Public Health Needs the Public Trust: A Pandemic Retrospective
Abstract
1. Introduction: A Loss of Trust
Objectives
2. Reasons for Distrust
2.1. Censorship
2.2. Narrowness and Inflexibility of Public Health Response
2.3. Conflicts of Interest and Regulatory Capture
2.4. Bioethical Violations
2.5. The Price of Distrust
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saechang, O.; Yu, J.; Li, Y. Public Trust and Policy Compliance during the COVID-19 Pandemic: The Role of Professional Trust. Healthcare 2021, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Adamecz, A.; Szabó-Morvai, Á. Confidence in Public Institutions Is Critical in Containing the COVID-19 Pandemic. World Med. Health Policy 2023, 1–17. [Google Scholar] [CrossRef]
- Kennedy, B.; Tyson, A.; Funk, C. Americans’ Trust in Scientists, Other Groups Declines. Pew Res. Cent. Sci. Soc. 2022, 1–25. Available online: https://www.pewresearch.org/science/2022/02/09/increasing-public-criticism-confusion-over-covid-19-response-in-u-s/ (accessed on 21 October 2022).
- Aksoy, C.; Eichengreen, B.J.; Saka, O. COVID-19 and Trust among the Young. Financ. Dev. 2022, 6. Available online: https://www.imf.org/en/Publications/fandd/issues/2022/06/covid-19-and-trust-among-the-young-aksoy-eichengreen-saka (accessed on 21 October 2022).
- Trust, W. How COVID-19 Has Increased the World’s Trust in Science. Available online: https://phys.org/news/2021-11-covid-world-science.html (accessed on 2 October 2022).
- Bromme, R.; Mede, N.G.; Thomm, E.; Kremer, B.; Ziegler, R. An Anchor in Troubled Times: Trust in Science before and within the COVID-19 Pandemic. PLoS ONE 2022, 17, e0262823. [Google Scholar] [CrossRef]
- Goldstein, D.A.N.; Wiedemann, J. Who Do You Trust? The Consequences of Partisanship and Trust for Public Responsiveness to COVID-19 Orders. Perspect. Politics 2022, 20, 412–438. [Google Scholar] [CrossRef]
- The Public’s Perspective on the United States Public Health System. Available online: https://www.rwjf.org/en/library/research/2021/05/the-publics-perspective-on-the-united-states-public-health-system.html (accessed on 21 October 2022).
- 2022 Edelman Trust Barometer. Available online: https://www.edelman.com/trust/2022-trust-barometer (accessed on 21 October 2022).
- van der Cruijsen, C.; de Haan, J.; Jonker, N. Has the COVID-19 Pandemic Affected Public Trust? Evidence for the US and the Netherlands. J. Econ. Behav. Organ. 2022, 200, 1010–1024. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Niu, X. The COVID-19 Pandemic Reduces Trust Behavior. Econ. Lett. 2021, 199, 109700. [Google Scholar] [CrossRef]
- Freeman, D.; Waite, F.; Rosebrock, L.; Petit, A.; Causier, C.; East, A.; Jenner, L.; Teale, A.-L.; Carr, L.; Mulhall, S.; et al. Coronavirus Conspiracy Beliefs, Mistrust, and Compliance with Government Guidelines in England. Psychol. Med. 2022, 52, 251–263. [Google Scholar] [CrossRef]
- Sokol, R.L.; Grummon, A.H. COVID-19 and Parent Intention to Vaccinate Their Children Against Influenza. Pediatrics 2020, 146, e2020022871. [Google Scholar] [CrossRef]
- Lee, D.I.D.; Vanderhout, S.; Aglipay, M.; Birken, C.S.; Morris, S.K.; Piché-Renaud, P.-P.; Keown-Stoneman, C.D.G.; Maguire, J.L. Delay in Childhood Vaccinations during the COVID-19 Pandemic. Can. J. Public Health 2022, 113, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, G. Pandemic Drives Largest Drop in Childhood Vaccinations in 30 Years. Nature 2022, 608, 253. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque Veloso Machado, M.; Roberts, B.; Wong, B.L.H.; van Kessel, R.; Mossialos, E. The Relationship Between the COVID-19 Pandemic and Vaccine Hesitancy: A Scoping Review of Literature Until August 2021. Front. Public Health 2021, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Khubchandani, J.; Sharma, S.; Price, J.H.; Wiblishauser, M.J.; Sharma, M.; Webb, F.J. COVID-19 Vaccination Hesitancy in the United States: A Rapid National Assessment. J. Community Health 2021, 46, 270–277. [Google Scholar] [CrossRef] [PubMed]
- SeyedAlinaghi, S.; Karimi, A.; Mojdeganlou, H.; Alilou, S.; Mirghaderi, S.P.; Noori, T.; Shamsabadi, A.; Dadras, O.; Vahedi, F.; Mohammadi, P.; et al. Impact of COVID-19 Pandemic on Routine Vaccination Coverage of Children and Adolescents: A Systematic Review. Health Sci. Rep. 2022, 5, e00516. [Google Scholar] [CrossRef]
- He, K.; Mack, W.J.; Neely, M.; Lewis, L.; Anand, V. Parental Perspectives on Immunizations: Impact of the COVID-19 Pandemic on Childhood Vaccine Hesitancy. J. Community Health 2022, 47, 39–52. [Google Scholar] [CrossRef]
- Marques, M.D.; Kerr, J.R.; Williams, M.N.; Ling, M.; McLennan, J. Associations between Conspiracism and the Rejection of Scientific Innovations. Public Underst. Sci. 2021, 30, 854–867. [Google Scholar] [CrossRef]
- Winter, T.; Riordan, B.C.; Scarf, D.; Jose, P.E. Conspiracy Beliefs and Distrust of Science Predicts Reluctance of Vaccine Uptake of Politically Right-Wing Citizens. Vaccine 2022, 40, 1896–1903. [Google Scholar] [CrossRef]
- Johnson, N.; Sparks, G.; Sparks, C. Anti-Scientific Beliefs Predict Health Behaviors during the COVID-19 Pandemic. JCOM 2022, 21, A04. [Google Scholar] [CrossRef]
- Hornsey, M.J.; Lobera, J.; Díaz-Catalán, C. Vaccine Hesitancy Is Strongly Associated with Distrust of Conventional Medicine, and Only Weakly Associated with Trust in Alternative Medicine. Soc. Sci. Med. 2020, 255, 113019. [Google Scholar] [CrossRef]
- Paudyal, V.; Sun, S.; Hussain, R.; Abutaleb, M.H.; Hedima, E.W. Complementary and Alternative Medicines Use in COVID-19: A Global Perspective on Practice, Policy and Research. Res. Soc. Adm. Pharm. 2022, 18, 2524–2528. [Google Scholar] [CrossRef] [PubMed]
- Portella, C.F.S.; Ghelman, R.; Abdala, C.V.M.; Schveitzer, M.C. Evidence Map on the Contributions of Traditional, Complementary and Integrative Medicines for Health Care in Times of COVID-19. Integr. Med. Res. 2020, 9, 100473. [Google Scholar] [CrossRef] [PubMed]
- Charan, J.; Bhardwaj, P.; Dutta, S.; Kaur, R.; Bist, S.K.; Detha, M.D.; Kanchan, T.; Yadav, D.; Mitra, P.; Sharma, P. Use of Complementary and Alternative Medicine (CAM) and Home Remedies by COVID-19 Patients: A Telephonic Survey. Ind. J. Clin. Biochem. 2021, 36, 108–111. [Google Scholar] [CrossRef]
- Alyami, H.S.; Orabi, M.A.A.; Aldhabbah, F.M.; Alturki, H.N.; Aburas, W.I.; Alfayez, A.I.; Alharbi, A.S.; Almasuood, R.A.; Alsuhaibani, N.A. Knowledge about COVID-19 and Beliefs about and Use of Herbal Products during the COVID-19 Pandemic: A Cross-Sectional Study in Saudi Arabia. Saudi Pharm. J. 2020, 28, 1326–1332. [Google Scholar] [CrossRef]
