Visual Analogue Scale
Definition
1. Introduction
2. The Visual Analogue Scale: Core Concepts
2.1. History of the Visual Analogue Scale
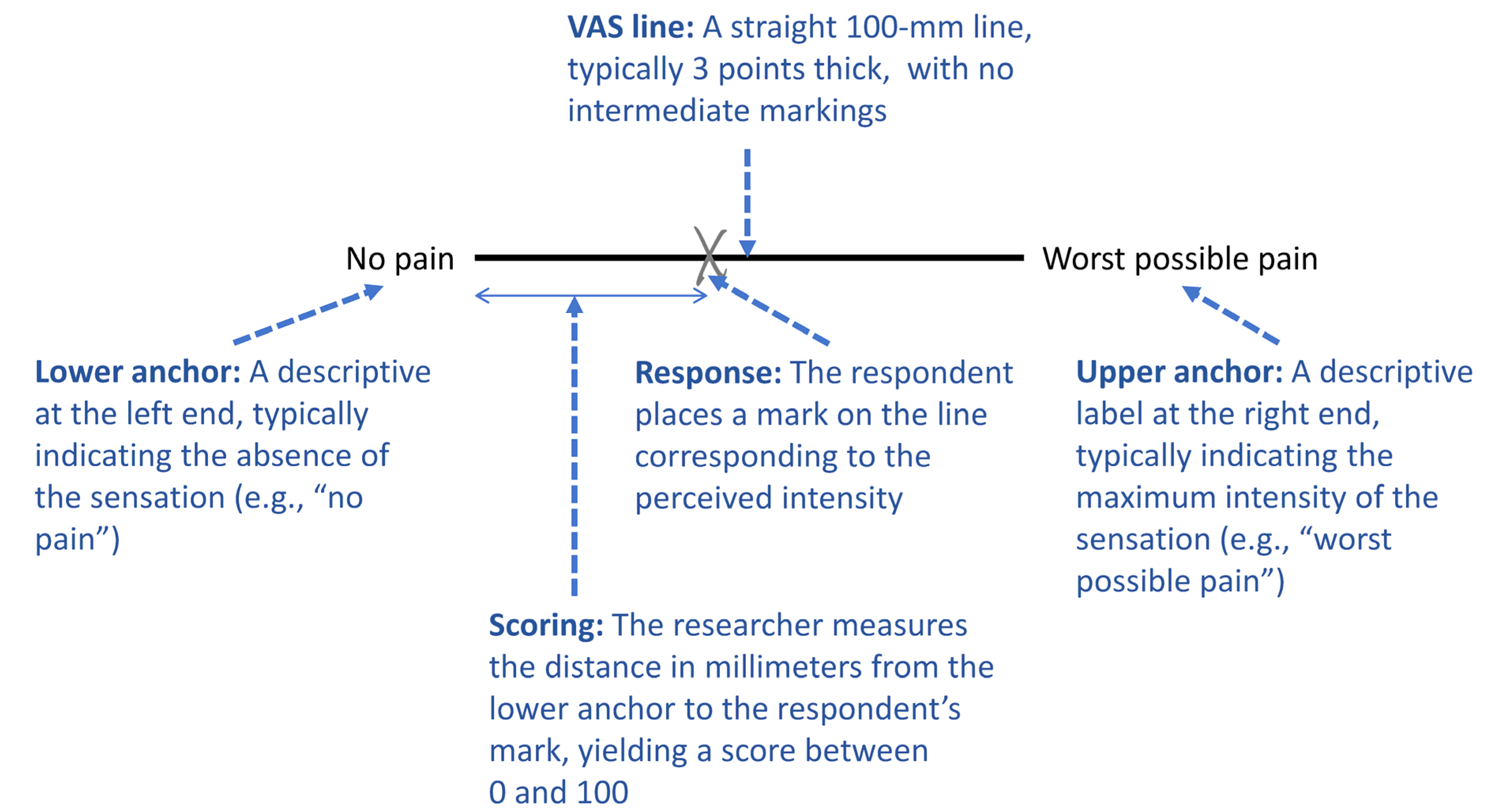
2.2. Design and Formatting
2.3. Comparison with Other Rating Scales
3. Administration, Scoring, and Analysis
3.1. Administration, Scoring and Data Handling
3.2. Statistical Analysis Considerations
3.3. Transparent Reporting of VAS Outcomes
4. Psychometric Properties of the Visual Analogue Scale
4.1. Reliability
4.2. Validity
4.3. Responsiveness
5. Limitations and Challenges of the Visual Analogue Scale
6. Current and Future Developments
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| ANOVA | Analysis of Variance |
| EMA | Ecological Momentary Assessment |
| FPS-R | Faces Pain Scale–Revised |
| GA-VAS | Generalized Anxiety Visual Analogue Scale |
| GRS | Graphic Rating Scale |
| MCID | Minimal Clinically Important Difference |
| MESH | Medical Subject Headings |
| NRS | Numeric Rating Scale |
| SF-36 | 36-Item Short Form Health Survey |
| VAS | Visual Analogue Scale |
| VRS | Verbal Rating Scale |
References
- Euasobhon, P.; Atisook, R.; Bumrungchatudom, K.; Zinboonyahgoon, N.; Saisavoey, N.; Jensen, M.P. Reliability and responsivity of pain intensity scales in individuals with chronic pain. Pain 2022, 163, e1184–e1191. [Google Scholar] [CrossRef]
- Dutheil, F.; Palgen, C.; Brousse, G.; Coste, A.; Saurin, J.; Lambert, C.; Pélissier, C. Validation of visual analog scales of mood and anxiety at the workplace. PLoS ONE 2024, 19, e0316159. [Google Scholar] [CrossRef]
- Åström, M.; Thet Lwin, Z.M.; Teni, F.S.; Burström, K.; Berg, J. Use of the visual analogue scale for health state valuation: A scoping review. Qual. Life Res. 2023, 32, 2719–2729. [Google Scholar] [CrossRef]
- Voutilainen, A.; Pitkäaho, T.; Kvist, T.; Vehviläinen-Julkunen, K. How to ask about patient satisfaction? The visual analogue scale is less vulnerable to confounding factors and ceiling effect than a symmetric Likert scale. J. Adv. Nurs. 2016, 72, 946–957. [Google Scholar] [CrossRef]
- Kindler, C.H.; Harms, C.; Amsler, F.; Ihde-Scholl, T.; Scheidegger, D. The visual analog scale allows effective measurement of pre-operative anxiety and detection of patients’ anesthetic concerns. Anesth. Analg. 2000, 90, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Evin, A.; Huon, J.F.; Thuaut, A.L.; Jego, P.; Nizet, P.; Victorri-Vigneau, C.; Bourdon, M.; Palliative Care Team. Use of brief, simple anxiety assessment tools in palliative care: Yes, we can. A cross-sectional observational study of anxiety visual analog scale and numeric rating scale. BMC Palliat. Care 2025, 24, 173. [Google Scholar] [CrossRef] [PubMed]
- Faasse, K.; Martin, L.R.; Grey, A.; Gamble, G.; Petrie, K.J. Impact of brand or generic labeling on medication effectiveness and side effects. Health Psychol. 2016, 35, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liang, S.; Jiang, T. Comparison of case-based and lecture-based learning in dental fluorosis diagnostic ability with visual analog scale assessment. BMC Med. Educ. 2024, 24, 761. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Wong, N.S.M. The historical roots of visual analog scale in psychology as revealed by reference publication year spectroscopy. Front. Hum. Neurosci. 2019, 13, 86. [Google Scholar] [CrossRef]
