Acute Vessel Closure or Major Adverse Cardiac Events of Drug-Coated Balloons and Stents: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
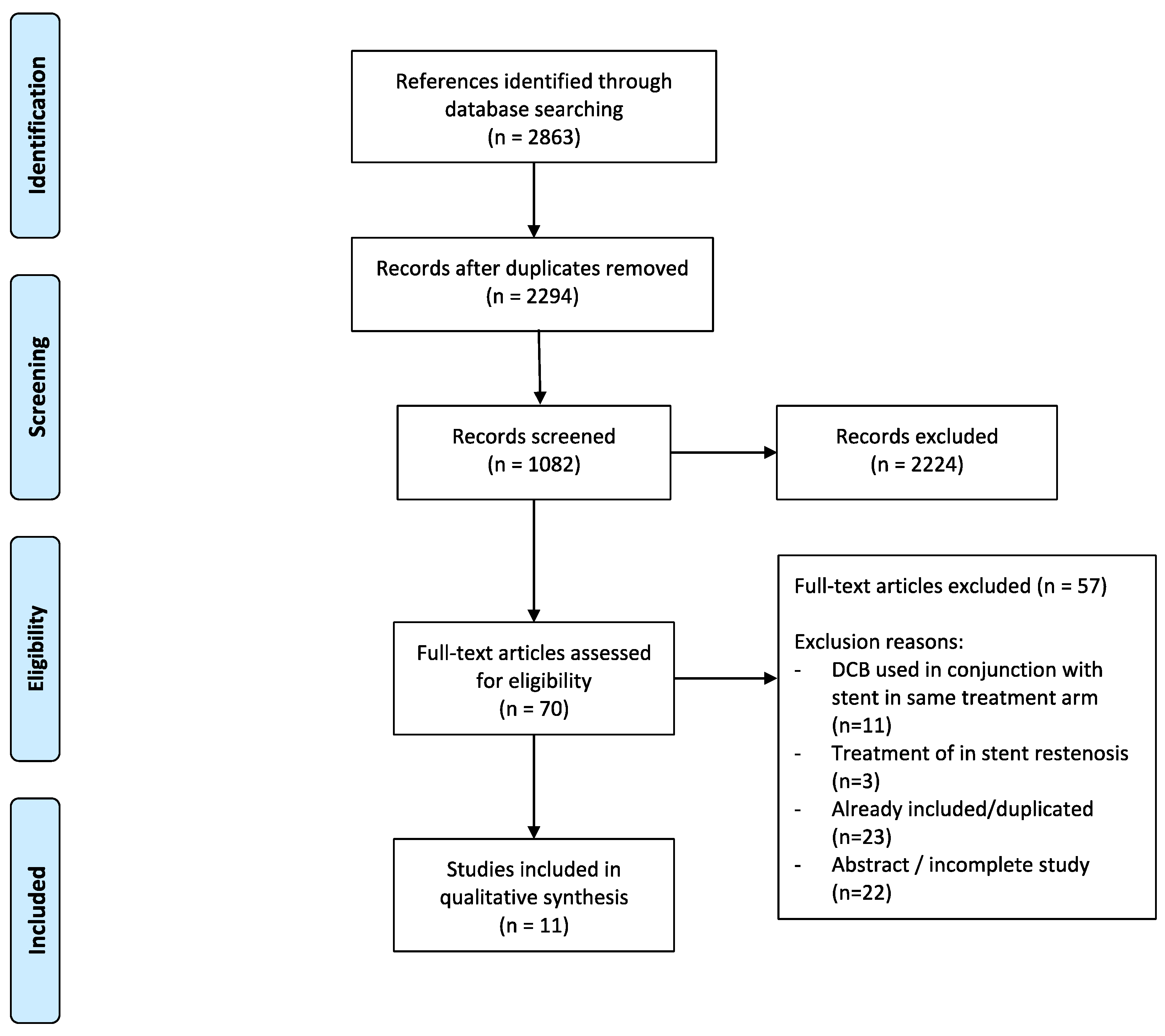
2. Materials and Methods
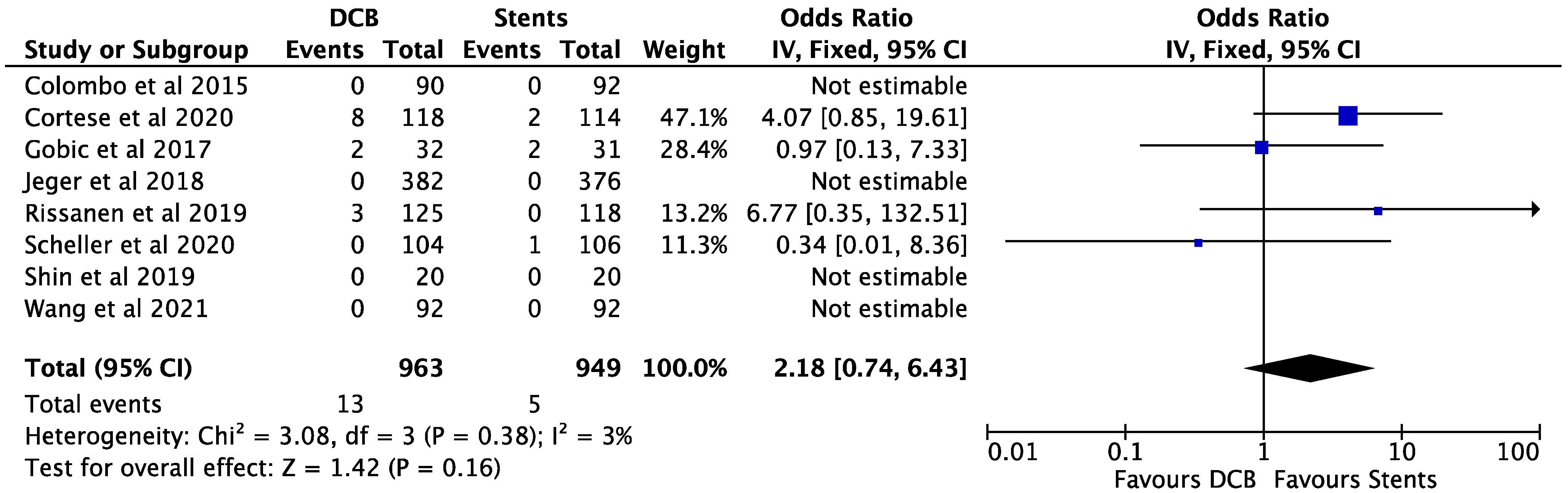
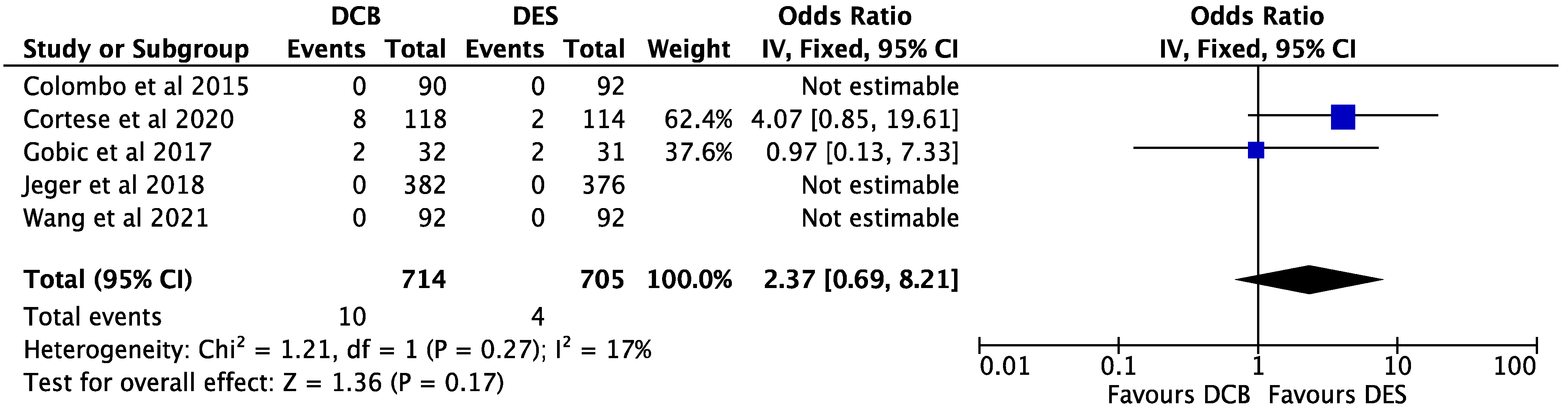
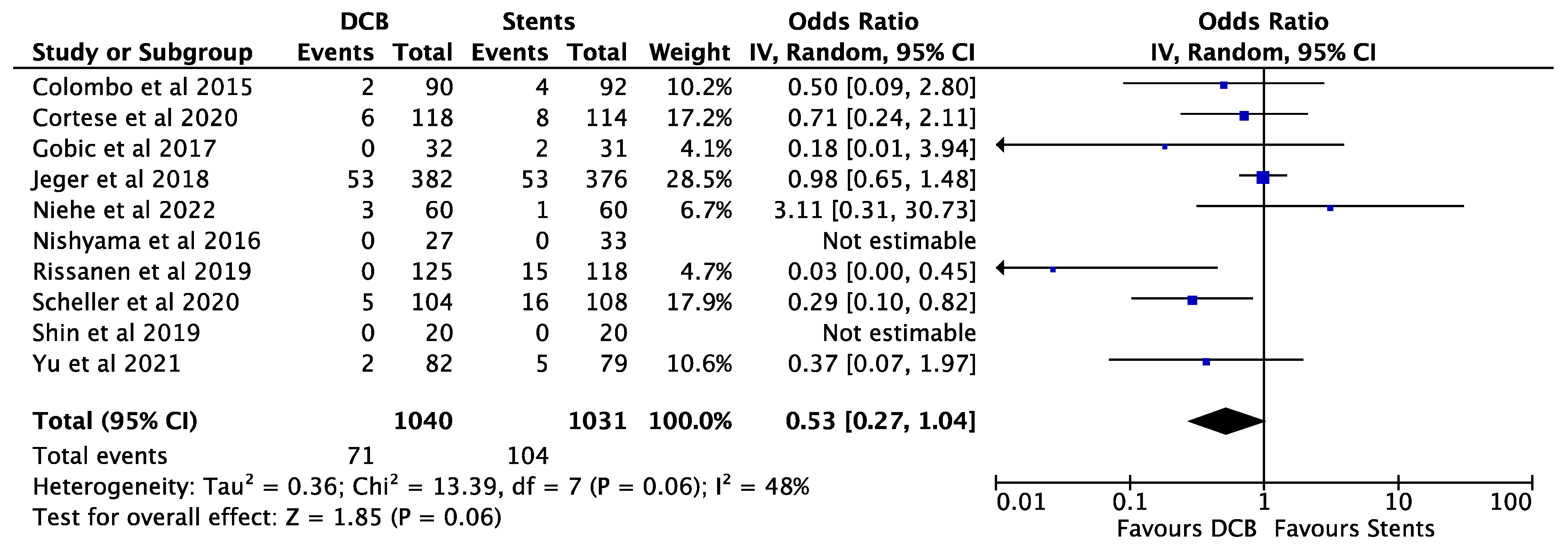
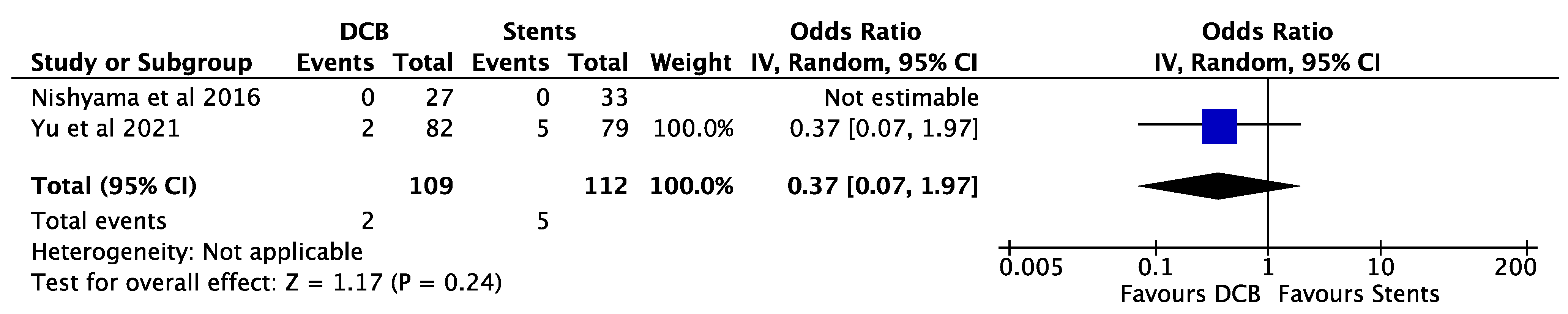
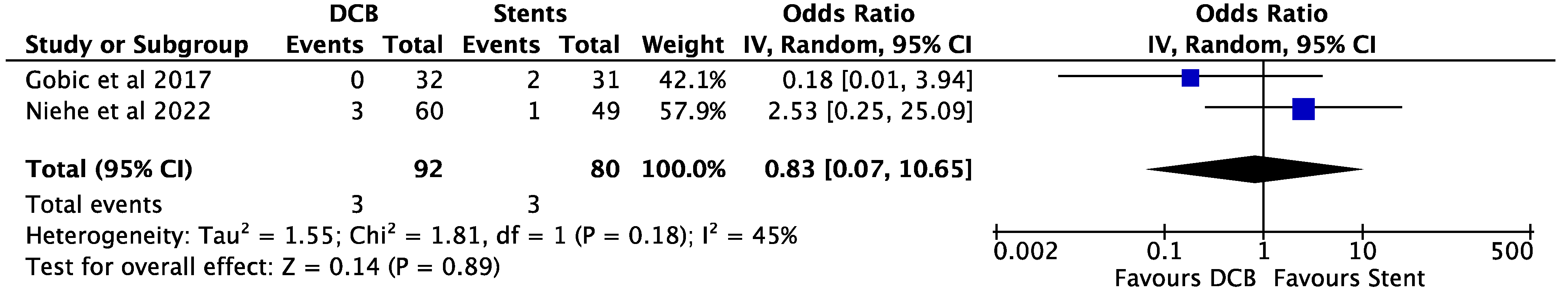
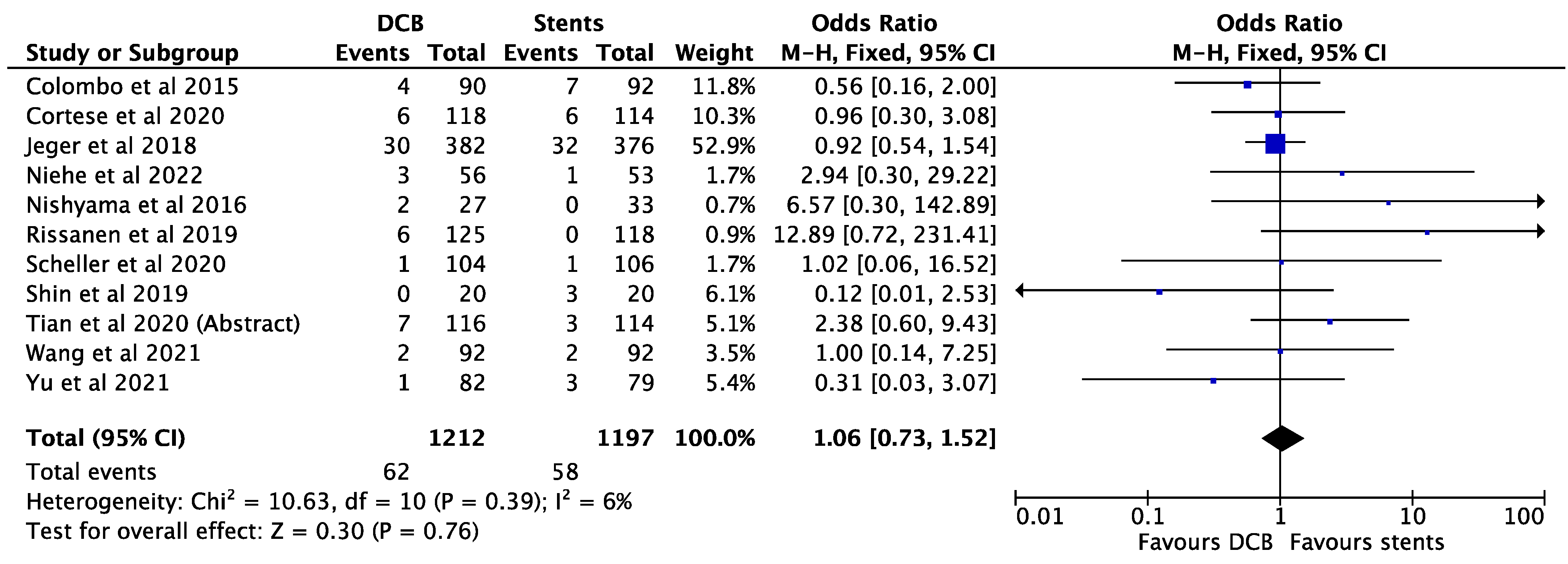
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lincoff, A.M.; Popma, J.J.; Ellis, S.G.; Hacker, J.A.; Topol, E.J. Abrupt vessel closure complicating coronary angioplasty: Clinical, angiographic and therapeutic profile. J. Am. Coll. Cardiol. 1992, 19, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, G.; Sonck, J.; Ferenc, M.; Chen, S.-L.; Colaiori, I.; Gallinoro, E.; Mizukami, T.; Kodeboina, M.; Nagumo, S.; Franco, D.; et al. Clinical Outcomes Following Coronary Bifurcation PCI Techniques: A Systematic Review and Network Meta-Analysis Comprising 5711 Patients. JACC Cardiovasc. Interv. 2020, 13, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-L.; Zhang, J.-J.; Han, Y.; Kan, J.; Chen, L.; Qiu, C.; Jiang, T.; Tao, L.; Zeng, H.; Li, L.; et al. Double Kissing Crush Versus Provisional Stenting for Left Main Distal Bifurcation Lesions: DKCRUSH-V Randomized Trial. J. Am. Coll. Cardiol. 2017, 70, 2605–2617. [Google Scholar] [CrossRef] [PubMed]
- Foin, N.; Sen, S.; Allegria, E.; Petraco, R.; Nijjer, S.; Francis, D.P.; Di Mario, C.; Davies, J.E. Maximal expansion capacity with current DES platforms: A critical factor for stent selection in the treatment of left main bifurcations? EuroIntervention 2013, 8, 1315–1325. [Google Scholar] [CrossRef]
