Complements from the Male Reproductive Tract: A Scoping Review
Abstract
1. Introduction
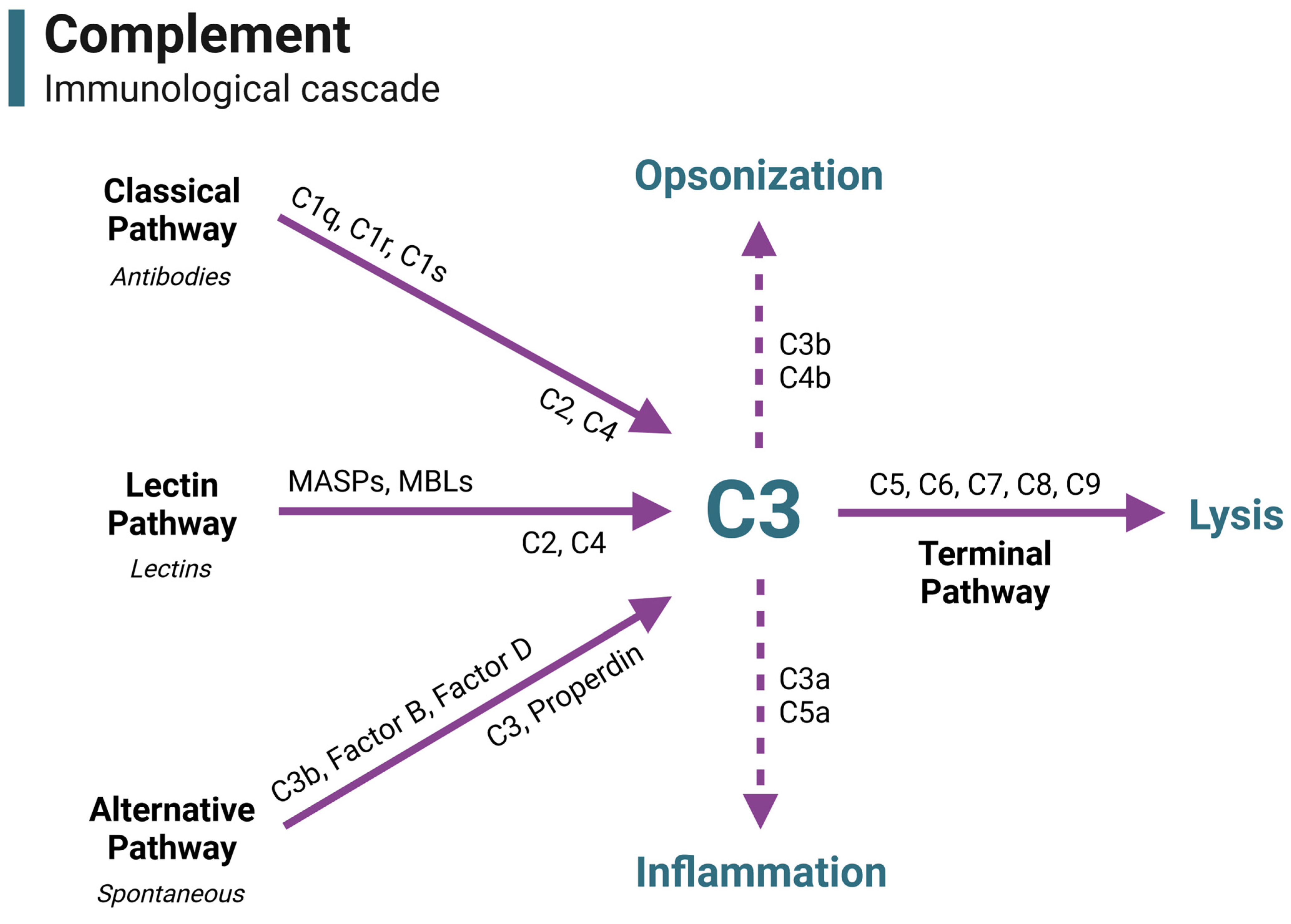
2. A Brief Overview of Complement
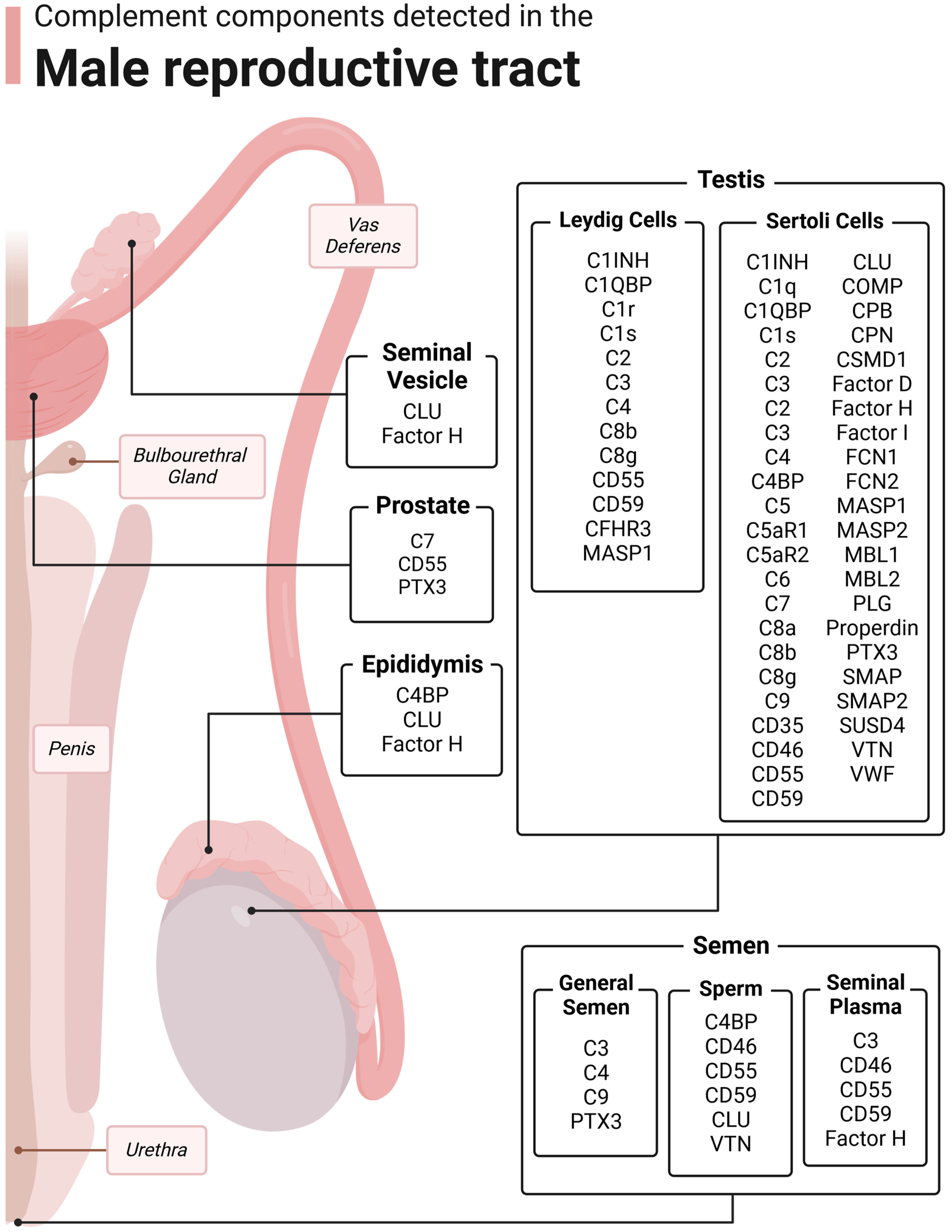
| Complement Component | Organ Expression (Male Reproductive) | Potential Functions in Male Reproductive Tract |
|---|---|---|
| C1 inhibitor (C1INH) 1,2 | Testis | Inhibition of female genital tract complement |
| C1q 1 | Testis | Classical pathway |
| C1q-binding protein (C1QBP) 1,2 | Testis | Inhibition of female genital tract complement |
| C1r 2 | Testis | Classical pathway |
| C1s 1,2 | Testis | Classical pathway |
| C2 1,2 | Testis | Classical and lectin pathways |
| C3 1,2,3 | Testis, semen | Alternative pathway and complement cascade, modulation of female reproductive immunity, sperm–oocyte interaction |
| C4 1,2,3 | Testis, semen | Classical and lectin pathways |
| C4-binding protein (C4BP) 1,4 | Testis, epididymis, semen | Inhibition of female genital tract complement, modulation of female reproductive immunity |
| C5 1 | Testis | Terminal pathway, immune cell modulation |
| C5a receptor 1 (C5aR1) 1 | Testis | Anaphylatoxin receptor, immune cell activation, inflammation |
| C5a receptor 2 (C5aR2) 1 | Testis | Anaphylatoxin receptor, immune cell modulation |
| C6 1 | Testis | Terminal pathway |
| C7 1,5 | Testis, prostate | Terminal pathway |
| C8a 1 | Testis | Terminal pathway |
| C8b 1,2 | Testis | Terminal pathway |
| C8c 1,2 | Testis | Terminal pathway |
| C9 1,3 | Testis, semen | Terminal pathway |
| CD35 1 | Testis | Inhibition of female genital tract complement, immune modulation |
| CD46 1,6,7,8 | Testis, semen | Inhibition of female genital tract complement, immune modulation, sperm–oocyte fusion |
| CD55 1,2,7,8 | Testis, prostate, semen | Inhibition of female genital tract complement, immune modulation |
| CD59 1,2,3,7,8 | Testis, semen | Inhibition of female genital tract complement |
| Complement Factor-H-related protein 3 (CFHR3)8 | Testis | Inhibition of female genital tract complement |
| Clusterin (CLU) 1,9 | Testis, epididymis, seminal vesicle | Inhibition of female genital tract complement, sperm capacitation, sperm viability, sperm maturation and development |
| Cartilage oligomeric matrix protein (COMP) 1 | Testis | Inhibition of female genital tract complement |
| Carboxypeptidase B (CPB) 1 | Testis | Inhibition of anaphylatoxins in female genital tract |