- Hayes, M.H.S.; Patterson, D.G. Experimental development of the graphic rating method. Psychol. Bull. 1921, 18, 98–99. [Google Scholar]
- Shiina, K. Commentary: The historical roots of visual analog scale in psychology as revealed by reference publication year spectroscopy. Front. Hum. Neurosci. 2021, 15, 711691. [Google Scholar] [CrossRef]
- Pilowsky, I.; Kaufman, A. An experimental study of atypical phantom pain. Br. J. Psychiatry 1965, 111, 1185–1187. [Google Scholar] [CrossRef]
- Aitken, R.C.B. A growing edge of measurement of feelings [Abridged]—Measurement of feelings using visual analogue scales. Proc. R. Soc. Med. 1969, 62, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Price, D.D.; McGrath, P.A.; Rafii, A.; Buckingham, B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 1983, 17, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.S. Agreement between horizontal and vertical visual analogue scales. Br. J. Rheumatol. 1986, 25, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Breivik, E.K.; Skoglund, L.A. Comparison of present pain intensity assessments on horizontally and vertically oriented visual analogue scales. Methods Find. Exp. Clin. Pharmacol. 1998, 20, 719–724. [Google Scholar] [CrossRef]
- Lundqvist, C.; Benth, J.S.; Grande, R.B.; Aaseth, K.; Russell, M.B. A vertical VAS is a valid instrument for monitoring headache pain intensity. Cephalalgia 2009, 29, 1034–1041. [Google Scholar] [CrossRef]
- Ogon, M.; Krismer, M.; Söllner, W.; Kantner-Rumplmair, W.; Lampe, A. Chronic low back pain measurement with visual analogue scales in different settings. Pain 1996, 64, 425–428. [Google Scholar] [CrossRef]
- Stephenson, N.L.; Herman, J.A. Pain measurement: A comparison using horizontal and vertical visual analogue scales. Appl. Nurs. Res. 2000, 13, 157–158. [Google Scholar] [CrossRef]
- Rabin, R.; de Charro, F. EQ-5D: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef]
- Yakut, E.; Bayar, B.; Meriç, A.; Bayar, K.; Yakut, Y. Reliability and validity of reverse visual analog scale (right to left) in different intensity of pain. Pain Clin. 2023, 15, 1–6. [Google Scholar] [CrossRef]
- Weigl, K.; Forstner, T. Design of paper-based visual analogue scale items. Educ. Psychol. Meas. 2021, 81, 595–611. [Google Scholar] [CrossRef]
- Shmueli, A. The visual analog rating scale of health-related quality of life: An examination of end-digit preferences. Health Qual. Life Outcomes 2005, 3, 71. [Google Scholar] [CrossRef]
- Abdel-Fattah, M.; Ramsay, I.; Barrington, J.W. A simple visual analogue scale to assess the quality of life in women with urinary incontinence. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 133, 86–89. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Renne, A.; Argandykov, D.; Convissar, D.; Lee, J. Comparison of an emoji-based visual analog scale with a numeric rating scale for pain assessment. JAMA 2022, 328, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Koo, M.; Yang, S.-W. Likert-type scale. Encyclopedia 2025, 5, 18. [Google Scholar] [CrossRef]
