable EC cartridges, which exempted tobacco and menthol flavors [87]. This was partly due to its capacity to suppress the harshness of nicotine and impart a cooling sensation via the activation of cold receptors of the skin and the oral cavity [88,89,90]. Low levels of menthol flavor have also been found in some ECs that were not labeled as menthol flavor [91]. The growing use of menthol flavor, together with the findings from this investigation demonstrating the preferential cytotoxicity of menthol towards LP cells, suggests a possibility for significant cytotoxic effects and unfavorable outcomes. There has been a growing trend in using a blend of menthol and fruity flavors inside JUUL devices [92]. Additionally, there has been a growing interest in using Puff Krush flavor-enhancing capsules inside JUUL pods, which enhance the overall taste experience. According to a previous study [19], it was found that 25% of youth under the age of 21 who used pod-based ECs also utilized the Puff Krush. A recent study [93] showed that youths aged 16–17 who used ECs engage in altering the e-liquid by combining different flavored e-liquids to produce novel tastes in the US. Based on the available evidence, it is likely that cells may be subjected to many varieties of flavored e-liquid simultaneously. The realistic situation involves the presence of a previously used e-liquid that remains in the cartridge, undergoing reheating and aging. Additionally, the user introduces fresh e-liquids with different flavors, potentially resulting in a mixture that might enhance cytotoxic effects. Subsequent investigations must focus on examining the effect of realistic flavored e-liquid compositions on cells since such mixtures are expected to induce elevated cytotoxic responses.
While the nicotine concentration of 18 mg/mL that was selected in the current study is considered moderate [94], it is worth noting that EC products may include greater amounts of nicotine. For instance, JUUL brand EC pods have been reported to have nicotine concentrations as high as 59 mg/mL [26,95]. Therefore, it is plausible that the cytotoxicity observed in our current study was heightened by certain flavors (specifically, cinnamon, vanilla, and menthol) as a result of the interaction between nicotine and these flavors when nicotine was present at a concentration of 18 mg/mL. It is conceivable that this cytotoxicity could be even more pronounced if the nicotine concentration in pure e-liquids were higher. However, further comprehensive investigations are necessary to examine the correlation between nicotine concentrations in e-liquids and the extent of flavor-induced toxicity. Because our unflavored nicotine base showed no significant cytotoxicity in DP cells over the concentration range of 0.5–2%, the increased cytotoxicity of flavor with nicotine can be ascribed to chemical interactions of nicotine with flavor chemicals. For example, Kerber et al. [96] have demonstrated that vapors generated by e-liquid containing a mixture of cinnamaldehyde with nicotine resulted in the generation of more toxic chemical products, as compared to cinnamaldehyde flavor e-liquid alone, indicative of chemical reaction of flavor with nicotine, although it was restricted to only specific flavors. Nevertheless, the interaction effects of nicotine and flavors can be complex.
The MTS assay is based on the conversion of tetrazolium salt to a purple-colored formazan product by the action of cellular mitochondrial dehydrogenases. The formation of the colored product is measured spectrophotometrically [97]. The MTS assay is widely recognized as the gold standard test for toxicological assessments because of its simplicity, rapid and sensitive detection of toxicity, and cost-efficiency [98]. Several previous studies have investigated the in vitro cytotoxicity of e-liquids or their chemical flavors using the MTS assay [67,99,100,101]. Our previous study [55] that examined the same panel of e-liquids but in human retinal pigment epithelial cells showed that four e-liquids, namely, menthol (−), menthol (+), banana (+), and vanilla (+), induced high lactate dehydrogenase (LDH) leakage, which is an indicator of necrotic cell death [102]. Although in the current study, the mode of cell death (early or late apoptosis or necrosis) induced by e-liquids in melanocytes was not examined, several other studies have documented that menthol-, cinnamon-, or vanilla-based e-liquids can trigger apoptosis and necrosis in cells [103,104,105]. For example, the flavoring chemical menthol induces apoptotic cell death in human gingival fibroblasts [103]. Elsewhere, a vapor extract of a vanilla custard e-liquid that contained cinnamaldehyde and vanillin flavor chemicals was shown to induce both apoptosis and necrosis in cardiomyocytes [105]. In another study, aerosols of a cinnamon-flavored e-liquid at 2% triggered apoptotic death pathways in neural retinal epithelial cells of chick embryos [104]. The evaluation of cell death mechanisms was not the focus of this study and warrants additional studies.
