Abstract
Research of post-stroke locomotion via musculoskeletal (MSK) modeling has offered an unprecedented insight into pathological muscle function and its interplay with skeletal geometry and external stimuli. Advances in solving the dynamical system of post-stroke effort and the generic MSK models used have triggered noticeable improvements in simulating muscle activation dynamics of stroke populations. However, a review of these advancements to inform the scientific community has yet to be made.: PubMed and Scopus databases were used to perform a thorough literature search to identify relevant articles since 2010. Here, we review MSK methods and practices—developed in the last ten years—that have been utilized to explore post-stroke locomotion and examine how their outcomes can inform clinical practice.: Out of the 44 articles that were initially found, 19 were reviewed. The articles were categorized with respect to the type of assessment the MSK methods were used for.: This review notes the considerable competence of existing methods to address post-stroke motion deficits. However, the drawbacks in the implementation of such methods by non-experts due to the high skill demand and the lack of mature software technology for further dissemination of practices and outcomes remain non-trivial.
1. Introduction
Post-stroke locomotion has been at the epicenter of clinical research [1,2,3] attempting to elucidate the neural and motor deficits underlying its deviation from the norm and to inform the rehabilitation process [4]. Hemiparesis—one of the most common signs of stroke—results mainly in muscle weakness, reduced peak muscle torque, a decrease in power generation and early fatigue [5], and an overall challenging postural stability [6]. Hence, stroke gait has been characterized as energetically expensive [7], with the lower limb joint power loss of the paretic leg mainly compensated by the increased mechanical output of the non-paretic side [8].
Methods to explore the pathophysiology of post-stroke movement involve clinical examinations and rigorous biomechanical analysis. The latter has mainly been addressed via the usage of traditional biomechanical models limited to the calculation of spatiotemporal parameters, joint angles, net joint moments, and, by extension, joint work [9,10,11,12]. Additional metrics include electromyography (EMG) [13,14] and muscle synergy analysis [15,16]. Their usability by non-experts made them applicable to many clinical settings, and they are widely used to assess patient function. However, they cannot sufficiently describe the interplay between external forces acting on the body and the internal muscle function to counteract it.
Musculoskeletal (MSK) modeling can build on the shortcomings of mainstream biomechanical analysis via incorporating mathematical formulations to solve the inherent muscle redundancy problem related to sharing the external loads amongst a joint’s musculature [17,18]. The computational biomechanics community has invested decades into simulating healthy human movement, of both the upper and lower body, in the pursuit of mathematical formulations that can accurately describe its mechanical etiology. Hence, three-dimensional (3D), cadaver-based computer models of muscle geometry and physiology [19,20,21,22] have been extensively produced and rigorously used to simulate human locomotion and have often been validated against synchronously measured muscle activity [23,24] or in vivo contact forces from joint replacement devices [25,26,27].
While these musculoskeletal models have provided reasonable results for healthy cohorts, pathological motion has proven more difficult to model. The central nervous system (CNS) intrinsically dictates muscle activation to result in healthy coordinated motion based on external and internal feedback. However, disease-driven impairment of this process substantially challenges our hypotheses of muscle coordination and CNS targets. Early efforts have accommodated a variety of methods to explain the observed motion [28], understand individual muscle roles [29], or predict a new motion by simulating a specific stroke-related symptom [30] to understand post-stroke motion. Furthermore, the personalization of muscle models according to individual measurements has also been incorporated in solving the muscle redundancy problem via the utilization of EMG signals to guide specific muscle activation levels [28]. User-friendly platforms, such as Opensim [31] or Anybody [32], have contributed to further dissemination of such modeling frameworks. Yet, their usage among medical experts has been highly discouraged, mainly due to (i) their complexity and their demand for highly technical skills, (ii) the poor dissemination of newly developed MSK models and modeling software, and (iii) the lack of knowledge of their possibilities to address clinical challenges.
Therefore, the main aim of this scoping review was—for the first time—to inspect current advancements in simulating post-stroke motion and to classify the most recent uses of MSK modeling in an effort to help appreciate its usage in the rehabilitation process of stroke recovery. The results will help to identify current trends and possible future pathways and to determine how the current work can be expanded to address the very restricted implementation of MSK modeling in clinical practice.
2. Materials and Methods
This review was conducted according to published guidelines for scoping reviews [33] and followed the population, concept, and context recommendations to formulate the eligibility criteria. The target population included post-stroke patients, regardless of stroke type and test timing after the stroke incident. The main focus of the review was a survey of MSK modeling methods used to address generic or patient-specific locomotion deficits, without excluding how they are implemented to guide the rehabilitation process. Taking these elements into account, we selected the appropriate studies accordingly.
2.1. Inclusion Criteria
Original studies written in English, using a variety of MSK modeling methods, were included that utilized MSK modeling for the understanding of post-stroke locomotion, either captured from patients through dedicated equipment or predicted in an effort to isolate specific deficits and study them separately.
2.2. Exclusion Criteria
Only peer-reviewed journal articles were considered. Due to significant recent advances in MSK modeling, articles before 2010 were excluded. Articles that made use of MSK models to assess methodological advancements but did not dwell on their implications for understanding stroke neuromuscular deficits were also excluded [34]. Finally, owing to vast variability in musculoskeletal models, we decided to include only studies using models incorporating individual muscle representations and respective anatomical characteristics, avoiding those that model muscle groups as single entities based on anatomical function [35,36].
2.3. Electronic Searches
A search of the literature was performed using the online databases Scopus and PubMed. The search criteria were formed as follows: identifying titles, keywords, and abstracts containing these terms. Papers were collated up to 25 January 2022. Search terms were as follows:
(“Musculoskeletal model*” OR “musculoskeletal simulation” OR “biomechanical model*” OR “biomechanical simulation” OR “inverse dynamics” OR “forward dynamics”) AND (“muscle” OR “tendon” OR “exoskeleton” OR “orthotic” OR “orthoses”) AND (“stroke” OR “hemiparetic”) NOT (“robot*” OR “robotic” OR “finite element”).
