Inhomogeneities in Glass: From Defects to Functional Nanostructures
Definition
1. Introduction
2. Conventional (“Faulty”) Inhomogeneities in Glass
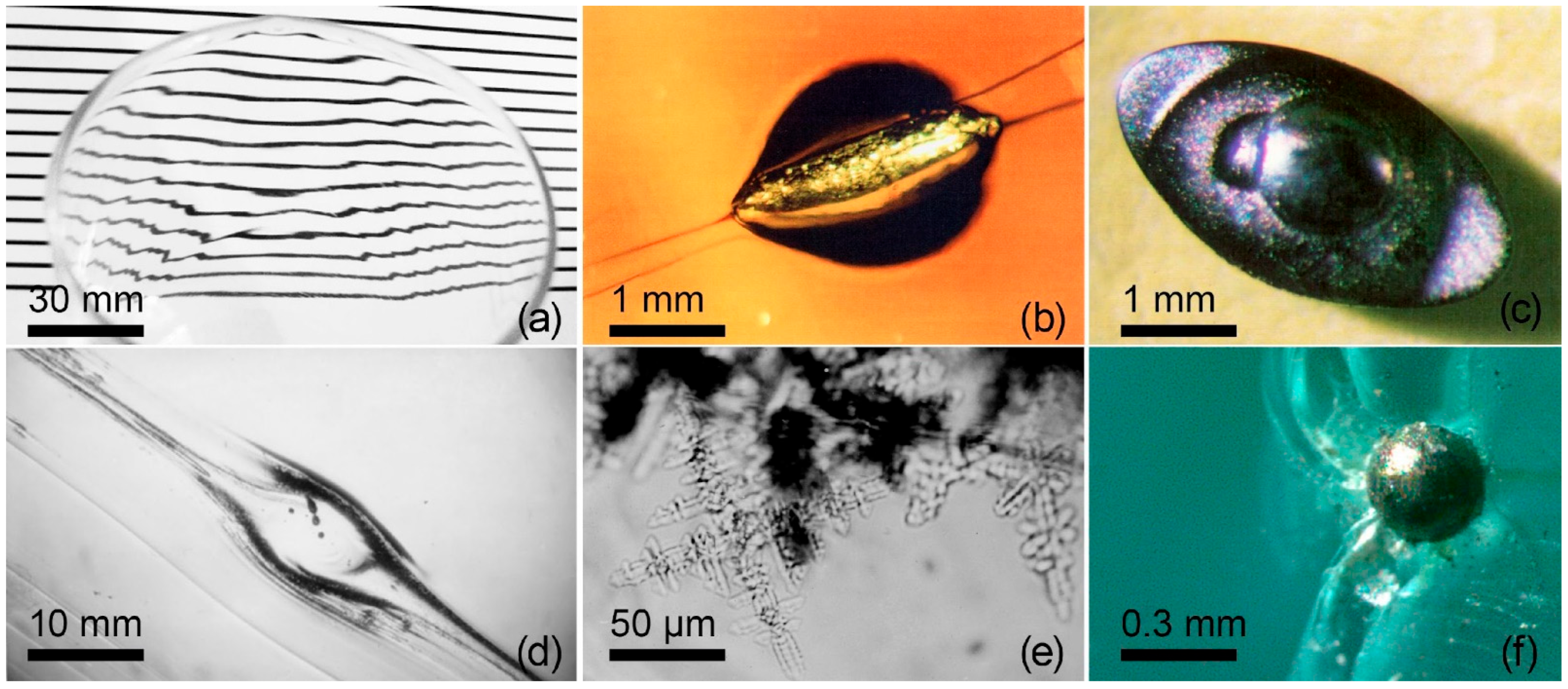
2.1. Main Types of Defects in Glass
2.2. Container Glass (Bottles and Jars)
2.3. Flat Glass (Window/Architectural Glass)
2.4. Optical Fibers
2.5. Optical Glasses (Precision Optics)
3. Functional (“Desired”) Nano-Inhomogeneities in Glass
3.1. Nanocrystals in Glass (Transparent Glass–Ceramics)
3.2. Metal Nanoparticles (Plasmonic Glasses)
3.3. Semiconductor and Perovskite Nanoparticles
3.4. Nanopores in Glass (Nanoporous Glasses)
3.5. Nuclear Waste Glasses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reitmayer, F.; Schuster, E. Homogeneity of optical glasses. Appl. Opt. 1972, 11, 1107–1111. [Google Scholar] [CrossRef]
- Richet, P.; Conradt, R.; Takada, A.; Dyon, J. Encyclopedia of Glass Science, Technology, History, and Culture; Wiley: Hoboken, NJ, USA, 2021; p. 1568. [Google Scholar]
- Hartmann, P.; Jedamzik, R.; Reichel, S.; Schreder, B. Optical glass and glass ceramic historical aspects and recent developments: A Schott view. Appl. Opt. 2010, 49, D157–D176. [Google Scholar] [CrossRef]
- Mishra, A.; Frechero, M.A.; Caron, A.; Singh, P.K.; Tiwari, A. Recent progress and future directions in nanoglass materials: A deep insight into synthesis, characterization, and application. Nanotechnol. Precis. Eng. 2025, 8, 015002. [Google Scholar] [CrossRef]
- Kumar, P.; Mathpal, M.C.; Prakash, J.; Jagannath, G.; Roos, W.D.; Swart, H.C. Plasmonic and nonlinear optical behavior of nanostructures in glass matrix for photonics application. Mater. Res. Bull. 2020, 125, 110799. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Petrov, V.A.; Yudintsev, S.V. Glass Crystalline Materials as Advanced Nuclear Wasteforms. Sustainability 2021, 13, 4117. [Google Scholar] [CrossRef]
