Machine Learning Techniques for the Prediction of Functional Outcomes in the Rehabilitation of Post-Stroke Patients: A Scoping Review
Abstract
:1. Introduction
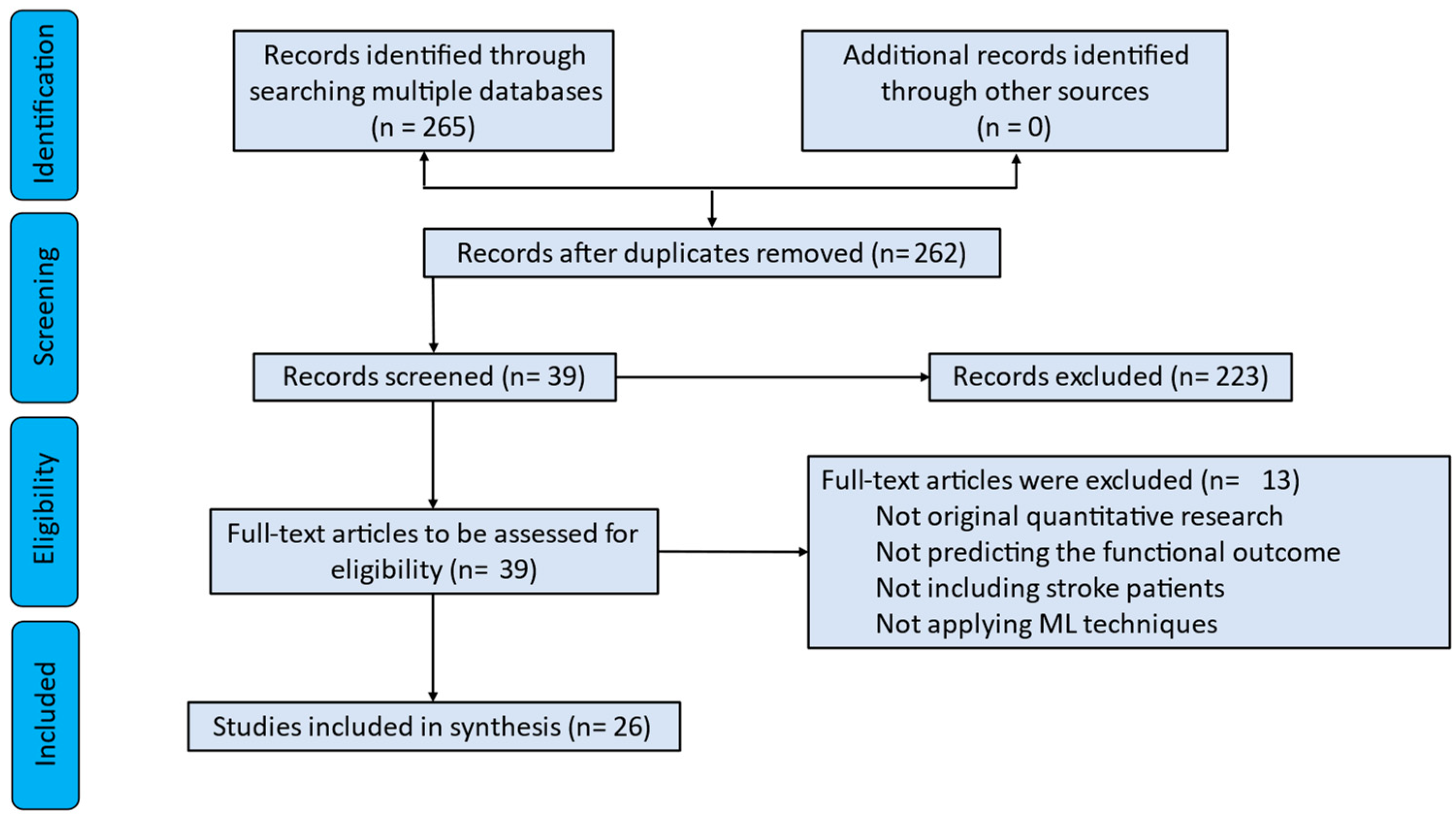
2. Materials and Methods
2.1. Literature Searches
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Data Extraction
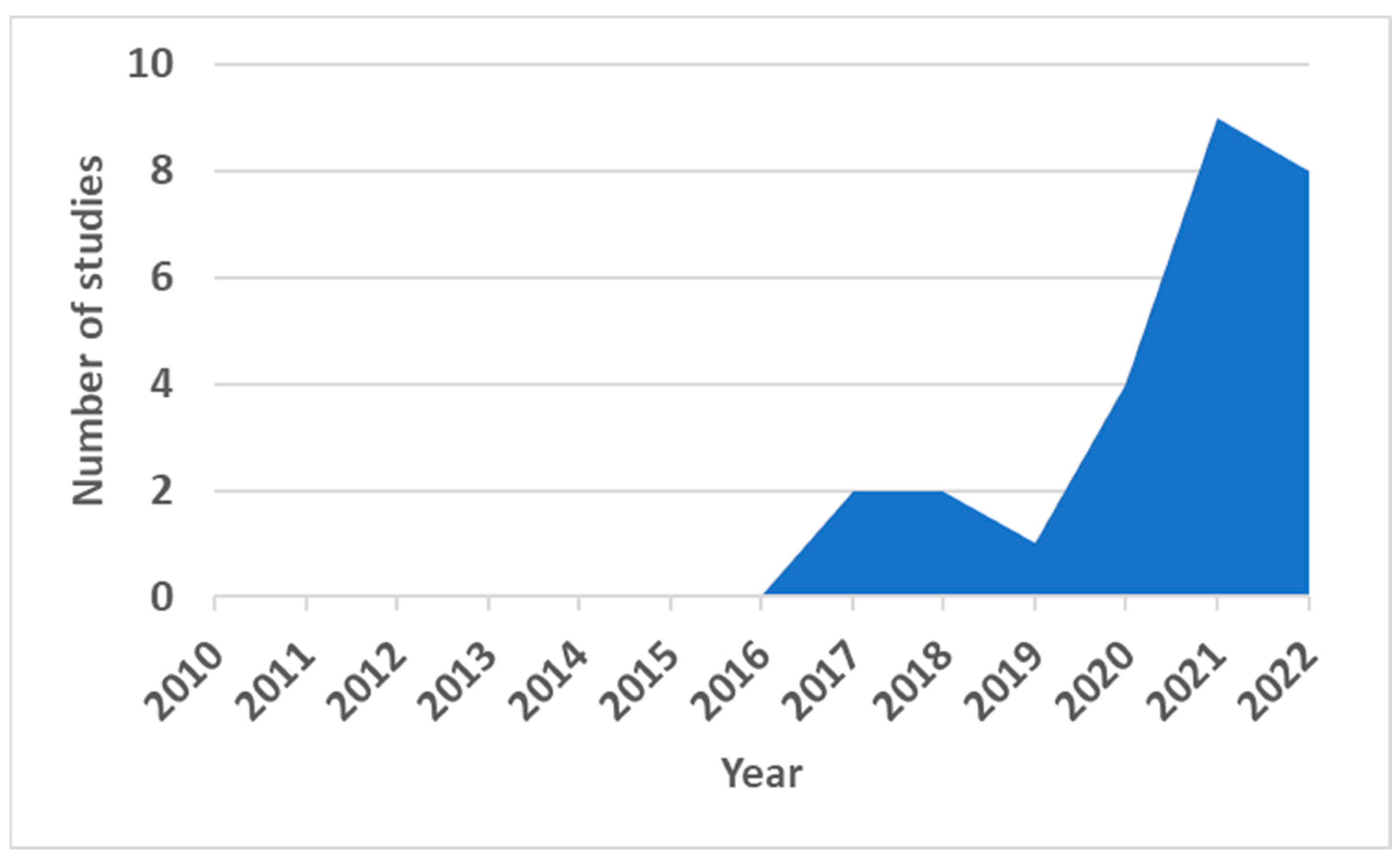
3. Results
3.1. Terminology and Definitions
3.1.1. Functional Outcomes
- Modified Rankin scale (mRS) is a measure for the degree of disability in post-stroke patients, and it is used to identify the level of functional independence [17];
- Functional independence measure (FIM) is an indicator of patient disability which is based on the International Classification of Impairment, Disabilities, and Handicaps for use in the medical system in the United States (McDowell and Newell, 1996) [20];
- Functional ambulation categories (FAC) test evaluates the ambulation ability. Specifically, FAC is a functional walking test [21];
- Fugl–Meyer assessment (FMA) scale assesses the sensorimotor impairment in post-stroke hemiparesis patients [22];
- Modified Brunnstrom classification (MBC) score is used to categorize the function of the affected hand [23];
- Wolf motor function test (WMFT) quantifies the motor ability for the upper extremities (UE) through functional and timed tasks [24];
- Fugl–Meyer assessment, upper extremity (FMA-UE) consists of items that reflect the motor function, and it is useful for an accurate evaluation of UE paresis and to rehabilitation practice [25];
- Ten-meter walk test (TMWT) measures the walking ability in post-stroke patients [26];
- Six-minute walk test (SMWT) assesses the aerobic capacity and walking endurance [27];
- Berg balance scale (BBS) evaluates fall risk and balance outcomes [28];
- Motor activity log (MAL) measures the functional upper limb performance in real life [29];
- National Institutes of Health stroke scale (NIHSS) rates stroke severity [30];
- Stroke impact scale (SIS) assesses physical functioning [31].
3.1.2. Prediction Horizon
3.1.3. Stages of Stroke Recovery