2.4. Selection of Studies
Two reviewers (G.G. and N.A.) separately screened all titles, abstracts, and full texts to ensure the suitability of the selected studies. No disagreements were reported. We outlined the selection method and completed a PRISMA flow diagram [37].
2.5. Data Extraction and Management
A standardized data extraction scheme for all selected studies was used by both reviewers, and specific details of each article were recorded, namely, type of intervention, number of stroke patients and healthy controls, type of movement studied, and the main findings of the conducted research. The latter was deemed necessary to showcase the strength of simulation studies in addressing a variety of research questions.
3. Results
An overview of the search methodology workflow is depicted in Figure 1. All included articles were read fully, and their subject matter was classified by use, as seen in Table 1
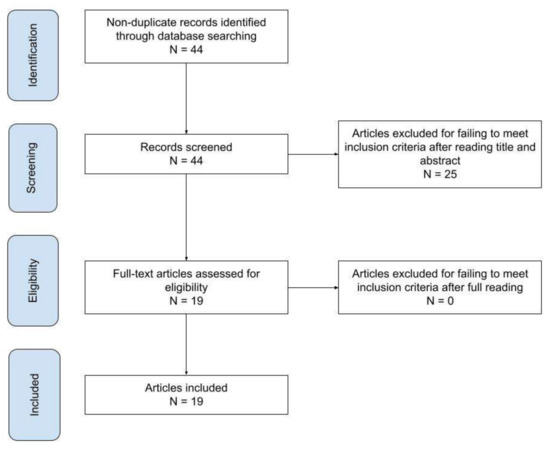
Figure 1.
Search methodology workflow.
Forty-four abstracts were initially found. After applying the exclusion criteria and deciding on the suitability of the studies to include, a sum of 19 papers were found appropriate to review (Table 1).
Studies that have made use of MSK modeling seemed to fall into three categories:
- Assessment of orthotic device and exoskeleton.
- Assessment of intervention.
- Assessment of movement deficits.
The software that was predominantly used was Opensim, except in the studies dealing with upper limb motion. Almost all the papers used captured motion from post-stroke chronic stroke patients of a different type. Some studies also included healthy motion, only to introduce and model specific stroke-induced deficits in isolation and their effect on locomotion. Different versions of the generic MSK model developed by Delp and colleagues [19] were mostly used, which is a cadaver-based computer model of healthy musculoskeletal function. One study [38] used the model developed by Rajahopal et al. [39]. In gait studies with motion captured data, the model’s segments were scaled to each subject’s anthropometric dimensions, based on a recorded standing trial (referred to as scaled models). Moreover, only three studies [40,41,42] targeted upper limb motion using in-house models, with only Li et al. [40] mentioning the use of a scaled model. To calibrate the models for simulating stroke motion and include patient-specific muscle characteristics, EMG analysis was dominantly incorporated, while external joint moments and ultrasound imaging were used in some cases and are described below (referred to as patient-specific models). Most of the studies calculated muscle forces using a mixture of inverse dynamics (based on a recorded motion and ground reaction forces) and/or forward dynamics approaches (based on predicted motion and calculated external forces), using an optimization process to minimize various objective functions. As an alternative, EMG-based muscle synergies were used to estimate muscle forces in some more recent studies. More details of this are discussed later. Finally, only two papers [43,44] did not need to use any kind of approach to solve the muscle redundancy problem. These papers are summarized below.
3.1. Assessment of Orthotic Device and Exoskeleton
Only 2 out of 19 papers used MSK modeling to assess the effect of an orthotic device on post-stroke gait. The effect of an ankle–foot orthosis (AFO) was tested using a scaled model [43] as well as the manipulation of its shank-to-vertical angle on the gastrocnemius muscle–tendon unit (MTU) length and its effect on the biomechanics of gait. Finally, advanced MSK modeling methods and patient-specific models were used to simulate external knee torque application and its effect on post-stroke gait during the pre-swing phase [45].


Table 1.
Characteristics of included studies in the scoping review. Study populations were both stroke patients (SP) and healthy controls (HC).
Table 1.
Characteristics of included studies in the scoping review. Study populations were both stroke patients (SP) and healthy controls (HC).
| Type of Assessment | Study | Study Population | Movement | MAIN Findings |
|---|---|---|---|---|
| Orthotic device | Akbas et al. [45] | 9 SP and 5 HC | Gait | Hip circumduction/hyperreflexia caused by knee external torque application and coupled muscle function. |
| Choi et al. [43] | 1 SP | Gait | Ankle–foot orthoses have successfully reduced gastrocnemius operating length during post-stroke gait, resulting in higher gait speed and reduced stiff-knee gait. | |
| Intervention | Lampire et al. [44] | 10 SP and 10 HC | Gait | Botulinum toxin injection increases rectus femoris maximal length during swing phase of gait. |
| Knarr, Kesar, et al. [46] | 8 SP | Gait | Positive ankle plantar flexor muscle function changes in pre-swing after gait retraining. | |
| Sauder et al. [47] | 1 SP | Gait | Personalized MSK models can allow prediction of optimal muscle electrical stimulation parameters to improve propulsive force symmetry during gait. | |
| Movement deficits | Peterson et al. [48] | 2 SP | Gait | Decreased forward propulsion and power generation by individual paretic muscles compared to healthy. |
| Hall et al. [49] | 10 HC | Gait | Non-paretic rectus femoris and vastii compensate for reduced paretic propulsion. Deficits in the walking subtasks of forward propulsion, swing initiation, and power generation are related to hemiparetic functional walking status. | |
| Peterson et al. [50] | 2 SP and 1 HC | Gait | Stroke gait is more metabolically expensive than healthy. | |
| Knarr, Reisman, et al. [51] | 10 HC | Gait | Simulated PF weakness relates to increased hamstring/hip flexor activation. Simulated hamstring weakness relates to extended knee during early stance. Simulated dorsiflexor weakness leads to foot drop during swing phase. | |
| Jansen et al. [52] | Not applicable | Simulated Gait | Equinus gait can be attributed to simulated ankle plantarflexor spasticity. | |
| Allen et al. [53] | 2 SP | Gait | Post-rehabilitation similar walking speeds can be the result of different coordination patterns related to paretic propulsion. | |
| Knarr et al. [54] | 4 SP | Gait | Subject-specific isometric force and activation data may affect the accuracy of model predictions and should be used when building musculoskeletal models of individuals after stroke. | |
| Meyer et al. [55] | 1 SP | Gait | Synergy-based muscle force prediction is a reliable method to simulate experimental data. | |
| L. Li and Tong [40] | 5 SP and 5 HC | Elbow flexion–extension | Better torque estimations with subject-specific muscle pennation angle and optimal length. | |
| Asghari et al. [41] | 6 SP and 2 HC | Elbow flexion–extension | Usage of subject-specific muscle weightings to update generic MSK models better predict experimental motion. | |