- Jensen, M.; Keding, R.; Fjendbo, S.; Poschwatta, H.H.; Yue, Y. Effect of bubbles on the characterisation of striae in glasses. Glass Technology-European. J. Glass Sci. Technol. Part A 2010, 51, 147–152. [Google Scholar]
- Trujillo-Sevilla, J.M.; Velasco-Ocana, M.; Bonaque-Gonzalez, S.; Belda-Para, C.; Rodriguez-Ramos, J.M. Wavefront phase measurement of striae in optical glass. Appl. Opt. 2010, 61, 3912–3918. [Google Scholar] [CrossRef]
- Stroud, J.S. Striae quality grades for optical glass. Opt. Eng. 2003, 42, 1618–1624. [Google Scholar] [CrossRef]
- Peng, X.; Chen, Y.; Yu, W.; Zhou, Z.; Sun, G. An online defects inspection method for float glass fabrication based on machine vision. Int. J. Adv. Manuf. Technol. 2008, 39, 1180–1189. [Google Scholar] [CrossRef]
- Rupp, F.; Jedamzik, R.; Dietrich, V.; Petzold, U. Improved Production of Large and Multi-Directional Homogeneous Optical Glass: SCHOTT N-BK7® for Challenging Applications. EPJ Web Conf. 2022, 266, 03018. [Google Scholar] [CrossRef]
- Saito, K.; Yamaguchi, M.; Kakiuchida, H.; Ikushima, A.J.; Ohsono, K.; Kurosawa, Y. Limit of the Rayleigh scattering loss in silica fiber. Appl. Phys. Lett. 2003, 83, 5175–5177. [Google Scholar] [CrossRef]
- Müller-Simon, H. Fining of glass melts. Rev. Mineral. Geochem. 2011, 73, 337–361. [Google Scholar] [CrossRef]
- Jensen, M.; Yue, Y. Effect of stirring on striae in glass melts. J. Non-Cryst. Solids 2012, 358, 349–353. [Google Scholar] [CrossRef]
- Sevast’yanov, R.I. The Role of Convection in Glass-Melting Furnaces. Glass Ceram. 2004, 61, 139–141. [Google Scholar] [CrossRef]
- SCHOTT AG. Bubbles and Inclusions in Optical Glass. Technical Information TIE-28, SCHOTT AG. 2004. Available online: https://wp.optics.arizona.edu/optomech/wp-content/uploads/sites/53/2016/10/tie-28_bubbles_and_inclusions_us.pdf (accessed on 23 July 2025).
