Definition
Photoremovable protecting groups (PPGs) (also often called photocages in the literature) are used for temporary inactivation of biologically active substrates. By photoirradiation the PPG could be cleaved off and the biological activity could be restored on-demand, with a high spatiotemporal precision. The on-site liberation of the biologically active substrate could be exploited for studying dynamic biological processes or for designing targeted pharmacological interventions in vitro or in vivo. Several chemical scaffolds have been described and tested as PPGs, operating at different wavelengths. The scope of potential substrates is very broad, spanning from small molecules to proteins. In a wider context, PPGs could be used for the design of various light-responsive materials as well, for diverse applications.
1. Introduction
Gaining spatiotemporal control over drug action or biological functions in a broader sense is a long sought for goal for therapeutic or experimental interventions. Thus, dynamic functions could be studied in vivo with high precision, ideally on a timescale relevant for the process studied, or in a clinical setting, deleterious side effects could be avoided or minimized. A possible approach towards this goal is to use materials/systems responding to a specific internal or external stimulus [1]. Chemical, physical and biological stimuli (e.g., pH, enzymes, ionic microenvironment, temperature, ultrasound, magnetic field, light) have been addressed in this respect. Of the various external stimuli, light has several potential benefits [2]. At appropriate wavelengths, phototoxicity could be avoided and light could be considered bioorthogonal (i.e., not interfering with biological signals or functioning). The light pulse can be precisely tuned in its duration and intensity and, with this external stimulus, extracellular regions or intracellular compartments could be selectively addressed, as necessary [3]. Moreover, the external activation is independent of the microenvironment vs. the case of endogenous approaches.
The operational mode of photoremovable protecting groups (PPGs, often referred to in the literature with the illustrative (photo)cage name, expressing the concept of the biological activity being trapped, although the term photolabile/photosensitive/photocleavable (protecting group) are also in use) is to temporarily inactivate the biological action of a given agent by linking a PPG to it. The action could be restored on-demand following a photoactivation step: the cleavage of the PPG via the dissociation of a covalent bond and the liberation of the parent biologically active compound (Figure 1). The activation step in the case of PPGs is typically a one-way, irreversible process [4]. (A reversible process, based on photoisomerisation occurs in the case of the so-called photoswitches [5]. The field of photoswitches—also referred to as photopharmacology—has seen a considerable expansion in the last decade with more elaborate applications emerging [6,7] that are, however, beyond the scope of the present entry). The relative simplicity of the PPG approach has its benefits, e.g., in terms of design, as the properties of an already optimized active agent could be further modified via a PPG linked to it. However, the approach has its limits as well, such as the on-site release of the PPG in stoichiometric amounts, the irreversibility of the process allowing only a one-time activation protocol, or the potential unwanted effects caused by the parent effector molecules upon diffusion from the intended site of action.
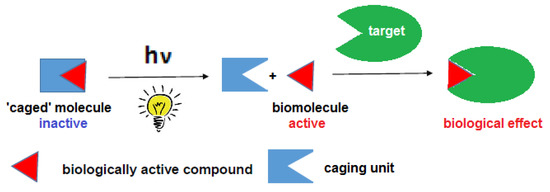
Figure 1.
A simplified overview of the operation of PPGs for the release of biologically active substrates (adapted from Korzycka et al. [8] and Piant et al. [9]).
The application of PPGs in the biological context dates back to the 1970s, to the first studies of Engels and Schlaeger and Kaplan et al. with caged cAMP and ATP [10,11], following an earlier report by Barltrop and Schofield on the photorelease of glycine (Figure 2) [12]. Although the present paper will focus on the biological applications of PPGs, synthetic applications (although relatively less common in the literature vs. the biological ones) could also be envisaged [13,14]. A deprotection step carried out under mild conditions and not requiring an additional reagent (i.e., light acting as a traceless reagent) is of considerable interest also for designing a (more complex/multi-step) synthesis pathway.
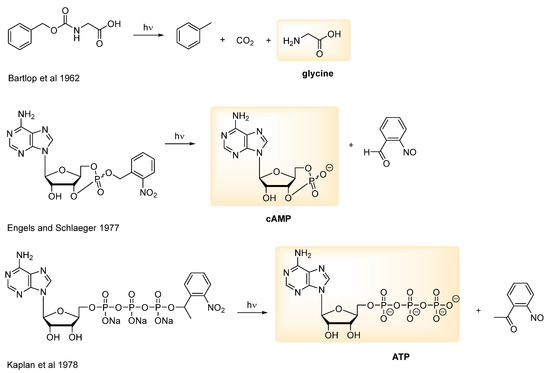
Figure 2.
First applications of PPGs for biologically active substrates: glycine [12], cAMP [10] and ATP [11].
2. Design and Applications of PPGs
2.1. The Substrate Scope of PPGs
Since the 1970s, a considerable effort has been dedicated both to the development of novel photoactivatable chemical probes and their applications in various experimental studies. Regarding the scope of PPG applications, the caged substrate could be as simple as a proton or an inorganic species or ion (e.g., Ca2+ [15], Zn2+ [16], CO [17], NO [18], H2S [19]), it could be a small molecule (e.g., second messenger (such as inositol-1,4,5-triphosphate (IP3) [20]), neurotransmitter (notably GABA and glutamate [21,22]), nucleotide [23], peptide [24], drug molecule [25] (such as antibiotics [26], analgesics [27] or anticancer agents [28]) or a more complex biomolecule (e.g., enzymes [29], RNA [30] or DNA [31]) (Figure 3).