- The acute phase corresponds to the first 7 days, the early subacute phase extends from 7 days to 3 months, the late subacute phase extends from 3 to 6 months, and the chronic phase extends to ≥6 months
3.1.4. ML Terminology
- Classification is the task that predicts the class (or label) of given input data. A common classification problem is the prediction of functional independence status based on mRS, which is a binary problem (class 1: mRS < 2 and class 2: mRS ≥ 2) [32]. Regression predicts a continuous outcome (target variable) on the basis of values of multiple predictor variables. For example, a regression task is the prediction of the Barthel index score at discharge from a rehabilitation unit [33].
3.1.5. Abbreviations of the Employed ML Models
- Support vector machines: SVMs,
- Decision trees: DTs,
- Logistic regression: LR,
- Regularized logistic regression: RLR,
- Extreme gradient boosting: XGB,
- Cox regression: COX,
- Naïve Bayes classifier: NBC,
- Random forest: RF,
- k-nearest neighbors: KNN,
- Linear discriminant analysis: LDA,
- Support vector regression: SVR,
- Epsilon regression: ER,
- Multiple linear regression: MLR,
- Chi-square automatic interaction detector: CHAID,
- Gradient boosting decision tree: GBDT,
- Convolutional neural network: CNN,
- Artificial neural network: ANN,
- Elastic net: EN,
- Enhanced probabilistic neural network: EPNN
- Neural dynamic classification: NDC
3.1.6. Performance Metrics
- Accuracy measures how often the classifier correctly predicts the label of an observation. Sensitivity provides how many of the actual positive cases are predicted correctly (true positive) from the ML model. Specificity gives the number of actual negative cases that are predicted correctly (true negative). Area under the receiver operating characteristic curve (AUC) is an effective metric to summarize the overall predictive accuracy.
- R2 or coefficient of determination is the proportion of the variation in the dependent (target) variable that is predictable from the multiple predictor variables. Mean absolute error (MAE) is the average of all absolute errors in a set of predictions. Root-mean-square error (RMSE) or root-mean-square deviation (RMSD) is the standard deviation of the prediction errors (residuals).
