The Rationale for the Automation of a New Diagnostic Thermography Protocol to Confirm a Chronic-Low-Back-Pain Subtype Related to Nociplastic Pain
Abstract
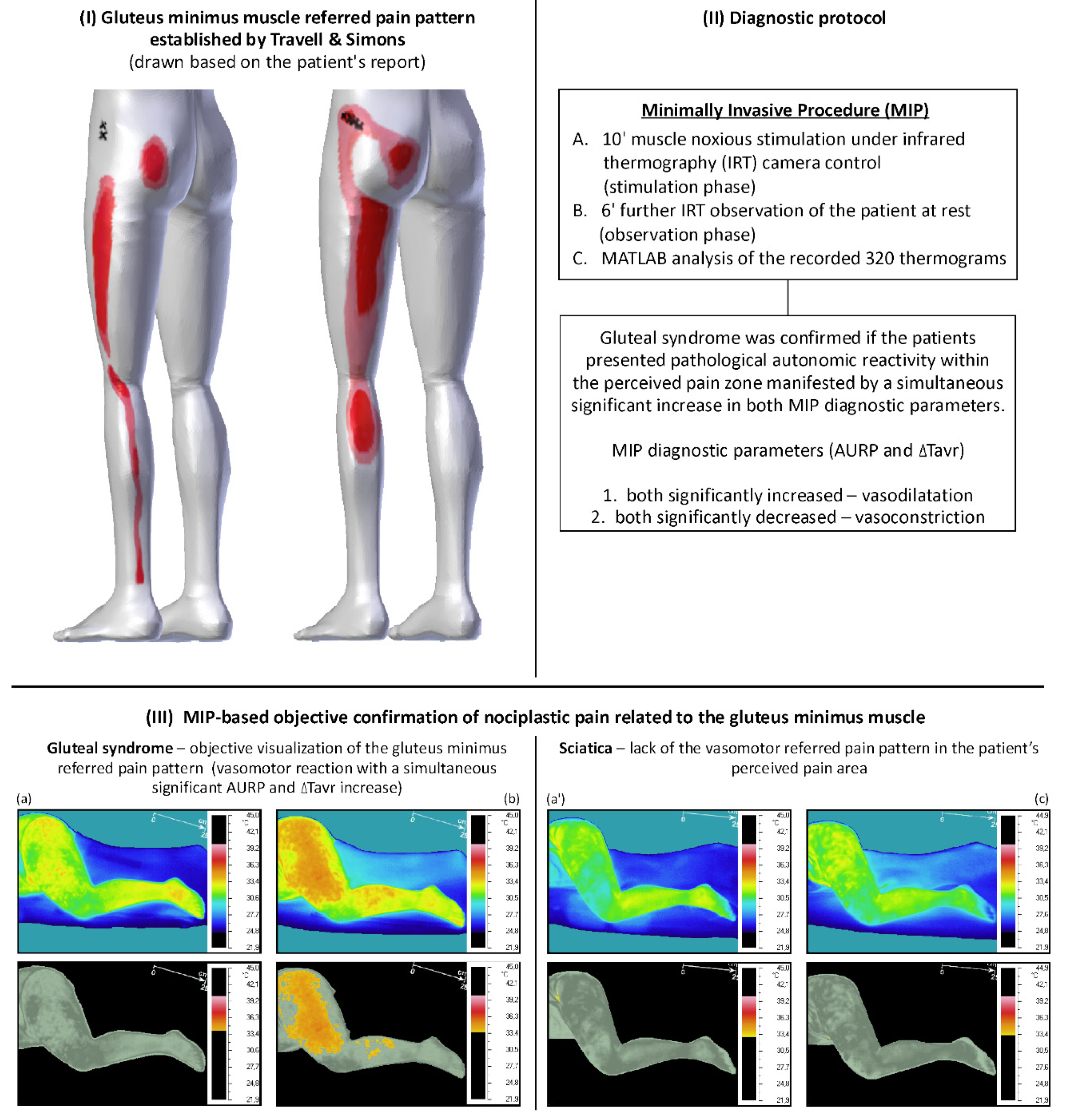
:1. Introduction
2. Autonomic Nervous System—A Possible Marker of Nociplastic Pain Involvement
3. The Background for the MIP Application
3.1. Central Sensitization Involvement in Muscles
3.2. Central Sensitization Involvement in Other Tissues Leading to Low Back Pain
4. The Rationale for the Automation of the Minimally Invasive Procedure for Confirming Gluteal Syndrome Objectively
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LBP | low back pain |
| GS | gluteal syndrome |
| IASP | the International Association for the Study of Pain |
| MIP | minimally invasive procedure |
| ANS | autonomic nervous system |
| CS | central sensitization |
| TrPs | trigger points |
| IRT | infrared thermography |
| ADT | active dynamic thermography |
| AURP | autonomic referred pain |
| ROI | region of interest |
References
- Wu, A.; March, L.; Zheng, X.; Huang, J.; Wang, X.; Zhao, J.; Blyth, F.M.; Smith, E.; Buchbinder, R.; Hoy, D. Global low back pain prevalence and years lived with disability from 1990 to 2017: Estimates from the Global Burden of Disease Study 2017. Ann. Transl. Med. 2020, 8, 299. [Google Scholar] [CrossRef]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-specific low back pain. Lancet 2012, 379, 482–491. [Google Scholar] [CrossRef] [Green Version]
- Maniadakis, N.; Gray, A. The economic burden of back pain in the UK. Pain 2000, 84, 95–103. [Google Scholar] [CrossRef]
- Lee, H.; Hübscher, M.; Moseley, G.L.; Kamper, S.J.; Traeger, A.C.; Mansell, G.; McAuley, J.H. How does pain lead to disability? A systematic review and meta-analysis of mediation studies in people with back and neck pain. Pain 2015, 156, 988–997. [Google Scholar] [CrossRef]
- Al Mazrou, S.H.; Elliott, R.A.; Knaggs, R.D.; Al Aujan, S.S. Cost-effectiveness of pain management services for chronic low back pain: A systematic review of published studies. BMC Health Serv. Res. 2020, 20, 194. [Google Scholar]
- Carregaro, R.L.; Tottoli, C.R.; Rodrigues, D.d.S.; Bosmans, J.E.; da Silva, E.N.; van Tulder, M. Low back pain should be considered a health and research priority in Brazil: Lost productivity and healthcare costs between 2012 to 2016. PLoS ONE 2020, 15, e0230902. [Google Scholar] [CrossRef] [PubMed]
