Synaptosomes: A Functional Tool for Studying Neuroinflammation
Definition
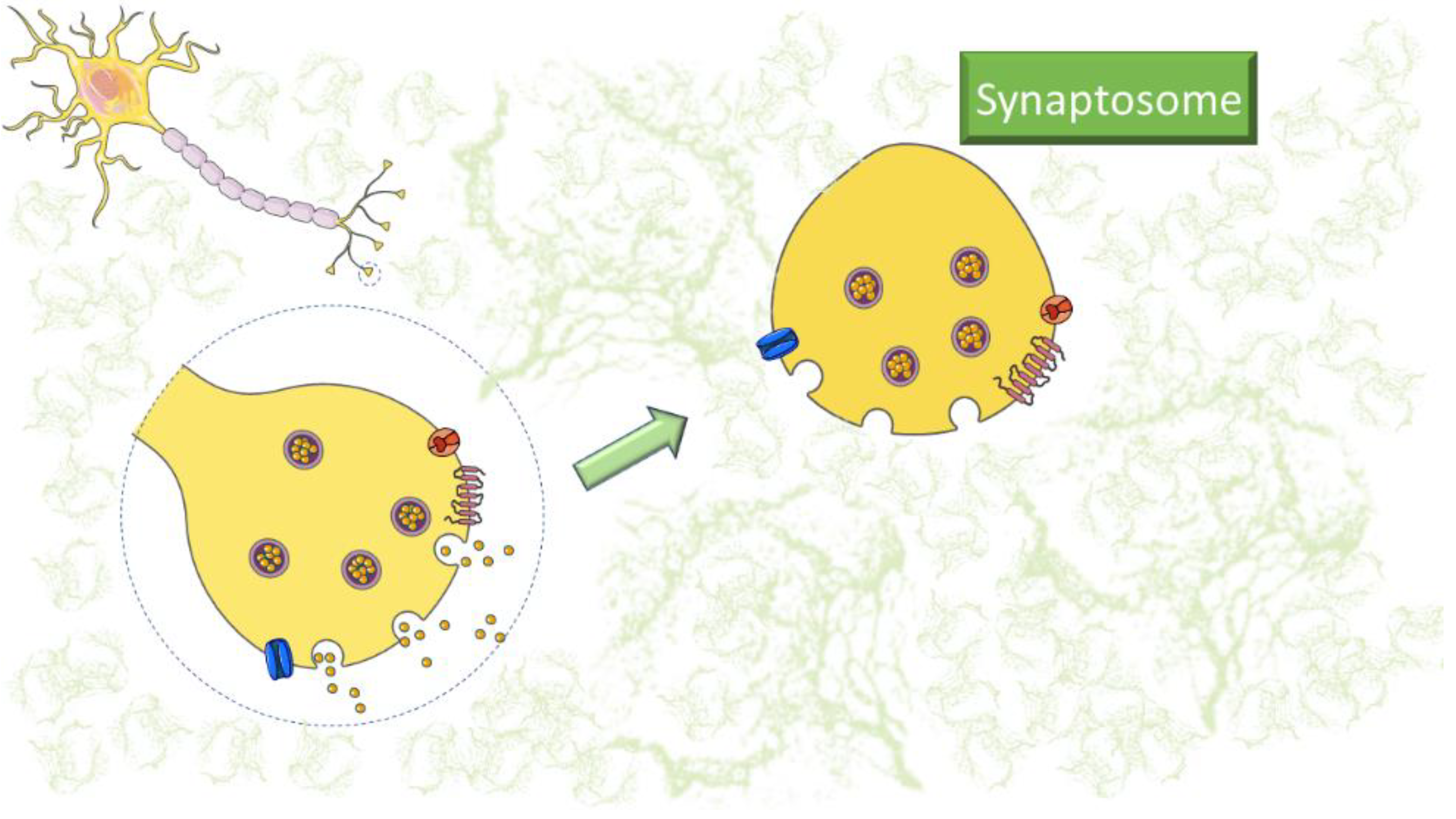
1. Introduction
2. Synaptosomes Applications
2.1. Synaptosomes and Western Blot Analysis
2.2. Synaptosomes in Flow Cytometry
2.3. Synaptosomes and Neurotransmitter Release
2.4. Synaptosome and Proteomic Analysis
2.5. Synaptosome and Confocal Microscopy
3. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rauf, A.; Badoni, H.; Abu-Izneid, T.; Olatunde, A.; Rahman, M.M.; Painuli, S.; Semwal, P.; Wilairatana, P.; Mubarak, M.S. Neuroinflammatory Markers: Key Indicators in the Pathology of Neurodegenerative Diseases. Molecules 2022, 27, 3194. [Google Scholar] [CrossRef]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xanthos, D.N.; Sandkühler, J. Neurogenic neuroinflammation: Inflammatory CNS reactions in response to neuronal activity. Nat. Rev. Neurosci. 2014, 15, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-R.; Xu, Z.-Z.; Gao, Y.-J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, F.; Cechova, K.; Amlerova, J.; Hort, J. Antibiotics, gut microbiota, and Alzheimer’s disease. J. Neuroinflammation 2019, 16, 108. [Google Scholar] [CrossRef]
- Zielinski, M.R.; Gibbons, A.J. Neuroinflammation, Sleep, and Circadian Rhythms. Front. Cell. Infect. Microbiol. 2022, 12, 853096. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef]
- Rao, J.S.; Kim, H.-W.; Kellom, M.; Greenstein, D.; Chen, M.; Kraft, A.D.; Harry, G.J.; Rapoport, S.I.; Basselin, M. Increased neuroinflammatory and arachidonic acid cascade markers, and reduced synaptic proteins, in brain of HIV-1 transgenic rats. J. Neuroinflammation 2011, 8, 101. [Google Scholar] [CrossRef]
- Mottahedin, A.; Ardalan, M.; Chumak, T.; Riebe, I.; Ek, J.; Mallard, C. Effect of Neuroinflammation on Synaptic Organization and Function in the Developing Brain: Implications for Neurodevelopmental and Neurodegenerative Disorders. Front. Cell. Neurosci. 2017, 11, 190. [Google Scholar] [CrossRef]
- Valcarcel-Ares, M.N.; Tucsek, Z.; Kiss, T.; Giles, C.B.; Tarantini, S.; Yabluchanskiy, A.; Balasubramanian, P.; Gautam, T.; Galvan, V.; Ballabh, P.; et al. Obesity in Aging Exacerbates Neuroinflammation, Dysregulating Synaptic Function-Related Genes and Altering Eicosanoid Synthesis in the Mouse Hippocampus: Potential Role in Impaired Synaptic Plasticity and Cognitive Decline. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 290–298. [Google Scholar] [CrossRef]
- Lauderback, C.M.; Kanski, J.; Hackett, J.M.; Maeda, N.; Kindy, M.S.; Butterfield, D.A. Apolipoprotein E modulates Alzheimer’s Abeta(1-42)-induced oxidative damage to synaptosomes in an allele-specific manner. Brain Res. 2002, 924, 90–97. [Google Scholar] [CrossRef] [PubMed]
- GRAY, E.G.; WHITTAKER, V.P. The isolation of nerve endings from brain: An electron-microscopic study of cell fragments derived by homogenization and centrifugation. J. Anat. 1962, 96, 79–88. [Google Scholar] [PubMed]
- Lin, T.Y.; Yang, T.-T.; Lu, C.W.; Wang, S.-J. Inhibition of glutamate release by bupropion in rat cerebral cortex nerve terminals. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 598–606. [Google Scholar] [CrossRef]
- Canas, P.M.; Duarte, J.M.N.; Rodrigues, R.J.; Köfalvi, A.; Cunha, R.A. Modification upon aging of the density of presynaptic modulation systems in the hippocampus. Neurobiol. Aging 2009, 30, 1877–1884. [Google Scholar] [CrossRef]
- Ahmad, F.; Jing, Y.; Lladó, A.; Liu, P. Chemical stimulation of rodent and human cortical synaptosomes: Implications in neurodegeneration. Cells 2021, 10, 1174. [Google Scholar] [CrossRef]
- Picone, P.; Porcelli, G.; Bavisotto, C.C.; Nuzzo, D.; Galizzi, G.; Biagio, P.L.S.; Bulone, D.; Carlo, M.D.; Di Carlo, M. Synaptosomes: New vesicles for neuronal mitochondrial transplantation. J. Nanobiotechnology 2021, 19, 6. [Google Scholar] [CrossRef]