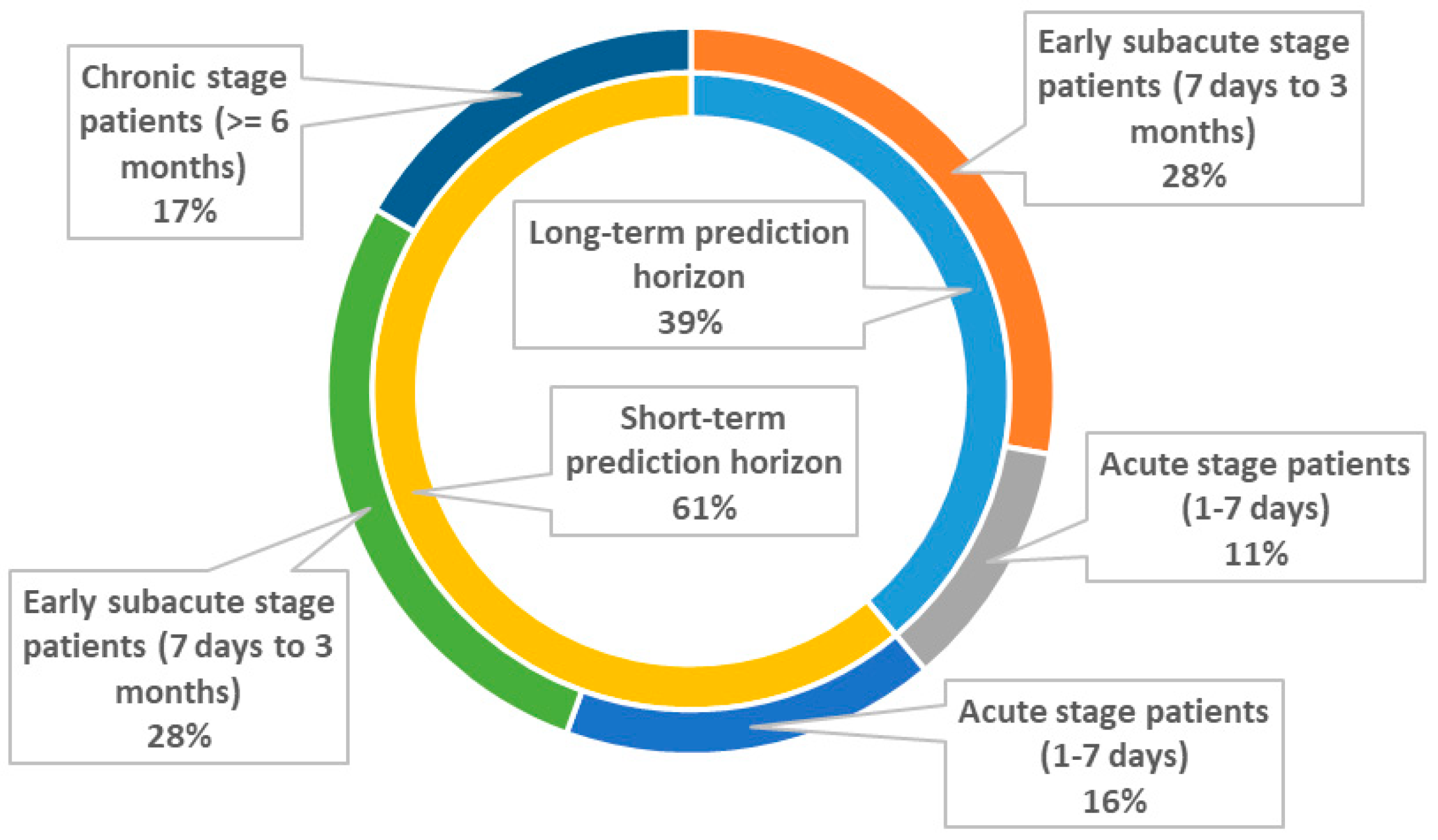
3.2. Motor Function
3.2.1. Long Term Prediction Horizon (≥3 Months)
3.2.2. Short-Term Prediction Horizon (<3 Months)
| Authors | Year | Application Domain | Intervention | Input Data | Outcome Assessment | Employed Subjects | Learning Type | Learning Algorithms | Validation | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Lin et al. [45] | 2022 | Prediction of a treatment’s outcome using a deep learning (DL) prognosis model | Robotic-assisted stretching training | Biomechanical measurement, clinical measurement, and EEG measurement | FMA of lower extremity at 2 weeks | 15 post-stroke (6 months) | Classification | Pretrained DL models and SHAP | Leave-one-out cross-validation (LOOCV) | 91.84% accuracy |
| Campagnini et al. [44] | 2022 | Development of predictive models for the functional prognosis of stroke patients | At least three hours of rehabilitation per day | Demographics and clinical, cognitive and functional evaluations | MBI at 40 days | 278 post-stroke at admission in intensive rehabilitation treatment | Classification | EN RLR, SVM, RF, KNN, and SHAP | Fivefold cross-validation loop was deployed for hyperparameter optimization, while an external 10-fold loop was adopted for test set identification | RF obtained the best overall results on the accuracy (76.2%), balanced accuracy (74.3%), sensitivity (0.80), and specificity (0.68) |
| Lai et al. [43] | 2022 | Prediction of functional outcomes of stroke | Hospitalization | MRI | mRS and NIHSS at admission and discharge (28 ± 3 days) | 44 stroke patients (acute phase) | Classification | Pretrained VGG-16 CNN | Training (90%)/testing (10%) | NHSS: mixed men and women, the diagnostic accuracy was 92.7%, the sensitivity was 88.9%, and the specificity was 96.4%. MRS: mixed men and women, the diagnostic accuracy was 93.2%, the sensitivity was 90.0%, and the specificity was 97.0%. |
| Chen et al. [42] | 2022 | Prediction of 30 day readmission for stroke patients | Post-acute care | Demographics, medical history, and outcome assessments | 30 day readmission | 1476 patients within the first 30 days of stoke onset | Classification | ANN, KNN, RF, SVM, NBC, and COX models | Training data set (70%) and a test dataset (30%); 167 patients were used for external validation | ANN 93% in external dataset |
| Liao et al. [40] | 2022 | Prediction of clinically significant HRQOL improvements of chronic stroke patients | Sensorimotor rehabilitation interventions | Demographics and baseline cognitive, motor, sensory, functional, and HRQOL attributes | SIS at 3 to 4 weeks | 132 people with chronic stroke | Classification | RF, KNN, ANN, SVM, and LR | Training data set (70%) and a test dataset (30%); 10-f cv in training | The accuracy of the RF model was 85%, precision was 0.88, recall was 0.85, the F1 score was 0.85, and the AUC-ROC was 0.86 |
| Park et al. [32] | 2021 | Prediction of functional outcome at 3 months | Hospitalization | Clinical data of personal and stroke-related factors (<7 days) | mRS | 1066 patients with acute ischemic stroke (mean age 65.8 ± 11.3 years favorable outcome group and 74.4 ± 11.4 years unfavorable outcome group) | Classification | RLR, SVM, RF, KNN, and XGB | Training data set (70%) and a test dataset (30%); 10-f cv in training | RLR achieved 86% AUC |
| Chang. et al. [35] | 2021 | Prediction of post-stroke functional outcomes at 3 months | Post-Acute Care-Cerebrovascular Disease (PAC-CVD) program | Demographic parameters, functional assessments, comorbidities, and complications (77 predictors, within 1 month post-stroke) | BI at discharge | 577 post-stroke patients (mean age 64.6 ± 12.6 years) | Classification | DTs, NBC, KNN, LDA, AdaBoost, SVM, LR, and RF | Fivefold cv | ML models AUC scores ranged from 0.83 to 0.887. RF achieved the best AUC score. |
| Katsuki et al. [38] | 2021 | Prediction models for total FIM scores at the discharge of KRW (up to 150 days) | Rehabilitation up to 150 days at Kaifukuki (convalescent) Rehabilitation Ward (KRW) system | Clinical data and CT at acute care ward and functional assessments at KRW (2–4 weeks after acute care ward) | FIM score at discharge | 122 stroke patients (mean age 71 years) | Regression | Prediction One (Sony Network Communications Inc., Tokyo, Japan) | Training (50%, inside 5-fold cv)/validation (50%). | R2 up to 0.972 |
| Rajashekar et al. [47] | 2021 | Prediction of the 30 day NIH stroke scale (NIHSS) at 30 days after stroke symptom onset | Hospitalization | Imaging features from MRI or CT scan (acquired between 18 h and 5 days from symptom onset) and measurable clinical data (obtained after stroke and up to 6 h post) | NIHSS | 221 acute ischemic stroke patients (mean age: 69 years) from ESCAPE and iKNOW trials | Regression | ER mode was implemented using in a radial kernel SVR framework. Three proposed models: an SVR, an SVR with Relief, and a SVR with lesion/symptom mapping technique | Training (80%)/ testing (20%) | Mrelief achieved MAE = 3.55, RMSE = 4.34, and R2 = 0.43 |
