Abstract
In the last few years, the world has experienced the impacts of climate change, such as elevated mean annual temperature, extreme weather events, drought, etc. Among living organisms, perennial plant species are the ones mostly exposed to climate change impacts, as they may experience different extreme events within the same year, such as flooding during some periods and drought in summer months, extremely low temperatures in winter but excessively high temperatures in summer, etc. Climate change affects a range of physiological functions of temperate fruit and nut tree species, such as their phenophases, bud dormancy release and vernalization, pollination and fruit set, fruit growth and quality, as well as bud sprouting and growth initiation. Besides these, the impact of climate change on pests, diseases, and weeds may generate significant negative interactions with tree physiology, threatening food production, food safety, and human welfare. In the present manuscript, a general aspect of climate change impacts on fruits’ and nut trees’ physiological functions is described and commented on.
1. Introduction
Climate change or, probably by now, climate crisis’s effects have been well documented in the last few years of human history [1]. According to NASA, climate change is defined as “a long-term change in the average weather patterns that have come to define Earth’s local, regional and global climates, having a broad range of observed effects that are synonymous with the term” [2]. According to the United Nations Framework Convention on Climate Change (UNFCCC), it is defined as “a change that is attributed directly or indirectly to human activity which alters the composition of the global atmosphere and that is in addition to natural climate variability observed over comparable periods” [3,4]. Even if the earth’s climate seemed quite stable during the pre-industrial period, changes have been taking place, mainly due to natural causes such as volcanic eruptions, solar storms, orbital activity, etc. [5,6,7]. After the industrial period, though, climate changes became mostly driven by anthropogenic activities, which, among others, include fossil fuel burn, vehicular and industrial emissions, land use change, and forestry, especially deforestation and degradation [8,9,10,11,12]. Such activities emit Greenhouse gases (GHGs), which can absorb infrared radiation and trap heat in the atmosphere [5], leading to global warming. The major GHGs are carbon dioxide, methane, nitrous oxide, and ozone, with CO2 being responsible for 70% of the potential of raising the earth’s temperature [8,9,12,13]. Among others, the receding of the arctic cycle diminishes its ability to reflect the sun’s irradiance, thereby cooling the earth, keeping the sun’s heat energy in the earth’s environment, contributing further to global warming [9].
Without implementing any significant mitigation measures (carbon trading seems a solution, but more drastic and direct measures need to be taken), the global temperature is likely to climb by 1.5 °C in the near future (between 2030 and 2052) [12,14,15,16,17], while an increase of approximately 1.4–5.8 °C till the end of the twenty-first century is a potential scenario, too [8,16]. Some authors indicate that the planet’s temperature has already risen by 0.6 °C [8], while others report an increase of 1.5 °C in the Mediterranean basin compared to the pre-industrial levels [18].
Climate change is expected to cause a series of phenomena with variable degrees of hazards other than the increase in temperature, such as erratic rainfall patterns and an increase in the frequency of extreme events such as heat or cold waves, frost days, droughts, floods, etc., even in the same area within the same year [3,8]. In fact, in 2023, Greece experienced two giga-forest fires and one extreme flood event, covering a vast area in water for months (Figure 1). The consequences of such disastrous phenomena are economic losses, social inequality, and ecological destruction which, all together, threaten human welfare.

Figure 1.
A giga-forest fire in “Dadia Forest” (northern Greece) in the summer of 2023 (above, left) and a flood event in Thessaly prefecture (central Greece) in the autumn of the same year (above, right). The remains of an olive tree trunk the spring after the summer fire. (The photo on the right above is courtesy of Mr Bartzialis Dimitrios; the rest of the photos are from personal archives).
In climate change research, vulnerability is referred to as “the degree to which a system is susceptible to and unable to cope with adverse effects of climate change variability and extremes” [19]. Among the sectors most vulnerable to climate change is agriculture, which has faced a lot of challenges in recent years [20]. As climate plays a vital role in defining the geographic distribution of the various plant species [21], climate change has impacted agricultural production all over the world [22]. This is mainly due to changes in the mean as well as the minimum and maximum temperatures, in rainfall pattern and height, as well as in newly presented biotic threats (pests and microorganisms) [16] threatening plants’ adaptability and survival. Recently, the term “winter weather whiplash” has been adopted by the scientific community to indicate the range of extreme as well as rapid shifts in weather conditions (from hot to cold, from drought to extreme rainfall, and vice versa) which may induce severe damages to all living organisms [12]. Unlike other organisms, plants are the most vulnerable to extreme climate events, as they are unable to escape from the stress factor by moving or migrating to the most favorable environments [16]. There are differences even among plant species, as perennials are experiencing multiple stress factors during their lifespan, either separately (even in the same year) or at the same time. On the other hand, farmers of annual crops have, to some extent, the power to overcome the negative impacts of climate change by sowing or transplanting either earlier or later. In any case, though, as global temperatures continue to increase, some crops are expected to face a yield decrease ranging from 30 to 80% by the end of this century, the extent of which depends on the pace of temperature increase [23].
Nonetheless, some areas may probably benefit, to a small extent, from climate change while others will not, but from a global perspective, the earth’s environment will face a great challenge. Any changes in the climatic suitability of an area for specific crops to grow and produce suggest that there will be no single “loser” or “winner” as a lot is expected to change regarding fruit tree production.
The objective of the present review focuses on presenting the possible effects of climate change on the physiological functions of temperate fruit trees, productivity, and fruit quality, as well as on both abiotic and abiotic threats.
2. Effects of Climate Change on Tree Phenology—Physiology
Fruit trees, as perennials, are annually experiencing differences in climate patterns in the microclimate of the area they are grown in. One of the best indicators to investigate the impact of climate change on fruit trees is its effect on tree phenology [16,24]. Phenology is the study of the seasonal appearance and timing of the growth stages of a plant during the annual life cycle [3,24]. Within the frame of phenology is the study of the effects of climatic changes on bud sprout, leaf and flower appearance, pollination and fertilization, fruit growth and maturation, dispersal of seeds, and leaf fall (where applicable, i.e., in deciduous plants). For plants, the seasonal timing of all these growth stages is critical, as it dictates their potential for survival and reproduction while, on the other hand, humanity must ensure enough food for the ever-growing earth’s population and preserve food security for all [24].
Physiological functions such as photosynthetic activity, respiration, transpiration, membrane stability, enzyme activity and protein coagulation, DNA stability, as well as plant growth regulators and secondary metabolites production are responsible for the proper growth and development of all plants [8,9,25].
Within the general frame of plant growth, one could say that within the range of 0–40 °C, plants’ physiological processes may function well (regarding temperate, sub-tropical and tropical plant species) [8], although, for each specific species, this range becomes narrower [9]. The optimum temperature for plant growth and development depends on many factors, such as the species and cultivar, the duration of exposure to extreme events, the age of the plant, as well as the stage of development, etc.
It must be noted here, though, that the severity of damages caused by elevated temperatures in plant species strongly depends on the phenological stage of the plant at that specific time [12] as well as on the conditions prevailing before and/or after the extreme incidence.
2.1. Effect of Climate Change on Bud Dormancy
Plant phenology is strongly connected with air temperature [21] as well as photoperiod. Since photoperiod is not expected to change due to climate change, temperature seems to play a crucial role in influencing sequential plant growth stages as well as the geographical distribution of fruit crops [21].
All plants show advanced timing of phenophases, which is mainly observed in spring, while, at the same time, a late cessation of shoot growth occurs in autumn [24].
Temperate deciduous fruit crops complete their yearly cycle by dropping their leaves in autumn when the photoperiod changes and temperatures gradually decrease [4,8]. To survive the forthcoming low temperatures, temperate fruit trees fall into a kind of cessation, which is called dormancy [15,26,27]. Dormancy is the physiological state of the visual suspension of a plant’s metabolic activity and occurs both in vegetative as well as in floral buds [4,26,27]. During this period, trees can tolerate freezing temperatures occurring in their natural environment [17,27]. Trees gradually enter a dormancy state by late autumn and early winter, and then re-growth begins as soon as the spring temperature increases enough, only after buds have fulfilled their chilling requirements [26,27]. Bud dormancy has been categorized into three distinct phases: para-dormancy, endo-dormancy, and eco-dormancy [4,17,28]. Para-dormancy is growth suppression due to the inhibiting action of factors outside of the affected part (i.e., apical dominance) [4,17]. Endo-dormancy refers to the inhibition of bud sprout due to endogenous factors (within the bud itself) inhibiting bud outgrowth even if growth conditions are favorable. Eco-dormancy, on the other hand, is attributed to unfavorable-for-growth weather conditions [4], and, sometimes, the period of endo-dormancy may overlap that of eco-dormancy [29].
For the buds to be released from endo-dormancy, their chilling requirements must be fulfilled each year [15,17,30], while right after, they must be exposed to enough heat to sprout (forcing) [21,31]. Once both the chill and heat requirements are fulfilled, endogenous hormone levels change (auxin, gibberellins, and cytokinins increase while abscisic acid decreases; the overall oxidation status of the bud changes; enzyme activities are altered; carbohydrate metabolism and nutrient levels within the buds are altered, etc.), and bud burst and subsequent bloom occur [29]. These chilling requirements are specific for each species and cultivar and must be known upon the selection of a cultivar to be grown in a specific area [15,26]. For the tree to thrive in this specific area, buds should be exposed to enough chilling hours to break dormancy and later on to enough heat to sprout, while the growing period should be long enough to ensure fruit ripeness [26]. Various models have been used to calculate the chilling requirements of a specific cultivar (the Chilling Hours Model, the Utah Model, the Positive Utah Model, the Dynamic Model, etc.) [15,26,30,32].
In the era of climate change, there is a risk in some areas of the world that the chilling requirements of buds (either flower or vegetative buds) will not be fulfilled, leading to erratic flowering or no flowering at all, if the chilling accumulation falls below a threshold limit [10,28] prolonged bud burst, bud drop, bad pollination, elongated fruit with protruding tips (in peach) [33], yield reduction, and economic losses [3,33]. Eventually, this will lead to the replacement of the vulnerable cultivars with new ones with low chilling requirements, something that is already taking place, although a long time is needed to breed new fruit tree cultivars with all of the desirable characteristics [3]. Nonetheless, even for such cultivars, there is the risk of early frost damage, as they might bloom early due to “false spring” conditions and be exposed to devastating low temperatures for the flowers [33]. Therefore, the selection of suitable cultivars for a specific area is crucial for achieving sustainable production levels throughout the lifespan of the cultivar [26,30].
As already stated above, apart from the fulfillment of chilling requirements, buds also need several growing degree hours (GDH—hourly mean temperatures minus 4.5 °C) [34] to leave eco-dormancy. There are cases where the chilling requirements were fulfilled and the days followed were characterized by temperatures high enough for the advancing of flowering [3] compared to the historical flowering time in the specific area [10,21,26], exposing sprouts and flowers to possible early spring frosts [3]. This is mostly true for early flowering cultivars, which have been found to change their phenology of flowering more than the later-flowering cultivars [10]. An advancement in spring phenology by 2 to 14 days has been recorded in some areas [3], while an increase in atmospheric CO2 also seems to promote early flowering in some species [8]. Nevertheless, there have been reports where the slow accumulation of chilling hours has resulted in delayed vegetative and flower bud sprouting, thus delaying spring phenology [21]. This could be partly beneficial in avoiding spring frosts, but on the other hand, it may shorten the growing period or may generate problems in effective pollination. A similar reduction of the growing period may be caused by prolonged chill temperatures during the warm season in spring, which delays bud opening and flowering, with a subsequent fruit set delay and a shorter period for the fruit to grow [12]. For an area to be considered suitable for the cultivation of a species, it is not only necessary for the fruit tree to avoid damage to its flowers but to allow all the processes thereafter to be completed properly to reach full fruit ripeness [21].
