Mast Cell Tryptase and Implications for SARS-CoV-2 Pathogenesis
Abstract
:1. Introduction
2. Mast Cell Ontogeny and Heterogeneity
3. MC-Derived Tryptase and Chymase in Inflammation
4. MC Tryptase and Chymase in Viral Infections
4.1. Flaviviruses
4.2. Respiratory Viruses
4.3. Other Viruses
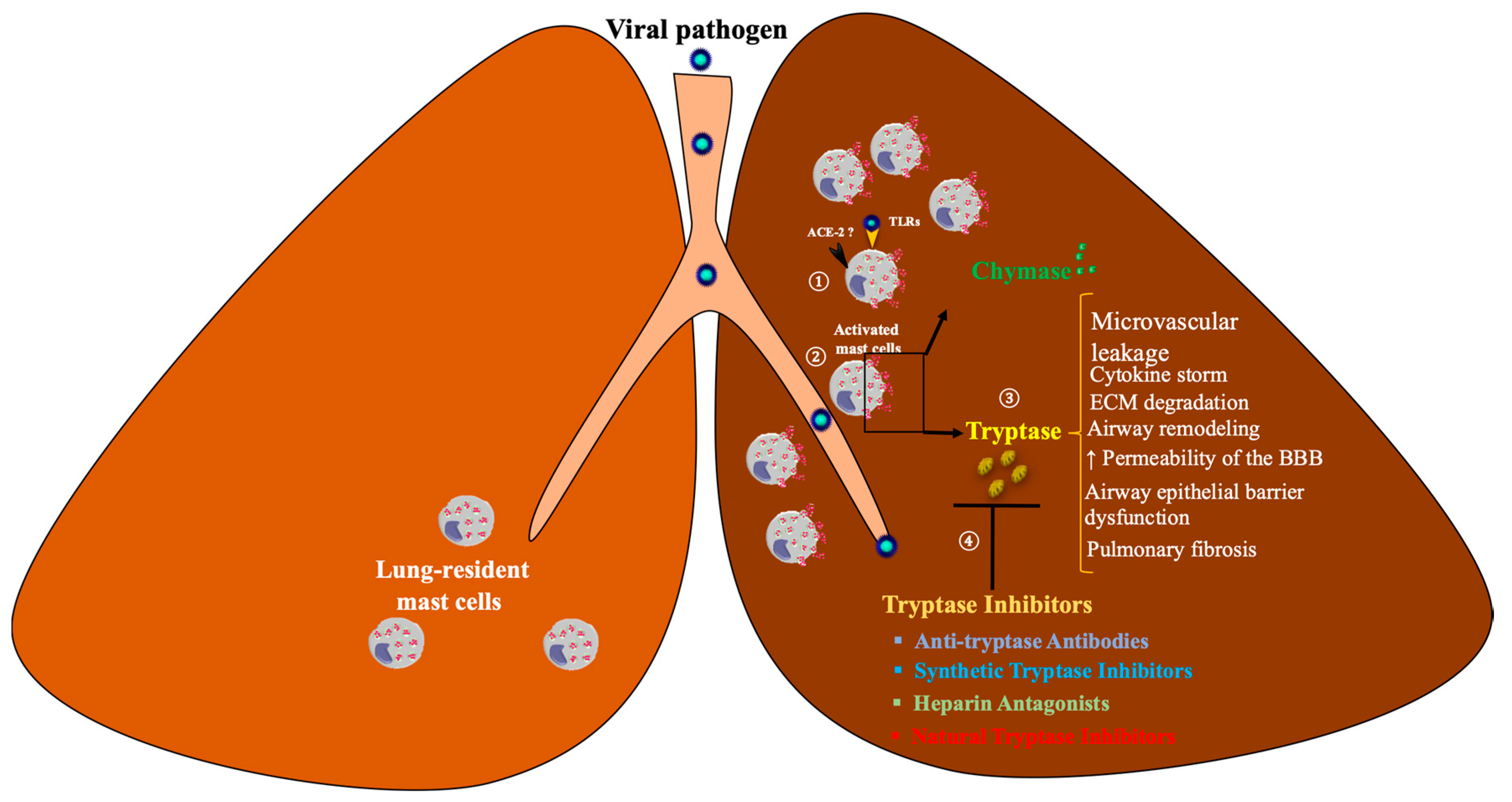
5. MC Tryptase in COVID-19
6. Drug Therapies
6.1. MC Stabilizers
6.2. Tryptase Inhibitors
6.2.1. Heparin Antagonists
6.2.2. Natural Tryptase Inhibitors
6.2.3. Synthetic Tryptase Inhibitors
Canonical Inhibitors
Zinc-Mediated Inhibitors
Dibasic and Bivalent Inhibitors
6.2.4. Tryptase-Specific Antibodies: Next Generation Tryptase Inhibitors
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galli, S.J.; Gaudenzio, N.; Tsai, M. Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns. Annu. Rev. Immunol. 2020, 38, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, J.; Pejler, G. Biology of mast cell tryptase. An inflammatory mediator. FEBS J. 2006, 273, 1871–1895. [Google Scholar] [CrossRef] [PubMed]
- Frossi, B.; Mion, F.; Sibilano, R.; Danelli, L.; Pucillo, C.E.M. Is it time for a new classification of mast cells? What do we know about mast cell heterogeneity? Immunol. Rev. 2018, 282, 35–46. [Google Scholar] [CrossRef]
- Graham, A.C.; Temple, R.M.; Obar, J.J. Mast cells and influenza a virus: Association with allergic responses and beyond. Front. Immunol. 2015, 6, 238. [Google Scholar] [CrossRef] [Green Version]
- Aoki, R.; Kawamura, T.; Goshima, F.; Ogawa, Y.; Nakae, S.; Nakao, A.; Moriishi, K.; Nishiyama, Y.; Shimada, S. Mast cells play a key role in host defense against herpes simplex virus infection through TNF-α and IL-6 production. J. Investig. Derm. 2013, 133, 2170–2179. [Google Scholar] [CrossRef] [Green Version]
- Gebremeskel, S.; Schanin, J.; Coyle, K.M.; Butuci, M.; Luu, T.; Brock, E.C.; Xu, A.; Wong, A.; Leung, J.; Korver, W.; et al. Mast Cell and Eosinophil Activation Are Associated With COVID-19 and TLR-Mediated Viral Inflammation: Implications for an Anti-Siglec-8 Antibody. Front. Immunol. 2021, 12, 650331. [Google Scholar] [CrossRef] [PubMed]
- St John, A.L.; Rathore, A.P.; Raghavan, B.; Ng, M.L.; Abraham, S.N. Contributions of mast cells and vasoactive products, leukotrienes and chymase, to dengue virus-induced vascular leakage. Elife 2013, 2, e00481. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, J.; Ouedrani, A.; Livideanu, C.B.; Barete, S.; Terriou, L.; Launay, D.; Lemal, R.; Greco, C.; Frenzel, L.; Meni, C.; et al. Absence of severe COVID-19 in patients with clonal mast cells activation disorders: Effective anti-SARS-CoV-2 immune response. bioRxiv 2021. [Google Scholar] [CrossRef]
- Hafezi, B.; Chan, L.; Knapp, J.P.; Karimi, N.; Alizadeh, K.; Mehrani, Y.; Bridle, B.W.; Karimi, K. Cytokine Storm Syndrome in SARS-CoV-2 Infections: A Functional Role of Mast Cells. Cells 2021, 10, 1761. [Google Scholar] [CrossRef]
- Crivellato, E.; Ribatti, D.; Mallardi, F.; Beltrami, C.A. The mast cell: A multifunctional effector cell. Adv. Clin. Pathol. 2003, 7, 13–26. [Google Scholar]
- Ribatti, D. Mast Cell Ontogeny. In The Mast Cell: A Multifunctional Effector Cell; Springer International Publishing: Cham, Switzerland, 2019; pp. 5–14. [Google Scholar]
- Moon, T.C.; St Laurent, C.D.; Morris, K.E.; Marcet, C.; Yoshimura, T.; Sekar, Y.; Befus, A.D. Advances in mast cell biology: New understanding of heterogeneity and function. Mucosal. Immunol. 2010, 3, 111–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cildir, G.; Yip, K.H.; Pant, H.; Tergaonkar, V.; Lopez, A.F.; Tumes, D.J. Understanding mast cell heterogeneity at single cell resolution. Trends Immunol. 2021, 42, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kurashima, Y. Two Sides of the Coin: Mast Cells as a Key Regulator of Allergy and Acute/Chronic Inflammation. Cells 2021, 10, 1615. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Nath, S.; Meininger, C.J.; Gashev, A.A. Emerging Roles of Mast Cells in the Regulation of Lymphatic Immuno-Physiology. Front. Immunol. 2020, 11, 1234. [Google Scholar] [CrossRef]
