Abstract
Untreated early childhood caries (ECC) is a global public health concern. In the short term, untreated ECC can lead to pain, infection, and disrupted sleep, among other issues. In the long term, it is associated with poor oral health in later life, increased risk of caries in permanent teeth, and adverse effects on physical and psychological development. There may be a link between untreated ECC and adverse cognitive and neurodevelopmental outcomes in young children, although the exact pathways are not fully understood. One possible pathway is through the relationship between mastication and brain stimulation. Impaired masticatory function due to ECC can affect the hippocampus, a key region responsible for memory and learning. Furthermore, untreated ECC can cause chronic inflammation, leading to the release of pro-inflammatory cytokines that may damage the brain. Sleep disturbances resulting from ECC-related pain and discomfort can also impact brain development and cognitive functioning. Additionally, frequent use of antibiotics and analgesics to address ECC-related infections can disrupt the gut microbiome, potentially affecting the brain through the gut–brain axis. Untreated ECC can cause nutritional deficiencies and elevated nutritional risk, and can further hinder brain development. Addressing ECC comprehensively with early childhood health initiatives can help mitigate potential long-term consequences and promote optimal brain development in young children.
Keywords:
dental caries; children; preschooler; cognitive function; nutrition; inflammation; microbiome 1. Introduction
Early childhood caries (ECC) is a prevalent oral health condition that affects children worldwide [1]. ECC is characterized by the presence of dental caries in primary teeth [2]. This condition has significant implications for children’s physical, mental, and social well-being. In the short term, untreated ECC results in pain, infection, poor appetite, disturbed sleep, hospitalization, loss of school days, reduced ability to learn, poor concentration, and eventual premature loss of the tooth [3], which causes poor mastication [4]. In the long term, untreated ECC is linked to poor oral health in later life, high risk for caries in the permanent dentition, poor physical growth and development, poor psychological development, and poor quality of life [3,5]. Although ECC has long been recognized as a major public health concern, recent research has highlighted potential links between untreated ECC and adverse cognitive and neurodevelopmental outcomes in young children [6,7,8,9,10]. This is because ECC occurs in the first few years of life when the brain is undergoing rapid and sensitive development of its neural systems. The exact pathways connecting ECC to poor brain development are not yet fully understood. However, the conceptualization of the pathway is represented in Figure 1.
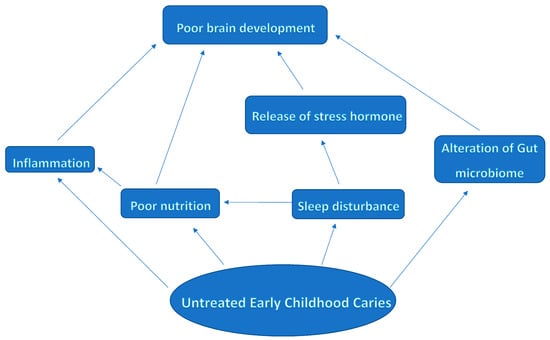
Figure 1.
Conceptual framework of the pathways associating untreated early childhood caries with poor brain development.
1.1. Mastication and Brain Development
One possible pathway is the relationship between masticatory force and brain stimulation. Animal and human studies have suggested a potential causal link between mastication and cognitive function [11,12]. Mastication plays a role in maintaining cognitive functions in the hippocampus by providing peripheral sensory input, which supports and enhances cognitive function [13]. The hippocampus is the central component of a large neural network involved in memory processes, especially spatial memory and learning in the central nervous system [14]. Neurons in the hippocampus connect to neural circuits controlling cognition, memory, and motor functions. A huge percentage of brain development occurs postnatally, a period characterized by the formation of masticatory habits. Therefore, impaired masticatory function can lead to morphological and functional changes in the hippocampus, resulting in deficits in hippocampus-dependent spatial memory [15,16].
Unfortunately, untreated ECC can cause pain, which leads to avoidance of using the affected tooth, resulting in a reduction in masticatory force [3,4]. Moreover, the presence of untreated ECC can lead to tooth loss, further impairing masticatory function [17]. The loss of teeth has been associated with specific changes in grey matter volume in various brain regions. These changes include an increase in grey matter volume in regions responsible for the fear response, learning, memory consolidation, and the trigeminal spinal tract nucleus. Conversely, there is a decrease in grey matter volume in regions controlling movement, motor processing/control, emotions, working memory, problem-solving behavior, and the trigeminal motor nucleus [18].