- Koskinas, K.C.; Chatzizisis, Y.S.; Antoniadis, A.P.; Giannoglou, G.D. Role of endothelial shear stress in stent restenosis and thrombosis: Pathophysiologic mechanisms and implications for clinical translation. J. Am. Coll. Cardiol. 2012, 59, 1337–1349. [Google Scholar] [CrossRef]
- Van der Heiden, K.; Gijsen, F.J.H.; Narracott, A.; Hsiao, S.; Halliday, I.; Gunn, J.; Wentzel, J.J.; Evans, P.C. The effects of stenting on shear stress: Relevance to endothelial injury and repair. Cardiovasc. Res. 2013, 99, 269–275. [Google Scholar] [CrossRef]
- Hamilos, M.; Sarma, J.; Ostojic, M.; Cuisset, T.; Sarno, G.; Melikian, N.; Ntalianis, A.; Muller, O.; Barbato, E.; Beleslin, B.; et al. Interference of drug-eluting stents with endothelium-dependent coronary vasomotion: Evidence for device-specific responses. Circ. Cardiovasc. Interv. 2008, 1, 193–200. [Google Scholar] [CrossRef]
- Puricel, S.; Kallinikou, Z.; Espinola, J.; Arroyo, D.; Goy, J.-J.; Stauffer, J.-C.; Baeriswyl, G.; Smits, P.C.; Cook, S.; Togni, M. Comparison of endothelium-dependent and -independent vasomotor response after abluminal biodegradable polymer biolimus-eluting stent and persistent polymer everolimus-eluting stent implantation (COMPARE-IT). Int. J. Cardiol. 2016, 202, 525–531. [Google Scholar] [CrossRef]
- Jeger, R.V.; Eccleshall, S.; Wan Ahmad, W.A.; Ge, J.; Poerner, T.C.; Shin, E.-S.; Alfonso, F.; Latib, A.; Ong, P.J.; Rissanen, T.T.; et al. Drug-Coated Balloons for Coronary Artery Disease: Third Report of the International DCB Consensus Group. JACC Cardiovasc. Interv. 2020, 13, 1391–1402. [Google Scholar] [CrossRef]