| Kim et al. [23] | 2021 | Prediction of motor function outcome in stroke patients at 6 months | Rehabilitation center | Clinical data (14 input variables, from early stage at 7–30 days after stroke onset) | MBC for upper limbs and FAC for lower limbs | 1056 consecutive stroke patients (mean age 59.92 ± 13.94 years) | Classification | DNN, LR, and RF | Training (70%)/validation set (21%)/testing set (9%) | Upper-limb function: DNN model achieved AUC = 0.906 Lower-limb function: DNN, LR, and RF models achieved AUC scores 0.822, 0.768, and 0.802, respectively |
| Sohn et al. [41] | 2021 | Prediction of rehabilitation prognosis in stroke patients using Brainstem Auditory Evoked Potential (BAEP) at 8 to 114 days | Brainstem auditory evoked potential A proper rehabilitation program was applied during hospitalization | Basal K-MBI score, age, and three IPLs (2 weeks of admission on average) | Korean version of the modified Barthel index (K-MBI) | 181 subjects with ischemic stroke (mean age 68.15 ± 11.68 years) | Classification and regression | ANN and SVM | Training (70%, inside 5-fold cv)/testing (30%) | ANN exploiting the BAEP IPLs together with the basal K-MBI score and age achieved 92% sensitivity, 90% specificity, and 90% AUC |
| Jiang et al. [37] | 2021 | A new index for multiple chronic conditions (MCCs) was proposed for prediction of post-stroke functional outcome (FO) at 90 days | Hospitalization | Pre-stroke functional, cognitive, and psychosocial impairments was ascertained from the baseline interview (after 24 h) | MCCs | 1035 patients with ischemic stroke (mean age 68 ± 12.1 years) | Regression | MLR | Training (90%, inside 5-fold cv)/validation (10%) | MCC by MLR: R2 = 0.32 |
| Thakkar et al. [39] | 2020 | Prediction of motor function improvements in chronic stroke patients after 3–4 weeks of rehabilitation | Contemporary task-oriented intervention | Age, side of lesion, gender, baseline functional status, motor function, time since stroke, and quality of life (more than 6 months post stroke) | FMA | 239 chronic stroke patients (mean age 54.72 ± 11.12 years) | Classification | KNN and ANN | Training (80%, inside 10-fold cv)/testing (20%) | KNN model accuracy = 85.42% and AUC = 0.89 |
| Harari et al. [46] | 2020 | Development of predictive models for four standardized clinical outcome measures at discharge (acute phase) | Acute inpatient rehabilitation | Demographics, stroke characteristics, and scores of clinical tests at first week of admission | FIM, TMWT, SMWT, and BBS | 50 stroke survivors (mean age 57.5 ± 14.15 years) | Regression | Lasso regression | LOOCV | R2 = 70–77% and MAEn = 13–15% for predicting the outcomes of new patients |
| Lin et al. [34] | 2020 | Prediction of functional mRS outcome at 90 days after stroke | Hospitalization | Clinical data (impatient elements and data from 30 days follow-up visit) | mRS | 5328 females and 24,965 males (mean onset age 69.71 ± 12.62 years and 65.40 ± 12.64 years) | Classification | SVM, RF, ANN, and hybrid ANN | 10-time repeated hold-out (30%) with 10-fold cv in training | Baseline and follow-up data achieved improved AUC scores to 0.97 |
| Sale et al. [33] | 2018 | Prediction of functional outcomes at discharge in ischemic stroke patients in early stage after rehabilitation treatment | Daily 3 h physiotherapy session | Functional and clinical data (24 h from the admission at the rehabilitation unit) | BI and FIM | 55 sub-acute stroke patients (15 ± 10 days from stroke onset) | Regression | SVM | 20 repetitions-training (70%, inside 5-fold cv)/testing (30%) | RMSE ranged from 4.28 for discharge (T1) cognitive FIM to 22.6 for discharge (T1) BI. |
| Lin et al. [36] | 2018 | Predicting the BI statuses of the patients at discharge after PAC-CVD program at 3 months | PAC-CVD program | MRS, BI, FOIS, MNA, QoL, IADL, BBT, gait speed, 6MWT, FuglUE, FuglSEN, MMSE, MAL, CCAT, age, and length of stay in the acute stroke ward prior to admission to the PAC-CVD ward (stroke onset time within 1 month) | BI status at discharge | 313 post-stroke patients (mean age for patients with high BI at discharge 58.25 ± 13.44, with medium BI 63.20 ± 11.34, and low BI 69.77 ± 11.89) | Classification and regression | LR, SVM, and RF (classification task) SVM with linear kernel and linear regression (regression task) | Fivefold cv | AUC score for LR and RF algorithms was 0.79 and for SVM algorithm was 0.77 SVM and linear regression models achieved mean absolute errors of 9.86 and 9.95, respectively |
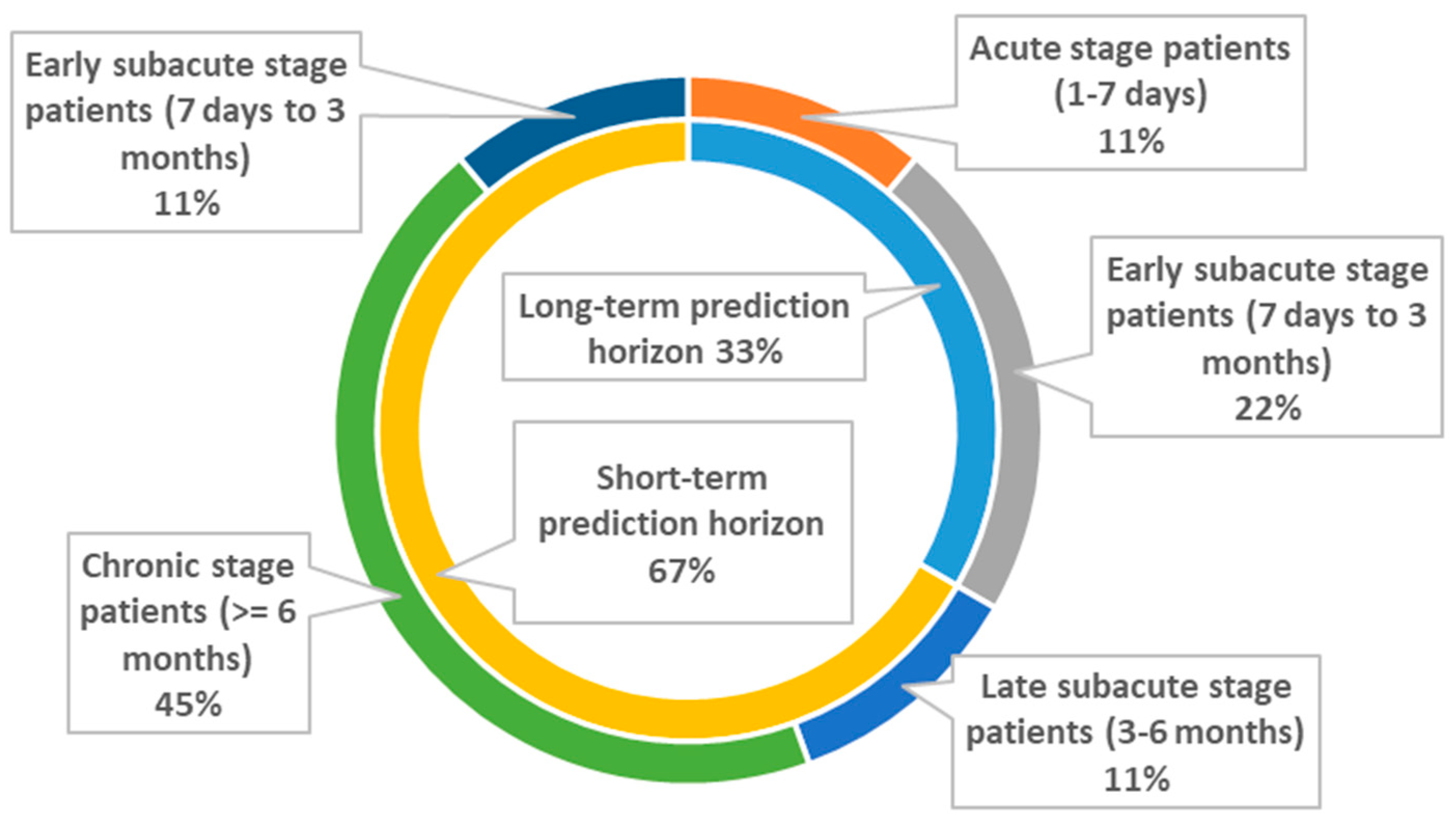
3.3. Upper Extremities
3.3.1. Long-Term Prediction Horizon (≥3 Months)
3.3.2. Short-Term Prediction Horizon (<3 Months)
