Dual-Modular Stems for Primary Total Hip Arthroplasty
Definition
:1. History
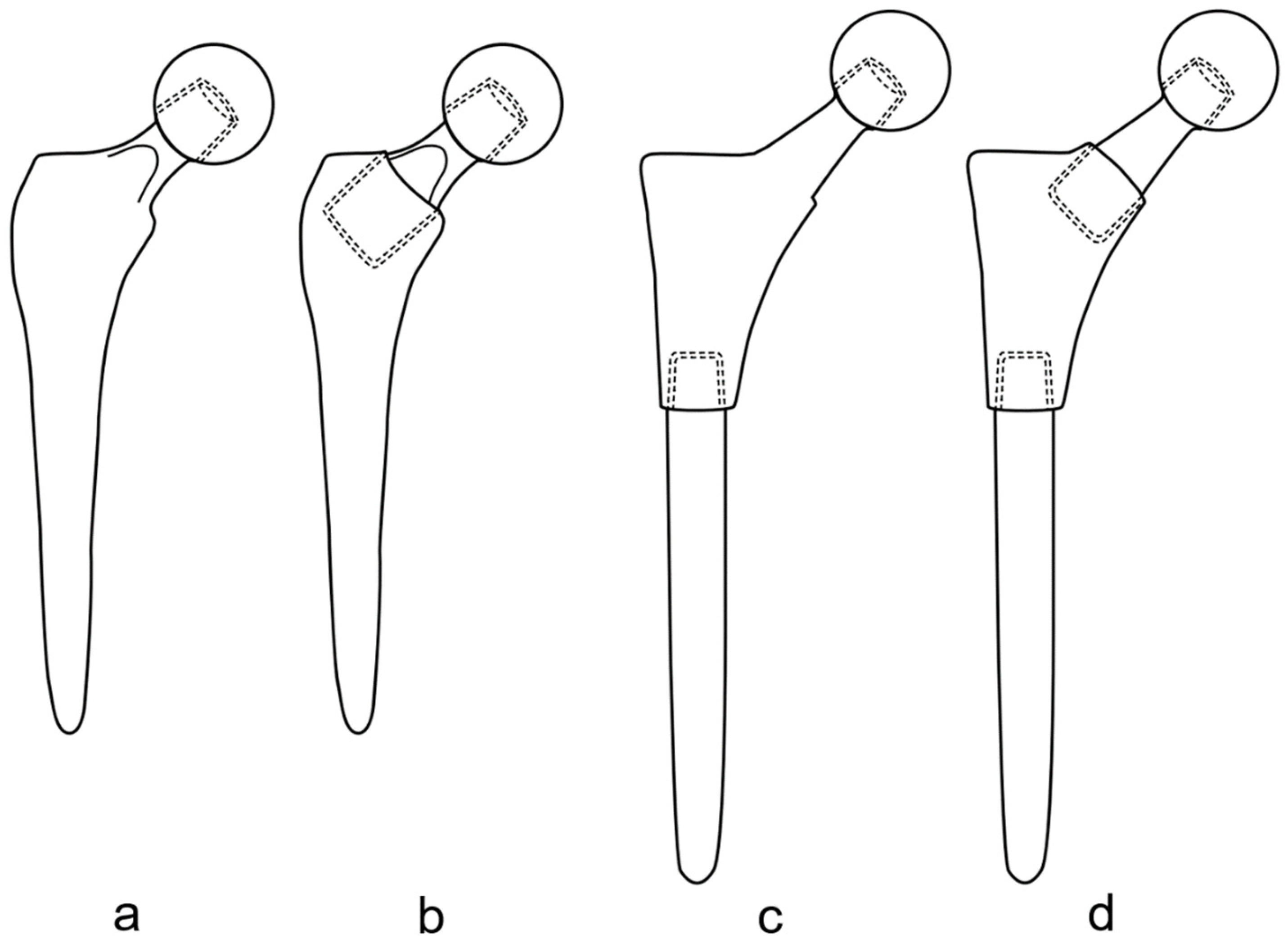
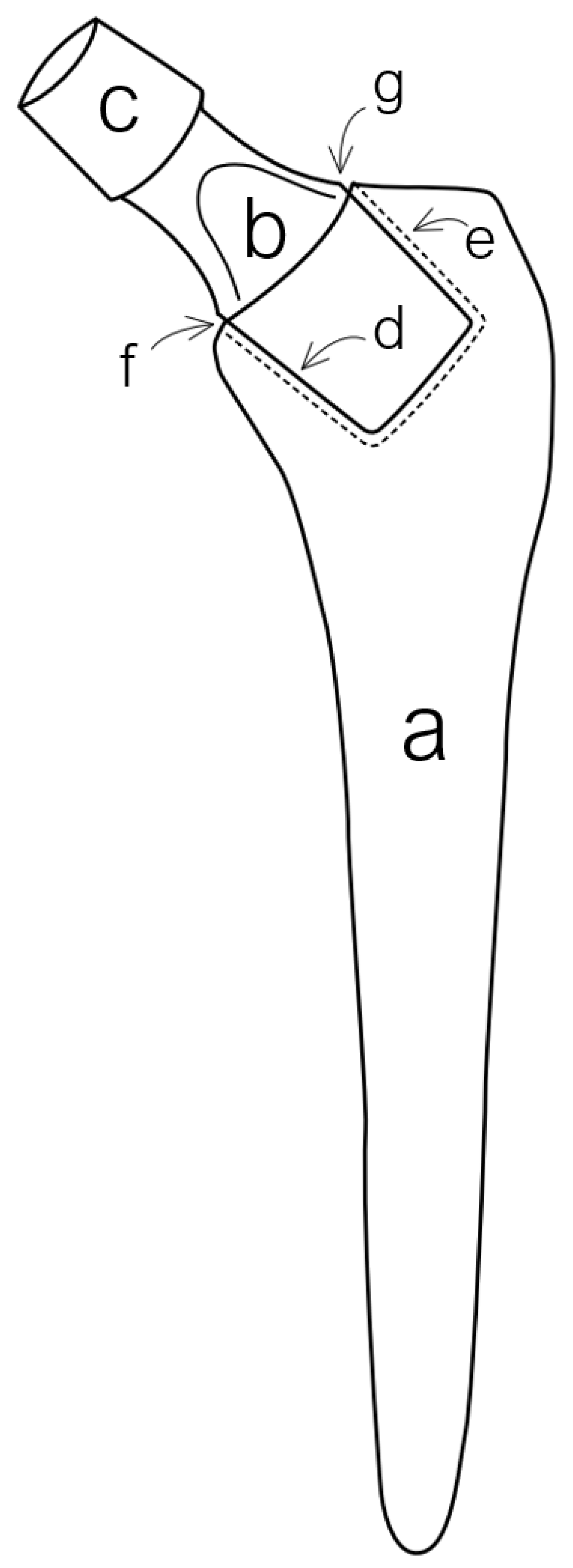
2. Types and Variations of Dual-Modular Implants
3. Examination and Diagnostics
- Ischaemic or trophic skin changes, scars, sinuses;
- Swelling/mass (lipoma, trauma, tumour, infection, hernia);
- Muscle atrophy (especially gluteal) or hypertrophy.