- Meucci, R.D.; Fassa, A.G.; Faria, N.M. Prevalence of chronic low back pain: Systematic review. Rev. Saude Publica 2015, 49, 73. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Classification of Chronic Pain, Second Edition (Revised). Available online: https://www.iasp-pain.org/PublicationsNews/Content.aspx?ItemNumber=1673&navItemNumber=677 (accessed on 15 August 2021).
- Allegri, M.; Montella, S.; Salici, F.; Valente, A.; Marchesini, M.; Compagnone, C.; Baciarello, M.; Manferdini, M.E.; Fanelli, G. Mechanisms of low back pain: A guide for diagnosis and therapy. F1000Research 2016, 5, 1530. [Google Scholar] [CrossRef]
- Tawa, N.; Rhoda, A.; Diener, I. Accuracy of clinical neurological examination in diagnosing lumbo-sacral radiculopathy: A systematic literature review. BMC Musculoskelet. Disord. 2017, 18, 93. [Google Scholar] [CrossRef] [Green Version]
- Al Nezari, N.H.; Schneiders, A.G.; Hendrick, P.A. Neurological examination of the peripheral nervous system to diagnose lumbar spinal disc herniation with suspected radiculopathy: A systematic review and meta-analysis. Spine J. 2013, 13, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Travell, J.G.; Simons, D.G. Myofascial Pain and Dysfunction: The Trigger Point Manual, 2nd ed.; Williams & Wilkins: Baltimore, MD, USA, 1999. [Google Scholar]
- Li, L.; Stoop, R.; Clijsen, R.; Hohenauer, E.; Fernández-de-Las-Peñas, C.; Huang, Q.; Barbero, M. Criteria Used for the Diagnosis of Myofascial Trigger Points in Clinical Trials on Physical Therapy: Updated Systematic Review. Clin. J. Pain 2020, 36, 955–967. [Google Scholar] [CrossRef] [PubMed]
- van Griensven, H.; Schmid, A.; Trendafilova, T.; Low, M. Central Sensitization in Musculoskeletal Pain: Lost in Translation? J. Orthop. Sports Phys. Ther. 2020, 50, 592–596. [Google Scholar] [CrossRef] [PubMed]
- El-Badawy, M.A.; El Mikkawy, D.M. Sympathetic Dysfunction in Patients with Chronic Low Back Pain and Failed Back Surgery Syndrome. Clin. J. Pain 2016, 32, 226–231. [Google Scholar] [CrossRef]
- Prim, J.H.; Ahn, S.; Davila, M.I.; Alexander, M.L.; McCulloch, K.L.; Fröhlich, F. Targeting the Autonomic Nervous System Balance in Patients with Chronic Low Back Pain Using Transcranial Alternating Current Stimulation: A Randomized, Crossover, Double-Blind, Placebo-Controlled Pilot Study. J. Pain Res. 2019, 12, 3265–3277. [Google Scholar] [CrossRef] [Green Version]
- Bruehl, S.; Chung, O.Y. Interactions between the cardiovascular and pain regulatory systems: An updated review of mechanisms and possible alterations in chronic pain. Neurosci. Biobehav. Rev. 2004, 28, 395–414. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.; Jarczok, M.N.; Ellis, R.J.; Hillecke, T.K.; Thayer, J.F. Heart rate variability and experimentally induced pain in healthy adults: A systematic review. Eur. J. Pain 2014, 18, 301–314. [Google Scholar] [CrossRef]