- Kretchy, I.A.; Boadu, J.A.; Kretchy, J.-P.; Agyabeng, K.; Passah, A.A.; Koduah, A.; Opuni, K.F.M. Utilization of Complementary and Alternative Medicine for the Prevention of COVID-19 Infection in Ghana: A National Cross-Sectional Online Survey. Prev. Med. Rep. 2021, 24, 101633. [Google Scholar] [CrossRef]
- Lam, C.S.; Koon, H.K.; Chung, V.C.-H.; Cheung, Y.T. A Public Survey of Traditional, Complementary and Integrative Medicine Use during the COVID-19 Outbreak in Hong Kong. PLoS ONE 2021, 16, e0253890. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.W.; Li, Q.Y.; Liu, J.; Efferth, T. Traditional Chinese Herbal Medicine at the Forefront Battle against COVID-19: Clinical Experience and Scientific Basis. Phytomedicine 2021, 80, 153337. [Google Scholar] [CrossRef]
- Yimer, G.; Ekuadzi, E.; Fasinu, P.; de Melo, A.C.; Pillai, G. Traditional Medicines for COVID-19: Perspectives from Clinical Pharmacologists. Br. J. Clin. Pharmacol. 2021, 87, 3455–3458. [Google Scholar] [CrossRef]
- Kolhe, R.; Pushpan, R.; Prasad, G.P.; Gurav, A.; Srikanth, N. A Survey among Ayurveda Wholesalers and Retailers in Pune City for Understanding the Demand for Ayurvedic Medicines during the COVID-19 Pandemic. J. Indian Syst. Med. 2021, 9, 191. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Tran, V.D.; Pham, D.T.; Dao, T.N.P.; Dewey, R.S. Use of and Attitudes towards Herbal Medicine during the COVID-19 Pandemic: A Cross-Sectional Study in Vietnam. Eur. J. Integr. Med. 2021, 44, 101328. [Google Scholar] [CrossRef]
- Chaachouay, N.; Douira, A.; Zidane, L. COVID-19, Prevention and Treatment with Herbal Medicine in the Herbal Markets of Salé Prefecture, North-Western Morocco. Eur. J. Integr. Med. 2021, 42, 101285. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.; Bhamra, S.K.; Pendry, B.; Heinrich, M. COVID-19 and Herbal Practice: A United Kingdom Practitioner Survey. Adv. Integr. Med. 2021, 8, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R. Dietary Supplements and Nutraceuticals Market Growth during the Coronavirus Pandemic–Implications for Consumers and Regulatory Oversight. PharmaNutrition 2021, 18, 100282. [Google Scholar] [CrossRef] [PubMed]
- Karbownik, M.S.; Dobielska, M.; Paul, E.; Kowalczyk, R.P.; Kowalczyk, E. Health-, Medication- and Dietary Supplement-Related Behaviors and Beliefs Relatively Unchanged during the COVID-19 Pandemic Lockdown. Res. Soc. Adm. Pharm. 2021, 17, 1501–1506. [Google Scholar] [CrossRef]
- Stub, T.; Jong, M.C.; Kristoffersen, A.E. The Impact of COVID-19 on Complementary and Alternative Medicine Providers: A Cross-Sectional Survey in Norway. Adv. Integr. Med. 2021, 8, 247–255. [Google Scholar] [CrossRef]
- Therapeutic Products Bill-New Zealand Parliament. Available online: https://www.parliament.nz/en/pb/sc/make-a-submission/document/53SCHE_SCF_BILL_130084/therapeutic-products-bill (accessed on 15 January 2023).
- Joshi, G.; Thakur, S.; Mayank; Poduri, R. Exploring Insights of Hydroxychloroquine, a Controversial Drug in COVID-19: An Update. Food Chem. Toxicol. 2021, 151, 112106. [Google Scholar] [CrossRef]
- Castillejos-López, M.; Torres-Espíndola, L.M.; Huerta-Cruz, J.C.; Flores-Soto, E.; Romero-Martinez, B.S.; Velázquez-Cruz, R.; Higuera-Iglesias, A.; Camarena, Á.; Torres-Soria, A.K.; Salinas-Lara, C.; et al. Ivermectin: A Controversial Focal Point during the COVID-19 Pandemic. Life 2022, 12, 1384. [Google Scholar] [CrossRef]
- Australian Department of Health and Aged Care Therapeutic Goods Administration. New Restrictions on Prescribing Ivermectin for COVID-19. Available online: https://www.tga.gov.au/news/media-releases/new-restrictions-prescribing-ivermectin-covid-19 (accessed on 15 January 2023).
- Bryant, A.; Lawrie, T.A.; Dowswell, T.; Fordham, E.J.; Mitchell, S.; Hill, S.R.; Tham, T.C. Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Meta-Analysis, and Trial Sequential Analysis to Inform Clinical Guidelines. Am. J. Ther. 2021, 28, e434–e460. [Google Scholar] [CrossRef]
- Brata, A.M.; Chereji, A.I.; Brata, V.D.; Morna, A.A.; Tirpe, O.P.; Popa, A.; Arion, F.H.; Banszki, L.I.; Chereji, I.; Popa, D.; et al. Consumers’ Perception towards Organic Products before and after the COVID-19 Pandemic: A Case Study in Bihor County, Romania. Int. J. Environ. Res. Public Health 2022, 19, 12712. [Google Scholar] [CrossRef]
- Güney, O.I.; Sangün, L. How COVID-19 Affects Individuals’ Food Consumption Behaviour: A Consumer Survey on Attitudes and Habits in Turkey. Br. Food J. 2021, 123, 2307–2320. [Google Scholar] [CrossRef]
- Organic Foods Getting Coronavirus Boost. Available online: https://www.ecoviaint.com/organic-foods-getting-coronavirus-boost/ (accessed on 11 January 2023).
- Tariga, J.N.; Nolasco, D.P.; Barayuga, S.J.R. Food Consumption Habits of Consumers in the Philippines: Changes amidst the Pandemic. Int. J. Public Health Sci. (IJPHS) 2021, 10, 662–669. [Google Scholar] [CrossRef]
- Cong, L.; Bremer, P.; Kaye-Blake, W.; Mirosa, M. Chinese Consumers’ Perceptions of Immune Health and Immune-Boosting Remedies Including Functional Foods. J. Food Prod. Mark. 2020, 26, 55–78. [Google Scholar] [CrossRef]
- Koswatta, T.J.; Wingenbach, G.; Leggette, H.R.; Murphrey, T.P. Factors Affecting Public Perception of Scientific Information about Organic Foods. Br. Food J. 2022; ahead-of-print. [Google Scholar] [CrossRef]
- Okruszek, Ł.; Piejka, A.; Banasik-Jemielniak, N.; Jemielniak, D. Climate Change, Vaccines, GMO: The N400 Effect as a Marker of Attitudes toward Scientific Issues. PLoS ONE 2022, 17, e0273346. [Google Scholar] [CrossRef] [PubMed]
- Ackah, B.B.B.; Woo, M.; Stallwood, L.; Fazal, Z.A.; Okpani, A.; Ukah, U.V.; Adu, P.A. COVID-19 Vaccine Hesitancy in Africa: A Scoping Review. Glob. Health Res. Policy 2022, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Shah, Z.; Garfield, S. Causes of Vaccine Hesitancy in Adults for the Influenza and COVID-19 Vaccines: A Systematic Literature Review. Vaccines 2022, 10, 1518. [Google Scholar] [CrossRef]
- Peterson, C.J.; Lee, B.; Nugent, K. COVID-19 Vaccination Hesitancy among Healthcare Workers—A Review. Vaccines 2022, 10, 948. [Google Scholar] [CrossRef]
- Schwarzinger, M.; Watson, V.; Arwidson, P.; Alla, F.; Luchini, S. COVID-19 Vaccine Hesitancy in a Representative Working-Age Population in France: A Survey Experiment Based on Vaccine Characteristics. Lancet Public Health 2021, 6, e210–e221. [Google Scholar] [CrossRef]
- Ward, J.K.; Alleaume, C.; Peretti-Watel, P.; COCONEL Group. The French Public’s Attitudes to a Future COVID-19 Vaccine: The Politicization of a Public Health Issue. Soc. Sci. Med. 2020, 265, 113414. [Google Scholar] [CrossRef]