- Deformity (leg length discrepancy, pes cavus, scoliosis), position and degree of rotation of the leg [17].
4. Indications
4.1. Indications for Primary THA in General
4.2. Indications for Using Dual-Modular Stem
5. Surgical Technique
Primary Implantation
6. Risks with THA in General
- Deep vein thrombosis (DVT) [28];
- Pain;
- Stiffness;
- Luxation of artificial joint [29];
- Nerve damage [30];
- Bone damage;
- Bleeding [31];
- Periprosthetic fractures (intra-, postoperative) [32];
- Implant fracture;
- Myositis ossificans;
- Polyethylene liner wear;
- Infection;
- Aseptic loosening;
- Pulmonary embolism;
- Loss of limb;
- Death [32].
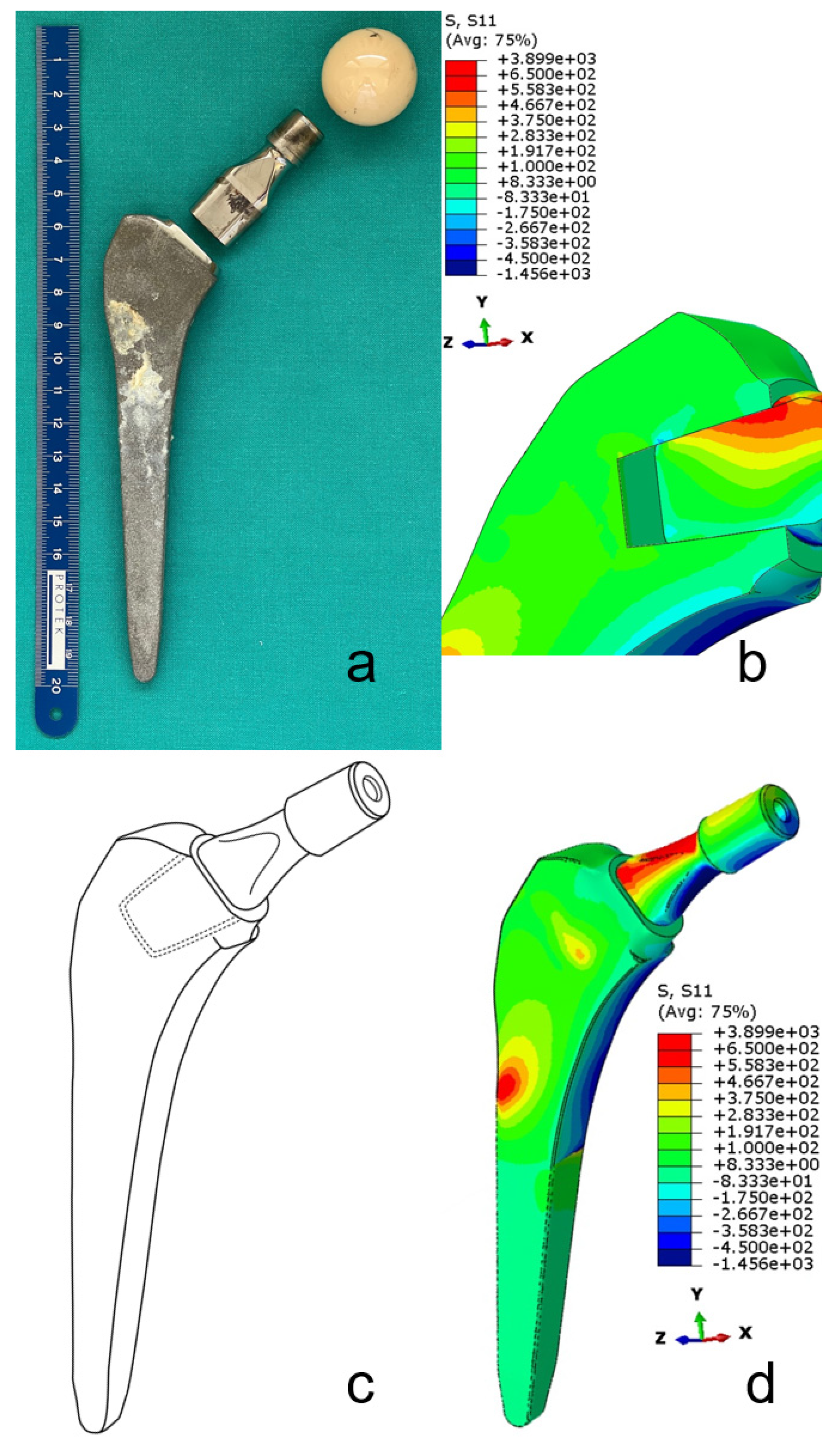
7. Complications with Dual-Modular Implants
7.1. Classification of Complications
7.1.1. Mechanical Complications
7.1.2. Inflammatory Complications
7.2. Diagnostics of Complications
- Focused patient history;
- Detailed physical examination;
- Serum metal ion levels;
- Serum inflammatory markers levels;
- Radiograph and cross-sectional imaging data;
7.2.1. Mechanical
7.2.2. Inflammatory
7.3. Therapeutic Approach to Complications
Revision
8. Alternatives to Dual-Modular Implants
8.1. "Monoblock” Systems with a Wide Range of Options
8.2. 3D CAD Scan and Additive Manufacturing
8.3. Evolving Modular Systems
9. Patient Rehabilitation
- Painless motion on the operated side;
- Independent patient mobility without any gait dysfunction;
- Functional patient independence with daily activities.