| Carboxypeptidase N (CPN) 1 | Testis | Inhibition of anaphylatoxins in female genital tract |
| Cub and Sushi Multiple Domains 1 (CSMD1) 1 | Testis | Inhibition of female genital tract complement |
| Factor D 1 | Testis | Alternative pathway |
| Factor H 1,10 | Testis, epididymis, seminal vesicles, semen | Inhibition of female genital tract complement |
| Factor I 1 | Testis | Inhibition of female genital tract complement |
| Ficolin 1/2 (FCN1/2) 1 | Testis | Lectin pathway |
| MBL-associated serine protease 1/2 (MASP1/2) 1,2 | Testis | Lectin pathway |
| Mannose-binding lectin 1/2 (MBL1/2) 1 | Testis | Lectin pathway |
| Plasminogen (PLG) 1 | Testis | Inhibition of female genital tract complement |
| Properdin 1 | Testis | Alternative pathway |
| Pentraxin (PTX3) 1,11 | Testis, prostate, semen | Inhibition of female genital tract complement |
| Soluble MBL-associated protein 1/2 (SMAP1/2) 1 | Testis | Inhibition of female genital tract complement |
| Sushi domain-containing protein 4 (SUSD4) 1 | Testis | Inhibition of female genital tract complement, may play a role in immune tolerance |
| Vitronectin (VTN) 1,12 | Testis, semen | Inhibition of female genital tract complement, may influence acrosome reaction but needs investigation |
| Von Willebrand Factor (VWF) 1 | Testis | Inhibition of female genital tract complement |
3. Complement in the Testis
3.1. Sertoli Cells
3.2. Leydig Cells
3.3. Macrophages
3.4. Germ Cells
4. Complement in the Epididymis
5. Complement in the Prostate
6. Complement in the Seminal Vesicles
7. Complement in Semen
7.1. Whole Semen
7.2. Seminal Plasma
7.3. Sperm/Germ Cells
8. Clinical Applications for Complement
8.1. Complement Action in Sperm Health and Fertilization
8.2. Complement Therapeutics in Brief
9. Discussion
- The roles of anaphylatoxin signaling in the gonads;
- Functional investigation into genes affected by robust complement exposure;
- Complement expressed by testicular macrophages;
- The effects of C4BP secreted by epididymal cells on sperm maturation;
- Complement in the healthy prostate;
- The specific function of seminal vesicle-derived Factor H;
- A role for PTX3 in sperm maturation;
- Noncanonical CLU functions in seminal plasma, sperm motility, and fertilization;
- The effects of C4BP on sperm in fertilization and sperm health;
- The function of vitronectin on the acrosome.