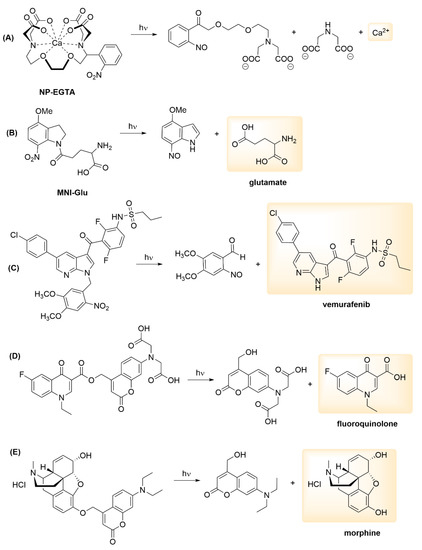
Figure 3.
Examples of PPG applications for small molecules/inorganic species: (A) calcium caging with photoactivatable EGTA [32], (B) neurotransmitter glutamate caging with MNI [21], (C) caging the anticancer agent vemurafenib with a nitrobenzyl PPG [33], (D) a coumarin PPG-caged antibiotic agent [34], (E) a coumarin PPG-caged analgesic [27].
2.2. Preparation of PPG-Substrate Constructs
With some exceptions, typically the PPG is linked with a covalent bond to the substrate. In the case of, e.g., Ca2+, instead of forming a covalent bond directly with the substrate, via photocleavage the affinity of calcium chelating agents (e.g., ethylene glycol tetraacetic acid (EGTA), 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)), is compromised irreversibly [15]. Binding the PPG to its substrate is possible via different functional groups, offering sufficient flexibility of design (binding is possible via, e.g., ester, ether, carbamate or amide bonds). Choosing the appropriate site (i.e., one where binding a PPG efficiently masks the biological activity) for introducing the PPG is often directed by computational studies [33]. Having sufficient structural and SAR information on the target concerned, the site(s) critical for biological activity could be judiciously addressed. However, more elaborate chemical modification(s) of the substrate could necessitate a collaboration of biologists and synthetic chemists and, therefore, the biological applications described are often based on a limited array of commercially available PPGs, despite the continuously growing number of alternative caging scaffolds (Figure 4 shows a non-exhaustive selection of the most important PPGs described so far) [35]. Besides a more efficient communication and collaboration between distinct scientific fields, application-driven photocage development could also contribute in the future to the transition from studies under laboratory settings towards (therapeutic) applications.
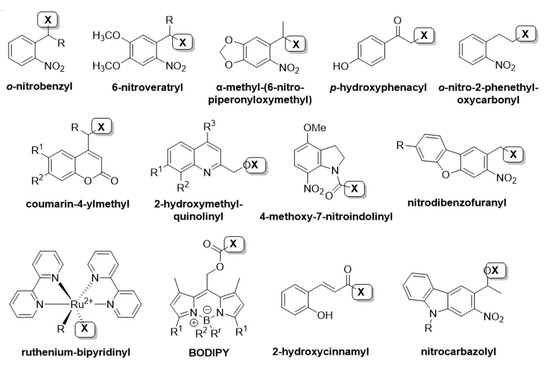
Figure 4.
PPG scaffolds used for biological applications [4,36,37], ‘X’ indicating the substrate to be liberated via photoactivation.
2.3. Application Criteria for PPGs
For their intended use, PPG-substrate constructs should comply with several criteria (such properties were considered by Lester and Nerbonne in 1982 [38]), some of them related to photophysics and photochemistry, some to the living system itself. Application criteria include good aqueous solubility (or solubility in the experimental medium); stability to hydrolysis (to avoid residual activity from the unmasked substrate, e.g., the often used ester bonds could be sensitive to hydrolysis); an efficient photorelease process (sufficiently rapid vs. the kinetics of the process to be studied, high yielding and not resulting in unwanted side products); photostability of the photolysis products; efficient masking of the biological activity with the PPG (the construct should be inactive prior to photoactivation); compatibility of the applied irradiation with the biological system in terms of wavelength and light intensity; and compatibility and non-reactivity of the PPG with the biological system to be studied/treated [35]. Additionally, a straightforward synthesis and purification process for the PPG itself and the PPG-substrate construct is necessary. To name a specific challenge, from a synthetic point of view, higher polarity molecules (e.g., molecules containing a number of hydrophilic groups such as sulfonates [39]) with better aqueous solubility could require aqueous synthesis and purification conditions, not typically used for organic synthesis. Upon choosing a chemical probe, often not all criteria of an ideal PPG are met. However, depending on the application, some shortcomings could still be tolerated.
2.4. UV and VIS (One-Photon) Activation of PPGs, Design Aspects
Regarding the activation signal used, phototoxicity of shorter (UV) wavelengths [40] and the tissue penetration of the light trigger are important factors when considering biological applications. The optical window of tissues (the wavelength region offering the best tissue penetration for light) spans the 650–900 nm (NIR) region [41,42], limited at the two extremities by the absorption of hemoglobin (<650 nm) and water (>900 nm). (Targeting this particular wavelength region is crucial also for in vivo optical imaging techniques.) Most of the PPGs used in experimental settings at present operate, however, outside this range (i.e., often at 350–450 nm). Work is ongoing for the development of PPGs operating at higher (visible/NIR) wavelengths (such as boron dipyrromethene (BODIPY) [36] or heptamethine cyanine derivatives [43]), that are more advantageous for in vivo operations. A straightforward approach is the modification of PPGs with moieties increasing the absorption wavelength (typically by creating systems with extended conjugation) [44]. To illustrate the optimization work around a specific scaffold, Figure 5 presents modifications of coumarin PPGs for obtaining probes operating at higher wavelengths. The modifications include extended conjugation with push-pull substituent patterns, application of conformationally locked electron-donating groups and a combination of π-extension with cationic moieties [45,46,47,48]. Of note, however, the different properties of PPGs are often difficult to fine-tune separately and the optimization of one characteristic could be detrimental for another (e.g., extended conjugation or the introduction of hydrophilic groups could alter the pharmacokinetics or the aqueous solubility of the construct).