- Wang, C.-C.; Hsieh, P.-W.; Kuo, J.-R.; Wang, S.-J. Rosmarinic Acid, a Bioactive Phenolic Compound, Inhibits Glutamate Release from Rat Cerebrocortical Synaptosomes through GABAA Receptor Activation. Biomolecules 2021, 11, 1029. [Google Scholar] [CrossRef] [PubMed]
- Sellgren, C.M.; Gracias, J.; Watmuff, B.; Biag, J.D.; Thanos, J.M.; Whittredge, P.B.; Fu, T.; Worringer, K.; Brown, H.E.; Wang, J.; et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat. Neurosci. 2019, 22, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, S.D.; Horng, J.E.; Perlis, R.H. Patient-Derived In Vitro Models of Microglial Function and Synaptic Engulfment in Schizophrenia. Biol. Psychiatry 2022, 92, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Jhou, J.-F.; Tai, H.-C. The Study of Postmortem Human Synaptosomes for Understanding Alzheimer’s Disease and Other Neurological Disorders: A Review. Neurol. Ther. 2017, 6, 57–68. [Google Scholar] [CrossRef]
- Györffy, B.A.; Tóth, V.; Török, G.; Gulyássy, P.; Kovács, R.; Vadászi, H.; Micsonai, A.; Tóth, M.E.; Sántha, M.; Homolya, L.; et al. Synaptic mitochondrial dysfunction and septin accumulation are linked to complement-mediated synapse loss in an Alzheimer’s disease animal model. Cell. Mol. Life Sci. 2020, 77, 5243–5258. [Google Scholar] [CrossRef]
- Marcelli, S.; Ficulle, E.; Iannuzzi, F.; Kövari, E.; Nisticò, R.; Feligioni, M. Targeting SUMO-1ylation Contrasts Synaptic Dysfunction in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2017, 54, 6609–6623. [Google Scholar] [CrossRef]
- Gylys, K.H.; Fein, J.A.; Cole, G.M. Quantitative characterization of crude synaptosomal fraction (P-2) components by flow cytometry. J. Neurosci. Res. 2000, 61, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Hobson, B.D.; Sims, P.A. Critical analysis of particle detection artifacts in synaptosome flow cytometry. eNeuro 2019, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Gulyássy, P.; Puska, G.; Györffy, B.A.; Todorov-Völgyi, K.; Juhász, G.; Drahos, L.; Kékesi, K.A. Proteomic comparison of different synaptosome preparation procedures. Amino Acids 2020, 52, 1529–1543. [Google Scholar] [CrossRef] [PubMed]
- Plum, S.; Eggers, B.; Helling, S.; Stepath, M.; Theiss, C.; Leite, R.E.P.P.; Molina, M.; Grinberg, L.T.; Riederer, P.; Gerlach, M.; et al. Proteomic Characterization of Synaptosomes from Human Substantia Nigra Indicates Altered Mitochondrial Translation in Parkinson’s Disease. Cells 2020, 9, 2580. [Google Scholar] [CrossRef]
- Gogoi, P.; Shiozaki, M.; Gouaux, E. Isolation, cryo-laser scanning confocal microscope imaging and cryo-FIB milling of mouse glutamatergic synaptosomes. PLoS ONE 2022, 17, e0271799. [Google Scholar] [CrossRef]
- Massaro Tieze, S.; Chandra, S.S.; Vidyadhara, D.J. Subcellular Fractionation for the Isolation of Synaptic Components from the Murine Brain. J. Vis. Exp. 2022, 2022, 64574. [Google Scholar] [CrossRef]
- Miyoshi, E.; Bilousova, T.; Melnik, M.; Fakhrutdinov, D.; Poon, W.W.; Vinters, H.V.; Miller, C.A.; Corrada, M.; Kawas, C.; Bohannan, R.; et al. Exosomal tau with seeding activity is released from Alzheimer’s disease synapses, and seeding potential is associated with amyloid beta. Lab. Investig. 2021, 101, 1605–1617. [Google Scholar] [CrossRef]
- Sapp, E.; Seeley, C.; Iuliano, M.; Weisman, E.; Vodicka, P.; DiFiglia, M.; Kegel-Gleason, K.B. Protein changes in synaptosomes of Huntington’s disease knock-in mice are dependent on age and brain region. Neurobiol. Dis. 2020, 141, 104950. [Google Scholar] [CrossRef]
- Fonseca-Ornelas, L.; Viennet, T.; Rovere, M.; Jiang, H.; Liu, L.; Nuber, S.; Ericsson, M.; Arthanari, H.; Selkoe, D.J. Altered conformation of α-synuclein drives dysfunction of synaptic vesicles in a synaptosomal model of Parkinson’s disease. Cell Rep. 2021, 36, 109333. [Google Scholar] [CrossRef]