- Ferreira-Valente, M.A.; Pais-Ribeiro, J.L.; Jensen, M.P. Validity of four pain intensity rating scales. Pain 2011, 152, 2399–2404. [Google Scholar] [CrossRef]
- Bieri, D.; Reeve, R.A.; Champion, D.G.; Addicoat, L.; Ziegler, J.B. The faces pain scale for the self-assessment of the severity of pain experienced by children: Development, initial validation, and preliminary investigation for ratio scale properties. Pain 1990, 41, 139–150. [Google Scholar] [CrossRef]
- Heller, G.Z.; Manuguerra, M.; Chow, R. How to analyze the visual analogue scale: Myths, truths and clinical relevance. Scand. J. Pain 2016, 13, 67–75. [Google Scholar] [CrossRef]
- Kersten, P.; White, P.J.; Tennant, A. Is the pain visual analogue scale linear and responsive to change? An exploration using Rasch analysis. PLoS ONE 2014, 9, e99485. [Google Scholar] [CrossRef]
- Dexter, F.; Chestnut, D.H. Analysis of statistical tests to compare visual analog scale measurements among groups. Anesthesiology 1995, 82, 896–902. [Google Scholar] [CrossRef]
- Manuguerra, M.; Heller, G.Z. Ordinal regression models for continuous scales. Int. J. Biostat. 2010, 6, 14. [Google Scholar] [CrossRef]
- Alghadir, A.H.; Anwer, S.; Iqbal, A.; Iqbal, Z.A. Test-retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J. Pain Res. 2018, 11, 851–856. [Google Scholar] [CrossRef]
- Williams, S.A.; Sharma, S.; Cashin, A.G.; Jones, M.D.; Chiarotto, A.; Hansford, H.J.; Venter, M.; Wewege, M.A.; Ferraro, M.C.; Devonshire, J.J.; et al. Test-retest reliability and measurement error of the numerical rating scale and visual analogue scale in people with low back pain. J. Pain 2025, 35, 105528. [Google Scholar] [CrossRef]
- Ferraz, M.B.; Quaresma, M.R.; Aquino, L.R.; Atra, E.; Tugwell, P.; Goldsmith, C.H. Reliability of pain scales in the assessment of literate and illiterate patients with rheumatoid arthritis. J. Rheumatol. 1990, 17, 1022–1024. [Google Scholar] [PubMed]
- Alacreu-Crespo, A.; Innamorati, M.; Courtet, P.; Fiorillo, A.; Pompili, M. Are visual analogue scales valid instruments to measure psychological pain in psychiatric patients? J. Affect. Disord. 2024, 358, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Williams, V.S.; Morlock, R.J.; Feltner, D. Psychometric evaluation of a visual analog scale for the assessment of anxiety. Health Qual. Life Outcomes 2010, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.L.; Pignatello, G.; Park, S.; Lipson, A.R. Psychometric properties of a single-item visual analog scale measuring goals of care in patients with advanced cancer. Qual. Life Res. 2020, 29, 1999–2005. [Google Scholar] [CrossRef]
- Kelly, A.M. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg. Med. J. 2001, 18, 205–207. [Google Scholar] [CrossRef]