- Merinopoulos, I.; Gunawardena, T.; Wickramarachchi, U.; Richardson, P.; Maart, C.; Sreekumar, S.; Sawh, C.; Wistow, T.; Sarev, T.; Ryding, A.; et al. Long-term safety of paclitaxel drug-coated balloon-only angioplasty for de novo coronary artery disease: The SPARTAN DCB study. Clin. Res. Cardiol. 2020, 1–8. [Google Scholar] [CrossRef]
- Cortese, B.; Testa, L.; Di Palma, G.; Heang, T.M.; Bossi, I.; Nuruddin, A.A.; Ielasi, A.; Tespili, M.; Perez, I.S.; Milazzo, D.; et al. Clinical performance of a novel sirolimus-coated balloon in coronary artery disease: EASTBOURNE registry. J. Cardiovasc. Med. 2021, 22, 94–100. [Google Scholar] [CrossRef]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Merinopoulos, I.; Gunawardena, T.; Corballis, N.; Bhalraam, U.; Gilbert, T.; Maart, C.; Richardson, P.; Ryding, A.; Sarev, T.; Sawh, C.; et al. Paclitaxel drug-coated balloon-only angioplasty for de novo coronary artery disease in elective clinical practice. Clin. Res. Cardiol. 2022. [Google Scholar] [CrossRef]
- Jeger, R.V.; Farah, A.; Ohlow, M.-A.; Mangner, N.; Möbius-Winkler, S.; Leibundgut, G.; Weilenmann, D.; Wöhrle, J.; Richter, S.; Schreiber, M.; et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): An open-label randomised non-inferiority trial. Lancet 2018, 392, 849–856. [Google Scholar] [CrossRef]
- Vos, N.S.; Fagel, N.D.; Amoroso, G.; Herrman, J.-P.R.; Patterson, M.S.; Piers, L.H.; van der Schaaf, R.J.; Slagboom, T.; Vink, M.A. Paclitaxel-Coated Balloon Angioplasty Versus Drug-Eluting Stent in Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2019, 4408. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Naganuma, T.; Latib, A.; Sgueglia, G.A.; Menozzi, A.; Castriota, F.; Micari, A.; Cremonesi, A.; De Felice, F.; Marchese, A.; Tespili, M.; et al. A 2-year follow-up of a randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels the BELLO study. Int. J. Cardiol. 2015, 184, 17–21. [Google Scholar] [CrossRef]