- Kasper, A. Spontaneous cracking of thermally toughened safety glass. Part one: Properties of nickel sulphide inclusions. Glass Struct. Eng. 2019, 4, 279–313. [Google Scholar]
- Stachel, D. Einschlüsse im Glas: Eine kleine Glasfehlerkunde für Praktiker, Studierende und andere Neugierige; Eigenverlag: Jena, Germany, 2012; Available online: https://www.db-thueringen.de/receive/dbt_mods_00047978 (accessed on 23 July 2025).
- Emhart Glass. Container Defect Causes and Remedies. Emhart Glass. 2020. Available online: https://www.scribd.com/document/474794629/BEG-BR0060-Defect-Guide-0-pdf (accessed on 23 July 2025).
- Peters, T.; Schneider, J. Appearance and prevalence of float glass inclusions with a diameter smaller than 500 µm, focused on the identification of nickel sulphide. Glass Struct. Eng. 2024, 9, 471–481. [Google Scholar] [CrossRef]
- Tsujikawa, K.; Tajima, K.; Zhou, J. Intrinsic loss of optical fibers. Opt. Fiber Technol. 2005, 11, 319–331. [Google Scholar] [CrossRef]
- Chen, C.; Jaluria, Y. Effects of doping on the optical fiber drawing process. Int. J. Heat Mass Transf. 2009, 52, 4812–4822. [Google Scholar] [CrossRef]
- Bufetova, G.; Kosolapov, A.; Yashkov, M.; Umnikov, A.; Velmiskin, V.; Tsvetkov, V.; Bufetov, I. Extra-High Pressure in the Core of Silica-Based Optical Fiber Preforms during the Manufacturing Process. Photonics 2023, 10, 335. [Google Scholar] [CrossRef]
- SCHOTT AG. Homogeneity of Optical Glass. Technical Information TIE-26, February 2016. Available online: https://www.us.schott.com/shop/medias/schott-tie-26-homogeneity-of-optical-glass-eng.pdf?context=bWFzdGVyfHJvb3R8MTQ0NDM2M3xhcHBsaWNhdGlvbi9wZGZ8aDFiL2g4NS84ODE3NDA5MzkyNjcwLnBkZnwwNWMyMTJiMmMyNzkyYjY1ZjQxNTViZjllMjVjZWFiZGY0NWZhMTUwOGI3ZmIzNGRiYTY2NGEwZmFkMWM3MzIx (accessed on 23 July 2025).
- Ohara Inc. Optical Glass Characteristics and Use. Ohara Technical Catalog. 2021. Available online: https://oharacorp.com/wp-content/uploads/2022/11/clearerem.pdf (accessed on 23 July 2025).
- SCHOTT AG. ZERODUR®–The Material for Extreme Precision. 2022. Available online: https://www.schott.com/en-us/products/zerodur (accessed on 23 July 2025).
- Naumov, A.S.; Shakhgildyan, G.Y.; Golubev, N.V.; Lipatiev, A.S.; Fedotov, S.S.; Alekseev, R.O.; Sigaev, V.N. Tuning the Coefficient of Thermal Expansion of Transparent Lithium Aluminosilicate Glass-Ceramics by a Two-Stage Heat Treatment. Ceramics 2023, 7, 1–14. [Google Scholar] [CrossRef]
- Singh, S.P.; Sontakke, A.D. Transparent glass ceramics. Crystals 2021, 11, 156. [Google Scholar] [CrossRef]
- Sigaev, V.N.; Savinkov, V.I.; Lotarev, S.V.; Shakhgildyan, G.Y.; Lorenzi, R.; Paleari, A. Spatially selective Au nanoparticle growth in laser-quality glass controlled by UV-induced phosphate-chain cross-linkage. Nanotechnology 2013, 24, 225302. [Google Scholar] [CrossRef]
- Shakhgildyan, G.Y.; Lipatiev, A.S.; Vetchinnikov, M.P.; Popova, V.V.; Lotarev, S.V.; Golubev, N.V.; Sigaev, V.N. One-step micro-modification of optical properties in silver-doped zinc phosphate glasses by femtosecond direct laser writing. J. Non-Cryst. Solids 2018, 481, 634–642. [Google Scholar] [CrossRef]