At the same time, many cultivars of important species (apple, pear, walnut, etc.) require pollinizers. It is evident by now that the partial fulfillment of chilling requirements of either the main cultivar or the pollinizer leads to bad pollination and poor fruit set due to a lack of bloom synchronization in areas where, during the last decades, these two cultivars were effectively cross-pollinated [33]. In this sense, the previous successful combination of the main cultivar and pollinizer has become useless by now due to climate change. Furthermore, especially in wind-pollinated species such as walnut, pistachio, hazelnut, etc., a sudden rainfall or increased air temperature at the time of pollen release may impose severe problems in the process of successful pollination, as pollen may be depleted by rain or stigma and pollen may dry out due to elevated temperatures, resulting in poor pollination and fruit set. In the same sense, low temperatures may also affect pollination, either due to pollen or ovule necrosis or due to delayed ovule fertilization as a result of slow pollen tube growth.
It is not the negative effect of the partial fulfillment of chilling requirements only on dormancy breaking that poses severe problems in fruit production, but also its effects on vernalization. Some fruit trees, such as kiwifruit, need winter chilling to both break bud dormancy as well as to complete bud differentiation. A major reduction of flowering in the Hayward kiwifruit cultivar was noticed after a relatively mild winter some years ago in Greece, leading to a devastating reduction in yield. The olive tree, the iconic tree of the Mediterranean basin, is another example of the negative impacts of climate change on tree physiology. In recent years, some areas in Spain as well as in Italy and Greece have experienced quite mild winter conditions (the temperature did not fall to the usual levels), leading to partial floral bud differentiation (completion of reproductive organs) and, therefore, extremely low olive fruit production (approximately 10–40% of the usual yields) (Figure 2). This is of utmost importance, especially when one takes into account that the olive tree thrives in Mediterranean-type climates, characterized by mild winters and hot summers. Nonetheless, climate change has driven, even this well-adapted tree species, to low production due to the incomplete fulfillment of winter chill requirements for floral bud reproductive organ completion (needing a mean of 1500 h below 13–16 °C). One must not forget that the Mediterranean region is considered a climate change hotspot where the impact is expected to be quite severe, making any fruit crop species cultivated there quite vulnerable to the forthcoming new climatic conditions [29,31].

Figure 2.
Olive staminate flower on the left—the pistil is missing as the red arrow indicates (a result of a mild winter) and a hermaphrodite flower with a well-developed pistil on the right, indicated by the right arrow (from personal archive).
2.2. Impact of Climate Change on Pollination
Many fruit trees require cross-pollination to bear a substantial yield. In some of them, pollen is transferred by bees or other insects, while others are wind-pollinated. Climate change may affect pollination in both categories since non-optimal temperatures for bee flying during tree flowering will lead to reduced pollination and, therefore, a lower fruit set and yield [3], while reduced nectar production can minimize bees’ flower visitation [35]. On the other hand, strong rainfalls during the flowering period inhibit bee flying, wash off pollen, and, therefore, diminish fruit set [36]. In recent years, extremely high temperatures during the flowering of the olive tree have led to reduced fruit set in the Mediterranean basin due to pollen fertility loss and/or stigma desiccation. Similarly, in papaya flowers, stigma and stamen sterility are observed under high temperatures, leading, eventually, to flower drops as well as sex changes in female and hermaphrodite flowers [3]. An insufficient winter chill influences the flowering time, which is particularly critical for trees such as walnuts and pistachios that depend on male and female flowering synchronization to ensure pollination and a normal yield. High temperatures during differentiation or an insufficient chilling amount during the winter may also lead to abnormal flower formation (for example, in cherry trees), with subsequent poor fruit set and low yields [12].
Furthermore, incomplete pollination may lead to either extended fruit drop (pome and stone fruits) and/or a small fruit size (kiwifruit). On the other hand, in tropical and subtropical species, when the temperature during flowering is unusually low, excessive flower drop is observed, and this is mostly encountered in mango, guava, and litchi as well as in other fruit tree species [3,8].
Climate change surely has an impact not only on plants but also on every living organism. Among them, queen bumble bees (Bombus sp.) overwinter underground and emerge in the spring as soon as the temperatures are favorable enough to start the development of new colonies. Bumble bees are very effective pollinators for many fruit tree species needing cross-pollination, and the synchronization of insect emergence and flowering time is crucial. In some areas, though, as air temperature rises faster than soil temperature, the flowering of trees is advanced compared to bumble bees’ emergence, leading to poor pollination and, therefore, lower fruit set [10].
2.3. Impact of Climate Change on Fruit Growth and Quality
A temperature increase of 0.7–1.0 °C is expected to affect photosynthesis and dark respiration, leading to changes in fruit quality indexes. As temperature rises during the growing period, the photosynthetic apparatus is severely affected in multiple ways. There may be stomatal as well as non-stomatal limitations of photosynthesis, leading to a reduced production of assimilates [37]. The most common reaction of plants to increased temperature (above the upper threshold limit), which, in most cases, is accompanied by water shortage as well (due to increased evaporative demand), is the closure of stomata. A direct effect of this closure, apart from restriction in water losses, is the reduction of CO2 entering the sub-stomatal areas. Under a continuous high-temperature exposure, the RuBisCO (Ribulose Bisphosphate Carboxylase Oxygenase) enzyme activity (the enzyme primarily responsible for carbon dioxide fixation and carbohydrate synthesis) declines, so the production of carbohydrates diminishes [37]. At the same time, photosystem II, the key determinant of electron transport during the photochemical reaction of photosynthesis, exhibits severe malfunctions, leading to the formation of reactive oxygen species (ROS) which may severely affect the physiological and metabolic processes by damaging the lipids, proteins, and nucleic acids [16,38].
As night temperature increases, photosynthates may be used to support shoot growth against fruit quality. Generally, the temperature rise has a significant impact on the relationship between primary and secondary metabolites [12]. Negative impacts of climate change on fruit quality have been documented regarding nutritional, sensorial, as well as nutraceutical properties in pomegranate, citrus, fig, apple, etc., while, in strawberry, higher night temperatures result in higher antioxidant capacity and flavonoid content, thus improving the nutraceutical properties of the fruit [23]. The main effects have been determined concerning the carbohydrate, flavonoid, protein, carotenoid, chlorophyll, and ascorbic acid contents of the fruits, the mineral nutrient concentration, the volatile constituents, as well as the total antioxidant capacity [23]. While in many cases climate change had a negative impact on all the aforementioned fruit quality parameters, there were also cases where positive effects were found [23]. On the other hand, though, in some cases, a rise in secondary metabolites may be positive, improving the functional properties of a fruit, without excluding a possible reduction in crop quality if the changes in secondary metabolites are related to off-flavors or food toxicity [12]. One must expect that the proper selection of species and cultivars in a specific region will eventually lead to improved fruit characteristics compared to those of old cultivars that are no longer suited for cultivation in that specific area.
Both temperate as well as tropical and sub-tropical tree species may experience heat stress during the summer months under a changing environment, where temperature rises and rainfall diminishes [6]. Fruit growth in many species is quite susceptible to water deprivation. The result of water shortage during the period of fast fruit growth is fruit drops, a small fruit weight, and changes in fruit organoleptic properties as well as nutraceuticals, which may, on the other hand, be accompanied by a higher phenolic compound content, which have beneficial effects on human health.
High solar irradiance may lead to fruit skin sunburns (apple sun scald), thus diminishing the quality of the fruit and making it susceptible to secondary pathogen attacks, while at the same time shortening its post-harvest life [22,23]. Among the factors that increase the propensity for sunburn are the high light intensity, the elevated air temperature, the heavy pruning (exposing extended fruit skin to solar irradiance), water stress, etc. At the same time, elevated temperatures in summer, when the early stages of bud differentiation take place, can lead to abnormal flower buds the following year, giving rise to commercially unacceptable fruits (double or even triple cherries, abnormal peach fruits, etc.) (Figure 3). High temperatures have also been found to induce spongy tissue in mangoes, even though the fruit is grown in hot climates [6].

Figure 3.
Abnormal (double) cherry fruit (double fruitlets in a single flower on the left and double mature fruit on the right) due to elevated temperatures during the previous summer when the bud differentiation was taking place (from personal archive).
Apart from the rising summer temperatures that may damage production (quantitatively and qualitatively), out-of-season rainfalls may also have a negative impact on fruit quality. Rainfalls at the later stages of fruit development (especially in stone fruits) may deteriorate fruit quality by either softening the fruit pulp or inducing fruit cracking (in cherries and pomegranate) (Figure 4) or skin pitting (citrus) [3]. At the early stages of fruitlet development, rainfall may have a beneficial effect, since it can support nutrient uptake from the soil to support the growing fruit and shoot and alleviate possible high temperatures during that time. On the other hand, rainfall followed by high temperatures may induce pest infestations (Mediterranean fruit fly, olive fruit fly, etc.) and/or disease outbreaks [6].

Figure 4.
Cherry fruit cracking due to rainfall at a fully ripe stage shortly before harvest, on the left, and orange fruit split due to irregular irrigation after heavy rainfall (from personal archive).
Climate change in some areas (especially those characterized as hotspots, such as the Mediterranean basin) is expected to increase drought periods, which may affect fruit or nut quality characteristics. In almonds, drought stress decreased the oleic-to-linoleic ratio while a sustained deficit irrigation increased the concentrations of organic acids, sugars, monounsaturated and polyunsaturated fatty acids, as well as some phenolic compounds in the skin [12]. In olive trees, total phenols in the oil have been found to change with the degree of drought stress while high temperatures seem to reduce the oil percentage in the fruits as well as oleic acid and phenolic compounds contents in the oil [37].
The possible earlier snowmelt in some areas may, however, result in earlier bud sprout, thus extending the growing period [10], especially in the mid- and high latitudes [39] with putative positive effects on both yield and fruit quality, as well as on increasing the number of cultivars and species suitable for this specific area. At the moment, the strongest advancement in plant development was recorded for the very early spring phases, while the late spring and summer phases were less reactive to the elevated mean temperature [39].
On the other hand, the CO2 enrichment of the atmosphere results in higher photosynthetic activities when all other factors do not pose limitations [8]. Positive responses to such CO2 increase have been reported in several species, achieving higher biomass as well as higher fruit production and improved quality [8]. High temperatures may also induce faster maturation [27], which may be beneficial for the early maturing of cultivars, coming into the market earlier, and, thus, achieving higher prices.