9.1. Pre-Operative Procedures
9.2. Post-Operative Procedures
9.2.1. Rehabilitation after Primary THA
- The patient positioning properly in bed;
- Respiratory exercises;
- Learning to sit- and stand-up;
- Kinesiotherapy;
- Learning to walk with a technical aid;
- Learning to climb the stairs;
- Learning how to perform daily activities;
- Measuring Range of motion and limb length (consider need for leg raise);
- Learning of precautionary measures [107].
9.2.2. Rehabilitation after Revision Surgery
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Traina, F.; De Clerico, M.; Biondi, F.; Pilla, F.; Tassinari, E.; Toni, T. Sex Differences in Hip Morphology: Is Stem Modularity Effective for Total Hip Replacement? J. Bone Jt. Surg. Ser. A 2009, 91 (Suppl. S6), 121–128. [Google Scholar] [CrossRef] [PubMed]
- Vierra, B.M.; Blumenthal, S.R.; Amanatullah, D.F. Modularity in Total Hip Arthroplasty: Benefits, Risks, Mechanisms, Diagnosis, and Management. Orthopedics 2017, 40, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Viceconti, M.; Baleani, M.; Squarzoni, S.; Toni, A. Fretting Wear in a Modular Neck Hip Prosthesis. J. Biomed. Mater. Res. 1997, 35, 207–216. [Google Scholar] [CrossRef]
- Traina, F.; De Fine, M.; Tassinari, E.; Sudanese, A.; Calderoni, P.P.; Toni, A. Modular neck prostheses in DDH patients: 11-year results. J. Orthop. Sci. 2011, 16, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Toni, A.; Giardina, F.; Guerra, G.; Sudanese, A.; Montalti, M.; Stea, S.; Bordini, B. 3rd Generation Alumina-on-Alumina in Modular Hip Prosthesis: 13 to 18 Years Follow-up Results. HIP Int. 2017, 27, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Zajc, J.; Moličnik, A.; Fokter, S. Dual Modular Titanium Alloy Femoral Stem Failure Mechanisms and Suggested Clinical Approaches. Materials 2021, 14, 3078. [Google Scholar] [CrossRef]
- Weiser, M.C.; Chen, D.D. Revision for taper corrosion at the neck-body junction following total hip arthroplasty: Pearls and pitfalls. Curr. Rev. Musculoskelet. Med. 2016, 9, 75–83. [Google Scholar] [CrossRef]
- Wright, C.G.; Sporer, S.; Urban, R.; Jacobs, J. Fracture of a Modular Femoral Neck After Total Hip Arthroplasty: A Case Report. J. Bone Jt. Surg. 2010, 92, 1518–1521. [Google Scholar] [CrossRef]
- Vucajnik, I.; Fokter, S.K. Modular Femoral Neck Fracture After Total Hip Arthroplasty. In Recent Advances in Hip and Knee Arthroplasty; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Grupp, T.M.; Weik, T.; Bloemer, W.; Knaebel, H.-P. Modular titanium alloy neck adapter failures in hip replacement—Failure mode analysis and influence of implant material. BMC Musculoskelet. Disord. 2010, 11, 3. [Google Scholar] [CrossRef]
- Meftah, M.; Haleem, A.M.; Burn, M.B.; Smith, K.M.; Incavo, S.J. Early Corrosion-Related Failure of the Rejuvenate Modular Total Hip Replacement. J. Bone Jt. Surg. Am. 2014, 96, 481–487. [Google Scholar] [CrossRef]
- Bernstein, D.T.; Meftah, M.; Paranilam, J.; Incavo, S.J. Eighty-six Percent Failure Rate of a Modular-Neck Femoral Stem Design at 3 to 5 Years: Lessons Learned. J. Bone Jt. Surg. 2016, 98, e49. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, A.; Jansson, V.; Melsheimer, O.; Steinbrück, A. The German Arthroplasty Registry Annual Report 2019; EPRD Deutsche Endoprothesenregister gGmbH: Berlin, Germany, 2020. [Google Scholar] [CrossRef]
- Mertl, P.; Dehl, M. Femoral stem modularity. Orthop. Traumatol. Surg. Res. 2020, 106, S35–S42. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-W.; Lim, S.-J.; Park, Y.-S. Modular Stems: Advantages and Current Role in Primary Total Hip Arthroplasty. Hip Pelvis 2018, 30, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.; Krishnan, S.P.; Blunn, G.; Skinner, J.A.; Hart, A.J. Modular neck femoral stems. Bone Jt. J. 2013, 95, 1011–1021. [Google Scholar] [CrossRef]
- Bowden, G.; McNally, M.A.; Thomas, S.R.Y.W.; Gibson, A. Oxford Handbook of Orthopaedics and Trauma. Oxford Handb. Orthop. Trauma 2010, 1, 16–17. [Google Scholar] [CrossRef]
- Zagra, L.; Benazzo, F.; Dallari, D.; Falez, F.; Solarino, G.; D’Apolito, R.; Castelli, C.C. Current concepts in hip–spine relationships: Making them practical for total hip arthroplasty. EFORT Open Rev. 2022, 7, 59–69. [Google Scholar] [CrossRef]
- Quintana, J.M.; Arostegui, I.; Azkarate, J.; Goenaga, J.I.; Elexpe, X.; Letona, J.; Arcelay, A. Evaluation of explicit criteria for total hip joint replacement. J. Clin. Epidemiol. 2000, 53, 1200–1208. [Google Scholar] [CrossRef]
- Gademan, M.G.J.; Hofstede, S.N.; Vlieland, T.P.M.V.; Nelissen, R.G.H.H.; De Mheen, P.J.M.-V. Indication criteria for total hip or knee arthroplasty in osteoarthritis: A state-of-the-science overview. BMC Musculoskelet. Disord. 2016, 17, 463. [Google Scholar] [CrossRef]