- Lipatiev, A.S.; Shakhgildyan, G.Y.; Vetchinnikov, M.P.; Lee, H.; Heo, J.; Lotarev, S.V.; Sigaev, V.N. Direct precipitation of CdS nanocrystals in glass by ultrafast laser pulses. Mater. Lett. 2022, 307, 130974. [Google Scholar] [CrossRef]
- Sigaeva, V.N.; Naumov, A.S.; Lipat’ev, A.S.; Shakhgil’dyan, G.Y.; Lotarev, S.V.; Fedotov, S.S.; Karateev, I.A. Phase transformations under the action of femtosecond pulses in ZnO–MgO–Al2O3–SiO2 sitalls. Glass Ceram. 2023, 80, 3–8. [Google Scholar] [CrossRef]
- Shakhgil’dyan, G.Y.; Piyanzina, K.I.; Stepko, A.A.; Natyrov, A.N.; Mikhailov, A.M.; Savinkov, V.I.; Sigaev, V.N. Nanoporous glass with controlled pore size for high-efficiency synthesis of oligonucleotides. Glass Ceram. 2019, 75, 377–382. [Google Scholar] [CrossRef]
- Hendy, S. Light scattering in transparent glass ceramics. Appl. Phys. Lett. 2002, 81, 1171–1173. [Google Scholar] [CrossRef]
- Pan, Q.; Yang, D.; Dong, G.; Qiu, J.; Yang, Z. Nanocrystal-in-glass composite (NGC): A powerful pathway from nanocrystals to advanced optical materials. Prog. Mater. Sci. 2022, 130, 100998. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Zhu, Y.; Zhong, J.; Chen, D. Upconversion of transparent glass ceramics containing β-NaYF 4: Yb 3+, Er 3+ nanocrystals for optical thermometry. RSC Adv. 2019, 9, 7948–7954. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, S.; Zhou, S.; Jiang, N.; Qiu, J. Enhanced upconversion luminescence of transparent Eu 3+-doped glass–ceramics containing nonlinear optical microcrystals. Opt. Lett. 2007, 32, 653–655. [Google Scholar] [CrossRef]
- Liu, Q.; Ran, P.; Chen, W.; Shi, N.; Zhang, W.; Qiao, X.; Fan, X. Bright transparent scintillators with high fraction BaCl2: Eu2+ nanocrystals precipitation: An ionic-covalent hybrid network strategy toward superior X-ray imaging glass-ceramics. Adv. Sci. 2023, 10, 2304889. [Google Scholar] [CrossRef]
- Shepilov, M.P.; Dymshits, O.S.; Zhilin, A.A. Light scattering in glass-ceramics: Revision of the concept. J. Opt. Soc. Am. B 2018, 35, 1717–1724. [Google Scholar] [CrossRef]
- Lee, G.; Struebing, C.; Wagner, B.; Summers, C.; Ding, Y.; Bryant, A.; Thadhani, N.; Shedlock, D.; Star-Lack, J.; Kang, Z. Synthesis and characterization of a BaGdF5: Tb glass ceramic as a nanocomposite scintillator for x-ray imaging. Nanotechnology 2016, 27, 205203. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Zhou, S.; Yue, Y.; Qiu, J. Transparent glass-ceramics functionalized by dispersed crystals. Prog. Mater. Sci. 2018, 97, 38–96. [Google Scholar] [CrossRef]
- Ruivo, A.; Gomes, C.; Lima, A.; Botelho, M.L.; Melo, R.; Belchior, A.; de Matos, A.P. Gold nanoparticles in ancient and contemporary ruby glass. J. Cult. Herit. 2008, 9, e134–e137. [Google Scholar] [CrossRef]
- Lafait, J.; Berthier, S.; Andraud, C.; Reillon, V.; Boulenguez, J. Physical colors in cultural heritage: Surface plasmons in glass. Comptes Rendus Phys. 2009, 10, 649–659. [Google Scholar] [CrossRef]
- Nath, N.; Chilkoti, A. Label-free biosensing by surface plasmon resonance of nanoparticles on glass: Optimization of nanoparticle size. Anal. Chem. 2004, 76, 5370–5378. [Google Scholar] [CrossRef] [PubMed]