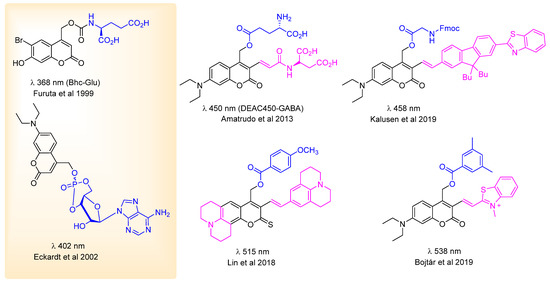
Figure 5.
A selection of modified coumarin PPGs operating at higher wavelengths [45,46,47,48]—with the parent coumarin PPG structures shown in the left panel, the cargos indicated in blue and the structural modifications vs. the parent structures [49,50] in violet.
2.5. NIR (Two-Photon) Activation of PPGs, Design Aspects. Characterization of PPGs
Alternatively, NIR wavelengths could be exploited for the photoactivation via a nonlinear optical process, the two-photon absorption (TPA) [51]. Whereas in this case the simultaneous absorption of two lower energy photons leads to excitation and consequent photoreaction, the process has a quadratic dependence on the light intensity (Figure 6). The excitation in this case necessitates on the one hand a maximum light intensity typically achieved at the focal point of the irradiation and, on the other hand, for practical purposes, a specific instrumentation. Two-photon (TP) activation has several advantages over the conventional one-photon (OP) activation. The longer wavelength used for TP activation is less phototoxic, has a deeper tissue penetration and the activation could be confined more efficiently, due to the inherent criteria of the process (i.e., a better resolution could be achieved).
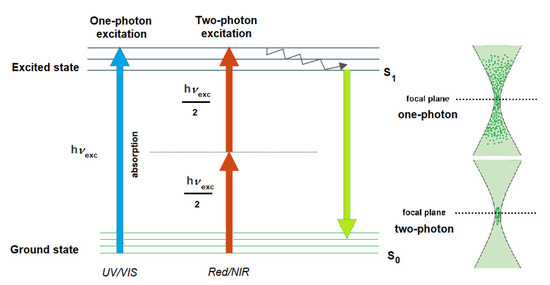
Figure 6.
Simplified Jablonski diagram of OP and TP activation (adapted from Klausen and Blanchard-Desce [51]).
PPGs are typically characterized by their absorption maxima and molar extinction coefficients (i.e., their light absorption properties), fluorescence, hydrolytic stability (crucial for avoiding unwanted release of the substrate prior to photoirradiation) and aqueous solubility. The quantum yield of the photolysis reaction (Φu—‘uncaging’ quantum yield) expresses the number of molecules liberated from the PPG-substrate construct vs. the photons absorbed. The overall photocleavage efficiency at a given wavelength depends also on the light-absorbing property of the construct (extinction coefficient—ε—in the case of a OP process or two-photon absorption cross-section—σ2—for a TP process). The OP and TP uncaging cross sections could be expressed as the product of the quantum yield and the respective extinction coefficient (ε, in M−1 cm−1 or two-photon absorption cross-section σ2, in GM (Göppert–Mayer), where 1 GM = 10−50 cm4 s photons−1) [51]. The quantitative cross section values reported in the literature could be used for a first evaluation and comparison of the novel PPGs disclosed. One-photon quantum yield measurements necessitate a monochromatic light source, that is often a mercury lamp or a LED/OLED source. Two-photon uncaging measurements require femtosecond pulsed infrared (Ti:Sapphire) lasers. The photolysis is often quantified by HPLC monitoring of the liberated substrate and the remaining fraction of the PPG-substrate construct [52]. With more sensitive chemical probes, a lower light intensity could be used, avoiding thereby phototoxicity. The minimal requirements regarding the uncaging performance of the probe are governed, however, by the studied system and the intended effect [53].
Particularly for TP PPGs, rational engineering and optimization of TP uncaging efficiency, i.e., predicting the effect of structural modifications on the uncaging process is still problematic, despite successful strategies for increasing the TP absorption itself [51,53]. To circumvent the issues related to the design of novel TP PPGs, modular approaches were suggested [54]: the light-harvesting and the cargo-releasing moieties of the probe are decoupled, by designing antenna-sensitized tandem systems operating via different mechanisms (such as triplet-energy transfer, photoinduced electron transfer or Förster resonance energy transfer sensitization—Figure 7) in which the two units could be optimized independently from each other.
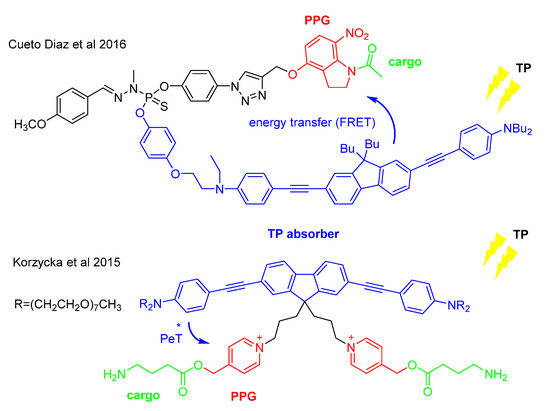
Figure 7.