One of the possible earliest signs of the impact of tooth loss on the brain is the resultant speech disorder due to the modification of occlusal forces [19]. Tooth loss, especially in the anterior region of the oral cavity, can lead to speech distortion and difficulties in pronouncing certain sounds, known as “labial” or “dental” sounds, that involve the lips as active articulators [20]. These “labial”, “bilabial”, and “labio-dental” sounds are produced when the tongue interacts with or comes close to the upper and/or lower anterior teeth in a coordinated action with the tongue [21]. Additionally, there are various other types of speech sounds related to the position and involvement of the teeth and tongue. These include interdental sounds, where the teeth act as passive articulators; alveolar sounds, produced just behind the teeth by placing the tip of the tongue against the alveolar ridge; palatal sounds, formed when the tongue approaches the hard palate; velar sounds, created when the back of the tongue contacts the soft palate or velum; and glottal sounds, produced in the region of the glottis. These different types of sounds contribute to the complexity and diversity of human speech [22] that are developed early in life.
The process of producing these sounds involves intricate neural connections that coordinate the interactions between the brain and various oral structures, such as the teeth, tongue, muscles of mastication, lips, and vocal cords [23]. These neural pathways develop and strengthen during childhood and adolescence, a critical period when language skills are rapidly evolving [24]. When tooth loss occurs during this crucial phase of brain development, the neural connections involved in speech production may face challenges [25]. Consequently, children with missing teeth may experience difficulties in articulating speech clearly and fluently [26]. In response, the brain may undergo adaptation and reorganization of its neural pathways to compensate for the altered oral structure and the resulting speech disorders [27]. This happens because tooth loss causes the modifications of neuronal activity that can lead to alterations in neurotransmission and synaptic strength known as synaptic plasticity [28]. The phenomenon brings about adjustments in intracortical microcircuitry, thereby impacting the organization of cortical maps and cytoarchitecture. These changes serve as the foundation for long-term modifications in motor performance [29].
1.2. Inflammation and Brain Development
Untreated ECC leads to infection and chronic inflammation resulting in elevated levels of pro-inflammatory cytokines and a resultant arithmetic progression with the severity of ECC [30]. The pro-inflammatory cytokines produce free radicals that generate peroxides, Prostaglandin E2, interleukin 6, tumor necrotizing factor-alpha, and cysteinyl leukotrienes that are powerful agents in the inflammatory response [31,32,33]. Infection and agents of inflammation can damage the brain and ECC may cause the dissemination of bacteria from distant sources with associated damage to the white brain matter [34,35] whose maturation is associated with the development of cognitive functions in early childhood [36]. The released endotoxins can initiate a brain-damaging process, and the inflammatory proteins released play a role in mediating the damage [37,38]. A corollary potential is that maternal untreated caries may be a risk factor for chorioamnionitis, a risk factor for intrapartum brain damage [39,40,41], which is also associated with host dental caries microbacteria [42].
In addition, pain associated with untreated ECC can contribute to elevated levels of stress, which may have a negative impact on brain development. Prolonged exposure to stress hormones, such as cortisol, activates the hypothalamic–pituitary–adrenal axis [43]. Cortisol can disrupt the delicate balance of neurochemicals in the brain and hinder the growth of neural connections necessary for optimal cognitive functioning [44,45]. The effect is a decrease in memory and cognitive flexibility, which is reversible in a healthy resilient brain [46]. Specifically, cortisol impairs the process of memory retrieval in a non-linear fashion [47,48] and it affects the working memory [49].
Moreover, untreated ECC can result in chronic systemic inflammation, leading to disruptions in the functional connectivity within the parieto-occipital lobe [50]. This chronic inflammation causes structural remodeling in brain regions crucial for achieving developmental milestones [50]. During early years of life, oligodendrocyte progenitor cells undergo proliferation and differentiation, contributing to the development of astrocytes and oligodendrocytes. This process plays a vital role in establishing neuronal connections, ensuring proper neural signaling [51]. Additionally, myelination processes continue in early childhood, but chronic inflammatory processes can disrupt these processes, affecting the number of synapses and the integrity of the myelin sheath [50]. Consequently, ECC-induced chronic inflammation can lead to issues such as poor memory, impaired functional connectivity within the brain, and adverse cognitive functions.
