Understanding the Gut-Heart Axis in Roemheld Syndrome: Mechanisms and Clinical Insights
Definition
1. Introduction
1.1. Literature Search Strategy
1.2. Medical Case History and Prevalence
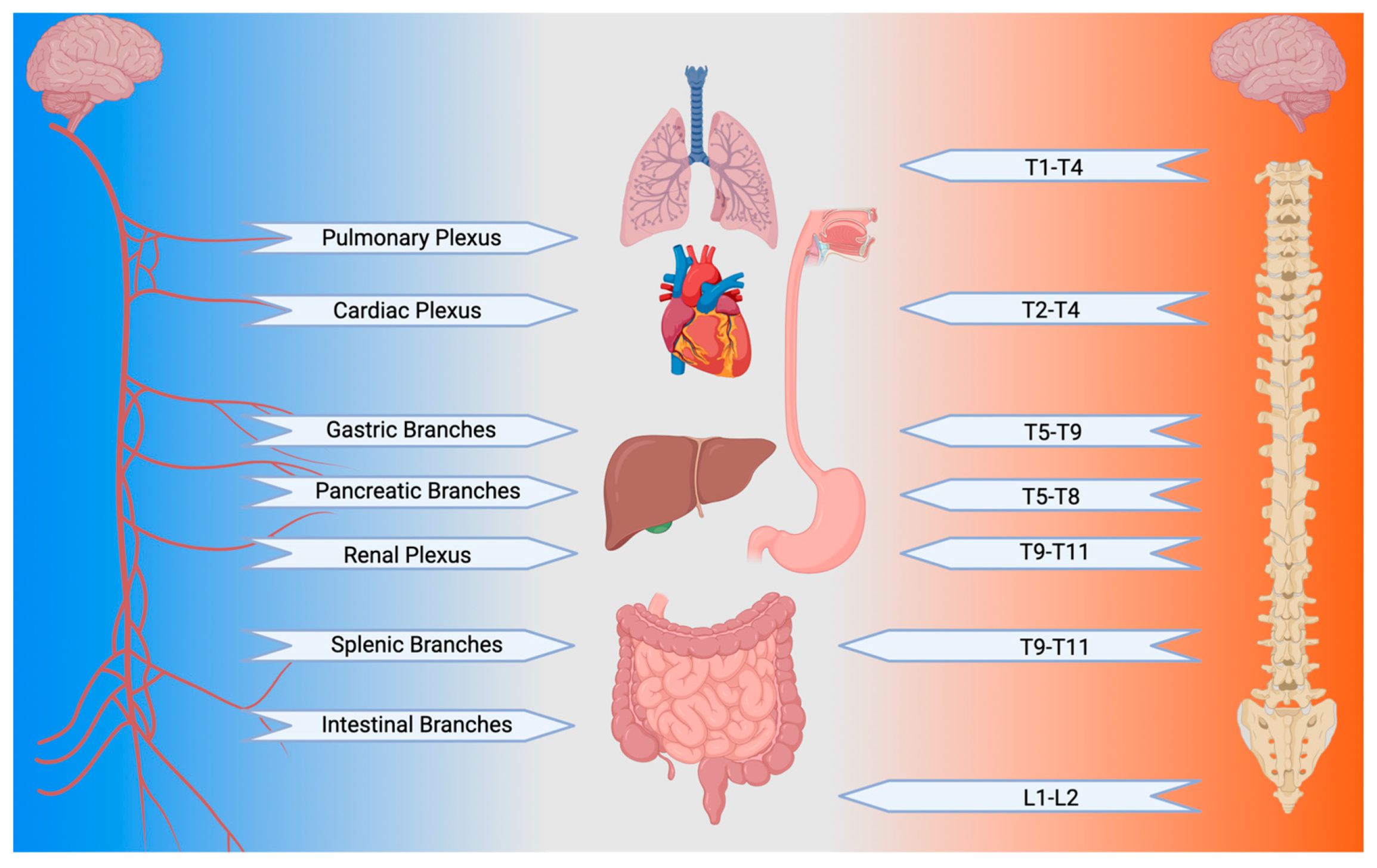
1.3. The Vagus Nerve: One Nerve to Rule Them All
1.4. The Gut as the Birthplace of GCS
1.5. Modulating the Gut Brain
1.6. Special Case: Hiatal Hernia Pathology as Causative for GCS
1.7. The Heart as the GCS Victim: A Fear-Amplification System
1.8. Cardiovascular Reflexes and GCS
1.8.1. The Baroreceptor Reflex
1.8.2. The Chemoreceptor Reflex
1.8.3. The Bainbridge and Bezoid-Jarsich Reflexes
1.8.4. The Valsalva Reflex
2. Gastrocardiac Syndrome as a Model System
2.1. Risk Factors
2.2. Symptoms and Their Roots
2.3. Causes
3. Treatment of Non-Surgical GCS: Multi-Pronged by Design
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bishop, L.F. Gastro-Enterology in the Practice of Cardiology. J. Am. Med. Assoc. 1939, 112, 33–36. [Google Scholar] [CrossRef]
- Khreshi, S.; Khraishi, R.; Pak, D.; Nguyen, T.; Assaassa, A.; Patel, R.B. Heartburn to Heart Skips: Gastric or Cardiac? Chest 2024, 166, A815–A816. [Google Scholar] [CrossRef]
- Natale, F.; Molinari, R.; Covino, S.; Alfieri, R.; Cimmino, G. The cardiac paradox of losing weight: A case of gastro-cardiac syndrome. Monaldi Arch. Chest Dis. 2022, 93. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, A.; Woodruff, R.; Murthy, A. A large hiatal hernia causing frequent premature ventricular contractions with bigeminy: A case report and review of literature. J. Cardiol. Cases 2023, 28, 36–39. [Google Scholar] [CrossRef]
- Noom, M.J.; Dunham, A.; DuCoin, C.G. Resolution of Roemheld Syndrome After Hiatal Hernia Repair and LINX Placement: Case Review. Cureus 2023, 15, e37429. [Google Scholar] [CrossRef]
- John, S.P.; Adabala, V.; Dhar, M. Successful Management of Roemheld Syndrome: A Diagnosis of Exclusion. Bali J. Anesthesiol. 2022, 6, 196–197. [Google Scholar] [CrossRef]