Antenna-sensitized tandem systems for TP uncaging based on photoinduced electron transfer (PET) or Förster resonance energy transfer (FRET) between the branches of the construct [8,55]. In the former case, upon TP excitation the TP-absorbing unit donates an electron to the pyridinium PPG unit (* referring to the photoexcited state), leading to a photochemical reaction and the release of the cargo [8]. The latter example is based on an appropriately selected FRET donor-acceptor pair (emission of the donor in sufficient overlap with the absorption of the acceptor) linked by a thiophosphoryl unit [55].
2.6. Selected Applications of PPGs
PPGs via masking the toxicity or altering the physicochemical properties could enable harnessing drug molecules with a suboptimal ADMET profile. Dcona et al. attached a cell impermeable (sulfonated) small molecule to an anticancer drug (doxorubicin) via a nitroveratryl PPG linker [56]. While the construct itself could not enter the cells, upon photorelease the cell permeability as well as the consequent cytotoxic effect were restored (called ‘photocaged permeability’ by the authors).
Besides the on-site activation of a biological agent (i.e., prodrug applications), PPG-substrate constructs could be endowed with further (e.g., theranostic) functions, such as monitoring the distribution of the probe or the photorelease process (typically via a turn-on fluorescence phenomenon). In this respect, Wu et al. designed a construct composed of a coumarin PPG, an anticancer drug as a cargo (camptothecin), a cleavable linker unit and a NIR fluorescent dye (dicyanomethylene-4H-pyran) [57]. In the construct, the fluorescence of the drug molecule and the PPG is quenched by the dye unit via fluorescence energy transfer. The cellular uptake of the construct and its intracellular distribution could be monitored using the fluorescence of the NIR dye (red emission). Upon OP or TP activation, following a reaction cascade, the release of the drug molecule could be visualized via its restored fluorescence (besides observing its pharmacological–cytotoxic–action). Additionally, cell- or site-selective targeting units could be integrated into the construct. Singh et al. developed a mitochondria-targeting system (using an alkyltriphenylphosphonium (TPP) targeting moiety) with an o-hydroxycinnamate PPG, releasing doxorubicin upon light irradiation [58] (Figure 8). Although the o-hydroxycinnamate PPG itself is not fluorescent, upon its photoinduced isomerization and cyclization a fluorescent coumarin derivative is formed, that could be exploited for the real-time monitoring of the release process. In an in vivo setting, drug release monitoring options (besides a targeting functionality) could consequently allow more precise (local) dosing and optimization of the side effect profile.
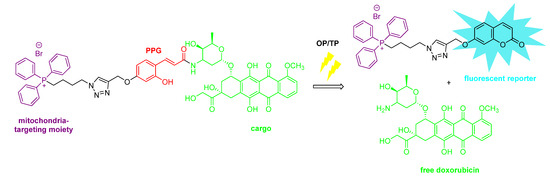
Figure 8.
Mitochondria-targeted light-activatable drug delivery system offering real-time fluorescent monitoring of the release process [58].
PPGs allow the design of more elaborate photorelease scenarios, with the liberation of several substrates. Namely, by applying a selection of PPGs activatable at different wavelengths, a sequential photorelease protocol could be devised (‘chromatic orthogonality’ [59,60]), as illustrated on Figure 9 with a wavelength-selective uncaging approach for oligonucleotides using four different PPGs [61].
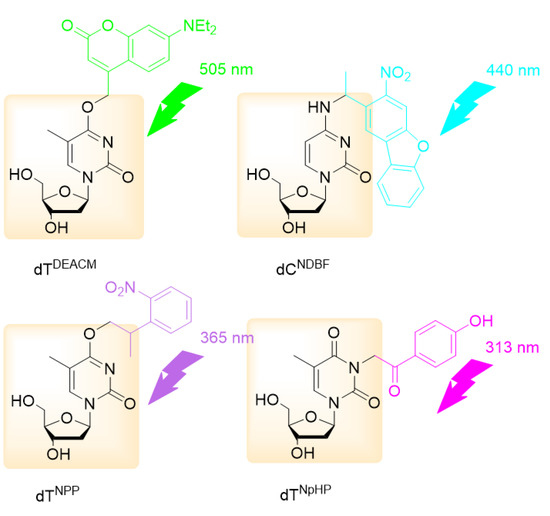
Figure 9.
Four layer wavelength-selective uncaging approach for oligonucleotides using four different PPGs: 7-diethylaminocoumarin-4-yl)methyl (DEACM), 1-(3-nitrodibenzofuran-1-yl)ethyl (NDBF), 2-(o-nitrophenyl)propyl (NPP) and p-hydroxyphenacyl (pHP) (adapted from Rodrigues-Correia et al. [61]).
PPGs could be used in the design of more complex, nanoparticle-based drug delivery systems as well. In their structure, a PPG could be integrated in different manners to orchestrate the targeting or the drug release [62]. To address targeting, a straightforward approach is to modify directly the targeting ligands (anchored typically on the surface of the nanoparticles) with PPGs to block their interactions with their target. Alternatively, targeting ligands could be anchored within nanoparticles using PPG units, their photolysis resulting in the surface exposure of the ligands. Integrating the PPGs into the structure of nanocarriers, the photolysis could lead to their dissociation and the consequent release of their payload. The dissociation could be the result of various processes, as hydrophobicity change or photocleavage at the block junction of block copolymers [62]. Of the potential nanocarrier systems, photodisruption of liposomes was studied by several groups, using various photocleavable synthetic amphiphilic lipids. Bayer et al. placed a 2-nitrobenzyl PPG into the sn-2 acyl chain of the naturally occurring phosphatidylcholine, the photolysis of which leads to a nonbilayer lipid (upon release of the succinimide linker) and consequent membrane destabilization [63]. An analogue system was reported, based on a NIR-sensitive coumarin PPG (Figure 10), better suited for in vivo applications [64].