| Authors | Year | Application Domain | Intervention | Input Data | Outcome Assessment | Employed Subjects | Learning Type | Learning Algorithms | Validation | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Iwamoto et al. [54] | 2022 | Identification of stroke patients who will obtain clinically important improvement at 30 days | Single-joint hybrid assistive limb (HAL-SJ) rehabilitation | Sex, age, days from stroke onset to the initiation of HAL-SJ rehabilitation, cognitive functions, and upper-limb motor functions | Difference of the FMA-UE at 30 days | 71 patients with subacute stroke | Classification | DT analysis CHAID model | - | Acc = 81.7% and AUC = 0.89 |
| Razak et al. [50] | 2022 | Prediction of ipsilateral hand (ILH) impairment at 9 months | Hospitalization | Demographic variables, behavioral, clinical, and neuropsychological data, medical history, thrombolysis treatment, stroke delay, and NIHSS discharge | mRS at 9 months | 209 subacute patients | Classification | LR, LR-bootstrapping, and cv with LASSO and RF | Training (70%)/testing (30%) for the LR | LR achieved AUC = 0.98 |
| Liu et al. [48] | 2022 | Prediction of motor improvement outcome at 3 months | Routine rehabilitation therapies | Baseline whole-brain volumes and motor data (FMA-UE scores) 1 week after symptom onset (<7 days) | Differences between FMA-UE from Week 1 to Week 12 (84 ± 4 days) | 56 patients with subcortical infarction (mean age 53.5 years at proportional group and 53 years at poor group) | Classification | Bagging and GBDT | Fivefold cv | Bagging classifier achieved 87.71% accuracy, 93.77% sensitivity, and 89.74% AUC |
| Lin et al. [55] | 2021 | Prediction of the outcome of BCI training for upper limbs at 2 weeks | Brain-computer interaction (BCI) training | EEG functional connectivity and EEG power spectrum (first-ever stroke within 6 months) | Recovery rate (FMA-UE score after the 2 weeks of BCI training minus the baseline score and then divided by the baseline FMA-UE score minus maximum score) | 11 stroke patients (aged 20–80 years) | Regression | CNN | LOOCV | R2 = 0.98 and RMSE = 0.89 |
| Koch et al. [49] | 2021 | Prediction of structural connectome and motor recovery for upper limbs in natural recovery at 3 months | Natural recovery | SEOUL dataset (3T MRI), GENEVA dataset (3T MRI), and PARIS dataset (3T MRI) were recorded at 2–4 weeks after stroke | Proportional recovery (the change in FMU over time was related to the maximal amount of potential recovery) | 92 after stroke patients (SEOUL: mean age 58 ± 12.6 years, GENEVA mean age 58 ± 12.2 years, and PARIS mean age 55 ± 16.7 years) | Classification | SVM (classification task) | SEOUL dataset in 5-fold cv as internal validation/GENEVA dataset as external validation | At GENEVA dataset (external validation) an accuracy = 60% and precision = 53% were achieved |
| Tozlu et al. [56] | 2020 | Prediction of the individual upper-limb motor impairment in chronic stroke at 6 weeks | 18 therapeutic sessions over a 6-week period | Demographic, clinical, neurophysiological, and imaging variables (3T MRI) with first-time hemorrhagic or ischemic stroke within 3 to 12 months | FMU | 102 stroke patients (aged 54–66 years) | Regression | EN, SVM, ANN, classification and regression trees, and RF | 10-fold cv | R2EN = 0.91, R2RF = 0.88, R2ANN = 0.83, R2SVM = 0.79, R2CART = 0.70 |
| Rafiei et al. [51] | 2019 | Prediction of the improved use of the more affected arm at 3 weeks | Constraint-induced movement therapy | Demographics and baseline clinical characteristics (MAL, WMFT, SWMT, and MCA) (admission date: >6 months of stroke onset) | MAL | 47 people with chronic (>6 months) mild to moderate upper-extremity hemiparesis | Classification | EPNN and NDC algorithm | Leave-one-out approach | Accuracy ≈ 100% |
| George et al. [52] | 2017 | Prediction of the response at 2 weeks of upper-extremity rehabilitation | Virtual-reality gaming (constraint-induced movement therapy was incorporated) | Data from motor and somatosensory (at admission >6 months after stroke) | WMFT | 19 patients with chronic stroke (>6 months) with mild to moderate upper-extremity hemiparesis (aged 14.1–69.6 years) | Classification | EPNN | Leave-one-out approach | Accuracy for the gaming (94.7%) and combined datasets (94.5%) |