- Qureshi, K.; Naeem, N.; Saleem, S.; Chaudry, M.S.; Pasha, F. Recurrent Episodes of Paroxysmal Supraventricular Tachycardia Triggered by Dyspepsia: A Rare Case of Gastrocardiac Syndrome. Cureus 2021, 13, e17966. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Bath, A.; Ahmed, M.U.; Kalavakunta, J.K. Rare and unusual presentation of gastrocardiac syndrome. BMJ Case Rep. 2020, 13, e236910. [Google Scholar] [CrossRef]
- Saeed, M.; Bhandohal, J.S.; Visco, F.; Pekler, G.; Mushiyev, S. Gastrocardiac syndrome: A forgotten entity. Am. J. Emerg. Med. 2018, 36, 1525.e5–1525.e7. [Google Scholar] [CrossRef]
- Cerritelli, F.; Frasch, M.G.; Antonelli, M.C.; Viglione, C.; Vecchi, S.; Chiera, M.; Manzotti, A. A Review on the Vagus Nerve and Autonomic Nervous System During Fetal Development: Searching for Critical Windows. Front. Neurosci. 2021, 15, 721605. [Google Scholar] [CrossRef]
- Premchand, R.K.; Sharma, K.; Mittal, S.; Monteiro, R.; Dixit, S.; Libbus, I.; DiCarlo, L.A.; Ardell, J.L.; Rector, T.A.; Amurthur, B.; et al. Autonomic Regulation Therapy via Left or Right Cervical Vagus Nerve Stimulation in Patients with Chronic Heart Failure: Results of the ANTHEM-HF Trial. J. Card. Fail. 2014, 20, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Konstam, M.A.; Mann, D.L.; Udelson, J.J.E.; Ardell, J.L.; DeFerrari, G.M.; Cowie, M.R.; Klein, H.U.; Gregory, D.D.; Massaro, J.M.; Libbus, I.; et al. Advances in Our Clinical Understanding of Autonomic Regulation Therapy Using Vagal Nerve Stimulation in Patients Living with Heart Failure. Front. Physiol. 2022, 13, 857538. [Google Scholar] [CrossRef] [PubMed]
- Ostendorf, D.M.; Caldwell, A.E.; Creasy, S.A.; Pan, Z.; Lyden, K.; Bergouignan, A.; MacLean, P.S.; Wyatt, H.R.; Hill, J.O.; Melanson, E.L.; et al. Physical Activity Energy Expenditure and Total Daily Energy Expenditure in Successful Weight Loss Maintainers. Obesity 2019, 27, 496–504. [Google Scholar] [CrossRef]
- Flynn, W.; Vickerton, P. Anatomy, Abdomen and Pelvis: Abdominal Wall. In Stat Pearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551649/ (accessed on 6 September 2024).