| Ang et al. [42] | 15 SP | Elbow flexion–extension | Model predicts severity ranking of spasticity. | |
| Ong et al. [56] | Not applicable | Simulated Gait | PF weakness leads to heel-strike gait. PF contracture leads to equinus gait. | |
| Arones et al. [38] | 2 SP | Gait | EMG-driven simulations combined with Barghava metabolic cost model correlated best with slopes from experimental data. | |
| Santos et al. [57] | 1 SP | Gait | Passive moment generation explains knee hyperextension. Increase in knee extensor strength predicted a reduction in knee hyperextension. Weakening of the knee extensors and strengthening of the knee flexors can correct stiff-knee gait. Weak ankle plantar flexors and strong ankle dorsiflexors predict a reduced drop foot. FES improves gait speed and reduces circumduction. |
3.2. Assessment of Intervention
Three out of nineteen papers used MSK modeling to evaluate the effect of a treatment prescription on post-stroke gait. The effect of botulinum toxin (BOTOX) injection on the length and lengthening velocity of rectus femoris pre- and post-intervention were investigated [44]. The rest explored the adaptations in stroke gait after a functional electrical stimulation (FES) intervention via MSK modeling [46,47]. Patient-specific models were used in these cases.
3.3. Assessment of Movement Deficits
The largest part of the literature, namely, 14 out of 19 papers, used MSK modeling to describe post-stroke gait deficits with mechanistic terms. The majority of those were based on recorded patient or healthy motion. Detailed muscle function during different portions of the gait phase was explored through MSK modeling in four studies [48,49,50,53]. EMG-informed, patient-specific MSK modeling to personalize certain muscle-specific properties and simulate post-stroke gait was used in three studies [38,54,55]. Moreover, three studies utilized predictive MSK modeling to generate motions and study specific stroke symptoms in isolation, using a scaled model [52] or a patient-specific one [51,56]. One recent study [57] implemented both motion prediction to study the cause–effect relationship of stroke-induced gait deficits and a scaled model of a single stroke patient to study the effect of FES treatment. The latter falls also into the previous section; however, the authors chose to include this study in this section as it was more relevant to motion and its underlying impairments. Finally, three studies focused on upper limb movement and especially elbow flexion–extension, with two implementing a personalization strategy of muscle properties [40] or recruitment pattern [41] based on experimental data (ultrasound and EMG, respectively). The third one used an inverse dynamics approach to explore dynamic stretch reflex properties and objectively assess spasticity during passive motion [42].
4. Discussion
As a novel contribution to the existing literature, the present scoping review highlighted the advancement in applications of MSK modeling combined with motion capture to explore post-stroke motion. Information on individual muscle function—mostly force output—was the main output of the reviewed studies, highlighting the importance of MSK methods to offer such insight otherwise not available. Biomechanical outcome measures from MSK modeling can provide an objective manner to assess post-stroke motion as an alternative to clinical scales of functional assessment which have been criticized for increased subjectivity and limited validity [58]. Such knowledge can drive their usage in settings outside the confined walls of a scientific lab, fostering novel methods of post-stroke evaluation and treatment. As stated before, detailed remarks on modeling choices and comparisons were outside the scope of this review. For further reference, the reader is advised to read the respective articles.
The vast majority of reviewed papers—16 out of 19—studied only stroke gait. Walking remains one of the instrumental functional activities, and its optimal performance is related to a high quality of life amongst post-stroke patients [59]; hence, research has mainly focused on this activity and a great deal of expertise has been built around it. Although scaled models have been used to simulate healthy gait, this review has found that post-stroke muscle deficits are suitably modeled when scaled models are updated with patient-specific information on muscle physiology from various sources, such as EMG or ultrasound. This necessity makes the modeling process even more complex and labor-intensive, and future research should minimize such demands to allow clinical usage.
Common symptoms of stroke have also been evaluated regarding their effect on walking performance, and individual muscle force calculations via MSK modeling have contributed to this end. Many studies have explored the confounding role of specific lower limb muscle weakness in common patterns of post-stroke gait, such as hip circumduction and equinus and stiff-knee gait, and have offered guidelines for training programs to focus on a particular muscle or muscle group that needs strengthening. Neurological symptoms often seen in stroke patients have been less modeled; nevertheless, MSK modeling could support decision making on orthotic device prescription to counteract contracture-related muscle shortening based on muscle length estimations or provide an objective, muscle force-based method to assess upper limb spasticity. Altogether, clinical practice has much insight to gain from modern MSK modeling methods and patient-specific models to design tailored interventions and address post-stroke muscle deficits.
The rest of the papers focused on a simple upper limb planar movement, namely, elbow flexion–extension, indicating the difficulty to model upper extremities and their muscular function during more complicated, multi-articular movements. MSK models of upper limbs were custom and not publicly available as opposed to Opensim models used in gait, limiting their usage by a broad scientific audience. Expanding the list of tasks analyzed involving both the upper and lower body remains a necessity for a better assessment of a patient’s motor abilities and enhanced rehabilitation outcomes. Although MSK models have been mostly validated for walking tasks or simple reaching tasks, it is highly recommended that a similar validation process be conducted for other functional tasks to enable their usage in pathological motion as well.
Notably, very little has been done—or at least published and undergone the peer-review process—to apply MSK modeling to the early stages of stroke recovery as part of the decision-making process for optimal rehabilitation plans. It seems its standard use revolves primarily around the assessment of functional deficits and secondarily around the treatment effect during the post-acute phase, without being used to inform early prescription.