2.4. Impact of Climate Change on Plant Growth
The rise in temperature would be beneficial under certain circumstances for plant growth, especially in areas at high latitudes, where the growing period is extended [24]. It is not only temperature that has a great effect on shoot growth, though, as water availability plays an equally important role. Unfortunately, climate change leads to increased air temperature and rather sporadic rainfalls, increasing water shortage stress. Under such conditions, shoot growth is severely affected, or it even ceases. When some fruit tree species bear fruits in a year’s shoots (peach, olive, etc.), it is understandable that one should not expect a high yield the following year due to the low number of flower buds formed in the previous year’s shoot. At the same time, high summer temperatures may easily lead to severe leaf scorching and dying, as has been observed in kiwifruit as well as in mango [8], reducing the leaf photosynthetic capacity, the production and the availability to support shoot growth, bud differentiation, and smooth fruit development. Periods of water shortage along with rising temperatures may severely affect physiological processes such as bud initiation and differentiation (in olive, pome trees, and stone fruits), winter dormancy (in almost all deciduous trees), flowering, fruit set, and fruit growth [29,40,41,42]. These fruit tree stages are extremely important for the sustainability of the profits of an orchard. For this reason alone, any assessment of the impacts of climate change on temperate as well as on tropical and sub-tropical fruit production is a crucial task for future orchard planning to be resilient to forthcoming climatic changes.
On the other hand, as global warming is 70% attributable to the high levels of CO2 (elevated CO2 (eCO2)), an increase in photosynthetic activity should not be excluded [43,44]. It is reported that if CO2 levels rise to 600–750 ppm from the approximately 400 ppm of today, most of the C3 plants will experience an increase in growth rate of approximately 30%, which is mainly attributed to the increased carboxylation efficiency of RuBisCO, which is low under the current ambient CO2 concentration [45]. This, of course, is subject to limitations due to the necessary availability of other sources for photosynthesis to function properly, such as minerals, water, etc., as well as atmospheric vapor pressure deficit (VPD) [46]. It is clear that at present, the CO2 deficiency (400 ppm instead of 600–750 ppm) is the limiting factor for achieving greater photosynthesis and crop production [45]. Therefore, the increase in CO2 levels in the atmosphere has been found to increase shoot growth and production, which is often referred to as “CO2 fertilization effect” [45,47,48] and improves the sugar accumulation in the fruits as well as their weight and size, while it may alter the nutraceutical composition of the fruit [45,48]. Orange trees grown for many years at eCO2 levels exhibited a 70% increase in total biomass compared to those grown under ambient conditions due to increased fruit production, branching, and trunk and branch thickness [49]. It should be noted here, though, that as with most biochemical processes in plants, photosynthesis is subject to feedback inhibition when the plant is unable to use the carbohydrates produced. Under these conditions, the surplus of carbohydrates is directed to C-based secondary metabolites such as phenolic compounds. According to Pritchard et al. [50], plant species’ response to eCO2 levels may not only be based on plant photosynthetic capacity and sink strength but also on the efficiency of the transport system to provide assimilates to the sinks. This means that species, cultivars, or rootstocks which are characterized by narrow vessels (many dwarf fruit tree rootstocks) may not be able to transport the surplus of carbohydrates produced under eCO2 to the sinks and may, thus, be prone to photosynthesis feedback inhibition.
Furthermore, photosynthetic acclimation to eCO2 has also been reported [48,51,52,53]. It seems that after a long-term exposure to eCO2 levels, leaf photosynthetic capacity decreases [43,48], in addition to all the possible negative effects it may have on fruit tree growth, productivity, and fruit quality [48]. This may be attributed to several factors such as the following:
- Altered photosynthesis-related gene expression,
- The feedback inhibition phenomenon described above,
- A limitation in the regeneration of ribulose-1,5 bisphosphate (RuBP) due to a conjugation of inorganic phosphate with the accumulated carbohydrates resulting in lower phosphorus availability,
- The progressive accumulation of starch in the chloroplasts,
- The limited utilization of triose phosphate,
- The decrease in stomatal density along with increased leaf thickness (due to increased mesophyll and vascular tissue cross-sectional area) which leads to reduced CO2 diffusion rates,
- The progressive limitation of nitrogen supply, as shoots accumulate carbohydrates faster than they can acquire nitrogen, resulting in deficient leaf nitrogen levels and, thus, decreased CO2 assimilation rates [46,48,50,52,53,54,55,56,57].
Under eCO2 levels, plant leaf size and anatomy are also altered [46,50]. Larger leaves and/or more leaves per plant have been reported as a consequence of eCO2, with the increase in the total leaf area of tree species being calculated to be approximately 14% [46,50,51,58]. The leaf area index has also been found to increase with eCO2 in various tree species, including Citrus species [46]. An increased number and size of xylem vessels due to eCO2 and consequently elevated hydraulic conductance have also been reported in some species but not in others such as Prunus [50]. It should be noted here, though, that although eCO2 induces a decrease in stomatal conductance and, therefore, offers better WUE at the leaf level, when this is extrapolated to the whole tree level, things may be different. The observed decrease in stomatal conductance should outweigh the potentially greater atmospheric evaporative demand due to the larger whole-tree leaf area to observe a significant water-saving function of eCO2 instead of greater water losses through transpiration [46]. Nonetheless, increased stomatal conductance has also been reported in Chinese pear trees subjected to eCO2 levels [56], complicating the overall effect of eCO2 on photosynthetic machinery.
At the same time, tree height is increased under eCO2, which is mostly attributed to the increased internode length and not to the increased node number, while the root growth is also enhanced (not in all species and not in all depths within the same species), which, in many cases, results in an increased root-to-shoot ratio [50,51]. These increases are mainly attributed to an increased cell division of the apical meristem due to enhanced carbohydrate availability through the elevated photosynthesis rates [50,51]. Nonetheless, eCO2 has been found to induce cell wall loosening and extensibility which result in larger cells, further supporting the increased shoot and root lengths reported as well as increased fruit biomass [46,51,58]. At this point, though, it must be noted that an increases in shoot length and branching may be beneficial in young orchards to speed up canopy development and the entrance into the reproductive phase, but in mature orchards, this may lead to increased pruning expenses, as well as to reduced flower bud differentiation, if shading pockets develop within the canopy. Furthermore, the height increase in modern fruit tree cultivation is considered a disadvantage, as it increases labor, phytosanitary protection, and overall cultivation costs. Furthermore, the increase in fruit tree height may pose a threat to tree survival in areas where strong winds are prevalent, as they may lead to severe tree damage due to the reductions in the wood density and mechanical strength of permanent parts [53].
An increased branching effect has been reported in some species, thus altering tree canopy shape, and it is attributed to a reduced apical dominance [50]. Apical dominance is known to be controlled by auxins (indole-3-acetic acid—IAA) produced at the apical meristem and basipetally transported to lower plant parts. On the other hand, several studies on various species have indicated that eCO2 may increase the number or the size of buds per node, thus partly justifying the observed increased branching [50,59,60]. Plant hormones (auxins, cytokinins, gibberellins, abscisic acid (ABA), ethylene, jasmonic acid (JA), and other plant growth-regulating compounds) control major developmental processes such as cell division, expansion and elongation, protein synthesis, etc. [50,51,58,61]. Based on this, the growth promotion by eCO2 may be either direct (through the enhanced substrate—carbohydrate supply, reduced dark respiration and photorespiration), indirect (based on chemical signals), or both [50,53,56]. As eCO2 has been found to enhance root growth [57] and roots are the primary sources of cytokinins in plants, it seems logical to assume that eCO2 will alter the cytokinin supply to the above-ground plant part, thus modifying its development, probably inducing extended branching. At the same time, eCO2 seems to enhance cytokinin production through the increased photosynthetically generated sugar supply to the roots [62]. According to Leibar-Porcel and Dodd [58], while eCO2 was found to increase the concentration of IAA, gibberellic acid (GA3), zeatin riboside (ZR), and ethylene, the concentration of ABA and JA decreased. ABA has a crucial role in controlling the stomatal aperture as a first line of defense against water stress, and eCO2 may interfere with this function by delaying the stomatal response to drought [61]. This may bring fruit trees growing under rainfed conditions to a difficult state of not being able to adequately control stomatal water loss under eCO2. As these plant growth regulators play a significant role in all phases of fruit tree growth and development and each species and cultivar responds differently to the exogenous application of growth regulators, it becomes obvious that altering endogenous hormone balance due to eCO2 produces an equation of uncertain results for the pomological sector.
All these changes, though, strongly depend on many other factors, such as necessary sources availability. Among these, water and nitrogen play the most significant role, as they influence the photosynthetic capacity of the leaves, among many others. According to Toreti et al. [43], under an adequate supply of water and nutrients, yield increases of up to 30% have been documented due to eCO2, while under nutrient-deficient conditions, such increases are either small or insignificant, strengthening the role of source availability. eCO2 has been found to attenuate the impact of drought stress on stomatal conductance and photosynthesis through an enhanced water use efficiency, although there is also evidence that eCO2 may impair the functionality of stomatal closure, thus increasing the vulnerability of plants against severe drought and/or heat stress [61]. The differential responses of plants grown under eCO2 along with different nitrogen levels have been reported in the literature [51], while it seems that under limited N supply, plants would increase its redistribution from vegetative to reproductive organs and this would be further enhanced under eCO2 levels [57]. In papaya seedlings, eCO2 at 700 ppm resulted in increased intrinsic WUE and assimilation rates as well as in increased dry mass, especially under high nitrogen levels, but it decreased N concentration in all plant organs, irrespective of the level of N applied [63]. According to Li et al. [61], though, eCO2 may restrain N acquisition and uptake, leading to N deficiency and growth limitation. As eCO2 decreases transpiration rates, it may cause a decrease in the mass flow, thus reducing nutrient absorption and transport to the upper plant parts. eCO2 seems to have beneficial effects under low nitrogen availability, increasing cytokinin concentration in the leaves [62,64]. Furthermore, it seems that N uptake under eCO2 functions under a nitrate dose-dependent scheme, while also depending on the pedoclimatic conditions prevailing in the region [61]. Besides plant growth and yield, long-term exposure to eCO2 has been found to affect mineral concentration in plants and the fruits or seeds, with most minerals declining (such as Ca, K, P, and Mg), which is probably due to the dilution effect and/or mass flow limitations [43,45,53,64], which may affect fruit quality and post-harvest behavior. Additionally, eCO2 has been reported to reduce the concentration of Co, Fe, Mn, Ni, S, and Zn in plants, too [44,45,64]. Generally, climate change and, more specifically, temperature increase, eCO2, and erratic precipitation have an influence on plant nutrition through their effects on mineralization, decomposition, availability, leaching, and loss of nutrients in deeper soil layers [64]. Under eCO2 and high-temperature soil micronutrient concentrations, such as Cu, Fe and Zn were found to increase, even though their concentration in the plant did not follow the same pattern [64]. Climate change has been found to alter the annual and seasonal nutrient availability and cycling, thus interfering with plant nutrition [64]. Nitrogen availability and cycling have a crucial role in plant development as they play a significant part in many physiological and biochemical processes and may regulate the responses of plants to eCO2, as low N levels do not support increased biomass production even under eCO2 [45]. Fruit tree species greatly rely on N availability and uptake, as it regulates several important functions regarding root growth, flower bud differentiation, fruit set, and growth. Similarly, phosphorus plays a significant role in water-use efficacy [64], root growth, energy status, and photosynthesis, which all affect fruit tree growth and fruit set [53]. There is evidence that the positive effects of eCO2 may not persist over time under low N and/or P conditions [53], strengthening the role of these two macronutrients in the new era of climate change. Increasing nitrogen fertilization under these conditions seems a logical approach with potential benefits, but one must keep in mind the economic and environmental impact of such a fertilization scheme. In any case, the adaptation of sophisticated fertilization strategies is necessary for economically and environmentally friendly fruit production. Therefore, and due to the genetic variability among fruit tree species and cultivars (within the same species), a careful, specialized, and individually planned fertilization strategy is deemed necessary for eCO2 to achieve sustainable growth and production.