- Nishiyama, N.; Komatsu, T.; Kuroyanagi, T.; Fujikake, A.; Komatsu, S.; Nakamura, H.; Yamada, K.; Nakahara, S.; Kobayashi, S.; Taguchi, I. Clinical value of drug-coated balloon angioplasty for de novo lesions in patients with coronary artery disease. Int. J. Cardiol. 2016, 222, 113–118. [Google Scholar] [CrossRef]
- Gobić, D.; Tomulić, V.; Lulić, D.; Židan, D.; Brusich, S.; Jakljević, T.; Zaputović, L. Drug-Coated Balloon Versus Drug-Eluting Stent in Primary Percutaneous Coronary Intervention: A Feasibility Study. Am. J. Med. Sci. 2017, 354, 553–560. [Google Scholar] [CrossRef]
- Jeger, R.V.; Farah, A.; Ohlow, M.-A.; Mangner, N.; Möbius-Winkler, S.; Weilenmann, D.; Wöhrle, J.; Stachel, G.; Markovic, S.; Leibundgut, G.; et al. Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease (BASKET-SMALL 2): 3-year follow-up of a randomised, non-inferiority trial. Lancet 2020, 396, 1504–1510. [Google Scholar] [CrossRef]
- Shin, E.S.; Lee, J.M.; Her, A.Y.; Chung, J.H.; Lee, K.E.; Garg, S.; Nam, C.W.; Doh, J.H.; Koo, B.K. Prospective randomized trial of paclitaxel-coated balloon versus bare-metal stent in high bleeding risk patients with de novo coronary artery lesions. Coron. Artery Dis. 2019, 30, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, T.T.; Uskela, S.; Eränen, J.; Mäntylä, P.; Olli, A.; Romppanen, H.; Siljander, A.; Pietilä, M.; Minkkinen, M.J.; Tervo, J.; et al. Drug-coated balloon for treatment of de-novo coronary artery lesions in patients with high bleeding risk (DEBUT): A single-blind, randomised, non-inferiority trial. Lancet 2019, 394, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; Di Palma, G.; Guimaraes, M.G.; Piraino, D.; Orrego, P.S.; Buccheri, D.; Rivero, F.; Perotto, A.; Zambelli, G.; Alfonso, F. Drug-Coated Balloon Versus Drug-Eluting Stent for Small Coronary Vessel Disease: PICCOLETO II Randomized Clinical Trial. JACC. Cardiovasc. Interv. 