Three categories of MSK modeling usages were found and are discussed in the following sections, providing details on the different methods used and the various research questions being answered.
4.1. Assessment of Orthotic Device and Exoskeleton
Documented usage of MSK modeling in the assessment of orthotic devices is scarce yet very informative regarding the effect of external devices on body function. Simulating the mechanical interplay between a device and the human body can be very challenging. In the study by Akbas et al. [45], different prescribed external knee torques driven by a wearable exoskeleton to maximize knee flexion during the pre-swing phase of post-stroke gait were simulated as coupled forces acting on the thigh and tibia, using 3D musculoskeletal models and a combination of inverse and forward dynamics analysis, namely, computed muscle control—CMC. In brief, CMC calculates the model accelerations that best match the experimental ones, estimates muscle forces for each instance that will achieve those accelerations using inverse dynamics, and performs a forward dynamics simulation that uses the forces to drive the MSK model to the next instant. Calculated muscle forces and fiber stretch velocities showed that such induced external torques may trigger rectus femoris hyperreflexia in stroke patients and consequent stiff-knee gait, coupled simultaneously with gluteus medius activation, mainly responsible for apparent hip circumduction.
Choi et al. [43] used a 3D musculoskeletal model to model the muscle function of a stroke patient when walking with ankle–foot orthosis in different prescribed shank-to-vertical angles. MSK modeling and standard inverse kinematics (i.e., calculation of joint angles based on recorded motion) facilitated the estimation of gastrocnemius musculotendon unit operating length, which enabled improved control of gait kinematics and the kinetics for maximum ankle plantar flexor moment and toe clearance during gait.
Both studies highlighted the usefulness of MSK modeling to present valuable information on muscle mechanics as influenced by external devices and guide their configuration to match the patient’s needs and improve biomechanical factors. In the past, MSK modeling has been used to test various “what if” scenarios and predict the best assistive devices’ parameters to optimize walking performance [60,61] or to avoid ankle injuries [62]; hence, future studies should build on this acquired knowledge to develop an a priori design optimized for assistive devices in silico and tailored to stroke patients’ specific needs in order to avoid a potentially detrimental prescription.
4.2. Assessment of Intervention
Evaluating the effect of treatment is very crucial to designing and implementing rehabilitation plans for stroke patients; however, the present review has found very limited usage of MSK modeling to that end. The study by Lampire et al. [44] has found that BOTOX injection on spastic rectus femoris can increase peak knee flexion during swing phase as well as gait velocity, with MSK modeling used to show that the maximum normalized length and lengthening velocity of the specific muscle increases post intervention and improves stiff-knee gait.
Fast functional electrical stimulation (FastFES) treatment—a gait retraining intervention combining fast treadmill gait and electrical stimulation—on post-stroke ankle PF function has been the focus of both the following studies. Knarr et al. [46] used a mix of inverse and forward dynamics analysis (CMC and induced acceleration analysis (IAA)) to demonstrate that MSK modeling can detect the improvements witnessed in clinical practice by calculating increased ankle PF activation and consequent contribution to center-of-mass (COM) acceleration during the double support phase of gait, leading to an overall gait velocity increase. The next study by Sauder et al. [47] simulated specific muscle stimulation settings to predict the functional outcome post-intervention, thus finding optimal muscle stimulation settings for non-responders to standard FastFES treatment. This particular workflow included a rigorous and highly complex personalization of the musculoskeletal model, optimizing joint positions, muscle–tendon parameters, ground–foot contact model settings, and muscle synergy factors based on muscle EMG, kinematics, and kinetics data to most accurately reproduce the experimental marker position and ground reaction forces during gait. The muscle redundancy problem was solved through a direct collocation method.
From the above, we can infer that MSK modeling can help clinicians objectively quantify the effect of intervention and provide biomarkers of muscle function pre- and post- treatment. The ability to predict a patient’s response to treatment can heavily optimize prescription.
4.3. Assessment of Movement Deficits
The vast majority of studies researched post-stroke gait and the accompanying muscle deficits, usually incorporating some level of personalization in the MSK model used. The work of Hall et al. [48] used CMC and IAA methods to identify limited forward propulsion in stroke patients, while Allen et al. [53] found that the asymmetry in forward propulsion is coupled with PF-impaired coordination. Both Hall et al. [49] and Henderson et al. [50] found that non-paretic enhanced muscle output functions as compensation for paretic limb weakness. A forward dynamic approach to explore muscle impairment was implemented by Knarr et al. [51] to predict how normal gait would be changed if specific limited muscle capacity was introduced in the model. An alternative method to determine which motor deficits drive stroke gait—initially generated motions of normal gait that can later be perturbed based on simulated muscle length and muscle lengthening velocity feedback gains (reflexes)—was implemented by Jansen et al. [52], while Ong et al. performed a similar analysis using a model of muscle force reflex [56]. This workflow allowed the authors to reproduce basic patterns of stroke gait, such as equinus or foot-drop gait, via modulating feedback gains on these reflexes, hence simulating spasticity. A recent study by Santos et al. [57] employed predictive simulations and personalized muscle–tendon parameters to study the effect of isolated muscle impairments on the motion of one stroke patient and how FES can attenuate them. The knee extensors’ weakness was related to knee hyperextension, and stiff-knee gait could be corrected by coupled knee flexor strengthening and knee extensor weakening. They later predicted and validated that FES on spastic rectus femoris can correct knee hyperextension and drop-foot but not stiff-knee gait. Finally, similar to work by Sauder et al. [47], EMG, kinematics, and kinetics data were used by Meyer et al. [55] to optimize joint center locations and muscle parameters that best follow recorded motion in an effort to best simulate patient-specific neuromuscular deficits or calculate the metabolic cost of stroke gait [38]. This method consisted of different complex simulation steps, a large number of which comprised a rigorous utilization of muscle synergy analysis to build dynamically consistent MSK models.