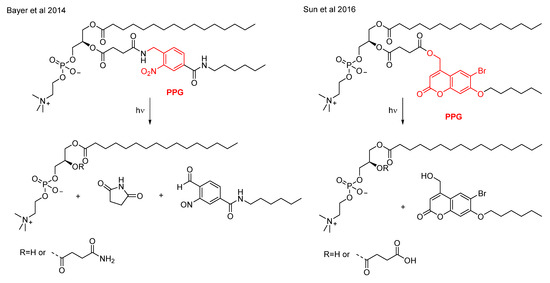
Figure 10.
Photocleavable amphihilic lipids for constructing photoresponsive liposomes [63,64].
3. Practical Issues and Future Directions
The previous section aimed to elucidate the essential features of PPGs, besides highlighting some of their interesting applications. Despite the growing number of elegant studies using PPGs, there are still several obstacles to overcome to be able to exploit their full potential even in therapeutic/clinical settings. To address the therapeutic potential of photoresponsive systems, Lerch et al. recently introduced the concept of ‘photodruggability’ [65]. As for other externally addressable systems, critical aspects encompass tissue penetration of the trigger, delivery to and retention at the active site of the light-responsive constructs (small molecules or nanocarriers), precise control of the activation signal under in vivo conditions, availability and complexity of the necessary instrumentation, availability of translational models and safety [66]. For addressing the tissue penetration issue (less relevant however for superficial locations, such as the skin or the eyes) in the case of PPGs, structural modifications leading to higher operational wavelengths [44], exploiting TP activation with specifically designed probes [51] or specific formulations/alternative light sources have been studied (e.g., upconversion nanoparticles [67] or Cherenkov-radiation initiated uncaging [68]). Alternatively, endoscopy or surgical interventions could be envisaged for targeting otherwise not accessible, deeper lying organs [65]. Regarding the instrumentation, the field of PPGs could build on systems developed for other light-driven approaches, such as sophisticated implantable devices for photoswitches [69,70] or light sources for photodynamic therapy [71], as well as the previously cited optical fibers or endoscopic setups. The availability of in vivo results is a major bottleneck of the field at present. Many studies discussing novel PPGs are focusing on the organic synthesis and the photophysical-photochemical properties of the novel probes and are not going beyond proof of concept studies. Although not yet imminent, the regulation and approval for therapy of PPG-substrate constructs would need further consideration and experimental (safety) data vs. conventional small-molecule drugs. However, given the well-established application of PPGs particularly in neurophysiological studies and their potential for diverse research areas (e.g., materials science), a further expansion and diversification of the field could be expected, offering novel chemical probes with improved properties and innovative systems for addressing research and clinical needs.
Funding
This research was funded by the National Research, Development and Innovation Office (NKFIH) grant number SNN 135825 and by the ÚNKP-20-5 New National Excellence Program of the Ministry of Innovation and Technology. P.D. is a recipient of the János Bolyai Research Scholarship of the Hungarian Academy of Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H.M.N. Endogenous and Exogenous Stimuli-Responsive Drug Delivery Systems for Programmed Site-Specific Release. Molecules 2019, 24, 1117. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, D.C.F.; Amoah, E.; Rogers, C.; Pearson, A.R. Using photocaging for fast time-resolved structural biology studies. Acta Crystallogr. Sect. D Struct. Biol. 2021, 77, 1218–1232. [Google Scholar] [CrossRef] [PubMed]
- Ellis-Davies, G.C.R. Caged compounds: Photorelease technology for control of cellular chemistry and physiology. Nat. Methods 2007, 4, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Klán, P.; Solomek, T.; Bochet, C.G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2013, 113, 119–191. [Google Scholar] [CrossRef]
- Szymański, W.; Beierle, J.M.; Kistemaker, H.A.V.; Velema, W.A.; Feringa, B.L. Reversible Photocontrol of Biological Systems by the Incorporation of Molecular Photoswitches. Chem. Rev. 2013, 113, 6114–6178. [Google Scholar] [CrossRef]
- Hüll, K.; Morstein, J.; Trauner, D. In Vivo Photopharmacology. Chem. Rev. 2018, 118, 10710–10747. [Google Scholar] [CrossRef]
- Fuchter, M.J. On the Promise of Photopharmacology Using Photoswitches: A Medicinal Chemist’s Perspective. J. Med. Chem. 2020, 63, 11436–11447. [Google Scholar] [CrossRef]
- Korzycka, K.A.; Bennett, P.M.; Cueto-Diaz, E.J.; Wicks, G.; Drobizhev, M.; Blanchard-Desce, M.; Rebane, A.; Anderson, H.L. Two-photon sensitive protecting groups operating via intramolecular electron transfer: Uncaging of GABA and tryptophan. Chem. Sci. 2015, 6, 2419–2426. [Google Scholar] [CrossRef]