It is noteworthy that a tree will respond differently as a single unit compared to when grown with other trees, and especially with other species [50,53]. This is mostly attributed to the differences in height growth and branching patterns. At the orchard level, this is not a common situation, since orchards are planted as single-species plantations. Nonetheless, different responses to eCO2 among cultivars of the same species have been reported in many plant species [43,65] and must be taken into account concerning future orchards planning. Many fruit tree species are budded on rootstocks to exploit their pedoclimatic adaptation potential as well as their growth regulation dynamic. Modern orchards are designed based on the rootstock’s ability to reduce the final tree size (dwarf rootstocks). At the same time, different cultivars within the same species present different vigor degrees. Since eCO2 levels affect tree growth rate and final tree size (in most cases, as already mentioned above, the tree size increases under eCO2), it is safe to assume that different cultivars of the same species, grafted on different rootstocks with variable vigor, will respond differently under the same eCO2 levels. Thus, the ability of a rootstock–cultivar combination to thrive under eCO2 conditions will depend on their competitive ability and interactions with the adjacent trees for the finite amount of available resources (such as space, nutrients, water, light, etc.) [53].
Although all these are logical outcomes of the eCO2 levels, one must keep in mind that for the photosynthetic apparatus to work properly, other factors such as water availability and favorable air temperature should come hand in hand. Trees grown in the field often experience multiple and simultaneous stresses (i.e., drought and heat stress, salt and heat stress, high light intensities, drought and heat stress, etc.) [66]. Under these conditions, the extent of the combined stress is not the sum of each stress factor (although the effects are, to some extent, additive), as it often results in the same physiological, molecular, and biochemical responses, while novel responses have also been reported under combinational stress [66]. Under mild abiotic stress conditions, the eCO2 could partially alleviate some of the impacts of the stress factors [65]. eCO2 has been found to decrease stomatal conductance, since there is a surplus of CO2 for supporting photosynthesis, while at the same time reducing the transpiration rate and, thus, improving the water use efficiency (WUE) [43,45,56,64,66]. This, many times, leads to improved plant biomass production compared to ambient CO2 conditions. Nonetheless, as eCO2 induces a rise in global temperature, and heat stress is among the factors limiting plant productivity, their combined effect is expected to induce significant changes in fruit tree physiology and productivity. As eCO2 reduces stomatal conductance, it simultaneously reduces the cooling potential of transpiration, which, under elevated air temperatures (supra-optimal temperature), will lead to increased leaf temperatures and reductions in growth rates [47]. Under a mild temperature increase, though, it may confer tolerance to drying, as it reduces water stomatal losses [45,65]. This could be an advantage for fruit trees cultivated in elevated longitudes and latitudes as the rise in air temperature is milder there. Furthermore, eCO2 can partially compensate for losses of net carbon gain due to photorespiration under high temperatures, as it decreases photorespiration and increases photosynthesis [45,53]. The extent of this compensation, of course, depends on the level of temperature rise in relation to the optimum growing temperature of the species involved. On the other hand, though, if water stress continues and becomes more severe, eCO2 may pose a disadvantage, as it has been found to increase xylem vessel diameter and/or reduce wood density, thus making trees more susceptible to xylem embolism and hydraulic failure [46,53,56]. Furthermore, under severe drought stresses, any benefits of eCO2 on photosynthetic activity may disappear, as stomatal diffusional efficiency is reduced along with the carboxylation and photochemical efficiency of RuBisCO [56].
The growth of all plants depends on CO2 supply along with water supply from the roots to sustain photosynthetic rates as well as on the sufficient inorganic nutrients absorbed from the root system. The availability of nutrients in soil solution depends on many factors, some of which can be significantly affected by climate change and eCO2 [64]. Besides the inorganic nutrients found freely in the soil solution and those provided by the input of fertilizers inorganic nutrients found in soil organic matter (SOM) in unavailable for the plant forms [64]. Soil fauna and microflora are responsible for the degradation of litter debris produced by plants (leaves, shoots, roots, fruits) into SOM, which will later, through decomposition, will liberate into soil solution readily available nutrients [64]. Soil microbes (the primary mediators of below-ground processes) play a pivotal role in the degradation of SOM and the overall soil fertility. Climate change inevitably affects their growth and action, which, in turn, has an impact on fruit tree growth. Temperature as well as soil moisture play a significant role in the survival and activity of soil microbes, and as climate changes, the soil microbe’s population undergoes significant changes [67]. As the soil temperature increases, it enhances soil microbial activity, resulting in higher levels of N mineralization and decomposition of SOM. Under eCO2 and limited nitrogen supply, the mass of arbuscular mycorrhiza fungi (AMF) has been found to increase and alleviate nitrogen limitation [68]. Of course, AMF also alleviates any P limitations, and as soil CO2 and temperature increase may boost their population dynamics, plants may benefit from the enhanced P availability under eCO2 [53]. Ectomycorrhizal fungi have been found to increase soil N availability and plant uptake through the degradation of organic N compounds in the soil by the production of extracellular enzymes [53]. Furthermore, as eCO2 may increase N fixation in N-fixers (Leguminosae plants), it may also increase its availability and levels in surrounding non-N fixers, such as fruit trees [53]. This might lead to increased nutrient concentration and availability in the soil under an adequate soil moisture, assuming there will be minimum rainstorms, which may increase nutrient leaching and loss. The increase (at least during the first years under continuous eCO2 levels) in photosynthesis and the accumulation of carbohydrates will benefit symbiotic organisms such as mycorrhizal fungi, rhizospheric bacteria, and endophytic microorganisms, too, which may contribute to the further increase in the plant biomass [45].
Based on all the above, it would be unwise to try to oversimplify and extend predictions from experiments examining one or two factors of climate change (i.e., eCO2 and/or heat or salt stress) to a global scale, as there are many factors involved in the reaction of a plant to climate change constituents, and this reaction cannot be calculated as the sum of each factor, as it is the result of their interactions [45,53]. Furthermore, as already stated above, the genetic variability of species and cultivars within the same species; the use of rootstocks; the dynamic seasonal variability of fruit trees’ responses during their life span, as well as within the growing period (same year) [46]; and the differences in pedoclimatic conditions from region to region, as well as from orchard to orchard, make it almost impossible to make an accurate prediction of the trend and magnitude of tree response to eCO2 and, overall, to climate change (Figure 5).
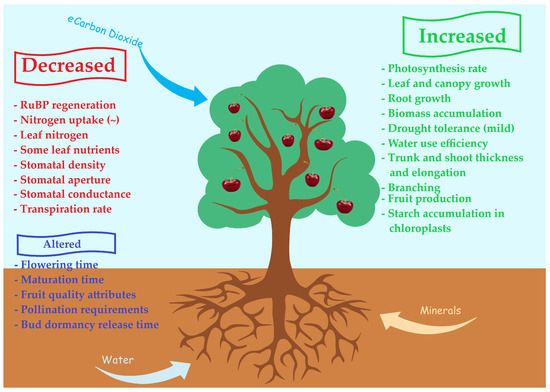
Figure 5.
Simplified possible effects of eCO2 on a fruit tree.
2.5. Effect of Erratic Precipitation or Sudden Rainstorms
Climate change has a strong negative effect on precipitation patterns. Agricultural areas in arid and semi-arid regions are expected to suffer from extreme drought episodes as the available water gets low while, at the same time, plants experience a high atmospheric evaporative demand [69]. Furthermore, the decreased precipitation may create another problem regarding soil properties, as soil salinity is expected to increase, due to either the use of brackish, saline water, and/or fertilizer build-up, thus affecting fruit tree productivity while simultaneously threatening the survival of some salt-sensitive species or cultivars. At the same time, soil salinity may induce severe osmotic stress as well as symptoms of nutrient imbalances and toxicities, impacting fruit yield and fruit quality indexes [23,38].
Although it has been referred to earlier that climate change has brought a limitation of rainfall, this does not stand for all areas. As climate changes, the pattern of rainfall changes, too. In some areas, this could mean a shortage of water, while in others, strong rainfalls could induce flooding and anoxia [37]. The limitation of water in an area is certain to drive the limited available amounts to urban uses to cover the city’s needs, leaving the agricultural sector struggling with drought stress. On the other hand, extreme rainfall incidents may lead to flooding phenomena (as the one that happened in Thessaly, Greece, in September 2023), soil erosion, and land degradation. As fruit trees are quite susceptible to anaerobic soil conditions, many of them will probably decay due to either limited oxygen availability to the root system and/or pathogenic attacks.
Furthermore, rainstorms have led to severe land degradation in hilly terrains due to severe soil erosion [70]. Apart from rainstorms, the steep slopes of the terrain, the unregulated changes in land use, the wrong agricultural practices, as well as deforestation (either anthropogenic conversion of forests into cropland—or due to wild-forest fires) are the main causes of land degradation. Erosion causes the loss of upper fertile soil layers to lower altitudes, leaving behind infertile soils with altered bio-hydro-thermal functions unsuitable for cultivation [70].
3. Impact on Pest, Disease, and Weed Incidence
As already mentioned, climate change does not influence only plant species or human welfare, but also all living organisms. Within this context, climate change may influence the stages and rate of development of pests and pathogens while, at the same time, altering the host tolerance as well as the physiology of host–pathogen interactions [3,24,71]. Weeds, pests, and diseases may also benefit from the elevation of temperature and higher carbon dioxide concentrations [9]. Climate change is expected to change the geographical distribution of plant pathogens, the number of generations (which by now is able to complete a higher number of reproductive cycles, thus resulting in increased populations), the ability or sensitivity of overwintering in specific areas (allowing the larvae or overwintering structures to survive at higher rates), as well as the synchronization between fruit tree vulnerability and pest infestation or disease infection [3,6,22]. All of the above lead to increased problems regarding plant protection [25,72]. The globalization of commerce has also helped in the expansion of pests and diseases in areas that were not traditionally their habitat, which, by now, due to climate change, can offer a secure environment for the development and growth of this invasive species [6,22]. Examples of pest infestation are the Aleurocanthus spiniferus (Quintance) Hemiptera, Aleurodidae, originating from South East Asia, now invading Mediterranean countries as a significant threat to citrus as well as grapevine production. Furthermore, the olive fly (Bactrocera oleae Rossi) in both Europe and North America is expected to retreat southward due to the higher summer temperatures’ effects on adult flies [73]. At the same time, it will migrate northward due to warmer winters, which allow for higher insect survival rates [73]. The changes in the pattern of precipitation along with the warmer autumns have increased the incidence of anthracnose disease in olive trees (caused by Colletotrichum spp.), reaching, in some years, epidemic levels, having significant socio-economic impacts [74]. Similarly, the outbreaks of Pseudomonas syringae pv. actinidiae (Psa) have caused significant economic losses, and several climatic models have been tested to evaluate the risk of global expansion in kiwifruit-growing areas [75]. Apart from pests and diseases, invasive plant species have also arisen as a problem for the agricultural sector [76,77,78,79]. One of the most pronounced is the tree of heaven (Ailanthus altissima (Mill.) Swingle) [76] (Figure 5), an invasive tree species with rapid growth, tolerant to many abiotic stress factors, and with a high degree of allelopathy due to the production and into-the-soil exudation of the toxic compound ailanthone [78] (Figure 6).