Upper extremity muscle deficits were modeled in three studies. Ultrasound images from stroke patients were used to update a generic arm musculoskeletal model of elbow extensors [40], indicating better compliance with measured external torques and the need for individualization of MSK modeling workflows. Such necessity was also outlined by Ashgari et al. [41] who used optimal control to distribute muscle weights to minimize experimental and predicted hand trajectory, thus exploring patient-specific neural control in hand movements. Last, MSK modeling was used to explore and quantify upper limb spasticity during passive planar elbow movements through inverse dynamics methods [42]. More specifically, Ang et al. predicted the angles in different angular velocities of elbow extension where spasticity sets in, that is, where abnormal muscle forces are elicited during motion. Thus, the angle at zero velocity was calculated and suggested as an objective biomarker of spasticity level.
To sum up, MSK modeling can serve as a means to objectively quantify muscle deficits and understand patient-specific motion strategy, which may enable clinicians to infer safer assumptions about the underlying neurological impairments. Recently, the Stroke Recovery and Rehabilitation Roundtable has strongly recommended that conventional biomechanical analysis should be incorporated in standard stroke motion evaluation [63], adding that kinematic and kinetic movement quantification is much needed. MSK modeling can build upon the availability of such data. The calculation of post-stroke muscle forces can help evaluate specific motion deficits and design treatments that focus on individual muscle force output.
4.4. Limitations
The current scoping review only included studies written in the English language and published in peer-reviewed journals, thus possibly excluding important studies written in other languages or available in pre-print form (for example, published on arXiv.org as accessed on 25 January 2022). All conclusions are based on data derived from simulations which are just an approximation of how stroke patients’ MSK systems work. Hence, our results should be interpreted with caution.
5. Conclusions
This scoping review found only 19 published studies that utilize MSK modeling to explore stroke locomotion. This finding is striking considering the vast literature on this specific disease and its high impact on society. Moreover, our findings also contradict the wealth of articles on MSK modeling that primarily targets healthy populations. Considering the above, a lack of mature software technology that can be easily utilized by a range of scientists and clinicians could be the determinant factor of such low usage of MSK modeling approaches for stroke management. Nevertheless, conclusions based on widely used biomechanical measurements of joint kinematics and kinetics can be further enhanced if combined with MSK modeling methods. Findings from the reviewed studies show that computational biomechanical modeling seems to be a unique tool for estimating unmeasured quantities of interest and characterizing stroke motion, yet still much development is needed for it to be utilized in a clinical setting.
A major shortcoming of MSK modeling is the lack of direct validation of estimated muscle forces; hence, conclusions made in most of the reviewed papers should be considered with caution. Indirect methods usually include comparisons with experimental data, such as EMG [64,65] or joint contact forces [26,66]; however, the literature has shown that not a single algorithm to solve the muscle redundancy problem has been proven accurate [67]. To address this issue and increase the personalization of the modeling process needed to simulate pathological motion [68], two distinct methods were used: (i) implementing a variety of cost functions to be minimized that incorporated error terms between model outputs and experimental data, and (ii) updating tendon–muscle parameters of the generic MSK models. However, the gathering and analyses of various signals, usually EMG and less often ultrasound, increases the burden of motion capture protocols and the complexity of the modeling process, which may be not feasible in a clinical setting where technical skill is limited. This drawback prevents the reproducibility of the research and hinders researchers from applying such methods for further patient stratification. Moreover, fully calibrated models’ output may be more difficult to interpret than simply scaled ones, since force estimations are highly sensitive to model parameters [69]. Finally, recent studies have shown subtly added value in using more complex methods, such as those involving CMC [70,71,72,73], EMG [67], or muscle synergies [74], to describe healthy or pathological motion, but no studies have made similar comparisons for stroke yet.
The assumptions and simplifications of the modeling methods were generally found to be inconsistent with stroke pathology. Optimization of some form of neuromuscular function probably contradicts the impaired state of the CNS of a stroke patient, which will try to compensate for specific muscle deficits to achieve a task goal [75] rather than minimize effort to do so. Alternatively, two studies [38,47] suggested a synergy-based muscle force estimation, although the need to acquire EMG data from a large number of muscles from each leg may render this technique unfeasible in a clinical setting. Furthermore, generic MSK models did not include any changes in the musculoskeletal system that come with stroke and age; hence, further research should systematically include such parameters. Last, the variability in the methods does not permit safe conclusions on which is the best to recommend, so further research must prove the advantages of each and its feasibility in clinical motion analysis.
To conclude, the authors believe that the computational biomechanics of the human body and its function can provide the means to explore stroke motion. However, much work is needed to simulate the variety of neuromuscular deficits present in stroke patients. Even though an understanding of the current motor state of a stroke patient is highly precious, future studies should also target predicting functional outcomes pre-intervention, thus helping clinicians make better choices of rehabilitation plans [76].