- Srabionyan, V.V.; Vetchinnikov, M.P.; Rubanik, D.S.; Durymanov, V.A.; Viklenko, I.A.; Avakyan, L.A.; Bugaev, L.A. Local electric field enhancement in the vicinity of aggregates of Ag, Au, Rb containing nanoparticles in oxide glasses. J. Non-Cryst. Solids 2024, 631, 122927. [Google Scholar] [CrossRef]
- Piotrowski, P.; Buza, M.; Nowaczyński, R.; Kongsuwan, N.; Surma, H.B.; Osewski, P.; Gajc, M.; Ryba-Romanowski, W.; Hess, O.; Pawlak, D.A. Ultrafast photoluminescence and multiscale light amplification in nanoplasmonic cavity glass. Nat. Commun. 2024, 15, 3309. [Google Scholar] [CrossRef]
- Berneschi, S.; Righini, G.C.; Pelli, S. Towards a glass new world: The role of ion-exchange in modern technology. Appl. Sci. 2021, 11, 4610. [Google Scholar] [CrossRef]
- Shakhgildyan, G.; Avakyan, L.; Atroshchenko, G.; Vetchinnikov, M.; Zolikova, A.; Ignat’eva, E.; Sigaev, V. Ultra-broadband plasmon resonance in gold nanoparticles precipitated in ZnO-Al2O3-SiO2 glass. Ceramics 2024, 7, 562–578. [Google Scholar] [CrossRef]
- Stepanov, A.L.; Hole, D.E.; Townsend, P.D. Formation of silver nanoparticles in soda–lime silicate glass by ion implantation near room temperature. J. Non-Cryst. Solids 1999, 260, 65–74. [Google Scholar] [CrossRef]
- Wei, Y.; Ebendorff-Heidepriem, H.; Zhao, J. Recent advances in hybrid optical materials: Integrating nanoparticles within a glass matrix. Adv. Opt. Mater. 2019, 7, 1900702. [Google Scholar] [CrossRef]
- Jagannath, G.; Eraiah, B.; NagaKrishnakanth, K.; Rao, S.V. Linear and nonlinear optical properties of gold nanoparticles doped borate glasses. J. Non-Cryst. Solids 2018, 482, 160–169. [Google Scholar] [CrossRef]
- Heinz, M.; Srabionyan, V.V.; Avakyan, L.A.; Bugaev, A.L.; Skidanenko, A.V.; Kaptelinin, S.Y.; Bugaev, L.A. Formation of bimetallic gold-silver nanoparticles in glass by UV laser irradiation. J. Alloys Compd. 2018, 767, 1253–1263. [Google Scholar] [CrossRef]
- Shakhgildyan, G.; Avakyan, L.; Ziyatdinova, M.; Atroshchenko, G.; Presnyakova, N.; Vetchinnikov, M.; Sigaev, V. Tuning the plasmon resonance of gold nanoparticles in phase-separated glass via the local refractive index change. J. Non-Cryst. Solids 2021, 566, 120893. [Google Scholar] [CrossRef]
- Lipatiev, A.S.; Fedotov, S.S.; Lotarev, S.V.; Lipateva, T.O.; Shakhgildyan, G.Y.; Sigaev, V.N. Single-pulse laser-induced Ag nanoclustering in silver-doped glass for high-density 3D-rewritable optical data storage. ACS Appl. Nano Mater. 2022, 5, 6750–6756. [Google Scholar] [CrossRef]
- Zhang, Q.; Xia, Z.; Cheng, Y.B.; Gu, M. High-capacity optical long data memory based on enhanced Young’s modulus in nanoplasmonic hybrid glass composites. Nat. Commun. 2018, 9, 1183. [Google Scholar] [CrossRef]
- Bai, X.; Wu, L.; Magan, J.J.; Jennings, B.; Zhou, W.; Wang, S.; Wang, G. Dense and Nanoporous Glasses as Host Matrices to Grow Quantum Dots for Optical and Photonic Applications. Small 2025, 21, 2410564. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Qiu, J.; Liu, X. Nonlinear Photonics in Glasses Doped with Quantum Dots and Plasmonic Nanoparticles. Nanoscale 2025.