It is imperative to acknowledge the presence of limitations within this study. For the e-liquids used in the current study, the label provided by the manufacturer listed four components: PG, VG, nicotine, and flavor name, along with the amounts of nicotine content (in mg/mL) and PG/VG (in % v/v) provided with each e-liquid. Previous studies have reported the use of gas chromatography–mass spectrometry (GC-MS) to analyze the chemical composition of e-liquids [106,107]. In addition, e-liquids have also been found to contain trace metals, as revealed by the analysis using ICP-MS [108]. We recognize that having a comprehensive compositional analysis from the manufacturer would have been advantageous. However, the absence of precise composition and accurate labeling has been recognized as a potential limitation in EC research [109]. In our study, the chemical analysis of e-liquids using GC-MS was not performed to determine the specific flavor chemicals, which is a limitation. However, the use of GC-MS can be time-consuming, especially when dealing with a large number of e-liquids. Furthermore, the use of GC-MS relies on pre-established flavor chemical standards, which may prevent the detection of newly formed chemicals that emerge in e-liquids as time progresses. Over time, e-liquids often form unique chemical adducts [110]. Moreover, several prior studies on the cytotoxicity of neat e-liquids [55,108,111] or their vapor condensates [112] did not include a GC-MS analysis of their composition. We employed unvaped e-liquids instead of aerosolized e-liquids to evaluate melanocyte cytotoxic responses. Despite this, recent publications [76,113] have demonstrated that the utilization of neat e-liquid treatments exhibits a high degree of accuracy in predicting the toxicity levels of associated aerosols. This substantiates using unvaped e-liquid treatments as an initial screening paradigm for in vitro experiments on ECs. Moreover, we only used one nicotine concentration (18 mg/mL) in the e-liquids utilized in this study, although nicotine concentrations in many popular EC products can be higher. For instance, JUUL EC products used by teenagers had nicotine concentrations ranging from 21.8 to 56.2 mg/mL [114], and in some cases, even higher, reaching up to 69 mg/mL [115]. Mr. Fog is another famous EC brand used by youth that markets its products with flavors containing 50 mg/mL nicotine [116]. Nevertheless, Mr. Fog flavored ECs are also available in 20 mg/mL nicotine [117], which matches the concentration ranges of this study. Our emphasis on in vitro models is insufficient in replicating the intricate nature of human physiological reactions to the use of e-liquids in real-world environments. Another limitation is that a limited assortment of flavored e-liquids that were acquired from a single vendor were selected in this study. Moreover, a single donor for each ethnicity type was used in cell culture experiments. Future studies should examine different brands of the same flavored e-liquid in LP and DP cells from multiple donors to validate the findings of the present study conclusively. Even though we evaluated the potential melanocytotoxicity of a limited assortment of ten flavors, these flavors are currently preferred in many unique e-liquid formulations by school-aged children and young adolescents. Yet, we must exercise caution in acknowledging that our present findings do not necessarily suggest an elevated risk associated with the real-world usage of menthol-, vanilla-, cinnamon-, tobacco-, and banana-flavored ECs. Moreover, this study focused on short-term exposures and cytotoxicity, offering a restricted understanding of the long-term consequences of e-liquid utilization. Additional studies are underway to further explore the potential of e-liquids to disrupt melanocyte functions at concentrations that do not induce cellular toxicity.
5. Conclusions
In summary, our results showed that different flavors exhibited a heterogeneous cellular response. Among the 20 e-liquids examined, 16 e-liquids exhibited cytotoxic effects on DP cells, with 10 being highly cytotoxic and 6 being moderately cytotoxic. Likewise, 12 e-liquids showed cytotoxic effects on LP cells, with 10 being highly cytotoxic and 2 being moderately cytotoxic. We demonstrated that cytotoxicity was solely due to flavorings, not the vehicle or nicotine bases, in both cells. Out of the 10 flavors that were evaluated, 5 of them (menthol, cinnamon, vanilla, tobacco, and banana) demonstrated the capacity to achieve the IC50 value of cytotoxicity within the concentration ranges of 0.5–2% that were examined. LP cells exhibited greater susceptibility to the cytotoxic effects of menthol (−), menthol (+), and cinnamon (−) e-liquids than DP cells. In contrast, DP cells were more sensitive to cytotoxicity by vanilla (+) e-liquid than LP cells. Nicotine enhanced the cytotoxicity of menthol e-liquid only in LP cells and the cytotoxicity of cinnamon e-liquid only in DP cells, but it enhanced the cytotoxicity of vanilla e-liquid in both LP and DP cells. These results suggest the interaction between nicotine and specific flavors in eliciting melanocyte damage. The findings indicate that frequently used flavors in e-liquids may induce cytotoxicity to melanocytes even without nicotine. These results suggest that ECs are not entirely safe and may help inform EC users in identifying refill liquid flavors that may be melanocytotoxic. Exploring the ethnic/racial differences and a better understanding of nicotine metabolism may also lead to more individualized and effective smoking cessation programs. Although our findings cannot be directly extrapolated to in vivo human exposure, they raise concerns over the possibility of these flavored e-liquids causing damage to EC users to the amounts used in some refill fluids. Furthermore, prolonged exposure to elevated levels of these e-liquids might potentially cause far greater harm compared to the results shown in our short-term experiments.
Funding
This research was partly funded by the Research Foundation for The State University of New York (85184–1155067).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.