- Sánchez-Sarasúa, S.; Fernández-Pérez, I.; Espinosa-Fernández, V.; Sánchez-Pérez, A.M.; Ledesma, J.C. Can we treat neuroinflammation in alzheimer’s disease? Int. J. Mol. Sci. 2020, 21, 8751. [Google Scholar] [CrossRef] [PubMed]
- Lanni, C.; Fagiani, F.; Racchi, M.; Preda, S.; Pascale, A.; Grilli, M.; Allegri, N.; Govoni, S. Beta-amyloid short- and long-term synaptic entanglement. Pharmacol. Res. 2019, 139, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.N.; Kumar, M.; Fedele, E.; Bonanno, G.; Bonifacino, T. MicroRNA Alteration, Application as Biomarkers, and Therapeutic Approaches in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 4718. [Google Scholar] [CrossRef] [PubMed]
- Obrador, E.; Salvador, R.; López-Blanch, R.; Jihad-Jebbar, A.; Vallés, S.L.; Estrela, J.M. Oxidative Stress, Neuroinflammation and Mitochondria in the Pathophysiology of Amyotrophic Lateral Sclerosis. Antioxidants 2020, 9, 901. [Google Scholar] [CrossRef] [PubMed]
- Raiteri, L.; Raiteri, M. Synaptosomes Still Viable after 25 Years of Superfusion. Neurochem. Res. 2000, 25, 1265–1274. [Google Scholar] [CrossRef]
- Pittaluga, A. Acute functional adaptations in isolated presynaptic terminals unveil synaptosomal learning and memory. Int. J. Mol. Sci. 2019, 20, 3641. [Google Scholar] [CrossRef]
- Raiteri, M.; Sala, R.; Fassio, A.; Rossetto, O.; Bonanno, G. Entrapping of impermeant probes of different size into nonpermeabilized synaptosomes as a method to study presynaptic mechanisms. J. Neurochem. 2000, 74, 423–431. [Google Scholar] [CrossRef]
- Grilli, M.; Summa, M.; Salamone, A.; Olivero, G.; Zappettini, S.; Prisco, S.D.; Feligioni, M.; Usai, C.; Pittaluga, A.; Marchi, M.; et al. In vitro exposure to nicotine induces endocytosis of presynaptic AMPA receptors modulating dopamine release in rat nucleus accumbens nerve terminals. Neuropharmacology 2012, 63, 916–926. [Google Scholar] [CrossRef]
- Trebesova, H.; Olivero, G.; Marchi, M.; Grilli, M. The Anti-Aggregative Peptide KLVFF Mimics Aβ1-40 in the Modulation of Nicotinic Receptors: Implications for Peptide-Based Therapy. Biomedicines 2022, 10, 2231. [Google Scholar] [CrossRef]
- Olivero, G.; Cisani, F.; Marimpietri, D.; Di Paolo, D.; Gagliani, M.C.; Podestà, M.; Cortese, K.; Pittaluga, A. The Depolarization-Evoked, Ca2+-Dependent Release of Exosomes From Mouse Cortical Nerve Endings: New Insights Into Synaptic Transmission. Front. Pharmacol. 2021, 12, 670158. [Google Scholar] [CrossRef]
- Bilousova, T.; Melnik, M.; Miyoshi, E.; Gonzalez, B.L.; Poon, W.W.; Vinters, H.V.; Miller, C.A.; Corrada, M.M.; Kawas, C.; Hatami, A.; et al. Apolipoprotein E/Amyloid-β Complex Accumulates in Alzheimer Disease Cortical Synapses via Apolipoprotein E Receptors and Is Enhanced by APOE4. Am. J. Pathol. 2019, 189, 1621–1636. [Google Scholar] [CrossRef]
- Wang, S.; Li, B.; Solomon, V.; Fonteh, A.; Rapoport, S.I.; Bennett, D.A.; Arvanitakis, Z.; Chui, H.C.; Sullivan, P.M.; Yassine, H.N. Calcium-dependent cytosolic phospholipase A2 activation is implicated in neuroinflammation and oxidative stress associated with ApoE4. Mol. Neurodegener. 2022, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sisqués, L.; Sancho-Balsells, A.; Solana-Balaguer, J.; Campoy-Campos, G.; Vives-Isern, M.; Soler-Palazón, F.; Anglada-Huguet, M.; López-Toledano, M.-Á.; Mandelkow, E.-M.; Alberch, J.; et al. RTP801/REDD1 contributes to neuroinflammation severity and memory impairments in Alzheimer’s disease. Cell Death Dis. 2021, 12, 616. [Google Scholar] [CrossRef] [PubMed]
- Marcatti, M.; Fracassi, A.; Montalbano, M.; Natarajan, C.; Krishnan, B.; Kayed, R.; Taglialatela, G. Aβ/tau oligomer interplay at human synapses supports shifting therapeutic targets for Alzheimer’s disease. Cell. Mol. Life Sci. 2022, 79, 222. [Google Scholar] [CrossRef] [PubMed]