- Sonawane, R.S.; Naik, S.D.; Apte, S.K.; Kulkarni, M.V.; Kale, B.B. CdS/CdSSe quantum dots in glass matrix. Bull. Mater. Sci. 2008, 31, 495–499. [Google Scholar] [CrossRef][Green Version]
- Al-Douri, Y.; Khan, M.M.; Jennings, J.R. Synthesis and optical properties of II–VI semiconductor quantum dots: A review. J. Mater. Sci. Mater. Electron. 2023, 34, 993. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, Y.; Han, X.; Li, L.; Xu, X.; Qin, S.; Han, L. Luminescent CdS quantum dot-embedded glass for W-LED lighting and X-ray imaging. Ceram. Int. 2024, 50, 13155–13165. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Huang, F.; Hua, Y.; Tian, Y.; Zhang, X.; Xu, S. Multifunctional optical materials based on transparent inorganic glasses embedded with PbS QDs. J. Alloys Compd. 2023, 942, 169040. [Google Scholar] [CrossRef]
- Malyarevich, A.M.; Denisov, I.A.; Savitsky, V.G.; Yumashev, K.V.; Lipovskii, A.A. Glass doped with PbS quantum dots for passive Q switching of a 1.54-µm laser. Appl. Opt. 2000, 39, 4345–4347. [Google Scholar] [CrossRef]
- Duan, Y.; Gu, K.; Li, S.; Du, J.; Zhang, J. Recent Progress in CsPbX3 (X= Cl, Br, and I) Perovskite Quantum Dot Glasses: Synthesis, Matrix Optimization, and Photonic Application Potentials. Laser Photonics Rev. 2025, 19, 2402245. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, W.; Zhao, Z.; Wang, J.; Liu, C.; Deng, Z.; Han, J. Highly luminescent cesium lead halide perovskite nanocrystals stabilized in glasses for light-emitting applications. Adv. Opt. Mater. 2019, 7, 1801663. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Rainò, G.; Kovalenko, M.V.; Manna, L. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals. Nat. Mater. 2018, 17, 394–405. [Google Scholar] [CrossRef]
- Miao, C.; Chen, X.; Zeng, X.; Sun, D.; Fu, Y.; Xiong, Z.; Yu, L. Dual-emission and enhanced stable nanocomposites by encapsulating CsPbBr3 quantum dots in mesoporous GdF3: Eu3+ spheres for LED applications. J. Alloys Compd. 2025, 1022, 179795. [Google Scholar] [CrossRef]
- Kulebyakina, E.V.; Skorikov, M.L.; Kolobkova, E.V.; Kuznetsova, M.S.; Bataev, M.N.; Yakovlev, D.R.; Belykh, V.V. Temperature-dependent photoluminescence dynamics of CsPbBr 3 and CsPb (Cl, Br) 3 perovskite nanocrystals in a glass matrix. Phys. Rev. B 2024, 109, 235301. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, X.; Xia, M.; Zhang, X. Perovskite nanocrystal doped all-inorganic glass for X-ray scintillators. J. Mater. Chem. C 2021, 9, 5452–5459. [Google Scholar] [CrossRef]
- Li, X.; Deng, X.; Hong, J.; Lin, J.; Lv, J.; Yu, M.; Guan, X.; Du, S.; Yu, Y.; Chen, D. Color adjustable CsPbX3 (X= Cl, Br, I) perovskite quantum dots germanate glass. J. Lumin. 2024, 266, 120256. [Google Scholar] [CrossRef]
- Wiltzius, P.; Bates, F.S.; Dierker, S.B.; Wignall, G.D. Structure of porous Vycor glass. Phys. Rev. A 1987, 36, 2991. [Google Scholar] [CrossRef]
- Vasilevskaya, T.N.; Antropova, T.V. Small-angle X-ray scattering study of the structure of glassy nanoporous matrices. Phys. Solid State 2009, 51, 2537–2545. [Google Scholar] [CrossRef]
- Viter, R.; Geveluk, S.; Smyntyna, V.; Doycho, I.; Rysiakiewicz-Pasek, E.; Buk, J.; Kordás, K. Optical properties of nanoporous glass filled with TiO. Opt. Appl. 2012, 42, 307–313. [Google Scholar]
- Shakhgil’dyan, G.Y.; Mikhailov, A.A.; Lipat’eva, T.O.; Piyanzina, K.I.; Kolesnikov, E.A.; Chereuta, O.S.; Sigaev, V.N. Effect of heat treatment conditions on the properties of nanoporous glasses activated by gold nanoparticles. Glass Ceram. 2021, 77, 419–421. [Google Scholar] [CrossRef]