- Debas, H.T.; Carvajal, S.H. Vagal regulation of acid secretion and gastrin release. Yale J. Biol. Med. 1994, 67, 145–151. [Google Scholar]
- Patel, K.S.; Thavamani, A. Physiology, Peristalsis. In Stat Pearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556137/ (accessed on 6 September 2024).
- Smith, T.K.; Gershon, M.D. CrossTalk proposal: 5-HT is necessary for peristalsis. J. Physiol. 2015, 593, 3225–3227. [Google Scholar] [CrossRef]
- Capilupi, M.J.; Kerath, S.M.; Becker, L.B. Vagus Nerve Stimulation and the Cardiovascular System. Cold Spring Harb. Perspect. Med. 2020, 10, a034173. [Google Scholar] [CrossRef]
- Linz, D.; Hohl, M.; Vollmar, J.; Ukena, C.; Mahfoud, F.; Böhm, M. Atrial fibrillation and gastroesophageal reflux disease: The cardiogastric interaction. EP Eur. 2017, 19, 16–20. [Google Scholar] [CrossRef]
- Michel, K.; Kuch, B.; Dengler, S.; Demir, I.E.; Zeller, F.; Schemann, M. How big is the little brain in the gut? Neuronal numbers in the enteric nervous system of mice, Guinea pig, and human. Neurogastroenterol. Motil. 2022, 34, e14440. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Roelofs, K. Freeze for action: Neurobiological mechanisms in animal and human freezing. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160206. [Google Scholar] [CrossRef]
- Schmidt, N.B.; Richey, J.A.; Zvolensky, M.J.; Maner, J.K. Exploring human freeze responses to a threat stressor. J. Behav. Ther. Exp. Psychiatry 2008, 39, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Fanselow, M.S. Fear and Anxiety Take a Double Hit From Vagal Nerve Stimulation. Biol. Psychiatry 2013, 73, 1043–1044. [Google Scholar] [CrossRef] [PubMed]
- Fukudo, S.; Nomura, T.; Hongo, M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut 1998, 42, 845. [Google Scholar] [CrossRef]
- Wang, D.; Meng, Q.; Leech, C.A.; Yepuri, N.; Zhang, L.; Holz, G.G.; Wang, C.; Cooney, R.N. α7 Nicotinic Acetylcholine Receptor Regulates the Function and Viability of L Cells. Endocrinology 2018, 159, 3132–3142. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, J.; Ren, X.; Song, Y.; Xu, J.; Xuan, S.; Jiang, X.; Kuang, Z.; Tang, Z. Vagus Nerve Stimulation Relives Irritable Bowel Syndrome and the Associated Depression via α7nAChR-mediated Anti-inflammatory Pathway. Neuroscience 2023, 530, 26–37. [Google Scholar] [CrossRef]
- Bonaz, B.; Sinniger, V.; Pellissier, S. Vagus Nerve Stimulation at the Interface of Brain–Gut Interactions. Cold Spring Harb. Perspect. Med. 2019, 9, a034199. [Google Scholar] [CrossRef]
- Canavan, C.; West, J.; Card, T. The epidemiology of irritable bowel syndrome. Clin. Epidemiol. 2014, 6, 71–80. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Black, C.J.; Teasdale, S.B.; Mikocka-Walus, A.; Keefer, L. Irritable bowel syndrome and mental health comorbidity—Approach to multidisciplinary management. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 582–596. [Google Scholar] [CrossRef]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28–32. [Google Scholar]
- Wang, T.; Dijk, L.; van Rijnaarts, I.; Hermes, G.D.A.; DeRoos, N.M.; Witteman, B.J.M.; DeWit, N.J.W.; Govers, C.; Smidt, H.; Zoetendal, E.G. Methanogen Levels Are Significantly Associated with Fecal Microbiota Composition and Alpha Diversity in Healthy Adults and Irritable Bowel Syndrome Patients. Microbiol. Spectr. 2022, 10, e01653-22. [Google Scholar] [CrossRef]
- Volokitin, M.; Song, A.; Peck, M.T.; Milani, S. Reduction and Resolution of a Hiatal Hernia Using Osteopathic Manipulative Treatment: A Case Report. Cureus 2022, 14, e26558. [Google Scholar] [CrossRef]
- Roy, R.R.; Sagar, S.; Bunch, T.J.; Aman, W.; Crusan, D.J.; Srivasthan, K.; Asirvatham, S.J.; Shen, W.K.; Jahangir, A. Hiatal Hernia Is Associated with an Increased Prevalence of Atrial Fibrillation in Young Patients. J. Atr. Fibrillation 2013, 6, 894. [Google Scholar] [CrossRef]