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia, P.C.; Rubio, E.M.; Pereira, O.C.M. Antisperm antibodies in infertile men and their correlation with seminal parameters. Reprod. Med. Biol. 2007, 6, 33–38. [Google Scholar] [CrossRef]
- Washburn, R.L.; Hibler, T.; Kaur, G.; Dufour, J.M. Sertoli Cell Immune Regulation: A Double-Edged Sword. Front. Immunol. 2022, 13, 913502. [Google Scholar] [CrossRef]
- Harris, C.L.; Mizuno, M.; Morgan, B.P. Complement and complement regulators in the male reproductive system. Mol. Immunol. 2006, 43, 57–67. [Google Scholar] [CrossRef]
- Dierich, M.P.; Erdei, A.; Huemer, H.; Petzer, A.; Stauder, R.; Schulz, T.F.; Gergely, J. Involvement of complement in B-cell, T-cell and monocyte/macrophage activation. Immunol. Lett. 1987, 14, 235–242. [Google Scholar] [CrossRef]
- Wagner, C.; Ochmann, C.; Schoels, M.; Giese, T.; Stegmaier, S.; Richter, R.; Hug, F.; Hänsch, G.M. The complement receptor 1, CR1 (CD35), mediates inhibitory signals in human T-lymphocytes. Mol. Immunol. 2006, 43, 643–651. [Google Scholar] [CrossRef]
- Kemper, C.; Atkinson, J.P. T-cell regulation: With complements from innate immunity. Nat. Rev. Immunol. 2007, 7, 9–18. [Google Scholar] [CrossRef]
- Heeger, P.S.; Lalli, P.N.; Lin, F.; Valujskikh, A.; Liu, J.; Muqim, N.; Xu, Y.; Medof, M.E. Decay-accelerating factor modulates induction of T cell immunity. J. Exp. Med. 2005, 201, 1523–1530. [Google Scholar] [CrossRef]
- Cardone, J.; Le Friec, G.; Vantourout, P.; Roberts, A.; Fuchs, A.; Jackson, I.; Suddason, T.; Lord, G.; Atkinson, J.P.; Cope, A.; et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat. Immunol. 2010, 11, 862–871. [Google Scholar] [CrossRef]
- West, E.E.; Kolev, M.; Kemper, C. Complement and the Regulation of T Cell Responses. Annu. Rev. Immunol. 2018, 36, 309–338. [Google Scholar] [CrossRef]
- Merle, N.S.; Noe, R.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part II: Role in Immunity. Front. Immunol. 2015, 6, 257. [Google Scholar] [CrossRef]
- Guo, R.F.; Ward, P.A. Role of C5a in inflammatory responses. Annu. Rev. Immunol. 2005, 23, 821–852. [Google Scholar] [CrossRef]
- Peng, Q.; Li, K.; Patel, H.; Sacks, S.H.; Zhou, W. Dendritic cell synthesis of C3 is required for full T cell activation and development of a Th1 phenotype. J. Immunol. 2006, 176, 3330–3341. [Google Scholar] [CrossRef]
- Strainic, M.G.; Shevach, E.M.; An, F.; Lin, F.; Medof, M.E. Absence of signaling into CD4⁺ cells via C3aR and C5aR enables autoinductive TGF-β1 signaling and induction of Foxp3⁺ regulatory T cells. Nat. Immunol. 2013, 14, 162–171. [Google Scholar] [CrossRef]
- Arbore, G.; West, E.E.; Rahman, J.; Le Friec, G.; Niyonzima, N.; Pirooznia, M.; Tunc, I.; Pavlidis, P.; Powell, N.; Li, Y.; et al. Complement receptor CD46 co-stimulates optimal human CD8(+) T cell effector function via fatty acid metabolism. Nat. Commun. 2018, 9, 4186. [Google Scholar] [CrossRef]
- Stephan, A.H.; Barres, B.A.; Stevens, B. The Complement System: An Unexpected Role in Synaptic Pruning During Development and Disease. Annu. Rev. Neurosci. 2012, 35, 369–389. [Google Scholar] [CrossRef]
- Veerhuis, R.; Nielsen, H.M.; Tenner, A.J. Complement in the brain. Mol. Immunol. 2011, 48, 1592–1603. [Google Scholar] [CrossRef]
- West, E.E.; Kemper, C. Complosome—The intracellular complement system. Nat. Rev. Nephrol. 2023, 19, 426–439. [Google Scholar] [CrossRef]
- Washburn, R.L.; Martinez-Marin, D.; Korać, K.; Sniegowski, T.; Rodriguez, A.R.; Chilton, B.S.; Hibler, T.; Pruitt, K.; Bhutia, Y.D.; Dufour, J.M. The Sertoli Cell Complement Signature: A Suspected Mechanism in Xenograft Survival. Int. J. Mol. Sci. 2023, 24, 1890. [Google Scholar] [CrossRef]
- Li, Y.; Mi, P.; Wu, J.; Tang, Y.; Liu, X.; Cheng, J.; Huang, Y.; Qin, W.; Cheng, C.Y.; Sun, F. High Throughput scRNA-Seq Provides Insights Into Leydig Cell Senescence Induced by Experimental Autoimmune Orchitis: A Prominent Role of Interstitial Fibrosis and Complement Activation. Front. Immunol. 2021, 12, 771373. [Google Scholar] [CrossRef]
- Blenk, H.; Hofstetter, A. Complement C3, coeruloplasmin and PMN-elastase in the ejaculate in chronic prostato-adnexitis and their diagnostic value. Infection 1991, 19 (Suppl. S3), S138–S140. [Google Scholar] [CrossRef]
- Rahimi, A.; Sepehri, H.; Pakravesh, J.; Bahar, K. Quantification of C3 and C4 in infertile men with antisperm antibody in their seminal plasma. Am. J. Reprod. Immunol. 1999, 41, 330–336. [Google Scholar] [CrossRef]