2020, 13, 2840–2849. [Google Scholar] [CrossRef] [PubMed]
- Scheller, B.; Ohlow, M.-A.; Ewen, S.; Kische, S.; Rudolph, T.K.; Clever, Y.P.; Wagner, A.; Richter, S.; El-Garhy, M.; Böhm, M.; et al. Bare metal or drug-eluting stent versus drug-coated balloon in non-ST-elevation myocardial infarction: The randomised PEPCAD NSTEMI trial. EuroIntervention 2020, 15, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yin, Y.; Li, J.; Qi, W.; Yu, B.; Xu, Z.; Zhu, W.; Yang, F.; Cao, M.; Zhang, H. New Ultrasound-Controlled Paclitaxel Releasing Balloon vs. Asymmetric Drug-Eluting Stent in Primary ST-Segment Elevation Myocardial Infarction—A Prospective Randomized Trial. Circ. J. 2022, 86, 642–650. [Google Scholar] [CrossRef]
- Yu, X.; Wang, X.; Ji, F.; Zhang, W.; Yang, C.; Xu, F.; Wang, F. A Non-inferiority, Randomized Clinical Trial Comparing Paclitaxel-Coated Balloon Versus New-Generation Drug-Eluting Stents on Angiographic Outcomes for Coronary De Novo Lesions. Cardiovasc. Drugs Ther. 2022, 36, 655–664. [Google Scholar] [CrossRef]
- Niehe, S.R.; Vos, N.S.; Van der Schaaf, R.J.; Amoroso, G.; Herrman, J.R.; Patterson, M.S.; Slagboom, T.; Vink, M.A. Two-Year Clinical Outcomes of the REVELATION Study: Sustained Safety and Feasibility of Paclitaxel-Coated Balloon Angioplasty Versus Drug-Eluting Stent in Acute Myocardial Infarction. J. Invasive Cardiol. 2022, 34, 39–42. [Google Scholar]