- Lucchini, S.; Castagnini, F.; Giardina, F.; Tentoni, F.; Masetti, C.; Tassinari, E.; Bordini, B.; Traina, F. Cementless ceramic-on-ceramic total hip arthroplasty in post-traumatic osteoarthritis after acetabular fracture: Long-term results. Arch. Orthop. Trauma. Surg. 2021, 141, 683–691. [Google Scholar] [CrossRef]
- Fokter, S.K.; Levašič, V.; Kovač, S. The Innovation Trap: Modular Neck in Total Hip Arthroplasty. ZdravVestn. 2017, 86, pp. 115–126. [CrossRef]
- Aljenaei, F.; Catelas, I.; Louati, H.; Beaulé, P.E.; Nganbe, M. Effects of hip implant modular neck material and assembly method on fatigue life and distraction force. J. Orthop. Res. 2017, 35, 2023–2030. [Google Scholar] [CrossRef]
- Jauch, S.Y.; Huber, G.; Hoenig, E.; Baxmann, M.; Grupp, T.M.; Morlock, M.M. Influence of material coupling and assembly condition on the magnitude of micromotion at the stem–neck interface of a modular hip endoprosthesis. J. Biomech. 2011, 44, 1747–1751. [Google Scholar] [CrossRef] [PubMed]
- Jauch, S.Y.; Huber, G.; Haschke, H.; Sellenschloh, K.; Morlock, M. Design parameters and the material coupling are decisive for the micromotion magnitude at the stem–neck interface of bi-modular hip implants. Med. Eng. Phys. 2014, 36, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Panagiotidou, A.; Cobb, T.; Meswania, J.; Skinner, J.; Hart, A.; Haddad, F.; Blunn, G. Effect of impact assembly on the interface deformation and fretting corrosion of modular hip tapers: An in vitro study. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2018, 36, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Frisch, N.B.; Lynch, J.R.; Banglmaier, R.F.; Silverton, C.D. The stability of dual-taper modular hip implants: A biomechanical analysis examining the effect of impact location on component stability. Arthroplast. Today 2017, 3, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Norris, S.L.; Schulman, S.; Hirsh, J.; Eckman, M.; Akl, E.A.; Crowther, M.; Vandvik, P.O.; Eikelboom, J.; McDonagh, M.S.; et al. Methodology for the Development of Antithrombotic Therapy and Prevention of Thrombosis Guidelines: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, 53S–70S. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Lau, E.C.; Ong, K.L.; Adler, E.M.; Kolisek, F.R.; Manley, M.T. Hospital, Patient, and Clinical Factors Influence 30- and 90-Day Readmission After Primary Total Hip Arthroplasty. J. Arthroplast. 2016, 31, 2130–2138. [Google Scholar] [CrossRef]
- Barrack, R.L. Neurovascular injury: Avoiding catastrophe. J. Arthroplast. 2004, 19, 104–107. [Google Scholar] [CrossRef]
- Wasielewski, R.C.; Cooperstein, L.A.; Kruger, M.P.; Rubash, H.E. Acetabular anatomy and the transacetabular fixation of screws in total hip arthroplasty. J. Bone Jt. Surg. Am. 1990, 72, 501–508. [Google Scholar] [CrossRef]
- Sandiford, N.A.; Mahendra, M.; Wickramarachchi, L.; Back, D.; Bansal, M. Informed Consent in Patients Undergoing Primary Hip and Knee Arthroplasty: What Do Patients Want to Know? Cureus 2020, 12, e8457. [Google Scholar] [CrossRef]
- Kop, A.M.; Swarts, E. Corrosion of a Hip Stem With a Modular Neck Taper Junction: A Retrieval Study of 16 Cases. J. Arthroplast. 2009, 24, 1019–1023. [Google Scholar] [CrossRef]
- Gilbert, J.L.; Buckley, C.A.; Jacobs, J.J. In Vivo Corrosion of Modular Hip Prosthesis Components in Mixed and Similar Metal Combinations. The Effect of Crevice, Stress, Motion, and Alloy Coupling. J. Biomed. Mater. Res. 1993, 27, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Atwood, S.A.; Patten, E.W.; Bozic, K.J.; Pruitt, L.A.; Ries, M.D. Corrosion-Induced Fracture of a Double-Modular Hip Prosthesis: A Case Report. J. Bone Jt. Surg. Ser. A 2010, 92, 1522–1525. [Google Scholar] [CrossRef]
- Ellman, M.B.; Levine, B.R. Fracture of the Modular Femoral Neck Component in Total Hip Arthroplasty. J. Arthroplast. 2013, 28, 196.e1–196.e5. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, G.; Graichen, F.; Rohlmann, A. Hip joint loading during walking and running, measured in two patients. J. Biomech. 1993, 26, 969–990. [Google Scholar] [CrossRef]
- Bergmann, G.; Graichen, F.; Rohlmann, A.; Bender, A.; Heinlein, B.; Duda, G.N.; Heller, M.O.; Morlock, M.M. Realistic loads for testing hip implants. Bio-Med. Mater. Eng. 2010, 20, 65–75. [Google Scholar] [CrossRef]
- Zajc, J.; Predan, J.; Gubeljak, N.; Moličnik, A.; Fokter, S.K. Modular femoral neck failure after revision of a total hip arthroplasty: A finite element analysis. Eur. J. Orthop. Surg. Traumatol. 2019, 29, 717–723. [Google Scholar] [CrossRef]
- Keršič, M.; Dolinar, D.; Antolič, V.; Mavčič, B. Shear Force in the Femoral Neck Affects Clinical Outcome of Total Hip Arthroplasty. Acta Orthop. Belg. 2020, 86, 109–116. [Google Scholar]