- Ud-Din, S.; Wilgus, T.A.; Bayat, A. Mast Cells in Skin Scarring: A Review of Animal and Human Research. Front. Immunol. 2020, 11, 552205. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Peng, Q.; Walls, A.F. Potent induction of a neutrophil and eosinophil-rich infiltrate in vivo by human mast cell tryptase: Selective enhancement of eosinophil recruitment by histamine. J. Immunol. 1997, 159, 6216–6225. [Google Scholar]
- He, S.; Walls, A.F. Human mast cell chymase induces the accumulation of neutrophils, eosinophils and other inflammatory cells in vivo. Br. J. Pharmacol. 1998, 125, 1491–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, G.; Ziyadeh, F.N.; Thaiss, F.; Tomaszewski, J.; Caron, R.J.; Wenzel, U.; Zahner, G.; Helmchen, U.; Stahl, R. Angiotensin II stimulates expression of the chemokine RANTES in rat glomerular endothelial cells. Role of the angiotensin type 2 receptor. J. Clin. Investig. 1997, 100, 1047–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, Y.; Yamamguchi, T.; Shimamura, M.; Hazato, T. Angiotensin III is a new chemoattractant for polymorphonuclear leukocytes. Biochem. Biophys. Res. Commun. 1993, 193, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, J.; Kalkkinen, N.; Welgus, H.G.; Kovanen, P.T. Activation of human interstitial procollagenase through direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J. Biol. Chem. 1994, 269, 18134–18140. [Google Scholar] [CrossRef]
- Sampson, A. The role of eosinophils and neutrophils in inflammation. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2000, 30, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Terakawa, M.; Tomimori, Y.; Goto, M.; Fukuda, Y. Mast cell chymase induces expression of chemokines for neutrophils in eosinophilic EoL-1 cells and mouse peritonitis eosinophils. Eur. J. Pharmacol. 2006, 538, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Tani, K.; Ogushi, F.; Kido, H.; Kawano, T.; Kunori, Y.; Kamimura, T.; Cui, P.; Sone, S. Chymase is a potent chemoattractant for human monocytes and neutrophils. J. Leukoc. Biol. 2000, 67, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Temkin, V.; Kantor, B.; Weg, V.; Hartman, M.-L.; Levi-Schaffer, F. Tryptase activates the mitogen-activated protein kinase/activator protein-1 pathway in human peripheral blood eosinophils, causing cytokine production and release. J. Immunol. 2002, 169, 2662–2669. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, L.B. Clinical utility of tryptase levels in systemic mastocytosis and associated hematologic disorders. Leuk. Res. 2001, 25, 553–562. [Google Scholar] [CrossRef]
- Thakurdas, S.M.; Melicoff, E.; Sansores-Garcia, L.; Moreira, D.C.; Petrova, Y.; Stevens, R.L.; Adachi, R. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J. Biol. Chem. 2007, 282, 20809–20815. [Google Scholar] [CrossRef] [Green Version]
- Cairns, J.A.; Walls, A.F. Mast cell tryptase is a mitogen for epithelial cells. Stimulation of IL-8 production and intercellular adhesion molecule-1 expression. J. Immunol. 1996, 156, 275–283. [Google Scholar]
- Compton, S.J.; Cairns, J.A.; Holgate, S.T.; Walls, A.F. The role of mast cell tryptase in regulating endothelial cell proliferation, cytokine release, and adhesion molecule expression: Tryptase induces expression of mRNA for IL-1β and IL-8 and stimulates the selective release of IL-8 from human umbilical vein endothelial cells. J. Immunol. 1998, 161, 1939–1946. [Google Scholar]
- Douaiher, J.; Succar, J.; Lancerotto, L.; Gurish, M.F.; Orgill, D.P.; Hamilton, M.J.; Krilis, S.A.; Stevens, R.L. Development of mast cells and importance of their tryptase and chymase serine proteases in inflammation and wound healing. Adv. Immunol. 2014, 122, 211–252. [Google Scholar] [PubMed] [Green Version]