Acknowledgments
The author would like to acknowledge Sanford R. Simon (Department of Biochemistry and Cell Biology, Stony Brook University) for access to resources. The funder had no role in study design, data collection, analysis, the decision to publish, or the preparation of the manuscript.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Rom, O.; Pecorelli, A.; Valacchi, G.; Reznick, A.Z. Are E-cigarettes a safe and good alternative to cigarette smoking? Ann. N. Y. Acad. Sci. 2015, 1340, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Breland, A.; Soule, E.; Lopez, A.; Ramôa, C.; El-Hellani, A.; Eissenberg, T. Electronic cigarettes: What are they and what do they do? Ann. N. Y. Acad. Sci. 2017, 1394, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Bertholon, J.; Becquemin, M.; Annesi-Maesano, I.; Dautzenberg, B. Electronic cigarettes: A short review. Respiration 2013, 86, 433–438. [Google Scholar] [CrossRef]
- Tierney, P.A.; Karpinski, C.D.; Brown, J.E.; Luo, W.; Pankow, J.F. Flavour chemicals in electronic cigarette fluids. Tob. Control 2016, 25, e10–e15. [Google Scholar] [CrossRef] [PubMed]
- Yoong, S.L.; Hall, A.; Leonard, A.; McCrabb, S.; Wiggers, J.; d’Espaignet, E.T.; Stockings, E.; Gouda, H.; Fayokun, R.; Commar, A. Prevalence of electronic nicotine delivery systems and electronic non-nicotine delivery systems in children and adolescents: A systematic review and meta-analysis. Lancet Public Health 2021, 6, e661–e673. [Google Scholar] [CrossRef]
- Birdsey, J. Tobacco Product Use among US Middle and High School Students—National Youth Tobacco Survey, 2023. MMWR. Morb. Mortal. Wkly. Rep. 2023, 72, 1173–1182. [Google Scholar] [CrossRef]
- Kong, G.; Morean, M.E.; Cavallo, D.A.; Camenga, D.R.; Krishnan-Sarin, S. Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine Tob. Res. 2015, 17, 847–854. [Google Scholar] [CrossRef]
- Harrell, M.B.; Weaver, S.R.; Loukas, A.; Creamer, M.; Marti, C.; Jackson, C.D.; Heath, J.; Nayak, P.; Perry, C.; Pechacek, T. Flavored e-cigarette use: Characterizing youth, young adult, and adult users. Prev. Med. Rep. 2017, 5, 33–40. [Google Scholar] [CrossRef]
- Kechter, A.; Wong, M.; Mason, T.B.; Tackett, A.P.; Smith, C.E.; Leventhal, A.M.; Dunton, G.F.; Barrington-Trimis, J.L. E-cigarette weight and appetite control beliefs and e-cigarette initiation in young adults. Health Psychol. 2023, 42, 668. [Google Scholar] [CrossRef]
- Morean, M.E.; Bold, K.W.; Kong, G.; Camenga, D.R.; Simon, P.; Jackson, A.; Cavallo, D.A.; Krishnan-Sarin, S. High school students’ use of flavored e-cigarette e-liquids for appetite control and weight loss. Addict. Behav. 2020, 102, 106139. [Google Scholar] [CrossRef]
- Sapru, S.; Vardhan, M.; Li, Q.; Guo, Y.; Li, X.; Saxena, D. E-cigarettes use in the United States: Reasons for use, perceptions, and effects on health. BMC Public Health 2020, 20, 1518. [Google Scholar] [CrossRef] [PubMed]
- Vasiljevic, M.; Petrescu, D.C.; Marteau, T.M. Impact of advertisements promoting candy-like flavoured e-cigarettes on appeal of tobacco smoking among children: An experimental study. Tob. Control 2016, 25, e107–e112. [Google Scholar] [CrossRef] [PubMed]
- Gorukanti, A.; Delucchi, K.; Ling, P.; Fisher-Travis, R.; Halpern-Felsher, B. Adolescents’ attitudes towards e-cigarette ingredients, safety, addictive properties, social norms, and regulation. Prev. Med. 2017, 94, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, B.K.; Rostron, B.L.; Johnson, S.E.; Portnoy, D.B.; Apelberg, B.J.; Kaufman, A.R.; Choiniere, C.J. Perceptions of the relative harm of cigarettes and e-cigarettes among US youth. Am. J. Prev. Med. 2014, 47, S53–S60. [Google Scholar] [CrossRef] [PubMed]
- Roditis, M.; Delucchi, K.; Cash, D.; Halpern-Felsher, B. Adolescents’ perceptions of health risks, social risks, and benefits differ across tobacco products. J. Adolesc. Health 2016, 58, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Amrock, S.M.; Lee, L.; Weitzman, M. Perceptions of e-cigarettes and noncigarette tobacco products among US youth. Pediatrics 2016, 138, e20154306. [Google Scholar] [CrossRef]
- Choi, K.; Forster, J.L. Beliefs and experimentation with electronic cigarettes: A prospective analysis among young adults. Am. J. Prev. Med. 2014, 46, 175–178. [Google Scholar] [CrossRef] [PubMed]
- FDA Finalizes Enforcement Policy on Unauthorized Flavored Cartridge-Based e-Cigarettes That Appeal to Children, Including Fruit and Mint. Available online: https://www.fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-based-e-cigarettes-appeal-children (accessed on 5 December 2023).