1.3. Sleep Disturbances and Brain Development
Untreated dental caries can cause sleep deprivation [3]. The pain and discomfort resulting from untreated ECC can additionally disturb sleep patterns [52]. Sleep plays a vital role in the development of the brain and cognitive functioning [52]. Disrupted sleep can hinder the consolidation of memories, impede attention and concentration, and impair overall cognitive development [53,54]. Moreover, poor sleep quality may contribute to behavioral issues and impact a child’s emotional well-being, potentially affecting their social and cognitive growth [55]. Furthermore, sleep deprivation in children triggers the release of stress hormones [56]. Conversely, sleep disturbances can predispose children to the development of ECC; thus, the relationship between sleep disturbances and ECC can be described as bidirectional. Irregular sleep patterns, inadequate sleep duration, and going to bed late have been linked to decreased self-regulation of appetite, potentially leading to late-night eating habits [57,58,59], which can contribute to the risk of ECC through increased intake of a sugary diet at bedtime (although increased intake of sugar before bedtime causes a delay in sleeping [60]) [61] or indirectly through obesity [62]. In addition, inadequate sleep alters the immune status and may predispose children to carious activity via Streptococcus mutans [63]. Untreated ECC can also lead to sleep disturbances which can in turn affect cortisol production [64].
1.4. Gut Microbiome and Brain Development
Moreover, the frequent administration of antibiotics and analgesics to address infections and pain related to untreated ECC can disrupt the balance of the gut microbiome [65]. The gut microbiome plays a vital role in the development and functioning of the brain, and emerging research indicates that diverse gut microbiota can have either a positive or a negative influence on cognitive processes and mental well-being [66].
The brain–gut axis forms a complex, two-way communication network linking the enteric and central nervous systems [67]. Through this connection, sensory signals from the gut are transmitted to the central nervous system via the Vagus nerve, influencing reflexes and impacting mood, cognition, and mental well-being. At the same time, the brain sends signals that regulate gut anatomy and physiology. This bidirectional communication allows the gut–brain axis to monitor and integrate gut functions while establishing connections between the emotional and cognitive centers of the brain. Additionally, gut bacteria have the capability to produce and release neurotransmitters that can communicate with and affect the central nervous system via the Vagus nerve [68,69]. The gut microbiome is sensitive to various factors, including stress and medication usage [70], which can lead to an imbalance known as dysbiosis [71,72]. Dysbiosis triggers an increase in the secretion of pro-inflammatory cytokines, which can have a detrimental impact on the brain [73,74].
1.5. Nutritional Implications
Untreated ECC leads to the generation of pro-inflammatory cytokines, which are strongly linked to an elevated nutritional risk [75] and can hinder brain development [76]. Severe ECC is also connected to challenges in eating, decreased food consumption, and deficiencies in iron, vitamin A, vitamin D, and albumin [77,78,79,80]. These nutritional deficiencies can potentially impede brain development and compromise cognitive capabilities [81,82,83], as the developing brain relies on adequate nutrition, especially protein deficiencies, which facilitate its growth, optimal functioning, and plasticity [84,85].
2. Discussion
Early childhood caries is a preventable condition; however, regrettably, it continues to be highly prevalent among children [4]. Moreover, the risk of ECC is disproportionately higher among individuals with a low socio-economic status [86], who often face various challenges that can negatively impact their brain health. Children from low socio-economic backgrounds are more likely to exhibit brain maturation patterns characterized by lower volume and slower rates of change as they develop [87]. This review suggests that untreated ECC may compound the risk for poor brain development in children with a low socio-economic status.
Early intervention and preventive strategies are crucial in mitigating the potential impact of untreated ECC on brain development. Promoting good oral hygiene practices, such as regular toothbrushing with fluoride toothpaste, along with limiting sugary foods and beverages, are essential preventive measures. Regular dental check-ups from an early age can facilitate early detection and prompt treatment of ECC, reducing the risk of complications. Country-level policies and regulations with advocacy for the addition of dental treatment into the National Health Insurance Scheme to assist children from the low socio-economic class to have access ECC treatment, and an active drive to monitor these regulations, can facilitate improved ECC management and control.
Currently, many oral healthcare programs targeting children are school-based. However, it is ironic that a significant number of pre-school children, who are at risk of developing ECC, are not part of formal school systems [88]. As a result, they are unable to benefit from these school-based interventions. Nevertheless, there is evidence suggesting that health promotion efforts among pre-school children can have a lasting impact on their health behaviors [89], and there are models available for integrating health promotion programs for this age group [90,91]. Despite this, the process of generating and scaling up evidence to drive the implementation of ECC prevention programs has been exceedingly slow. What is lacking is the political willpower to drive the change.