Author Contributions
Conceptualization, G.G. and N.A.; methodology, G.G.; writing—original draft preparation, G.G., S.F. and E.G.; writing—review and editing, N.A., E.G., C.K., T.F., S.K. and K.V.; visualization, G.G.; supervision, N.A.; project administration, N.A.; funding acquisition, N.A. and K.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grant MIS 5047286 from Greek and European funds (EYD-EPANEK).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beyaert, C.; Vasa, R.; Frykberg, G.E. Gait post-stroke: Pathophysiology and rehabilitation strategies. Neurophysiol. Clin. 2015, 45, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Pollock, C.L.; Eng, J.J.; Garland, S.J. Clinical measurement of walking balance in people post stroke: A systematic review. Clin. Rehabil. 2011, 25, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Roelker, S.A.; Bowden, M.G.; Kautz, S.A.; Neptune, R.R. Paretic propulsion as a measure of walking performance and functional motor recovery post-stroke: A review. Gait Posture 2019, 68, 6–14. [Google Scholar] [CrossRef]
- Pollock, A.; Farmer, E.S.; Brady, M.C.; Langhorne, P.; Mead, E.G.; Mehrholz, J.; van Wijck, F. Interventions for improving upper limb function after stroke. Cochrane Database Syst. Rev. 2013, 2013, CD010820. [Google Scholar] [CrossRef]
- Rabelo, M.; Nunes, G.S.; Amante, N.M.d.; de Noronha, M.; Fachin-Martins, E. Reliability of muscle strength assessment in chronic post-stroke hemiparesis: A systematic review and meta-Analysis. Top. Stroke Rehabil. 2016, 23, 26–35. [Google Scholar] [CrossRef]
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Stoquart, G.; Detrembleur, C.; Lejeune, T.M. The reasons why stroke patients expend so much energy to walk slowly. Gait Posture 2012, 36, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, I.; Delp, S.; Patten, C. Capacity to increase walking speed is limited by impaired hip and ankle power generation in lower functioning persons post-stroke. Gait Posture 2009, 29, 129–137. [Google Scholar] [CrossRef]
- Ramlee, M.H.; Gan, K.B. Function and Biomechanics of Upper Limb in Post-Stroke Patients—A Systematic Review. J. Mech. Med. Biol. 2017, 17, 1750099. [Google Scholar] [CrossRef]
- Cruz, T.H.; Lewek, M.D.; Dhaher, Y.Y. Biomechanical impairments and gait adaptations post-stroke: Multi-factorial associations. J. Biomech. 2009, 42, 1673–1677. [Google Scholar] [CrossRef]
- Nadeau, S.; Betschart, M.; Bethoux, F. Gait analysis for poststroke rehabilitation: The relevance of biomechanical analysis and the impact of gait speed. Phys. Med. Rehabil. Clin. N. Am. 2013, 24, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Arya, K.N.; Sharma, P.; Garg, R.K. Understanding gait control in post-stroke: Implications for management. J. Bodyw. Mov. Ther. 2012, 16, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Park, C.I.; Chon, J.S.; Ohn, S.H.; Park, T.H.; Bang, I.K. Biomechanical assessment with electromyography of post-stroke ankle plantar flexor spasticity. Yonsei Med. J. 2005, 46, 546–554. [Google Scholar] [CrossRef]
- Disselhorst-Klug, C.; Williams, S. Surface Electromyography Meets Biomechanics: Correct Interpretation of sEMG-Signals in Neuro-Rehabilitation Needs Biomechanical Input. Front. Neurol. 2020, 11, 603550. [Google Scholar] [CrossRef]
- Barroso, F.O.; Torricelli, D.; Molina-Rueda, F.; Alguacil-Diego, I.M.; Cano-De-La-Cuerda, R.; Santos, C.; Moreno, J.C.; Miangolarra-Page, J.C.; Pons, J.L. Combining muscle synergies and biomechanical analysis to assess gait in stroke patients. J. Biomech. 2017, 63, 98–103. [Google Scholar] [CrossRef]
- Clark, D.J.; Ting, L.H.; Zajac, F.E.; Neptune, R.R.; Kautz, S.A. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J. Neurophysiol. 2010, 103, 844–857. [Google Scholar] [CrossRef]
- de Groote, F.; Kinney, A.L.; Rao, A.V.; Fregly, B.J. Evaluation of Direct Collocation Optimal Control Problem Formulations for Solving the Muscle Redundancy Problem. Ann. Biomed. Eng. 2016, 44, 2922–2936. [Google Scholar] [CrossRef] [PubMed]
- Thelen, D.G.; Anderson, F.C.; Delp, S.L. Generating dynamic simulations of movement using computed muscle control. J. Biomech. 2003, 36, 321–328. [Google Scholar] [CrossRef]
- Delp, S.L.; Loan, J.P.; Hoy, M.G.; Zajac, F.E.; Topp, E.L.; Rosen, J.M. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Trans. Biomed. Eng. 1990, 37, 757–767. [Google Scholar] [CrossRef]
- Thelen, D.G. Adjustment of muscle mechanics model parameters to simulate dynamic contractions in older adults. J. Biomech. Eng. 2003, 125, 70–77. [Google Scholar] [CrossRef]
- Arnold, E.M.; Ward, S.R.; Lieber, R.L.; Delp, S.L. A Model of the Lower Limb for Analysis of Human Movement. Ann. Biomed. Eng. 2010, 38, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Hoy, M.G.; Zajac, F.E.; Gordon, M.E. A musculoskeletal model of the human lower extremity: The effect of muscle, tendon, and moment arm on the moment-angle relationship of musculotendon actuators at the hip, knee, and ankle. J. Biomech. 1990, 23, 157–169. [Google Scholar] [CrossRef]
- Karimi, M.T.; Hemmati, F.; Mardani, M.A.; Sharifmoradi, K.; Hosseini, S.I.; Fadayevatan, R.; Esrafilian, A. Determination of the correlation between muscle forces obtained from OpenSim and muscle activities obtained from electromyography in the elderly. Phys. Eng. Sci. Med. 2021, 44, 243–251. [Google Scholar] [CrossRef]
- Żuk, M.; Syczewska, M.; Pezowicz, C. Use of the surface electromyography for a quantitative trend validation of estimated muscle forces. Biocybern. Biomed. Eng. 2018, 38, 243–250. [Google Scholar] [CrossRef]
- Giarmatzis, G.; Jonkers, I.; Wesseling, M.; van Rossom, S.; Verschueren, S. Loading of Hip Measured by Hip Contact Forces at Different Speeds of Walking and Running. J. Bone Miner. Res. 2015, 30, 1431–1440. [Google Scholar] [CrossRef]
- Fregly, B.J.; Besier, T.; Lloyd, D.; Delp, S.L.; Banks, S.; Pandy, M.; D’Lima, D. Grand challenge competition to predict in vivo knee loads. J. Orthop. Res. 2012, 30, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Modenese, L.; Phillips, A.T.M.; Bull, A.M.J. An open source lower limb model: Hip joint validation. J. Biomech. 2011, 44, 2185–2193. [Google Scholar] [CrossRef]