- Terkelsen, A.J.; Andersen, O.K.; Mølgaard, H.; Hansen, J.; Jensen, T.S. Mental stress inhibits pain perception and heart rate variability but not a nociceptive withdrawal reflex. Acta Physiol. Scand. 2004, 180, 405–414. [Google Scholar] [CrossRef]
- Ansuategui Echeita, J.; Schiphorst Preuper, H.R.; Dekker, R.; Stuive, I.; Timmerman, H.; Wolff, A.P.; Reneman, M.F. Central Sensitisation and functioning in patients with chronic low back pain: Protocol for a cross-sectional and cohort study. BMJ Open. 2020, 10, e031592. [Google Scholar] [CrossRef] [Green Version]
- Hohenschurz-Schmidt, D.J.; Calcagnini, G.; Dipasquale, O.; Jackson, J.B.; Medina, S.; O’Daly, O.; O’Muircheartaigh, J.; de Lara Rubio, A.; Williams, S.C.R.; McMahon, S.B.; et al. Linking Pain Sensation to the Autonomic Nervous System: The Role of the Anterior Cingulate and Periaqueductal Gray Resting-State Networks. Front. Neurosci. 2020, 14, 147. [Google Scholar] [CrossRef]
- Reyes del Paso, G.A.; Montoro, C.; Muñóz Ladrón de Guevara, C.; Duschek, S.; Jennings, J.R. The effect of baroreceptor stimulation on pain perception depends on the elicitation of the reflex cardiovascular response: Evidence of the interplay between the two branches of the baroreceptor system. Biol. Psychol. 2014, 101, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Nickel, M.M.; May, E.S.; Tiemann, L.; Postorino, M.; Ta Dinh, S.; Ploner, M. Autonomic responses to tonic pain are more closely related to stimulus intensity than to pain intensity. Pain 2017, 158, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Skorupska, E.; Rychlik, M.; Samborski, W. Validation and Test-Retest Reliability of New Thermographic Technique Called Thermovision Technique of Dry Needling for Gluteus Minimus Trigger Points in Sciatica Subjects and TrPs-Negative Healthy Volunteers. BioMed Res. Int. 2015, 2015, 546497. [Google Scholar] [CrossRef] [PubMed]
- Skorupska, E.; Jokiel, M.; Rychlik, M.; Łochowski, R.; Kotwicka, M. Female Overrepresentation in Low Back-Related Leg Pain: A Retrospective Study of the Autonomic Response to a Minimally Invasive Procedure. J. Pain Res. 2020, 13, 3427–3435. [Google Scholar] [CrossRef]
- Skorupska, E.; Rychlik, M.; Samborski, W. Intensive vasodilatation in the sciatic pain area after dry needling. BMC Complement. Altern. Med. 2015, 15, 72. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.iasp-pain.org/terminology?navItemNumber=576#Sensitization (accessed on 19 May 2021).