- Mumme, T.; Müller-Rath, R.; Jakobi, N.; Weißkopf, M.; Dott, W.; Marx, R.; Wirtz, D.-C. In vitro serum levels of metal ions released from orthopaedic implants. Eur. J. Orthop. Surg. Traumatol. 2005, 15, 83–89. [Google Scholar] [CrossRef]
- Gilbert, J.L.; Mali, S.; Urban, R.M.; Silverton, C.D.; Jacobs, J. In vivo oxide-induced stress corrosion cracking of Ti-6Al-4V in a neck-stem modular taper: Emergent behavior in a new mechanism of in vivo corrosion. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 584–594. [Google Scholar] [CrossRef]
- Higgs, G.B.; Hanzlik, J.A.; MacDonald, D.W.; Gilbert, J.L.; Rimnac, C.M.; Kurtz, S.M. Is Increased Modularity Associated With Increased Fretting and Corrosion Damage in Metal-On-Metal Total Hip Arthroplasty Devices?: A Retrieval Study. J. Arthroplast. 2013, 28, 2–6. [Google Scholar] [CrossRef]
- Fokter, S.K.; Rudolf, R.; Moličnik, A. Titanium alloy femoral neck fracture—Clinical and metallurgical analysis in 6 cases. Acta Orthop. 2016, 87, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Pour, A.E.; Borden, R.; Murayama, T.; Groll-Brown, M.; Blaha, D.J. High Risk of Failure With Bimodular Femoral Components in THA. Clin. Orthop. Relat. Res. 2016, 474, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Kovač, S.; Mavčič, B.; Kotnik, M.; Levašič, V.; Sirše, M.; Fokter, S.K. What Factors Are Associated With Neck Fracture in One Commonly Used Bimodular THA Design? A Multicenter, Nationwide Study in Slovenia. Clin. Orthop. Relat. Res. 2019, 477, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Wodecki, P.; Sabbah, D.; Kermarrec, G.; Semaan, I. New type of hip arthroplasty failure related to modular femoral components: Breakage at the neck-stem junction. Orthop. Traumatol. Surg. Res. 2013, 99, 741–744. [Google Scholar] [CrossRef]
- Fokter, S.K.; Moličnik, A.; Kavalar, R.; Pelicon, P.; Rudolf, R.; Gubeljak, N. Why do some titanium-alloy total hip arthroplasty modular necks fail? J. Mech. Behav. Biomed. Mater. 2017, 69, 107–114. [Google Scholar] [CrossRef]
- Swiontkowski, M.; Resnick, L. Concern About Femoral Neck Fractures in Long-Necked Modular Implants. JBJS Case Connect. 2014, 4, e7. [Google Scholar] [CrossRef]
- Lex, J.R.; Welch, M.D.; See, A.; Edwards, T.C.; Stavropoulos, N.A.; Babis, G.C. Systematic review of primary total hip arthroplasty using titanium-titanium modular-neck prostheses: The true risk of revision. HIP Int. 2020, 31, 295–303. [Google Scholar] [CrossRef]
- Toni, A.; Castagnini, F.; Stea, S. Reproducing the Proximal Femur Anatomy: Modular Femoral Component. In Personalized Hip and Knee Joint Replacement; Springer: Cham, Switzerland, 2020; pp. 75–84. [Google Scholar] [CrossRef]
- Silverton, C.D.; Jacobs, J.; Devitt, J.W.; Cooper, H.J. Midterm Results of a Femoral Stem With a Modular Neck Design: Clinical Outcomes and Metal Ion Analysis. J. Arthroplast. 2014, 29, 1768–1773. [Google Scholar] [CrossRef]
- Lombardi, A.V. Case Studies in Management of THA Failure Secondary to Taper Corrosion, Modular Junctions and Metal-on-Metal Bearings. J. Arthroplast. 2014, 29, 663–667. [Google Scholar] [CrossRef]
- De Martino, I.; Assini, J.B.; Elpers, M.E.; Wright, T.M.; Westrich, G.H. Corrosion and Fretting of a Modular Hip System: A Retrieval Analysis of 60 Rejuvenate Stems. J. Arthroplast. 2015, 30, 1470–1475. [Google Scholar] [CrossRef]
- Buente, D.; Huber, G.; Bishop, N.; Morlock, M. Quantification of material loss from the neck piece taper junctions of a bimodular primary hip prosthesis. A retrieval study from 27 failed Rejuvenate bimodular hip arthroplasties. Bone Jt. J. 2015, 97, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Talmo, C.; Nandi, S. Titanium neck-titanium stem taper corrosion in a modular neck stem. Arthroplast. Today 2019, 5, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Su, S.L.; Koch, C.N.; Nguyen, T.M.; Burket, J.C.; Wright, T.M.; Westrich, G.H. Retrieval Analysis of Neck-Stem Coupling in Modular Hip Prostheses. J. Arthroplast. 2017, 32, 2301–2306. [Google Scholar] [CrossRef] [PubMed]
- Wittenberg, R.H.; Steffen, R. Comparative 5-Year Results of Short Hip Total Hip Arthroplasty with Ti- or CoCr-Neck Adapters. Orthopedics 2015, 38, S33–S39. [Google Scholar] [CrossRef]
- Pourzal, R.; Hall, D.; Ehrich, J.; McCarthy, S.M.; Mathew, M.T.; Jacobs, J.J.; Urban, R.M. Alloy Microstructure Dictates Corrosion Modes in THA Modular Junctions. Clin. Orthop. Relat. Res. 2017, 475, 3026–3043. [Google Scholar] [CrossRef]
- Lanzutti, A.; Andreatta, F.; Rossi, L.; Di Benedetto, P.; Causero, A.; Magnan, M.; Fedrizzi, L. Corrosion fatigue failure of a high carbon CoCrMo modular hip prosthesis: Failure analysis and electrochemical study. Eng. Fail. Anal. 2019, 105, 856–868. [Google Scholar] [CrossRef]