- Goodwin, M.L.; Nishimura, J.M.; D’Souza, D.M. Atypical and typical manifestations of the hiatal hernia. Ann. Laparosc. Endosc. Surg. 2021, 6. [Google Scholar] [CrossRef]
- Menon, S.; Trudgill, N. Risk factors in the aetiology of hiatus hernia. Eur. J. Gastroenterol. Hepatol. 2011, 23, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Prystowsky, E.N.; Gilge, J.L. Atrioventricular Conduction Physiology and Autonomic Influences. Card. Electrophysiol. Clin. 2021, 13, 585–598. [Google Scholar] [CrossRef]
- Shanks, J.; Pachen, M.; Chang, J.W.-H.; George, B.; Ramchandra, R. Cardiac Vagal Nerve Activity Increases During Exercise to Enhance Coronary Blood Flow. Circ. Res. 2023, 133, 559–571. [Google Scholar] [CrossRef]
- Moore, J.P.; Simpson, L.L.; Drinkhill, M.J. Differential contributions of cardiac, coronary and pulmonary artery vagal mechanoreceptors to reflex control of the circulation. J. Physiol. 2022, 600, 4069–4087. [Google Scholar] [CrossRef]
- Amasi-Hartoonian, N.; Sforzini, L.; Cattaneo, A.; Pariante, C.M. Cause or consequence? Understanding the role of cortisol in the increased inflammation observed in depression. Curr. Opin. Endocr. Metab. Res. 2022, 24, 100356. [Google Scholar] [CrossRef]
- Roux, C.; Kachenoura, N.; Raissuni, Z.; Mousseaux, E.; Young, J.; Graves, M.J.; Jublanc, C.; Cluzel, P.; Chanson, P.; Kamenický, P.; et al. Effects of cortisol on the heart: Characterization of myocardial involvement in cushing’s disease by longitudinal cardiac MRI T1 mapping. J. Magn. Reson. Imaging 2017, 45, 147–156. [Google Scholar] [CrossRef]
- Gottfried-Blackmore, A.; Habtezion, A.; Nguyen, L. Noninvasive vagal nerve stimulation for gastroenterology pain disorders. Pain Manag. 2021, 11, 89–96. [Google Scholar] [CrossRef]
- Armstrong, M.; Kerndt, C.C.; Moore, R.A. Physiology, Baroreceptors. In Stat Pearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538172/ (accessed on 12 September 2024).
- Hladky, S.B.; Barrand, M.A. Fluid and ion transfer across the blood–brain and blood–cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS 2016, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Kara, T.; Narkiewicz, K.; Somers, V.K. Chemoreflexes—Physiology and clinical implications. Acta Physiol. Scand. 2003, 177, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Miao, J.H.; Yetiskul, E.; Anokhin, A.; Majmundar, S.H. Physiology, Carbon Dioxide Retention. In Stat Pearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482456/ (accessed on 14 September 2024).
- Sur, M.; Hashmi, M.F. Alkalosis. In Stat Pearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545269/ (accessed on 17 September 2024).
- Pakkam, M.L.; Moore, M.J. Physiology, Bainbridge Reflex. In Stat Pearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541017/ (accessed on 17 September 2024).
- Lovelace, J.W.; Ma, J.; Yadav, S.; Chhabria, K.; Shen, H.; Pang, Z.; Qi, T.; Sehgal, R.; Zhang, Y.; Bali, T.; et al. Vagal sensory neurons mediate the Bezold–Jarisch reflex and induce syncope. Nature 2023, 623, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Feigl, E.O. Reflex parasympathetic coronary vasodilation elicited from cardiac receptors in the dog. Circ. Res. 2018, 37, 175–182. [Google Scholar] [CrossRef]
- Takahashi, N.; Imai, S.; Saito, F.; Suzuki, K.; Tanaka, H.; Kushiro, T.; Yagi, H.; Hirayama, A. Alcohol Produces Imbalance of Adrenal and Neuronal Sympathetic Activity in Patients with Alcohol-Induced Neurocardiogenic Syncope. Circ. J. 2008, 72, 979–985. [Google Scholar] [CrossRef]
- Holland, E.M.; Stouffer, G.A. Bezold-Jarisch Reflex. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100029. [Google Scholar] [CrossRef]
- Srivastav, S.; Jamil, R.T.; Zeltser, R. Valsalva Maneuver. In Stat Pearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537248/ (accessed on 17 September 2024).