- Hacquin, A.-S.; Altay, S.; Araujo, E.D.; Chevallier, C.; Mercier, H. Sharp Rise in Vaccine Hesitancy in a Large and Representative Sample of the French Population: Reasons for Vaccine Hesitancy. PsyArXiv 2020. [Google Scholar] [CrossRef]
- Roozenbeek, J.; Schneider, C.R.; Dryhurst, S.; Kerr, J.; Freeman, A.L.J.; Recchia, G.; van der Bles, A.M.; van der Linden, S. Susceptibility to Misinformation about COVID-19 around the World. R. Soc. Open Sci. 2020, 7, 201199. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Wyka, K.; White, T.M.; Picchio, C.A.; Rabin, K.; Ratzan, S.C.; Parsons Leigh, J.; Hu, J.; El-Mohandes, A. Revisiting COVID-19 Vaccine Hesitancy around the World Using Data from 23 Countries in 2021. Nat. Commun. 2022, 13, 3801. [Google Scholar] [CrossRef] [PubMed]
- Qunaibi, E.A.; Helmy, M.; Basheti, I.; Sultan, I. A High Rate of COVID-19 Vaccine Hesitancy in a Large-Scale Survey on Arabs. eLife 2021, 10, e68038. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.C.; Comar, M.; Folan, J.; Williams, S.; Kola-Palmer, S. The Psychological and Behavioural Correlates of COVID-19 Vaccine Hesitancy and Resistance in Ireland and the UK. Acta Psychol. 2022, 225, 103550. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; Ackerman, M.S.; Laspra, B.; Polino, C.; Huffaker, J.S. Public Attitude toward COVID-19 Vaccination: The Influence of Education, Partisanship, Biological Literacy, and Coronavirus Understanding. FASEB J. 2022, 36, e22382. [Google Scholar] [CrossRef]
- Ruiz, J.B.; Bell, R.A. Parental COVID-19 Vaccine Hesitancy in the United States. Public Health Rep. 2022, 137, 1162–1169. [Google Scholar] [CrossRef]
- Stroope, S.; Kroeger, R.A.; Williams, C.E.; Baker, J.O. Sociodemographic Correlates of Vaccine Hesitancy in the United States and the Mediating Role of Beliefs about Governmental Conspiracies. Soc. Sci. Q. 2021, 102, 2472–2481. [Google Scholar] [CrossRef]
- Gilles, I.; Le Pogam, M.-A.; Perriraz, M.; Bangerter, A.; Green, E.G.T.; Staerklé, C.; Krings, F.; Wagner-Egger, P.; Peytremann-Bridevaux, I. Trust in Institutions and the COVID-19 Threat: A Cross-Sectional Study on the Public Perception of Official Recommendations and of Othering in Switzerland. Int. J. Public Health 2021, 66, 1604223. [Google Scholar] [CrossRef]
- Bogart, L.M.; Ojikutu, B.O.; Tyagi, K.; Klein, D.J.; Mutchler, M.G.; Dong, L.; Lawrence, S.J.; Thomas, D.R.; Kellman, S. COVID-19 Related Medical Mistrust, Health Impacts, and Potential Vaccine Hesitancy Among Black Americans Living With HIV. J. Acquir. Immune Defic. Syndr. 2021, 86, 200–207. [Google Scholar] [CrossRef]
- Bajaj, S.S.; Stanford, F.C. Beyond Tuskegee—Vaccine Distrust and Everyday Racism. N. Engl. J. Med. 2021, 384, e12. [Google Scholar] [CrossRef]
- Mosby, I.; Swidrovich, J. Medical Experimentation and the Roots of COVID-19 Vaccine Hesitancy among Indigenous Peoples in Canada. CMAJ 2021, 193, E381–E383. [Google Scholar] [CrossRef]
- Muhajarine, N.; Adeyinka, D.A.; McCutcheon, J.; Green, K.L.; Fahlman, M.; Kallio, N. COVID-19 Vaccine Hesitancy and Refusal and Associated Factors in an Adult Population in Saskatchewan, Canada: Evidence from Predictive Modelling. PLoS ONE 2021, 16, e0259513. [Google Scholar] [CrossRef] [PubMed]
- Criss, S.; Nguyen, T.T.; Norton, S.; Virani, I.; Titherington, E.; Tillmanns, E.L.; Kinnane, C.; Maiolo, G.; Kirby, A.B.; Gee, G.C. Advocacy, Hesitancy, and Equity: Exploring U.S. Race-Related Discussions of the COVID-19 Vaccine on Twitter. Int. J. Environ. Res. Public Health 2021, 18, 5693. [Google Scholar] [CrossRef] [PubMed]
- Willis, D.E.; Andersen, J.A.; Bryant-Moore, K.; Selig, J.P.; Long, C.R.; Felix, H.C.; Curran, G.M.; McElfish, P.A. COVID-19 Vaccine Hesitancy: Race/Ethnicity, Trust, and Fear. Clin. Transl. Sci. 2021, 14, 2200–2207. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Joshi, A.D.; Drew, D.A.; Merino, J.; Ma, W.; Lo, C.-H.; Kwon, S.; Wang, K.; Graham, M.S.; Polidori, L.; et al. Self-Reported COVID-19 Vaccine Hesitancy and Uptake among Participants from Different Racial and Ethnic Groups in the United States and United Kingdom. Nat. Commun. 2022, 13, 636. [Google Scholar] [CrossRef]
- Hart, P.S.; Chinn, S.; Soroka, S. Politicization and Polarization in COVID-19 News Coverage. Sci. Commun. 2020, 42, 679–697. [Google Scholar] [CrossRef]
- Loomba, S.; de Figueiredo, A.; Piatek, S.J.; de Graaf, K.; Larson, H.J. Measuring the Impact of COVID-19 Vaccine Misinformation on Vaccination Intent in the UK and USA. Nat. Hum. Behav. 2021, 5, 337–348. [Google Scholar] [CrossRef]
- Trotter, G. COVID-19 and the Authority of Science. HEC Forum 2021, 35, 111–138. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, W. Promoting COVID-19 Vaccination: The Interplay of Message Framing, Psychological Uncertainty, and Public Agency as a Message Source. Sci. Commun. 2021, 44, 3–29. [Google Scholar] [CrossRef]
- Nan, X.; Iles, I.A.; Yang, B.; Ma, Z. Public Health Messaging during the COVID-19 Pandemic and Beyond: Lessons from Communication Science. Health Commun. 2022, 37, 1–19. [Google Scholar] [CrossRef]
- Stolow, J.A.; Moses, L.M.; Lederer, A.M.; Carter, R. How Fear Appeal Approaches in COVID-19 Health Communication May Be Harming the Global Community. Health Educ. Behav. 2020, 47, 531–535. [Google Scholar] [CrossRef]
- Hase, V.; Engelke, K.M. Emotions in Crisis Coverage: How UK News Media Used Fear Appeals to Report on the Coronavirus Crisis. J. Media 2022, 3, 633–649. [Google Scholar] [CrossRef]
- Teye-Kwadjo, E. How Can We Better Frame COVID-19 Public Health Messages? Discov. Psychol. 2022, 2, 30. [Google Scholar] [CrossRef]
- Merton, R.K. The Sociology of Science: Theoretical and Empirical Investigations; University of Chicago Press: Chicago, IL, USA, 1973; ISBN 978-0-226-52092-6. [Google Scholar]
- Niemiec, E. COVID-19 and Misinformation: Is Censorship of Social Media a Remedy to the Spread of Medical Misinformation? EMBO Rep. 2020, 21, e51420. [Google Scholar] [CrossRef]
- Scanlon, T. Scientific Divisions on COVID-19: Time for Open Debate. BMJ 2020, 371, m4538. [Google Scholar] [CrossRef] [PubMed]
- Great Barrington Declaration and Petition. Available online: https://gbdeclaration.org/ (accessed on 2 October 2022).
- Select Subcommittee’s Year-End Staff Report Highlights Oversight Work, Releases New Findings from Ongoing Investigations. Available online: https://coronavirus.house.gov/news/press-releases/select-subcommittee-s-year-end-staff-report-highlights-oversight-work-releases (accessed on 2 October 2022).