In addition, it is crucial to foster collaboration between oral health professionals, neurodevelopmental pediatricians, and early childhood educators. Such collaboration can increase awareness about the significance of oral health in overall development, enable the implementation of preventive strategies, and provide appropriate care for children affected by ECC. It is also important to incorporate oral health into programs and interventions for early childhood development at national and international levels. Educating parents and caregivers about proper oral hygiene practices, the importance of a balanced diet, and the potential consequences of untreated ECC can empower them to take proactive comprehensive and wholistic measures to safeguard their children’s oral health and support their brain development.
This opinion piece has its limitations. We acknowledge that this was a consensus review that drew on our expert knowledge of the subject matter, and the evaluation of research to reach our collective conclusions. However, the main weakness lies in the potential for bias during article selection as we may have selected articles that confirm own views and, thereby, unintentionally introduced bias. The information aligns better with the specifics of the focus of our discussion, and potentially offers more relevant guidance for further work on the topic of interest. There are no established standards or guidelines governing the process of generating articles for an opinion piece and thus we share some insights into the issue of understanding that our thorough grasp of the overall state of the science concerning the problem is limited and further studies are needed. The group will build on the conceptual framework from this study to inform our future studies.
3. Conclusions
Untreated ECC may have plausible links with disturbances in brain development, highlighting the need for early intervention and preventive measures. The chronic inflammation, pain, nutritional implications, and sleep disruptions associated with untreated ECC can adversely affect neurodevelopmental outcomes in young children. Recognizing the connections between untreated ECC and disturbances in brain development underscores the importance of a comprehensive approach that integrates oral health care within early childhood health and development initiatives. By prioritizing oral health for pre-school children and implementing timely interventions, we can strive to mitigate the potential long-term consequences of untreated ECC and promote optimal brain development in young children.
Author Contributions
Conceptualization, M.O.F.; writing—original draft preparation, M.O.F., O.M.F.-A., B.A. and O.E.O.; writing—review and editing, M.O.F., O.M.F.-A., B.A. and O.E.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meyer, F.; Enax, J. Early Childhood Caries: Epidemiology, Aetiology, and Prevention. Int. J. Dent. 2018, 2018, 1415873. [Google Scholar] [CrossRef] [PubMed]
- Kazeminia, M.; Abdi, A.; Shohaimi, S.; Jalali, R.; Vaisi-Raygani, A.; Salari, N.; Mohammadi, M. Dental caries in primary and permanent teeth in children worldwide, 1995 to 2019: A systematic review and meta-analysis. Head Face Med. 2020, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Colak, H.; Dülgergil, C.T.; Dalli, M.; Hamidi, M.M. Early childhood caries update: A review of causes, diagnoses, and treatments. J. Nat. Sci. Biol. Med. 2013, 4, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Anil, S.; Anand, P.S. Early Childhood Caries: Prevalence, Risk Factors, and Prevention. Front. Pediatr. 2017, 5, 157. [Google Scholar] [CrossRef] [PubMed]
- Sheiham, A. Dental caries affects body weight, growth and quality of life in pre-school children. Br. Dent. J. 2006, 201, 625–626. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.G.; Shieh, T.Y.; Teng, A.Y. Is caries an independent risk factor for the child’s psychomotor development?—A new insight to potentially shed the underlying mechanisms. J. Dent. Sci. 2018, 13, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.Y.; Liu, Y.C.; Shieh, T.Y.; Lin, J.R.; Tseng, Y.C.; Teng, A.Y. Experience of Early Childhood Caries May Positively Correlate with Psychomotor Development. Oral Health Prev. Dent. 2015, 13, 365–375. [Google Scholar] [PubMed]