- Sullivan, H.; Quinlivan, W.L.G. Immunoglobulins in the Semen of Men with Azoospermia, Oligospermia, or Self-Agglutination of Spermatozoa. Fertil. Steril. 1980, 34, 465–468. [Google Scholar] [CrossRef]
- Esther Bozas, S.; Kirszbaum, L.; Sparrow, R.L.; Walker, I.D. Several Vascular Complement Inhibitors are Present on Human Sperm1. Biol. Reprod. 1993, 48, 503–511. [Google Scholar] [CrossRef][Green Version]
- Nonaka, M.I.; Hishikawa, Y.; Moriyama, N.; Koji, T.; Ogata, R.T.; Kudo, A.; Kawakami, H.; Nonaka, M. Complement C4b-binding protein as a novel murine epididymal secretory protein. Biol. Reprod. 2003, 69, 1931–1939. [Google Scholar] [CrossRef]
- Ronca, R.; Alessi, P.; Coltrini, D.; Di Salle, E.; Giacomini, A.; Leali, D.; Corsini, M.; Belleri, M.; Tobia, C.; Garlanda, C.; et al. Long pentraxin-3 as an epithelial-stromal fibroblast growth factor-targeting inhibitor in prostate cancer. J. Pathol. 2013, 230, 228–238. [Google Scholar] [CrossRef]
- Anderson, D.J.; Abbott, A.F.; Jack, R.M. The role of complement component C3b and its receptors in sperm-oocyte interaction. Proc. Natl. Acad. Sci. USA 1993, 90, 10051–10055. [Google Scholar] [CrossRef]
- Hsu, E.C.; Dörig, R.E.; Sarangi, F.; Marcil, A.; Iorio, C.; Richardson, C.D. Artificial mutations and natural variations in the CD46 molecules from human and monkey cells define regions important for measles virus binding. J. Virol. 1997, 71, 6144–6154. [Google Scholar] [CrossRef]
- Cummerson, J.A.; Flanagan, B.F.; Spiller, D.G.; Johnson, P.M. The complement regulatory proteins CD55 (decay accelerating factor) and CD59 are expressed on the inner acrosomal membrane of human spermatozoa as well as CD46 (membrane cofactor protein). Immunology 2006, 118, 333–342. [Google Scholar] [CrossRef]
- Geller, A.; Yan, J. The Role of Membrane Bound Complement Regulatory Proteins in Tumor Development and Cancer Immunotherapy. Front. Immunol. 2019, 10, 1074. [Google Scholar] [CrossRef]
- Jenne, D.E.; Tschopp, J. Clusterin: The intriguing guises of a widely expressed glycoprotein. Trends Biochem. Sci. 1992, 17, 154–159. [Google Scholar] [CrossRef]
- Han, Z.; Wang, Z.; Cheng, G.; Liu, B.; Li, P.; Li, J.; Wang, W.; Yin, C.; Zhang, W. Presence, localization, and origin of clusterin in normal human spermatozoa. J. Assist. Reprod. Genet. 2012, 29, 751–757. [Google Scholar] [CrossRef]
- O’Bryan, M.K.; Baker, H.W.; Saunders, J.R.; Kirszbaum, L.; Walker, I.D.; Hudson, P.; Liu, D.Y.; Glew, M.D.; d’Apice, A.J.; Murphy, B.F. Human seminal clusterin (SP-40,40). Isolation and characterization. J. Clin. Investig. 1990, 85, 1477–1486. [Google Scholar] [CrossRef]
- Janiszewska, E.; Kratz, E.M. Could the glycosylation analysis of seminal plasma clusterin become a novel male infertility biomarker? Mol. Reprod. Dev. 2020, 87, 515–524. [Google Scholar] [CrossRef]
- Sakaue, T.; Takeuchi, K.; Maeda, T.; Yamamoto, Y.; Nishi, K.; Ohkubo, I. Factor H in porcine seminal plasma protects sperm against complement attack in genital tracts. J. Biol. Chem. 2010, 285, 2184–2192. [Google Scholar] [CrossRef]
- Doni, A.; Paffoni, A.; Nebuloni, M.; Ragni, G.; Pasqualini, F.; Valentino, S.; Bonetti, S.; Mantovani, A.; Somigliana, E.; Garlanda, C. The long pentraxin 3 is a soluble and cell-associated component of the human semen. Int. J. Androl. 2009, 32, 255–264. [Google Scholar] [CrossRef]
- Bronson, R.; Peresleni, T.; Golightly, M.; Preissner, K. Vitronectin is sequestered within human spermatozoa and liberated following the acrosome reaction. Mol. Hum. Reprod. 2000, 6, 977–982. [Google Scholar] [CrossRef][Green Version]
- Washburn, R.L.; Dufour, J.M. Complementing Testicular Immune Regulation: The Relationship between Sertoli Cells, Complement, and the Immune Response. Int. J. Mol. Sci. 2023, 24, 3371. [Google Scholar] [CrossRef]
- Chowdhury, N.A.; Kamada, M.; Takikawa, M.; Mori, H.; Gima, H.; Aono, T. Complement-inhibiting activity of human seminal plasma and semen quality. Arch. Androl. 1996, 36, 109–118. [Google Scholar] [CrossRef]
- Luo, C.; Chen, M.; Madden, A.; Xu, H. Expression of Complement Components and Regulators by Different Subtypes of Bone Marrow-Derived Macrophages. Inflammation 2012, 35, 1448–1461. [Google Scholar] [CrossRef]
- Kaur, G.; Wright, K.; Verma, S.; Haynes, A.; Dufour, J.M. The Good, the Bad and the Ugly of Testicular Immune Regulation: A Delicate Balance Between Immune Function and Immune Privilege. Adv. Exp. Med. Biol. 2021, 1288, 21–47. [Google Scholar] [CrossRef]