- Trouvin, A.P.; Perrot, S. New concepts of pain. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101415. [Google Scholar] [CrossRef]
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiologic Approach to Pain Therapy for Complex Pain Entities: A Narrative Review. Pain Ther. 2020, 9, 7–21. [Google Scholar] [CrossRef] [Green Version]
- Arendt-Nielsen, L.; Morlion, B.; Perrot, S.; Dahan, A.; Dickenson, A.; Kress, H.G.; Wells, C.; Bouhassira, D.; Mohr Drewes, A. Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur. J. Pain 2018, 22, 216–241. [Google Scholar] [CrossRef] [Green Version]
- Harte, S.E.; Harris, R.E.; Clauw, D.J. The neurobiology of central sensitization. J. Appl. Biobehav. Res. 2018, 23, e12137. [Google Scholar] [CrossRef] [Green Version]
- Price, T.J.; Gold, M.S. From Mechanism to Cure: Renewing the Goal to Eliminate the Disease of Pain. Pain Med. 2018, 19, 1525–1549. [Google Scholar] [CrossRef]
- Harper, D.E.; Schrepf, A.; Clauw, D.J. Pain Mechanisms and Centralized Pain in Temporomandibular Disorders. J. Dent. Res. 2016, 95, 1102–1108. [Google Scholar] [CrossRef] [Green Version]
- Nystrom, N.A.; Freeman, M.D. Central Sensitization Is Modulated Following Trigger Point Anesthetization in Patients with Chronic Pain from Whiplash Trauma. A Double-Blind, Placebo-Controlled, Crossover Study. Pain Med. 2018, 19, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152 (Suppl. 3), S2–S15. [Google Scholar] [CrossRef] [PubMed]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain. 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truini, A.; Tinelli, E.; Gerardi, M.C.; Calistri, V.; Iannuccelli, C.; La Cesa, S.; Tarsitani, L.; Mainero, C.; Sarzi-Puttini, P.; Cruccu, G.; et al. Abnormal resting state functional connectivity of the periaqueductal grey in patients with fibromyalgia. Clin. Exp. Rheumatol. 2016, 34 (Suppl. 96), S129–S133. [Google Scholar] [PubMed]
- Yu, R.; Gollub, R.L.; Spaeth, R.; Napadow, V.; Wasan, A.; Kong, J. Disrupted functional connectivity of the periaqueductal gray in chronic low back pain. Neuroimage Clin. 2014, 6, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Charkoudian, N. Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. J. Appl. Physiol. 2010, 109, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Schoenen, J. Chronic tension-type headache: What is new? Curr. Opin. Neurol. 2009, 22, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Phan, V.; Shah, J.; Tandon, H.; Srbely, J.; DeStefano, S.; Kumbhare, D.; Sikdar, S.; Clouse, A.; Gandhi, A.; Gerber, L. Myofascial Pain Syndrome: A Narrative Review Identifying Inconsistencies in Nomenclature. PM&R 2020, 12, 916–925. [Google Scholar] [CrossRef]
- Travell, J.G.; Simons, D. Travell, Simons & Simons’ Myofascial Pain and Dysfunction; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2018. [Google Scholar]
- Skorupska, E.; Zawadziński, J.; Bednarek, A.; Samborski, W. Skin Resistivity Value of Upper Trapezius Latent Trigger Points. BioMed Res. Int. 2015, 2015, 351726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, D.G. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J. Electromyogr. Kinesiol. 2004, 14, 95–107. [Google Scholar] [CrossRef]
- Kimura, Y.; Ge, H.-Y.; Zhang, M.; Kimura, H.; Sumikura, H.; Arendt-Nielsen, L. Evaluation of sympathetic vasoconstrictor response following nociceptive stimulation of latent myofascial trigger points in humans. Acta Physiol. 2009, 196, 411–417. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, H.-Y.; Yue, S.-W.; Kimura, Y.; Arendt-Nielsen, L. Attenuated skin blood flow response to nociceptive stimulation of latent myofascial trigger point. Arch. Phys. Med. Rehabil. 2009, 90, 325–332. [Google Scholar] [CrossRef]
- Mense, S. Muscle pain: Mechanisms and clinical significance. Dtsch. Ärzteblatt Int. 2008, 105, 214–219. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Svensson, P. Referred Muscle Pain: Basic and Clinical Findings. Clin. J. Pain 2001, 17, 11–19. [Google Scholar] [CrossRef]