- Apple, K. When The Shield Becomes The Sword: The Evolution of Section 230 From A Free Speech Shield To A Sword of Censorship. SSRN 2022. [Google Scholar] [CrossRef]
- Shir-Raz, Y.; Elisha, E.; Martin, B.; Ronel, N.; Guetzkow, J. Censorship and Suppression of COVID-19 Heterodoxy: Tactics and Counter-Tactics. Minerva 2022, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.; McCullough, P.A. WITHDRAWN: A Report on Myocarditis Adverse Events in the U.S. Vaccine Adverse Events Reporting System (VAERS) in Association with COVID-19 Injectable Biological Products. Curr. Probl. Cardiol. 2021, 101011. [Google Scholar] [CrossRef]
- Jiang, H.; Mei, Y.-F. SARS–CoV–2 Spike Impairs DNA Damage Repair and Inhibits V(D)J Recombination In Vitro. Available online: https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/covidwho-1470992 (accessed on 2 October 2022).
- Retraction: Comparative Immunogenicity of HIV-1 Gp160, Gp140 and Gp120 Expressed by Live Attenuated Newcastle Disease Virus Vector. PLoS ONE 2020, 15, e0244046. [CrossRef]
- COVID-19: Global Attack on Freedom of Expression Is Having a Dangerous Impact on Public Health Crisis. Available online: https://amnesty.ca/news/covid-19-global-attack-on-freedom-of-expression-is-having-a-dangerous-impact-on-public-health-crisis/ (accessed on 2 October 2022).
- Nord, L. No Lockdown Please, We Are Swedish: How the Middle Way Country Became an Extreme Case of Government Communication. In Manufacturing Government Communication on COVID-19: A Comparative Perspective; Maarek, P.J., Ed.; Springer Studies in Media and Political Communication; Springer International Publishing: Cham, Switzerland, 2022; pp. 107–121. ISBN 978-3-031-09230-5. [Google Scholar]
- Ludvigsson, J.F. How Sweden Approached the COVID-19 Pandemic: Summary and Commentary on the National Commission Inquiry. Acta Paediatr. 2023, 112, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Comparing Different International Measures of Excess Mortality-Office for National Statistics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/comparingdifferentinternationalmeasuresofexcessmortality/2022-12-20 (accessed on 17 April 2023).
- Levitt, M.; Zonta, F.; Ioannidis, J.P.A. Excess Death Estimates from Multiverse Analysis in 2009–2021. Eur. J. Epidemiol. 2023, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Levitt, M.; Zonta, F.; Ioannidis, J.P.A. Comparison of Pandemic Excess Mortality in 2020–2021 across Different Empirical Calculations. Environ. Res. 2022, 213, 113754. [Google Scholar] [CrossRef] [PubMed]
- Hale, T.; Angrist, N.; Goldszmidt, R.; Kira, B.; Petherick, A.; Phillips, T.; Webster, S.; Cameron-Blake, E.; Hallas, L.; Majumdar, S.; et al. A Global Panel Database of Pandemic Policies (Oxford COVID-19 Government Response Tracker). Nat. Hum. Behav. 2021, 5, 529–538. [Google Scholar] [CrossRef]
- Chu, T.H.; Yeo, T.E.D.; Su, Y. Effects of Exposure to COVID-19 News and Information: A Meta-Analysis of Media Use and Uncertainty-Related Responses During the Pandemic. J. Mass Commun. Q. 2022, 99, 89–112. [Google Scholar] [CrossRef]
- Melki, J.; Tamim, H.; Hadid, D.; Farhat, S.; Makki, M.; Ghandour, L.; Hitti, E. Media Exposure and Health Behavior during Pandemics: The Mediating Effect of Perceived Knowledge and Fear on Compliance with COVID-19 Prevention Measures. Health Commun. 2022, 37, 586–596. [Google Scholar] [CrossRef]
- Price, M.; Legrand, A.C.; Brier, Z.M.F.; van Stolk-Cooke, K.; Peck, K.; Dodds, P.S.; Danforth, C.M.; Adams, Z.W. Doomscrolling during COVID-19: The Negative Association between Daily Social and Traditional Media Consumption and Mental Health Symptoms during the COVID-19 Pandemic. Psychol. Trauma 2022, 14, 1338–1346. [Google Scholar] [CrossRef]
- Thacker, P.D. Conflicts of Interest among the UK Government’s COVID-19 Advisers. BMJ 2020, 371, m4716. [Google Scholar] [CrossRef]
- Omer, S.B.; Salmon, D.A.; Orenstein, W.A.; deHart, M.P.; Halsey, N. Vaccine Refusal, Mandatory Immunization, and the Risks of Vaccine-Preventable Diseases. N. Engl. J. Med. 2009, 360, 1981–1988. [Google Scholar] [CrossRef]
- King, W.C.; Rubinstein, M.; Reinhart, A.; Mejia, R. Time Trends, Factors Associated with, and Reasons for COVID-19 Vaccine Hesitancy: A Massive Online Survey of US Adults from January–May 2021. PLoS ONE 2021, 16, e0260731. [Google Scholar] [CrossRef]
- Lee, C.; Yang, T.; Inchoco, G.D.; Jones, G.M.; Satyanarayan, A. Viral Visualizations: How Coronavirus Skeptics Use Orthodox Data Practices to Promote Unorthodox Science Online. In Proceedings of the 2021 CHI Conference on Human Factors in Computing Systems, Online, 6 May 2021; Association for Computing Machinery: New York, NY, USA, 2021; pp. 1–18. [Google Scholar]
- Herby, J.; Jonung, L.; Hanke, S. A Literature Review and Meta-Analysis of the Effects of Lockdowns on COVID-19 Mortality. Stud. Appl. Econ. 2022, 200, 1–62. Available online: https://ideas.repec.org/p/ris/jhisae/0200.html (accessed on 21 October 2022).
- De Larochelambert, Q.; Marc, A.; Antero, J.; Le Bourg, E.; Toussaint, J.-F. COVID-19 Mortality: A Matter of Vulnerability Among Nations Facing Limited Margins of Adaptation. Front. Public Health 2020, 8, 782. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, T.; Rose, A.; Wei, D. The Impacts of the Coronavirus on the Economy of the United States. Econ. Disasters Clim. Chang. 2021, 5, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Asahi, K.; Undurraga, E.A.; Valdés, R.; Wagner, R. The Effect of COVID-19 on the Economy: Evidence from an Early Adopter of Localized Lockdowns. J. Glob. Health 2021, 11, 05002. [Google Scholar] [CrossRef]
- Verschuur, J.; Koks, E.E.; Hall, J.W. Global Economic Impacts of COVID-19 Lockdown Measures Stand out in High-Frequency Shipping Data. PLoS ONE 2021, 16, e0248818. [Google Scholar] [CrossRef]
- Fairlie, R.; Fossen, F. Sales Losses in the First Quarter of the COVID-19 Pandemic: Evidence from California Administrative Data; National Bureau of Economic Research: Cambridge, MA, USA, 2021; p. w28414. [Google Scholar]
- Evans, S.; Alkan, E.; Bhangoo, J.K.; Tenenbaum, H.; Ng-Knight, T. Effects of the COVID-19 Lockdown on Mental Health, Wellbeing, Sleep, and Alcohol Use in a UK Student Sample. Psychiatry Res. 2021, 298, 113819. [Google Scholar] [CrossRef]
- Birmingham, W.C.; Wadsworth, L.L.; Lassetter, J.H.; Graff, T.C.; Lauren, E.; Hung, M. COVID-19 Lockdown: Impact on College Students’ Lives. J. Am. Coll. Health 2021, 71, 879–893. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Vishwakarma, D.K.; Singh, N. COVID-19 and Its Impact on Education, Social Life and Mental Health of Students: A Survey. Child Youth Serv. Rev. 2021, 121, 105866. [Google Scholar] [CrossRef]
- PACE-Changing Patterns of Growth in Oral Reading Fluency During the COVID-19 Pandemic. Available online: https://edpolicyinca.org/publications/changing-patterns-growth-oral-reading-fluency-during-covid-19-pandemic (accessed on 2 October 2022).
- The Shadow Pandemic: Violence against Women during COVID-19|UN Women–Headquarters. Available online: https://www.unwomen.org/en/news/in-focus/in-focus-gender-equality-in-covid-19-response/violence-against-women-during-covid-19 (accessed on 2 October 2022).