- Shao, Q.; Bassett, D.N.; Manal, K.; Buchanan, T.S. Moments in Stroke Patients. Comput. Biol. Med. 2009, 39, 1083–1088. [Google Scholar] [CrossRef]
- Higginson, J.S.; Zajac, F.E.; Neptune, R.R.; Kautz, S.A.; Delp, S.L. Muscle contributions to support during gait in an individual with post-stroke hemiparesis. J. Biomech. 2006, 39, 1769–1777. [Google Scholar] [CrossRef]
- Higginson, J.S.; Zajac, F.E.; Neptune, R.R.; Kautz, S.A.; Burgar, C.G.; Delp, S.L. Effect of equinus foot placement and intrinsic muscle response on knee extension during stance. Gait Posture 2006, 23, 32–36. [Google Scholar] [CrossRef]
- Seth, A.; Hicks, J.L.; Uchida, T.K.; Habib, A.; Dembia, C.L.; Dunne, J.J.; Ong, C.F.; Demers, M.S.; Rajagopal, A.; Millard, M.; et al. OpenSim: Simulating musculoskeletal dynamics and neuromuscular control to study human and animal movement. PLoS Comput. Biol. 2018, 14, e1006223. [Google Scholar] [CrossRef]
- Damsgaard, M. Analysis of musculoskeletal systems in the AnyBody Modeling System. Simul. Model. Pract. Theory 2006, 14, 1100–1111. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. Int. J. Evid. Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Shourijeh, M.S.; Ao, D.; Patten, C.; Fregly, B.J. How Well Do Commonly Used Co-contraction Indices Approximate Lower Limb Joint Stiffness Trends During Gait for Individuals Post-stroke? Front. Bioeng. Biotechnol. 2021, 8, 588908. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, P.G.; Gäverth, J.; Islam, M.; Fagergren, A.; Borg, J.; Forssberg, H. Validation of a new biomechanical model to measure muscle tone in spastic muscles. Neurorehabil. Neural Repair 2011, 25, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Gäverth, J.; Herman, P.A. Neural and non-neural related properties in the spastic wrist flexors: An optimization study. Med. Eng. Phys. 2017, 47, 198–209. [Google Scholar] [CrossRef]
- Aromataris, E.; Riitano, D. Constructing a search strategy and searching for evidence. A guide to the literature search for a systematic review. Am. J. Nurs. 2014, 114, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Arones, M.M.; Shourijeh, M.S.; Patten, C.; Fregly, B.J. Musculoskeletal Model Personalization Affects Metabolic Cost Estimates for Walking. Front. Bioeng. Biotechnol. 2020, 8, 588925. [Google Scholar] [CrossRef]
- Rajagopal, A.; Dembia, C.L.; DeMers, M.S.; Delp, D.D.; Hicks, J.L.; Delp, S.L. Full-Body Musculoskeletal Model for Muscle-Driven Simulation of Human Gait. IEEE Trans. Biomed. Eng. 2016, 63, 2068–2079. [Google Scholar] [CrossRef]
- Li, L.; Tong, R.K.Y. Combined Ultrasound Imaging and Biomechanical Modeling to Estimate Triceps Brachii Musculotendon Changes in Stroke Survivors. Biomed Res. Int. 2016, 2016, 5275768. [Google Scholar] [CrossRef]
- Asghari, M.; Behzadipour, S.; Taghizadeh, G. A planar neuro-musculoskeletal arm model in post-stroke patients. Biol. Cybern. 2018, 112, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Ang, W.S.; Geyer, H.; Chen, I.M.; Ang, W.T. Objective Assessment of Spasticity with a Method Based on a Human Upper Limb Model. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Bjornson, K.; Fatone, S.; Steele, K.M. Using musculoskeletal modeling to evaluate the effect of ankle foot orthosis tuning on musculotendon dynamics: A case study. Disabil. Rehabil. Assist. Technol. 2016, 11, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Lampire, N.; Roche, N.; Carne, P.; Cheze, L.; Pradon, D. Effect of botulinum toxin injection on length and lengthening velocity of rectus femoris during gait in hemiparetic patients. Clin. Biomech. 2013, 28, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Akbas, T.; Neptune, R.R.; Sulzer, J. Neuromusculoskeletal simulation reveals abnormal rectus femoris-gluteus medius coupling in post-stroke gait. Front. Neurol. 2019, 10, 301. [Google Scholar] [CrossRef]
- Knarr, B.A.; Kesar, T.M.; Reisman, D.S.; Binder-Macleod, S.A.; Higginson, J.S. Changes in the activation and function of the ankle plantar flexor muscles due to gait retraining in chronic stroke survivors. J. Neuroeng. Rehabil. 2013, 10, 12. [Google Scholar] [CrossRef]
- Sauder, N.R.; Meyer, A.J.; Allen, J.L.; Ting, L.H.; Kesar, T.M.; Fregly, B.J. Computational Design of FastFES Treatment to Improve Propulsive Force Symmetry during Post-stroke Gait: A feasibility study. Front. Neurorobot. 2019, 13, 80. [Google Scholar] [CrossRef]
- Peterson, C.L.; Hall, A.L.; Kautz, S.A.; Neptune, R.R. Pre-swing deficits in forward propulsion, swing initiation and power generation by individual muscles during hemiparetic walking. J. Biomech. 2010, 43, 2348–2355. [Google Scholar] [CrossRef]
- Hall, A.L.; Peterson, C.L.; Kautz, S.A.; Neptune, R.R. Relationships between muscle contributions to walking subtasks and functional walking status in persons with post-stroke hemiparesis. Clin. Biomech. 2011, 26, 509–515. [Google Scholar] [CrossRef]
- Peterson, C.L.; Kautz, S.A.; Neptune, R.R. Muscle work is increased in pre-swing during hemiparetic walking. Clin. Biomech. 2011, 26, 859–866. [Google Scholar] [CrossRef]
- Knarr, B.A.; Reisman, D.S.; Binder-Macleod, S.A.; Higginson, J.S. Understanding compensatory strategies for muscle weakness during gait by simulating activation deficits seen post-stroke. Gait Posture 2013, 38, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.; de Groote, F.; Aerts, W.; de Schutter, J.; Duysens, J.; Jonkers, I. Altering length and velocity feedback during a neuro-musculoskeletal simulation of normal gait contributes to hemiparetic gait characteristics. J. Neuroeng. Rehabil. 2014, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; Kautz, S.A.; Neptune, R.R. Forward propulsion asymmetry is indicative of changes in plantarflexor coordination during walking in individuals with post-stroke hemiparesis. Clin. Biomech. 2014, 29, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Knarr, B.A.; Reisman, D.S.; Binder-Macleod, S.A.; Higginson, J.S. Changes in predicted muscle coordination with subject-specific muscle parameters for individuals after stroke. Stroke Res. Treat. 2014, 2014, 321747. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.J.; Eskinazi, I.; Jackson, J.N.; Rao, A.V.; Patten, C.; Fregly, B.J. Muscle synergies facilitate computational prediction of subject-specific walking motions. Front. Bioeng. Biotechnol. 2016, 4, 77. [Google Scholar] [CrossRef]