- Gualdoni, G.S.; Jacobo, P.V.; Sobarzo, C.M.; Pérez, C.V.; Matzkin, M.E.; Höcht, C.; Frungieri, M.B.; Hill, M.; Anegon, I.; Lustig, L.; et al. Role of indoleamine 2,3-dioxygenase in testicular immune-privilege. Sci. Rep. 2019, 9, 15919. [Google Scholar] [CrossRef]
- Skinner, M.K.; Moses, H.L. Transforming growth factor beta gene expression and action in the seminiferous tubule: Peritubular cell-Sertoli cell interactions. Mol. Endocrinol. 1989, 3, 625–634. [Google Scholar] [CrossRef]
- França, L.R.; Hess, R.A.; Dufour, J.M.; Hofmann, M.C.; Griswold, M.D. The Sertoli cell: One hundred fifty years of beauty and plasticity. Andrology 2016, 4, 189–212. [Google Scholar] [CrossRef]
- Dufour, J.M.; Hamilton, M.; Rajotte, R.V.; Korbutt, G.S. Neonatal Porcine Sertoli Cells Inhibit Human Natural Antibody-Mediated Lysis1. Biol. Reprod. 2005, 72, 1224–1231. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, L.; Xiang, Y.; Ruan, Y.; Li, J.; Wang, X.; Ichim, T.E.; Chen, S.; Chen, G. Resistance of neonatal porcine Sertoli cells to human xenoantibody and complement-mediated lysis is associated with low expression of alpha-Gal and high production of clusterin and CD59. Xenotransplantation 2010, 17, 215–223. [Google Scholar] [CrossRef]
- Doyle, T.J.; Kaur, G.; Putrevu, S.M.; Dyson, E.L.; Dyson, M.; McCunniff, W.T.; Pasham, M.R.; Kim, K.H.; Dufour, J.M. Immunoprotective Properties of Primary Sertoli Cells in Mice: Potential Functional Pathways that Confer Immune Privilege1. Biol. Reprod. 2012, 86, 6. [Google Scholar] [CrossRef]
- Washburn, R.L.; Kaur, G.; Dufour, J.M. Mouse Sertoli Cells Inhibit Humoral-Based Immunity. Int. J. Mol. Sci. 2022, 23, 12760. [Google Scholar] [CrossRef]
- Wright, K.; Dziuk, R.; Mital, P.; Kaur, G.; Dufour, J.M. Xenotransplanted Pig Sertoli Cells Inhibit Both the Alternative and Classical Pathways of Complement-Mediated Cell Lysis While Pig Islets Are Killed. Cell Transplant. 2016, 25, 2027–2040. [Google Scholar] [CrossRef]
- Yang, W.; Liu, L.-B.; Liu, F.-L.; Wu, Y.-H.; Zhen, Z.-D.; Fan, D.-Y.; Sheng, Z.-Y.; Song, Z.-R.; Chang, J.-T.; Zheng, Y.-T.; et al. Single-cell RNA sequencing reveals the fragility of male spermatogenic cells to Zika virus-induced complement activation. Nat. Commun. 2023, 14, 2476. [Google Scholar] [CrossRef]
- Pandey, S.; Maharana, J.; Li, X.X.; Woodruff, T.M.; Shukla, A.K. Emerging Insights into the Structure and Function of Complement C5a Receptors. Trends Biochem. Sci. 2020, 45, 693–705. [Google Scholar] [CrossRef]
- Rudilla, F.; Fayolle, C.; Casares, N.; Durantez, M.; Arribillaga, L.; Lozano, T.; Villanueva, L.; Pio, R.; Sarobe, P.; Leclerc, C.; et al. Combination of a TLR4 ligand and anaphylatoxin C5a for the induction of antigen-specific cytotoxic T cell responses. Vaccine 2012, 30, 2848–2858. [Google Scholar] [CrossRef]
- Kwan, W.H.; van der Touw, W.; Paz-Artal, E.; Li, M.O.; Heeger, P.S. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J. Exp. Med. 2013, 210, 257–268. [Google Scholar] [CrossRef]
- Washburn, R.L.; Martinez-Marin, D.; Sniegowski, T.; Korać, K.; Rodriguez, A.R.; Miranda, J.M.; Chilton, B.S.; Bright, R.K.; Pruitt, K.; Bhutia, Y.D.; et al. Sertoli Cells Express Accommodation, Survival, and Immunoregulatory Factors When Exposed to Normal Human Serum. Biomedicines 2023, 11, 1650. [Google Scholar] [CrossRef]
- Kolev, M.; Le Friec, G.; Kemper, C. The role of complement in CD4⁺ T cell homeostasis and effector functions. Semin. Immunol. 2013, 25, 12–19. [Google Scholar] [CrossRef]
- Kemper, C.; Chan, A.C.; Green, J.M.; Brett, K.A.; Murphy, K.M.; Atkinson, J.P. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature 2003, 421, 388–392. [Google Scholar] [CrossRef]
- Liu, J.; Miwa, T.; Hilliard, B.; Chen, Y.; Lambris, J.D.; Wells, A.D.; Song, W.C. The complement inhibitory protein DAF (CD55) suppresses T cell immunity in vivo. J. Exp. Med. 2005, 201, 567–577. [Google Scholar] [CrossRef]
- Zirkin, B.R.; Papadopoulos, V. Leydig cells: Formation, function, and regulation. Biol. Reprod. 2018, 99, 101–111. [Google Scholar] [CrossRef]
- Mossadegh-Keller, N.; Sieweke, M.H. Testicular macrophages: Guardians of fertility. Cell Immunol. 2018, 330, 120–125. [Google Scholar] [CrossRef]
- Bohlson, S.S.; O’Conner, S.D.; Hulsebus, H.J.; Ho, M.-M.; Fraser, D.A. Complement, C1q, and C1q-Related Molecules Regulate Macrophage Polarization. Front. Immunol. 2014, 5, 402. [Google Scholar] [CrossRef]
- Chaumonnot, K.; Masson, S.; Sikner, H.; Bouchard, A.; Baverel, V.; Bellaye, P.-S.; Collin, B.; Garrido, C.; Kohli, E. The HSP GRP94 interacts with macrophage intracellular complement C3 and impacts M2 profile during ER stress. Cell Death Dis. 2021, 12, 114. [Google Scholar] [CrossRef]