- Serruys, P.W.; de Jaegere, P.; Kiemeneij, F.; Macaya, C.; Rutsch, W.; Heyndrickx, G.; Emanuelsson, H.; Marco, J.; Legrand, V.; Materne, P.; et al. A Comparison of Balloon-Expandable-Stent Implantation with Balloon Angioplasty in Patients with Coronary Artery Disease. N. Engl. J. Med. 1994, 331, 489–495. [Google Scholar] [CrossRef]
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; Chambers, C.E.; Ellis, S.G.; Guyton, R.A.; Hollenberg, S.M.; et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011, 124, e574–e651. [Google Scholar] [CrossRef]
- Grove, E.C.L.; Kristensen, S.D. Stent thrombosis: Definitions, mechanisms and prevention. e-J. ESC Counc. Cardiol. Pract. 2007, 5. [Google Scholar]
- Palmerini, T.; Biondi-Zoccai, G.; Della Riva, D.; Stettler, C.; Sangiorgi, D.; D’Ascenzo, F.; Kimura, T.; Briguori, C.; Sabatè, M.; Kim, H.-S.; et al. Stent thrombosis with drug-eluting and bare-metal stents: Evidence from a comprehensive network meta-analysis. Lancet 2012, 379, 1393–1402. [Google Scholar] [CrossRef]
- Fischman, D.L.; Leon, M.B.; Baim, D.S.; Schatz, R.A.; Savage, M.P.; Penn, I.; Detre, K.; Veltri, L.; Ricci, D.; Nobuyoshi, M.; et al. A Randomized Comparison of Coronary-Stent Placement and Balloon Angioplasty in the Treatment of Coronary Artery Disease. N. Engl. J. Med. 1994, 331, 496–501. [Google Scholar] [CrossRef]
- Ormiston, J.A.; De Vroey, F.; Serruys, P.W.; Webster, M.W.I. Bioresorbable polymeric vascular scaffolds: A cautionary tale. Circ. Cardiovasc. Interv. 2011, 4, 535–538. [Google Scholar] [CrossRef][Green Version]
- Mukherjee, D. Device Thrombosis with Bioresorbable Scaffolds. N. Engl. J. Med. 2017, 376, 2388–2389. [Google Scholar] [CrossRef]