- Cefaliello, C.; Penna, E.; Barbato, C.; Di Ruberto, G.; Mollica, M.P.; Trinchese, G.; Cigliano, L.; Borsello, T.; Chun, J.T.; Giuditta, A.; et al. Deregulated Local Protein Synthesis in the Brain Synaptosomes of a Mouse Model for Alzheimer’s Disease. Mol. Neurobiol. 2020, 57, 1529–1541. [Google Scholar] [CrossRef]
- Megur, A.; Baltriukien, D.; Bukelskien, V.; Burokas, A. The Microbiota-Gut-Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame? Nutrients 2020, 1, 37. [Google Scholar] [CrossRef]
- Kovács, R.A.; Vadászi, H.; Bulyáki, E.; Török, G.; Tóth, V.; Mátyás, D.; Kun, J.; Hunyadi-Gulyás, E.; Fedor, F.Z.; Csincsi, A.; et al. Identification of Neuronal Pentraxins as Synaptic Binding Partners of C1q and the Involvement of NP1 in Synaptic Pruning in Adult Mice. Front. Immunol. 2021, 11, 599771. [Google Scholar] [CrossRef]
- Bhatti, G.K.; Reddy, A.P.; Reddy, P.H.; Bhatti, J.S. Lifestyle Modifications and Nutritional Interventions in Aging-Associated Cognitive Decline and Alzheimer’s Disease. Front. Aging Neurosci. 2020, 11, 369. [Google Scholar] [CrossRef]
- Wijasa, T.S.; Sylvester, M.; Brocke-Ahmadinejad, N.; Schwartz, S.; Santarelli, F.; Gieselmann, V.; Klockgether, T.; Brosseron, F.; Heneka, M.T. Quantitative proteomics of synaptosome S-nitrosylation in Alzheimer’s disease. J. Neurochem. 2020, 152, 710–726. [Google Scholar] [CrossRef]
- Largo-Barrientos, P.; Apóstolo, N.; Creemers, E.; Callaerts-Vegh, Z.; Swerts, J.; Davies, C.; McInnes, J.; Wierda, K.; De Strooper, B.; Spires-Jones, T.; et al. Lowering Synaptogyrin-3 expression rescues Tau-induced memory defects and synaptic loss in the presence of microglial activation. Neuron 2021, 109, 767–777.e5. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.; Grant, S.G.N.N. Synapse pathology in Alzheimer’s disease. Semin. Cell Dev. Biol. 2022, 139, 13–23. [Google Scholar] [CrossRef]
- Sharman, M.J.; Verdile, G.; Kirubakaran, S.; Parenti, C.; Singh, A.; Watt, G.; Karl, T.; Chang, D.; Li, C.G.; Münch, G. Targeting Inflammatory Pathways in Alzheimer’s Disease: A Focus on Natural Products and Phytomedicines. CNS Drugs 2019, 33, 457–480. [Google Scholar] [CrossRef] [PubMed]
- Castelletto, V.; Ryumin, P.; Cramer, R.; Hamley, I.W.; Taylor, M.; Allsop, D.; Reza, M.; Ruokolainen, J.; Arnold, T.; Hermida-Merino, D.; et al. Self-assembly and anti-amyloid cytotoxicity activity of amyloid beta peptide derivatives. Sci. Rep. 2017, 7, 43637. [Google Scholar] [CrossRef] [PubMed]
- Saliba, S.W.; Bonifacino, T.; Serchov, T.; Bonanno, G.; de Oliveira, A.C.P.; Fiebich, B.L. Neuroprotective Effect of AM404 Against NMDA-Induced Hippocampal Excitotoxicity. Front. Cell. Neurosci. 2019, 13, 566. [Google Scholar] [CrossRef]
- Marogianni, C.; Sokratous, M.; Dardiotis, E.; Hadjigeorgiou, G.M.; Bogdanos, D.; Xiromerisiou, G. Neurodegeneration and inflammation—An interesting interplay in parkinson’s disease. Int. J. Mol. Sci. 2020, 21, 8421. [Google Scholar] [CrossRef]
- Lin, C.H.; Hsieh, C.L. Chinese Herbal Medicine for Treating Epilepsy. Front. Neurosci. 2021, 15, 682821. [Google Scholar] [CrossRef]
- Gorski, K.; Spoljaric, A.; Nyman, T.A.; Kaila, K.; Battersby, B.J.; Lehesjoki, A.E. Quantitative Changes in the Mitochondrial Proteome of Cerebellar Synaptosomes From Preclinical Cystatin B-Deficient Mice. Front. Mol. Neurosci. 2020, 13, 570640. [Google Scholar] [CrossRef]
- Manabe, T.; Rácz, I.; Schwartz, S.; Oberle, L.; Santarelli, F.; Emmrich, J.V.; Neher, J.J.; Heneka, M.T. Systemic inflammation induced the delayed reduction of excitatory synapses in the CA3 during ageing. J. Neurochem. 2021, 159, 525–542. [Google Scholar] [CrossRef]