- Tao, J.; Zhao, J.; Qi, X.-M.; Wu, Y.-G. Complement-mediated M2/M1 macrophage polarization may be involved in crescent formation in lupus nephritis. Int. Immunopharmacol. 2021, 101, 108278. [Google Scholar] [CrossRef]
- James, E.R.; Carrell, D.T.; Aston, K.I.; Jenkins, T.G.; Yeste, M.; Salas-Huetos, A. The Role of the Epididymis and the Contribution of Epididymosomes to Mammalian Reproduction. Int. J. Mol. Sci. 2020, 21, 5377. [Google Scholar] [CrossRef]
- Ali Hassan, H.; Domain, G.; Luvoni, G.C.; Chaaya, R.; Van Soom, A.; Wydooghe, E. Canine and Feline Epididymal Semen—A Plentiful Source of Gametes. Animals 2021, 11, 2961. [Google Scholar] [CrossRef]
- Calvel, P.; Rolland, A.D.; Jégou, B.; Pineau, C. Testicular postgenomics: Targeting the regulation of spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1481–1500. [Google Scholar] [CrossRef][Green Version]
- Mann, T. Secretory function of the prostate, seminal vesicle and other male accessory organs of reproduction. J. Reprod. Fertil. 1974, 37, 179–188. [Google Scholar] [CrossRef]
- Zhou, Z.; Jia, D.; Kwon, O.; Li, S.; Sun, H.; Roudier, M.P.; Lin, D.W.; True, L.; Morrissey, C.; Creighton, C.J.; et al. Androgen-regulated stromal complement component 7 (C7) suppresses prostate cancer growth. Oncogene 2023, 42, 2428–2438. [Google Scholar] [CrossRef]
- Samanta, L.; Parida, R.; Dias, T.R.; Agarwal, A. The enigmatic seminal plasma: A proteomics insight from ejaculation to fertilization. Reprod. Biol. Endocrinol. 2018, 16, 41. [Google Scholar] [CrossRef]
- McKay, A.C.; Odeluga, N.; Jiang, J.; Sharma, S. Anatomy, Abdomen and Pelvis, Seminal Vesicle. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Mann, T.; Lutwak-Mann, C. Male Reproductive Function and the Composition of Semen: General Considerations. In Male Reproductive Function and Semen: Themes and Trends in Physiology, Biochemistry and Investigative Andrology; Mann, T., Lutwak-Mann, C., Eds.; Springer: London, UK, 1981; pp. 1–37. [Google Scholar] [CrossRef]
- Qin, X.; Krumrei, N.; Grubissich, L.; Dobarro, M.; Aktas, H.; Perez, G.; Halperin, J.A. Deficiency of the mouse complement regulatory protein mCd59b results in spontaneous hemolytic anemia with platelet activation and progressive male infertility. Immunity 2003, 18, 217–227. [Google Scholar] [CrossRef]
- Talluri, T.R.; Mal, G.; Ravi, S.K. Biochemical components of seminal plasma and their correlation to the fresh seminal characteristics in Marwari stallions and Poitou jacks. Vet. World 2017, 10, 214–220. [Google Scholar] [CrossRef]
- Cross, N.L.; Mahasreshti, P. Prostasome fraction of human seminal plasma prevents sperm from becoming acrosomally responsive to the agonist progesterone. Arch. Androl. 1997, 39, 39–44. [Google Scholar] [CrossRef]
- Rooney, I.A.; Heuser, J.E.; Atkinson, J.P. GPI-anchored complement regulatory proteins in seminal plasma. An analysis of their physical condition and the mechanisms of their binding to exogenous cells. J. Clin. Investig. 1996, 97, 1675–1686. [Google Scholar] [CrossRef]
- Seya, T.; Hara, T.; Matsumoto, M.; Kiyohara, H.; Nakanishi, I.; Kinouchi, T.; Okabe, M.; Shimizu, A.; Akedo, H. Membrane cofactor protein (MCP, CD46) in seminal plasma and on spermatozoa in normal and “sterile” subjects. Eur. J. Immunol. 1993, 23, 1322–1327. [Google Scholar] [CrossRef]
- Janiszewska, E.; Kokot, I.; Gilowska, I.; Faundez, R.; Kratz, E.M. The possible association of clusterin fucosylation changes with male fertility disorders. Sci. Rep. 2021, 11, 15674. [Google Scholar] [CrossRef]
- Milardi, D.; Grande, G.; Vincenzoni, F.; Messana, I.; Pontecorvi, A.; De Marinis, L.; Castagnola, M.; Marana, R. Proteomic approach in the identification of fertility pattern in seminal plasma of fertile men. Fertil. Steril. 2012, 97, 67–73.e1. [Google Scholar] [CrossRef]
- Merlotti, A.; Dantas, E.; Remes Lenicov, F.; Ceballos, A.; Jancic, C.; Varese, A.; Rubione, J.; Stover, S.; Geffner, J.; Sabatté, J. Fucosylated clusterin in semen promotes the uptake of stress-damaged proteins by dendritic cells via DC-SIGN. Hum. Reprod. 2015, 30, 1545–1556. [Google Scholar] [CrossRef]
- Brooks, G.F.; Lammel, C.J.; Petersen, B.H.; Stites, D.P. Human seminal plasma inhibition of antibody complement-mediated killing and opsonization of Neisseria gonorrhoeae and other gram-negative organisms. J. Clin. Investig. 1981, 67, 1523–1531. [Google Scholar] [CrossRef]