- Zhang, Q.; Qiao, Y.; Qian, B.; Dong, G.; Ruan, J.; Liu, X.; Zhou, Q.; Chen, Q.; Qiu, J.; Chen, D. Luminescence properties of the Eu-doped porous glass and spontaneous reduction of Eu3+ to Eu2+. J. Lumin. 2009, 129, 1393–1397. [Google Scholar] [CrossRef]
- Hu, Z.; Jiang, Y.; Zhou, F.; Chen, C.; He, J.; Zhan, Z.; Leng, Y. Nano-Confined Growth of Perovskite Quantum Dots in Transparent Nanoporous Glass for Luminescent Chemical Sensing. Adv. Opt. Mater. 2023, 11, 2202131. [Google Scholar] [CrossRef]
- Kieu, K.; Schneebeli, L.; Norwood, R.A.; Peyghambarian, N. Integrated liquid-core optical fibers for ultra-efficient nonlinear liquid photonics. Opt. Express 2012, 20, 8148–8154. [Google Scholar] [CrossRef] [PubMed]
- Lipatiev, A.S.; Fedotov, S.S.; Okhrimchuk, A.G.; Lotarev, S.V.; Vasetsky, A.M.; Stepko, A.A.; Sigaev, V.N. Multilevel data writing in nanoporous glass by a few femtosecond laser pulses. Appl. Opt. 2018, 57, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Shakhgil’dyan, G.Y.; Lipat’eva, T.O.; Gavrilova, N.N.; Sergeev, I.I.; Tyulagin, P.E.; Chereuta, O.S.; Sigaev, V.N. Silver-Activated Nanoporous Glass Substrates for Giant Raman Scattering Spectroscopy. Glass Ceram. 2022, 79, 205–209. [Google Scholar] [CrossRef]
- Li, B.; Li, Z.; Cooperstein, I.; Shan, W.; Wang, S.; Jiang, B.; He, J. Additive Manufacturing of Transparent Multi-Component Nanoporous Glasses. Adv. Sci. 2023, 10, 2305775. [Google Scholar] [CrossRef]
- Fujima, T.; Futakuchi, E.; Tomita, T.; Orai, Y.; Sunaoshi, T. Hierarchical nanoporous glass with antireflectivity and superhydrophilicity by one-pot etching. Langmuir 2014, 30, 14494–14497. [Google Scholar] [CrossRef]
- Ghosh, A.; Patra, C.R.; Mukherjee, P.; Sastry, M.; Kumar, R. Preparation and stabilization of gold nanoparticles formed by in situ reduction of aqueous chloroaurate ions within surface-modified mesoporous silica. Microporous Mesoporous Mater. 2003, 58, 201–211. [Google Scholar] [CrossRef]
- Baruah, R.; Dilshad, M.; Diegel, M.; Dellith, J.; Plentz, J.; Undisz, A.; Wächtler, M. Deposition of CdSe Nanocrystals in Highly Porous SiO2 Matrices—In Situ Growth vs. Infiltration Methods. Materials 2024, 17, 4379. [Google Scholar] [PubMed]
- Ojovan, M.I. Vitrification as a Key Solution for Immobilisation Within Nuclear Waste Management. Arab. J. Sci. Eng. 2025, 50, 3253–3261. [Google Scholar] [CrossRef]
- McCloy, J.S.; Riley, B.J.; Wilkins, M.C.D.; Evarts, J.S.; Bussey, J.; Vienna, J.D.; Bingham, P.A.; Gregg, D.J.; Ojovan, M.; Schuller, S.; et al. International perspectives on glass waste form development for low-level and intermediate-level radioactive waste. Mater. Today 2024, 80, 594–618. [Google Scholar] [CrossRef]
- Rose, P.B.; Woodward, D.I.; Ojovan, M.I.; Hyatt, N.C.; Lee, W.E. Crystallisation of a simulated borosilicate high-level waste glass produced on a full-scale vitrification line. J. Non-Cryst. Solids 2011, 357, 2989–3001. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Yudintsev, S.V. Glass, ceramic, and glass-crystalline matrices for HLW immobilisation. Open Ceram. 2023, 14, 100355. [Google Scholar] [CrossRef]

| Defect Type | Description | Cause | Occurrence | Impact |
|---|---|---|---|---|
| Striae/Cords | Thread-like streaks of differing refractive index in glass. Visually appear as veins or “cords” in the glass. Essentially regions of abruptly varying density in an otherwise uniform matrix. | Compositional inhomogeneity caused by incomplete mixing or localized evaporation during glass formation. | Found especially in optical glass if the melt is not well-stirred. Also occurs in other glass types with inadequate mixing. | Even slight striae can distort optical wavefronts, making high-end optics require striae-free glass. In less critical applications, may only cause cosmetic issues. |