| George et al. [53] | 2017 | Prediction of the extent of motor recovery for upper-limb motor ability at 3 weeks | Constraint-induced movement therapy | BKT, SWTM, 15 timed items of the WMFT, and stroke-affected side (at admission >6 months after stroke) | WMFT | 35 post-stroke patients (mean age 60 years) | Classification | EPNN, KNN, and PNN | Leave-one-out approach | EPPN yielded 100% accuracy |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ovbiagele, B.; Nguyen-Huynh, M.N. Stroke Epidemiology: Advancing Our Understanding of Disease Mechanism and Therapy. Neurotherapeutics 2011, 8, 319–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avan, A.; Hachinski, V. Stroke and Dementia, Leading Causes of Neurological Disability and Death, Potential for Prevention. Alzheimer’s Dement. 2021, 17, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Jaracz, K.; Kozubski, W. Quality of Life in Stroke Patients. Acta Neurol. Scand. 2003, 107, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Inchai, P.; Tsai, W.-C.; Chiu, L.-T.; Kung, P.-T. Incidence, Risk, and Associated Risk Factors of Stroke among People with Different Disability Types and Severities: A National Population-Based Cohort Study in Taiwan. Disabil. Health J. 2021, 14, 101165. [Google Scholar] [CrossRef]
- Dobe, J.; Gustafsson, L.; Walder, K. Co-Creation and Stroke Rehabilitation: A Scoping Review. Disabil. Rehabil. 2022, 3, 1–13. [Google Scholar] [CrossRef]
- Chavva, I.R.; Crawford, A.L.; Mazurek, M.H.; Yuen, M.M.; Prabhat, A.M.; Payabvash, S.; Sze, G.; Falcone, G.J.; Matouk, C.C.; de Havenon, A.; et al. Deep Learning Applications for Acute Stroke Management. Ann. Neurol. 2022, 92, 574–587. [Google Scholar] [CrossRef]
- Umeonwuka, C.; Roos, R.; Ntsiea, V. Current Trends in the Treatment of Patients with Post-Stroke Unilateral Spatial Neglect: A Scoping Review. Disabil. Rehabil. 2022, 44, 2158–2185. [Google Scholar] [CrossRef]
- Malekloo, A.; Ozer, E.; AlHamaydeh, M.; Girolami, M. Machine Learning and Structural Health Monitoring Overview with Emerging Technology and High-Dimensional Data Source Highlights. Struct. Health Monit. 2022, 21, 1906–1955. [Google Scholar] [CrossRef]
- Capobianco, E. High-Dimensional Role of AI and Machine Learning in Cancer Research. Br. J. Cancer 2022, 126, 523–532. [Google Scholar] [CrossRef]
- Kokkotis, C.; Moustakidis, S.; Papageorgiou, E.; Giakas, G.; Tsaopoulos, D.E. Machine Learning in Knee Osteoarthritis: A Review. Osteoarthr. Cartil. Open 2020, 2, 100069. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Sakhare, S.R.; Wanjale, K.; Akter, F. Early Stroke Prediction Methods for Prevention of Strokes. Behav. Neurol. 2022, 2022, 7725597. [Google Scholar] [CrossRef]
- Nancy, N.R.S.; Priyadarshini, J. Machine Learning Algorithms for the Diagnosis of Alzheimer and Parkinson Disease. J. Med. Eng. Technol. 2022, 1–9. [Google Scholar] [CrossRef]
- Bivard, A.; Churilov, L.; Parsons, M. Artificial Intelligence for Decision Support in Acute Stroke—Current Roles and Potential. Nat. Rev. Neurol. 2020, 16, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Campagnini, S.; Arienti, C.; Patrini, M.; Liuzzi, P.; Mannini, A.; Carrozza, M.C. Machine Learning Methods for Functional Recovery Prediction and Prognosis in Post-Stroke Rehabilitation: A Systematic Review. J. Neuro Eng. Rehabil. 2022, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Banks, J.L.; Marotta, C.A. Outcomes Validity and Reliability of the Modified Rankin Scale: Implications for Stroke Clinical Trials: A Literature Review and Synthesis. Stroke 2007, 38, 1091–1096. [Google Scholar] [CrossRef] [Green Version]
- Quinn, T.J.; Langhorne, P.; Stott, D.J. Barthel Index for Stroke Trials: Development, Properties, and Application. Stroke 2011, 42, 1146–1151. [Google Scholar] [CrossRef] [Green Version]
- Ohura, T.; Hase, K.; Nakajima, Y.; Nakayama, T. Validity and Reliability of a Performance Evaluation Tool Based on the Modified Barthel Index for Stroke Patients. BMC Med. Res. Methodol. 2017, 17, 131. [Google Scholar] [CrossRef] [Green Version]
- McDowell, I.; Newell, C. Measuring Health: A Guide to Rating Scales and Questionnaires; Oxford University Press: New York, NY, USA, 1996. [Google Scholar]
- Mehrholz, J.; Wagner, K.; Rutte, K.; Meiβner, D.; Pohl, M. Predictive Validity and Responsiveness of the Functional Ambulation Category in Hemiparetic Patients after Stroke. Arch. Phys. Med. Rehabil. 2007, 88, 1314–1319. [Google Scholar] [CrossRef]
- Van Der Lee, J.H.; Beckerman, H.; Lankhorst, G.J.; Bouter, L.M. The Responsiveness of the Action Research Arm Test and the Fugl-Meyer Assessment Scale in Chronic Stroke Patients. J. Rehabil. Med. 2001, 33, 110–113. [Google Scholar] [PubMed] [Green Version]
- Kim, J.K.; Choo, Y.J.; Chang, M.C. Prediction of Motor Function in Stroke Patients Using Machine Learning Algorithm: Development of Practical Models. J. Stroke Cerebrovasc. Dis. 2021, 30, 105856. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.M.; Uswatte, G.; Crago, J.E.; Cook III, E.W.; Taub, E. The Reliability of the Wolf Motor Function Test for Assessing Upper Extremity Function after Stroke. Arch. Phys. Med. Rehabil. 2001, 82, 750–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundquist, C.B.; Maribo, T. The Fugl–Meyer Assessment of the Upper Extremity: Reliability, Responsiveness and Validity of the Danish Version. Disabil. Rehabil. 2017, 39, 934–939. [Google Scholar] [CrossRef] [PubMed]