- Petersen, B.H.; Lammel, C.J.; Stites, D.P.; Brooks, G.F. Human seminal plasma inhibition of complement. J. Lab. Clin. Med. 1980, 96, 582–591. [Google Scholar]
- Bouhlal, H.; Chomont, N.; Haeffner-Cavaillon, N.; Kazatchkine, M.D.; Belec, L.; Hocini, H. Opsonization of HIV-1 by semen complement enhances infection of human epithelial cells. J. Immunol. 2002, 169, 3301–3306. [Google Scholar] [CrossRef]
- Tomlinson, M.J.; White, A.; Barratt, C.L.; Bolton, A.E.; Cooke, I.D. The removal of morphologically abnormal sperm forms by phagocytes: A positive role for seminal leukocytes? Hum. Reprod. 1992, 7, 517–522. [Google Scholar] [CrossRef]
- Baalasubramanian, S.; Harris, C.L.; Donev, R.M.; Mizuno, M.; Omidvar, N.; Song, W.C.; Morgan, B.P. CD59a is the primary regulator of membrane attack complex assembly in the mouse. J. Immunol. 2004, 173, 3684–3692. [Google Scholar] [CrossRef]
- Sylvester, S.R.; Morales, C.; Oko, R.; Griswold, M.D. Localization of sulfated glycoprotein-2 (clusterin) on spermatozoa and in the reproductive tract of the male rat. Biol. Reprod. 1991, 45, 195–207. [Google Scholar] [CrossRef][Green Version]
- O’Bryan, M.K.; Mallidis, C.; Murphy, B.F.; Baker, H.W. Immunohistological localization of clusterin in the male genital tract in humans and marmosets. Biol. Reprod. 1994, 50, 502–509. [Google Scholar] [CrossRef][Green Version]
- Perricone, R.; Pasetto, N.; De Carolis, C.; Vaquero, E.; Piccione, E.; Baschieri, L.; Fontana, L. Functionally active complement is present in human ovarian follicular fluid and can be activated by seminal plasma. Clin. Exp. Immunol. 1992, 89, 154–157. [Google Scholar] [CrossRef]
- Kabut, J.; Kondera-Anasz, Z.; Sikora, J.; Mielczarek-Palacz, A. Levels of complement components iC3b, C3c, C4, and SC5b-9 in peritoneal fluid and serum of infertile women with endometriosis. Fertil. Steril. 2007, 88, 1298–1303. [Google Scholar] [CrossRef]
- Brucker, C.; Lipford, G.B. The human sperm acrosome reaction: Physiology and regulatory mechanisms. An update. Hum. Reprod. Update 1995, 1, 51–62. [Google Scholar] [CrossRef]
- Morohoshi, A.; Miyata, H.; Tokuhiro, K.; Iida-Norita, R.; Noda, T.; Fujihara, Y.; Ikawa, M. Testis-enriched ferlin, FER1L5, is required for Ca(2+)-activated acrosome reaction and male fertility. Sci. Adv. 2023, 9, eade7607. [Google Scholar] [CrossRef]
- Cervoni, F.; Oglesby, T.J.; Fénichel, P.; Dohr, G.; Rossi, B.; Atkinson, J.P.; Hsi, B.L. Expression of decay-accelerating factor (CD55) of the complement system on human spermatozoa. J. Immunol. 1993, 151, 939–948. [Google Scholar] [CrossRef]
- Lee, A.S.; Rusch, J.; Lima, A.C.; Usmani, A.; Huang, N.; Lepamets, M.; Vigh-Conrad, K.A.; Worthington, R.E.; Mägi, R.; Wu, X.; et al. Rare mutations in the complement regulatory gene CSMD1 are associated with male and female infertility. Nat. Commun. 2019, 10, 4626. [Google Scholar] [CrossRef]
- Kitamura, M.; Matsumiya, K.; Yamanaka, M.; Takahara, S.; Hara, T.; Matsumoto, M.; Namiki, M.; Okuyama, A.; Seya, T. Possible association of infertility with sperm-specific abnormality of CD46. J. Reprod. Immunol. 1997, 33, 83–88. [Google Scholar] [CrossRef]
- Inoue, N.; Ikawa, M.; Nakanishi, T.; Matsumoto, M.; Nomura, M.; Seya, T.; Okabe, M. Disruption of mouse CD46 causes an accelerated spontaneous acrosome reaction in sperm. Mol. Cell Biol. 2003, 23, 2614–2622. [Google Scholar] [CrossRef]
- Mizuno, M.; Harris, C.L.; Johnson, P.M.; Morgan, B.P. Rat membrane cofactor protein (MCP; CD46) is expressed only in the acrosome of developing and mature spermatozoa and mediates binding to immobilized activated C3. Biol. Reprod. 2004, 71, 1374–1383. [Google Scholar] [CrossRef]
- Choileain, S.N.; Astier, A.L. CD46 processing: A means of expression. Immunobiology 2012, 217, 169–175. [Google Scholar] [CrossRef]
- Zelek, W.M.; Xie, L.; Morgan, B.P.; Harris, C.L. Compendium of current complement therapeutics. Mol. Immunol. 2019, 114, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Mastellos, D.C.; Ricklin, D.; Lambris, J.D. Clinical promise of next-generation complement therapeutics. Nat. Rev. Drug Discov. 2019, 18, 707–729. [Google Scholar] [CrossRef]
- West, E.E.; Woodruff, T.; Fremeaux-Bacchi, V.; Kemper, C. Complement in human disease: Approved and up-and-coming therapeutics. Lancet 2023. [Google Scholar] [CrossRef]
- Griffith, B.P.; Goerlich, C.E.; Singh, A.K.; Rothblatt, M.; Lau, C.L.; Shah, A.; Lorber, M.; Grazioli, A.; Saharia, K.K.; Hong, S.N.; et al. Genetically Modified Porcine-to-Human Cardiac Xenotransplantation. N. Engl. J. Med. 2022, 387, 35–44. [Google Scholar] [CrossRef]