| Swirls | Large-scale wavy patterns of refractive index variation. Similar to striae but broader and more diffuse with no sharp edges. | Result from convection currents that are frozen during the cooling process. | Historically seen in old window glass and large cast blocks. Modern float glass processes have largely eliminated these. | Can blur transmitted images or produce distortion (“lens” effects). Modern controlled cooling and flow processes minimize these effects. |
| Bubbles/Seeds | Gas-filled voids in glass. Seeds refer to small bubbles (<~1 mm) while bubbles refer to larger ones. | Result from gases released during melting (CO2 from carbonates, O2 from nitrates/sulfates) or air entrapment during insufficient melting. | Can occur in any glass type. More common when melting conditions are inadequate or when using certain raw materials. | In container and float glass, small seeds are usually cosmetic but large bubbles weaken products and can cause leaks or breakage. In optical glass, even tiny bubbles scatter light and must be tightly controlled. |
| Stones/Inclusions | Solid defects including unmelted raw batch grains, devitrification crystals, or bits of furnace refractory. | Often high-melting particles (e.g., quartz grain or alumina ceramic) that remained undissolved or re-crystallized out of the melt. | More common in commodity glasses (containers, float) due to large-scale production and recycled cullet use. | Act as stress concentrators—hard inclusions can initiate cracks under thermal or mechanical load. In flat glass, tiny stones or NiS inclusions can cause tempered glass to spontaneously shatter. |
| Unmelt/Cord (chemical) | A subtype of inclusion consisting of streaks of incompletely melted batch, often high-silica threads called cord when they extend in a line. | Essentially a glassy inclusion with different composition (e.g., silica-rich) than the bulk glass due to incomplete melting. | Seen in container glass and some optical glasses produced in pot melts. | Manifest as visible line defects. Chemically different cords can have thermal expansion mismatch, causing internal stresses. |
| Other Inhomogeneities | Include crizzle (sub-micron phase separation causing cloudiness) and other specialized defects. | Usually secondary effects of composition or furnace environment (e.g., sulfur deposits causing haze). Can result from improper cooling of certain glass compositions. | Generally rare and specific to certain glass compositions or processes. For instance, borosilicate glass can phase-separate if not cooled properly. | Can lead to opalescence, cloudiness, or surface haze. Manufacturers adjust compositions to avoid such effects or apply post-processing like fire-polishing to remove them. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakhgildyan, G.Y.; Ojovan, M.I. Inhomogeneities in Glass: From Defects to Functional Nanostructures. Encyclopedia 2025, 5, 136. https://doi.org/10.3390/encyclopedia5030136
Shakhgildyan GY, Ojovan MI. Inhomogeneities in Glass: From Defects to Functional Nanostructures. Encyclopedia. 2025; 5(3):136. https://doi.org/10.3390/encyclopedia5030136
Chicago/Turabian StyleShakhgildyan, Georgiy Yu., and Michael I. Ojovan. 2025. "Inhomogeneities in Glass: From Defects to Functional Nanostructures" Encyclopedia 5, no. 3: 136. https://doi.org/10.3390/encyclopedia5030136
APA StyleShakhgildyan, G. Y., & Ojovan, M. I. (2025). Inhomogeneities in Glass: From Defects to Functional Nanostructures. Encyclopedia, 5(3), 136. https://doi.org/10.3390/encyclopedia5030136