- Piant, S.; Bolze, F.; Specht, A. Two-photon uncaging, from neuroscience to materials. Opt. Mater. Express 2016, 6, 1679. [Google Scholar] [CrossRef]
- Engels, J.; Schlaeger, E.J. Synthesis, structure, and reactivity of adenosine cyclic 3′,5′-phosphate-benzyltriesters. J. Med. Chem. 1977, 20, 907–911. [Google Scholar] [CrossRef]
- Kaplan, J.H.; Forbush, I.B.; Hoffman, J.F. Rapid photolytic release of adenosine 5′-triphosphate from a protected analog: Utilization by the sodium:potassium pump of human red blood cell ghosts. Biochemistry 1978, 17, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Barltrop, J.; Schofield, P. Photosensitive Protecting Groups. Tetrahedron Lett. 1962, 3, 697–699. [Google Scholar] [CrossRef]
- Hurevich, M.; Samarasimhareddy, M.; Alshanski, I.; Mervinetsky, E. Photodeprotection of up to Eight Photolabile Protecting Groups from a Single Glycan. Synlett 2018, 29, 880–884. [Google Scholar] [CrossRef]
- Kessler, M.; Glatthar, R.; Giese, B.; Bochet, C.G. Sequentially Photocleavable Protecting Groups in Solid-Phase Synthesis. Org. Lett. 2003, 5, 1179–1181. [Google Scholar] [CrossRef]
- Agarwal, H.K.; Janicek, R.; Chi, S.-H.; Perry, J.W.; Niggli, E.; Ellis-Davies, G.C.R. Calcium Uncaging with Visible Light. J. Am. Chem. Soc. 2016, 138, 3687–3693. [Google Scholar] [CrossRef]
- Basa, P.N.; Barr, C.A.; Oakley, K.M.; Liang, X.; Burdette, S.C. Zinc Photocages with Improved Photophysical Properties and Cell Permeability Imparted by Ternary Complex Formation. J. Am. Chem. Soc. 2019, 141, 12100–12108. [Google Scholar] [CrossRef]
- Venkatesh, Y.; Vangala, V.; Mengji, R.; Chaudhuri, A.; Bhattacharya, S.; Datta, P.K.; Banerjee, R.; Jana, A.; Singh, N.D.P. One- and Two-Photon Uncaging of Carbon Monoxide (CO) with Real-Time Monitoring: On-Demand Carbazole-Based Dual CO-Releasing Platform to Test over Single and Combinatorial Approaches for the Efficient Regression of Orthotopic Murine Melanoma In Vivo. J. Med. Chem. 2022, 65, 1822–1834. [Google Scholar] [CrossRef]
- Fraix, A.; Parisi, C.; Seggio, M.; Sortino, S. Nitric Oxide Photoreleasers with Fluorescent Reporting. Chem. Eur. J. 2021, 27, 12714–12725. [Google Scholar] [CrossRef]
- Štacko, P.; Muchová, L.; Vítek, L.; Klán, P. Visible to NIR Light Photoactivation of Hydrogen Sulfide for Biological Targeting. Org. Lett. 2018, 20, 4907–4911. [Google Scholar] [CrossRef]
- Li, W.-H.; Llopis, J.; Whitney, M.A.; Zlokarnik, G.; Tsien, R.Y. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature 1998, 392, 936–941. [Google Scholar] [CrossRef]
- Ellis-Davies, G.C.R. Useful Caged Compounds for Cell Physiology. Accounts Chem. Res. 2020, 53, 1593–1604. [Google Scholar] [CrossRef]
- Chiovini, B.; Pálfi, D.; Majoros, M.; Juhász, G.; Szalay, G.; Katona, G.; Szőri, M.; Frigyesi, O.; Haveland, L.C.; Szabó, G.; et al. Theoretical Design, Synthesis, and In Vitro Neurobiological Applications of a Highly Efficient Two-Photon Caged GABA Validated on an Epileptic Case. ACS Omega 2021, 6, 15029–15045. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.K.; Zhai, S.; Surmeier, D.J.; Ellis-Davies, G.C.R. Intracellular Uncaging of cGMP with Blue Light. ACS Chem. Neurosci. 2017, 8, 2139–2144. [Google Scholar] [CrossRef] [PubMed]
- Mangubat-Medina, A.E.; Ball, Z.T. Triggering biological processes: Methods and applications of photocaged peptides and proteins. Chem. Soc. Rev. 2021, 50, 10403–10421. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; Silva, E.; Reis, R.L. Light-triggered release of photocaged therapeutics—Where are we now? J. Control. Release 2019, 298, 154–176. [Google Scholar] [CrossRef]
- Shchelik, I.S.; Tomio, A.; Gademann, K. Design, Synthesis, and Biological Evaluation of Light-Activated Antibiotics. ACS Infect. Dis. 2021, 7, 681–692. [Google Scholar] [CrossRef]
- López-Cano, M.; Font, J.; Aso, E.; Sahlholm, K.; Cabré, G.; Giraldo, J.; De Koninck, Y.; Hernando, J.; Llebaria, A.; Fernández-Dueñas, V.; et al. Remote local photoactivation of morphine produces analgesia without opioid-related adverse effects. Br. J. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Dunkel, P.; Ilaš, J. Targeted Cancer Therapy Using Compounds Activated by Light. Cancers 2021, 13, 3237. [Google Scholar] [CrossRef]
- Wang, X.; Feng, M.; Xiao, L.; Tong, A.; Xiang, Y. Postsynthetic Modification of DNA Phosphodiester Backbone for Photocaged DNAzyme. ACS Chem. Biol. 2015, 11, 444–451. [Google Scholar] [CrossRef]