Figure 6.
Rapid growth of Ailanthus altissima in a fig (on the left) and citrus orchard (on the right) (from personal archive).
Climate change is expected to have a direct effect on weed species composition, as some species, or even new species, in an area will be favored by the new climatic conditions, while others will decline, even if weeds exhibit greater adaptability to stress than fruit tree species [80,81]. Furthermore, one or two weed species may prevail in an area, which may be difficult to manage. Such an example is the prevalence of Solanum elaeagnifolium in many areas of the world and in Greece, of course, which is quite difficult to eradicate [82]. The number of thermophile species is expected to increase as well as the number of exotic, potentially invasive species, changing the weed dispersion and composition in such a grade, where weeds of formerly minor importance may become highly important species [80,83]. In areas where winter is milder and wetter, the survival of some winter weeds will be favored, while the same is true for thermophile summer annuals in areas where summer gets hotter and the growing period gets longer [80,81]. Such species are expected to extend further north, invading new areas. Under such conditions, species that were not able to produce viable seeds due to short growing periods (growing degree days) will be able to form seeds and disperse them, thus increasing the weed population [80]. In this sense, some opportunistic weed species will probably thrive and emerge as new enemies for the cultivation [80], increasing the need for the development of new weed management strategies, as the efficacy of the present herbicides may decrease [83].
4. Possible Mitigation Measures
Various measures have been proposed to overcome, to some extent, the negative impacts of climate change on fruit trees. Nonetheless, one must keep in mind that trees, as perennial species, are exposed to a variety of stress factors within the same year, making it difficult to fully alleviate all the impacts of climate change.
Among the most effective measures to be taken is the breeding of rootstocks and cultivars that are tolerant to the stress factors imposed by climate change [84,85,86]. This is a time- and money-consuming effort with uncertain results. Molecular techniques have facilitated and sped up the process of the evaluation of the new genotypes for desirable characteristics, but as some of these characteristics are the result of the expression of many genes and the interaction of many factors, it is still difficult to predict with a high degree of certainty the desirable outcome [86,87]. Still, breeding remains a reliable and significant tool that can offer solutions in our changing environment.
On the other hand, the perennial character of fruit trees makes it difficult to uproot old ones and plant new ones. For some species, such as stone fruits and pome fruits, this may be easy, as their productive lifespan in high-density orchards is by now quite short, and the farmers are used to and familiar with orchard renewal. In other species, though, such as citrus, olive, nuts, etc., this is not an easy task. Other measures must be developed and put into action to alleviate climate change impacts.
Starting from the beginning, the lack of enough chilling hour accumulation can be overcome in some cases with the application of bud-breaking agents (Figure 7), such as hydrogen cyanamide [88,89], potassium nitrate, urea, gibberellins [88,90,91,92], synthetic cytokinins (thidiazuron) [93] and other plant growth regulators [88], emulsified vegetable oils [89], overhead irrigation, early defoliation (in deciduous species), early pruning, etc.

Figure 7.
Efficacy of a bud dormancy-breaking agent applied on half of a tree canopy in late winter. On the photo on the left-hand side, there is an earlier flower bud sprouting, and on the right-hand side, there is an earlier leaf emergence compared to the untreated control on the same apricot tree (from personal archive).
The lack of flowering synchronization can also be overcome by the previous bud-breaking agents, while in some species, artificial pollination may be the solution too. A high solar irradiance in summer (heat stress) can be alleviated by overhead net installation (net shading) [84,94,95] (Figure 8), melatonin [96,97,98], jasmonates [99], brassinosteroids [100,101], silicon [102], as well as sugars and sugar alcohols [103,104], and reflecting agent spraying (such as talc, kaolin clay particles, attapoulgite, calcium carbonate) [84,105,106] (Figure 9). Heat stress along with water stress can be also alleviated by the use of products containing reflecting agents as well as osmolytes (such as proline, glycine betaine, trehalose, mannitol, sorbitol, potassium products, etc.) [85,103,104,105,106,107,108], strigolactones [100,109], salicylic acid [84,100,110,111], seaweed extracts [112], polyamines [84,113], abscisic acid (ABA) and other plant growth regulators [84,107,114], jasmonates [84,99], silicon [102], arbuscular mycorrhizal fungi (AMFs) along with endophytes, plant growth-promoting rhizobacteria (PGPR) and fungi (PGPF) (against drought stress) [94,112,115,116], superabsorbent polymers (SAP) or hydrophilic polymer gels in the soil [84,85], biochar as well as humate compounds incorporation in the soil (against water shortage) [107,117], and melatonin foliar application [96,97,98,118].
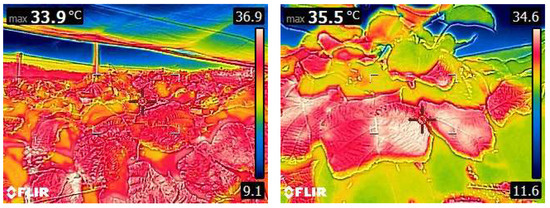

Figure 8.
Overhead net installation on a kiwifruit orchard resulting in a reduced canopy heat load. On the left is the thermal image (above, left) of the net-covered kiwifruit canopy (below, left), and on the right is the thermal image of the uncovered canopy (above, right) in late summer (below, right) (from personal archive).

Figure 9.
Kaolin clay particles deposited on olive leaves for protection against heat and drought stress (from personal archive).
Plants exposed to a stress factor often undergo oxidation stress, and this may be, to some extent, overcome by the use of antioxidants such as ascorbic acid, glutathione, tocopherol, Ambiol, etc. [106,119,120,121]. At the same time, salinity stress may impose toxicity, ionic imbalance, and osmotic stress, which can be overcome by the use of osmolytes as described earlier [85,103,104,108]; seaweed extracts [112]; melatonin [96,97,118]; salicylic acid [110,111,122]; ABA [85,114]; polyamines [85,113,123]; brassinosteroids [94,100,101]; silicon [102]; PGPRs and PGPFs [85,94,115,116]; calcium, potassium, as well as nitrates (if sodium chloride is the saline factor) [84,85,107,122] added into the soil; biochar as well as humate compounds’ incorporation in the soil [117]; jasmonic acid [99,107,122]; ascorbic acid; and other antioxidants [119,122] to reduce oxidation stress. In winter, low temperatures may jeopardize plant survival, and countermeasures have to be taken. Osmolytes [104], copper-containing products, ABA [84,114], antioxidants [121], brassinosteroids [94,100,101], melatonin [98], potassium [84], salicylic acid [100,110,111,124], proline [84], and polyamines [113,123] may confer some tolerance as well as overhead irrigation (spraying) (Figure 10). The reduced flower bud differentiation (observed both in olive and kiwifruit during the last few years) is a significant factor for the economic viability of the cultivation. Cultivars with low chilling requirements (such as the yellow and red-fleshed kiwifruit cultivars) seem to be the solution. In olive cultivation, though, it is difficult for farmers to uproot the trees or to graft them with new cultivars, as there are not many cultivars available that can satisfy all the desirable traits an olive cultivar should have for a specific region. However, there are some actions which may help, such as early pruning, spraying with reflecting agents, lowering canopy height, etc., but with a high degree of uncertain efficacy.

Figure 10.
Canopy sprinkler application result for the protection of citrus orchard against frost (from personal archive).
5. Conclusions and Prospects
Climate change has “shown its teeth” in many sectors of human activities and in various ways and severity. The increasing population of the earth makes the demand for food security non-negotiable, but under the influence of climate change, this does not seem as sure as it was decades before. As the global temperature is rising, precipitation is getting erratic, and land degradation is increasing, the need to understand how climate change impacts fruit tree physiology is deemed an imperative. Fruit trees, as woody perennials, are exposed to all extreme weather events throughout all seasons, not being able to escape them. Therefore, fruit tree physiology is affected, starting from bud dormancy release and floral bud differentiation, flowering, pollination, and fruit set, as well as fruit growth and quality (Table 1). At the same time, pests and diseases experience the impacts of climate change, which, in many cases, leads to the expansion of new enemies in areas that previously could not thrive and survive. The breeding of cultivars that are tolerant to the evolving climatic conditions in a specific area and the adaptation of cultural management to these new conditions is vital, as human welfare and food security could be jeopardized under the changing environment.

Table 1.
Summary of the primary impacts of climate change on fruit and nut tree physiology (for a more detailed per-species summary, please see Medda et al. [12]).