- Gaiha, S.M.; Lempert, L.K.; McKelvey, K.; Halpern-Felsher, B. E-Cigarette devices, brands, and flavors attract youth: Informing FDA’s policies and priorities to close critical gaps. Addict. Behav. 2022, 126, 107179. [Google Scholar] [CrossRef] [PubMed]
- Williams, R. The rise of disposable JUUL-type e-cigarette devices. Tob. Control 2020, 29, e134–e135. [Google Scholar] [CrossRef]
- Amalia, B.; Fu, M.; Tigova, O.; Ballbè, M.; Paniello-Castillo, B.; Castellano, Y.; Vyzikidou, V.K.; O’donnell, R.; Dobson, R.; Lugo, A. Exposure to secondhand aerosol from electronic cigarettes at homes: A real-life study in four European countries. Sci. Total Environ. 2023, 854, 158668. [Google Scholar] [CrossRef]
- Ballbè, M.; Fu, M.; Masana, G.; Pérez-Ortuño, R.; Gual, A.; Gil, F.; Olmedo, P.; García-Algar, Ó.; Pascual, J.A.; Fernández, E. Passive exposure to electronic cigarette aerosol in pregnancy: A case study of a family. Environ. Res. 2023, 216, 114490. [Google Scholar] [CrossRef] [PubMed]
- Quintana, P.J.; Lopez-Galvez, N.; Dodder, N.G.; Hoh, E.; Matt, G.E.; Zakarian, J.M.; Vyas, M.; Chu, L.; Akins, B.; Padilla, S. Nicotine, cotinine, and tobacco-specific nitrosamines measured in children’s silicone wristbands in relation to secondhand smoke and E-cigarette vapor exposure. Nicotine Tob. Res. 2021, 23, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Goniewicz, M.L.; Lee, L. Electronic cigarettes are a source of thirdhand exposure to nicotine. Nicotine Tob. Res. 2015, 17, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, P.; Spiller, H.A.; Casavant, M.J.; Chounthirath, T.; Smith, G.A. E-cigarette and liquid nicotine exposures among young children. Pediatrics 2018, 141, e20173361. [Google Scholar] [CrossRef] [PubMed]
- Jackler, R.K.; Ramamurthi, D. Nicotine arms race: JUUL and the high-nicotine product market. Tob. Control 2019, 28, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Maina, G.; Castagnoli, C.; Ghione, G.; Passini, V.; Adami, G.; Filon, F.L.; Crosera, M. Skin contamination as pathway for nicotine intoxication in vapers. Toxicol. Vitr. 2017, 41, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Plonka, P.M.; Passeron, T.; Brenner, M.; Tobin, D.J.; Shibahara, S.; Thomas, A.; Slominski, A.; Kadekaro, A.L.; Hershkovitz, D.; Peters, E. What are melanocytes really doing all day long…? Exp. Dermatol. 2009, 18, 799–819. [Google Scholar] [CrossRef] [PubMed]
- ElObeid, A.S.; Kamal-Eldin, A.; Abdelhalim, M.A.K.; Haseeb, A.M. Pharmacological properties of melanin and its function in health. Basic Clin. Pharmacol. Toxicol. 2017, 120, 515–522. [Google Scholar] [CrossRef]
- Tolleson, W.H. Human melanocyte biology, toxicology, and pathology. J. Environ. Sci. Health Part C 2005, 23, 105–161. [Google Scholar] [CrossRef]
- Jin, W.; Stehbens, S.J.; Barnard, R.T.; Blaskovich, M.A.; Ziora, Z.M. Dysregulation of tyrosinase activity: A potential link between skin disorders and neurodegeneration. J. Pharm. Pharmacol. 2023, 76, 13–22. [Google Scholar] [CrossRef]
- Whittington, J.R.; Simmons, P.M.; Phillips, A.M.; Gammill, S.K.; Cen, R.; Magann, E.F.; Cardenas, V.M. The use of electronic cigarettes in pregnancy: A review of the literature. Obstet. Gynecol. Surv. 2018, 73, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Li, G.E.; Chen, H.; Cranfield, C.G.; McGrath, K.C.; Gorrie, C.A. Maternal e-cigarette exposure results in cognitive and epigenetic alterations in offspring in a mouse model. Chem. Res. Toxicol. 2018, 31, 601–611. [Google Scholar] [CrossRef] [PubMed]
- England, L.J.; Bunnell, R.E.; Pechacek, T.F.; Tong, V.T.; McAfee, T.A. Nicotine and the developing human: A neglected element in the electronic cigarette debate. Am. J. Prev. Med. 2015, 49, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, P.T.; Holloway, A.C.; Vijayan, M.M. Vape flavourants dull sensory perception and cause hyperactivity in developing zebrafish embryos. Biol. Lett. 2020, 16, 20200361. [Google Scholar] [CrossRef] [PubMed]