- IJmker, T.; Houdijk, H.; Lamoth, C.J.; Jarbandhan, A.V.; Rijntjes, D.; Beek, P.J.; van der Woude, L.H. Effect of Balance Support on the Energy Cost of Walking after Stroke. Arch. Phys. Med. Rehabil. 2013, 94, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, N.; Thanikachalam, S.; Satyanarayana, M.; Maiya, A.; Senthil, K.; Sridevi, S. Six Minute Walk Test: A Literary Review. Sri Ramachandra J. Med. 2011, 4, 2–6. [Google Scholar]
- Blum, L.; Korner-Bitensky, N. Usefulness of the Berg Balance Scale in Stroke Rehabilitation: A Systematic Review. Phys. Ther. 2008, 88, 559–566. [Google Scholar] [CrossRef]
- Uswatte, G.; Taub, E.; Morris, D.; Light, K.; Thompson, P. The Motor Activity Log-28: Assessing Daily Use of the Hemiparetic Arm after Stroke. Neurology 2006, 67, 1189–1194. [Google Scholar] [CrossRef]
- Kwah, L.K.; Diong, J. National Institutes of Health Stroke Scale (NIHSS). J. Physiother. 2014, 60, 61. [Google Scholar] [CrossRef] [Green Version]
- Lai, S.-M.; Studenski, S.; Duncan, P.W.; Perera, S. Persisting Consequences of Stroke Measured by the Stroke Impact Scale. Stroke 2002, 33, 1840–1844. [Google Scholar] [CrossRef] [Green Version]
- Park, D.; Jeong, E.; Kim, H.; Pyun, H.W.; Kim, H.; Choi, Y.-J.; Kim, Y.; Jin, S.; Hong, D.; Lee, D.W. Machine Learning-Based Three-Month Outcome Prediction in Acute Ischemic Stroke: A Single Cerebrovascular-Specialty Hospital Study in South Korea. Diagnostics 2021, 11, 1909. [Google Scholar] [CrossRef] [PubMed]
- Sale, P.; Ferriero, G.; Ciabattoni, L.; Cortese, A.M.; Ferracuti, F.; Romeo, L.; Piccione, F.; Masiero, S. Predicting Motor and Cognitive Improvement through Machine Learning Algorithm in Human Subject That Underwent a Rehabilitation Treatment in the Early Stage of Stroke. J. Stroke Cerebrovasc. Dis. 2018, 27, 2962–2972. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Hsu, K.-C.; Johnson, K.R.; Fann, Y.C.; Tsai, C.-H.; Sun, Y.; Lien, L.-M.; Chang, W.-L.; Chen, P.-L.; Lin, C.-L. Evaluation of Machine Learning Methods to Stroke Outcome Prediction Using a Nationwide Disease Registry. Comput. Methods Programs Biomed. 2020, 190, 105381. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-C.; Chu, C.-L.; Chen, C.-K.; Chang, H.-N.; Wong, A.M.; Chen, Y.-P.; Pei, Y.-C. The Comparison and Interpretation of Machine-Learning Models in Post-Stroke Functional Outcome Prediction. Diagnostics 2021, 11, 1784. [Google Scholar] [CrossRef]
- Lin, W.-Y.; Chen, C.-H.; Tseng, Y.-J.; Tsai, Y.-T.; Chang, C.-Y.; Wang, H.-Y.; Chen, C.-K. Predicting Post-Stroke Activities of Daily Living through a Machine Learning-Based Approach on Initiating Rehabilitation. Int. J. Med. Inform. 2018, 111, 159–164. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, L.; Morgenstern, L.B.; Cigolle, C.T.; Claflin, E.S.; Lisabeth, L.D. New Index for Multiple Chronic Conditions Predicts Functional Outcome in Ischemic Stroke. Neurology 2021, 96, e42–e53. [Google Scholar] [CrossRef]
- Katsuki, M.; Narita, N.; Ozaki, D.; Sato, Y.; Jia, W.; Nishizawa, T.; Kochi, R.; Sato, K.; Kawamura, K.; Ishida, N. Deep Learning-Based Functional Independence Measure Score Prediction After Stroke in Kaifukuki (Convalescent) Rehabilitation Ward Annexed to Acute Care Hospital. Cureus 2021, 13, e16588. [Google Scholar] [CrossRef]
- Thakkar, H.K.; Liao, W.; Wu, C.; Hsieh, Y.-W.; Lee, T.-H. Predicting Clinically Significant Motor Function Improvement after Contemporary Task-Oriented Interventions Using Machine Learning Approaches. J. Neuro Eng. Rehabil. 2020, 17, 131. [Google Scholar] [CrossRef]
- Liao, W.-W.; Hsieh, Y.-W.; Lee, T.-H.; Chen, C.; Wu, C. Machine Learning Predicts Clinically Significant Health Related Quality of Life Improvement after Sensorimotor Rehabilitation Interventions in Chronic Stroke. Sci. Rep. 2022, 12, 11235. [Google Scholar] [CrossRef]
- Sohn, J.; Jung, I.-Y.; Ku, Y.; Kim, Y. Machine-Learning-Based Rehabilitation Prognosis Prediction in Patients with Ischemic Stroke Using Brainstem Auditory Evoked Potential. Diagnostics 2021, 11, 673. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chung, J.-H.; Yeh, Y.-J.; Lou, S.-J.; Lin, H.-F.; Lin, C.-H.; Hsien, H.-H.; Hung, K.-W.; Yeh, S.-C.J.; Shi, H.-Y. Predicting 30-Day Readmission for Stroke Using Machine Learning Algorithms: A Prospective Cohort Study. Front. Neurol. 2022, 13, 875491. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-L.; Wu, Y.-D.; Yeh, H.-J.; Wu, Y.-T.; Tsai, H.-Y.; Chen, J.-C. Using Convolutional Neural Network to Analyze Brain MRI Images for Predicting Functional Outcomes of Stroke. Med. Biol. Eng. Comput. 2022, 60, 2841–2849. [Google Scholar] [CrossRef] [PubMed]
- Campagnini, S.; Liuzzi, P.; Mannini, A.; Basagni, B.; Macchi, C.; Carrozza, M.C.; Cecchi, F. Cross-Validation of Predictive Models for Functional Recovery after Post-Stroke Rehabilitation. J. NeuroEng. Rehabil. 2022, 19, 96. [Google Scholar] [CrossRef]