- Jing, G.; Zuo, J.; Fang, Q.; Yuan, M.; Xia, Y.; Jin, Q.; Liu, Y.; Wang, Y.; Zhang, Z.; Liu, W.; et al. Erbin protects against sepsis-associated encephalopathy by attenuating microglia pyroptosis via IRE1α/Xbp1s-Ca2+ axis. J. Neuroinflammation 2022, 19, 237. [Google Scholar] [CrossRef]
- Düsedau, H.P.; Steffen, J.; Figueiredo, C.A.; Boehme, J.D.; Schultz, K.; Erck, C.; Korte, M.; Faber-Zuschratter, H.; Smalla, K.-H.; Dieterich, D.; et al. Influenza A Virus (H1N1) Infection Induces Microglial Activation and Temporal Dysbalance in Glutamatergic Synaptic Transmission. MBio 2021, 12, e01776-21. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, G.; Trinchese, G.; Penna, E.; Cimmino, F.; Pirozzi, C.; Lama, A.; Annunziata, C.; Catapano, A.; Mattace Raso, G.; Meli, R.; et al. High-Fat Diet Induces Neuroinflammation and Mitochondrial Impairment in Mice Cerebral Cortex and Synaptic Fraction. Front. Cell. Neurosci. 2019, 13, 509. [Google Scholar] [CrossRef] [PubMed]
- Han, T.K.; Leem, Y.H.; Kim, H.S. Treadmill exercise restores high fat diet-induced disturbance of hippocampal neurogenesis through β2-adrenergic receptor-dependent induction of thioredoxin-1 and brain-derived neurotrophic factor. Brain Res. 2019, 1707, 154–163. [Google Scholar] [CrossRef]
- Marte, A.; Cavallero, A.; Morando, S.; Uccelli, A.; Raiteri, M.; Fedele, E. Alterations of glutamate release in the spinal cord of mice with experimental autoimmune encephalomyelitis. J. Neurochem. 2010, 115, 343–352. [Google Scholar] [CrossRef]
- Di Prisco, S.; Merega, E.; Lanfranco, M.; Casazza, S.; Uccelli, A.; Pittaluga, A. Acute desipramine restores presynaptic cortical defects in murine experimental autoimmune encephalomyelitis by suppressing central CCL5 overproduction. Br. J. Pharmacol. 2014, 171, 2457–2467. [Google Scholar] [CrossRef]
- Vallarino, G.; Salis, A.; Lucarini, E.; Turrini, F.; Olivero, G.; Roggeri, A.; Damonte, G.; Boggia, R.; Mannelli, L.D.C.; Ghelardini, C.; et al. Healthy Properties of a New Formulation of Pomegranate-Peel Extract in Mice Suffering from Experimental Autoimmune Encephalomyelitis. Molecules 2022, 27, 914. [Google Scholar] [CrossRef]
- Chanaday, N.L.; Vilcaes, A.A.; de Paul, A.L.; Torres, A.I.; Degano, A.L.; Roth, G.A. Glutamate release machinery is altered in the frontal cortex of rats with experimental autoimmune encephalomyelitis. Mol. Neurobiol. 2015, 51, 1353–1367. [Google Scholar] [CrossRef]
- Vilcaes, A.A.; Furlan, G.; Roth, G.A. Inhibition of Ca2+-dependent glutamate release from cerebral cortex synaptosomes of rats with experimental autoimmune encephalomyelitis. J. Neurochem. 2009, 108, 881–890. [Google Scholar] [CrossRef]
- Fernández Hurst, N.; Chanaday, N.L.; Roth, G.A. GABAergic Agonists Modulate the Glutamate Release from Frontal Cortex Synaptosomes of Rats with Experimental Autoimmune Encephalomyelitis. Inflamm. Allergy Drug Targets 2015, 14, 105–110. [Google Scholar] [CrossRef]
- Di Filippo, M.; De Iure, A.; Giampà, C.; Chiasserini, D.; Tozzi, A.; Orvietani, P.L.; Ghiglieri, V.; Tantucci, M.; Durante, V.; Quiroga-Varela, A.; et al. Persistent activation of microglia and NADPH drive hippocampal dysfunction in experimental multiple sclerosis. Sci. Rep. 2016, 6, 20926. [Google Scholar] [CrossRef]
- Ravera, S.; Torazza, C.; Bonifacino, T.; Provenzano, F.; Rebosio, C.; Milanese, M.; Usai, C.; Panfoli, I.; Bonanno, G. Altered glucose catabolism in the presynaptic and perisynaptic compartments of SOD1G93A mouse spinal cord and motor cortex indicates that mitochondria are the site of bioenergetic imbalance in ALS. J. Neurochem. 2019, 151, 336–350. [Google Scholar] [CrossRef] [PubMed]
- Raiteri, L.; Paolucci, E.; Prisco, S.; Raiteri, M.; Bonanno, G. Activation of a glycine transporter on spinal cord neurons causes enhanced glutamate release in a mouse model of amyotrophic lateral sclerosis. Br. J. Pharmacol. 2003, 138, 1021–1025. [Google Scholar] [CrossRef]