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Kunz, A.; Blanke, M.M. Effects of climate change on fruit tree physiology—Based on 55 years of meteorological and phenological data at Klein-Altendorf. Acta Hortic. 2016, 1130, 49–54. [Google Scholar] [CrossRef]
- Roussos, P.A. Climate change impacts on fruit trees and mitigation strategies of adverse effects. AgroLife Sci. J. 2020, 9, 269–276. [Google Scholar]
- Haokip, S.W.; Shankar, K.; Lalrinngheta, J. Climate change and its impact on fruit crops. J. Pharmacogn. Phytochem. 2020, 9, 435–438. [Google Scholar]
- Salama, A.-M.; Ezzat, A.; El-Ramady, H.; Alam-Eldein, S.M.; Okba, S.K.; Elmenofy, H.M.; Hassan, I.F.; Illés, A.; Holb, I.J. Temperate Fruit Trees under Climate Change: Challenges for Dormancy and Chilling Requirements in Warm Winter Regions. Horticulturae 2021, 7, 86. [Google Scholar] [CrossRef]
- Naz, S.; Fatima, Z.; Iqbal, P.; Khan, A.; Zakir, I.; Ullah, H.; Abbas, G.; Ahmed, M.; Mubeen, M.; Hussain, S.; et al. An Introduction to Climate Change Phenomenon. In Building Climate Resilience in Agriculture: Theory, Practice and Future Perspective; Jatoi, W.N., Mubeen, M., Ahmad, A., Cheema, M.A., Lin, Z., Hashmi, M.Z., Eds.; Springer Nature: Cham, Switzerland, 2022; pp. 3–16. [Google Scholar] [CrossRef]
- Rajatiya, J. Climate Change: Impact, Mitigation and Adaptation in Fruit Crops. Intern. J. Pure Appl. Biosci. 2018, 6, 6161. [Google Scholar] [CrossRef]
- Lee, H.; Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.; Trisos, C.; Romero, J.; Aldunce, P.; Barret, K. Climate Change 2023: Synthesis Report, Summary for Policymakers. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Saqib, M.; Anjum, M.A.; Ali, M.; Ahmad, R.; Sohail, M.; Zakir, I.; Ahmad, S.; Hussain, S. Horticultural Crops as Affected by Climate Change. In Building Climate Resilience in Agriculture: Theory, Practice and Future Perspective; Jatoi, W.N., Mubeen, M., Ahmad, A., Cheema, M.A., Lin, Z., Hashmi, M.Z., Eds.; Springer Nature: Cham, Switzerland, 2022; pp. 95–109. [Google Scholar] [CrossRef]
- Sthapit, S.R.; Scherr, S.J. Tropical fruit trees and opportunities for adaptation and mitigation. In Tropical Fruit Tree Species and Climate Change; Sthapit, B.R., Ramanatha Rao, V., Sthapit, S.R., Eds.; Biodiversity International: New Delhi, India, 2012; pp. 129–137. [Google Scholar]
- Inouye, D.W. Climate change and phenology. WIREs Clim. Chang. 2022, 13, e764. [Google Scholar] [CrossRef]
- IPCC. 2021: Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V.P., Zhai, A., Pirani, S.L., Connors, C., Péan, S., Berger, N., Caud, Y., Chen, L., Goldfarb, M.I., Gomis, M., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 3–32. [Google Scholar] [CrossRef]
- Medda, S.; Fadda, A.; Mulas, M. Influence of Climate Change on Metabolism and Biological Characteristics in Perennial Woody Fruit Crops in the Mediterranean Environment. Horticulturae 2022, 8, 273. [Google Scholar] [CrossRef]
- Lu, L.-C.; Chiu, S.-Y.; Chiu, Y.-h.; Chang, T.-H. Sustainability efficiency of climate change and global disasters based on greenhouse gas emissions from the parallel production sectors–A modified dynamic parallel three-stage network DEA model. J. Environ. Manag. 2022, 317, 115401. [Google Scholar] [CrossRef]
- Beck, S.; Mahony, M. The IPCC and the new map of science and politics. Wiley Interdiscip. Rev. Clim. Chang. 2018, 9, e547. [Google Scholar] [CrossRef]
- Rodríguez, A.; Pérez-López, D.; Centeno, A.; Ruiz-Ramos, M. Viability of temperate fruit tree varieties in Spain under climate change according to chilling accumulation. Agric. Syst. 2021, 186, 102961. [Google Scholar] [CrossRef]
- Aslam, M.; Ahmed, M.; Hassan, F.-U.; Afzal, O.; Mehmood, M.Z.; Qadir, G.; Asif, M.; Komal, S.; Hussain, T. Impact of Temperature Fluctuations on Plant Morphological and Physiological Traits. In Building Climate Resilience in Agriculture; Jatoi, W.N., Mubeen, M., Ahmad, A., Cheema, M.A., Lin, Z., Hashmi, M.Z., Eds.; Springer Nature: Cham, Switzerland, 2022; pp. 25–52. [Google Scholar] [CrossRef]
- Rodríguez, A.; Pérez-López, D.; Sánchez, E.; Centeno, A.; Gómara, I.; Dosio, A.; Ruiz-Ramos, M. Chilling accumulation in fruit trees in Spain under climate change. Nat. Hazards Earth Syst. Sci. 2019, 19, 1087–1103. [Google Scholar] [CrossRef]
- Cherif, S.; Doblas-Miranda, E.; Lionello, P.; Borrego, C.; Giorgi, F.; Iglesias, A.; Jebari, S.; Mahmoudi, E.; Moriondo, M.; Pringault, O.; et al. Drivers of Change. In Climate and Environmental Change in the Mediterranean Basin—Current Situation and Risks for the Future. First Mediterranean Assessment Report; Cramer, W., Guiot, J., Marini, K., Eds.; Union for the Mediterranean, Plan Bleu, UNEP/MAP: Marseille, France, 2020; pp. 59–180. [Google Scholar]
- Derbile, E.K.; Kanlisi, S.K.; Dapilah, F. Mapping the vulnerability of indigenous fruit trees to environmental change in the fragile savannah ecological zone of Northern Ghana. Heliyon 2022, 8, e09796. [Google Scholar] [CrossRef]
- Abd El-Rahman, M.M.A. Effect of using bio-stimulants and foliar spraying of anti-stressors for counteract the negative effects of climate changes on growth and fruiting of Balady mandarin trees. SVU-Intern. J. Agric. Sci. 2022, 4, 153–167. [Google Scholar] [CrossRef]
- Vanalli, C.; Casagrandi, R.; Gatto, M.; Bevacqua, D. Shifts in the thermal niche of fruit trees under climate change: The case of peach cultivation in France. Agric. For. Meteorol. 2021, 300, 108327. [Google Scholar] [CrossRef]
- Vujadinović Mandić, M.; Vuković Vimić, A.; Ranković-Vasić, Z.; Đurović, D.; Ćosić, M.; Sotonica, D.; Nikolić, D.; Đurđević, V. Observed Changes in Climate Conditions and Weather-Related Risks in Fruit and Grape Production in Serbia. Atmosphere 2022, 13, 948. [Google Scholar] [CrossRef]
- Stewart, A.L.; Ahmed, S. Chapter 7—Effects of climate change on fruit nutrition. In Fruit Crops; Srivastava, A.K., Hu, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 77–93. [Google Scholar] [CrossRef]
- Paul, V.; Pandey, V.; Singh, A. Vulnerability of trees and fruit crops to climate change. In Climate Change: Impacts and Adaptations in Crop Plants; Singh, M.P., Ed.; Today’s and Tomorrow’s Printer and Publishers: New Delhi, India, 2011. [Google Scholar] [CrossRef]
- Stockle, C.; Marsal, J.; Villar, J.M. Impact of climate change on irrigated tree fruit production. Acta Hortic. 2011, 889, 41–52. [Google Scholar] [CrossRef]
- Luedeling, E. Climate change impacts on winter chill for temperate fruit and nut production: A review. Sci. Hortic. 2012, 144, 218–229. [Google Scholar] [CrossRef]
- Hribar, J.; Vidrih, R. Impacts of climate change on fruit physiology and quality. In Proceedings of the 50th Croatian and 10th International Symposium on Agriculture, Opatija, Croatia, 16–20 February 2015; pp. 42–45. [Google Scholar]
- Tominaga, A.; Ito, A.; Sugiura, T.; Yamane, H. How Is Global Warming Affecting Fruit Tree Blooming? “Flowering (Dormancy) Disorder” in Japanese Pear (Pyrus pyrifolia) as a Case Study. Front. Plant Sci. 2022, 12, 787638. [Google Scholar] [CrossRef]
- Fernandez, E.; Mojahid, H.; Fadón, E.; Rodrigo, J.; Ruiz, D.; Egea, J.A.; Ben Mimoun, M.; Kodad, O.; El Yaacoubi, A.; Ghrab, M.; et al. Climate change impacts on winter chill in Mediterranean temperate fruit orchards. Reg. Environ. Chang. 2022, 23, 7. [Google Scholar] [CrossRef]
- Luedeling, E.; Zhang, M.; Luedeling, V.; Girvetz, E.H. Sensitivity of winter chill models for fruit and nut trees to climatic changes expected in California’s Central Valley. Agric. Ecosyst. Environ. 2009, 133, 23–31. [Google Scholar] [CrossRef]
- Fraga, H.; Santos, J.A. Assessment of Climate Change Impacts on Chilling and Forcing for the Main Fresh Fruit Regions in Portugal. Front. Plant Sci. 2021, 12, 689121. [Google Scholar] [CrossRef] [PubMed]
- Drogoudi, P.; Cantín, C.M.; Brandi, F.; Butcaru, A.; Cos-Terrer, J.; Cutuli, M.; Foschi, S.; Galindo, A.; García-Brunton, J.; Luedeling, E.; et al. Impact of Chill and Heat Exposures under Diverse Climatic Conditions on Peach and Nectarine Flowering Phenology. Plants 2023, 12, 584. [Google Scholar] [CrossRef] [PubMed]
- Pantelidis, G.; Drogoudi, P. Exploitation of genotypic variation in chilling and heat requirements for flowering in Prunus armeniaca and Prunus persica (L.) Batsch cultivars. Sci. Hortic. 2023, 321, 112287. [Google Scholar] [CrossRef]
- Naseri, S.; Gholami, M.; Baninasab, B. Chilling and heat requirements in the flower and vegetative buds of some local almond cultivars. Theor. Appl. Clim. 2023, 154, 337–347. [Google Scholar] [CrossRef]
- Alzate-Marin, A.L.; Rivas, P.M.S.; Galaschi-Teixeira, J.S.; Bonifácio-Anacleto, F.; Silva, C.C.; Schuster, I.; Nazareno, A.G.; Giuliatti, S.; da Rocha Filho, L.C.; Garófalo, C.A.; et al. Warming and elevated CO2 induces changes in the reproductive dynamics of a tropical plant species. Sci. Total Environ. 2021, 768, 144899. [Google Scholar] [CrossRef] [PubMed]
- Nath, V.; Kumar, G.; Pandey, S.; Pandey, S. Impact of climate change on tropical fruit production systems and its mitigation strategies. In Climate Change and Agriculture in India: Impact and Adaptation; Mahdi, S.S., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 129–146. [Google Scholar]
- Dias, M.C.; Araújo, M.; Silva, S.; Santos, C. Sustainable Olive Culture under Climate Change: The Potential of Biostimulants. Horticulturae 2022, 8, 1048. [Google Scholar] [CrossRef]
- Denaxa, N.-K.; Damvakaris, T.; Roussos, P.A. Antioxidant defense system in young olive plants against drought stress and mitigation of adverse effects through external application of alleviating products. Sci. Hortic. 2020, 259, 108812. [Google Scholar] [CrossRef]
- Chmielewski, F.-M.; Müller, A.; Bruns, E. Climate changes and trends in phenology of fruit trees and field crops in Germany, 1961–2000. Agric. For. Meteorol. 2004, 121, 69–78. [Google Scholar] [CrossRef]
- Campoy, J.A.; Ruiz, D.; Egea, J. Dormancy in temperate fruit trees in a global warming context: A review. Sci. Hortic. 2011, 130, 357–372. [Google Scholar] [CrossRef]
- El Yaacoubi, A.; Malagi, G.; Oukabli, A.; Hafidi, M.; Legave, J.-M. Global warming impact on floral phenology of fruit trees species in Mediterranean region. Sci. Hortic. 2014, 180, 243–253. [Google Scholar] [CrossRef]