- Liang, C.Y.; Liu, Y.G.; Shieh, T.Y.; Tseng, Y.C.; Teng, A.Y. Higher Levels of Early Childhood Caries (ECC) Is Associated with Developing Psychomotor Deficiency: The Cross-Sectional Bi-Township Analysis for The New Hypothesis. Int. J. Environ. Res. Public Health 2019, 16, 3082. [Google Scholar] [CrossRef]
- Teng, A.Y.; Liang, C.Y.; Liu, Y.C.G. Socio-Economic Status May Associate Different Risk(s) with Early Childhood Caries (ECC) That Can Cause the Development of Psychomotor Deficiency in Preschool Children Aged 3-6 Years Old: The Results of Preliminary Analysis from a Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 9011. [Google Scholar] [CrossRef]
- Liang, C.-Y.; Teng, A.Y.-T.; Liu, Y.C. Early Childhood Caries Is Causally Attributed to Developing Psychomotor Deficiency in Pre-School Children: The Resultant Covariate and Confounder Analyses in a Longitudinal Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 6831. [Google Scholar] [CrossRef]
- Ono, Y.; Yamamoto, T.; Kubo, K.Y.; Onozuka, M. Occlusion and brain function: Mastication as a prevention of cognitive dysfunction. J. Oral Rehabil. 2010, 37, 624–640. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.B.; Fernandes, L.M.; Noronha, P.A.; dos Santos, M.A.R.; Gomes-Leal, W.; Maia, C.D.S.F.; Lima, R.R. Masticatory deficiency as a risk factor for cognitive dysfunction. Int. J. Med. Sci. 2014, 11, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Lexomboon, D.; Trulsson, M.; Wårdh, I.; Parker, M.G. Chewing ability and tooth loss: Association with cognitive impairment in an elderly population study. J. Am. Geriatr. Soc. 2012, 60, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Henke, K. A model for memory systems based on processing modes rather than consciousness. Nat. Rev. Neurosci. 2010, 11, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Kawahata, M.; Ono, Y.; Ohno, A.; Kawamoto, S.; Kimoto, K.; Onozuka, M. Loss of molars early in life develops behavioral lateralization and impairs hippocampus-dependent recognition memory. BMC Neurosci. 2014, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Obata, T.; Takahashi, H.; Tachibana, A.; Kuroiwa, D.; Takahashi, T.; Ikehira, H.; Onozuka, M. Effects of chewing on cognitive processing speed. Brain Cogn. 2013, 81, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Moynihan, P. The interrelationship between diet and oral health. Proc. Nutr. Soc. 2005, 64, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Velundandi, S.; Chitre, S. The Effects of Tooth Loss on the Brain. J. Oral Med. 2017, 1, 5. [Google Scholar]
- Takagi, M.; Takahashi, M.; Narita, E.; Shimooka, S. Comparison between children with missing anterior deciduous teeth and posterior deciduous teeth by analysis of speech sounds. Shoni Shikagaku Zasshi 1989, 27, 144–152. [Google Scholar]
- Rai, A.K.; Rozario, J.E.; Ganeshkar, S.V. Comparison of speech performance in labial and lingual orthodontic patients: A prospective study. Dent. Res. J. 2014, 11, 663–675. [Google Scholar]
- Dan, M. English Phonetics and Phonological Theory-20th Century Approaches. Bucureşti: [Monograph Online] Universitatea din Bucureşti. 2003. [Last cited on 20 November 2010]. Available online: http://www.ebooks.unibuc.ro/filologie/mateescu/cuprins.htm (accessed on 26 September 2023).
- Speech. 1995. [Last Updated on 1995; Last cited on 20 October 2010]. Available online: http://www.uv.es/EBRIT/macro/macro_5005_97_1.html (accessed on 26 September 2023).
- Indefrey, P.; Levelt, W.J.M. The spatial and temporal signatures of word production components. Cognition 2004, 92, 101–144. [Google Scholar] [CrossRef] [PubMed]
- Rosselli, M.; Ardila, A.; Matute, E.; Vélez-Uribe, I. Language Development across the Life Span: A Neuropsychological/Neuroimaging Perspective. Neurosci. J. 2014, 2014, 585237. [Google Scholar] [CrossRef] [PubMed]
- Larson, C. Neurophysiology of Speech and Swallowing. Semin. Speech Lang. 1985, 6, 275–291. [Google Scholar] [CrossRef]
- Hyde, A.C.; Moriarty, L.; Morgan, A.G.; Elsharkasi, L.M.; Deery, C. Speech and the dental interface. Dent. Update 2018, 45, 795–803. [Google Scholar] [CrossRef]
- Ludlow, C.L.; Hoit, J.; Kent, R.; Ramig, L.O.; Shrivastav, R.; Strand, E.; Yorkston, K.; Sapienza, C.M. Translating principles of neural plasticity into research on speech motor control recovery and rehabilitation. J. Speech Lang. Hear. Res. 2008, 51, S240–S258. [Google Scholar] [CrossRef] [PubMed]