- Boserup, B.; McKenney, M.; Elkbuli, A. Alarming Trends in US Domestic Violence during the COVID-19 Pandemic. Am. J. Emerg. Med. 2020, 38, 2753–2755. [Google Scholar] [CrossRef]
- Bhavsar, V.; Kirkpatrick, K.; Calcia, M.; Howard, L.M. Lockdown, Domestic Abuse Perpetration, and Mental Health Care: Gaps in Training, Research, and Policy. Lancet Psychiatry 2021, 8, 172–174. [Google Scholar] [CrossRef]
- Darius, P.; Urquhart, M. Disinformed Social Movements: A Large-Scale Mapping of Conspiracy Narratives as Online Harms during the COVID-19 Pandemic. Online Soc. Netw. Media 2021, 26, 100174. [Google Scholar] [CrossRef]
- CDC Director Rochelle Walensky: Too Little Caution and Too Much Optimism. Available online: https://www.youtube.com/watch?v=8DPS4nBFXBo (accessed on 21 October 2022).
- Gibson, J. Public Misunderstanding of Pivotal COVID-19 Vaccine Trials May Contribute to New Zealand’s Adoption of a Costly and Economically Inefficient Vaccine Mandate. N. Z. Econ. Pap. 2023, 57, 31–40. [Google Scholar] [CrossRef]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of SARS-CoV-2 Reinfection and COVID-19 Hospitalisation in Individuals with Natural and Hybrid Immunity: A Retrospective, Total Population Cohort Study in Sweden. Lancet Infect. Dis. 2022, 22, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Siddle, K.J.; Krasilnikova, L.A.; Moreno, G.K.; Schaffner, S.F.; Vostok, J.; Fitzgerald, N.A.; Lemieux, J.E.; Barkas, N.; Loreth, C.; Specht, I.; et al. Transmission from Vaccinated Individuals in a Large SARS-CoV-2 Delta Variant Outbreak. Cell 2022, 185, 485–492.e10. [Google Scholar] [CrossRef] [PubMed]
- Gharpure, R.; Sami, S.; Vostok, J.; Johnson, H.; Hall, N.; Foreman, A.; Sabo, R.T.; Schubert, P.L.; Shephard, H.; Brown, V.R.; et al. Multistate Outbreak of SARS-CoV-2 Infections, Including Vaccine Breakthrough Infections, Associated with Large Public Gatherings, United States. Emerg. Infect. Dis. 2022, 28, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Mastrovito, B.; Naimi, C.; Kouam, L.; Naudot, X.; Fournier, L.; Spaccaferri, G.; Plantier, J.-C.; Soares, A.; Oliveira, F.D.; Gueudin, M.; et al. Investigation of Outbreak Cases Infected with the SARS-CoV-2 B.1.640 Variant in a Fully Vaccinated Elderly Population, Normandy, France, November to December 2021. Eurosurveillance 2022, 27, 2200078. [Google Scholar] [CrossRef]
- Aarstad, J.; Kvitastein, O.A. Is There a Link between the 2021 COVID-19 Vaccination Uptake in Europe and 2022 Excess All-Cause Mortality? Asian Pac. J. Health Sci. 2023, 10, 25–31. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Emergency Use Authorization (EUA) for an Unapproved Product Review Memorandum. 2020. Available online: https://www.fda.gov/media/144416/download (accessed on 21 October 2022).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Moline, H.L. Effectiveness of COVID-19 Vaccines in Preventing Hospitalization Among Adults Aged ≥ 65 Years—COVID-NET, 13 States, February–April 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1088–1093. [Google Scholar] [CrossRef]
- Leung, G.; Verma, A. Epidemiological Study of COVID-19 Fatalities and Vaccine Uptake: Insight From a Public Health Database in Ontario, Canada. Cureus 2021, 13, e16160. [Google Scholar] [CrossRef]
- Bardosh, K.; Krug, A.; Jamrozik, E.; Lemmens, T.; Keshavjee, S.; Prasad, V.; Makary, M.A.; Baral, S.; Høeg, T.B. COVID-19 Vaccine Boosters for Young Adults: A Risk-Benefit Assessment and Five Ethical Arguments against Mandates at Universities. J. Med. Ethics 2022. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Esposito, D.; de los Campos, G. Age-Specific Rate of Severe and Critical SARS-CoV-2 Infections Estimated with Multi-Country Seroprevalence Studies. BMC Infect. Dis. 2022, 22, 311. [Google Scholar] [CrossRef] [PubMed]
- Gazit, S.; Shlezinger, R.; Perez, G.; Lotan, R.; Peretz, A.; Ben-Tov, A.; Herzel, E.; Alapi, H.; Cohen, D.; Muhsen, K.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Naturally Acquired Immunity versus Vaccine-Induced Immunity, Reinfections versus Breakthrough Infections: A Retrospective Cohort Study. Clin. Infect. Dis. 2022, 75, e545–e551. [Google Scholar] [CrossRef] [PubMed]
- Erener, S. Diabetes, Infection Risk and COVID-19. Mol. Metab. 2020, 39, 101044. [Google Scholar] [CrossRef]
- Demeulemeester, F.; de Punder, K.; van Heijningen, M.; van Doesburg, F. Obesity as a Risk Factor for Severe COVID-19 and Complications: A Review. Cells 2021, 10, 933. [Google Scholar] [CrossRef]
- UC COVID-19 Vaccine and Booster Requirements|University Health Services. Available online: https://uhs.berkeley.edu/requirements/covid19 (accessed on 1 October 2022).
- Coronavirus Vaccinations for Children and Young People-THL. Available online: https://thl.fi/en/web/infectious-diseases-and-vaccinations/what-s-new/coronavirus-covid-19-latest-updates/vaccines-and-coronavirus/coronavirus-vaccinations-for-children-and-young-people (accessed on 1 October 2022).
- Áframhaldandi Notkun COVID-19 Bóluefnis Moderna á Íslandi. Available online: https://www.landlaeknir.is/um-embaettid/frettir/frett/item47722/aframhaldandi-notkun-covid-19-boluefnis-moderna-a-islandi (accessed on 1 October 2022).
- Vaccination against COVID-19. Available online: https://www.sst.dk/en/english/corona-eng/vaccination-against-covid-19 (accessed on 1 October 2022).
- Switzerland Not Recommending COVID Vaccines, Even for High Risk Individuals, during Spring and Summer|WLOS. Available online: https://wlos.com/news/nation-world/switzerland-not-recommending-covid-vaccines-even-for-high-risk-individuals-during-spring-and-summer (accessed on 21 April 2023).
- Almashat, S.; Wolfe, S.M.; Carome, M. Twenty-Five Years of Pharmaceutical Industry Criminal and Civil Penalties: 1991 through 2015. Public Citiz. 2016. Available online: https://www.citizen.org/article/twenty-five-years-of-pharmaceutical-industry-criminal-and-civil-penalties-1991-through-2015/ (accessed on 21 October 2022).
- Lenzer, J. US Drug Company Executives Could Face Criminal Charges for Off-Label Promotion. BMJ 2010, 341, c5808. [Google Scholar] [CrossRef]
- Justice Department Announces Largest Health Care Fraud Settlement in Its History. Available online: https://www.justice.gov/opa/pr/justice-department-announces-largest-health-care-fraud-settlement-its-history (accessed on 1 October 2022).