- Olsen, M.F.; Bjerre, E.; Hansen, M.D.; Tendal, B.; Hilden, J.; Hróbjartsson, A. Minimum clinically important differences in chronic pain vary considerably by baseline pain and methodological factors: Systematic review of empirical studies. J. Clin. Epidemiol. 2018, 101, 87–106.e2. [Google Scholar] [CrossRef]
- Young, I.; Dunning, J.; Mourad, F.; Escaloni, J.; Bliton, P.; Fernández-de-Las-Peñas, C. Clinimetric analysis of the visual analogue scale and pain-free mouth opening in patients with muscular temporomandibular disorder. Cranio, 2025; in press. [Google Scholar] [CrossRef]
- Hilari, K.; Boreham, L.D. Visual analogue scales in stroke: What can they tell us about health-related quality of life? BMJ Open 2013, 3, e003309. [Google Scholar] [CrossRef][Green Version]
- Bringuier, S.; Dadure, C.; Raux, O.; Dubois, A.; Picot, M.C.; Capdevila, X. The perioperative validity of the visual analog anxiety scale in children: A discriminant and useful instrument in routine clinical practice to optimize postoperative pain management. Anesth. Analg. 2009, 109, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Kremer, E.; Atkinson, H.J.; Ignelzi, R.J. Measurement of pain: Patient preference does not confound pain measurement. Pain 1981, 10, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Tin, A.L.; Austria, M.; Ogbennaya, G.; Chimonas, S.; Andréll, P.; Atkinson, T.M.; Vickers, A.J.; Carlsson, S.V. Pain as bad as you can imagine or extremely severe pain? A randomized controlled trial comparing two pain scale anchors. J. Patient Rep. Outcomes 2023, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.G.; Harris, J.M.; Benstock, S.E.; Ales, J.M. The reliability of pseudoneglect is task dependent. Neuropsychologia 2020, 148, 107618. [Google Scholar] [CrossRef]
- Brokelman, R.B.; Haverkamp, D.; van Loon, C.; Hol, A.; van Kampen, A.; Veth, R. The validation of the visual analogue scale for patient satisfaction after total hip arthroplasty. Eur. Orthop. Traumatol. 2012, 3, 101–105. [Google Scholar] [CrossRef]
- Leknes, S.; Berna, C.; Lee, M.C.; Snyder, G.D.; Biele, G.; Tracey, I. The importance of context: When relative relief renders pain pleasant. Pain 2013, 154, 402–410. [Google Scholar] [CrossRef]
- Gracely, R.H.; McGrath, P.; Dubner, R. Ratio scales of sensory and affective verbal pain descriptors. Pain 1978, 5, 5–18. [Google Scholar] [CrossRef]
- Gaston-Johansson, F.; Albert, M.; Fagan, E.; Zimmerman, L. Similarities in pain descriptions of four different ethnic-culture groups. J. Pain Symptom. Manag. 1990, 5, 94–100. [Google Scholar] [CrossRef]
- Cervera-Garvi, P.; Galan-Hurtado, M.H.; Marchena-Rodriguez, A.; Chicharro-Luna, E.; Guerra-Marmolejo, C.; Diaz-Miguel, S.; Ortega-Avila, A.B. Transcultural adaptation and validation of the Spanish version of the Visual Analogue Scale for the Foot and Ankle (VASFA). J. Clin. Med. 2023, 13, 213. [Google Scholar] [CrossRef]