- Massarsky, A.; Abdel, A.; Glazer, L.; Levin, E.D.; Di Giulio, R.T. Neurobehavioral effects of 1, 2-propanediol in zebrafish (Danio rerio). Neurotoxicology 2018, 65, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Massarsky, A.; Abdel, A.; Glazer, L.; Levin, E.D.; Di Giulio, R.T. Exposure to 1, 2-propanediol impacts early development of zebrafish (Danio rerio) and induces hyperactivity. Zebrafish 2017, 14, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Delijewski, M.; Wrześniok, D.; Beberok, A.; Rok, J.; Otręba, M.; Buszman, E. The effect of simultaneous exposure of HEMn-DP and HEMn-LP melanocytes to nicotine and UV-radiation on the cell viability and melanogenesis. Environ. Res. 2016, 151, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Delijewski, M.; Wrześniok, D.; Otręba, M.; Beberok, A.; Buszman, E. Nicotine impact on melanogenesis and antioxidant defense system in HEMn-DP melanocytes. Mol. Cell. Biochem. 2014, 395, 109–116. [Google Scholar] [CrossRef]
- Delijewski, M.; Beberok, A.; Otręba, M.; Wrześniok, D.; Rok, J.; Buszman, E. Effect of nicotine on melanogenesis and antioxidant status in HEMn-LP melanocytes. Environ. Res. 2014, 134, 309–314. [Google Scholar] [CrossRef]
- Goenka, S. Biological impact of the ratio of e-cigarette liquid base constituents, propylene glycol and vegetable glycerin, on primary human melanocytes. Oral 2023, 3, 40–56. [Google Scholar] [CrossRef]
- Cambron, C. Racial/Ethnic Differences in Vaping Product Use among Youth: A State-Level Analysis. Int. J. Environ. Res. Public Health 2023, 20, 5729. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Ramos, A.K.; Faseru, B.; Hill, J.L.; Sussman, S.Y. Racial disparities of e-cigarette use among US youths: 2014–2019. Am. J. Public Health 2021, 111, 2050–2058. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.; Bluestein, M.; Chen, B.; Perry, C.L.; Harrell, M.B. Prospectively estimating the age of initiation of e-cigarettes among US youth: Findings from the population assessment of tobacco and health (PATH) study, 2013–2017. J. Biom. Biostat. 2020, 11. [Google Scholar]
- Harlow, A.F.; Stokes, A.; Brooks, D.R. Socioeconomic and racial/ethnic differences in e-cigarette uptake among cigarette smokers: Longitudinal analysis of the population assessment of tobacco and health (PATH) study. Nicotine Tob. Res. 2019, 21, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Lippert, A.M. Do adolescent smokers use e-cigarettes to help them quit? The sociodemographic correlates and cessation motivations of US adolescent e-cigarette use. Am. J. Health Promot. 2015, 29, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Dutra, L.M.; Glantz, S.A. Electronic cigarettes and conventional cigarette use among US adolescents: A cross-sectional study. JAMA Pediatr. 2014, 168, 610–617. [Google Scholar] [CrossRef]
- Baumann, A.W.; Kohler, C.; Kim, Y.-i.; Cheong, J.; Hendricks, P.; Bailey, W.C.; Harrington, K.F. Differences in electronic cigarette awareness, use history, and advertisement exposure between black and white hospitalized cigarette smokers. J. Cancer Educ. 2015, 30, 648–654. [Google Scholar] [CrossRef]
- Muscat, J.; Richie, J.; Stellman, S. Mentholated cigarettes and smoking habits in whites and blacks. Tob. Control 2002, 11, 368. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Marumoto, S.; Takahashi, T.; Nakahashi, H.; Haigou, R.; Nakanishi, K. Metabolism of (+)-and (−)-menthols by CYP2A6 in human liver microsomes. J. Oleo Sci. 2011, 60, 127–132. [Google Scholar] [CrossRef]
- Winters, B.R.; Kochar, T.K.; Clapp, P.W.; Jaspers, I.; Madden, M.C. Impact of e-cigarette liquid flavoring agents on activity of microsomal recombinant CYP2A6, the primary nicotine-metabolizing enzyme. Chem. Res. Toxicol. 2020, 33, 1689–1697. [Google Scholar] [CrossRef]
- King, G.; Yerger, V.B.; Whembolua, G.-L.; Bendel, R.B.; Kittles, R.; Moolchan, E.T. Link between facultative melanin and tobacco use among African Americans. Pharmacol. Biochem. Behav. 2009, 92, 589–596. [Google Scholar] [CrossRef]
- Yerger, V.B.; Malone, R.E. Melanin and nicotine: A review of the literature. Nicotine Tob. Res. 2006, 8, 487–498. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Larsson, B.S. Interaction between chemicals and melanin. Pigment Cell Res. 1993, 6, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. Effects of e-cigarette refill liquid flavorings with and without nicotine on human retinal pigment epithelial cells: A preliminary study. Int. J. Environ. Res. Public Health 2021, 18, 11655. [Google Scholar] [CrossRef]
- Human Epidermal Melanocytes, Neonatal, Lightly Pigmented Donor, (HEMn-LP). Available online: https://www.thermofisher.com/order/catalog/product/C0025C?SID=srch-srp-C0025C (accessed on 23 October 2023).
- Human Epidermal Melanocytes, Neonatal, Darkly Pigmented Donor, (HEMn-DP). Available online: https://www.thermofisher.com/order/catalog/product/C2025C (accessed on 19 December 2023).