- Gruber, B.L.; Marchese, M.J.; Suzuki, K.; Schwartz, L.B.; Okada, Y.; Nagase, H.; Ramamurthy, N.S. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J. Clin. Investig. 1989, 84, 1657–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kettelhut, B.V.; Metcalfe, D.D. Pediatric mastocytosis. J. Investig. Derm. 1991, 96, 15S–18S. [Google Scholar] [CrossRef] [Green Version]
- Shaker, S.A.; Ayuob, N.N.; Hajrah, N.H. Cell talk: A phenomenon observed in the keloid scar by immunohistochemical study. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 153–159. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Brown, M.G.; McAlpine, S.M.; Huang, Y.Y.; Haidl, I.D.; Al-Afif, A.; Marshall, J.S.; Anderson, R. RNA sensors enable human mast cell anti-viral chemokine production and IFN-mediated protection in response to antibody-enhanced dengue virus infection. PLoS ONE 2012, 7, e34055. [Google Scholar] [CrossRef]
- Chang, T.-H.; Liao, C.-L.; Lin, Y.-L. Flavivirus induces interferon-beta gene expression through a pathway involving RIG-I-dependent IRF-3 and PI3K-dependent NF-κB activation. Microbes Infect. 2006, 8, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Harris, E. Interferon inhibits dengue virus infection by preventing translation of viral RNA through a PKR-independent mechanism. Virology 2001, 289, 297–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St John, A.L.; Rathore, A.P.; Yap, H.; Ng, M.L.; Metcalfe, D.D.; Vasudevan, S.G.; Abraham, S.N. Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT-cell recruitment and viral clearance. Proc. Natl. Acad. Sci. USA 2011, 108, 9190–9195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, C.-F.; Zhao, H.; Liu, Z.-Y.; Jiang, T.; Deng, Y.-Q.; Yu, X.-D.; Yu, M.; Qin, E.-D. Retinoic acid inducible gene-I and melanoma differentiation-associated gene 5 are induced but not essential for dengue virus induced type I interferon response. Mol. Biol. Rep. 2011, 38, 3867–3873. [Google Scholar] [CrossRef]
- Muñoz-Jordán, J.L.; Fredericksen, B.L. How flaviviruses activate and suppress the interferon response. Viruses 2010, 2, 676–691. [Google Scholar] [CrossRef]
- Chen, J.-P.; Lu, H.-L.; Lai, S.-L.; Campanella, G.S.; Sung, J.-M.; Lu, M.-Y.; Wu-Hsieh, B.A.; Lin, Y.-L.; Lane, T.E.; Luster, A.D. Dengue virus induces expression of CXC chemokine ligand 10/IFN-γ-inducible protein 10, which competitively inhibits viral binding to cell surface heparan sulfate. J. Immunol. 2006, 177, 3185–3192. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, M.-F.; Lai, S.-L.; Chen, J.-P.; Sung, J.-M.; Lin, Y.-L.; Wu-Hsieh, B.A.; Gerard, C.; Luster, A.; Liao, F. Both CXCR3 and CXCL10/IFN-inducible protein 10 are required for resistance to primary infection by dengue virus. J. Immunol. 2006, 177, 1855–1863. [Google Scholar] [CrossRef] [Green Version]
- John, A.L.S.; Abraham, S.N.; Gubler, D.J. Barriers to preclinical investigations of anti-dengue immunity and dengue pathogenesis. Nat. Rev. Microbiol. 2013, 11, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.G.; King, C.A.; Sherren, C.; Marshall, J.S.; Anderson, R. A dominant role for FcγRII in antibody-enhanced dengue virus infection of human mast cells and associated CCL5 release. J. Leukoc. Biol. 2006, 80, 1242–1250. [Google Scholar] [CrossRef]
- King, C.A.; Anderson, R.; Marshall, J.S. Dengue virus selectively induces human mast cell chemokine production. J. Virol. 2002, 76, 8408–8419. [Google Scholar] [CrossRef] [Green Version]
- King, C.A.; Marshall, J.S.; Alshurafa, H.; Anderson, R. Release of vasoactive cytokines by antibody-enhanced dengue virus infection of a human mast cell/basophil line. J. Virol. 2000, 74, 7146–7150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malasit, P. Complement and dengue haemorrhagic fever/shock syndrome. Southeast Asian J. Trop. Med. Public Health 1987, 18, 316–320. [Google Scholar]