- Meftahi, G.H.; Bahari, Z.; Zarei Mahmoudabadi, A.; Iman, M.; Jangravi, Z. Applications of western blot technique: From bench to bedside. Biochem. Mol. Biol. Educ. A Bimon. Publ. Int. Union Biochem. Mol. Biol. 2021, 49, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.W.; Choe, K.; Park, J.S.; Lee, H.J.; Kang, M.H.; Ahmad, R.; Kim, M.O. Pharmacological Inhibition of Spleen Tyrosine Kinase Suppressed Neuroinflammation and Cognitive Dysfunction in LPS-Induced Neurodegeneration Model. Cells 2022, 11, 1777. [Google Scholar] [CrossRef]
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef]
- Lu, C.W.; Lin, T.Y.; Huang, S.K.; Wang, S.J. 5-HT1B receptor agonist CGS12066 presynaptically inhibits glutamate release in rat hippocampus. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 86, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Polishchuk, A.; Cilleros-Mañé, V.; Just-Borràs, L.; Balanyà-Segura, M.; Vandellòs Pont, G.; Silvera Simón, C.; Tomàs, M.; Garcia, N.; Tomàs, J.; Lanuza, M.A. Synaptic retrograde regulation of the PKA-induced SNAP-25 and Synapsin-1 phosphorylation. Cell. Mol. Biol. Lett. 2023, 28, 17. [Google Scholar] [CrossRef] [PubMed]
- Morató, X.; López-Cano, M.; Canas, P.M.; Cunha, R.A.; Ciruela, F. Brain Membrane Fractionation: An Ex Vivo Approach to Assess Subsynaptic Protein Localization. J. Vis. Exp. 2017, 123, 55661. [Google Scholar] [CrossRef]
- Martín-Flores, N.; Romaní-Aumedes, J.; Rué, L.; Canal, M.; Sanders, P.; Straccia, M.; Allen, N.D.; Alberch, J.; Canals, J.M.; Pérez-Navarro, E.; et al. RTP801 Is Involved in Mutant Huntingtin-Induced Cell Death. Mol. Neurobiol. 2016, 53, 2857–2868. [Google Scholar] [CrossRef]
- Romaní-Aumedes, J.; Canal, M.; Martín-Flores, N.; Sun, X.; Pérez-Fernández, V.; Wewering, S.; Fernández-Santiago, R.; Ezquerra, M.; Pont-Sunyer, C.; Lafuente, A.; et al. Parkin loss of function contributes to RTP801 elevation and neurodegeneration in Parkinson’s disease. Cell Death Dis. 2014, 5, e1364. [Google Scholar] [CrossRef]
- Postupna, N.O.; Keene, C.D.; Latimer, C.; Sherfield, E.E.; Van Gelder, R.D.; Ojemann, J.G.; Montine, T.J.; Darvas, M. Flow cytometry analysis of synaptosomes from post-mortem human brain reveals changes specific to Lewy body and Alzheimer’s disease. Lab. Investig. 2014, 94, 1161–1172. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, F.; Mao, L.; Feng, T.; Wang, K.; Xu, M.; Lv, B.; Wang, X. Bifico relieves irritable bowel syndrome by regulating gut microbiota dysbiosis and inflammatory cytokines. Eur. J. Nutr. 2023, 62, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Postupna, N.O.; Latimer, C.S.; Keene, C.D.; Montine, K.S.; Thomas, J.; Darvas, M. Synaptosomes; Neuromethods; Murphy, K.M., Ed.; Springer: New York, NY, USA, 2018; Volume 141, ISBN 978-1-4939-8738-2. [Google Scholar]
- Sokolow, S.; Henkins, K.M.; Williams, I.A.; Vinters, H.V.; Schmid, I.; Cole, G.M.; Gylys, K.H. Isolation of synaptic terminals from Alzheimer’s disease cortex. Cytom. Part A 2012, 81A, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Gylys, K.H.; Fein, J.A.; Wiley, D.J.; Cole, G.M. Rapid annexin-V labeling in synaptosomes. Neurochem. Int. 2004, 44, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Centonze, D.; Muzio, L.; Rossi, S.; Cavasinni, F.; Chiara, V.D.; Bergami, A.; Musella, A.; D’Amelio, M.; Cavallucci, V.; Martorana, A.; et al. Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J. Neurosci. 2009, 29, 3442–3452. [Google Scholar] [CrossRef]
- Schaller, S.; Buttigieg, D.; Alory, A.; Jacquier, A.; Barad, M.; Merchant, M.; Gentien, D.; Grange, P.D.L.; Haase, G.; De La Grange, P.; et al. Novel combinatorial screening identifies neurotrophic factors for selective classes of motor neurons. Proc. Natl. Acad. Sci. USA 2017, 114, E2486–E2493. [Google Scholar] [CrossRef]