- Uskela, S.; Kärkkäinen, J.M.; Eränen, J.; Siljander, A.; Mäntylä, P.; Mustonen, J.; Rissanen, T.T. Percutaneous coronary intervention with drug-coated balloon-only strategy in stable coronary artery disease and in acute coronary syndromes: An all-comers registry study. Catheter. Cardiovasc. Interv. 2019, 93, 893–900. [Google Scholar] [CrossRef]
- Li, M.; Guo, C.; Lv, Y.-H.; Zhang, M.-B.; Wang, Z.-L. Drug-coated balloon versus drug-eluting stent in de novo small coronary vessel disease: A systematic review and meta-analysis. Medicine 2019, 98, e15622. [Google Scholar] [CrossRef]
- Sanz Sánchez, J.; Chiarito, M.; Cortese, B.; Moretti, A.; Pagnotta, P.; Reimers, B.; Stefanini, G.G.; Ferrante, G. Drug-Coated balloons vs drug-eluting stents for the treatment of small coronary artery disease: A meta-analysis of randomized trials. Catheter. Cardiovasc. Interv. 2021, 98, 66–75. [Google Scholar] [CrossRef]

| Study ID | Year of Publication | Patients, n DCBs | Patients, n DESs | Clinical Presentation | Age (Mean) | Male Sex n(%) | DCB Used | Stent Used | Follow-Up Time (Months) |
|---|---|---|---|---|---|---|---|---|---|
| Colombo et al. [17] | 2015 | 90 | 92 | Stable/unstable angina Silent ischaemia | 65.6 | 143 (78.6) | In.pact falcon | DES | 36 |
| Nishiyama et al. [18] | 2016 | 27 | 33 | Stable angina/silent ischaemia | SeQuent PLEASE | DES | 8 | ||
| Gobic et al. [19] | 2017 | 32 | 31 | STEMI | 55.5 | 46 (72.0) | SeQuent PLEASE | DES | 6 |
| Jeger et al. [20] | 2018 | 382 | 376 | All comers | 67.8 | 557 (73.4) | SeQuent PLEASE | DES | 36 |
| Shin et al. [21] | 2019 | 20 | 20 | High bleeding risk | SeQuent PLEASE | BMS | 9 | ||
| Rissanen et al. [22] | 2019 | 125 | 118 | High bleeding risk | 76.8 | 131 (62.9) | SeQuent PLEASE | BMS | 9 |
| Cortese et al. [23] | 2020 | 118 | 114 | All comers | 65.0 | 170 (73.3) | Elutax SV | DES | 6 |