- Ferreira, R.A.X.; de Oliveira, S.A.; Gandini, M.; da Cunha Ferreira, L.; Correa, G.; Abiraude, F.M.; Reid, M.M.; Cruz, O.G.; Kubelka, C.F. Circulating cytokines and chemokines associated with plasma leakage and hepatic dysfunction in Brazilian children with dengue fever. Acta Trop. 2015, 149, 138–147. [Google Scholar] [CrossRef]
- Tissera, H.; Rathore, A.P.; Leong, W.Y.; Pike, B.L.; Warkentien, T.E.; Farouk, F.S.; Syenina, A.; Eong Ooi, E.; Gubler, D.J.; Wilder-Smith, A. Chymase level is a predictive biomarker of dengue hemorrhagic fever in pediatric and adult patients. J. Infect. Dis. 2017, 216, 1112–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathore, A.P.; Senanayake, M.; Athapathu, A.S.; Gunasena, S.; Karunaratna, I.; Leong, W.Y.; Lim, T.; Mantri, C.K.; Wilder-Smith, A.; John, A.L.S. Serum chymase levels correlate with severe dengue warning signs and clinical fluid accumulation in hospitalized pediatric patients. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- He, S.; Walls, A.F. The induction of a prolonged increase in microvascular permeability by human mast cell chymase. Eur. J. Pharmacol. 1998, 352, 91–98. [Google Scholar] [CrossRef]
- Goldstein, S.; Leong, J.; Schwartz, L.; Cooke, D. Protease composition of exocytosed human skin mast cell protease-proteoglycan complexes. Tryptase resides in a complex distinct from chymase and carboxypeptidase. J. Immunol. 1992, 148, 2475–2482. [Google Scholar]
- Sayama, S.; Iozzo, R.; Lazarus, G.; Schechter, N. Human skin chymotrypsin-like proteinase chymase. Subcellular localization to mast cell granules and interaction with heparin and other glycosaminoglycans. J. Biol. Chem. 1987, 262, 6808–6815. [Google Scholar] [CrossRef]
- Rathore, A.P.; Mantri, C.K.; Aman, S.A.; Syenina, A.; Ooi, J.; Jagaraj, C.J.; Goh, C.C.; Tissera, H.; Wilder-Smith, A.; Ng, L.G.; et al. Dengue virus-elicited tryptase induces endothelial permeability and shock. J. Clin. Investig. 2019, 129, 4180–4193. [Google Scholar] [CrossRef] [Green Version]
- Shubin, N.J.; Glukhova, V.A.; Clauson, M.; Truong, P.; Abrink, M.; Pejler, G.; White, N.J.; Deutsch, G.H.; Reeves, S.R.; Vaisar, T. Proteome analysis of mast cell releasates reveals a role for chymase in the regulation of coagulation factor XIIIA levels via proteolytic degradation. J. Allergy Clin. Immunol. 2017, 139, 323–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molino, M.; Barnathan, E.S.; Numerof, R.; Clark, J.; Dreyer, M.; Cumashi, A.; Hoxie, J.A.; Schechter, N.; Woolkalis, M.; Brass, L.F. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J. Biol. Chem. 1997, 272, 4043–4049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fresh, J.W.; Reyes, V.; Clarke, E.J.; Uylangco, C.V. Philippine hemorrhagic fever: A clinical, laboratory, and necropsy study. J. Lab. Clin. Med. 1969, 73, 451–458. [Google Scholar]
- Srichaikul, T.; Nimmanitaya, S.; Artchararit, N.; Siriasawakul, T.; Sungpeuk, P. Fibrinogen metabolism and disseminated intravascular coagulation in dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 1977, 26, 525–532. [Google Scholar] [CrossRef]
- Hsieh, J.T.; Rathore, A.P.; Soundarajan, G.; John, A.L.S. Japanese encephalitis virus neuropenetrance is driven by mast cell chymase. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Dai, J.; Bai, F.; Kong, K.-F.; Wong, S.J.; Montgomery, R.R.; Madri, J.A.; Fikrig, E. Matrix metalloproteinase 9 facilitates West Nile virus entry into the brain. J. Virol. 2008, 82, 8978–8985. [Google Scholar] [CrossRef] [Green Version]
- Schwarze, J.; Johnston, S. Unravelling synergistic immune interactions between respiratory virus infections and allergic airway inflammation. Clin. Exp. Allergy 2004, 34, 1153. [Google Scholar] [CrossRef]
- Bradding, P. Human lung mast cell heterogeneity. Thorax 2009, 64, 278–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laitinen, A.; Karjalainen, E.-M.; Altraja, A.; Laitinen, L.A. Histopathologic features of early and progressive asthma. J. Allergy Clin. Immunol. 2000, 105, S509–S513. [Google Scholar] [CrossRef]