- Fokter, S.K.; Gubeljak, N.; Predan, J.; Sevšek, J.; Zajc, J.; Krajnc, Z. Bilateral neck fracture in bimodular femoral stem after primary total hip arthroplasty: A case report. BMC Musculoskelet. Disord. 2021, 22, 356. [Google Scholar] [CrossRef]
- Werner, S.D.; Bono, J.V.; Nandi, S.; Ward, D.M.; Talmo, C.T. Adverse Tissue Reactions in Modular Exchangeable Neck Implants: A Report of Two Cases. J. Arthroplast. 2013, 28, 543.e13–543.e15. [Google Scholar] [CrossRef]
- Hsu, A.R.; Gross, C.E.; Levine, B.R. Pseudotumor from modular neck corrosion after ceramic-on-polyethylene total hip arthroplasty. Am. J. Orthop. 2012, 41, 422–426. [Google Scholar]
- Cooper, H.; Urban, R.M.; Wixson, R.L.; Meneghini, R.; Jacobs, J.J. Adverse Local Tissue Reaction Arising from Corrosion at the Femoral Neck-Body Junction in a Dual-Taper Stem with a Cobalt-Chromium Modular Neck. J. Bone Jt. Surg. Ser. A 2013, 95, 865–872. [Google Scholar] [CrossRef]
- Ghanem, E.; Ward, D.M.; Robbins, C.E.; Nandi, S.; Bono, J.V.; Talmo, C. Corrosion and Adverse Local Tissue Reaction in One Type of Modular Neck Stem. J. Arthroplast. 2015, 30, 1787–1793. [Google Scholar] [CrossRef]
- Walsh, C.P.; Hubbard, J.C.; Nessler, J.P.; Markel, D.C. Revision of Recalled Modular Neck Rejuvenate and ABG Femoral Implants. J. Arthroplast. 2015, 30, 822–826. [Google Scholar] [CrossRef]
- Perino, G.; Ricciardi, B.F.; Jerabek, S.A.; Martignoni, G.; Wilner, G.; Maass, D.; Goldring, S.R.; Purdue, P.E. Implant based differences in adverse local tissue reaction in failed total hip arthroplasties: A morphological and immunohistochemical study. BMC Clin. Pathol. 2014, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Iwata, H.; Ezaki, A.; Ishida, H.; Sakata, K.; Matsuoka, H.; Sogou, E.; Nannno, K.; Kuroda, S.; Nakamura, S.; et al. Adverse Reaction to Metal Debris Following Modular Neck Stem Metal-on-Metal Total Hip Arthroplasty Accompanied by a Dual-Modular Component. Orthop. Proc. 2020, 102, 277–281. [Google Scholar]
- Gkagkalis, G.; Mettraux, P.; Omoumi, P.; Mischler, S.; Rüdiger, H. Adverse tissue reaction to corrosion at the neck-stem junction after modular primary total hip arthroplasty. Orthop. Traumatol. Surg. Res. 2015, 101, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Ricciardi, B.F.; Liu, Z.; von Ruhland, C.; Ward, M.; Lord, A.; Hughes, L.; Goldring, S.R.; Purdue, E.; Murray, D.; et al. Nano-analyses of wear particles from metal-on-metal and non-metal-on-metal dual modular neck hip arthroplasty. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Kolatat, K.; Perino, G.; Wilner, G.; Kaplowitz, E.; Ricciardi, B.F.; Boettner, F.; Westrich, G.H.; Jerabek, S.A.; Goldring, S.R.; Purdue, P.E. Adverse local tissue reaction (ALTR) associated with corrosion products in metal-on-metal and dual modular neck total hip replacements is associated with upregulation of interferon gamma-mediated chemokine signaling. J. Orthop. Res. 2015, 33, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Della Valle, C.J.; Calkins, T.E.; Jacobs, J.J. Diagnosing Taper Corrosion: When Is It the Taper and When Is It Something Else? J. Arthroplast. 2018, 33, 2712–2715. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-M.; Fehring, T.K.; Lombardi, A.V.; Barnes, C.L.; Cabanela, M.E.; Jacobs, J. Risk Stratification Algorithm for Management of Patients with Dual Modular Taper Total Hip Arthroplasty: Consensus Statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons and the Hip Society. J. Arthroplast. 2014, 29, 2060–2064. [Google Scholar] [CrossRef]
- Pivec, R.; Meneghini, R.M.; Hozack, W.J.; Westrich, G.H.; Mont, M.A. Modular Taper Junction Corrosion and Failure: How to Approach a Recalled Total Hip Arthroplasty Implant. J. Arthroplast. 2014, 29, 1–6. [Google Scholar] [CrossRef]
- Kwon, Y.-M. Evaluation of the Painful Dual Taper Modular Neck Stem Total Hip Arthroplasty: Do They All Require Revision? J. Arthroplast. 2016, 31, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Vekaria, S.; Brander, V.; Hanses, M.; Stulberg, S.D. A Protocol to Asses for Adverse Local Tissue Reraction in Patients with Dual-Taper Femoral Stems. In Orthopaedic Proceedings. Bone Jt. J. 2018, 98, 30. [Google Scholar]
- Clohisy, J.; Haddad, F.S. The Hip Society 2020 Summer Meeting. In Orthopaedic Proceedings. Bone Jt. J. 2020, 102, 1. [Google Scholar]
- Laurençon, J.; Augsburger, M.; Faouzi, M.; Becce, F.; Hassani, H.; Rüdiger, H.A. Systemic Metal Ion Levels in Patients With Modular-Neck Stems: A Prospective Cohort Study. J. Arthroplast. 2016, 31, 1750–1755. [Google Scholar] [CrossRef]
- Barry, J.; Kiss, M.-O.; Massé, V.; Lavigne, M.; Matta, J.; Vendittoli, P.-A. Send Orders for Reprints to Reprints@benthamscience.Ae The Open Orthopaedics Journal Effect of Femoral Stem Modular Neck’s Material on Metal Ion Release. Open Orthop. J. 2017, 11, 1337–1344. [Google Scholar] [CrossRef]