- Parkman, H.P.; Sharkey, E.; McCallum, R.W.; Hasler, W.L.; Koch, K.L.; Sarosiek, I.; Abell, T.L.; Kuo, B.; Shulman, R.J.; Grover, M.; et al. Constipation in Patients with Symptoms of Gastroparesis: Analysis of Symptoms and Gastrointestinal Transit. Clin. Gastroenterol. Hepatol. 2022, 20, 546–558.e5. [Google Scholar] [CrossRef]
- Crudele, L.; Gadaleta, R.M.; Cariello, M.; Moschetta, A. Gut microbiota in the pathogenesis and therapeutic approaches of diabetes. eBioMedicine 2023, 97, 104821. [Google Scholar] [CrossRef] [PubMed]
- Aswath, G.S.; Foris, L.A.; Ashwath, A.K.; Patel, K. Diabetic Gastroparesis. In Stat Pearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430794/ (accessed on 18 September 2024).
- Waqar, S.H.B.; Rehan, A. Methane and Constipation-predominant Irritable Bowel Syndrome: Entwining Pillars of Emerging Neurogastroenterology. Cureus 2019, 11, e4764. [Google Scholar] [CrossRef]
- Kumpitsch, C.; Fischmeister, F.P.h.S.; Mahnert, A.; Lackner, S.; Wilding, M.; Sturm, C.; Springer, A.; Madl, T.; Holasek, S.; Högenauer, C.; et al. Reduced B12 uptake and increased gastrointestinal formate are associated with archaeome-mediated breath methane emission in humans. Microbiome 2021, 9, 193. [Google Scholar] [CrossRef]
- Leite, G.; Rezaie, A.; Mathur, R.; Barlow, G.M.; Rashid, M.; Hosseini, A.; Wang, J.; Parodi, G.; Villaneuva-Millan, M.J.; Sanchez, M.; et al. Defining Small Intestinal Bacterial Overgrowth by Culture and High Throughput Sequencing. Clin. Gastroenterol. Hepatol. 2024, 22, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pramanik, J.; Goyal, N.; Chauhan, D.; Sivamaruthi, B.S.; Prajapati, B.G.; Chaiyasut, C. Gut Microbiota in Anxiety and Depression: Unveiling the Relationships and Management Options. Pharmaceuticals 2023, 16, 565. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-J.; Hu, L.-Y.; Yeh, C.-M.; Hu, Y.-W.; Chen, P.-M.; Chen, T.-J.; Lu, T. Irritable Brain Caused by Irritable Bowel? A Nationwide Analysis for Irritable Bowel Syndrome and Risk of Bipolar Disorder. PLoS ONE 2015, 10, e0118209. [Google Scholar] [CrossRef]
- Zikos, T.A.; Kamal, A.N.; Neshatian, L.; Triadafilopoulos, G.; Clarke, J.O.; Nandwani, M.; Nguyen, L.A. High Prevalence of Slow Transit Constipation in Patients with Gastroparesis. J. Neurogastroenterol. Motil. 2019, 25, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Hahm, S. Gastroparesis: A Commonly Misdiagnosed Disease for Irritable Bowel Syndrome. Proc. UCLA Health 2020, 24. [Google Scholar]
- Watson, N.F.; Mystkowski, S.K. Aerophagia and gastroesophageal reflux disease in patients using continuous positive airway pressure: A preliminary observation. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep. Med. 2008, 4, 434–438. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Aerophagia and intestinal gas. Curr. Treat. Options Gastroenterol. 2002, 5, 259–265. [Google Scholar] [CrossRef]
- Novljan, U.; Pintar, T. Small Intestinal Bacterial Overgrowth in Patients with Roux-en-Y Gastric Bypass and One-Anastomosis Gastric Bypass. Obes. Surg. 2022, 32, 4102–4109. [Google Scholar] [CrossRef]