- Muthumalage, T.; Prinz, M.; Ansah, K.O.; Gerloff, J.; Sundar, I.K.; Rahman, I. Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front. Physiol. 2017, 8, 1130. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, D.; Minor, M.; Chu, H.W. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS ONE 2014, 9, e108342. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S. Effects of serotype and species dependency of bacterial lipopolysaccharides in human melanocytes from lightly and darkly-pigmented skin. BBA Adv. 2022, 2, 100042. [Google Scholar] [CrossRef] [PubMed]
- McAlinden, K.D.; Lu, W.; Ferdowsi, P.V.; Myers, S.; Markos, J.; Larby, J.; Chia, C.; Weber, H.C.; Haug, G.; Eapen, M.S. Electronic cigarette aerosol is cytotoxic and increases ACE2 expression on human airway epithelial cells: Implications for SARS-CoV-2 (COVID-19). J. Clin. Med. 2021, 10, 1028. [Google Scholar] [CrossRef]
- Golli, N.E.; Dallagi, Y.; Rahali, D.; Rejeb, I.; Fazaa, S.E. Neurobehavioral assessment following e-cigarette refill liquid exposure in adult rats. Toxicol. Mech. Methods 2016, 26, 425–432. [Google Scholar] [CrossRef]
- Thomas, A.J.; Erickson, C.A. The making of a melanocyte: The specification of melanoblasts from the neural crest. Pigment Cell Melanoma Res. 2008, 21, 598–610. [Google Scholar] [CrossRef]
- Sommer, L. Generation of melanocytes from neural crest cells. Pigment Cell Melanoma Res. 2011, 24, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.E.; Kandalam, S.; Olivares-Navarrete, R.; Dickinson, A.J. E-cigarette aerosol exposure can cause craniofacial defects in Xenopus laevis embryos and mammalian neural crest cells. PLoS ONE 2017, 12, e0185729. [Google Scholar] [CrossRef] [PubMed]
- Ween, M.P.; Moshensky, A.; Thredgold, L.; Bastian, N.A.; Hamon, R.; Badiei, A.; Nguyen, P.T.; Herewane, K.; Jersmann, H.; Bojanowski, C.M. E-cigarettes and health risks: More to the flavor than just the name. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2021, 320, L600–L614. [Google Scholar] [CrossRef]
- Sherwood, C.L.; Boitano, S. Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine. Respir. Res. 2016, 17, 57. [Google Scholar] [CrossRef]
- Kechter, A.; Ceasar, R.C.; Simpson, K.A.; Schiff, S.J.; Dunton, G.F.; Bluthenthal, R.N.; Barrington-Trimis, J.L. A chocolate cake or a chocolate vape? Young adults describe their relationship with food and weight in the context of nicotine vaping. Appetite 2022, 175, 106075. [Google Scholar] [CrossRef] [PubMed]
- Litt, M.D.; Duffy, V.; Oncken, C. Cigarette smoking and electronic cigarette vaping patterns as a function of e-cigarette flavourings. Tob. Control 2016, 25, ii67–ii72. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Narain, V.; Bondesson, M. E-cigarette vaping liquids and the flavoring chemical cinnamaldehyde perturb bone, cartilage and vascular development in zebrafish embryos. Aquat. Toxicol. 2021, 240, 105995. [Google Scholar] [CrossRef]
- Behar, R.; Davis, B.; Wang, Y.; Bahl, V.; Lin, S.; Talbot, P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol. Vitr. 2014, 28, 198–208. [Google Scholar] [CrossRef]
- Clapp, P.W.; Pawlak, E.A.; Lackey, J.T.; Keating, J.E.; Reeber, S.L.; Glish, G.L.; Jaspers, I. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L278–L292. [Google Scholar] [CrossRef]
- Cabello, C.M.; Bair III, W.B.; Lamore, S.D.; Ley, S.; Bause, A.S.; Azimian, S.; Wondrak, G.T. The cinnamon-derived Michael acceptor cinnamic aldehyde impairs melanoma cell proliferation, invasiveness, and tumor growth. Free Radic. Biol. Med. 2009, 46, 220–231. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, J.; Cheng, C.; Zhang, M.; Liu, W.; Ma, X.; Lei, W.; Hao, E.; Hou, X.; Hou, Y. Cinnamaldehyde enhances antimelanoma activity through covalently binding ENO1 and exhibits a promoting effect with dacarbazine. Cancers 2020, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Behar, R.Z.; Luo, W.; Lin, S.C.; Wang, Y.; Valle, J.; Pankow, J.F.; Talbot, P. Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols. Tob. Control 2016, 25, ii94–ii102. [Google Scholar] [CrossRef] [PubMed]
- Behar, R.Z.; Wang, Y.; Talbot, P. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob. Control 2018, 27, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Omaiye, E.E.; McWhirter, K.J.; Luo, W.; Tierney, P.A.; Pankow, J.F.; Talbot, P. High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci. Rep. 2019, 9, 2468. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lim, K.-M. Melanocytotoxic chemicals and their toxic mechanisms. Toxicol. Res. 2022, 38, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, B.; Wilkinson, D. Cinnamic aldehyde in toothpaste. 1. Clinical aspects and patch tests. Contact Dermat. 1975, 1, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Isaac-Renton, M.; Li, M.K.; Parsons, L.M. Cinnamon spice and everything not nice: Many features of intraoral allergy to cinnamic aldehyde. Dermatitis 2015, 26, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Mathias, C.T.; Maibach, H.I.; Conant, M.A. Perioral leukoderma simulating vitiligo from use of a toothpaste containing cinnamic aldehyde. Arch. Dermatol. 1980, 116, 1172–1173. [Google Scholar] [CrossRef]