- Si, Z.-Z.; Zou, C.-J.; Mei, X.; Li, X.-F.; Luo, H.; Shen, Y.; Hu, J.; Li, X.-X.; Wu, L.; Liu, Y. Targeting neuroinflammation in Alzheimer’s disease: From mechanisms to clinical applications. Neural Regen. Res. 2023, 18, 708–715. [Google Scholar] [CrossRef]
- Pedicone, C.; Fernandes, S.; Matera, A.; Meyer, S.T.; Loh, S.; Ha, J.H.; Bernard, D.; Chisholm, J.D.; Paolicelli, R.C.; Kerr, W.G. Discovery of a novel SHIP1 agonist that promotes degradation of lipid-laden phagocytic cargo by microglia. iScience 2022, 25, 104170. [Google Scholar] [CrossRef]
- Abg Abd Wahab, D.Y.; Gau, C.H.; Zakaria, R.; Muthu Karuppan, M.K.; A-Rahbi, B.S.; Abdullah, Z.; Alrafiah, A.; Abdullah, J.M.; Muthuraju, S. Review on Cross Talk between Neurotransmitters and Neuroinflammation in Striatum and Cerebellum in the Mediation of Motor Behaviour. Biomed Res. Int. 2019, 2019, 1767203. [Google Scholar] [CrossRef]
- Pozdnyakova, N.; Krisanova, N.; Pastukhov, A.; Tarasenko, A.; Dudarenko, M.; Chernykh, A.; Pashenko, A.; Ryabukhin, S.; Tolstanova, G.; Volochnyuk, D.; et al. Neuromodulation by selective angiotensin-converting enzyme 2 inhibitors. Neuroscience 2022, 498, 155–173. [Google Scholar] [CrossRef]
- Krisanova, N.; Pozdnyakova, N.; Pastukhov, A.; Dudarenko, M.; Shatursky, O.; Gnatyuk, O.; Afonina, U.; Pyrshev, K.; Dovbeshko, G.; Yesylevskyy, S.; et al. Amphiphilic anti-SARS-CoV-2 drug remdesivir incorporates into the lipid bilayer and nerve terminal membranes influencing excitatory and inhibitory neurotransmission. Biochim. Biophys. Acta. Biomembr. 2022, 1864, 183945. [Google Scholar] [CrossRef] [PubMed]
- Storchak, L.G.; Kravchuk, M.V.; Himmelreich, N.H. Okadaic acid and cyclosporin A modulate [(3)H]GABA release from rat brain synaptosomes. Neurochem. Int. 2001, 38, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Y.; Zheng, K. Microglial mitophagy integrates the microbiota-gut-brain axis to restrain neuroinflammation during neurotropic herpesvirus infection. Autophagy 2023, 19, 734–736. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.W.; Dixon, C.E. Lithium Improves Dopamine Neurotransmission and Increases Dopaminergic Protein Abundance in the Striatum after Traumatic Brain Injury. J. Neurotrauma 2018, 35, 2827–2836. [Google Scholar] [CrossRef] [PubMed]
- Nishiham, I.; Toshiaki, M.; Watanabe, Y.; Ito, S.; Hayaishi, O. Prostaglandin E2 stimulates glutamate release from synaptosomes of rat spinal cord. Neurosci. Lett. 1995, 196, 57–60. [Google Scholar] [CrossRef]
- Gagné, F.; Bérubé, E.; Fournier, M.; Blaise, C. Inflammatory properties of municipal effluents to Elliptio complanata mussels—Lack of effects from anti-inflammatory drugs. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 141, 332–337. [Google Scholar] [CrossRef]
- D’arcangelo, G.; Tancredi, V.; Onofri, F.; D’antuono, M.; Giovedì, S.; Benfenati, F. Interleukin-6 inhibits neurotransmitter release and the spread of excitation in the rat cerebral cortex. Eur. J. Neurosci. 2000, 12, 1241–1252. [Google Scholar] [CrossRef]
- Ferreras, S.; Singh, N.P.; Le Borgne, R.; Bun, P.; Binz, T.; Parton, R.G.; Verbavatz, J.-M.; Vannier, C.; Galli, T. A synthetic organelle approach to probe SNARE-mediated membrane fusion in a bacterial host. J. Biol. Chem. 2023, 299, 102974. [Google Scholar] [CrossRef]
- Yang, R.; Gao, Y.; Li, H.; Huang, W.; Tu, D.; Yang, M.; Liu, X.; Hong, J.-S.; Gao, H.-M. Posttranslational S-nitrosylation modification regulates HMGB1 secretion and promotes its proinflammatory and neurodegenerative effects. Cell Rep. 2022, 40, 111330. [Google Scholar] [CrossRef]
- Kummer, M.P.; Hermes, M.; Delekarte, A.; Hammerschmidt, T.; Kumar, S.; Terwel, D.; Walter, J.; Pape, H.-C.; König, S.; Roeber, S.; et al. Nitration of tyrosine 10 critically enhances amyloid β aggregation and plaque formation. Neuron 2011, 71, 833–844. [Google Scholar] [CrossRef]