- Maryanoff, B.E.; De Garavilla, L.; Greco, M.N.; Haertlein, B.J.; Wells, G.I.; Andrade-Gordon, P.; Abraham, W.M. Dual inhibition of cathepsin G and chymase is effective in animal models of pulmonary inflammation. Am. J. Respir. Crit. Care Med. 2010, 181, 247–253. [Google Scholar] [CrossRef]
- Krishna, M.T.; Chauhan, A.; Little, L.; Sampson, K.; Hawksworth, R.; Mant, T.; Djukanovic, R.; Lee, T.; Holgate, S. Inhibition of mast cell tryptase by inhaled APC 366 attenuates allergen-induced late-phase airway obstruction in asthma. J. Allergy Clin. Immunol. 2001, 107, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.C. Viruses in asthma exacerbations. Curr. Opin. Pulm. Med. 2005, 11, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Reeves, S.R.; Barrow, K.A.; Rich, L.M.; White, M.P.; Shubin, N.J.; Chan, C.K.; Kang, I.; Ziegler, S.F.; Piliponsky, A.M.; Wight, T.N. Respiratory syncytial virus infection of human lung fibroblasts induces a hyaluronan-enriched extracellular matrix that binds mast cells and enhances expression of mast cell proteases. Front. Immunol. 2020, 10, 3159. [Google Scholar] [CrossRef] [PubMed]
- Ramu, S.; Akbarshahi, H.; Mogren, S.; Berlin, F.; Cerps, S.; Menzel, M.; Hvidtfeldt, M.; Porsbjerg, C.; Uller, L.; Andersson, C.K. Direct effects of mast cell proteases, tryptase and chymase, on bronchial epithelial integrity proteins and anti-viral responses. BMC Immunol. 2021, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wei, T.; Cox, C.W.; Jiang, Y.; Roche, W.R.; Walls, A.F. Mast cell chymase impairs bronchial epithelium integrity by degrading cell junction molecules of epithelial cells. Allergy 2019, 74, 1266–1276. [Google Scholar] [CrossRef]
- Cho, S.H.; Lee, S.H.; Kato, A.; Takabayashi, T.; Kulka, M.; Shin, S.C.; Schleimer, R.P. Cross-talk between human mast cells and bronchial epithelial cells in plasminogen activator inhibitor-1 production via transforming growth factor-β1. Am. J. Respir. Cell Mol. Biol. 2015, 52, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmood, M.A.; Rashid, B.M.; Amin, K.A.; Tofiq, D.M.; Nore, B.F. Correlation between serum Tryptase level and disease severity in asthmatic patients in the Sulaimani governorate. Int. J. Med. Res. Health Sci. 2016, 5, 34–41. [Google Scholar]
- Franceschini, B.; Russo, C.; Dioguardi, N.; Grizzi, F. Increased liver mast cell recruitment in patients with chronic C virus-related hepatitis and histologically documented steatosis. J. Viral. Hepat. 2007, 14, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, A.E.; Price, P.; Robertson, T.; Papadimitriou, J.; Shellam, G. Replication of murine cytomegalovirus in mast cells. Arch. Virol. 1990, 115, 299–307. [Google Scholar] [CrossRef]
- Guhl, S.; Franke, R.; Schielke, A.; Johne, R.; Krüger, D.H.; Babina, M.; Rang, A. Infection of in vivo differentiated human mast cells with hantaviruses. J. Gen. Virol. 2010, 91, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, J.B.; Little, D.M.; Villinger, F.; Ellis, J.E.; Ansari, A.A. Signaling through Toll-like receptors triggers HIV-1 replication in latently infected mast cells. J. Immunol. 2004, 172, 4391–4401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Jin, Y.; Han, D.; Zhang, G.; Cao, S.; Xie, J.; Xue, J.; Li, Y.; Meng, D.; Fan, X. Mast cell-induced lung injury in mice infected with H5N1 influenza virus. J. Virol. 2012, 86, 3347–3356. [Google Scholar] [CrossRef] [Green Version]
- Sorden, S.D.; Castleman, W. Virus-induced increases in bronchiolar mast cells in Brown Norway rats are associated with both local mast cell proliferation and increases in blood mast cell precursors. Lab. Investig. J. Tech. Methods Pathol. 1995, 73, 197–204. [Google Scholar]
- Chen, Y.; Shiota, M.; Ohuchi, M.; Towatari, T.; Tashiro, J.; Murakami, M.; Yano, M.; Yang, B.; Kido, H. Mast cell tryptase from pig lungs triggers infection by pneumotropic Sendai and influenza A viruses: Purification and characterization. Eur. J. Biochem. 2000, 267, 3189–3197. [Google Scholar] [CrossRef]