- Vahav, I.; Thon, M.; van den Broek, L.J.; Spiekstra, S.W.; Atac, B.; Lindner, G.; Schimek, K.; Marx, U.; Gibbs, S. Proof-of-concept organ-on-chip study: Topical cinnamaldehyde exposure of reconstructed human skin with integrated neopapillae cultured under dynamic flow. Pharmaceutics 2022, 14, 1529. [Google Scholar] [CrossRef]
- Chang, K.; Zeng, N.; Ding, Y.; Zhao, X.; Gao, C.; Li, Y.; Wang, H.; Liu, X.; Niu, Y.; Sun, Y. Cinnamaldehyde causes developmental neurotoxicity in zebrafish via the oxidative stress pathway that is rescued by astaxanthin. Food Funct. 2022, 13, 13028–13039. [Google Scholar] [CrossRef]
- Holden, L.L.; Truong, L.; Simonich, M.T.; Tanguay, R.L. Assessing the hazard of E-Cigarette flavor mixtures using zebrafish. Food Chem. Toxicol. 2020, 136, 110945. [Google Scholar] [CrossRef] [PubMed]
- Berkelhamer, S.K.; Helman, J.M.; Gugino, S.F.; Leigh, N.J.; Lakshminrusimha, S.; Goniewicz, M.L. In vitro consequences of electronic-cigarette flavoring exposure on the immature lung. Int. J. Environ. Res. Public Health 2019, 16, 3635. [Google Scholar] [CrossRef] [PubMed]
- Herbert, J.; Kelty, J.S.; Laskin, J.D.; Laskin, D.L.; Gow, A.J. Menthol flavoring in e-cigarette condensate causes pulmonary dysfunction and cytotoxicity in precision cut lung slices. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2023, 324, L345–L357. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.C.; Donovan, E.M.; Schillo, B.A.; Vallone, D. Menthol e-cigarette sales rise following 2020 FDA guidance. Tob. Control 2021, 30, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Diomede, L. The soothing effect of menthol, eucalyptol and high-intensity cooling agents. Int. J. Nutraceuticals Funct. Foods Nov. Foods 2017, 16, 153–157. [Google Scholar]
- Davis, D.R.; Morean, M.E.; Bold, K.W.; Camenga, D.; Kong, G.; Jackson, A.; Simon, P.; Krishnan-Sarin, S. Cooling e-cigarette flavors and the association with e-cigarette use among a sample of high school students. PLoS ONE 2021, 16, e0256844. [Google Scholar] [CrossRef] [PubMed]
- Paschke, M.; Tkachenko, A.; Ackermann, K.; Hutzler, C.; Henkler, F.; Luch, A. Activation of the cold-receptor TRPM8 by low levels of menthol in tobacco products. Toxicol. Lett. 2017, 271, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Chen, H.; Zhang, X.; Liu, T.; Fu, Y.n. Levels of selected groups of compounds in refill solutions for electronic cigarettes. Nicotine Tob. Res. 2016, 18, 708–714. [Google Scholar] [CrossRef]
- Ramamurthi, D.; Chau, C.; Berke, H.Y.; Tolba, A.M.; Yuan, L.; Kanchan, V.; Santos, G.; Jackler, R.K. Flavour spectrum of the Puff family of disposable e-cigarettes. Tob. Control 2022, 32, e71–e77. [Google Scholar] [CrossRef] [PubMed]
- Churchill, V.; Fairman, R.T.; Brown, D.; Massey, Z.B.; Ashley, D.L.; Popova, L. “I get the flavors and it makes me love vaping more:” How and why youth users modify electronic nicotine delivery systems. Nicotine Tob. Res. 2023, 25, 1791–1797. [Google Scholar] [CrossRef]
- Bengalli, R.; Ferri, E.; Labra, M.; Mantecca, P. Lung toxicity of condensed aerosol from E-CIG liquids: Influence of the flavor and the in vitro model used. Int. J. Environ. Res. Public Health 2017, 14, 1254. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthi, D.; Chau, C.; Jackler, R.K. JUUL and other stealth vaporisers: Hiding the habit from parents and teachers. Tob. Control 2019, 28, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Kerber, P.J.; Duell, A.K.; Powers, M.; Strongin, R.M.; Peyton, D.H. Effects of common e-liquid flavorants and added nicotine on toxicant formation during vaping analyzed by 1H NMR spectroscopy. Chem. Res. Toxicol. 2022, 35, 1267–1276. [Google Scholar] [CrossRef]
- Barltrop, J.A.; Owen, T.C.; Cory, A.H.; Cory, J.G. 5-(3-carboxymethoxyphenyl)-2-(4, 5-dimethylthiazolyl)-3-(4-sulfophenyl) tetrazolium, inner salt (MTS) and related analogs of 3-(4, 5-dimethylthiazolyl)-2, 5-diphenyltetrazolium bromide (MTT) reducing to purple water-soluble formazans as cell-viability indicators. Bioorg. Med. Chem. Lett. 1991, 1, 611–614. [Google Scholar]
- Malich, G.; Markovic, B.; Winder, C. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology 1997, 124, 179–192. [Google Scholar] [CrossRef]