- Tchaikovskaya, T.; Fraifeld, V.; Urphanishvili, T.; Andorfer, J.H.; Davies, P.; Listowsky, I. Glutathione S-transferase hGSTM3 and ageing-associated neurodegeneration: Relationship to Alzheimer’s disease. Mech. Ageing Dev. 2005, 126, 309–315. [Google Scholar] [CrossRef]
- Giannoccaro, M.P.; La Morgia, C.; Rizzo, G.; Carelli, V. Mitochondrial DNA and primary mitochondrial dysfunction in Parkinson’s disease. Mov. Disord. 2017, 32, 346–363. [Google Scholar] [CrossRef]
- Kälviäinen, R.; Khyuppenen, J.; Koskenkorva, P.; Eriksson, K.; Vanninen, R.; Mervaala, E. Clinical picture of EPM1-Unverricht-Lundborg disease. Epilepsia 2008, 49, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, K.M.; Gardella, E.; Linnankivi, T.; Courage, C.; de Saint Martin, A.; Lehesjoki, A.-E.; Mignot, C.; Afenjar, A.; Lesca, G.; Abi-Warde, M.-T.; et al. Defining the phenotypic spectrum of SLC6A1 mutations. Epilepsia 2018, 59, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Marin-Dett, F.H.; Campanella, J.E.M.; Trovatti, E.; Bertolini, M.C.; Vergani, C.E.; Barbugli, P.A. Extracellular lipids of Candida albicans biofilm induce lipid droplet formation and decreased response to a topoisomerase I inhibitor in dysplastic and neoplastic oral cells. J. Appl. Oral Sci. 2023, 30, e20220319. [Google Scholar] [CrossRef] [PubMed]
- Györffy, B.A.; Kun, J.; Török, G.; Bulyáki, É.; Borhegyi, Z.; Gulyássy, P.; Kis, V.; Szocsics, P.; Micsonai, A.; Matkó, J.; et al. Local apoptotic-like mechanisms underlie complementmediated synaptic pruning. Proc. Natl. Acad. Sci. USA 2018, 115, 6303–6308. [Google Scholar] [CrossRef] [PubMed]
- Valbuena, G.N.; Tortarolo, M.; Bendotti, C.; Cantoni, L.; Keun, H.C. Altered Metabolic Profiles Associate with Toxicity in SOD1G93A Astrocyte-Neuron Co-Cultures. Sci. Rep. 2017, 7, 50. [Google Scholar] [CrossRef]
| Analytical Technique | Disease | Neuroinflammation Markers | References |
|---|---|---|---|
| Western Blot Flow Cytometry Neurotransmitter release Proteomics Confocal microscopy | Alzheimer’s disease | ROS | [1,5,11,21,29,32,42,43,44,45,46,47,48,49,50,51,52,53,54] |
| C1q | |||
| iNOS | |||
| TNF-α | |||
| IL-1β, IL-6, IL-1 | |||
| CCL2, CCL5, CXCL1 | |||
| Parkinson’s disease | TNFα | [1,26,49,55,56] | |
| IL-6 | |||
| NOS2 | |||
| ROS | |||
| Huntington’s disease | TNF-α | [1,30,44,55] | |
| IL-1β | |||
| IL-10 | |||
| NO | |||
| Epilepsy | ROS | [57,58] | |
| iNOS | |||
| NMDA-induced excitotoxicity | IL-1β, IL-6, TNFα, mPGES-1, COX-2 | [55,57] | |
| iNOS | |||
| SAE | IFN-γ | [59,60] | |
| IL-1β | |||
| IL-10 | |||
| TNF-α | |||
| IL-6 | |||
| CXCL1 | |||
| Influenza A Virus | CD80 | [61] | |
| CD36, CD68, | |||
| C1QA, C3 | |||
| HFD | BDNF | [62,63] | |
| IL-1β | |||
| TNF-α | |||
| NF-κB | |||
| Multiple Sclerosis | Iba-1 | [64,65,66,67,68,69,70] | |
| TNF-α | |||
| IL-17 | |||
| CCL5 | |||
| CXCR4 | |||
| Th1, Th17 | |||
| ROS | |||
| Amyotrophic Lateral Sclerosis | IL-1β, IL-12, | [35,71,72] | |
| IFN-γ, IL-1α | |||
| C1q | |||
| ROS | |||
| COX-2 | |||
| iNOS | |||
| TNF-α |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trebesova, H.; Grilli, M. Synaptosomes: A Functional Tool for Studying Neuroinflammation. Encyclopedia 2023, 3, 406-418. https://doi.org/10.3390/encyclopedia3020027
Trebesova H, Grilli M. Synaptosomes: A Functional Tool for Studying Neuroinflammation. Encyclopedia. 2023; 3(2):406-418. https://doi.org/10.3390/encyclopedia3020027
Chicago/Turabian StyleTrebesova, Hanna, and Massimo Grilli. 2023. "Synaptosomes: A Functional Tool for Studying Neuroinflammation" Encyclopedia 3, no. 2: 406-418. https://doi.org/10.3390/encyclopedia3020027
APA StyleTrebesova, H., & Grilli, M. (2023). Synaptosomes: A Functional Tool for Studying Neuroinflammation. Encyclopedia, 3(2), 406-418. https://doi.org/10.3390/encyclopedia3020027