- Stampanoni Bassi, M.; Iezzi, E.; Gilio, L.; Centonze, D.; Buttari, F. Synaptic Plasticity Shapes Brain Connectivity: Implications for Network Topology. Int. J. Mol. Sci. 2019, 20, 6193. [Google Scholar] [CrossRef] [PubMed]
- Monfils, M.H.; Plautz, E.J.; Kleim, J.A. In search of the motor engram: Motor map plasticity as a mechanism for encoding motor experience. Neuroscientist 2005, 11, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Leviton, A.; Gilles, F.; Neff, R.; Yaney, P. Multivariate analysis of risk of perinatal telencephalic leucoencephalopathy. Am. J. Epidemiol. 1976, 104, 621–626. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Hinson, R.M.; Williams, J.A.; Shacter, E. Elevated interleukin 6 is induced by prostaglandin E2 in a murine model of inflammation: Possible role of cyclooxygenase-2. Proc. Natl. Acad. Sci. USA 1996, 93, 4885–4890. [Google Scholar] [CrossRef]
- Tian, W.; Jiang, X.; Kim, D.; Guan, T.; Nicolls, M.R.; Rockson, S.G. Leukotrienes in Tumor-Associated Inflammation. Front. Pharmacol. 2020, 11, 1289. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.C.C.; Pachêco, C.D.J.B.; Costa, E.L.; Ladeira, L.L.C.; Costa, J.F.; da Silva, R.A.; Carmo, C.D.S. Proinflammatory cytokines in early childhood caries: Salivary analysis in the mother/children pair. Cytokine 2018, 107, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Dammann, O.; Leviton, A. Infection remote from the brain, neonatal white matter damage, and cerebral palsy in the preterm infant. Semin. Pediatr. Neurol. 1998, 5, 190–201. [Google Scholar] [CrossRef]
- Nagy, Z.; Westerberg, H.; Klingberg, T. Maturation of White Matter is Associated with the Development of Cognitive Functions during Childhood. J. Cogn. Neurosci. 2004, 16, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, F.S.; Barks, J.D.E.; Hagan, P.; Liu, X.H.; Ivacko, J.; Szaflarski, J. Cytokines and perinatal brain injury. Neurochem. Int. 1997, 30, 375–383. [Google Scholar] [CrossRef]
- Hagberg, H.; Mallard, C. Effect of inflammation on central nervous system development and vulnerability. Curr. Opin. Neurol. 2005, 18, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Colford, J.M. Chorioamnionitis as a risk factor for cerebral palsy—A meta-analysis. J. Am. Med. Assoc. 2000, 284, 1417–1424. [Google Scholar] [CrossRef]
- Wu, Y.W.; Escobar, G.J.; Grether, J.K.; Croen, L.A.; Greene, J.D.; Newman, T.B. Chorioamnionitis and cerebral palsy in term and near-term infants. J. Am. Med. Assoc. 2003, 290, 2677–2684. [Google Scholar] [CrossRef]
- Badawi, N.; Kurinczuk, J.J.; Keogh, J.M.; Alessandri, L.M.; O’Sullivan, F.; Burton, P.R.; Pemberton, P.J.; Stanley, F.J. Intrapartum risk factors for newborn encephalopathy: The Western Australian case-control study. Br. Med. J. 1998, 317, 1554–1558. [Google Scholar] [CrossRef]
- Mitchell, J. Streptococcus mitis: Walking the line between commensalism and pathogenesis. Mol. Oral Microbiol. 2011, 26, 89–98. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Panahi, Y.; Sahraei, H.; Johnston, T.P.; Sahebkar, A. The impact of stress on body function: A review. EXCLI J. 2017, 16, 1057–1072. [Google Scholar] [CrossRef] [PubMed]
- De Souza-Talarico, J.N.; Marin, M.F.; Sindi, S.; Lupien, S.J. Effects of stress hormones on the brain and cognition: Evidence from normal to pathological aging. Dement. Neuropsychol. 2011, 5, 8–16. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Effects of stress on the developing brain. In Cerebrum: The Dana Forum on Brain Science; Dana Foundation: New York, NY, USA, 2011; Volume 2011, p. 14. [Google Scholar]
- Het, S.; Ramlow, G.; Wolf, O.T. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology 2005, 30, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Lupien, S.J.; McEwen, B.S. The acute effects of corticosteroids on cognition: Integration of animal and human model studies. Brain Res. Rev. 1997, 24, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Oei, N.Y.; Everaerd, W.T.; Elzinga, B.M.; van Well, S.; Bermond, B. Psychosocial stress impairs working memory at high loads: An association with cortisol levels and memory retrieval. Stress 2006, 9, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.M.; Xie, W.; Piazzoli, L.; Jensen, S.K.G.; Afreen, S.; Haque, R.; Petri, W.A.; Nelson, C.A. Systemic inflammation during the first year of life is associated with brain functional connectivity and future cognitive outcomes. Dev. Cogn. Neurosci. 2022, 53, 101041. [Google Scholar] [CrossRef] [PubMed]