- Beard, J.M.; Collom, C.; Liu, J.Y.; Obiako, P.; Strongin, R.M.; Zavala, J.; Sayes, C.M. In vitro toxicity and chemical analysis of e-cigarette aerosol produced amid dry hitting. Toxicology 2024, 506, 153865. [Google Scholar] [CrossRef] [PubMed]
- Baldovinos, Y.; Archer, A.; Salamanca, J.; Strongin, R.M.; Sayes, C.M. Chemical interactions and cytotoxicity of terpene and diluent vaping ingredients. Chem. Res. Toxicol. 2022, 36, 589–597. [Google Scholar] [CrossRef]
- Rickard, B.P.; Ho, H.; Tiley, J.B.; Jaspers, I.; Brouwer, K.L. E-cigarette flavoring chemicals induce cytotoxicity in HepG2 cells. ACS Omega 2021, 6, 6708–6713. [Google Scholar] [CrossRef]
- Chan, F.K.-M.; Moriwaki, K.; De Rosa, M.J. Detection of necrosis by release of lactate dehydrogenase activity. In Immune Homeostasis: Methods and Protocols; Springer: New York, NY, USA, 2013; pp. 65–70. [Google Scholar]
- Puig-Herreros, C.; Sanz, J.L.; García-Bernal, D.; Rodríguez-Lozano, F.J.; Murcia, L.; Forner, L.; Ghilotti, J.; Oñate-Sánchez, R.E.; López-García, S. Comparative Cytotoxicity of Menthol and Eucalyptol: An In Vitro Study on Human Gingival Fibroblasts. Pharmaceutics 2024, 16, 521. [Google Scholar] [CrossRef]
- Alshareef, M.; Alrafiah, A.; Abed, S.; Basingab, F.; Alrofaidi, A. Effect of e-cigarette flavoring agents on the neural retina of chick embryo: Histological and gene expression study. Folia Histochem. Cytobiol. 2021, 59, 245–258. [Google Scholar] [CrossRef]
- Abouassali, O.; Chang, M.; Chidipi, B.; Martinez, J.L.; Reiser, M.; Kanithi, M.; Soni, R.; McDonald, T.V.; Herweg, B.; Saiz, J. In vitro and in vivo cardiac toxicity of flavored electronic nicotine delivery systems. Am. J. Physiol.-Heart Circ. Physiol. 2021, 320, H133–H143. [Google Scholar] [CrossRef] [PubMed]
- Girvalaki, C.; Tzatzarakis, M.; Kyriakos, C.N.; Vardavas, A.I.; Stivaktakis, P.D.; Kavvalakis, M.; Tsatsakis, A.; Vardavas, C. Composition and chemical health hazards of the most common electronic cigarette liquids in nine European countries. Inhal. Toxicol. 2018, 30, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Krüsemann, E.J.; Pennings, J.L.; Cremers, J.W.; Bakker, F.; Boesveldt, S.; Talhout, R. GC–MS analysis of e-cigarette refill solutions: A comparison of flavoring composition between flavor categories. J. Pharm. Biomed. Anal. 2020, 188, 113364. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Go, Y.Y.; Mun, J.Y.; Lee, S.; Im, G.J.; Kim, Y.Y.; Lee, J.H.; Chang, J. Effect of electronic cigarettes on human middle ear. Int. J. Pediatr. Otorhinolaryngol. 2018, 109, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Dunn, K.; Turfus, S. A review of nicotine-containing electronic cigarettes—Trends in use, effects, contents, labelling accuracy and detection methods. Drug Test. Anal. 2021, 13, 242–260. [Google Scholar] [CrossRef] [PubMed]
- Gschwend, G.; Jenkins, C.; Jones, A.; Kelso, C.; Morgan, J. A wide range of flavoring–carrier fluid adducts form in e-cigarette liquids. Chem. Res. Toxicol. 2023, 36, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Suarez, I.; Marescotti, D.; Martin, F.; Scotti, E.; Guedj, E.; Acali, S.; Dulize, R.; Baumer, K.; Peric, D.; Frentzel, S. In vitro systems toxicology assessment of nonflavored e-cigarette liquids in primary lung epithelial cells. Appl. Vitr. Toxicol. 2017, 3, 41–55. [Google Scholar] [CrossRef]
- De Martin, S.; Gabbia, D.; Bogialli, S.; Biasioli, F.; Boschetti, A.; Gstir, R.; Rainer, D.; Cappellin, L. Refill liquids for electronic cigarettes display peculiar toxicity on human endothelial cells. Toxicol. Rep. 2021, 8, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Rowell, T.R.; Reeber, S.L.; Lee, S.L.; Harris, R.A.; Nethery, R.C.; Herring, A.H.; Glish, G.L.; Tarran, R. Flavored e-cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L52–L66. [Google Scholar] [CrossRef]
- Goniewicz, M.L.; Boykan, R.; Messina, C.R.; Eliscu, A.; Tolentino, J. High exposure to nicotine among adolescents who use Juul and other vape pod systems (‘pods’). Tob. Control 2019, 28, 676–677. [Google Scholar] [CrossRef]
- Talih, S.; Salman, R.; El-Hage, R.; Karam, E.; Karaoghlanian, N.; El-Hellani, A.; Saliba, N.; Shihadeh, A. Characteristics and toxicant emissions of JUUL electronic cigarettes. Tob. Control 2019, 28, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Mr. Fog E-Liquids. Available online: https://www.mrfog.com/e-liquid/?f=nav&site=com (accessed on 12 December 2023).
- Marland Vape Store: Mr. Fog Switch 5500 E-Liquids (20 mg/mL). Available online: https://marlandvape.com/?product_cat=&s=Fog&post_type=product (accessed on 12 December 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).