- Rodrigues, D.C.; Urban, R.M.; Jacobs, J.J.; Gilbert, J.L. In Vivo Severe Corrosion and Hydrogen Embrittlement of Retrieved Modular Body Titanium Alloy Hip-Implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88B, 206–219. [Google Scholar] [CrossRef]
- Fokter, S.K.; Zajc, J.; Merc, M. Interchangeable neck failures of bi-modular femoral stems in primary total hip arthroplasty cannot be predicted from serum trace element analysis. Int. Orthop. 2020, 45, 877–881. [Google Scholar] [CrossRef]
- Reich, M.S.; Walker, R.H. Activity-related Changes in Cobalt Levels in a Total Hip with a Modular Femoral Stem: A Case Report. J. Orthop. Case Rep. 2018, 8, 44–47. [Google Scholar] [CrossRef]
- McGrory, B.J.; Payson, A.M.; MacKenzie, J.A. Elevated Intra-Articular Cobalt and Chromium Levels in Mechanically Assisted Crevice Corrosion in Metal-on-Polyethylene Total Hip Arthroplasty. J. Arthroplast. 2017, 32, 1654–1658. [Google Scholar] [CrossRef]
- Liow, M.H.L.; Urish, K.L.; Preffer, F.I.; Nielson, G.P.; Kwon, Y.-M. Metal Ion Levels Are Not Correlated With Histopathology of Adverse Local Tissue Reactions in Taper Corrosion of Total Hip Arthroplasty. J. Arthroplast. 2016, 31, 1797–1802. [Google Scholar] [CrossRef]
- Jacobs, J.J.; Cooper, H.J.; Urban, R.M.; Wixson, R.L.; Della Valle, C.J. What Do We Know About Taper Corrosion in Total Hip Arthroplasty? J. Arthroplast. 2014, 29, 668–669. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.J. The Local Effects of Metal Corrosion in Total Hip Arthroplasty. Orthop. Clin. N. Am. 2014, 45, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-M.; Khormaee, S.; Liow, M.H.L.; Tsai, T.-Y.; Freiberg, A.A.; Rubash, H.E. Asymptomatic Pseudotumors in Patients with Taper Corrosion of a Dual-Taper Modular Femoral Stem MARS-MRI and Metal Ion Study. J. Bone Jt. Surg. Am. 2016, 98, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-M.; Tsai, T.-Y.; Leone, W.A.; Liow, M.H.L. Sensitivity and Specificity of Metal Ion Levels in Predicting “Pseudotumors” due to Taper Corrosion in Patients With Dual Taper Modular Total Hip Arthroplasty. J. Arthroplast. 2017, 32, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.M.; Frisch, N.B.; Darrith, B.; Patel, I.; Silverton, C.D. Incidence of Pseudotumors in a Dual Modular Stem Construct With and Without Metal-on-Metal Bearing Surface. J. Am. Acad. Orthop. Surg. 2021, 29, e92–e97. [Google Scholar] [CrossRef]
- Mabry, T.M. Preventing Complications Associated With Operating on Taper Corrosion. J. Arthroplast. 2018, 33, 2720–2721. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, C.; Ross, D.; Restrepo, S.; Heller, S.; Goyal, N.; Moore, R.; Hozack, W.J. Adverse Clinical Outcomes in a Primary Modular Neck/Stem System. J. Arthroplast. 2014, 29, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Restrepo, C.; Nourie, B.; Restrepo, S.; Hozack, W.J. Patients With Modular-Neck Total Hip Arthroplasty: A Brief Five-Year Follow-Up Study. J. Arthroplast. 2020, 35, S268–S272. [Google Scholar] [CrossRef] [PubMed]
- Vendittoli, P.-A.; Massé, V.; Kiss, M.-O.; Lusignan, D.; Lavigne, M. Modular junction may be more problematic than bearing wear in metal-on-metal total hip arthroplasty. HIP Int. 2019, 29, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, A.C.; Nathani, A.; Weber, A.E.; Blaha, J.D. Femoral neck modularity: A bridge too far—Affirms. Semin. Arthroplast. 2014, 25, 93–98. [Google Scholar] [CrossRef]
- Kwon, Y.-M.; Antoci, V.; Eisemon, E.; Tsai, T.-Y.; Yan, Y.; Liow, M.H.L. “Top-Out” Removal of Well-Fixed Dual-Taper Femoral Stems: Surgical Technique and Radiographic Risk Factors. J. Arthroplast. 2016, 31, 2843–2849. [Google Scholar] [CrossRef]
- Laffosse, J.-M. Removal of well-fixed fixed femoral stems. Orthop. Traumatol. Surg. Res. 2016, 102, S177–S187. [Google Scholar] [CrossRef] [PubMed]
- Barlow, B.T.; Assini, J.; Boles, J.; Lee, Y.-Y.; Westrich, G.H. Short-Term Metal Ion Trends Following Removal of Recalled Modular Neck Femoral Stems. J. Arthroplast. 2015, 30, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Elmallah, R.K.; Cherian, J.J.; Meneghini, R.M.; Hozack, W.J.; Westrich, G.H.; Mont, M.A. How to Approach a Recalled Dual Modular Hip Implant: An Update. J. Arthroplast. 2016, 31, 2646–2652. [Google Scholar] [CrossRef] [PubMed]