- Li, X.X.; Yu, H.Y.; Li, J.J.; Liu, X.L.; Zheng, H.Y.; Li, Y.F.; Li, Q.; Liu, S.Y. Cross-cultural adaptation and construct validity of the Chinese Version of Visual Vertigo Analogue Scale by using structural equation modeling. J. Vestib. Res. 2024, 34, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Byrom, B.; Elash, C.A.; Eremenco, S.; Bodart, S.; Muehlhausen, W.; Platko, J.V.; Watson, C.; Howry, C. Measurement comparability of electronic and paper administration of visual analogue scales: A review of published studies. Ther. Innov. Regul. Sci. 2022, 56, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Pinto, B.; Ramanauskaite, A.; Neisinger, S.; Witte-Händel, E.; Gimenez-Arnau, A.M.; Guillet, C.; Parisi, C.A.S.; Katelaris, C.H.; Fomina, D.; Larenas-Linnemann, D.; et al. Validity, reliability and responsiveness of digital visual analogue scales for chronic spontaneous urticaria monitoring: A CRUSE® mobile health study. Allergy 2025, 80, 750–761. [Google Scholar] [CrossRef]
- Haslbeck, J.M.B.; Martínez, A.J.; Roefs, A.J.; Fried, E.I.; Lemmens, L.H.J.M.; Groot, E.; Edelsbrunner, P.A. Comparing Likert and visual analogue scales in ecological momentary assessment. Behav. Res. Methods 2025, 57, 217. [Google Scholar] [CrossRef]
- Ratti, P.L.; Faraci, F.; Hackethal, S.; Mascheroni, A.; Ferlito, C.; Caverzasio, S.; Amato, N.; Choe, E.K.; Luo, Y.; Nunes-Ferreira, P.E.; et al. A new prospective, home-based monitoring of motor symptoms in Parkinson’s disease. J. Park. Dis. 2019, 9, 803–809. [Google Scholar] [CrossRef]
- Shajari, S.; Kuruvinashetti, K.; Komeili, A.; Sundararaj, U. The emergence of AI-based wearable sensors for digital health technology: A review. Sensors 2023, 23, 9498. [Google Scholar] [CrossRef] [PubMed]
| Feature | Visual Analogue Scale (VAS) | Numeric Rating Scale (NRS) | Verbal Rating Scale (VRS) | Faces Pain Scale-Revised (FPS-R) |
|---|---|---|---|---|
| Format | 100 mm line without numerical markers | 0–10 or 0–11 numerical scale | Discrete verbal descriptors (e.g., “no pain” to “worst pain”) | Series of facial expressions |
| Measurement Type | Continuous (ratio scale) | Discrete (interval scale) | Ordinal scale | Ordinal scale |
| Scoring | Millimeter measurement from left end (0–100 mm) | Numeric score from 0 (no pain) to 10 (worst pain) | Predefined categories converted to numeric equivalents | Score of 0, 2, 4, 6, 8, or 10 |
| Advantages | High sensitivity; ratio scale properties | Simple; widely used | Easy for patients with cognitive or literacy challenges | Culturally neutral; suitable for young children |
| Limitations | Requires understanding of scale | Less sensitive to small changes | Limited sensitivity; fewer response options | Less precise; not suitable for adults |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, M.; Yang, S.-W. Visual Analogue Scale. Encyclopedia 2025, 5, 190. https://doi.org/10.3390/encyclopedia5040190
Koo M, Yang S-W. Visual Analogue Scale. Encyclopedia. 2025; 5(4):190. https://doi.org/10.3390/encyclopedia5040190
Chicago/Turabian StyleKoo, Malcolm, and Shih-Wei Yang. 2025. "Visual Analogue Scale" Encyclopedia 5, no. 4: 190. https://doi.org/10.3390/encyclopedia5040190
APA StyleKoo, M., & Yang, S.-W. (2025). Visual Analogue Scale. Encyclopedia, 5(4), 190. https://doi.org/10.3390/encyclopedia5040190