- Kitaghenda, F.K.; Hong, J.; Shao, Y.; Yao, L.; Zhu, X. The Prevalence of Small Intestinal Bacterial Overgrowth After Roux-en-Y Gastric Bypass (RYGB): A Systematic Review and Meta-analysis. Obes. Surg. 2024, 34, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, M.; Rezaeianzadeh, R.; Kezouh, A.; Etminan, M. Risk of Gastrointestinal Adverse Events Associated with Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss. JAMA 2023, 330, 1795–1797. [Google Scholar] [CrossRef]
- Johannesson, E.; Ringström, G.; Abrahamsson, H.; Sadik, R. Intervention to increase physical activity in irritable bowel syndrome shows long-term positive effects. World J. Gastroenterol. 2015, 21, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Noetel, M.; Sanders, T.; Gallardo-Gómez, D.; Taylor, P.; Cruz, B.d.P.; Van den Hoek, D.; Smith, J.J.; Mahoney, J.; Spathis, J.; Moresi, M.; et al. Effect of exercise for depression: Systematic review and network meta-analysis of randomised controlled trials. BMJ 2024, 384, e075847. [Google Scholar] [CrossRef]
- Stonerock, G.L.; Gupta, R.P.; Blumenthal, J.A. Is exercise a viable therapy for anxiety? Systematic review of recent literature and critical analysis. Prog. Cardiovasc. Dis. 2024, 83, 97–115. [Google Scholar] [CrossRef]
- Nanayakkara, W.S.; Skidmore, P.M.; O’Brien, L.; Wilkinson, T.J.; Gearry, R.B. Efficacy of the low FODMAP diet for treating irritable bowel syndrome: The evidence to date. Clin. Exp. Gastroenterol. 2016, 9, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Giacomelli, L.; Togni, S.; Francheschi, F.; Eggenhoffner, R.; Zuccarini, M.C.; Belcaro, G. Oral administration of a lecithin-based delivery form of boswellic acids (Casperome®) for the prevention of symptoms of irritable bowel syndrome: A randomized clinical study. Minerva Gastroenterol. Dietol. 2019, 65, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Shahbandeh, M. Estimated Value of Probiotics Market Worldwide from 2022 to 2027. In Statista; Statista: Hamburg, Germany, 2023; Available online: https://www.statista.com/statistics/821259/global-probioticsl-market-value/#:~:text=Global%20probiotics%20market%20value%202022%2D2027&text=The%20global%20market%20for%20probiotics,85%20billion%20dollars%20by%202027 (accessed on 20 September 2024).
- Amagase, H. Current Marketplace for Probiotics: A Japanese Perspective. Clin. Infect. Dis. 2008, 46, S73–S75. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, S.K.; Reyaz, I.; Anam, H.; Ijaz, O.; Attique, I.; Shahzad, Z.; Saleem, F. Effectiveness of Rifaximin on the Outcomes of Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cureus 2023, 15, e44807. [Google Scholar] [CrossRef]
- Redondo-Cuevas, L.; Belloch, L.; Martín-Carbonell, V.; Nicolás, A.; Alexandra, I.; Sanchis, L.; Ynfante, M.; Colmenares, M.; Mora, M.; Molés, J.R.; et al. Do Herbal Supplements and Probiotics Complement Antibiotics and Diet in the Management of SIBO? A Randomized Clinical Trial. Nutrients 2024, 16, 1083. [Google Scholar] [CrossRef]
- Isola, S.; Hussain, A.; Dua, A.; Singh, K.; Adams, N. Metoclopramide. In Stat Pearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519517/ (accessed on 20 September 2024).