- Hawkes, N. GlaxoSmithKline Pays $3bn to Settle Dispute over Rosiglitazone and Other Drugs. BMJ 2011, 343, d7234. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. Why Most Published Research Findings Are False. PLOS Med. 2005, 2, e124. [Google Scholar] [CrossRef]
- Diels, J.; Cunha, M.; Manaia, C.; Sabugosa-Madeira, B.; Silva, M. Association of Financial or Professional Conflict of Interest to Research Outcomes on Health Risks or Nutritional Assessment Studies of Genetically Modified Products. Food Policy 2011, 36, 197–203. [Google Scholar] [CrossRef]
- Porter, J.; Jick, H. Addiction Rare in Patients Treated with Narcotics. N. Engl. J. Med. 1980, 302, 123. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.T.M.; Macdonald, E.M.; Stanbrook, M.B.; Dhalla, I.A.; Juurlink, D.N. A 1980 Letter on the Risk of Opioid Addiction. N. Engl. J. Med. 2017, 376, 2194–2195. [Google Scholar] [CrossRef] [PubMed]
- Gale, A.H. Drug Company Compensated Physicians Role in Causing America’s Deadly Opioid Epidemic: When Will We Learn? Mo. Med. 2016, 113, 244–246. [Google Scholar] [PubMed]
- Vowles, K.E.; McEntee, M.L.; Julnes, P.S.; Frohe, T.; Ney, J.P.; van der Goes, D.N. Rates of Opioid Misuse, Abuse, and Addiction in Chronic Pain: A Systematic Review and Data Synthesis. Pain 2015, 156, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Raad, R.; Appelbaum, P.S. Relationships between Medicine and Industry: Approaches to the Problem of Conflicts of Interest. Annu. Rev. Med. 2012, 63, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Lexchin, J.; Bero, L.A.; Djulbegovic, B.; Clark, O. Pharmaceutical Industry Sponsorship and Research Outcome and Quality: Systematic Review. BMJ 2003, 326, 1167. [Google Scholar] [CrossRef]
- Sinha, M.S.; Kesselheim, A.S.; Darrow, J.J. Pharmaceutical Advertising in Medical Journals: Revisiting a Long-Standing Relationship. CHEST 2018, 153, 9–11. [Google Scholar] [CrossRef]
- Smith, R. Medical Journals Are an Extension of the Marketing Arm of Pharmaceutical Companies. PLOS Med. 2005, 2, e138. [Google Scholar] [CrossRef]
- Glauser, W. Pharma Influence Widespread at Medical Schools: Study. CMAJ 2013, 185, 1121–1122. [Google Scholar] [CrossRef]
- Colombo, C.; Mosconi, P.; Villani, W.; Garattini, S. Patient Organizations’ Funding from Pharmaceutical Companies: Is Disclosure Clear, Complete and Accessible to the Public? An Italian Survey. PLoS ONE 2012, 7, e34974. [Google Scholar] [CrossRef]
- Ozieranski, P.; Rickard, E.; Mulinari, S. Exposing Drug Industry Funding of UK Patient Organisations. BMJ 2019, 365, l1806. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Fact Sheet: FDA at a Glance. 2022. Available online: https://www.fda.gov/about-fda/fda-basics/fact-sheet-fda-glance (accessed on 21 October 2022).
- Gresham, G.K.; Ehrhardt, S.; Meinert, J.L.; Appel, L.J.; Meinert, C.L. Characteristics and Trends of Clinical Trials Funded by the National Institutes of Health between 2005 and 2015. Clin. Trials 2018, 15, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Hvistendahl, M. Corruption and Research Fraud Send Big Chill Through Big Pharma in China. Science 2013, 341, 445–446. [Google Scholar] [CrossRef]
- Kim, M.S.; Jung, S.Y.; Ahn, J.G.; Park, S.J.; Shoenfeld, Y.; Kronbichler, A.; Koyanagi, A.; Dragioti, E.; Tizaoui, K.; Hong, S.H.; et al. Comparative Safety of MRNA COVID-19 Vaccines to Influenza Vaccines: A Pharmacovigilance Analysis Using WHO International Database. J. Med. Virol. 2022, 94, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A. Curing the Pandemic of Misinformation on COVID-19 MRNA Vaccines through Real Evidence-Based Medicine-Part 1. J. Insul. Resist. 2022, 5, 8. [Google Scholar] [CrossRef]
- Langmuir, A.D. Guillain-Barré Syndrome: The Swine Influenza Virus Vaccine Incident in the United States of America, 1976–1977: Preliminary Communication. J. R. Soc. Med. 1979, 72, 660–669. [Google Scholar] [CrossRef]
- Langmuir, A.D.; Bregman, D.J.; Kurland, L.T.; Nathanson, N.; Victor, M. An Epidemiologic and Clinical Evaluation of Guillain-Barré Syndrome Reported in Association with the Administration of Swine Influenza Vaccines. Am. J. Epidemiol. 1984, 119, 841–879. [Google Scholar] [CrossRef]
- Shao, S.-C.; Wang, C.-H.; Chang, K.-C.; Hung, M.-J.; Chen, H.-Y.; Liao, S.-C. Guillain-Barré Syndrome Associated with COVID-19 Vaccination. Emerg. Infect. Dis. 2021, 27, 3175–3178. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ozaki, A.; Bhandari, D.; Sawano, T.; Sah, R.; Tanimoto, T. High Anaphylaxis Rates Following Vaccination with the Pfizer BNT162b2 MRNA Vaccine against COVID-19 in Japanese Healthcare Workers: A Secondary Analysis of Initial Post-Approval Safety Data. J. Travel Med. 2021, 28, taab090. [Google Scholar] [CrossRef]
- de Gregorio, C.; Colarusso, L.; Calcaterra, G.; Bassareo, P.P.; Ieni, A.; Mazzeo, A.T.; Ferrazzo, G.; Noto, A.; Koniari, I.; Mehta, J.L.; et al. Cerebral Venous Sinus Thrombosis Following COVID-19 Vaccination: Analysis of 552 Worldwide Cases. Vaccines 2022, 10, 232. [Google Scholar] [CrossRef]
- Frontera, J.A.; Tamborska, A.A.; Doheim, M.F.; Garcia-Azorin, D.; Gezegen, H.; Guekht, A.; Yusof Khan, A.H.K.; Santacatterina, M.; Sejvar, J.; Thakur, K.T.; et al. Neurological Events Reported after COVID-19 Vaccines: An Analysis of Vaccine Adverse Event Reporting System. Ann. Neurol. 2022, 91, 756–771. [Google Scholar] [CrossRef] [PubMed]
- Halma, M.T.J.; Rose, J.; Lawrie, T. The Novelty of MRNA Viral Vaccines and Potential Harms: A Scoping Review. J 2023, 6, 220–235. [Google Scholar] [CrossRef]
- Gøtzsche, P.C.; Demasi, M. Serious Harms of the COVID-19 Vaccines: A Systematic Review. medRxiv 2023. [Google Scholar] [CrossRef]
- Montano, D. Frequency and Associations of Adverse Reactions of COVID-19 Vaccines Reported to Pharmacovigilance Systems in the European Union and the United States. Front. Public Health 2022, 9, 2237. [Google Scholar] [CrossRef]
- Sun, C.L.F.; Jaffe, E.; Levi, R. Increased Emergency Cardiovascular Events among Under-40 Population in Israel during Vaccine Rollout and Third COVID-19 Wave. Sci. Rep. 2022, 12, 6978. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, D.; Smith, M.K.; Faden, R. Informed Consent and Medical Ethics. Arch. Ophthalmol. 1993, 111, 324–326. [Google Scholar] [CrossRef]
- Drew, L. Did COVID Vaccine Mandates Work? What the Data Say. Nature 2022, 607, 22–25. [Google Scholar] [CrossRef]
- Chuan Voo, T.; Savulescu, J.; Schaefer, O.; Ho Zhi Ling, A.; Tam, C.C. COVID-19 Differentiated Measures for Unvaccinated Individuals: The Need for Clear Goals and Strong Justifications. Vaccine 2022, 40, 5333–5337. [Google Scholar] [CrossRef]
- Vogel, L.; Duong, D. What’s the Evidence for Fining the Unvaccinated? CMAJ 2022, 194, E132–E133. [Google Scholar] [CrossRef]
- Mavridis, C. Mandatory Vaccinations, the Imposing of Fines, the Segregation of Citizens and Promotion of Inequality in the Modern Democracy of Greece. Is Science Allowed to “Enforce” or Silently Back-up Such Policies? Qeios 2022, H4XGP2. [Google Scholar] [CrossRef]
- Shuster, E. Fifty Years Later: The Significance of the Nuremberg Code. N. Engl. J. Med. 1997, 337, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Laws Are Not the Only Way to Boost Immunization. Nature 2018, 553, 249–250. [CrossRef] [PubMed]
- Helps, C.; Leask, J.; Barclay, L. “It Just Forces Hardship”: Impacts of Government Financial Penalties on Non-Vaccinating Parents. J. Public Health Pol. 2018, 39, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Bardosh, K.; Figueiredo, A.D.; Gur-Arie, R.; Jamrozik, E.; Doidge, J.C.; Lemmens, T.; Keshavjee, S.; Graham, J.; Baral, S. The Unintended Consequences of COVID-19 Vaccine Policy: Why Mandates, Passports, and Segregated Lockdowns May Cause More Harm than Good. BMJ Glob. Health 2022, 7, e008684. [Google Scholar] [CrossRef] [PubMed]
- Pennings, S.; Symons, X. Persuasion, Not Coercion or Incentivisation, Is the Best Means of Promoting COVID-19 Vaccination. J. Med. Ethics 2021, 47, 709–711. [Google Scholar] [CrossRef]
- AP-NORC/USAFacts Poll: US Trust in COVID-19 Information Down. Available online: https://apnews.com/article/virus-outbreak-donald-trump-pandemics-media-social-media-d3c50f0479f8ac123c8cf548c33282be (accessed on 21 October 2022).