- Lopez, G.; Mata, M.; Arbones, A.; Solans, J.R.; Girona, J.; Marsal, J. Mitigation of effects of extreme drought during stage III of peach fruit development by summer pruning and fruit thinning. Tree Physiol. 2006, 26, 469–477. [Google Scholar] [CrossRef]
- Toreti, A.; Deryng, D.; Tubiello, F.N.; Müller, C.; Kimball, B.A.; Moser, G.; Boote, K.; Asseng, S.; Pugh, T.A.M.; Vanuytrecht, E.; et al. Narrowing uncertainties in the effects of elevated CO2 on crops. Nat. Food 2020, 1, 775–782. [Google Scholar] [CrossRef]
- Alae-Carew, C.; Nicoleau, S.; Bird, F.A.; Hawkins, P.; Tuomisto, H.L.; Haines, A.; Dangour, A.D.; Scheelbeek, P.F.D. The impact of environmental changes on the yield and nutritional quality of fruits, nuts and seeds: A systematic review. Environ. Res. Lett. 2020, 15, 023002. [Google Scholar] [CrossRef]
- Fischer, G.; Melgarejo, L.M.; Balaguera-López, H.E. Review on the impact of elevated CO2 concentrations on fruit species in the face of climate change. Cienc. Tecnol. Agropecu. 2022, 23, e2475. [Google Scholar]
- Lauriks, F.; Salomón, R.L.; Steppe, K. Temporal variability in tree responses to elevated atmospheric CO2. Plant Cell Environ. 2021, 44, 1292–1310. [Google Scholar] [CrossRef]
- Zhu, C.; Wolf, J.; Zhang, J.; Anderegg, W.R.L.; Bunce, J.A.; Ziska, L.H. Rising temperatures can negate CO2 fertilization effects on global staple crop yields: A meta-regression analysis. Agric. For. Meteorol. 2023, 342, 109737. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, Y.; Ha, R.; Cao, B.; Song, L. Effects of Elevated CO2 on Photosynthetic Accumulation, Sucrose Metabolism-Related Enzymes, and Genes Identification in Goji Berry (Lycium barbarum L.). Front. Plant Sci. 2021, 12, 643555. [Google Scholar] [CrossRef]
- Kimball, B.A.; Idso, S.B.; Johnson, S.; Rillig, M.C. Seventeen years of carbon dioxide enrichment of sour orange trees: Final results. Glob. Chang. Biol. 2007, 13, 2171–2183. [Google Scholar] [CrossRef]
- Pritchard, S.G.; Rogers, H.H.; Prior, S.A.; Peterson, C.M. Elevated CO2 and plant structure: A review. Glob. Chang. Biol. 1999, 5, 807–837. [Google Scholar] [CrossRef]
- Yong, J.W.; Wong, S.C.; Letham, D.S.; Hocart, C.H.; Farquhar, G.D. Effects of elevated CO2 and nitrogen nutrition on cytokinins in the xylem sap and leaves of cotton. Plant Physiol. 2000, 124, 767–780. [Google Scholar] [CrossRef]
- Bloom, A.J.; Burger, M.; Asensio, J.S.R.; Cousins, A.B. Carbon Dioxide Enrichment Inhibits Nitrate Assimilation in Wheat and ‘Arabidopsis’. Science 2010, 328, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Maschler, J.; Bialic-Murphy, L.; Wan, J.; Andresen, L.C.; Zohner, C.M.; Reich, P.B.; Lüscher, A.; Schneider, M.K.; Müller, C.; Moser, G.; et al. Links across ecological scales: Plant biomass responses to elevated CO2. Glob. Chang. Biol. 2022, 28, 6115–6134. [Google Scholar] [CrossRef]
- Wang, F.; Gao, J.; Yong, J.W.H.; Wang, Q.; Ma, J.; He, X. Higher Atmospheric CO2 Levels Favor C3 Plants Over C4 Plants in Utilizing Ammonium as a Nitrogen Source. Front. Plant Sci. 2020, 11, 537443. [Google Scholar] [CrossRef]
- Ramírez, F.; Kallarackal, J. Response of trees to CO2 Increase. In Responses of Fruit Trees to Global Climate Change; Springer International Publishing: Cham, Switzerland, 2015; pp. 3–7. [Google Scholar] [CrossRef]
- Hao, L.; Chang, Z.; Lu, Y.; Tian, Y.; Zhou, H.; Wang, Y.; Liu, L.; Wang, P.; Zheng, Y.; Wu, J. Drought dampens the positive acclimation responses of leaf photosynthesis to elevated CO2 by altering stomatal traits, leaf anatomy, and Rubisco gene expression in Pyrus. Environ. Exp. Bot. 2023, 211, 105375. [Google Scholar] [CrossRef]
- Yu, M.; Sun, P.; Huang, X.; Zha, Z.; Wang, X.; Mantri, N.; Lou, H.; Jiang, B.; Shen, Z.; Sun, Y.; et al. Interacting Effects of CO2, Temperature, and Nitrogen Supply on Photosynthetic, Root Growth, and Nitrogen Allocation of Strawberry at the Fruiting Stage. Agronomy 2023, 13, 1353. [Google Scholar] [CrossRef]
- Leibar-Porcel, E.; Dodd, I.C. Role of Plant Hormones in Plant Response to Elevated CO2 Concentrations: Above- and Below-ground Interactions. In Plant Hormones and Climate Change; Ahammed, G.J., Yu, J., Eds.; Springer Nature: Singapore, 2023; pp. 55–74. [Google Scholar] [CrossRef]
- Norby, R.J.; O’Neill, E.G.; Luxmoore, R.J. Effects of Atmospheric CO2 Enrichment on the Growth and Mineral Nutrition of Quercus alba Seedlings in Nutrient-Poor Soil. Plant Physiol. 1986, 82, 83–89. [Google Scholar] [CrossRef]
- Conroy, J.P.; Milham, P.J.; Mazur, M.; Barlow, E.W.R. Growth, dry weight partitioning and wood properties of Pinus radiata D. Don after 2 years of CO2 enrichment. Plant Cell Environ. 1990, 13, 329–337. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Wei, Z.; Liu, F. ABA-mediated modulation of elevated CO2 on stomatal response to drought. Curr. Opin. Plant Biol. 2020, 56, 174–180. [Google Scholar] [CrossRef]
- Kiba, T.; Takebayashi, Y.; Kojima, M.; Sakakibara, H. Sugar-induced de novo cytokinin biosynthesis contributes to Arabidopsis growth under elevated CO2. Sci. Rep. 2019, 9, 7765. [Google Scholar] [CrossRef]
- Cruz, J.L.; Alves, A.A.C.; LeCain, D.R.; Ellis, D.D.; Morgan, J.A. Interactive effects between nitrogen fertilization and elevated CO2 on growth and gas exchange of papaya seedlings. Sci. Hortic. 2016, 202, 32–40. [Google Scholar] [CrossRef]
- Elbasiouny, H.; El-Ramady, H.; Elbehiry, F.; Rajput, V.D.; Minkina, T.; Mandzhieva, S. Plant Nutrition under Climate Change and Soil Carbon Sequestration. Sustainability 2022, 14, 914. [Google Scholar] [CrossRef]
- Lahive, F.; Handley, L.R.; Hadley, P.; Daymond, A.J. Climate Change Impacts on Cacao: Genotypic Variation in Responses of Mature Cacao to Elevated CO2 and Water Deficit. Agronomy 2021, 11, 818. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Ruan, Y.; Kuzyakov, Y.; Liu, X.; Zhang, X.; Xu, Q.; Guo, J.; Guo, S.; Shen, Q.; Yang, Y.; Ling, N. Elevated temperature and CO2 strongly affect the growth strategies of soil bacteria. Nat. Comm. 2023, 14, 391. [Google Scholar] [CrossRef]
- Cheng, L.; Booker, F.L.; Tu, C.; Burkey, K.O.; Zhou, L.; Shew, H.D.; Rufty, T.W.; Hu, S. Arbuscular Mycorrhizal Fungi Increase Organic Carbon Decomposition Under Elevated CO2. Science 2012, 337, 1084–1087. [Google Scholar] [CrossRef]
- Ammar, A.; Aissa, I.B.; Gouiaa, M.; Mars, M. Fig (Ficus carica L.) vulnerability to climate change: Combined effects of water stress and high temperature on ecophysiological behaviour of different cultivars. S. Afric J. Bot. 2022, 147, 482–492. [Google Scholar] [CrossRef]
- Choudhury, B.U.; Nengzouzam, G.; Islam, A. Evaluation of climate change impact on soil erosion in the integrated farming system based hilly micro-watersheds using Revised Universal Soil Loss Equation. Catena 2022, 214, 106306. [Google Scholar] [CrossRef]
- Gutierrez, A.P.; Ponti, L. Analysis of Invasive Insects: Links to Climate Change. In Invasive Species and Global Climate Change; Ziska, L.H., Dukes, J.S., Eds.; CABI Publishing: Wallingford, UK, 2014; pp. 45–61. ISBN 978-1780641645. [Google Scholar] [CrossRef]
- Jawo, T.O.; Kyereh, D.; Lojka, B. The impact of climate change on coffee production of small farmers and their adaptation strategies: A review. Clim. Dev. 2023, 15, 93–109. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Graniti, A.; Faedda, R.; Cacciola, S.O.; Magnano di San Lio, G. Olive diseases in a changing ecosystem. In Olive Diseases and Disorders; Transworld Research Network: Kerala, India, 2011; pp. 403–433. [Google Scholar]
- Narouei-Khandan, H.A.; Worner, S.P.; Viljanen, S.L.H.; van Bruggen, A.H.C.; Balestra, G.M.; Jones, E. The Potential Global Climate Suitability of Kiwifruit Bacterial Canker Disease (Pseudomonas syringae pv. actinidiae (Psa)) Using Three Modelling Approaches: CLIMEX, Maxent and Multimodel Framework. Climate 2022, 10, 14. [Google Scholar] [CrossRef]
- Nunes, L.J. Creation of Value Chains for the Sustainability of Control and Eradication Actions on Ailanthus altissima (Mill.) Swingle. Environments 2022, 9, 64. [Google Scholar] [CrossRef]
- Chuang, L.; Liu, S.; Biedermann, D.; Franke, J. Identification of early quassinoid biosynthesis in the invasive tree of heaven (Ailanthus altissima) confirms evolutionary origin from protolimonoids. Front. Plant Sci. 2022, 13, 958138. [Google Scholar] [CrossRef]
- Valiño, A.; Pardo-Muras, M.; López-Periago, J.E.; Puig, C.G.; Pedrol, N. Biomass from Allelopathic Agroforestry and Invasive Plant Species as Soil Amendments for Weed Control—A Review. Agronomy 2023, 13, 2880. [Google Scholar] [CrossRef]
- Khamare, Y.; Chen, J.; Marble, S.C. Allelopathy and its application as a weed management tool: A review. Front. Plant Sci. 2022, 13, 1034649. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.; Breitsameter, L.; Gerowitt, B. Impact of climate change on weeds in agriculture: A review. Agron. Sustain. Dev. 2014, 34, 707–721. [Google Scholar] [CrossRef]
- Korres, N.E.; Norsworthy, J.K.; Tehranchian, P.; Gitsopoulos, T.K.; Loka, D.A.; Oosterhuis, D.M.; Gealy, D.R.; Moss, S.R.; Burgos, N.R.; Miller, M.R.; et al. Cultivars to face climate change effects on crops and weeds: A review. Agron. Sustain. Dev. 2016, 36, 12. [Google Scholar] [CrossRef]
- Tataridas, A.; Moreira, M.; Frazão, L.; Kanatas, P.; Ota, N.; Travlos, I. Biology of Invasive Plants 5. Solanum elaeagnifolium Cav. Invasive Plant Sci. Manag. 2023, 16, 139–159. [Google Scholar] [CrossRef]
- Ramesh, K.; Matloob, A.; Aslam, F.; Florentine, S.K.; Chauhan, B.S. Weeds in a Changing Climate: Vulnerabilities, Consequences, and Implications for Future Weed Management. Front. Plant Sci. 2017, 8, 95. [Google Scholar] [CrossRef]
- Zeinab, R.-R.; Majid, M.; Ahmad, G.; Taqi, R.; Neal, S.E. Abiotic Stresses Management in Citrus. In Citrus Research; Mateus Perei-ra, G., Júlia Scherer, S., Eds.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- Ziogas, V.; Tanou, G.; Morianou, G.; Kourgialas, N. Drought and Salinity in Citriculture: Optimal Practices to Alleviate Salinity and Water Stress. Agronomy 2021, 11, 1283. [Google Scholar] [CrossRef]