- Casey, J.P.; Blidner, R.A.; Monroe, W.T. Caged siRNAs for Spatiotemporal Control of Gene Silencing. Mol. Pharm. 2009, 6, 669–685. [Google Scholar] [CrossRef]
- Vaníková, Z.; Hocek, M. Polymerase Synthesis of Photocaged DNA Resistant against Cleavage by Restriction Endonucleases. Angew. Chem. Int. Ed. 2014, 53, 6734–6737. [Google Scholar] [CrossRef] [PubMed]
- Ellis-Davies, G.C.; Kaplan, J.H. Nitrophenyl-EGTA, a photolabile chelator that selectively binds Ca2+ with high affinity and releases it rapidly upon photolysis. Proc. Natl. Acad. Sci. USA 1994, 91, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Horbert, R.; Pinchuk, B.; Davies, P.; Alessi, D.; Peifer, C. Photoactivatable Prodrugs of Antimelanoma Agent Vemurafenib. ACS Chem. Biol. 2015, 10, 2099–2107. [Google Scholar] [CrossRef]
- Velema, W.A.; van der Berg, J.P.; Szymanski, W.; Driessen, A.J.M.; Feringa, B.L. Orthogonal Control of Antibacterial Activity with Light. ACS Chem. Biol. 2014, 9, 1969–1974. [Google Scholar] [CrossRef]
- Ellis-Davies, G.C. A chemist and biologist talk to each other about caged neurotransmitters. Beilstein J. Org. Chem. 2013, 9, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.A.; Wijesooriya, C.; Gehrmann, E.J.; Mahoney, K.M.; Goswami, P.P.; Albright, T.R.; Syed, A.; Dutton, A.S.; Smith, E.A.; Winter, A.H. Family of BODIPY Photocages Cleaved by Single Photons of Visible/Near-Infrared Light. J. Am. Chem. Soc. 2018, 140, 7343–7346. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Martin, S.F. Design, Synthesis and Evaluation of Novel Carbazole-Derived Photocages. Chem. Eur. J. 2022, 28, e202200311. [Google Scholar] [CrossRef]
- Lester, H.A.; Nerbonne, J.M. Physiological and Pharmacological Manipulations with Light Flashes. Annu. Rev. Biophys. Bioeng. 1982, 11, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Pauff, S.M.; Miller, S.C. A Trifluoroacetic Acid-labile Sulfonate Protecting Group and Its Use in the Synthesis of a Near-IR Fluorophore. J. Org. Chem. 2012, 78, 711–716. [Google Scholar] [CrossRef][Green Version]
- McMillan, T.J.; Leatherman, E.; Ridley, A.; Shorrocks, J.; Tobi, S.E.; Whiteside, J.R. Cellular effects of long wavelength UV light (UVA) in mammalian cells. J. Pharm. Pharmacol. 2008, 60, 969–976. [Google Scholar] [CrossRef]
- Cheong, W.-F.; Prahl, S.A.; Welch, A.J. A review of the optical properties of biological tissues. IEEE J. Quantum Electron. 1990, 26, 2166–2185. [Google Scholar] [CrossRef]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Gorka, A.P.; Nani, R.R.; Zhu, J.; Mackem, S.; Schnermann, M.J. A Near-IR Uncaging Strategy Based on Cyanine Photochemistry. J. Am. Chem. Soc. 2014, 136, 14153–14159. [Google Scholar] [CrossRef] [PubMed]
- Josa-Culleré, L.; Llebaria, A. In the Search for Photocages Cleavable with Visible Light: An Overview of Recent Advances and Chemical Strategies. ChemPhotoChem 2020, 5, 296–314. [Google Scholar] [CrossRef]
- Amatrudo, J.M.; Olson, J.P.; Lur, G.; Chiu, C.Q.; Higley, M.J.; Ellis-Davies, G.C.R. Wavelength-Selective One- and Two-Photon Uncaging of GABA. ACS Chem. Neurosci. 2013, 5, 64–70. [Google Scholar] [CrossRef]
- Klausen, M.; Dubois, V.; Clermont, G.; Tonnelé, C.; Castet, F.; Blanchard-Desce, M. Dual-wavelength efficient two-photon photorelease of glycine by π-extended dipolar coumarins. Chem. Sci. 2019, 10, 4209–4219. [Google Scholar] [CrossRef]
- Lin, Q.; Yang, L.; Wang, Z.; Hua, Y.; Zhang, D.; Bao, B.; Bao, C.; Gong, X.; Zhu, L. Coumarin Photocaging Groups Modified with an Electron-Rich Styryl Moiety at the 3-Position: Long-Wavelength Excitation, Rapid Photolysis, and Photobleaching. Angew. Chem. Int. Ed. 2018, 57, 3722–3726. [Google Scholar] [CrossRef]
- Bojtár, M.; Kormos, A.; Kis-Petik, K.; Kellermayer, M.; Kele, P. Green-Light Activatable, Water-Soluble Red-Shifted Coumarin Photocages. Org. Lett. 2019, 21, 9410–9414. [Google Scholar] [CrossRef]
- Furuta, T.; Wang, S.S.-H.; Dantzker, J.L.; Dore, T.M.; Bybee, W.J.; Callaway, E.M.; Denk, W.; Tsien, R.Y. Brominated 7-hydroxycoumarin-4-ylmethyls: Photolabile protecting groups with biologically useful cross-sections for two photon photolysis. Proc. Natl. Acad. Sci. USA 1999, 96, 1193–1200. [Google Scholar] [CrossRef]
- Eckardt, T.; Hagen, V.; Schade, B.; Schmidt, R.; Schweitzer, C.; Bendig, J. Deactivation Behavior and Excited-State Properties of (Coumarin-4-yl)methyl Derivatives. 2. Photocleavage of Selected (Coumarin-4-yl)methyl-Caged Adenosine Cyclic 3‘,5‘-Monophosphates with Fluorescence Enhancement. J. Org. Chem. 2002, 67, 703–710. [Google Scholar] [CrossRef]