- Wang, D.; Xiong, J.; She, R.; Liu, L.; Zhang, Y.; Luo, D.; Li, W.; Hu, Y.; Wang, Y.; Zhang, Q. Mast cell mediated inflammatory response in chickens after infection with very virulent infectious bursal disease virus. Vet. Immunol. Immunopathol. 2008, 124, 19–28. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, D.; She, R.; Li, W.; Liu, S.; Han, D.; Wang, Y.; Ding, Y. Increased mast cell density during the infection with velogenic Newcastle disease virus in chickens. Avian Pathol. 2008, 37, 579–585. [Google Scholar] [CrossRef]
- Kitaura-Inenaga, K.; Hara, M.; Higuchi, K.; Yamamoto, K.; Yamaki, A.; Ono, K.; Nakano, A.; Kinoshita, M.; Sasayama, S.; Matsumori, A. Gene expression of cardiac mast cell chymase and tryptase in a murine model of heart failure caused by viral myocarditis. Circ. J. 2003, 67, 881–884. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, S.F.; Ho, Y.-C. SARS-CoV-2: A storm is raging. J. Clin. Investig. 2020, 130, 2202–2205. [Google Scholar] [CrossRef] [PubMed]
- Afrin, L.B.; Weinstock, L.B.; Molderings, G.J. Covid-19 hyperinflammation and post-Covid-19 illness may be rooted in mast cell activation syndrome. Int. J. Infect. Dis. 2020, 100, 327–332. [Google Scholar] [CrossRef]
- Motta Junior, J.d.S.; Miggiolaro, A.F.R.d.S.; Nagashima, S.; de Paula, C.B.V.; Baena, C.P.; Scharfstein, J.; de Noronha, L. Mast cells in alveolar septa of COVID-19 patients: A pathogenic pathway that may link interstitial edema to immunothrombosis. Front. Immunol. 2020, 11, 2369. [Google Scholar] [CrossRef]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Uzunismail, H. Increased Mast Cell Activation may be Responsible for the Critical Conditions in COVID-19 and Targeting Mast Cells and Their Mediators can Bring New Treatment Prospects. Microbiol. Infect. Dis. 2020, 4, 1–6. [Google Scholar] [CrossRef]
- Lin, H.; Cherukupalli, S.; Feng, D.; Gao, S.; Kang, D.; Zhan, P.; Liu, X. SARS-CoV-2 Entry inhibitors targeting virus-ACE2 or virus-TMPRSS2 interactions. Curr. Med. Chem. 2021. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; de Gregorio, C.; Velissaris, D.; Petalas, K.; Brinia, A.; Assimakopoulos, S.F.; Gogos, C.; Kouni, S.N.; Kounis, G.N. Allergic reactions to current available COVID-19 vaccinations: Pathophysiology, causality, and therapeutic considerations. Vaccines 2021, 9, 221. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Cholevas, C.; Polyzoidis, K.; Politis, A. Long-COVID syndrome-associated brain fog and chemofog: Luteolin to the rescue. Biofactors 2021, 47, 232–241. [Google Scholar] [CrossRef]
- Matias-Guiu, J.A.; Delgado-Alonso, C.; Yus, M.; Polidura, C.; Gómez-Ruiz, N.; Valles-Salgado, M.; Ortega-Madueño, I.; Cabrera-Martín, M.N.; Matias-Guiu, J. “Brain Fog” by COVID-19 or Alzheimer’s Disease? A Case Report. Front. Psychol. 2021, 12, 724022. [Google Scholar] [CrossRef] [PubMed]
- Tale, S.; Ghosh, S.; Meitei, S.P.; Kolli, M.; Garbhapu, A.K.; Pudi, S. Post-COVID-19 pneumonia pulmonary fibrosis. QJM Int. J. Med. 2020, 113, 837–838. [Google Scholar] [CrossRef]
- Bagher, M.; Larsson-Callerfelt, A.-K.; Rosmark, O.; Hallgren, O.; Bjermer, L.; Westergren-Thorsson, G. Mast cells and mast cell tryptase enhance migration of human lung fibroblasts through protease-activated receptor 2. Cell Commun. Signal. 2018, 16, 1–13. [Google Scholar] [CrossRef]
- Kazama, I. Stabilizing mast cells by commonly used drugs: A novel therapeutic target to relieve post-COVID syndrome? Drug Discov. Ther. 2020, 14, 259–261. [Google Scholar] [CrossRef]
- Sommerhoff, C.P.; Schaschke, N. Mast cell tryptase beta as a target in allergic inflammation: An evolving story. Curr. Pharm. Des. 2007, 13, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, J.; Estrada, S.; Karlson, U.; Alving, K.; Pejler, G. Heparin antagonists are potent inhibitors of mast cell tryptase. Biochemistry 2001, 40, 7342–7349. [Google Scholar] [CrossRef] [PubMed]