- Graven-Nielsen, T.; Arendt-Nielsen, L. Induction and assessment of muscle pain, referred pain, and muscular hyperalgesia. Curr. Pain Headache Rep. 2003, 7, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Mense, S.; Gerwin, R.D. Introduction. In Muscle Pain: Understanding the Mechanisms; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Fernández-de-Las-Peñas, C.; Dommerholt, J. International Consensus on Diagnostic Criteria and Clinical Considerations of Myofascial Trigger Points: A Delphi Study. Pain Med. 2018, 19, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Will, J.S.; Bury, D.C.; Miller, J.A. Mechanical Low Back Pain. Am. Fam. Physician 2018, 98, 421–428. [Google Scholar]
- Havelin, J.; King, T. Mechanisms Underlying Bone and Joint Pain. Curr. Osteoporos. Rep. 2018, 16, 763–771. [Google Scholar] [CrossRef]
- Eitner, A.; Hofmann, G.O.; Schaible, H.G. Mechanisms of Osteoarthritic Pain. Studies in Humans and Experimental Models. Front. Mol. Neurosci. 2017, 10, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.; Lee, Y.C. Mechanisms for Joint Pain in Rheumatoid Arthritis (RA): From Cytokines to Central Sensitization. Curr. Osteoporos. Rep. 2018, 16, 603–610. [Google Scholar] [CrossRef]
- Di Stefano, G.; Celletti, C.; Baron, R.; Castori, M.; Di Franco, M.; La Cesa, S.; Leone, C.; Pepe, A.; Cruccu, G.; Truini, A.; et al. Central sensitization as the mechanism underlying pain in joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility type. Eur. J. Pain 2016, 20, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, T.W.; Felson, D.T. Mechanisms of Osteoarthritis (OA) Pain. Curr. Osteoporos. Rep. 2018, 16, 611–616. [Google Scholar] [CrossRef] [Green Version]
- Arendt-Nielsen, L.; Fernández-de-Las-Peñas, C.; Graven-Nielsen, T. Basic aspects of musculoskeletal pain: From acute to chronic pain. J. Man. Manip. Ther. 2011, 19, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Soni, A.; Wanigasekera, V.; Mezue, M.; Cooper, C.; Javaid, M.K.; Price, A.J.; Tracey, I. Both profiling and subgrouping patients with knee osteoarthritis pain seem important. A specific group with mild to moderate joint damage, but severe pain, might be a particularly sensitized group of patients. Arthritis Rheumatol. 2019, 71, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Baert, I.A.; Lluch, E.; Mulder, T.; Nijs, J.; Noten, S.; Meeus, M. Does pre-surgical central modulation of pain influence outcome after total knee replacement? A systematic review. Osteoarthr. Cartil. 2016, 24, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falowski, S.; Sayed, D.; Pope, J.; Patterson, D.; Fishman, M.; Gupta, M.; Mehta, P. A Review and Algorithm in the Diagnosis and Treatment of Sacroiliac Joint Pain. J. Pain Res. 2020, 13, 3337–3348. [Google Scholar] [CrossRef]
- Eloqayli, H. Clinical Decision-Making in Chronic Spine Pain: Dilemma of Image-Based Diagnosis of Degenerative Spine and Generation Mechanisms for Nociceptive, Radicular, and Referred Pain. BioMed Res. Int. 2018, 2018, 8793843. [Google Scholar] [CrossRef] [Green Version]
- Moss, P.; Benson, H.A.E.; Will, R.; Wright, A. Patients with Knee Osteoarthritis Who Score Highly on the PainDETECT Questionnaire Present with Multimodality Hyperalgesia, Increased Pain, and Impaired Physical Function. Clin. J. Pain 2018, 34, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Walsh, D.A.; McWilliams, D.F.; Turley, M.J.; Dixon, M.R.; Fransès, R.E.; Mapp, P.I.; Wilson, D. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology 2010, 49, 1852–1861. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, M.; Nowakowski, A. Active Dynamic Thermography in Medical Diagnostics. In Application of Infrared to Biomedical Sciences; Springer: Singapore, 2017; pp. 291–310. [Google Scholar]
- Vardasca, R.; Ring, F.; Plassmann, P.; Jones, C. Thermal symmetry of the upper and lower extremities in healthy subjects. Thermol. Int. 2012, 22, 53–60. [Google Scholar]
- Ismail, E.; Merla, A. Modeling Thermal Infrared Imaging Data for Differential Diagnosis. In Application of Infrared to Biomedical Sciences; Etehadtavakol, M., Ed.; Series in BioEngineering; Springer: Singapore, 2017. [Google Scholar]
- Saxena, A.; Ng, E.; Lim, S.T. Infrared (IR) thermography as a potential screening modality for carotid artery stenosis. Comput. Biol. Med. 2019, 113, 103419. [Google Scholar] [CrossRef]