- Jalil, S.U.; Ansari, M.I. Biotechnology Strategies to Combat Plant Abiotic Stress. In Nanobiotechnology: Mitigation of Abiotic Stress in Plants; Al-Khayri, J.M., Ansari, M.I., Singh, A.K., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 61–76. [Google Scholar] [CrossRef]
- Kisku, K.; Naik, U.C. Nanobiotechnology: A Process to Combat Abiotic Stress in Crop Plants. In Nanobiotechnology: Mitigation of Abiotic Stress in Plants; Al-Khayri, J.M., Ansari, M.I., Singh, A.K., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 139–163. [Google Scholar] [CrossRef]
- Barba-Espín, G.; Hernández, J.A.; Díaz-Vivancos, P. Antioxidant system: The hub of bud dormancy regulation in Prunus sp. Sci. Hortic. 2022, 305, 111396. [Google Scholar] [CrossRef]
- Bound, S.A.; Foo, E.; Gélinas-Marion, A.; Nichols, D.S.; Nissen, R. The impact of dormancy breakers on hormone profiles, Fruit Growth and Quality in Sweet Cherry. Agriculture 2022, 12, 270. [Google Scholar] [CrossRef]
- Yamane, H.; Singh, A.K.; Cooke, J.E.K. Plant dormancy research: From environmental control to molecular regulatory networks. Tree Physiol. 2021, 41, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Lloret, A.; Quesada-Traver, C.; Ríos, G. Models for a molecular calendar of bud-break in fruit trees. Sci. Hortic. 2022, 297, 110972. [Google Scholar] [CrossRef]
- Hernandez, J.A.; Díaz-Vivancos, P.; Martínez-Sánchez, G.; Alburquerque, N.; Martínez, D.; Barba-Espín, G.; Acosta-Motos, J.R.; Carrera, E.; García-Bruntón, J. Physiological and biochemical characterization of bud dormancy: Evolution of carbohydrate and antioxidant metabolisms and hormonal profile in a low chill peach variety. Sci. Hortic. 2021, 281, 109957. [Google Scholar] [CrossRef]
- Wang, S.Y.; Steffens, G.L.; Faust, M. Breaking bud dormancy in apple with a plant bioregulator, thidiazuron. Phytochemistry 1986, 25, 311–317. [Google Scholar] [CrossRef]
- Bera, K.; Dutta, P.; Sadhukhan, S. Plant Responses Under Abiotic Stress and Mitigation Options Towards Agricultural Sustainability. In Plant Stress: Challenges and Management in the New Decade; Roy, S., Mathur, P., Chakraborty, A.P., Saha, S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 3–28. [Google Scholar] [CrossRef]
- Ntanos, E.; Tsafouros, A.; Denaxa, N.-K.; Kosta, A.; Bouchagier, P.; Roussos, P.A. Mitigation of high solar irradiance and heat stress in kiwifruit during summer via the use of alleviating products with different modes of action—Part 1 Effects on leaf physiology and biochemistry. Agriculture 2022, 12, 2121. [Google Scholar] [CrossRef]
- Khattak, W.; Sun, J.; Abbas, A.; Hameed, R.; Jalal, D.; Niaz, N.; Anwar, S.; Liu, Y.; Wang, Y. Melatonin alleviating drought stress in plants: A review. S. Afr. J. Bot. 2023, 161, 192–201. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Naga, K.C.; Kumar, R.; Chourasia, K.N.; Subhash, S.; Kumar, D.; Sharma, S. Emerging roles of melatonin in mit-igating abiotic and biotic stresses of horticultural crops. Sci. Hortic. 2020, 272, 109592. [Google Scholar] [CrossRef]
- Sajjad, N.; Bhat, E.A.; Hassan, S.; Ali, R.; Shah, D. Role of Melatonin in Amelioration of Abiotic Stress-induced Damages. In Pro-Tective Chemical Agents in the Amelioration of Plant Abiotic Stress. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress; Roychoudhury, K., Tripathi, D.K., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 306–317. [Google Scholar] [CrossRef]
- Parankusam, S.; Jalli, L.; Bhatnagar-Mathur, P.; Sharma, K. Emerging Roles of Salicylic Acid and Jasmonates in Plant Abiotic Stress Responses. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress; Roychoudhury, K., Tripathi, D.K., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 342–373. [Google Scholar] [CrossRef]
- Zahid, G.; Iftikhar, S.; Shimira, F.; Ahmad, H.M.; Aka Kaçar, Y. An overview and recent progress of plant growth regulators (PGRs) in the mitigation of abiotic stresses in fruits: A review. Sci. Hortic. 2023, 309, 111621. [Google Scholar] [CrossRef]
- Vardhini, B. Brassinosteroids and Salicylic Acid as Chemical Agents to Ameliorate Diverse Environmental Stresses in Plants. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress; Roychoudhury, K., Tripathi, D.K., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 389–412. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; Bala, M.; Pareek, A.; Singla-Pareek, S.L.; Singh, A.K. Silicon-mediated abiotic and biotic stress mitigation in plants: Underlying mechanisms and potential for stress resilient agriculture. Plant Physiol. Biochem. 2021, 163, 15–25. [Google Scholar] [CrossRef]
- Ahmad, F.; Singh, A.; Kamal, A. Osmoprotective Role of Sugar in Mitigating Abiotic Stress in Plants. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress; Roychoudhury, K., Tripathi, D.K., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 53–70. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Kundu, A. Sugars and Sugar Polyols in Overcoming Environmental Stresses. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress; Roychoudhury, K., Tripathi, D.K., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 71–101. [Google Scholar] [CrossRef]
- Graziani, G.; Cirillo, A.; Giannini, P.; Conti, S.; El-Nakhel, C.; Rouphael, Y.; Ritieni, A.; Di Vaio, C. Biostimulants improve plant growth and bioactive compounds of young olive trees under abiotic stress conditions. Agriculture 2022, 12, 227. [Google Scholar] [CrossRef]
- Denaxa, N.-K.; Roussos, P.A.; Damvakaris, T.; Stournaras, V. Comparative effects of exogenous glycine betaine, kaolin clay particles and Ambiol on photosynthesis, leaf sclerophylly indexes and heat load of olive cv. Chondrolia Chalkidikis under drought. Sci. Hortic. 2012, 137, 87–94. [Google Scholar] [CrossRef]
- Abobatta, w. Fruit orchards under climate change conditions. J. Appl. Bio. Bioeng. 2021, 8, 99–102. [Google Scholar] [CrossRef]
- Denaxa, N.-K.; Tsafouros, A.; Ntanos, E.; Roussos, P.A. Chapter 8—Role of glycine betaine in the protection of plants against environmental stresses. In Plant Stress Mitigators; Ghorbanpour, M., Adnan Shahid, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 127–158. [Google Scholar] [CrossRef]
- Bhatt, M.D.; Bhatt, D. Strigolactones in Overcoming Environmental Stresses. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress; Roychoudhury, K., Tripathi, D.K., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 327–341. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, C.-B.; Ren, R.-M.; Jiang, J.-H. Salicylic acid had the potential to enhance tolerance in horticultural crops against abiotic stress. Front. Plant Sci. 2023, 14, 1141918. [Google Scholar] [CrossRef]
- Khalid, M.F.; Saleem, M.S.; Zakir, I.; Khan, R.I.; Sohail, M.; Ejaz, S.; Anjum, M.A.; Sabir, S.; Ali, S.; Ahmad, S.; et al. Chapter 3—Salicylic acid induced abiotic stress tolerance in plants. In Plant Stress Mitigators; Ghorbanpour, M., Adnan Shahid, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 57–67. [Google Scholar] [CrossRef]
- Reddy, K.S.; Wakchaure, G.; Khapte, P.; Changan, S. Plant Bio-stimulants for Mitigating Abiotic Stresses in Agriculture. Indian J. Fertil. 2023, 19, 788–800. [Google Scholar]
- Biondi, S.; Antognoni, F.; Marincich, L.; Mariacaterina, L.; Tejos, R.; Ruiz Carrasco, K. The polyamine “multiverse” and stress mit-igation in crops: A case study with seed priming in quinoa. Sci. Hortic. 2022, 304, 111292. [Google Scholar] [CrossRef]
- Sajjad, N.; Bhat, E.A.; Shah, D.; Wani, A.; Nazir, N.; Ali, R.; Hassan, S. Abscisic Acid Application and Abiotic Stress Ameliora-tion. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress; Roychoudhury, K., Tripathi, D.K., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 280–290. [Google Scholar] [CrossRef]
- Munir, N.; Hanif, M.; Abideen, Z.; Sohail, M.; El-Keblawy, A.; Radicetti, E.; Mancinelli, R.; Haider, G. Mechanisms and strategies of plant microbiome interactions to mitigate abiotic stresses. Agronomy 2022, 12, 2069. [Google Scholar] [CrossRef]
- Singh, A.; Mazahar, S.; Chapadgaonkar, S.S.; Giri, P.; Shourie, A. Phyto-microbiome to mitigate abiotic stress in crop plants. Front. Microbiol. 2023, 14, 1210890. [Google Scholar] [CrossRef] [PubMed]
- Imran, S.; Sarker, P.; Hoque, M.N.; Paul, N.C.; Mahamud, M.A.; Chakrobortty, J.; Tahjib-Ul-Arif, M.; Latef, A.A.H.A.; Hasanuz-zaman, M.; Rhaman, M.S. Biochar actions for the mitigation of plant abiotic stress. Crop Pasture Sci. 2023, 74, 6–20. [Google Scholar] [CrossRef]
- Huang, L.; Fu, W.; Zhang, Y.; Liu, X.; Wang, Q.; Wang, L.; Tanveer, M. The role of melatonin in regulating horticultural crop production under various abiotic stresses. Sci. Hortic. 2024, 323, 112508. [Google Scholar] [CrossRef]
- Koh, Y.S.; Wong, S.K.; Ismail, N.H.; Zengin, G.; Duangjai, A.; Saokaew, S.; Phisalprapa, P.; Tan, K.W.; Goh, B.H.; Tang, S.Y. Mit-igation of Environmental Stress-Impacts in Plants: Role of Sole and Combinatory Exogenous Application of Glutathione. Front. Plant Sci. 2021, 12, 791205. [Google Scholar] [CrossRef] [PubMed]
- Das, K. Ascorbate and Tocopherols in Mitigating Oxidative Stress. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress; Roychoudhury, K., Tripathi, D.K., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 102–121. [Google Scholar] [CrossRef]
- Amist, N.; Singh, N.B. Role of Glutathione Application in Overcoming Environmental Stress. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress; Roychoudhury, K., Tripathi, D.K., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 122–146. [Google Scholar] [CrossRef]
- Machado, R.; Serralheiro, R. Chapter 33—Salt stress alleviation through fertilization in fruit crops. In Fruit Crops; Srivastava, A.K., Hu, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 465–480. [Google Scholar] [CrossRef]
- Ali, R.; Hassan, S.; Shah, D.; Sajjad, N.; Bhat, E.A. Role of Polyamines in Mitigating Abiotic Stress. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress; Roychoudhury, K., Tripathi, D.K., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 291–305. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, G.; Liu, C.; Li, H.; Chen, X.; Fu, Q.; Lin, Y.; Guo, B. Salicylic Acid, a multifaceted hormone, combats abiotic stresses in plants. Life 2022, 12, 886. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).