- Rhodes, A.; Hoq, M.; Measey, M.-A.; Danchin, M. Intention to Vaccinate against COVID-19 in Australia. Lancet Infect. Dis. 2021, 21, e110. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A Global Survey of Potential Acceptance of a COVID-19 Vaccine. Nat. Med. 2021, 27, 225–228. [Google Scholar] [CrossRef]
- Majid, U.; Ahmad, M. The Factors That Promote Vaccine Hesitancy, Rejection, or Delay in Parents. Qual. Health Res. 2020, 30, 1762–1776. [Google Scholar] [CrossRef]
- Fan, J.; Wang, X.; Du, S.; Mao, A.; Du, H.; Qiu, W. Discussion of the Trust in Vaccination against COVID-19. Vaccines 2022, 10, 1214. [Google Scholar] [CrossRef]
- Latkin, C.A.; Dayton, L.; Yi, G.; Konstantopoulos, A.; Boodram, B. Trust in a COVID-19 Vaccine in the U.S.: A Social-Ecological Perspective. Soc. Sci. Med. 2021, 270, 113684. [Google Scholar] [CrossRef]
- Warren, R.C.; Forrow, L.; Hodge, D.A.; Truog, R.D. Trustworthiness before Trust—COVID-19 Vaccine Trials and the Black Community. N. Engl. J. Med. 2020, 383, e121. [Google Scholar] [CrossRef] [PubMed]
- Batelaan, K. ‘It’s Not the Science We Distrust; It’s the Scientists’: Reframing the Anti-Vaccination Movement within Black Communities. Glob. Public Health 2022, 17, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- LaCour, M.; Davis, T. Vaccine Skepticism Reflects Basic Cognitive Differences in Mortality-Related Event Frequency Estimation. Vaccine 2020, 38, 3790–3799. [Google Scholar] [CrossRef] [PubMed]
- Fuhrer, J.; Cova, F. “Quick and Dirty”: Intuitive Cognitive Style Predicts Trust in Didier Raoult and His Hydroxychloroquine-Based Treatment against COVID-19. Judgm. Decis. Mak. 2020, 15, 889–908. [Google Scholar] [CrossRef]
- Besley, J.C. The National Science Foundation’s Science and Technology Survey and Support for Science Funding, 2006–2014. Public Underst. Sci. 2018, 27, 94–109. [Google Scholar] [CrossRef]
- Muñoz, A.; Moreno, C.; Luján, J.L. Who Is Willing to Pay for Science? On the Relationship between Public Perception of Science and the Attitude to Public Funding of Science. Public Underst. Sci. 2012, 21, 242–253. [Google Scholar] [CrossRef]
- Petty, R.E.; Cacioppo, J.T. Attitudes and Persuasion: Classic and Contemporary Approaches; Routledge: New York, NY, USA, 2019; ISBN 978-0-429-50215-6. [Google Scholar]
- Gilbert, D.T.; Fiske, S.T.; Lindzey, G. The Handbook of Social Psychology; McGraw-Hill: New York, NY, USA, 1998; ISBN 978-0-19-521376-8. [Google Scholar]
- Besley, J.C.; Dudo, A.D.; Yuan, S.; Abi Ghannam, N. Qualitative Interviews With Science Communication Trainers About Communication Objectives and Goals. Sci. Commun. 2016, 38, 356–381. [Google Scholar] [CrossRef]
- Kahan, D.M.; Peters, E.; Wittlin, M.; Slovic, P.; Ouellette, L.L.; Braman, D.; Mandel, G. The Polarizing Impact of Science Literacy and Numeracy on Perceived Climate Change Risks. Nat. Clim. Chang. 2012, 2, 732–735. [Google Scholar] [CrossRef]
- Miller, J.M. Booster Doses of Moderna COVID-19 Vaccines in Adults, Adolescents & Children. Advis. Comm. Immun. Pract. 2022, 2022, 11. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-09-01/06-COVID-Miller-508.pdf (accessed on 1 April 2023).
- Blastland, M.; Freeman, A.L.J.; van der Linden, S.; Marteau, T.M.; Spiegelhalter, D. Five Rules for Evidence Communication. Nature 2020, 587, 362–364. [Google Scholar] [CrossRef]