- Jin, C.; He, Z.-Z.; Liu, J. Finite element method based three-dimensional thermal tomography for disease diagnosis of human body. J. Heat Transfer. 2016, 138, 104501. [Google Scholar] [CrossRef]
- Nowakowski, A. Quantitative active dynamic thermal IR-imaging and thermal tomography in medical diagnostics. In Medical Devices System, 3rd ed.; Bronzino, J.D., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 1–30. [Google Scholar]
- Ammer, K. The Glamorgan protocol for recording and evaluation of thermal images of the human body. Thermol. Int. 2008, 18, 125–129. [Google Scholar]
- Paszkiel, S.Z. Control, Computer Engineering and Neuroscience; Springer Nature: Chan, Germany, 2021. [Google Scholar]
- Skorupska, E.; Dybek, T.; Rychlik, M.; Jokiel, M.; Dobrakowski, P. The Automatization of a New Thermography Method Using Invasive Nociceptive Stimulation to Confirm an Autonomic Phenomenon within a Trigger Point Referred Pain Zone. Brain Sci. 2021, 11, 893. [Google Scholar] [CrossRef] [PubMed]
- Maniar, N.; Bach, A.J.E.; Stewart, I.B.; Costello, J.T. The effect of using different regions of interest on local and mean skin temperature. J. Therm. Biol. 2015, 49–50, 33–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quesada, J.I.P.; Lucas-Cuevas, A.G.; Palmer, R.S.; Pérez-Soriano, P.; de Anda, R.M.C.O. Definition of the thermographic regions of interest in cycling by using a factor analysis. Infrared Phys. Technol. 2016, 75, 180–186. [Google Scholar] [CrossRef]
- Fournet, D.; Ross, L.; Voelcker, T.; Redortier, B.; Havenith, G. Body mapping of thermoregulatory and perceptual responses of males and females running in the cold. J. Therm. Biol. 2013, 38, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Cuevas, I.; Marins, J.C.; Carmona, P.G.; García-Concepción, M.A.; Lastras, J.A.; Quintana, M.S. Reliability and reproducibility of skin temperature of overweight subjects by an infrared thermography software designed for human beings. Thermol. Int. 2012, 22, 130–137. [Google Scholar]
- Duarte, A.; Carrão, L.; Espanha, M.; Viana, T.; Freitas, D.; Bártolo, P.; Faria, P.; Almeida, H. Segmentation algorithms for thermal images. Procedia Technol. 2014, 16, 1560–1569. [Google Scholar] [CrossRef]
- Barcelos, E.Z.; Caminhas, W.M.; Ribeiro, E.; Pimenta, E.M.; Palhares, R.M. A combined method for segmentation and registration for an advanced and progressive evaluation of thermal images. Sensors 2014, 14, 21950–21967. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, N.; Formenti, D.; Gargano, M.; Alberti, G. Skin temperature evaluation by infrared thermography: Comparison of image analysis methods. Infrared Phys. Technol. 2014, 62, 1–6. [Google Scholar] [CrossRef]
| “Top-Down” Subtype | “Bottom-Up” Subtype | Trigger Points | |
|---|---|---|---|
| Scientific opinion | hypothesis | hypothesis | some scientific evidence exists |
| Sex ratio | significant female domination | female domination | female domination |
| Age | young | any age | any age |
| Family history of pain | yes | no | no |
| Psychological co-morbidity | high | moderate | some correlation with depression; sleep deprivation |
| Therapy | a multimodal approach | not defined | manual pressure, dry needling, and TENS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skorupska, E.; Dybek, T. The Rationale for the Automation of a New Diagnostic Thermography Protocol to Confirm a Chronic-Low-Back-Pain Subtype Related to Nociplastic Pain. BioMed 2021, 1, 99-111. https://doi.org/10.3390/biomed1020009
Skorupska E, Dybek T. The Rationale for the Automation of a New Diagnostic Thermography Protocol to Confirm a Chronic-Low-Back-Pain Subtype Related to Nociplastic Pain. BioMed. 2021; 1(2):99-111. https://doi.org/10.3390/biomed1020009
Chicago/Turabian StyleSkorupska, Elzbieta, and Tomasz Dybek. 2021. "The Rationale for the Automation of a New Diagnostic Thermography Protocol to Confirm a Chronic-Low-Back-Pain Subtype Related to Nociplastic Pain" BioMed 1, no. 2: 99-111. https://doi.org/10.3390/biomed1020009
APA StyleSkorupska, E., & Dybek, T. (2021). The Rationale for the Automation of a New Diagnostic Thermography Protocol to Confirm a Chronic-Low-Back-Pain Subtype Related to Nociplastic Pain. BioMed, 1(2), 99-111. https://doi.org/10.3390/biomed1020009