- Klinovska, K.; Sebkova, N.; Dvorakova-Hortova, K. Sperm-egg fusion: A molecular enigma of mammalian reproduction. Int. J. Mol. Sci. 2014, 15, 10652–10668. [Google Scholar] [CrossRef]
- Kemper, C.; Verbsky, J.W.; Price, J.D.; Atkinson, J.P. T-Cell stimulation and regulation: With complements from CD46. Immunol. Res. 2005, 32, 31–43. [Google Scholar] [CrossRef]
- Yamamoto, H.; Fara, A.F.; Dasgupta, P.; Kemper, C. CD46: The ‘multitasker’ of complement proteins. Int. J. Biochem. Cell Biol. 2013, 45, 2808–2820. [Google Scholar] [CrossRef]
- Verhaagh, S.; de Jong, E.; Goudsmit, J.; Lecollinet, S.; Gillissen, G.; de Vries, M.; van Leuven, K.; Que, I.; Ouwehand, K.; Mintardjo, R.; et al. Human CD46-transgenic mice in studies involving replication-incompetent adenoviral type 35 vectors. J. Gen. Virol. 2006, 87, 255–265. [Google Scholar] [CrossRef]

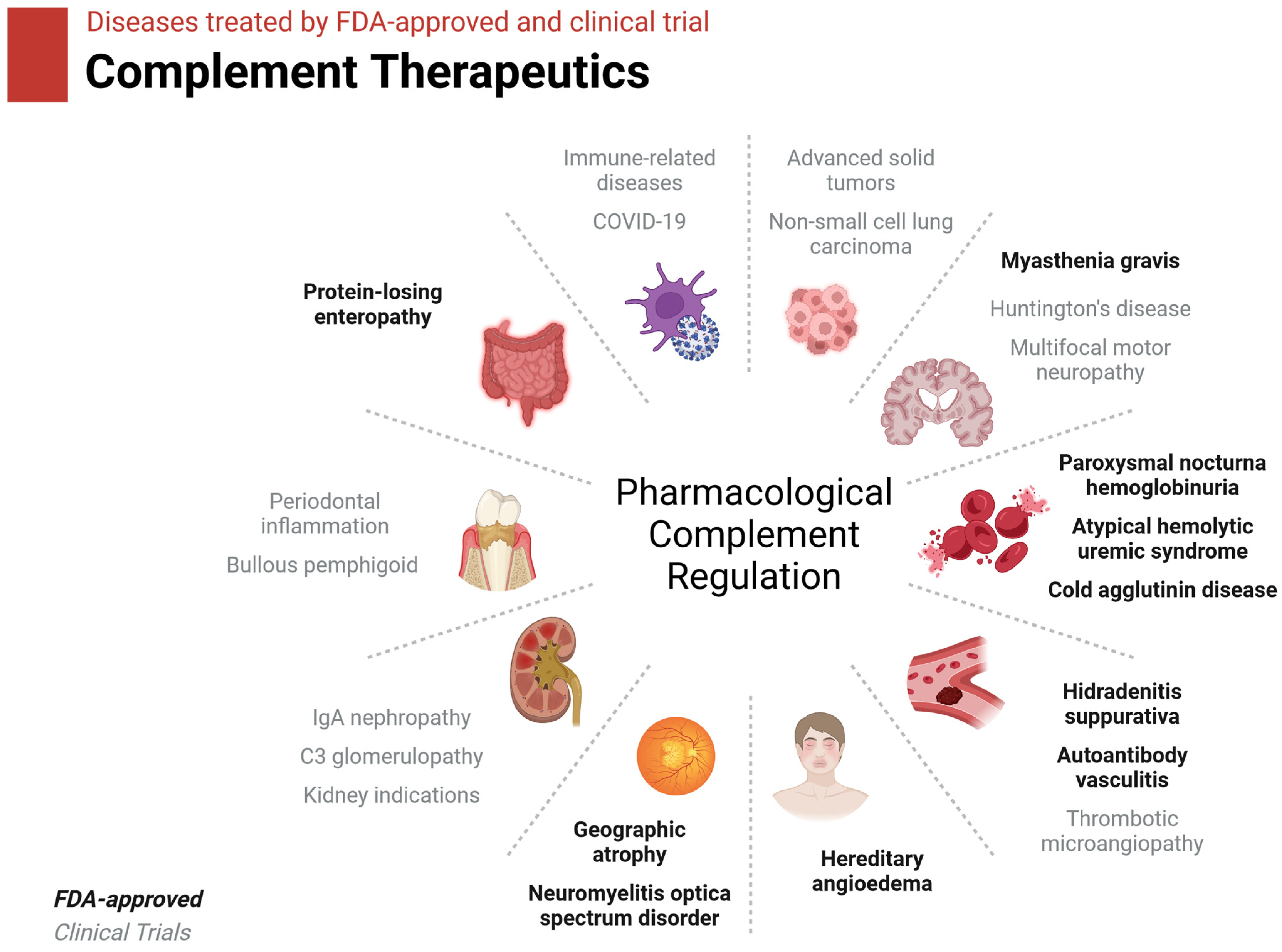
| Drug | Complement Target | Disease | Status |
|---|---|---|---|
| ACH-5548 | Factor D | Immune-related disease | Phase I |
| ALXN1720 | C5/C5a/C5aR1 | Myasthenia gravis | Phase I |
| ALXN1820 | Properdin | Sickle cell disease | Phase I |
| AMY-101 | C3 | Periodontal inflammation | Phase II |
| ANX005 | C1/C1q | Huntington’s disease | Phase II |
| ANX007 | C1/C1q | Geographic atrophy | Phase II |
| ARGX-117 | C2 | Multifocal motor neuropathy, kidney interactions | Phase I |
| Avacincaptad pegol | C5 | Geographic atrophy | FDA-approved |
| Avacopan | C5aR1 | Autoantibody vasculitis | FDA-approved |
| Avdoralimab | C5/C5a/C5aR1 | Bullous pemphigoid | Phase II |
| BCX-9930 | Factor D | Paroxysmal nocturnal hemoglobinuria | Phase I |
| BDB-001 | C5/C5a/C5aR1 | COVID-19, hidradenitis suppurativa | Phase II/III |
| Berinert | C1r/s, MASP | Hereditary angioedema | FDA-approved |
| Cemdisiran | C5/C5a/C5aR1 | Hemolytic uremic syndrome, IgA nephropathy, myasthenia gravis | Phase II |
| Cinryze | C1r/s, MASP | Hereditary angioedema | FDA-approved |
| CLG561 | Properdin | Geographic atrophy | Phase II |
| Crovalimab | C5/C5a/C5aR1 | Paroxysmal nocturnal hemoglobinuria | Phase III |
| Danicopan | Factor D | Paroxysmal nocturnal hemoglobinuria | Phase III |
| Eclulizumab | C5 | Paroxysmal nocturnal hemoglobinuria, myasthenia gravis, neuromyelitis optica spectrum disorder | FDA-approved |
| GT103 | Factor H | Non-small-cell lung cancer | Phase I |
| IONIS-FB-liuc | Factor B | IgA nephropathy | Phase II |
| Iptacopan | Factor B | Paroxysmal nocturnal hemoglobinuria | FDA-approved |
| Iptacopan | Factor B | Paroxysmal nocturnal hemoglobinuria, C3 glomerulopathy, IgA nephropathy | Phase III |
| MOR210 | C5/C5a/C5aR1 | Advanced solid tumors | Phase I |
| Narsoplimab | MASP2 | Thrombotic microangiopathy | Phase III |
| Nomacopan | C5/C5a/C5aR1 | Thrombotic microangiopathy | Phase III |
| OCTA-C1-INH | C1s/C1r/MASP | Hereditary angioedema | Phase II |
| Pegcetacoplan | C3 | Paroxysmal nocturnal hemoglobinuria | FDA-approved |
| Pegcetacoplan injection | C3 | Geographic atrophy | FDA-approved |
| Pozelimab | C5 | Protein-losing enteropathy | FDA-approved |
| Pozelimab | C5/C5a/C5aR1 | Paroxysmal nocturnal hemoglobinuria | Phase III |
| Ravulizumab | C5 | Paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome | FDA-approved |
| Ruconest | C1r/s, MASP | Hereditary angioedema | FDA-approved |
| Sutimlimab | C1s | Cold agglutinin disease | FDA-approved |
| Tesidolumab | C5/C5a/C5aR1 | Paroxysmal nocturnal hemoglobinuria | Phase II |
| Vemircopan | Factor D | Paroxysmal nocturnal hemoglobinuria | Phase II |
| Vilobelimab | C5/C5a/C5aR1 | COVID-19, hidradenitis suppurativa | Phase III |
| Zilucoplan | C5 | Myasthenia gravis | FDA-approved |
| Zilucoplan | C5/C5a/C5aR1 | Myasthenia gravis | Phase III |
| Zimura | C5/C5a/C5aR1 | Geographic atrophy | Phase III |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Washburn, R.L. Complements from the Male Reproductive Tract: A Scoping Review. BioMed 2024, 4, 19-38. https://doi.org/10.3390/biomed4010002
Washburn RL. Complements from the Male Reproductive Tract: A Scoping Review. BioMed. 2024; 4(1):19-38. https://doi.org/10.3390/biomed4010002
Chicago/Turabian StyleWashburn, Rachel L. 2024. "Complements from the Male Reproductive Tract: A Scoping Review" BioMed 4, no. 1: 19-38. https://doi.org/10.3390/biomed4010002
APA StyleWashburn, R. L. (2024). Complements from the Male Reproductive Tract: A Scoping Review. BioMed, 4(1), 19-38. https://doi.org/10.3390/biomed4010002