- Scheerlinck, T.; De Winter, E.; Sas, A.; Kolk, S.; Van Gompel, G.; Vandemeulebroucke, J. Hip implants can restore anatomical and medialized rotation centres in most cases. Bone Jt. Open 2021, 2, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Castagnini, F.; Caternicchia, F.; Biondi, F.; Masetti, C.; Faldini, C.; Traina, F. Off-the-shelf 3D printed titanium cups in primary total hip arthroplasty. World J. Orthop. 2021, 12, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, M.; Small, I.; Restrepo, C.; Hozack, W. A New Additive-Manufactured Cementless Highly Porous Titanium Acetabular Cup for Primary Total Hip Arthroplasty—Early Two-Year Follow Up. Surg. Technol. Int. 2021, 38, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, O.; Darwish, S.; El-Hofy, H.; Saito, Y. Patient-specific design process and evaluation of a hip prosthesis femoral stem. Int. J. Artif. Organs 2019, 42, 271–290. [Google Scholar] [CrossRef]
- Grunert, R.; Schleifenbaum, S.; Möbius, R.; Kopper, M.; Rotsch, C.; Drossel, W.-G.; Hammer, N.; Prietzel, T. Novel concept of a modular hip implant could contribute to less implant failure in THA: A hypothesis. Patient Saf. Surg. 2018, 12, 1. [Google Scholar] [CrossRef]
- Skou, S.T.; Roos, E.M. Physical therapy for patients with knee and hip osteoarthritis: Supervised, active treatment is current best practice. Clin. Exp. Rheumatol. 2019, 37, 112–117. [Google Scholar]
- Goh, S.-L.; Persson, M.S.M.; Stocks, J.; Hou, Y.; Welton, N.J.; Lin, J.; Hall, M.C.; Doherty, M.; Zhang, W. Relative Efficacy of Different Exercises for Pain, Function, Performance and Quality of Life in Knee and Hip Osteoarthritis: Systematic Review and Network Meta-Analysis. Sports Med. 2019, 49, 743–761. [Google Scholar] [CrossRef] [PubMed]
- Juhakoski, R.; Tenhonen, S.; Malmivaara, A.; Kiviniemi, V.; Anttonen, T.; Arokoski, J.P. A pragmatic randomized controlled study of the effectiveness and cost consequences of exercise therapy in hip osteoarthritis. Clin. Rehabil. 2011, 25, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Bennell, K. Physiotherapy management of hip osteoarthritis. J. Physiother. 2013, 59, 145–157. [Google Scholar] [CrossRef]
- Beard, D.J.; Palan, J.; Andrew, J.G.; Nolan, J.; Murray, D.W. Incidence and effect of leg length discrepancy following total hip arthroplasty. Physiotherapy 2008, 94, 91–96. [Google Scholar] [CrossRef]
- Nouri, F.; Coole, C.; Baker, P.; Drummond, A. Return to driving after total hip and knee arthroplasty—The perspective of employed patients. Disabil. Rehabil. 2021, 1–7. [Google Scholar] [CrossRef]
- Rougereau, G.; Rabot, C.; de Thomasson, E.; Tourabaly, I.; Mazel, C.; Langlais, T.; Ollat, D. Sexual activity resumption after total hip arthroplasty: A satisfaction survey in 101 patients. Orthop. Traumatol. Surg. Res. 2022, 108, 103171. [Google Scholar] [CrossRef]
- Berry, D.J. Postoperative Care Following Revision Total Hip and Knee Arthroplasty. In Revision Total Hip and Knee Arthroplasty; Berry, D.J., Trousdale, R.T., Dennis, D.A., Paprosky, W.G., Eds.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; pp. 419–422. [Google Scholar]
- Hailer, N.P. The innovation trap. Acta Orthop. 2016, 87, 91–92. [Google Scholar] [CrossRef]
- Baleani, M.; Erani, P.; Acciaioli, A.; Toni, A. In Vitro Comparative Study of Fretting-Corrosion Resistance of Ti6Al4V and Co28Cr6Mo in a Taper Joint Subjected to High Bending Moment. Corrosion 2017, 73, 1520–1529. [Google Scholar] [CrossRef]
- Podlipec, R.; Punzón-Quijorna, E.; Pirker, L.; Kelemen, M.; Vavpetič, P.; Kavalar, R.; Hlawacek, G.; Štrancar, J.; Pelicon, P.; Fokter, S. Revealing Inflammatory Indications Induced by Titanium Alloy Wear Debris in Periprosthetic Tissue by Label-Free Correlative High-Resolution Ion, Electron and Optical Microspectroscopy. Materials 2021, 14, 3048. [Google Scholar] [CrossRef]

| Pain | Loss of Function | Suitability for THA |
|---|---|---|
| Severe | Severe | Suitable |
| Severe | Moderate | Suitable |
| Severe (after conservative treatment) | Mild | Suitable |
| Severe (with no conservative treatment) | Mild | Not suitable |
| Moderate | Severe | Suitable |
| Moderate | Moderate | Uncertain |
| Moderate | Mild | Not suitable |
| Mild | Severe (after conservative treatment) | Suitable |
| Mild | Severe (with no conservative treatment) | Suitable |
| Mild | Moderate | Not suitable |
| Mild | Mild | Not suitable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zajc, J.; Fokter, S.K. Dual-Modular Stems for Primary Total Hip Arthroplasty. Encyclopedia 2022, 2, 893-911. https://doi.org/10.3390/encyclopedia2020059
Zajc J, Fokter SK. Dual-Modular Stems for Primary Total Hip Arthroplasty. Encyclopedia. 2022; 2(2):893-911. https://doi.org/10.3390/encyclopedia2020059
Chicago/Turabian StyleZajc, Jan, and Samo Karel Fokter. 2022. "Dual-Modular Stems for Primary Total Hip Arthroplasty" Encyclopedia 2, no. 2: 893-911. https://doi.org/10.3390/encyclopedia2020059
APA StyleZajc, J., & Fokter, S. K. (2022). Dual-Modular Stems for Primary Total Hip Arthroplasty. Encyclopedia, 2(2), 893-911. https://doi.org/10.3390/encyclopedia2020059