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2022, 51, D1373–D1380. [Google Scholar] [CrossRef]

| Case | Year | Author | Age/Sex | Symptoms | History | Cause | Treatment | Resolved? | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2024 | Khreshi | 53/M | Bradycardia, GERD | T2D, gout | Vagal tone | PPI | No | [2] |
| 2 | 2023 | Natale | 40/M | Syncope, bradycardia | None | Intragastric balloon | Excretion | Yes | [3] |
| 3 | 2023 | Bhandari | 57/F | Chest pain, ventricular bigeminy, bradycardia, mitral regurgitation | Takotsubo cardiomyopathy, hiatal hernia, hypertension, hyperlipidemia | Hiatal hernia | Surgery | Yes | [4] |
| 4 | 2023 | Noom | 60/M | SVT with PVCs, hypertensive urgency, tachycardia | Esophageal stricture, hiatal hernia, GERD, arrythmias | Hiatal hernia | Surgery | Yes | [5] |
| 5 | 2022 | John | 65/M | Abdomen pain, dyspnea, tachycardia | None Listed | Stomach compression | Surgery | Yes | [6] |
| 6 | 2021 | Qureshi | 54/F | Tachycardia, bloating, distention, nausea | Hypertension, dyspepsia, PSVT | Dyspepsia | Lifestyle change, omeprezole | Yes | [7] |
| 7 | 2020 | Mehta | 62/F | Palpitations, PVC, GERD | Obesity, hiatal hernia, dyslipidemia | Hiatal hernia | Surgery | Yes | [8] |
| 8 | 2018 | Saeed | 75/F | Dizziness, gut distention, bradycardia | Hypertension, COPD, constipation, dementia | Hiatal Hernia | Nasogastric tube, pacemaker | Yes | [9] |
| Location | Symptom |
|---|---|
| Chest | Tachycardia |
| Bradycardia | |
| Arrythmia | |
| PVC | |
| Dyspnea | |
| Abdomen | Bloating |
| GERD | |
| Fullness | |
| Dyspepsia | |
| Vomiting | |
| Pain | |
| Constipation | |
| Meteorism | |
| Psychological | Anxiety |
| Panic | |
| Depression | |
| Insomnia | |
| Dizziness | |
| Nausea |
| Drug | PubChem ID | Indication | Class | Side Effect | Ref. |
|---|---|---|---|---|---|
| Activated charcoal | 481108125 | Gas | Inert Substance | Intestinal blockage, lung ingress | - |
| Pancrelipase | - | Dyspepsia | Enzyme | Constipation, nausea | - |
| Protease | - | Dyspepsia | Enzyme | Diarrhea, nausea | - |
| Magnesium | Various | Constipation | Osmotic laxative | Diarrhea | - |
| Sennosides | 656822 | Constipation | Stimulant laxative | Diarrhea, stomach cramps | - |
| Bromopride | 3016754 | Gastroparesis | Dopamine antagonist | Drowsiness, fatigue, dry mouth, diarrhea or constipation | [78,79] |
| Metoclopramide | 4168 | Gastroparesis | Dopamine antagonist | Drowsiness, kidney problems (in elderly), fatigue, dry mouth, diarrhea or constipation | [78] |
| Levosulpiride | 688272 | Gastroparesis, anxiety | Dopamine antagonist | Headache, fatigue, altered heart rate | [79] |
| Prucalopride | 3052762 | Constipation | Dopamine antagonist | Stomach pain, diarrhea, dizziness, suicidal thoughts | [79] |
| Domperidone | 3151 | Vomiting, constipation | Dopamine antagonist | Edema in feet, fatigue, hot flashes, cardiac QT prolongation | [79] |
| Cinitapride | 68867 | Constipation, gastroparesis | 5-HT Prokinetic | Headache, dizziness, nausea, vomiting, hypertension if aspirin is taken | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathis, B.J.; Suzuki, R.; Kuroda, Y.; Kato, H.; Hiramatsu, Y. Understanding the Gut-Heart Axis in Roemheld Syndrome: Mechanisms and Clinical Insights. Encyclopedia 2024, 4, 1721-1738. https://doi.org/10.3390/encyclopedia4040113
Mathis BJ, Suzuki R, Kuroda Y, Kato H, Hiramatsu Y. Understanding the Gut-Heart Axis in Roemheld Syndrome: Mechanisms and Clinical Insights. Encyclopedia. 2024; 4(4):1721-1738. https://doi.org/10.3390/encyclopedia4040113
Chicago/Turabian StyleMathis, Bryan J., Ryuji Suzuki, Yukihito Kuroda, Hideyuki Kato, and Yuji Hiramatsu. 2024. "Understanding the Gut-Heart Axis in Roemheld Syndrome: Mechanisms and Clinical Insights" Encyclopedia 4, no. 4: 1721-1738. https://doi.org/10.3390/encyclopedia4040113
APA StyleMathis, B. J., Suzuki, R., Kuroda, Y., Kato, H., & Hiramatsu, Y. (2024). Understanding the Gut-Heart Axis in Roemheld Syndrome: Mechanisms and Clinical Insights. Encyclopedia, 4(4), 1721-1738. https://doi.org/10.3390/encyclopedia4040113