- Lin, P.-J.; Zhai, X.; Li, W.; Li, T.; Cheng, D.; Li, C.; Pan, Y.; Ji, L. A Transferable Deep Learning Prognosis Model for Predicting Stroke Patients’ Recovery in Different Rehabilitation Trainings. IEEE J. Biomed. Health Inform. 2022, 26, 6003–6011. [Google Scholar] [CrossRef] [PubMed]
- Harari, Y.; O’Brien, M.K.; Lieber, R.L.; Jayaraman, A. Inpatient Stroke Rehabilitation: Prediction of Clinical Outcomes Using a Machine-Learning Approach. J. Neuroeng. Rehabil. 2020, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Rajashekar, D.; Hill, M.D.; Demchuk, A.M.; Goyal, M.; Fiehler, J.; Forkert, N.D. Prediction of Clinical Outcomes in Acute Ischaemic Stroke Patients: A Comparative Study. Front. Neurol. 2021, 12, 663899. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wu, J.; Dang, C.; Tan, S.; Peng, K.; Guo, Y.; Xing, S.; Xie, C.; Zeng, J.; Tang, X. Machine Learning for Predicting Motor Improvement after Acute Subcortical Infarction Using Baseline Whole Brain Volumes. Neurorehabilit. Neural Repair 2022, 36, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.J.; Park, C.-H.; Girard, G.; Beanato, E.; Egger, P.; Evangelista, G.G.; Lee, J.; Wessel, M.J.; Morishita, T.; Koch, G. The Structural Connectome and Motor Recovery after Stroke: Predicting Natural Recovery. Brain 2021, 144, 2107–2119. [Google Scholar] [CrossRef]
- Razak, R.A.; Hannanu, F.F.; Naegele, B.; Hommel, M.J.; Detante, O.; Jaillard, A. Ipsilateral Hand Impairment Predicts Long-term Outcome in Patients with Subacute Stroke. Eur. J. Neurol. 2022, 29, 1983–1993. [Google Scholar] [CrossRef]
- Rafiei, M.H.; Kelly, K.M.; Borstad, A.L.; Adeli, H.; Gauthier, L.V. Predicting Improved Daily Use of the More Affected Arm Poststroke Following Constraint-Induced Movement Therapy. Phys. Ther. 2019, 99, 1667–1678. [Google Scholar] [CrossRef]
- George, S.H.; Rafiei, M.H.; Borstad, A.; Adeli, H.; Gauthier, L.V. Gross Motor Ability Predicts Response to Upper Extremity Rehabilitation in Chronic Stroke. Behav. Brain Res. 2017, 333, 314–322. [Google Scholar] [CrossRef] [PubMed]
- George, S.H.; Rafiei, M.H.; Gauthier, L.; Borstad, A.; Buford, J.A.; Adeli, H. Computer-Aided Prediction of Extent of Motor Recovery Following Constraint-Induced Movement Therapy in Chronic Stroke. Behav. Brain Res. 2017, 329, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Imura, T.; Tanaka, R.; Mitsutake, T.; Jung, H.; Suzukawa, T.; Taki, S.; Imada, N.; Inagawa, T.; Araki, H. Clinical Prediction Rule for Identifying the Stroke Patients Who Will Obtain Clinically Important Improvement of Upper Limb Motor Function by Robot-Assisted Upper Limb. J. Stroke Cerebrovasc. Dis. 2022, 31, 106517. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-J.; Jia, T.; Li, C.; Li, T.; Qian, C.; Li, Z.; Pan, Y.; Ji, L. CNN-Based Prognosis of BCI Rehabilitation Using EEG From First Session BCI Training. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1936–1943. [Google Scholar] [CrossRef]
- Tozlu, C.; Edwards, D.; Boes, A.; Labar, D.; Tsagaris, K.Z.; Silverstein, J.; Pepper Lane, H.; Sabuncu, M.R.; Liu, C.; Kuceyeski, A. Machine Learning Methods Predict Individual Upper-Limb Motor Impairment Following Therapy in Chronic Stroke. Neurorehabilit. Neural Repair 2020, 34, 428–439. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokkotis, C.; Moustakidis, S.; Giarmatzis, G.; Giannakou, E.; Makri, E.; Sakellari, P.; Tsiptsios, D.; Karatzetzou, S.; Christidi, F.; Vadikolias, K.; et al. Machine Learning Techniques for the Prediction of Functional Outcomes in the Rehabilitation of Post-Stroke Patients: A Scoping Review. BioMed 2023, 3, 1-20. https://doi.org/10.3390/biomed3010001
Kokkotis C, Moustakidis S, Giarmatzis G, Giannakou E, Makri E, Sakellari P, Tsiptsios D, Karatzetzou S, Christidi F, Vadikolias K, et al. Machine Learning Techniques for the Prediction of Functional Outcomes in the Rehabilitation of Post-Stroke Patients: A Scoping Review. BioMed. 2023; 3(1):1-20. https://doi.org/10.3390/biomed3010001
Chicago/Turabian StyleKokkotis, Christos, Serafeim Moustakidis, Georgios Giarmatzis, Erasmia Giannakou, Evangelia Makri, Paraskevi Sakellari, Dimitrios Tsiptsios, Stella Karatzetzou, Foteini Christidi, Konstantinos Vadikolias, and et al. 2023. "Machine Learning Techniques for the Prediction of Functional Outcomes in the Rehabilitation of Post-Stroke Patients: A Scoping Review" BioMed 3, no. 1: 1-20. https://doi.org/10.3390/biomed3010001
APA StyleKokkotis, C., Moustakidis, S., Giarmatzis, G., Giannakou, E., Makri, E., Sakellari, P., Tsiptsios, D., Karatzetzou, S., Christidi, F., Vadikolias, K., & Aggelousis, N. (2023). Machine Learning Techniques for the Prediction of Functional Outcomes in the Rehabilitation of Post-Stroke Patients: A Scoping Review. BioMed, 3(1), 1-20. https://doi.org/10.3390/biomed3010001