- Cregar, L.; Elrod, K.C.; Putnam, D.; Moore, W.R. Neutrophil myeloperoxidase is a potent and selective inhibitor of mast cell tryptase. Arch. Biochem. Biophys. 1999, 366, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Elrod, K.C.; Moore, W.R.; Abraham, W.M.; Tanaka, R.D. Lactoferrin, a potent tryptase inhibitor, abolishes late-phase airway responses in allergic sheep. Am. J. Respir. Crit. Care Med. 1997, 156, 375–381. [Google Scholar] [CrossRef]
- Alter, S.C.; Kramps, J.A.; Janoff, A.; Schwartz, L.B. Interactions of human mast cell tryptase with biological protease inhibitors. Arch. Biochem. Biophys. 1990, 276, 26–31. [Google Scholar] [CrossRef]
- Bae, K.S.; Kim, S.Y.; Park, S.Y.; Jeong, A.J.; Lee, H.H.; Lee, J.; Cho, Y.S.; Leem, S.H.; Kang, T.H.; Bae, K.H.; et al. Identification of lactoferrin as a human dedifferentiation factor through the studies of reptile tissue regeneration mechanisms. J. Microbiol. Biotechnol. 2014, 24, 869–878. [Google Scholar] [CrossRef]
- Ni, W.W.; Cao, M.D.; Huang, W.; Meng, L.; Wei, J.F. Tryptase inhibitors: A patent review. Expert. Opin. Pat. 2017, 27, 919–928. [Google Scholar] [CrossRef]
- He, S.; McEuen, A.R.; Blewett, S.A.; Li, P.; Buckley, M.G.; Leufkens, P.; Walls, A.F. The inhibition of mast cell activation by neutrophil lactoferrin: Uptake by mast cells and interaction with tryptase, chymase and cathepsin G. Biochem. Pharmacol. 2003, 65, 1007–1015. [Google Scholar] [CrossRef]
- Lundequist, A.; Juliano, M.A.; Juliano, L.; Pejler, G. Polycationic peptides as inhibitors of mast cell serine proteases. Biochem. Pharmacol. 2003, 65, 1171–1180. [Google Scholar] [CrossRef]
- Stubbs, M.T.; Morenweiser, R.; Stürzebecher, J.; Bauer, M.; Bode, W.; Huber, R.; Piechottka, G.P.; Matschiner, G.; Sommerhoff, C.P.; Fritz, H.; et al. The three-dimensional structure of recombinant leech-derived tryptase inhibitor in complex with trypsin. Implications for the structure of human mast cell tryptase and its inhibition. J. Biol. Chem. 1997, 272, 19931–19937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohlig, G.; Fendrich, G.; Knecht, R.; Eder, B.; Piechottka, G.; Sommerhoff, C.P.; Heim, J. Purification, characterization and biological evaluation of recombinant leech-derived tryptase inhibitor (rLDTI) expressed at high level in the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 1996, 241, 619–626. [Google Scholar] [CrossRef]
- Bronsoms, S.; Pantoja-Uceda, D.; Gabrijelcic-Geiger, D.; Sanglas, L.; Aviles, F.X.; Santoro, J.; Sommerhoff, C.P.; Arolas, J.L. Oxidative folding and structural analyses of a Kunitz-related inhibitor and its disulfide intermediates: Functional implications. J. Mol. Biol. 2011, 414, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Paesen, G.C.; Siebold, C.; Harlos, K.; Peacey, M.F.; Nuttall, P.A.; Stuart, D.I. A tick protein with a modified Kunitz fold inhibits human tryptase. J. Mol. Biol. 2007, 368, 1172–1186. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, S.; Sönnichsen, F.D.; Polte, T. Therapeutic potential of the peptide leucine arginine as a new nonplant bowman-birk-like serine protease inhibitor. J. Med. Chem. 2013, 56, 6732–6744. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Chen, X.; Ye, T.; Zeng, B.; Zeng, Q.; Wu, J.; Kascakova, B.; Martins, L.A.; Prudnikova, T.; Smatanova, I.K.; et al. Characterization and functional analysis of cathelicidin-MH, a novel frog-derived peptide with anti-septicemic properties. Elife 2021, 10, e64411. [Google Scholar] [CrossRef]
- Kolmar, H. Natural and engineered cystine knot miniproteins for diagnostic and therapeutic applications. Curr. Pharm. Des. 2011, 17, 4329–4336. [Google Scholar] [CrossRef]
- Bateman, K.S.; James, M.N. Plant protein proteinase inhibitors: Structure and mechanism of inhibition. Curr. Protein. Pept. Sci. 2011, 12, 340–347. [Google Scholar] [CrossRef]
- Sommerhoff, C.P.; Avrutina, O.; Schmoldt, H.U.; Gabrijelcic-Geiger, D.; Diederichsen, U.; Kolmar, H. Engineered cystine knot miniproteins as potent inhibitors of human mast cell tryptase beta. J. Mol. Biol. 2010, 395, 167–175. [Google Scholar] [CrossRef]