- Stiles, J.; Jernigan, T.L. The basics of brain development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef]
- Finan, P.H.; Goodin, B.R.; Smith, M.T. The association of sleep and pain: An update and a path forward. J. Pain 2013, 14, 1539–1552. [Google Scholar] [CrossRef]
- Alhola, P.; Polo-Kantola, P. Sleep deprivation: Impact on cognitive performance. Neuropsychiatr. Dis. Treat. 2007, 3, 553–567. [Google Scholar]
- Csipo, T.; Lipecz, A.; Owens, C.; Mukli, P.; Perry, J.W.; Tarantini, S.; Balasubramanian, P.; Nyúl-Tóth, Á.; Yabluchanska, V.; Sorond, F.A.; et al. Sleep deprivation impairs cognitive performance, alters task-associated cerebral blood flow and decreases cortical neurovascular coupling-related hemodynamic responses. Sci. Rep. 2021, 11, 20994. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, K.; Reid, G.J.; Morton, J.B. Behavioral Sleep Problems and their Potential Impact on Developing Executive Function in Children. Sleep 2013, 36, 1077–1084. [Google Scholar] [CrossRef]
- Hirotsu, C.; Tufik, S.; Andersen, M.L. Interactions between sleep, stress, and metabolism: From physiological to pathological conditions. Sleep Sci. 2015, 8, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Sardana, D.; Galland, B.; Wheeler, B.J.; Yiu, C.K.Y.; Ekambaram, M. Effect of sleep on development of early childhood caries: A systematic review. Eur. Arch. Paediatr. Dent. 2023, 24, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L.; Miller, S.E.; LeBourgeois, M.K.; Sturza, J.; Rosenblum, K.L.; Lumeng, J.C. Sleep duration and quality are associated with eating behavior in low-income toddlers. Appetite 2019, 135, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Arun, R.; Pina, P.; Rubin, D.; Erichsen, D. Association between sleep stages and hunger scores in 36 children. Pediatr. Obes. 2016, 11, e9–e11. [Google Scholar] [CrossRef] [PubMed]
- Burt, J.; Dube, L.; Thibault, L.; Gruber, R. Sleep and eating in childhood: A potential behavioral mechanism underlying the relationship between poor sleep and obesity. Sleep Med. 2014, 15, 71–75. [Google Scholar] [CrossRef]
- Hermes, F.N.; Nunes, E.E.M.; Melo, C.M. Sleep, nutritional status and eating behavior in children: A review study. Rev. Paul. Pediatr. 2022, 40, e2020479. [Google Scholar] [CrossRef]
- Mohamed, R.N.; Basha, S.; Al-Thomali, Y.; AlZahrani, F.S.; Ashour, A.A.; Almutair, N.E. Association Between Early Childhood Caries and Obesity among Preschool Children. Oral Health Prev. Dent. 2022, 20, 113–118. [Google Scholar] [CrossRef]
- Greer, S.M.; Goldstein, A.N.; Walker, M.P. The impact of sleep deprivation on food desire in the human brain. Nat. Commun. 2013, 4, 2259. [Google Scholar] [CrossRef]
- Tikhonova, S.; Booij, L.; D’Souza, V.; Crosara, K.T.B.; Siqueira, W.L.; Emami, E. Investigating the association between stress, saliva and dental caries: A scoping review. BMC Oral Health 2018, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Du Teil Espina, M.; Gabarrini, G.; Harmsen, H.J.M.; Westra, J.; van Winkelhoff, A.J.; van Dijl, J.M. Talk to your gut: The oral-gut microbiome axis and its immunomodulatory role in the etiology of rheumatoid arthritis. FEMS Microbiol. Rev. 2019, 43, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Tooley, K.L. Effects of the Human Gut Microbiota on Cognitive Performance, Brain Structure and Function: A Narrative Review. Nutrients 2020, 12, 3009. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. A Clin. J. 2018, 17, 28. [Google Scholar]
- Osadchiy, V.; Labus, J.S.; Gupta, A.; Jacobs, J.; Ashe-McNalley, C.; Hsiao, E.Y.; Mayer, E.A. Correlation of tryptophan metabolites with connectivity of extended central reward network in healthy subjects. PLoS ONE 2018, 13, e0201772. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Mackos, A.R.; Maltz, R.; Bailey, M.T. The role of the commensal microbiota in adaptive and maladaptive stressor-induced immunomodulation. Horm. Behav. 2017, 88, 70–78. [Google Scholar] [CrossRef]