| Scheller et al. [24] | 2020 | 104 | 106 | NSTEMI | 66.5 | 141 (67.1) | SeQuent PLEASE+/-NEO | BMS + DES | 9 |
| Wang et al. [25] | 2021 | 92 | 92 | STEMI | Vasoguard DCB | DES | 9 | ||
| Yu et al. [26] | 2021 | 84 | 79 | Stable angina | 63.3 | 118 (72.4) | SeQuent PLEASE | DES | 9 |
| Niehe et al. [27] | 2022 | 60 | 49 | STEMI | 57.4 | 104 (87) | Biotronik Pantera Lux | DES | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunawardena, T.; Corballis, N.; Merinopoulos, I.; Tsampasian, V.; Reinhold, J.; Eccleshall, S.; Vassiliou, V.S. Acute Vessel Closure or Major Adverse Cardiac Events of Drug-Coated Balloons and Stents: A Systematic Review and Meta-Analysis. BioMed 2022, 2, 442-451. https://doi.org/10.3390/biomed2040035
Gunawardena T, Corballis N, Merinopoulos I, Tsampasian V, Reinhold J, Eccleshall S, Vassiliou VS. Acute Vessel Closure or Major Adverse Cardiac Events of Drug-Coated Balloons and Stents: A Systematic Review and Meta-Analysis. BioMed. 2022; 2(4):442-451. https://doi.org/10.3390/biomed2040035
Chicago/Turabian StyleGunawardena, Tharusha, Natasha Corballis, Ioannis Merinopoulos, Vasiliki Tsampasian, Johannes Reinhold, Simon Eccleshall, and Vassilios S. Vassiliou. 2022. "Acute Vessel Closure or Major Adverse Cardiac Events of Drug-Coated Balloons and Stents: A Systematic Review and Meta-Analysis" BioMed 2, no. 4: 442-451. https://doi.org/10.3390/biomed2040035
APA StyleGunawardena, T., Corballis, N., Merinopoulos, I., Tsampasian, V., Reinhold, J., Eccleshall, S., & Vassiliou, V. S. (2022). Acute Vessel Closure or Major Adverse Cardiac Events of Drug-Coated Balloons and Stents: A Systematic Review and Meta-Analysis. BioMed, 2(4), 442-451. https://doi.org/10.3390/biomed2040035