- Klausen, M.; Blanchard-Desce, M. Two-photon uncaging of bioactive compounds: Starter guide to an efficient IR light switch. J. Photochem. Photobiol. C Photochem. Rev. 2021, 48, 100423. [Google Scholar] [CrossRef]
- Specht, A.; Bolze, F.; Nicoud, J.F.; Goeldner, M. Characterization of One- and Two-Photon Photochemical Uncaging Efficiency. Methods Mol. Biol. 2013, 995, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Bort, G.; Gallavardin, T.; Ogden, D.; Dalko, P.I. From One-Photon to Two-Photon Probes: “Caged” Compounds, Actuators, and Photoswitches. Angew. Chem. Int. Ed. 2013, 52, 4526–4537. [Google Scholar] [CrossRef]
- Klausen, M.; Dubois, V.; Verlhac, J.; Blanchard-Desce, M. Tandem Systems for Two-Photon Uncaging of Bioactive Molecules. ChemPlusChem 2019, 84, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Diaz, E.J.C.; Picard, S.; Klausen, M.; Hugues, V.; Pagano, P.; Genin, E.; Blanchard-Desce, M. Cooperative Veratryle and Nitroindoline Cages for Two-Photon Uncaging in the NIR. Chem. Eur. J. 2016, 22, 10848–10859. [Google Scholar] [CrossRef] [PubMed]
- Dcona, M.; Mitra, D.; Goehe, R.W.; Gewirtz, D.A.; Lebman, D.A.; Hartman, M.C.T. Photocaged permeability: A new strategy for controlled drug release. Chem. Commun. 2012, 48, 4755–4757. [Google Scholar] [CrossRef]
- Liu, P.; Li, B.; Zhan, C.; Zeng, F.; Wu, S. A two-photon-activated prodrug for therapy and drug release monitoring. J. Mater. Chem. B 2017, 5, 7538–7546. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, A.; Prasad, P.; Sah, R.K.; Singh, A.; Kumar, S.; Singh, S.; Gupta, S.; Sasmal, P.K. Mitochondria-Targeted Photoactivatable Real-Time Monitoring of a Controlled Drug Delivery Platform. J. Med. Chem. 2021, 64, 17813–17823. [Google Scholar] [CrossRef]
- Bochet, C.G. Two Decades of Chromatic Orthogonality. Isr. J. Chem. 2021, 61, 486–495. [Google Scholar] [CrossRef]
- Hansen, M.J.; Velema, W.A.; Lerch, M.M.; Szymanski, W.; Feringa, B.L. Wavelength-selective cleavage of photoprotecting groups: Strategies and applications in dynamic systems. Chem. Soc. Rev. 2015, 44, 3358–3377. [Google Scholar] [CrossRef]
- Rodrigues-Correia, A.; Weyel, X.M.M.; Heckel, A. Four Levels of Wavelength-Selective Uncaging for Oligonucleotides. Org. Lett. 2013, 15, 5500–5503. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kang, W.; Wang, W. Photocleavage-based Photoresponsive Drug Delivery. Photochem. Photobiol. 2021, 98, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.M.; Alam, S.; Mattern-Schain, S.I.; Best, M.D. Triggered Liposomal Release through a Synthetic Phosphatidylcholine Analogue Bearing a Photocleavable Moiety Embedded within thesn-2 Acyl Chain. Chem. Eur. J. 2014, 20, 3350–3357. [Google Scholar] [CrossRef]
- Sun, Y.; Ji, Y.; Yu, H.; Wang, D.; Cao, M.; Wang, J. Near-infrared light-sensitive liposomes for controlled release. RSC Adv. 2016, 6, 81245–81249. [Google Scholar] [CrossRef]
- Lerch, M.M.; Hansen, M.J.; Van Dam, G.M.; Szymanski, W.; Feringa, B.L. Emerging Targets in Photopharmacology. Angew. Chem. Int. Ed. 2016, 55, 10978–10999. [Google Scholar] [CrossRef]
- Said, S.S.; Campbell, S.; Hoare, T. Externally Addressable Smart Drug Delivery Vehicles: Current Technologies and Future Directions. Chem. Mater. 2019, 31, 4971–4989. [Google Scholar] [CrossRef]
- Yang, Y.; Shao, Q.; Deng, R.; Wang, C.; Teng, X.; Cheng, K.; Cheng, Z.; Huang, L.; Liu, Z.; Liu, X.; et al. In Vitro and In Vivo Uncaging and Bioluminescence Imaging by Using Photocaged Upconversion Nanoparticles. Angew. Chem. Int. Ed. 2012, 51, 3125–3129. [Google Scholar] [CrossRef]
- Ran, C.; Zhang, Z.; Hooker, J.; Moore, A. In Vivo Photoactivation Without “Light”: Use of Cherenkov Radiation to Overcome the Penetration Limit of Light. Mol. Imaging Biol. 2012, 14, 156–162. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, M.; Vázquez-Guardado, A.; Wegener, A.J.; Grajales-Reyes, J.G.; Deng, Y.; Wang, T.; Avila, R.; Moreno, J.A.; Minkowicz, S.; et al. Wireless multilateral devices for optogenetic studies of individual and social behaviors. Nat. Neurosci. 2021, 24, 1035–1045. [Google Scholar] [CrossRef]
- Park, S.I.; Brenner, D.S.; Shin, G.; Morgan, C.D.; Copits, B.A.; Chung, H.U.; Pullen, M.Y.; Noh, K.N.; Davidson, S.; Oh, S.J.; et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat. Biotechnol. 2015, 33, 1280–1286. [Google Scholar] [CrossRef]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in Photosensitizers and Light Delivery for Photodynamic Therapy. Clin. Endosc. 2013, 46, 7–23. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).