- Karl, J.P.; Margolis, L.M.; Madslien, E.H.; Murphy, N.E.; Castellani, J.W.; Gundersen, Y.; Hoke, A.V.; Levangie, M.W.; Kumar, R.; Chakraborty, N.; et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G559–G571. [Google Scholar] [CrossRef]
- Galley, J.D.; Bailey, M.T. Impact of stressor exposure on the interplay between commensal microbiota and host inflammation. Gut Microbes 2014, 5, 390–396. [Google Scholar] [CrossRef]
- Sarkar, A.; Harty, S.; Dunbar, R.I.M.; Lehto, S.M.; Moeller, A.H.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. The Microbiome in Psychology and Cognitive Neuroscience. Trends Cogn. Sci. 2018, 22, 611–636. [Google Scholar] [CrossRef]
- Schmidt, K.; Cowen, P.; Harmer, C.; Tzortzis, G.; Errington, S.; Burnet, P. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 2015, 232, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, M.; Delaleu, N.; Du, Y.; Bickel, M. Cytokine gene expression--part of host defence in pulpitis. Cytokine 2003, 22, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Zoico, E.; Roubenoff, R. The role of cytokines in regulating protein metabolism and muscle function. Nutr. Rev. 2002, 60, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, A.; Bahrololoomi, Z.; Salarian, S. Relationship between early childhood caries and anemia: A systematic review. Iran. J. Pediatr. Hematol. Oncol. 2018, 8, 126–138. [Google Scholar]
- Sheetal, A.; Hiremath, V.K.; Patil, A.G.; Sajjansetty, S.; Kumar, S.R. Malnutrition and its oral outcome—A review. J. Clin. Diagn. Res. 2013, 7, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Schroth, R.; Jeal, N.; Kliewer, E.; Sellers, A.C. The relationship between vitamin D and severe early childhood caries: A pilot study. Int. J. Vitam. Nutr. Res. 2012, 82, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Schroth, R.J.; Levi, J.A.; Sellers, E.A.; Friel, J.; Kliewer, E.; Moffatt, M.E. Vitamin D status of children with severe early childhood caries: A case-control study. BMC Pediatr. 2013, 13, 174. [Google Scholar] [CrossRef]
- Georgieff, M.K. The role of iron in neurodevelopment: Fetal iron deficiency and the developing hippocampus. Biochem. Soc. Trans. 2008, 36 Pt 6, 1267–1271. [Google Scholar] [CrossRef]
- Tafti, M.; Ghyselinck, N.B. Functional implication of the vitamin A signaling pathway in the brain. Arch. Neurol. 2007, 64, 1706–1711. [Google Scholar] [CrossRef]
- Anjum, I.; Jaffery, S.S.; Fayyaz, M.; Samoo, Z.; Anjum, S. The Role of Vitamin D in Brain Health: A Mini Literature Review. Cureus 2018, 10, e2960. [Google Scholar] [CrossRef]
- Llewellyn, D.J.; Langa, K.M.; Friedland, R.P.; Lang, I.A. Serum albumin concentration and cognitive impairment. Curr. Alzheimer Res. 2010, 7, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, L.M. Immune proteins in brain development and synaptic plasticity. Neuron 2009, 64, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Reisine, S.T.; Psoter, W. Socioecnomic status and selected behavioural determinants as risk factors for dental caries. J. Dent. Educ. 2001, 65, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, D.; Whittle, S.; Sheridan, M.A.; McLaughlin, K.A. Childhood socioeconomic status and the pace of structural neurodevelopment: Accelerated, delayed, or simply different? Trends Cogn. Sci. 2023, 27, 833–851. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. 175 Million Children Are Not Enrolled in Pre-Primary Education. Available online: https://www.unicef.org/press-releases/175-million-children-are-not-enrolled-pre-primary-education-unicef (accessed on 30 July 2023).
- Santos-Beneit, G.; Fernández-Jiménez, R.; de Cos-Gandoy, A.; Rodríguez, C.; Carral, V.; Bodega, P.; de Miguel, M.; Orrit, X.; Haro, D.; Peñalvo, J.L.; et al. Lessons Learned From 10 Years of Preschool Intervention for Health Promotion: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Riggs, E.; Kilpatrick, N.; Slack-Smith, L.; Chadwick, B.; Yelland, J.; Muthu, M.S.; Gomersall, J.C. Interventions with pregnant women, new mothers and other primary caregivers for preventing early childhood caries. Cochrane Database Syst. Rev. 2019, 2019, CD012155. [Google Scholar] [CrossRef] [PubMed]
- Weber-Gasparoni, K.; Warren, J.J.; Reeve, J.; Drake, D.R.; Kramer, K.W.; Marshall, T.A.; Dawson, D.V. An effective psychoeducational intervention for early childhood caries prevention: Part II. Pediatr. Dent. 2013, 35, 247–251. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).