- Thongyoo, P.; Bonomelli, C.; Leatherbarrow, R.J.; Tate, E.W. Potent inhibitors of beta-tryptase and human leukocyte elastase based on the MCoTI-II scaffold. J. Med. Chem. 2009, 52, 6197–6200. [Google Scholar] [CrossRef]
- Jones, P.M.; George, A.M. Computational analysis of the MCoTI-II plant defence knottin reveals a novel intermediate conformation that facilitates trypsin binding. Sci. Rep. 2016, 6, 23174. [Google Scholar] [CrossRef] [Green Version]
- Ocak, U.; Eser Ocak, P.; Huang, L.; Xu, W.; Zuo, Y.; Li, P.; Gamdzyk, M.; Zuo, G.; Mo, J.; Zhang, G.; et al. Inhibition of mast cell tryptase attenuates neuroinflammation via PAR-2/p38/NFκB pathway following asphyxial cardiac arrest in rats. J. Neuroinflamm. 2020, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J.A. Inhibitors of mast cell tryptase beta as therapeutics for the treatment of asthma and inflammatory disorders. Pulm Pharm. 2005, 18, 55–66. [Google Scholar] [CrossRef]
- Bachelet, I.; Munitz, A.; Levi-Schaffer, F. Tryptase as an inflammatory marker in allergic disease and asthma. Expert Rev. Clin. Immunol. 2005, 1, 63–73. [Google Scholar] [CrossRef]
- Carreira, E.M.; Hisashi, Y. Comprehensive Chirality; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; Volume 1. [Google Scholar]
- Galletti, P.; Giacomini, D. Monocyclic β-lactams: New structures for new biological activities. Curr. Med. Chem. 2011, 18, 4265–4283. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, W.; Yoneda, T.; Koba, H.; Ueda, T.; Tsuji, N.; Ogawa, H.; Asakura, H. Potential mechanisms of nafamostat therapy for severe COVID-19 pneumonia with disseminated intravascular coagulation. Int. J. Infect. Dis. 2021, 102, 529–531. [Google Scholar] [CrossRef]
- Oh, S.W.; Pae, C.I.; Lee, D.K.; Jones, F.; Chiang, G.K.; Kim, H.O.; Moon, S.H.; Cao, B.; Ogbu, C.; Jeong, K.W.; et al. Tryptase inhibition blocks airway inflammation in a mouse asthma model. J. Immunol. 2002, 168, 1992–2000. [Google Scholar] [CrossRef] [Green Version]
- Fukuoka, Y.; Schwartz, L.B. The B12 anti-tryptase monoclonal antibody disrupts the tetrameric structure of heparin-stabilized beta-tryptase to form monomers that are inactive at neutral pH and active at acidic pH. J. Immunol. 2006, 176, 3165–3172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maun, H.R.; Jackman, J.K.; Choy, D.F.; Loyet, K.M.; Staton, T.L.; Jia, G.; Dressen, A.; Hackney, J.A.; Bremer, M.; Walters, B.T.; et al. An Allosteric Anti-tryptase Antibody for the Treatment of Mast Cell-Mediated Severe Asthma. Cell 2020, 180, 406. [Google Scholar] [CrossRef] [Green Version]
- Rymut, S.M.; Sukumaran, S.; Sperinde, G.; Bremer, M.; Galanter, J.; Yoshida, K.; Smith, J.; Banerjee, P.; Sverkos, V.; Cai, F.; et al. Dose-dependent inactivation of airway tryptase with a novel dissociating anti-tryptase antibody (MTPS9579A) in healthy participants: A randomized trial. Clin. Transl. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimi, N.; Morovati, S.; Chan, L.; Napoleoni, C.; Mehrani, Y.; Bridle, B.W.; Karimi, K. Mast Cell Tryptase and Implications for SARS-CoV-2 Pathogenesis. BioMed 2021, 1, 136-149. https://doi.org/10.3390/biomed1020013
Karimi N, Morovati S, Chan L, Napoleoni C, Mehrani Y, Bridle BW, Karimi K. Mast Cell Tryptase and Implications for SARS-CoV-2 Pathogenesis. BioMed. 2021; 1(2):136-149. https://doi.org/10.3390/biomed1020013
Chicago/Turabian StyleKarimi, Negar, Solmaz Morovati, Lily Chan, Christina Napoleoni, Yeganeh Mehrani, Byram W. Bridle, and Khalil Karimi. 2021. "Mast Cell Tryptase and Implications for SARS-CoV-2 Pathogenesis" BioMed 1, no. 2: 136-149. https://doi.org/10.3390/biomed1020013
APA StyleKarimi, N., Morovati, S., Chan, L., Napoleoni, C., Mehrani, Y., Bridle, B. W., & Karimi, K. (2021). Mast Cell Tryptase and Implications for SARS-CoV-2 Pathogenesis. BioMed, 1(2), 136-149. https://doi.org/10.3390/biomed1020013