- Ong, C.F.; Geijtenbeek, T.; Hicks, J.L.; Delp, S.L. Predicting gait adaptations due to ankle plantarflexor muscle weakness and contracture using physics-based musculoskeletal simulations. PLoS Comput. Biol. 2019, 15, e1006993. [Google Scholar] [CrossRef]
- Santos, G.F.; Jakubowitz, E.; Pronost, N.; Bonis, T.; Hurschler, C. Predictive simulation of post-stroke gait with functional electrical stimulation. Sci. Rep. 2021, 11, 21351. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.K.; McArthur, K.S.; Quinn, T.J. Assessment scales in stroke: Clinimetric and clinical considerations. Clin. Interv. Aging 2013, 8, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Hong, E. Comparison of quality of life according to community walking in stroke patients. J. Phys. Ther. Sci. 2015, 27, 2391–2393. [Google Scholar] [CrossRef] [PubMed]
- Dembia, C.L.; Silder, A.; Uchida, T.K.; Hicks, J.L.; Delp, S.L. Simulating ideal assistive devices to reduce the metabolic cost of walking with heavy loads. PLoS ONE 2017, 12, e0180320. [Google Scholar] [CrossRef]
- Afschrift, M.; de Groote, F.; de Schutter, J.; Jonkers, I. The effect of muscle weakness on the capability gap during gross motor function: A simulation study supporting design criteria for exoskeletons of the lower limb. Biomed. Eng. Online 2014, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, J. Analysis of the Human Musculoskeletal System and Simulation-Based Design of Assistive Devices Using OpenSim; Birla Institute of Technology and Science: Pilani, India, 2014; p. 45. Available online: http://jalajmaheshwari.com/files/OpenSIM.pdf (accessed on 19 October 2022).
- Kwakkel, G.; Lannin, N.; Borschmann, K.; English, C.; Ali, M.; Churilov, L.; Saposnik, G.; Winstein, C.; van Wegen, E.; Wolf, S.L.; et al. Standardized Measurement of Sensorimotor Recovery in Stroke Trials: Consensus-Based Core Recommendations from the Stroke Recovery and Rehabilitation Roundtable. Neurorehabil. Neural Repair 2017, 31, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Monaco, V.; Coscia, M.; Micera, S. Cost function tuning improves muscle force estimation computed by static optimization during walking. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 8263–8266. [Google Scholar] [CrossRef]
- Trinler, U.; Leboeuf, F.; Hollands, K.; Jones, R.; Baker, R. Estimation of muscle activation during different walking speeds with two mathematical approaches compared to surface EMG. Gait Posture 2018, 64, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Zargham, A.; Afschrift, M.; de Schutter, J.; Jonkers, I.; de Groote, F. Inverse dynamic estimates of muscle recruitment and joint contact forces are more realistic when minimizing muscle activity rather than metabolic energy or contact forces. Gait Posture 2019, 74, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Michaud, F.; Lamas, M.; Lugrís, U.; Cuadrado, J. A fair and EMG-validated comparison of recruitment criteria, musculotendon models and muscle coordination strategies, for the inverse-dynamics based optimization of muscle forces during gait. J. Neuroeng. Rehabil. 2021, 18, 17. [Google Scholar] [CrossRef]
- Veerkamp, K.; Schallig, W.; Harlaar, J.; Pizzolato, C.; Carty, C.P.; Lloyd, D.; van der Krogt, M. The effects of electromyography-assisted modelling in estimating musculotendon forces during gait in children with cerebral palsy. J. Biomech. 2019, 92, 45–53. [Google Scholar] [CrossRef]
- Trinler, U.; Hollands, K.; Jones, R.; Baker, R. A systematic review of approaches to modelling lower limb muscle forces during gait: Applicability to clinical gait analyses. Gait Posture 2018, 61, 353–361. [Google Scholar] [CrossRef]
- Roelker, S.A.; Caruthers, E.J.; Hall, R.K.; Pelz, N.C.; Chaudhari, A.M.W.; Siston, R.A. Effects of optimization technique on simulated muscle activations and forces. J. Appl. Biomech. 2020, 36, 259–278. [Google Scholar] [CrossRef]
- Mateus, R.; João, F.; Veloso, A.P. Differences between static and dynamical optimization methods in musculoskeletal modeling estimations to study elite athletes. In Lecture Notes in Computational Vision and Biomechanics; Springer: Berlin/Heidelberg, Germany, 2020; Volume 36, pp. 624–631. [Google Scholar] [CrossRef]
- Lucareli, P.; Menegaldo, L. Muscle forces estimation during the single leg triple hop test using opensim. In Proceedings of the ENEBI 2018 – 6º Encontro Nacional de Engenharia Biomecânica, Águas de Lindóia, SP, Brasil, 8–11 May 2018. [Google Scholar]
- Kainz, H.; Wesseling, M.; Jonkers, I. Generic scaled versus subject-specific models for the calculation of musculoskeletal loading in cerebral palsy gait: Effect of personalized musculoskeletal geometry outweighs the effect of personalized neural control. Clin. Biomech. 2021, 87, 105402. [Google Scholar] [CrossRef]
- Shuman, B.R.; Goudriaan, M.; Desloovere, K.; Schwartz, M.H.; Steele, K.M. Muscle synergy constraints do not improve estimates of muscle activity from static optimization during gait for unimpaired children or children with cerebral palsy. Front. Neurorobot. 2019, 13, 102. [Google Scholar] [CrossRef]
- Kutch, J.J.; Valero-Cuevas, F.J. Muscle redundancy does not imply robustness to muscle dysfunction. J. Biomech. 2011, 44, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Fregly, B.J. A Conceptual Blueprint for Making Neuromusculoskeletal Models Clinically Useful. Appl. Sci. 2021, 11, 2037. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).