- Zhang, Y.; Suhaimi, N.; Yongsatianchot, N.; Gaggiano, J.D.; Kim, M.; Patel, S.A.; Sun, Y.; Marsella, S.; Griffin, J.; Parker, A.G. Shifting Trust: Examining How Trust and Distrust Emerge, Transform, and Collapse in COVID-19 Information Seeking. In Proceedings of the 2022 CHI Conference on Human Factors in Computing Systems, New Orleans, LA, USA, 27 April 2022; Association for Computing Machinery: New York, NY, USA, 2022; pp. 1–21. [Google Scholar]
- Doshi, P.; Godlee, F.; Abbasi, K. COVID-19 Vaccines and Treatments: We Must Have Raw Data, Now. BMJ 2022, 376, o102. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.D.; Dumontier, M.; Aalbersberg, I.J.; Appleton, G.; Axton, M.; Baak, A.; Blomberg, N.; Boiten, J.W.; da Silva Santos, L.B.; Bourne, P.E.; et al. The FAIR Guiding Principles for Scientific Data Management and Stewardship. Sci. Data 2016, 3, 160018. [Google Scholar] [CrossRef] [PubMed]
- Piwowar, H.A.; Vision, T.J. Data Reuse and the Open Data Citation Advantage. PeerJ 2013, 1, e175. [Google Scholar] [CrossRef] [PubMed]
- Rosman, T.; Bosnjak, M.; Silber, H.; Koßmann, J.; Heycke, T. Open Science and Public Trust in Science: Results from Two Studies. Public Underst. Sci. 2022, 31, 09636625221100686. [Google Scholar] [CrossRef] [PubMed]
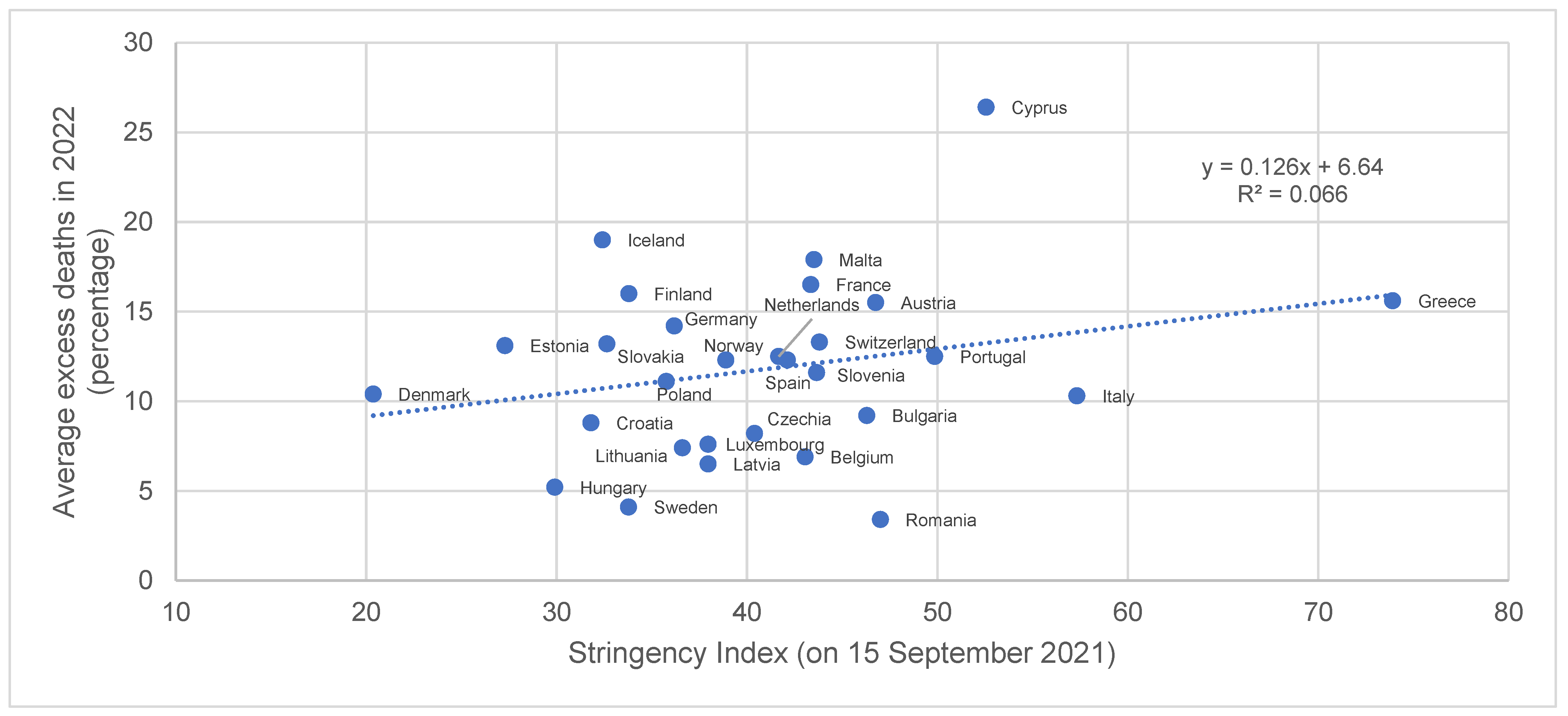
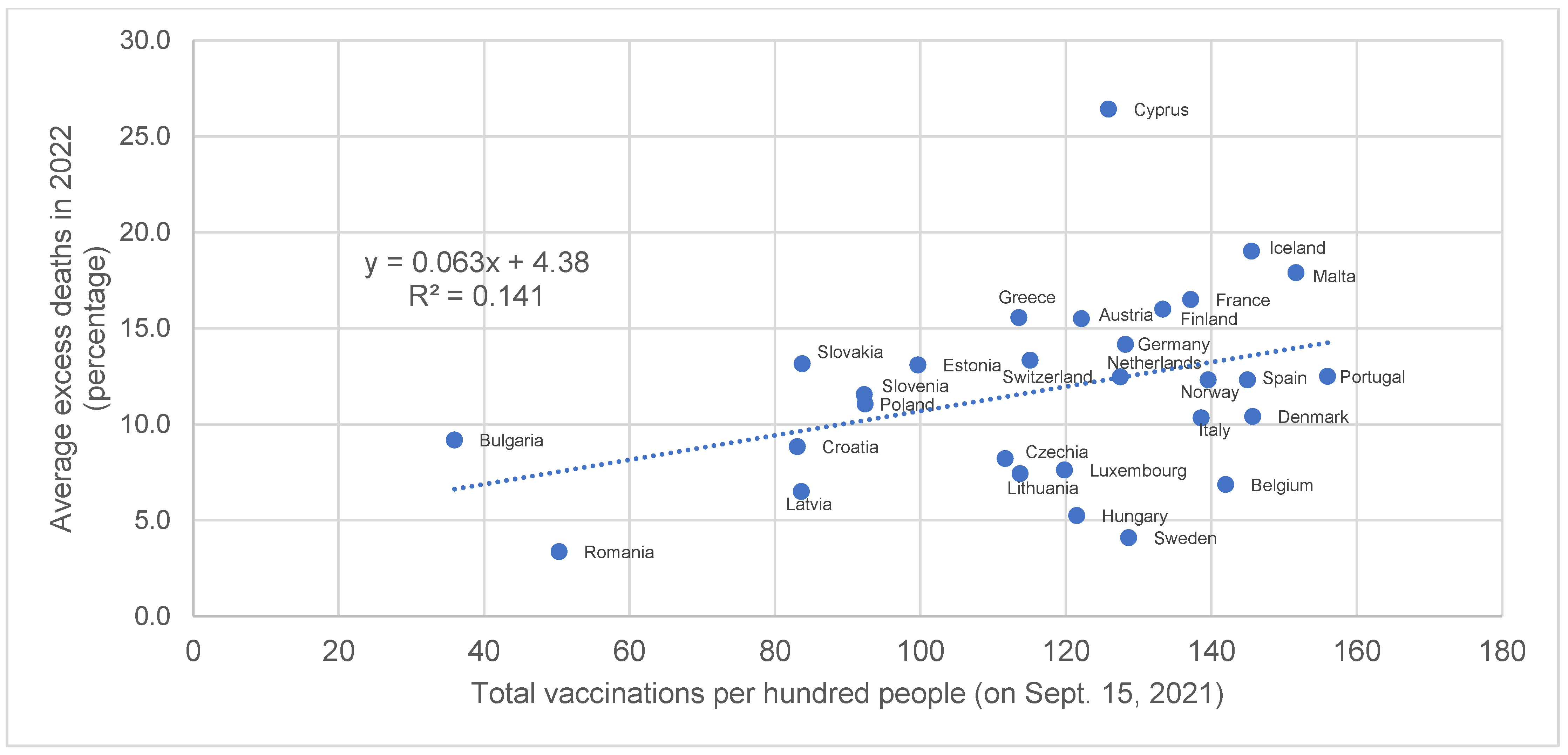
| Country | Average Excess Deaths in 2022 (Percentage of Total Deaths) | Total Vaccinations per Hundred People (on 15 September 2021) | Stringency Index (on 15 September 2021) |
|---|---|---|---|
| Romania | 3.4 | 50.34 | 47.01 |
| Sweden | 4.1 | 128.65 | 33.78 |
| Hungary | 5.2 | 121.51 | 29.91 |
| Latvia | 6.5 | 83.64 | 37.96 |
| Belgium | 6.9 | 142.02 | 43.06 |
| Lithuania | 7.4 | 113.73 | 36.61 |
| Luxembourg | 7.6 | 119.83 | 37.96 |
| Czechia | 8.2 | 111.69 | 40.4 |
| Croatia | 8.8 | 83.09 | 31.81 |
| Bulgaria | 9.2 | 35.94 | 46.3 |
| Italy | 10.3 | 138.64 | 57.33 |
| Denmark | 10.4 | 145.72 | 20.37 |
| Poland | 11.1 | 92.43 | 35.77 |
| Slovenia | 11.6 | 92.3 | 43.66 |
| Norway | 12.3 | 139.59 | 38.89 |
| Spain | 12.3 | 144.99 | 42.13 |
| Netherlands | 12.5 | 127.52 | 41.67 |
| Portugal | 12.5 | 156.01 | 49.86 |
| Estonia | 13.1 | 99.69 | 27.29 |
| Slovakia | 13.2 | 83.78 | 32.65 |
| Switzerland | 13.3 | 115.12 | 43.81 |
| Germany | 14.2 | 128.23 | 36.18 |
| Austria | 15.5 | 122.16 | 46.76 |
| Greece | 15.6 | 113.56 | 73.92 |
| Finland | 16 | 133.36 | 33.8 |
| France | 16.5 | 137.18 | 43.35 |
| Malta | 17.9 | 151.69 | 43.52 |
| Iceland | 19 | 145.56 | 32.41 |
| Cyprus | 26.4 | 125.89 | 52.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halma, M.T.J.; Guetzkow, J. Public Health Needs the Public Trust: A Pandemic Retrospective. BioMed 2023, 3, 256-271. https://doi.org/10.3390/biomed3020023
Halma MTJ, Guetzkow J. Public Health Needs the Public Trust: A Pandemic Retrospective. BioMed. 2023; 3(2):256-271. https://doi.org/10.3390/biomed3020023
Chicago/Turabian StyleHalma, Matthew T. J., and Joshua Guetzkow. 2023. "Public Health Needs the Public Trust: A Pandemic Retrospective" BioMed 3, no. 2: 256-271. https://doi.org/10.3390/biomed3020023
APA StyleHalma, M. T. J., & Guetzkow, J. (2023). Public Health Needs the Public Trust: A Pandemic Retrospective. BioMed, 3(2), 256-271. https://doi.org/10.3390/biomed3020023






